In 2018, a patient received capecitabine without prior testing for dihydropyrimidine dehydrogenase (DPYD) and later presented with vomiting, rash, and diarrhea. The hospital failed to provide uridine triacetate in a timely fashion, and the patient died. The patient's widow filed a wrongful death lawsuit against Oregon Health Sciences University (OHSU) and assisted in the formation of a nonprofit organization to advocate for DPYD testing for fluoropyrimidines. A settlement for $1 million US dollars was reached requiring OHSU oncologists to undergo education about DPYD testing and inform their patients about its availability.1 Clinical practice guidelines from the National Comprehensive Cancer Network (NCCN) and ASCO still do not support testing for DPYD genetic variants before fluoropyrimidine chemotherapy. The US Food and Drug Administration (FDA) package inserts for capecitabine and fluorouracil (FU) acknowledge patients with dihydropyrimidine dehydrogenase protein (DPD) deficiency have increased risk of life-threatening toxicity; however, instead of recommending preemptive testing, they posit an unlikely scenario in which patients who have known DPD deficiency should discuss it with their physicians.2,3 The European Medicines Agency, the French National Agency for the Safety of Medicines and Health Products, and the Medicines and Healthcare products Regulatory Agency have each approved guidelines for preemptive DPYD testing for patients treated with fluoropyrimidines.4
Like all genetic tests, levels of evidence vary for each allele, but sufficient data are now published in the literature to conclude that individuals harboring certain DPYD variants are at increased risk of toxicity or death when administered standard doses of fluoropyrimidines. The genetic basis for the association between slow FU metabolism, pharmacokinetics, and toxicity was established in 1988.5,6 In 1990, DPD activity was associated with FU plasma concentrations.7 DPYD*2A, exon skipping IVS14G>A variant (c.1905+1G>A, rs3918290), was identified in 1995 and associated with FU toxicity in 1996.8,9 A large number of studies are now published establishing the relationship between DPYD variants and fluoropyrimidine pharmacokinetics.10 This commentary will establish that pretreatment DPYD testing is well justified and recommend dose reduction in those patients with a decreased function variant. We recommend an immediate modification to the oncology treatment guidelines that include a fluoropyrimidine.
Severe Fluoropyrimidine-Associated Toxicity
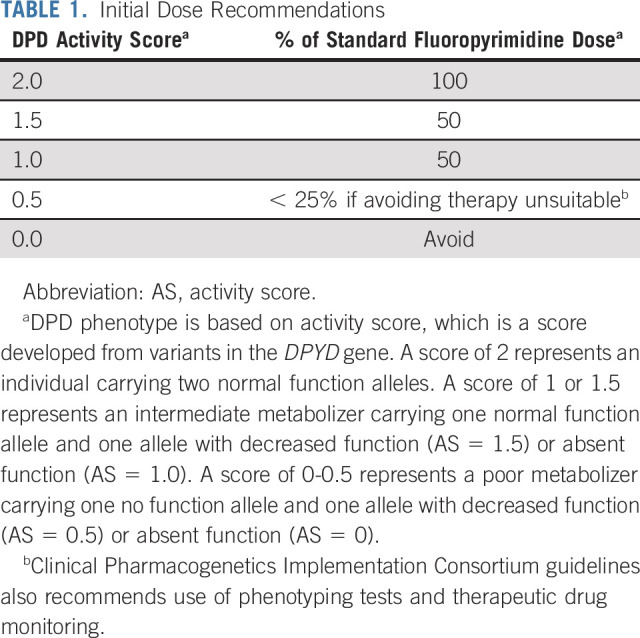
A recent meta-analysis of 13,929 patients in 35 studies found that patients carrying DPYD*2A were much more likely to experience severe life-threatening toxicity from fluoropyrimidine therapy than those carrying only wild-type alleles.11 The NCCN colon cancer guideline discusses some of these studies, and we agree with the view presented therein: “Pretreatment DPYD testing of all patients has the potential to identify the estimated 1%-2% of the population with truncating alleles that may herald an increased risk of severe toxicity.” However, the NCCN statement is not broad enough: Other DPYD variants have sufficient levels of evidence to justify testing (eg, c.1679T>G, rs55886062, DPYD*13; c.1129-5923C>G, rs75017182, DPYD HapB3; and c.2846A>T, rs67376798, p.D949V), raising the number of at-risk patients to approximately 9% of the US population.10 Table 1 shows the recommended initial dose based on DPYD genotype-predicted phenotype.10
TABLE 1.
