Oncology drug development has benefited from the ability in many cases to directly measure disease burden by clinical and radiographic measures. Tumor-based end points including objective response rate (ORR) and progression-free survival (PFS) have been implemented as early clinical end points and have been extensively used in the evaluation of anticancer agents. However, recent oncology trials have highlighted a lack of correlation between these early efficacy end points and overall survival (OS). Six randomized controlled trials (RCTs) in either non-Hodgkin lymphomas (NHLs) or chronic lymphocytic leukemia (CLL) that demonstrated PFS improvements were associated with potential detriments in OS.1-4 Similarly, three trials in recurrent ovarian cancer with initial PFS benefit were associated with potential OS detriment with longer follow-up.5-8 Moreover, several immunotherapy trials demonstrated improvements in OS without improvements in PFS and/or ORR.9-13
EARLY AND LATE ONCOLOGY END POINTS
OS is considered a gold standard for oncology drug approvals and is a clinically meaningful objective measure assessing both safety and efficacy. Because end points including PFS and ORR can be assessed earlier than OS, these end points have been used to expedite drug approvals for patients with life-threatening diseases.14 These early end points are commonly used in indolent hematologic malignancies and in solid tumors where randomized trials with an OS primary end point would result in significant delays or be impractical because of limited patient numbers. The relationship between these early end points and OS has not been formally established, and this relationship may vary based on multiple factors, including the drug class, the natural history of the disease, available subsequent therapies, the effect size on the end point, and the safety profile of the drug or regimen. Early tumor-based end points are heavily weighted to assess efficacy, and the impacts of late toxicity and other postprogression events are either not captured or minimally assessed by these end points. Although these earlier end points have been used in accelerated approvals in oncology, they have also supported regular approvals based on the understanding that delaying disease progression (as measured by PFS or time to progression) or reducing tumor bulk (assessed by ORR) may represent a clinical benefit to patients and, if of sufficient magnitude with acceptable tolerability, may lead to an improvement in OS.
Time-to-event end points—PFS and OS—cannot be accurately interpreted in nonrandomized trials, as the comparison with an external control introduces bias. ORR can be used where RCTs may not be feasible, including rare diseases or where prior clinical data indicating superior efficacy of a drug compared with currently available drugs lead to lack of clinical equipoise. ORR is unique because it can be directly attributed to treatment, since tumors generally do not regress spontaneously. The demonstration of objective response is also clinically relevant, as evaluation of tumor response is integral to guiding therapeutic decisions in routine practice. In terms of PFS, long periods of response without disease recurrence or progression can be meaningful to patients.
Despite their clinical relevance and utility in drug development, ORR and PFS have not been established as surrogates for OS. Prentice15 proposed operational criteria to validate potential surrogates, including that the surrogate must be a correlate of the true clinical end point irrespective of the intervention and the treatment effect on the surrogate should capture the full effect of treatment on the clinical end point. Other statistical methods, such as meta-analyses, have been developed to characterize the relationship of potential surrogates to OS.16 Most early end points have not been rigorously assessed using patient-level data from meta-analyses.
REASONS FOR DISCORDANCE BETWEEN EARLY END POINTS AND OS
Discordance between early end points and OS may occur for several reasons, and the risk of discordance is likely to increase in settings with a modest magnitude of effect on ORR or PFS in the context of significant toxicity. Even larger effects on PFS or ORR could be accompanied by unfavorable OS findings in settings of substantive toxicity causing treatment-related deaths. Adverse events need not be fatal to have a negative effect on OS. Side effects can affect the ability of a patient to receive full exposure to the known effective components of a combination regimen or impact a patient's ability to receive effective subsequent therapies.
Trial design can also impact the relationship between early end points and OS. Many trials are designed with a PFS primary end point that allows crossover of the control arm to the investigational treatment. In this setting, control arm patients can receive the investigational drug as a subsequent therapy which can lead to a trial result with superior PFS results without demonstrating a statistically significant improvement in OS. Even without crossover, trials with a PFS primary end point may not have adequate statistical power to detect statistically significant improvements in OS. A large PFS benefit with an attenuated OS trend toward improvement is not necessarily an example of discordance, but rather a lack of comparable improvement. Nonetheless, while crossover and lack of power may attenuate the ability to detect an OS difference, they should not result in a negative effect on the observed OS.
