Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
In the longest follow-up, to our knowledge, for a KRASG12C inhibitor, we assessed the long-term efficacy, safety, and biomarkers of sotorasib in patients with KRAS G12C-mutated advanced non–small-cell lung cancer (NSCLC) from the CodeBreaK 100 clinical trial (ClinicalTrials.gov identifier: NCT03600883). This multicenter, single-group, open-label phase I/phase II trial enrolled 174 patients with KRAS G12C-mutated, locally advanced or metastatic NSCLC after progression on prior therapies. Patients (N = 174) received sotorasib 960 mg once daily with the primary end points for phase I of safety and tolerability and for phase II of objective response rate (ORR). Sotorasib produced an ORR of 41%, median duration of response of 12.3 months, progression-free survival (PFS) of 6.3 months, overall survival (OS) of 12.5 months, and 2-year OS rate of 33%. Long-term clinical benefit (PFS ≥ 12 months) was observed in 40 (23%) patients across PD-L1 expression levels, in a proportion of patients with somatic STK11 and/or KEAP1 alterations, and was associated with lower baseline circulating tumor DNA levels. Sotorasib was well tolerated, with few late-onset treatment-related toxicities, none of which led to treatment discontinuation. These results demonstrate the long-term benefit of sotorasib, including in subgroups with poor prognosis.
Long-term OS benefit with sotorasib in KRAS G12Cmut NSCLC: Experience from largest prospective cohort (CB100 study).
INTRODUCTION
Overall survival (OS) remains poor for molecularly unselected, advanced non–small-cell lung cancer (NSCLC),1,2 with 2-5 months of median progression-free survival (PFS) on second-line plus chemotherapy or immunotherapy.3-6
CONTEXT
Key Objective
To determine the long-term safety, tolerability, and efficacy of sotorasib 960 mg once daily in patients with KRAS G12C-mutated, locally advanced or metastatic non–small-cell lung cancer from the CodeBreaK 100 clinical trial (ClinicalTrials.gov identifier: NCT03600883). Exploratory analyses assessed the relationship of various biomarkers, such as PD-L1 expression level and genomic alterations, with efficacy.
Knowledge Generated
This 2-year pooled analysis of CodeBreaK 100, which is, to our knowledge, the largest clinical data set with the longest follow-up reported for patients treated with any KRASG12C inhibitor to date, showed that sotorasib treatment provided long-term efficacy and was well tolerated, with no new safety signals detected. Long-term benefit with sotorasib (defined as progression-free survival of at least 12 months) was associated with lower baseline circulating tumor DNA levels and was observed across KRAS G12C variant allele frequency levels, PD-L1 expression levels, and in a proportion of patients with STK11 and/or KEAP1 comutations.
Relevance
The findings from this analysis with over 2-year follow-up data demonstrate that nearly a quarter of previously treated advanced stage KRAS G12C-mutated NSCLC patients treated with sotorasib derived long-term benefit, with few late-onset treatment-related toxicities, supporting not only its use in this treatment setting but also additional studies investigating its therapeutic role in earlier lines of therapy.
Sotorasib specifically and irreversibly inhibits KRASG12C,7-10 with approval in over 40 countries11-13 for adults with KRAS G12C-mutated advanced NSCLC after prior systemic therapy.14,15 In CodeBreaK 100 phase II, sotorasib demonstrated an objective response rate (ORR) of 37%, a median duration of response (DOR) of 11.1 months, a median PFS of 6.8 months, a median OS of 12.5 months, and a manageable safety profile in KRAS G12C-mutated advanced NSCLC.16
We report CodeBreaK 100 phase I/II 2-year pooled analyses representing, to our knowledge, the longest KRASG12C inhibitor treatment follow-up to date.
METHODS
Patients
The multicenter, single-group, open-label phase I/II CodeBreaK 100 trial (ClinicalTrials.gov identifier: NCT03600883) enrolled patients age 18 years and older with KRAS G12C-mutated locally advanced or metastatic NSCLC after progression on prior therapies (Data Supplement, online only).16 Institutional review board approval before study initiation and participating country regulatory authority approval were received; all patients provided written informed consent.