Initial Dose Recommendations
Fluoropyrimidine Efficacy
A prospective DPYD genotype-guided dose reduction (53% dose intensity) study resulted in similar efficacy in 40 DPYD*2A carriers versus matched controls.12 Retrospective studies involving standard dosing found no relationship between DPYD SNPs and progression-free survival or overall survival in spite of a 50% dose reduction in DPYD*2A carriers.13 Seven more clinical studies examining DPYD polymorphisms with a dose reduction did not observe a difference in response, time to progression, progression-free survival, and/or overall survival.14 We were unable to find a study demonstrating a decrease in efficacy in patients with DPYD variants who were treated with a reduced dose. Thus, there is no evidence that a priori dose adjustments for DPYD carriers decreases fluoropyrimidine efficacy, and low-activity variant carriers treated with standard of care appear to have similar efficacy once an acceptable dose is found.
Pharmacokinetic Considerations
One study prospectively recruited heterozygous DPYD*2A carriers (n = 8) and wild-type carriers (n = 5) and demonstrated a significant difference in terminal half-life (T1/2) between the two groups for single FU doses of 300 and 450 mg/m2 (mean T1/2 was 60% longer for those with a variant).15 A larger study found a statistically significant 1.5-fold and 1.3-fold higher area under the concentration versus time curve (AUC) in patients with a DPYD*2A variant receiving a single FU dose of 300 mg/m2 and 450 mg/m2, respectively.16 The mean FU clearance of DPYD*2A heterozygotes was 53% for controls.17 Two case reports are published in which a heterozygous DPYD*2A carrier had a 2.5-fold higher AUC0-3 hours and another had 66% lower FU clearance normalized for bioavailability than control patients.18,19 Two genotype-guided dosing studies found no difference in the AUCs of DPYD*2A variant carriers receiving reduced doses compared with wild-type control patients receiving standard doses.20,21 Thus, DPYD*2A carriers have greater exposure when provided standard dosing, and adjusting fluoropyrimidine dose on the basis of DPYD*2A genotype normalizes exposure across genotypic groups.
Practical Basis for a Study Involving Randomized Genotype-Guided Dosing
Although randomized clinical trial evidence is the gold standard for justifying clinical validity and clinical utility of genetic testing, obtaining such evidence is highly impractical, potentially delaying testing implementation for several years.
An ideal study design to prospectively validate DPYD genotyping before fluoropyrimidine administration would randomly assign a cohort to receive standard therapy despite a DPYD*2A, DPYD*13, HapB3, and D949V genotypes, which presents ethical and legal concerns because physicians may be obligated to act on this information to avoid severe toxicity in their patients. Another design could randomly assign patients to a nongenotyped cohort versus a genotyped cohort, with the genotyped cohort then receiving treatment with standard or reduced fluoropyrimidine dosing depending on genotype. Such a study likely would suffer from difficulty recruiting. Given these constraints, it is doubtful whether a randomized genotype-directed study will ever be conducted.
NCCN board members acknowledge the question of whether genotype testing should be implemented as standard of care is probably impossible to answer with traditional randomized studies, and they suggest a real-world study would be sufficient.22 Yet, real-world studies published for the past 27 years in the scientific literature consistently demonstrate the relationship between DPYD variant carriers and toxicity. The NCCN and ASCO guidelines should act on these data to mitigate the incidence of ongoing life-threatening toxicity in the United States.11
Position of the Group
The NCCN guideline for the treatment of colon cancer (Table 2) states that DPYD variant carriers have significant risk of life-threatening toxicity and that DPYD testing is a cost-effective method to reduce such toxicity. NCCN's primary objection to testing DPD activity involves uncertainty that every patient with low DPD activity is at risk and the degree to which DPYD variants confer such risk. In public commentary, NCCN board members state further studies are required to mitigate the possibility that dose reduction would reduce fluoropyrimidine efficacy in some patients.22 Current evidence suggests that dose adjustments do not alter efficacy; thus, a requirement for additional efficacy research should not supersede established concerns of unacceptable rates of life-threatening toxicity in DPYD low-activity variant carriers because the practice of medicine is guided by primum non nocere.
TABLE 2.
