Small-cell lung cancer (SCLC) comprises approximately 14% of all lung cancer diagnoses and is exceptionally lethal. Approximately two thirds of patients with SCLC present with extra-thoracic metastases, and the median overall survival of patients with extensive stage disease remains only slightly over 1 year.1,2 Although addition of PD-L1 inhibitory antibodies to the first-line treatment for patients with SCLC was an important advance,1,2 the limited survival benefit emphasizes the urgent need for additional strategies to better treat this disease.
THE TAKEAWAY
In the article that accompanies this editorial, Paz-Ares et al10 present the tolerability and initial efficacy data for the bispecific T-cell engager, tarlatamab, in 107 patients with metastatic small-cell lung cancer (SCLC). In addition to introducing a novel therapeutic agent and strategy, the study offers encouraging durable responses in a subgroup of patients with heavily pretreated SCLC.
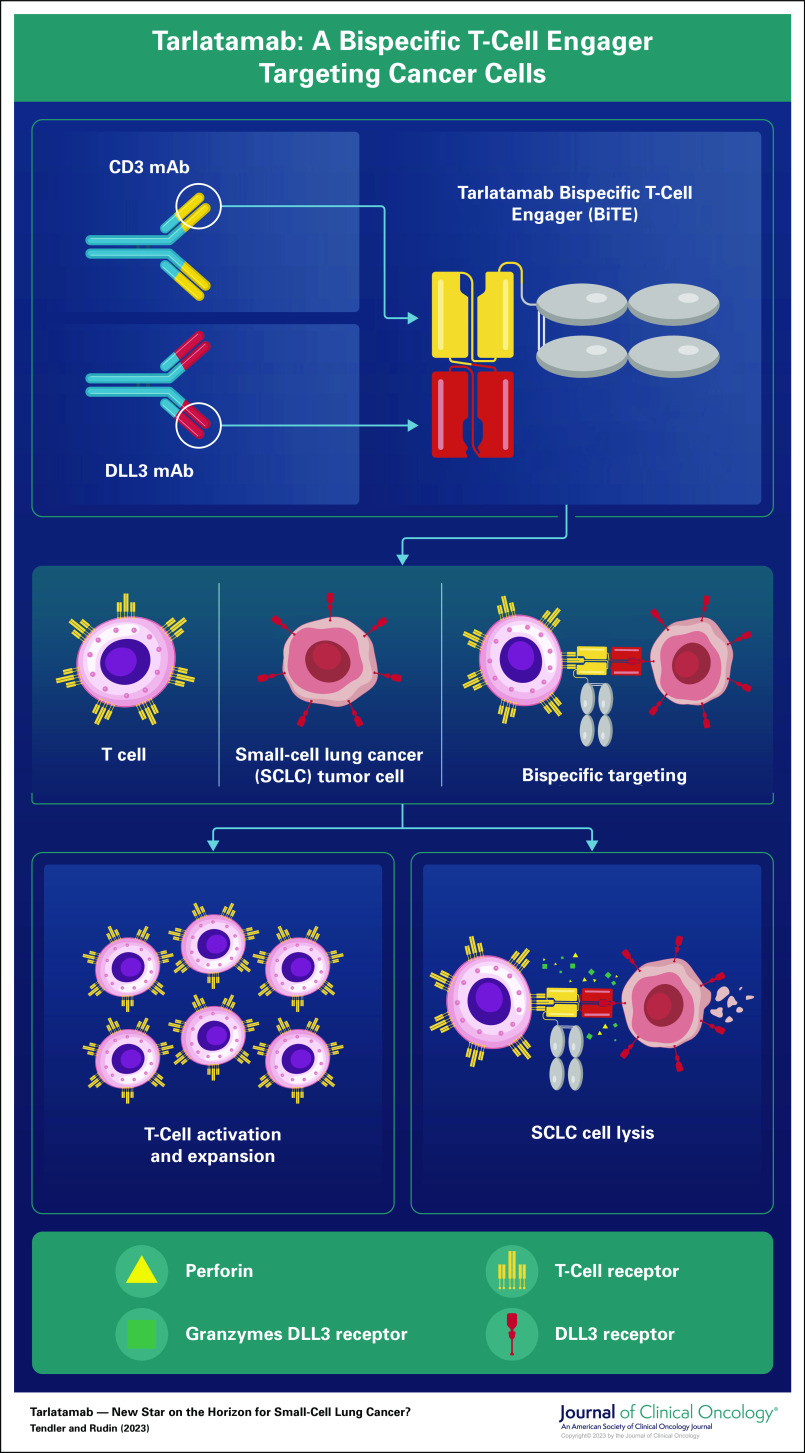
An important contributor to the lack of immune checkpoint blockade efficacy in most patients with SCLC may be tumor-intrinsic suppression of Major Histocompatibility Complex class I (MHC-I), required for antigen presentation to cytolytic CD8+ T cells.3 While many investigators are focusing on epigenetic strategies to restore antigen presentation in SCLC, an alternative strategy is to bypass canonical antigen presentation machinery entirely through the use of a bispecific T-cell engager (BiTE)—an antibody-derived hybrid molecular construct that binds tightly to a surface determinant on the cancer cell, and another on the T cell, enforcing interaction and triggering T-cell activation and target cell lysis. Tarlatamab is a BiTE targeting the Notch ligand DLL3 on SCLC cells and the CD3 complex on T cells (Fig 1). Tarlatamab activates T cells when incubated in vitro with DLL3-positive SCLC cells, resulting in SCLC lysis.4 Tarlatamab also produces impressive tumor regression in vivo in a disseminating orthotopic model of SCLC.5 Tarlatamab is the first DLL3-targeted immune therapy to be evaluated in a clinical trial.
FIG 1.
Tarlatamab: A bispecific T-cell engager targeting cancer cells.