Study Design
Phase I primary end point was safety and tolerability (key secondary: DOR and PFS). Phase II primary end point was ORR (blinded independent central review; key secondary: DOR, PFS, OS, and safety). Late-onset toxicities were assessed (treatment-related adverse events [TRAEs] occurring after 1 year on treatment).
Exploratory analyses evaluated molecular correlates with efficacy (Data Supplement).16 PD-L1 and genomic alterations were correlated with long-term benefit (PFS ≥ 12 months) versus early progression (nonresponders with PFS ≤ 3 months).
RESULTS
Patients
As of February 22, 2022, 174 patients (phase I, N = 48; phase II, N = 126) received sotorasib 960 mg once daily (Table 1). Median treatment duration was 5.6 months (range, 0.2-35.9); 13 patients remained on treatment at cutoff. Median prior lines of therapy was 2.0 (range, 0 to 4+). Prior therapies included anti–PD-(L)1 (157 [90%]) and platinum-based chemotherapy plus anti–PD-(L)1 (144 [83%]).
TABLE 1.
Patient Demographics and Baseline Characteristics
Safety
Any-grade TRAEs were observed in 121 (70%) patients (Data Supplement), with grade 3 in 34 (20%), grade 4 in 2 (1%), and no fatal TRAEs; TRAEs led to treatment reduction or interruption in 39 (22%) and treatment discontinuation in 11 (6%). Most common TRAEs were diarrhea (53 [30%]), increased alanine aminotransferase level (31 [18%]), and increased aspartate aminotransferase level (31 [18%]). Median (range) time to grade ≥ 3 diarrhea and hepatotoxicity onset was 6.1 (1.7-11.1) and 9.1 (3.1-18.7) weeks. All grade ≥ 3 (median [range] duration, weeks) diarrhea resolved (2.9 [0.3-6.0]); grade ≥ 3 hepatotoxicity resolved in all but three of 19 (16%; 5.5 [0.4-39.1]). Trends toward increased hepatotoxicity in patients receiving checkpoint inhibitors ≤ versus >3 months before sotorasib initiation were observed (Data Supplement).
Of 45 patients who continued sotorasib beyond 1 year, 11 (24%) had any-grade TRAEs after 1 year on treatment (new-onset TRAEs), without trends in adverse event type. One grade 3 new-onset TRAE (2%; hemolytic anemia) resolved in 5 days (sotorasib discontinued after disease progression). No grade 4 or 5 new-onset TRAEs occurred. New-onset TRAEs led to dose reduction in one (2%) patient without treatment discontinuation.
Efficacy
ORR was 41% (95% CI, 33.3 to 48.4), and DCR was 84% (95% CI, 77.3 to 88.9; Data Supplement). Of patients with confirmed response, estimated 72.8% (95% CI, 60.0 to 82.2) and 50.6% (37.4 to 62.4) remained in response at 6 and 12 months, respectively. Median DOR was 12.3 months (95% CI, 7.1 to 15.0).