NCCN Guideline for DPYD Testing (version 1.2022—February 25, 2022)
Risk/benefit analysis includes integrating evidence and uncertainties within the context of unmet needs.23 Similar to most laboratory tests, DPYD testing has never been expected to provide certainty that a patient will develop drug toxicity; however, it does indicate a higher risk for severe or life-threatening toxicity that should be considered before treatment. The NCCN colorectal cancer guideline stipulates to the risk of life-threatening toxicity and then goes on to ignore it because “… it is not certain that every one of these patients is at risk.” If one were to demand 100% predictive value for every test involving selection of an appropriate cancer treatment, almost no individual test would meet this standard for use in clinical care. Other tumor type NCCN guidelines that use fluoropyrimidines as a treatment option fail to mention DPYD.
Evidence demonstrates that standard doses of FU and capecitabine are intolerable for most DPYD*2A carriers anyway.13,17,24-26 Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for dosing fluoropyrimidines in DPD intermediate or poor metabolizers explicitly recommend dose escalation as tolerated.10 We, therefore, believe DPYD genotyping should be recommended in the NCCN and ASCO guidelines for any patient diagnosed with cancer in which fluoropyrimidines are administered, provided testing does not interfere with clinical scenarios in which it does not add value (eg, the patient already tolerated a specific dose) or testing delays urgently needed therapy (eg, test results are available in 3-10 days).
Modification of these guidelines must be addressed immediately. OHSU originally denied fault in the wrongful death suit, justifying this claim by referring to national expert consensus.1 OHSU is clearly relying on guidelines set forth by NCCN and ASCO. Although the NCCN stance against routine DPYD genotyping may have been acceptable in the past, accumulating data regarding the strong association of DPYD gene variants with severe toxicity make that stance increasingly untenable and possibly leaves cancer centers vulnerable to claims of malpractice in cases of fatal toxicity. On the basis of a survey of 18.2% of US medical oncologists, DPYD testing is limited by a lack of guidelines or recommendations for dosing decisions,27 although some institutions are launching DPYD testing programs to avoid fluoropyrimidine toxicity.22 Updating the guidelines to promote testing would mitigate a significant amount of toxicity and would increase the likelihood that such toxicity would be readily identifiable.
Limitations
Several limitations are apparent.28 DPYD genotyping has a small up-front cost that will be applied to all patients while only benefiting the relatively small number of DPYD carriers. However, avoiding severe toxicity in the small population of DPYD variant carriers has been found to be ultimately cost-effective and possibly cost saving.20,29,30 Pharmacogenetic panel testing could spread the cost over multiple medications. Moreover, as genomic sequencing is performed more commonly in patients with cancer at diagnosis, incidental data on DPYD variants will be generated, and clinicians will be obligated to act on such data anyway. Treatment delays may occur because of the time taken to generate genotyping results.31 However, treatment delays and dose adjustments are already common in patients carrying DPYD variants who experience toxicity with standard dosing,13 and turnaround times for genetic sequencing are becoming increasingly more rapid. Preemptive pharmacogenetic testing also obviates this concern. Although dose reductions of anticancer agents are sometimes associated with reductions in efficacy, dose optimization strategies are commonly used in oncology to normalize systemic drug concentrations relative to a traditional patient while maintaining efficacy.32 In fact, a recent FDA initiative, Project Optimus, is designed to emphasize the selection of doses that maximize both efficacy and safety/tolerability of oncologics. To this end, the FDA should consider that evidence already shows accounting for DPYD genotype normalizes pharmacokinetics, toxicity, and outcome of fluoropyrimidines.33 The FDA has previously proposed a PGx Pyramid Framework and used this mechanism to assess the evidence for HLA/allopurinol, which eventually led to a package insert change to recommend HLA testing.34,35 Citizen petitions have also led to acknowledgment of the risk to DPYD variant carriers in the capecitabine and FU package insert, and other petitions are submitted to the FDA to include pretreatment testing.36,37 Thus, the FDA has several mechanisms in place to overcome barriers to recommending DPYD testing. It would also be reasonable to expect that avoiding fluoropyrimidine overdoses in variant carriers would reduce the use of highly expensive uridine triacetate and costly hospitalizations. Thus, we consider genotype-guided dosing to be dose optimization, not simple dose reduction. Finally, DPD activity is a function of several allelic variants in the gene, and the levels of evidence for these polymorphisms affecting fluoropyrimidine therapy vary. Although this limitation exists, there are those who are curating the strength of evidence for these alleles for public consumption.10 Opportunities to understand less common polymorphisms will be increasingly possible if pretreatment DPYD testing becomes standard practice.