Regulation of Notch signaling orchestrates many developmental processes, including the derivation of pulmonary neuroendocrine cells.6 DLL3 is a transmembrane inhibitor of Notch signaling, which, in healthy adult tissues, is confined to intracellular compartments including the Golgi apparatus. The DLL3 gene is a direct target of ASCL1, a transcription factor that induces neuroendocrine cell proliferation, which is critically implicated in the development of SCLC.7 In ASCL1-expressing SCLC, DLL3 is markedly upregulated and aberrantly trafficked to the cell surface, defining an attractive candidate for tumor-selective targeting.8 Relative to other cell surface proteins that have been exploited as cancer targets, expression of DLL3 on SCLC is notably of low prevalence, on the order of 10,000 molecules per cell.8,9 However, the unique tumor specificity of its cell surface exposure has prompted the development of a variety of DLL3-targeting agents.
In the article that accompanies this editorial, Paz-Ares et al10 report the safety and preliminary efficacy of tarlatamab in an international phase I dose-escalation study that included 107 patients with recurrent metastatic SCLC. Although the observed response rate of 23.4% is similar to that of many standard cytotoxics used for patients with recurrent SCLC, the median duration of response of 12.3 months, with 44% of these responding patients having ongoing responses at data cutoff, is exceptional, showing substantial promise for this experimental drug. Most patients with durable tumor responses had received the higher dosages of the study drug: 10, 30, or 100 mg once every 2 weeks. The median overall survival of 13.2 months (95% CI, 10.5 to not reached) is also encouraging in this context.
Cytokine release syndrome (CRS) was the most serious recurrent adverse event (AE) observed, occurring predominantly after the first dose. Eight patients received tocilizumab for CRS, with others typically receiving combinations of steroids, antipyretics, and fluids. All CRS-related toxicities were reversible, and none resulted in discontinuation of tarlatamab although 12 patients (11.2%) experienced grade ≥3 neurologic AEs. The pathophysiology and mechanisms underlying symptoms of CRS are not fully understood. Both immune effector cell–associated neurotoxicity syndrome and CRS have been associated with potent responses to immune-modulating therapies; high levels of circulating cytokines appear to trigger endothelial cell activation and blood-brain barrier disruption, resulting in an inflammatory cascade that can inflict diffuse cerebral edema.11,12 Informed management algorithms for CRS management would be of substantial utility for future investigation of tarlatamab and other emerging next-generation immunotherapies.