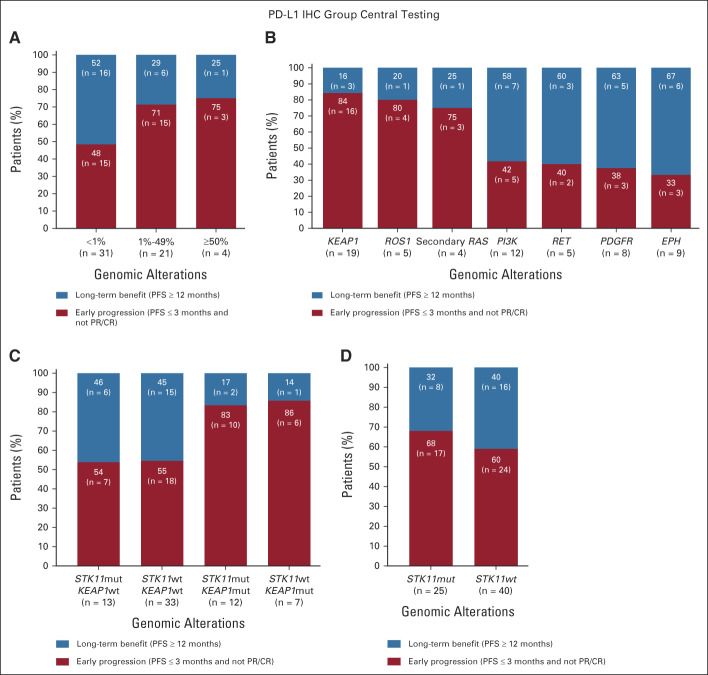
Median PFS was 6.3 months (95% CI, 5.3 to 8.2; Fig 1A); median OS was 12.5 months (10.0 to 17.8; Fig 1B). Kaplan-Meier OS estimate was 51% (95% CI, 42.8 to 58.2) and 33% (95% CI, 25.0 to 40.2) at 12 and 24 months, respectively. At data cutoff, nine of 70 (13%) patients with response remained on study without progression, including five receiving sotorasib for ≥2 years with continued response (Fig 1C).
FIG 1.
Long-term benefit and outcomes with sotorasib treatment. Kaplan-Meier plot of (A) PFS and (B) OS (median OS follow-up of 24.9 months [range, 0.7-35.9 months]) by central review, and (C) swimmer plot for phase I and phase II responders by central review (one patient with an ongoing response ended treatment because of patient request). BOR, best objective response; CR, complete response; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response.
Sixteen patients had evaluable brain metastases per central Response Assessment in exploratory Neuro-Oncology Brain Metastases review (Data Supplement); three (19%) had complete response and 11 (69%) had stable disease, with 14 (88%) having intracranial disease control overall (Data Supplement).
Across 172 efficacy-evaluable patients, 40 (23%) had long-term clinical benefit (PFS ≥ 12 months) and 62 (36%) had early progression (PFS ≤ 3 months). Baseline characteristics were similar; the latter had slightly higher proportions of patients with visceral metastasis (liver/bone), progressive disease on prior therapy, and received prior platinum-based chemotherapy and immunotherapy.
Biomarker Analysis
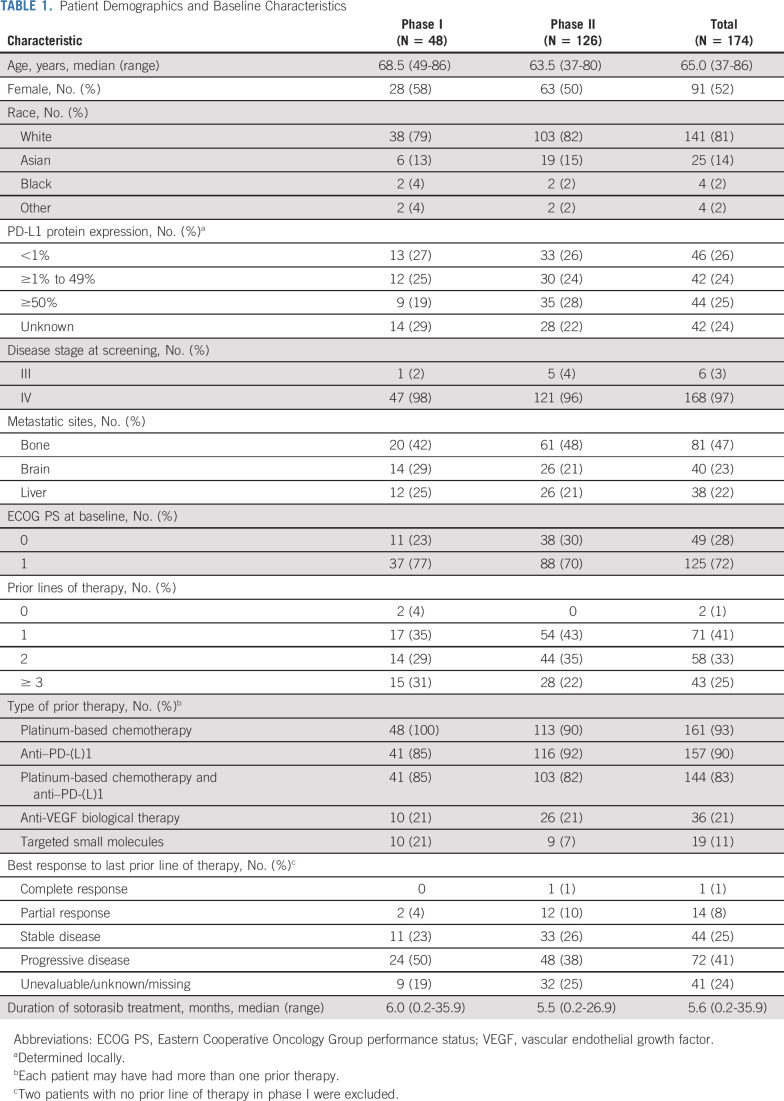
Centrally measured PD-L1 and/or genomic data were available for 114 phase II patients (Data Supplement). Most prevalent alterations were TP53 (46%), LRP1B (36%), KDM6A (32%), and STK11 (32%).
Albeit limited in sample size, long-term clinical benefit with sotorasib was observed across PD-L1 expression levels (Fig 2A). There was a nonsignificant trend toward enrichment of longer benefit with PD-L1 tumor proportion score (TPS) <1% versus ≥1% (odds ratio [OR], 0.36 [0.12 to 1.12]), without significant differences between PD-L1 TPS 1%-49% and ≥50% (OR, 0.83 [0.07 to 9.69]). Most significant enrichment in patients with early progression was with mutant KEAP1 (OR, 0.22 [0.06 to 0.87] long-term benefit v early progression); although not significant, these patients were more likely to have ROS1 (single-nucleotide