Recommendations
We recommend that pretreatment DPYD variant testing should be incorporated immediately into the standard of care for fluoropyrimidine regimens. Since fluoropyrimidine pharmacokinetic exposure is higher in reduced-function DPYD variant carriers, starting at a reduced dose and titrating upward to avoid undue toxicity should be adequate to maximize benefit while reducing risk in patients carrying heterozygous genotypes. Homozygous patients are at unacceptably high risk of fatal toxicity, and fluoropyrimidine therapy should be avoided unless it is absolutely necessary, in which case < 25% of the dose should be administered with DPD phenotyping tests and therapeutic drug monitoring. These methods are already recommended in the CPIC guidelines, and high evidence variants are included therein.10 Thus, we recommend oncologists order testing before initiating fluoropyrimidine chemotherapy and follow the CPIC dosing guidelines, and the FDA should require updates to the package insert. We recommend that NCCN and ASCO treatment guidelines be modified to reflect the relationship between fluoropyrimidine and toxicity and that a priori testing should be adopted as the standard of care.
ACKNOWLEDGMENT
Dr Peter O'Donnell was funded to conduct an ongoing clinical trial examining the question of DPYD genotyping. Dr O'Donnell participated and was paid as a retained expert in the OHSU case surrounding DPD testing. He received honoraria for speaking on the subject of pharmacogenomics at CME and educational conferences. He is a cosignee of a citizen petition to the FDA regarding this question. Finally, Dr O'Donnell is a compensated member of the DSMB of the NIH IGNITE II Network, which sponsors pharmacogenomic clinical trials (although not related to DPYD).
Author order is listed alphabetically.
Sharyn D. Baker
Research Funding: SPD Oncology (Inst)
Susan E. Bates
Honoraria: Merck (I)
Consulting or Advisory Role: Pegascy, ElmediX, Servier, Akita Biomedical (I), Secura Bio (I), OnKure, Ipsen, Progenics (I)
Research Funding: Merck (Inst), Amgen (Inst), RenovoRx (Inst), Ipsen (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Patent: Deacetylase Inhibitor Therapy (Inst), Patent: Depsipeptide for Therapy of Kidney Cancer (Inst)
Gabriel A. Brooks
Consulting or Advisory Role: CareCentrix, UnitedHealthcare, Ipsen
Research Funding: Roche/Genentech (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/197685
William L. Dahut
Leadership: Dexcom (I)
Stock and Other Ownership Interests: Dexcom (I)
Wafik S. El-Deiry
Stock and Other Ownership Interests: Oncoceutics, p53-Therapeutics, Chimerix, SMURF-Therapeutics
Consulting or Advisory Role: Rain Therapeutics
Research Funding: D&D Pharmatech, Chimerix
Patents, Royalties, Other Intellectual Property: Patent on TIC10 (ONC201), Patents pending on the use of small molecules to target mutant p53, Patent on therapeutic targeting on hypoxia-inducible factors
Other Relationship: Caris Life Sciences
Uncompensated Relationships: Rain Therapeutics, Caris Life Sciences
William E. Evans
Stock and Other Ownership Interests: BioSkryb (I)
William D. Figg
Research Funding: Celgene (Inst), Astellas Pharma (Inst), Nerviano Medical Sciences (Inst), Pfizer (Inst), NovaRX (Inst), TRACON Pharma (Inst), Biocompatibles (Inst), Propella Therapeutics (Inst)
Dan L. Hertz
Research Funding: Disarm Therapeutics
Other Relationship: Advocates for Universal DPD/DPYD Testing (AUDT)
Uncompensated Relationships: PEPID, Saladax Biomedical
J. Kevin Hicks
Consulting or Advisory Role: Quest Diagnostics, 23andMe, Jackson Laboratory for Genomic Medicine
Research Funding: OneOme
Suneel Kamath
Consulting or Advisory Role: OncLive, Exelixis, Tempus, Guardant Health, Seattle Genetics
Travel, Accommodations, Expenses: AstraZeneca, Bristol Myers Squibb, Merck, Foundation Medicine, Guardant Health, Ipsen, Janssen
Open Payments Link: https://openpaymentsdata.cms.gov/physician/711572
Pashtoon Murtaza Kasi
Stock and Other Ownership Interests: Elicio Therapeutics
Consulting or Advisory Role: Taiho Pharmaceutical (Inst), Ipsen (Inst), Natera, Foundation Medicine, MSD Oncology, Tempus, Bayer, Lilly, Delcath Systems, QED Therapeutics, Servier, Taiho Oncology, Exact Sciences, Daiichi Sankyo/Astra Zeneca, Eisai, Seattle Genetics, SAGA Diagnostics, Illumina, BostonGene, NeoGenomics Laboratories, Elicio Therapeutics