The discordance between early tumor-based end points and OS is bidirectional, and the US Food and Drug Administration (FDA) has evaluated multiple trials in which an OS advantage has been demonstrated without substantive improvements in PFS or ORR. Examples include recent approvals of immune checkpoint inhibitors for non–small-cell lung cancer, head and neck squamous cell cancer, and melanoma. In several trials, statistically significant and clinically relevant improvements in OS have been demonstrated; however, minimal or no improvements in PFS or ORR have been observed in the same trial (Table 1).10-13 Unlike conventional cytotoxic drugs where the relationship between early end points and OS has been more consistently observed, the unique mechanism of action of the immune checkpoint inhibitors and other treatments that may alter tumor growth kinetics rather than solely act via direct cytotoxicity may account for this disconnect.
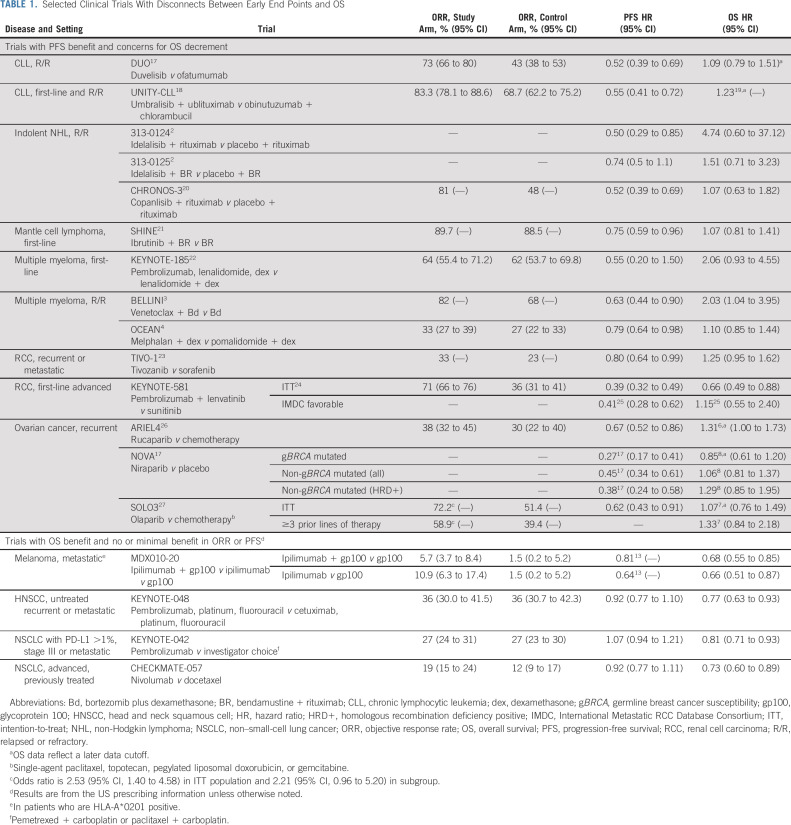
TABLE 1.
Selected Clinical Trials With Disconnects Between Early End Points and OS
Importantly, some trials have not only failed to show a significant improvement in OS but also demonstrated a potential detriment (Table 1).1-4,7,8,17-34 Many of these trials evaluate molecularly targeted drugs with prolonged use and highlight the need to evaluate mature OS data in establishing the final assessment of a drug's risks and benefits. The FDA's experience with the phosphatidylinositol 3-kinases (PI3K) inhibitor drug class in indolent NHL and CLL was presented at the April 2022 Oncologic Drugs Advisory Committee Meeting. FDA highlighted multiple randomized trials evaluating this drug class that raised concerns for potential OS detriments despite earlier PFS advantages. Several of these examples were in the setting of drugs with significant toxicities that resulted in a high incidence of dose interruptions and dose reductions.
The discordances seen are due in part to toxicity, and understanding the relationship between dosage, toxicity, and efficacy or dose optimization is not always adequately explored before initiating randomized trials for drug registration. Historically, the maximum tolerated dose has been selected for use in late-stage drug development. However, investigational drugs are increasingly delivered orally and for longer periods of time, and better dose optimization is critical to maximize adherence. Inadequate attention to dose optimization, for both monotherapy and combination regimens, may contribute to randomized trials with potential OS detriments. Toxicity can negatively impact OS by limiting the patient's ability to receive full exposure of both the investigational treatment and effective subsequent anticancer drugs. Inadequate exposure could also theoretically lead to more resistant disease after progression negatively affecting the efficacy of subsequent drugs. The FDA's Oncology Center of Excellence (OCE) has established Project Optimus to collaboratively work with sponsors to explore the relationship between dose schedule, safety, and efficacy.35,36 Project Optimus encourages interactions between drug developers and FDA review divisions early in development to discuss dose finding and development and communicates expectations for dose finding and dose optimization through guidance, workshops, and other public meetings.