variant [SNV]) and secondary RAS mutations (Fig 2B; Data Supplement). Patients with long-term benefit were more likely to harbor mutations in PI3K, PDGFR, and EPH receptor gene family and RET SNVs. Association of KEAP1 wild-type status with long-term benefit was independent of STK11 mutation status (Fig 2C). Patients with STK11 comutations were as likely to derive long-term benefit as patients with STK11 wild-type (OR, 0.71 [0.25 to 2.02]; Fig 2D).
FIG 2.
Association of long-term benefit and early progression with PD-L1 expression and genomic alterations: (A) PD-L1 tumor proportion score, (B) selected genomic alterations, (C) STK11 and KEAP1 mutations, and (D) Mutant versus wild-type STK11. CR, complete response; IHC, immunohistochemistry; PFS, progression-free survival; PR, partial response.
No difference in tumor tissue median KRAS G12C variant allele frequency (VAF) or tumor mutational burden was observed in long-term benefit or early progression groups (Data Supplement). Patients with long-term benefit tended to have lower baseline median plasma circulating tumor DNA (ctDNA; P = .01; Data Supplement).
DISCUSSION
In this analysis representing the most mature KRASG12C inhibitor clinical data, sotorasib demonstrated long-term efficacy, without new safety signals.16-18 A substantial proportion of patients derived long-term clinical benefit (1- and 2-year OS rates, 51% and 33%, respectively). Once-daily oral sotorasib 960 mg did not result in cumulative late-onset severe or chronic lower-grade toxicities.
Durable sotorasib benefit and safety profiles compare favorably with standard-of-care chemotherapy with docetaxel-based regimens, which historically yielded approximately 10%-23% response rates and a median PFS < 4.5 months.19-24 Two-year OS rate (33%) with sotorasib was higher versus docetaxel (historically 14%).2 In the phase III CodeBreaK 200 randomized controlled trial (ClinicalTrials.gov identifier: NCT04303780), sotorasib showed statistically significant improvement in PFS versus docetaxel in pretreated KRAS G12C-mutated advanced NSCLC, with a 34% decrease in the relative risk of disease progression or death with sotorasib (HR 0.66; P = .0017).25 There was no OS difference, although the study was not powered for OS, and docetaxel arm crossover was permitted25; improved quality of life with sotorasib was observed.25 These findings are encouraging, considering historically poor standard-of-care chemotherapy outcomes.