Research Funding: Advanced Accelerator Applications (Inst), Tersera (Inst), Boston Scientific (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Todd C. Knepper
Honoraria: North American Center for Continuing Medical Education
Consulting or Advisory Role: Jackson Laboratory for Genomic Medicine
Howard L. McLeod
Leadership: Cancer Genetics, Vyant Bio
Stock and Other Ownership Interests: Cancer Genetics, Interpares Biomedicine, Pharmazam, Total Dx Connect, Vyant Bio, PGx Accellerator, Genovation Holdings, Clarified Precision Medicine
Honoraria: Genentech/Roche, Illumina
Consulting or Advisory Role: Gentris, Cancer Genetics, Saladax Biomedical, NIH/NCI, Admera Health, eviCore healthcare, Pharmazam, Viecure, Total Dx Connect, VieCure, Illumina, Intermountain Precision Genomics
Speakers' Bureau: Genentech
Other Relationship: Northwestern University, Xiangya Hospital, Kansas University Medical Center, AGBT Precision Health Conference
Peter H. O'Donnell
Honoraria: Merck, Astellas Pharma, Pfizer, CLD Inc, Axiom Healthcare Strategies, EMD Serono, IntrinsiQ, ISMIE, NAMCP, Seattle Genetics, Curio Science, FirstWord, MedLearning Group, Research to Practice, Great Debates and Updates, MJH Life Sciences, Peerview, Vaniam Group, Institute for Enquiring Minds
Research Funding: Boehringer Ingelheim (Inst), Merck (Inst), Genentech/Roche (Inst), AstraZeneca/MedImmune (Inst), Acerta Pharma (Inst), Janssen (Inst), Seattle Genetics (Inst), Bristol Myers Squibb (Inst), Astellas Pharma (Inst)
Expert Testimony: Oregon Health & Science University (OHSU)
Travel, Accommodations, Expenses: Curio Science
Other Relationship: Janssen, Nektar, NIH, Dragonfly Therapeutics, G1 Therapeutics
Mary V. Relling
Stock and Other Ownership Interests: Bioskryb
Research Funding: Servier
Michelle A. Rudek
Employment: GlaxoSmithKline (I)
Leadership: American Society for Clinical Pharmacology & Therapeutics, Geminus Therapetuics
Stock and Other Ownership Interests: Geminus Therapetuics
Consulting or Advisory Role: Leidos, EMMES Corporation
Research Funding: RenovoRx (Inst)
Other Relationship: British Pharmacological Society, UpToDate, CMTx Biotech Inc
D. Max Smith
Research Funding: Kailos Genetics Inc (Inst)
Sandra M. Swain
Leadership: Seattle Genetics
Stock and Other Ownership Interests: Seattle Genetics
Consulting or Advisory Role: Genentech/Roche, Daiichi Sankyo, Molecular Templates, Athenex, AstraZeneca, Exact Sciences, Natera, Lilly, Merck, bioTheranostics, Aventis Pharma
Research Funding: Genentech (Inst), Kailos Genetics (Inst)
Travel, Accommodations, Expenses: Daichi Sankyo, Aventis Pharma
Other Relationship: AstraZeneca, Roche, AstraZeneca
Uncompensated Relationships: Genentech/Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/801195
Christine M. Walko
Employment: Mission Healthcare
Consulting or Advisory Role: Jackson Laboratory for Genomic Medicine, Intermountain Precision Genomics, Clarified Precision Medicine
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: William D. Figg, Howard McLeod, Tristan M. Sissung
Financial support: William D. Figg
Administrative support: Howard McLeod
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
DPYD Testing: Time to Put Patient Safety First
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sharyn D. Baker
Research Funding: SPD Oncology (Inst)
Susan E. Bates
Honoraria: Merck (I)
Consulting or Advisory Role: Pegascy, ElmediX, Servier, Akita Biomedical (I), Secura Bio (I), OnKure, Ipsen, Progenics (I)
Research Funding: Merck (Inst), Amgen (Inst), RenovoRx (Inst), Ipsen (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Patent: Deacetylase Inhibitor Therapy (Inst), Patent: Depsipeptide for Therapy of Kidney Cancer (Inst)
Gabriel A. Brooks
Consulting or Advisory Role: CareCentrix, UnitedHealthcare, Ipsen
Research Funding: Roche/Genentech (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/197685
William L. Dahut
Leadership: Dexcom (I)
Stock and Other Ownership Interests: Dexcom (I)
Wafik S. El-Deiry
Stock and Other Ownership Interests: Oncoceutics, p53-Therapeutics, Chimerix, SMURF-Therapeutics
Consulting or Advisory Role: Rain Therapeutics
Research Funding: D&D Pharmatech, Chimerix
Patents, Royalties, Other Intellectual Property: Patent on TIC10 (ONC201), Patents pending on the use of small molecules to target mutant p53, Patent on therapeutic targeting on hypoxia-inducible factors
Other Relationship: Caris Life Sciences