Discordance between early tumor-based end points and OS may be observed in specific subpopulations in randomized trials, and these analyses are usually exploratory. The clinical importance of a subgroup analysis for OS must be put in the context of the magnitude of the drug's effect on the subpopulation, the degree of subgroup's representation in the overall intention-to-treat (ITT) population, and the biologic plausibility of a differential effect in the subgroup, among other factors. For example, in relapsed or refractory ovarian cancer, despite a PFS advantage observed across subgroups with niraparib maintenance therapy compared with placebo, an OS detriment was observed in the nongermline breast cancer susceptibility (non-gBRCA) mutated, homologous recombination deficiency positive population, but not in the gBRCA-mutated patients.8 Discordance can also be observed between an early end point and OS in an ITT population while a subpopulation may demonstrate concordance. For example, in relapsed or refractory multiple myeloma, when venetoclax was added to bortezomib and dexamethasone, an OS detriment was observed in the ITT population, but not in patients with the t(11;14) translocation.3 While there is biologic plausibility, based on the anti-BCL2 activity of venetoclax, the small numbers of patients with this translocation coupled with a lack of a prespecified statistical plan limited any conclusions regarding this subgroup. Early attention in a drug's development should focus on patient subgroups who may be expected to have different outcomes, and adequate plans in both patient accrual and statistical analysis should be made to characterize this potential difference.
STATISTICAL AND REGULATORY CONSIDERATIONS
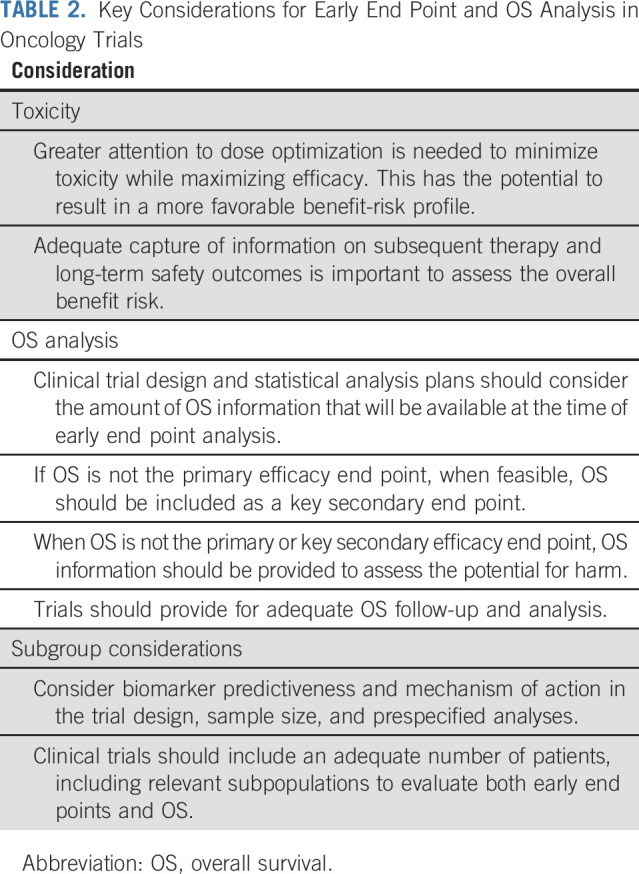
Early tumor-based end points may be meaningfully evaluated when few survival events have occurred, particularly in diseases where survival may be prolonged. Trials are often not powered to detect OS differences at the time of final PFS analysis; however, FDA relies on an OS analysis, even if descriptive, to assure there is no decrement. When formal statistical testing of OS is not specified, a rigorous plan for OS evaluation as a safety end point including monitoring for early safety signals that may adversely affect OS can allow for a more thorough assessment of OS. The number of OS events expected at the time of a PFS analysis and the expected time for mature survival analysis can help determine the appropriate design of interim and final analyses. Sponsors should provide plans for continuing to collect OS data after disease progression, minimize missing OS data, and outline their efforts to capture subsequent therapies and causes of death (Table 2). For situations where the OS analysis will be immature at the time of an FDA submission, plans for continued OS evaluation including a long-term OS analysis with more events may be requested as a postmarketing commitment or requirement.
TABLE 2.
Key Considerations for Early End Point and OS Analysis in Oncology Trials
The results and maturity of an OS analysis at the time of an FDA submission may affect the appropriate approval pathway for randomized trials with an early end point such as PFS. Where there may be uncertainty regarding the results of an OS analysis despite a meaningful effect on PFS, an accelerated approval rather than a traditional approval may be warranted. This may be particularly relevant if significant safety findings are discovered or previous discordance between PFS and OS have been observed in a similar drug class. The accelerated approval pathway allows approval in serious and life-threatening diseases for drugs that demonstrate an improvement over available therapy based on earlier clinical end points. In fact, the statute for accelerated approval was recently amended by congress to provide new procedures for expedited withdrawal of a product. If subsequent postmarketing data reveal an adverse effect on a mature OS analysis, the risk benefit must be reassessed, and when unfavorable, both parties should honor the regulatory prenuptial agreement of drug approval and seek expedited withdrawal of the indication.