Long-term benefit with sotorasib was associated with lower baseline ctDNA levels, consistent with ctDNA prognostic roles across therapeutics.26-28 Prolonged benefit was observed across KRAS G12C VAF levels, PD-L1 expression, and a proportion of patients with STK11 and/or KEAP1 comutations. However, consistent with studies of other therapies,29,30 KEAP1 mutation was negatively prognostic overall. Relatively small sample sizes with available biomarker data were challenging; additional analyses evaluating prognostic and predictive impact of baseline and postprogression genomic alterations are warranted. International collaboration and data sharing are key to uncovering KRAS-mutant cancer molecular complexities.31-33
In this long-term analysis, oral once-daily sotorasib demonstrated favorable safety profile and durable efficacy across subgroups in KRAS G12C-mutated NSCLC.
ACKNOWLEDGMENT
The authors thank the patients and their families for participating in this trial, as well as Lisa R. Denny, PhD, and Lee B. Hohaia, PharmD (ICON, Blue Bell, PA), whose work was funded by Amgen Inc, for medical writing assistance in the preparation of this manuscript. The authors thank Agnes Ang, PhD, who contributed to the biomarker analysis presented in this work, Maya Shehayeb, PharmD, and Jennifer Martucci for operational planning assistance, Bob Dawson for graphics assistance, Liz Leight, PhD, and Brittany L. Phillips, PhD, for medical writing support (all employed by Amgen Inc, Thousand Oaks, CA).
Grace K. Dy
Consulting or Advisory Role: AstraZeneca, Mirati Therapeutics, Lilly, Amgen
Research Funding: Amgen (Inst), AstraZeneca (Inst), Mirati Therapeutics (Inst), Lilly (Inst), Sanofi (Inst), Bioatla (Inst), Regeneron (Inst), Iovance Biotherapeutics (Inst), Revolution Medicines (Inst)
Ramaswamy Govindan
Consulting or Advisory Role: Merck, Jacobio, Precisca
Vamsidhar Velcheti
Honoraria: ITeos Therapeutics
Consulting or Advisory Role: Bristol Myers Squibb, Merck, AstraZeneca/MedImmune, GlaxoSmithKline, Amgen, Elevation Oncology, Merus, Taiho Oncology
Research Funding: Genentech (Inst), Trovagene (Inst), Eisai (Inst), OncoPlex Diagnostics (Inst), Alkermes (Inst), NantWorks (Inst), Genoptix (Inst), Altor BioScience (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Atreca (Inst), Heat Biologics (Inst), Leap Therapeutics (Inst), RSIP Vision (Inst), GlaxoSmithKline (Inst)
Gerald S. Falchook
Employment: Sarah Cannon Research Institute, HealthONE
Honoraria: Rocky Mountain Oncology Society
Consulting or Advisory Role: Fujifilm (Inst), EMD Serono, Silicon Therapeutics (Inst), Navire (Inst), Turning Point Therapeutics (Inst), Silicon Therapeutics (Inst), Predicine (Inst), Inspirna (Inst), Regeneron (Inst), Jubilant Pharmaceuticals (Inst), BostonGene (Inst), BostonGene (Inst), AbbVie (Inst), Teon Therapeutics (Inst), Merck (Inst)
Speakers' Bureau: Total Health Conferencing