Uncompensated Relationships: Rain Therapeutics, Caris Life Sciences
William E. Evans
Stock and Other Ownership Interests: BioSkryb (I)
William D. Figg
Research Funding: Celgene (Inst), Astellas Pharma (Inst), Nerviano Medical Sciences (Inst), Pfizer (Inst), NovaRX (Inst), TRACON Pharma (Inst), Biocompatibles (Inst), Propella Therapeutics (Inst)
Dan L. Hertz
Research Funding: Disarm Therapeutics
Other Relationship: Advocates for Universal DPD/DPYD Testing (AUDT)
Uncompensated Relationships: PEPID, Saladax Biomedical
J. Kevin Hicks
Consulting or Advisory Role: Quest Diagnostics, 23andMe, Jackson Laboratory for Genomic Medicine
Research Funding: OneOme
Suneel Kamath
Consulting or Advisory Role: OncLive, Exelixis, Tempus, Guardant Health, Seattle Genetics
Travel, Accommodations, Expenses: AstraZeneca, Bristol Myers Squibb, Merck, Foundation Medicine, Guardant Health, Ipsen, Janssen
Open Payments Link: https://openpaymentsdata.cms.gov/physician/711572
Pashtoon Murtaza Kasi
Stock and Other Ownership Interests: Elicio Therapeutics
Consulting or Advisory Role: Taiho Pharmaceutical (Inst), Ipsen (Inst), Natera, Foundation Medicine, MSD Oncology, Tempus, Bayer, Lilly, Delcath Systems, QED Therapeutics, Servier, Taiho Oncology, Exact Sciences, Daiichi Sankyo/Astra Zeneca, Eisai, Seattle Genetics, SAGA Diagnostics, Illumina, BostonGene, NeoGenomics Laboratories, Elicio Therapeutics
Research Funding: Advanced Accelerator Applications (Inst), Tersera (Inst), Boston Scientific (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Todd C. Knepper
Honoraria: North American Center for Continuing Medical Education
Consulting or Advisory Role: Jackson Laboratory for Genomic Medicine
Howard L. McLeod
Leadership: Cancer Genetics, Vyant Bio
Stock and Other Ownership Interests: Cancer Genetics, Interpares Biomedicine, Pharmazam, Total Dx Connect, Vyant Bio, PGx Accellerator, Genovation Holdings, Clarified Precision Medicine
Honoraria: Genentech/Roche, Illumina
Consulting or Advisory Role: Gentris, Cancer Genetics, Saladax Biomedical, NIH/NCI, Admera Health, eviCore healthcare, Pharmazam, Viecure, Total Dx Connect, VieCure, Illumina, Intermountain Precision Genomics
Speakers' Bureau: Genentech
Other Relationship: Northwestern University, Xiangya Hospital, Kansas University Medical Center, AGBT Precision Health Conference
Peter H. O'Donnell
Honoraria: Merck, Astellas Pharma, Pfizer, CLD Inc, Axiom Healthcare Strategies, EMD Serono, IntrinsiQ, ISMIE, NAMCP, Seattle Genetics, Curio Science, FirstWord, MedLearning Group, Research to Practice, Great Debates and Updates, MJH Life Sciences, Peerview, Vaniam Group, Institute for Enquiring Minds
Research Funding: Boehringer Ingelheim (Inst), Merck (Inst), Genentech/Roche (Inst), AstraZeneca/MedImmune (Inst), Acerta Pharma (Inst), Janssen (Inst), Seattle Genetics (Inst), Bristol Myers Squibb (Inst), Astellas Pharma (Inst)
Expert Testimony: Oregon Health & Science University (OHSU)
Travel, Accommodations, Expenses: Curio Science
Other Relationship: Janssen, Nektar, NIH, Dragonfly Therapeutics, G1 Therapeutics
Mary V. Relling
Stock and Other Ownership Interests: Bioskryb
Research Funding: Servier
Michelle A. Rudek
Employment: GlaxoSmithKline (I)
Leadership: American Society for Clinical Pharmacology & Therapeutics, Geminus Therapetuics
Stock and Other Ownership Interests: Geminus Therapetuics
Consulting or Advisory Role: Leidos, EMMES Corporation
Research Funding: RenovoRx (Inst)
Other Relationship: British Pharmacological Society, UpToDate, CMTx Biotech Inc
D. Max Smith
Research Funding: Kailos Genetics Inc (Inst)
Sandra M. Swain
Leadership: Seattle Genetics
Stock and Other Ownership Interests: Seattle Genetics
Consulting or Advisory Role: Genentech/Roche, Daiichi Sankyo, Molecular Templates, Athenex, AstraZeneca, Exact Sciences, Natera, Lilly, Merck, bioTheranostics, Aventis Pharma
Research Funding: Genentech (Inst), Kailos Genetics (Inst)
Travel, Accommodations, Expenses: Daichi Sankyo, Aventis Pharma
Other Relationship: AstraZeneca, Roche, AstraZeneca
Uncompensated Relationships: Genentech/Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/801195
Christine M. Walko
Employment: Mission Healthcare
Consulting or Advisory Role: Jackson Laboratory for Genomic Medicine, Intermountain Precision Genomics, Clarified Precision Medicine
No other potential conflicts of interest were reported.