In conclusion, tumor-based assessments and OS form critical drug development end points that are measured at different time points in a patient's disease, have different confounding factors, and may be impacted differently by specific drugs. OS is not a feasible primary efficacy end point in all trials, and reducing tumor burden (ORR) and delaying its progression (PFS) have clinical relevance in routine care and can be sufficient to provide evidence of efficacy. However, even a meaningful improvement in ORR or PFS is insufficient when a potential OS detriment is observed. Thus, regardless of primary end point used, OS must be carefully assessed and a reasonably mature descriptive OS analysis provided as part of the totality of data. The divorce between the efficacy findings of early end points like ORR and PFS and their complicated relationship to OS highlights possible irreconcilable differences. In 2023, the FDA's OCE plans a series of workshops to examine the role of early end points, their relationship to OS, and considerations around obtaining the information necessary to make informed decisions on the risks and benefits of a novel cancer therapy.
Margret Merino
Employment: Spectrum Primary Care Incorporated, Chenega Professional Services
No other potential conflicts of interest were reported.
DISCLAIMER
This is a US Government work. There are no restrictions on its use.
AUTHOR CONTRIBUTIONS
Conception and design: Richard Pazdur, Nicole Gormley
Collection and assembly of data: Margret Merino, Yvette Kasamon, Nicole Gormley
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Irreconcilable Differences: The Divorce Between Response Rates, Progression-Free Survival, and Overall Survival
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Margret Merino
Employment: Spectrum Primary Care Incorporated, Chenega Professional Services
No other potential conflicts of interest were reported.
REFERENCES
- 1.Richardson NC, Kasamon Y, Pazdur R, et al. : The saga of PI3K inhibitors in haematological malignancies: Survival is the ultimate safety endpoint. Lancet Oncol 23:563-566, 2022 [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration : FDA briefing document for the Oncologic Drugs Advisory Committee (ODAC) Meeting, April 21, 2022. Phosphatidylinositol 3-kinase (PI3K) inhibitors in hematologic malignancies. https://www.fda.gov/media/157762/download
- 3.Kumar SK, Harrison SJ, Cavo M, et al. : Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 21:1630-1642, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Schjesvold FH, Dimopoulos MA, Delimpasi S, et al. : Melflufen or pomalidomide plus dexamethasone for patients with multiple myeloma refractory to lenalidomide (OCEAN): A randomised, head-to-head, open-label, phase 3 study. Lancet Haematol 9:e98-e110, 2022 [DOI] [PubMed] [Google Scholar]
- 5.GlaxoSmithKline : Dear Health Care Provider Letter, May 2022. Important drug warning: Zejula (niraparib) important drug warning for the maintenance treatment in recurrent ovarian cancer (2L+). https://www.zejulahcp.com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US/pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter.pdf
- 6.Clovis Oncology : Dear Health Care Provider Letter, June 2022. Rubraca (rucaparib) for treatment of BRCA mutated ovarian cancer after 2 or more chemotherapies is voluntarily withdrawn in the US. https://clovisoncology.com/pdfs/US_DHCPL_final_signed.pdf
- 7.AstraZeneca : Dear Health Care Professional Letter, August 2022. Important prescribing information: Lunparza (olaparib) for treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy is voluntarily withdrawn in the US. https://www.lynparzahcp.com/content/dam/physician-services/us/590-lynparza-hcp-branded/hcp-global/pdf/solo3-dhcp-final-signed.pdf
- 8.GlaxoSmithKline : Dear Health Care Provider Letter, November 2022. Zejula (niraparib): Important prescribing information for the maintenance treatment of adult patients with non-gBRCAmut recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy in second or later line setting. https://www.zejulahcp.com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US/pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20September%202022.pdf
- 9.Nathan P, Hassel JC, Rutkowski P, et al. : Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 385:1196-1206, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Burtness B, Harrington KJ, Greil R, et al. : Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394:1915-1928, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Mok TSK, Wu YL, Kudaba I, et al. : Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393:1819-1830, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Borghaei H, Paz-Ares L, Horn L, et al. : Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627-1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, O'Day SJ, McDermott DF, et al. : Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711-723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration : Expedited programs for serious conditions—Drugs and biologics. Guidance for industry. https://www.fda.gov/media/86377/download