The trial had several limitations. Almost all patients had good performance status (0-1), and none had brain metastases; application in the more diverse population of typical patients with metastatic SCLC remains to be defined. The study includes a very limited analysis of potential predictive biomarkers, restricted to immunohistochemical staining for DLL3 in a subset of patients with available archival tumor biopsies. A robust effort to quantitate baseline (pretreatment, proximal to therapy) target expression on tumor would be of benefit in defining patients most likely to benefit from tarlatamab. Obtaining core biopsies admittedly may not be safe or feasible in all patients. One alternative, allowing pretreatment and on-treatment dynamic assessment, might be analysis of circulating tumor cells which are typically abundant in patients with SCLC.13 Another approach, avoiding the inherent sampling biases of immunohistochemistry and reflecting in vivo accessibility of the target in tumors, would be quantitative anti-DLL3 positron emission tomography scanning using a radioimmunoconjugate. The feasibility of this approach has been demonstrated in multiple patient-derived xenograft models, and clinical assessment is ongoing (ClinicalTrials.gov identifier: NCT04199741).14,15
Tarlatamab is one of several DLL3-targeted therapeutic agents that have been or are being investigated in human clinical trials. The first of these was the antibody-drug conjugate (ADC) rovalpituzumab tesirine (Rova-T), an anti-DLL3 antibody linked to the toxin pyrrolobenzodiazepine.8,16 Rova-T produced clinical responses in patients with SCLC, but significant toxicities including body cavity effusions precluded repetitive dosing, and the median overall survival for patients with recurrent SCLC was <6 months.17 Development of an anti-DLL3 ADC with a better tolerated therapeutic payload remains a viable strategy.
BI 764532 is an anti-DLL3/anti-CD3 BiTE, conceptually similar to tarlatamab. BI 764532 has demonstrated activity in a human T-cell–engrafted mouse model, including complete tumor regressions.18 A phase I study is ongoing in patients with SCLC and other neuroendocrine tumors expressing DLL3.19 HPN328 is a third anti-DLL3 T-cell engager, referred to as a trispecific T-cell activating construct. HPN328 contains three binding domains: anti-DLL3, anti-CD3, and antialbumin, promoting long half-life in circulation. An ongoing phase I/IIa study of HPN328 in patients with metastatic SCLC and other neuroendocrine cancers associated with DLL3 expression has shown preliminary evidence of efficacy, with no grade 3 CRS events observed in the first 16 patients.20
Taken together, preclinical and clinical data are pointing to DLL3 as an exciting new target for treatment of patients with SCLC. The initial safety and efficacy data of tarlatamab are an important milestone, further validating both DLL3 as a target and MHC-I bypass as an immunotherapeutic strategy for SCLC. The durable responses observed in this study should offer hope to patients with SCLC and other aggressive neuroendocrine cancers.
Charles M. Rudin
Consulting or Advisory Role: Harpoon Therapeutics ($5,000 USD or above in a single calendar year), Genentech/Roche (less than $5,000 USD in a single calendar year), AstraZeneca (less than $5,000 USD in a single calendar year), Ipsen (less than $5,000 USD in a single calendar year), Bridge Medicines ($5,000 USD or above in a single calendar year), Syros Pharmaceuticals (less than $5,000 USD in a single calendar year), Amgen (less than $5,000 USD in a single calendar year), Jazz Pharmaceuticals (less than $5,000 USD in a single calendar year), Epizyme (less than $5,000 USD in a single calendar year), Syros Pharmaceuticals (less than $5,000 USD in a single calendar year), Earli (less than $5,000 USD in a single calendar year), AbbVie (less than $5,000 USD in a single calendar year), Daiichi Sankyo/UCB Japan (less than $5,000 USD in a single calendar year), Kowa (less than $5,000 USD in a single calendar year), Merck (less than $5,000 USD in a single calendar year), D2G Oncology (less than $5,000 USD in a single calendar year)
Research Funding: Merck (Inst), Roche/Genentech (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/111056
No other potential conflicts of interest were reported.
Footnotes
See accompanying article on page 2893
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Salomon Tendler
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tarlatamab: New Star on the Horizon for Small-Cell Lung Cancer?