REFERENCES
- 1.Zarkhin F: OHSU to pay $1 million, promises change to settle lawsuit from widow of cancer patient, The Oregonian, 2022 [Google Scholar]
- 2.FDA : Capecitabine Package Insert. US Food and Drug Administration, Silver Spring, MD, 2021 [Google Scholar]
- 3.FDA : Fluorouracil Package Insert. US Food and Drug Administration, Silver Spring, MD, 2016 [Google Scholar]
- 4.Ciccolini J, Milano G, Guchelaar HJ: Detecting DPD deficiency: When perfect is the enemy of good. Cancer Chemother Pharmacol 87:717-719, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Diasio RB, Beavers TL, Carpenter JT: Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity. J Clin Invest 81:47-51, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson MR, Wang K, Diasio RB: Profound dihydropyrimidine dehydrogenase deficiency resulting from a novel compound heterozygote genotype. Clin Cancer Res 8:768-774, 2002 [PubMed] [Google Scholar]
- 7.Harris BE, Song R, Soong SJ, et al. : Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res 50:197-201, 1990 [PubMed] [Google Scholar]
- 8.Meinsma R, Fernandez-Salguero P, Van Kuilenburg AB, et al. : Human polymorphism in drug metabolism: Mutation in the dihydropyrimidine dehydrogenase gene results in exon skipping and thymine uracilurea. DNA Cell Biol 14:1-6, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Wei X, McLeod HL, McMurrough J, et al. : Molecular basis of the human dihydropyrimidine dehydrogenase deficiency and 5-fluorouracil toxicity. J Clin Invest 98:610-615, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amstutz U, Henricks LM, Offer SM, et al. : Clinical pharmacogenetics implementation Consortium (CPIC) guideline for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing: 2017 Update. Clin Pharmacol Ther 103:210-216, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma BB, Rai K, Blunt H, et al. : Pathogenic DPYD variants and treatment-related mortality in patients receiving fluoropyrimidine chemotherapy: A systematic review and meta-analysis. Oncologist 26:1008-1016, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henricks LM, van Merendonk LN, Meulendijks D, et al. : Effectiveness and safety of reduced-dose fluoropyrimidine therapy in patients carrying the DPYD*2A variant: A matched pair analysis. Int J Cancer 144:2347-2354, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Deenen MJ, Tol J, Burylo AM, et al. : Relationship between single nucleotide polymorphisms and haplotypes in DPYD and toxicity and efficacy of capecitabine in advanced colorectal cancer. Clin Cancer Res 17:3455-3468, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50:9-22, 2016 [DOI] [PubMed] [Google Scholar]
- 15.van Kuilenburg ABP, Maring JG, Schalhorn A, et al. : Pharmacokinetics of 5-fluorouracil in patients heterozygous for the IVS14+1G > A mutation in the dihydropyrimidine dehydrogenase gene. Nucleosides Nucleotides Nucleic Acids 27:692-698, 2008 [DOI] [PubMed] [Google Scholar]
- 16.van Kuilenburg ABP, Hausler P, Schalhorn A, et al. : Evaluation of 5-fluorouracil pharmacokinetics in cancer patients with a c.1905+1G>A mutation in DPYD by means of a Bayesian limited sampling strategy. Clin Pharmacokinet 51:163-174, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Morel A, Boisdron-Celle M, Fey L, et al. : Clinical relevance of different dihydropyrimidine dehydrogenase gene single nucleotide polymorphisms on 5-fluorouracil tolerance. Mol Cancer Ther 5:2895-2904, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Joerger M, Huitema ADR, Boot H, et al. : Germline TYMS genotype is highly predictive in patients with metastatic gastrointestinal malignancies receiving capecitabine-based chemotherapy. Cancer Chemother Pharmacol 75:763-772, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Maring JG, van Kuilenburg ABP, Haasjes J, et al. : Reduced 5-FU clearance in a patient with low DPD activity