- 15.Prentice RL: Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med 8:431-440, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Buyse M, Sargent DJ, Grothey A, et al. : Biomarkers and surrogate end points—The challenge of statistical validation. Nat Rev Clin Oncol 7:309-317, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Mirza MR, Monk BJ, Herrstedt J, et al. : Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 375:2154-2164, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Gribben JG, Jurczak W, Jacobs RW, et al. : Umbralisib plus ublituximab (U2) is superior to obinutuzumab plus chlorambucil (O+Chl) in patients with treatment naïve (TN) and relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): Results from the phase 3 unity-CLL study. Blood 136:37-39, 2020 [Google Scholar]
- 19.TG Therapeutics : Press release, November 30, 2021. Regulatory update conveying US Food and Drug Administration plan to host an Oncologic Drugs Advisory Committee (ODAC) Meeting regarding pending BLA for the combination of ublituximab and umbralisib for the treatment of adult patients with chronic lymphocytic leukemia (CLL) and small lymphocytic leukemia (SLL). https://ir.tgtherapeutics.com/news-releases/news-release-details/tg-therapeutics-provides-regulatory-update
- 20.Matasar MJ, Capra M, Ozcan M, et al. : Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 22:678-689, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Wang ML, Jurczak W, Jerkeman M, et al. : Ibrutinib plus bendamustine and rituximab in untreated mantle-cell lymphoma. N Engl J Med 386:2482-2494, 2022 [DOI] [PubMed] [Google Scholar]
- 22.Usmani SZ, Schjesvold F, Oriol A, et al. : Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): A randomised, open-label, phase 3 trial. Lancet Haematol 6:e448-e458, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Mehta A, Sonpavde G, Escudier B: Tivozanib for the treatment of renal cell carcinoma: Results and implications of the TIVO-1 trial. Future Oncol 10:1819-1826, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Merck Sharp & Dohme Corp : KEYTRUDA (pembrolizumab) injection for intravenous use: US prescribing information. June 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125514s131lbl.pdf
- 25.Motzer R, Alekseev B, Rha SY, et al. : Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384:1289-1300, 2021 [DOI] [PubMed] [Google Scholar]
- 26.Kristeleit R, Lisyanskaya A, Fedenko A, et al. : Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): An international, open-label, randomised, phase 3 trial. Lancet Oncol 23:465-478, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Penson RT, Valencia RV, Cibula D, et al. : Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): A randomized phase III trial. J Clin Oncol 38:1164-1174, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration : Safety alert, July 28, 2021. FDA alerts patients and health care professionals about clinical trial results showing an increased risk of death associated with Pepaxto (melphalan flufenamide). https://www.fda.gov/drugs/drug-safety-and-availability/fda-alerts-patients-and-health-care-professionals-about-clinical-trial-results-showing-increased#:∼:text=%5B7%2F28%2F2021%5D,an%20increased%20risk%20of%20death
- 29.Gilead Sciences : Dear Health Care Provider Letter, March 21, 2016. Important drug warning: decreased overall survival and increased risk of serious infections in patients receiving Zydelig (idelalisib). http://cllsociety.org/docs/Zydelig%20Safety%20Update.pdf
- 30.Secura Bio, Inc : Dear Healthcare Professional letter, May 1, 2022. Correction of drug information: Updated overall survival data for Copiktra® (duvelisib) in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). https://copiktrahcp.com/notice-to-hcps/
- 31.Flinn IW, Hillmen P, Montillo M, et al. : The phase 3 DUO trial: Duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 132:2446-2455, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gormley NJ, Pazdur R: Immunotherapy combinations in multiple myeloma—Known unknowns. N Engl J Med 379:1791-1795, 2018 [DOI] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration : NDA 211155 (duvelisib) multidisciplinary review and evaluation. September 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211155Orig1Orig2s000TOC.cfm
- 34.Penson R, Valencia RV, Colombo N, et al. : Final overall survival results from SOLO3: Phase III trial assessing olaparib monotherapy versus non-platinum chemotherapy in heavily pretreated patients with germline BRCA1- and/or BRCA2-mutated platinum-sensitive relapsed ovarian cancer. Gynecol Oncol 166:S19-S20, 2022 [Google Scholar]
- 35.US Food and Drug Adminstration, Oncology Center of Excellence : Project Optimus, Reforming the dose optimization and dose selection paradigm in oncology. https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus
- 36.Shah M, Rahman A, Theoret MR, et al. : The drug-dosing conundrum in oncology—When less is more. N Engl J Med 385:1445-1447, 2021 [DOI] [PubMed] [Google Scholar]