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Charles M. Rudin
Consulting or Advisory Role: Harpoon Therapeutics ($5,000 USD or above in a single calendar year), Genentech/Roche (less than $5,000 USD in a single calendar year), AstraZeneca (less than $5,000 USD in a single calendar year), Ipsen (less than $5,000 USD in a single calendar year), Bridge Medicines ($5,000 USD or above in a single calendar year), Syros Pharmaceuticals (less than $5,000 USD in a single calendar year), Amgen (less than $5,000 USD in a single calendar year), Jazz Pharmaceuticals (less than $5,000 USD in a single calendar year), Epizyme (less than $5,000 USD in a single calendar year), Syros Pharmaceuticals (less than $5,000 USD in a single calendar year), Earli (less than $5,000 USD in a single calendar year), AbbVie (less than $5,000 USD in a single calendar year), Daiichi Sankyo/UCB Japan (less than $5,000 USD in a single calendar year), Kowa (less than $5,000 USD in a single calendar year), Merck (less than $5,000 USD in a single calendar year), D2G Oncology (less than $5,000 USD in a single calendar year)
Research Funding: Merck (Inst), Roche/Genentech (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/111056
No other potential conflicts of interest were reported.
REFERENCES
- 1.Horn L, Mansfield AS, Szczesna A, et al. : First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220-2229, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Paz-Ares L, Chen Y, Reinmuth N, et al. : Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-Year overall survival update from CASPIAN. ESMO Open 7:100408, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahadevan NR, Knelson EH, Wolff JO, et al. : Intrinsic immunogenicity of small cell lung carcinoma revealed by its cellular plasticity. Cancer Discov 11:1952-1969, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giffin MJ, Cooke K, Lobenhofer EK, et al. : AMG 757, a half-life extended, DLL3-targeted bispecific T-cell engager, shows high potency and sensitivity in preclinical models of small-cell lung cancer. Clin Cancer Res 27:1526-1537, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Giffin MJ, Lobenhofer EK, Cooke K, et al. : Abstract 3632: BiTE® antibody constructs for the treatment of SCLC. Cancer Res 77:3632, 2017. 28446465 [Google Scholar]
- 6.Morimoto M, Nishinakamura R, Saga Y, et al. : Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 139:4365-4373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augustyn A, Borromeo M, Wang T, et al. : ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci U S A 111:14788-14793, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saunders LR, Bankovich AJ, Anderson WC, et al. : A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 7:302ra136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma SK, Pourat J, Abdel-Atti D, et al. : Noninvasive interrogation of DLL3 expression in metastatic small cell lung cancer. Cancer Res 77:3931-3941, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paz-Ares L, Champiat S, Lai WV, et al. : Tarlatamab, a first-in-class DLL3-targeted bispecific T-cell engager, in recurrent small cell lung cancer: An open-label, phase I study. J Clin Oncol 41:2893-2903, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, Santomasso BD, Locke FL, et al. : ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25:625-638, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Gust J, Hay KA, Hanafi LA, et al. : Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov 7:1404-1419, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackhall F, Frese KK, Simpson K, et al. : Will liquid biopsies improve outcomes for patients with small-cell lung cancer? Lancet Oncol 19:e470-e481, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Korsen JA, Gutierrez JA, Tully KM, et al. : Delta-like ligand 3-targeted radioimmunotherapy for neuroendocrine prostate cancer. Proc Natl Acad Sci U S A 119:e2203820119, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tully KM, Tendler S, Carter LM, et al. : Radioimmunotherapy targeting delta-like ligand 3 in small cell lung cancer exhibits antitumor efficacy with low toxicity. Clin Cancer Res 28:1391-1401, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudin CM, Pietanza MC, Bauer TM, et al. : Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 18:42-51, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgensztern D, Besse B, Greillier L, et al. : Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: Results from the phase II TRINITY study. Clin Cancer Res 25:6958-6966, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hipp S, Voynov V, Drobits-Handl B, et al. : A bispecific DLL3/CD3 IgG-like T-cell engaging antibody induces antitumor responses in small cell lung cancer. Clin Cancer Res 26:5258-5268, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Wermke M, Felip E, Gambardella V, et al. : Phase I trial of the DLL3/CD3 bispecific T-cell engager BI 764532 in DLL3-positive small-cell lung cancer and neuroendocrine carcinomas. Future Oncol 18:2639-2649, 2022 [DOI] [PubMed] [Google Scholar]
- 20.Johnson ML, Dy GK, Mamdani H, et al. : Interim results of an ongoing phase 1/2a study of HPN328, a tri-specific, half-life extended, DLL3-targeting, T-cell engager, in patients with small cell lung cancer and other neuroendocrine cancers. J Clin Oncol 40:8566, 2022 [Google Scholar]