due to heterozygosity for a mutant allele of the DPYD gene. Br J Cancer 86:1028-1033, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deenen MJ, Meulendijks D, Cats A, et al. : Upfront genotyping of DPYD*2A to individualize fluoropyrimidine therapy: A safety and cost analysis. J Clin Oncol 34:227-234, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Henricks LM, Lunenburg CATC, de Man FM, et al. : DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: A prospective safety analysis. Lancet Oncol 19:1459-1467, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Ray T: Cancer Centers Nudge Oncologists toward DPYD Testing as PGx Supporters Push for Guidelines Change, Genomeweb. genomeweb.com, 2022 [Google Scholar]
- 23.FDA : Benefit-Risk Assessment for New Drug and Biological Products. US Food and Drug Administration, Silver Spring, MD, 2021 [Google Scholar]
- 24.Braun MS, Richman SD, Thompson L, et al. : Association of molecular markers with toxicity outcomes in a randomized trial of chemotherapy for advanced colorectal cancer: The FOCUS trial. J Clin Oncol 27:5519-5528, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Jennings BA, Loke YK, Skinner J, et al. : Evaluating predictive pharmacogenetic signatures of adverse events in colorectal cancer patients treated with fluoropyrimidines. PLoS One 8:e78053, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakeel F, Fang F, Kwon JW, et al. : Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22:145-155, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Koo K, Pasternak AL, Henry NL, et al. : Survey of US medical oncologists' practices and beliefs regarding DPYD testing before fluoropyrimidine chemotherapy. JCO Oncol Pract 18:e958-e965, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ISMP : Screening for Dihydropyrimidine Dehydrogenase (DPD) Deficiency in Fluorouracil Patients: Why Not? Featured Article. Plymouth Meeting, PA, Institute for Safe Medication Practices, 2021 [Google Scholar]
- 29.Ontario Health (Quality) : DPYD genotyping in patients who have planned cancer treatment with fluoropyrimidines. Ont Health Technol Assess Ser 21:1-186, 2021 [PMC free article] [PubMed] [Google Scholar]
- 30.Brooks GA, Tapp S, Daly AT, et al. : Cost-effectiveness of DPYD genotyping prior to fluoropyrimidine-based adjuvant chemotherapy for colon cancer. Clin Colorectal Cancer 21:e189-e195, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau-Min KS, Varughese LA, Nelson MN, et al. : Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: A qualitative study of barriers to implementation. BMC Cancer 22:47, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavani O: 5-Fluorouracil response prediction and blood level-guided therapy in oncology: Existing evidence fundamentally supports instigation. Ther Drug Monit 42:660-664, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Dean L, Kane M: Fluorouracil therapy and DPYD genotype, in Pratt VM, Scott SA, Pirmohamed M, et al. (eds): Medical Genetics Summaries. National Center for Biotechnology Information (US) Bethesda, MD, 2012 [PubMed] [Google Scholar]
- 34.Zineh I, Lesko LJ: Pharmacogenetics in medicine: Barriers, critical factors and a framework for dialogue. Per Med 6:359-361, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Zineh I, Mummaneni P, Lyndly J, et al. : Allopurinol pharmacogenetics: Assessment of potential clinical usefulness. Pharmacogenomics 12:1741-1749, 2011 [DOI] [PubMed] [Google Scholar]
- 36.FDA : Docket Nos. FDA-20 14-P-0405 & FDA-2014-P-0497. US Food and Drug Administration, Silver Spring, MD, 2016 [Google Scholar]
- 37.Surprenant K: Citizen Petition from Kenneth E. Surprenant. US Food and Drug Administration, Silver Spring, MD, 2020 [Google Scholar]
- 38.CPIC. CPIC Guideline for Fluoropyrimidines and DPYD. https://cpicpgx.org/guidelines/guideline-for-fluoropyrimidines-and-dpyd/ [Google Scholar]