Research Funding: Millennium (Inst), EMD Serono (Inst), Celgene (Inst), MedImmune (Inst), Genmab (Inst), Vegenics (Inst), Novartis (Inst), AstraZeneca (Inst), Incyte (Inst), ARMO BioSciences (Inst), Kolltan Pharmaceuticals (Inst), 3-V Biosciences (Inst), AbbVie (Inst), Aileron Therapeutics (Inst), DelMar Pharmaceuticals (Inst), eFFECTOR Therapeutics (Inst), Strategia Therapeutics (Inst), Fujifilm (Inst), Hutchison MediPharma (Inst), Regeneron (Inst), Biothera (Inst), Curegenix (Inst), Curis (Inst), Lilly (Inst), Jounce Therapeutics (Inst), OncoMed (Inst), Precision Oncology (Inst), Syndax (Inst), Taiho Pharmaceutical (Inst), Tesaro (Inst), Takeda (Inst), BeiGene (Inst), Ignyta (Inst), Merck (Inst), Rgenix (Inst), Tarveda Therapeutics (Inst), Tocagen (Inst), Loxo (Inst), Jacobio (Inst), Ciclomed (Inst), miRNA Therapeutics (Inst), Celldex (Inst), ADC Therapeutics (Inst), Amgen (Inst), Exelixis (Inst), Bioatla (Inst), Turning Point Therapeutics (Inst), Ribon Therapeutics (Inst), Cyteir (Inst), Xencor (Inst), Daiichi (Inst), Epizyme (Inst), Abbisko (Inst), Prelude Therapeutics (Inst), Poseida (Inst), Oncorus (Inst), Synthorx (Inst), BioInvent (Inst), Sapience Therapeutics (Inst), Bicycle Therapeutics (Inst), Silicon Therapeutics (Inst), PureTech (Inst), Immunogen/MacroGenics (Inst), IgM Biosciences (Inst), Navire (Inst), TeneoBio (Inst), Erasca, Inc (Inst), RasCal (Inst), Boehringer Ingelheim (Inst), Pyramid Biosciences (Inst), Samumed (Inst), ABL Bio (Inst), Freenome (Inst), Artios (Inst), NiKang Therapeutics (Inst), Molecular Templates (Inst), Sirnaomics (Inst), Accutar Biotech (Inst), Relay Therapeutics (Inst), Simcha Therapeutics (Inst), Black Diamond Therapeutics (Inst), Seagen (Inst), Jubilant Pharmaceuticals (Inst), Metabomed (Inst), Agenus (Inst), Tallac Therapeutics (Inst), Zhuhai Yufan Biotechnologies (Inst), Mirati Therapeutics (Inst), Immunitas (Inst), Jazz Pharmaceuticals (Inst), Bayer (Inst)
Patents, Royalties, Other Intellectual Property: Handbook of Targeted Cancer Therapy
Travel, Accommodations, Expenses: Millennium, Sarah Cannon Research Institute, EMD Serono, Bristol Myers Squibb, Fujifilm, Amgen, Synthorx/Sanofi
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, Ipsen
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: Bristol Myers Squibb
Jürgen Wolf
Honoraria: AbbVie, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, MSD, Novartis, Roche, Amgen, Bayer, Blueprint Medicines, Chugai Pharma Europe, Daiichi Sankyo Europe GmbH, Ignyta, Janssen, Lilly, Loxo, Loxo/Lilly, Pfizer, Seagen, Takeda, Nuvalent, Inc
Consulting or Advisory Role: AbbVie, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Chugai Pharma, Ignyta, Lilly, MSD Oncology, Novartis, Pfizer, Roche, Janssen, Loxo/Lilly, Blueprint Medicines, Amgen, Takeda, Amgen, Bayer, Blueprint Medicines, Daiichi Sankyo Europe GmbH, Seagen, Nuvalent, Inc
Research Funding: Bristol Myers Squibb, Novartis, Pfizer, Janssen
Travel, Accommodations, Expenses: Bristol Myers Squibb, Janssen, Novartis, Pfizer
Adrian G. Sacher
Research Funding: AstraZeneca, Genentech/Roche, Bristol Myers Squibb
Uncompensated Relationships: Genentech/Roche, AstraZeneca
Toshiaki Takahashi
Honoraria: AstraZeneca Japan, Chugai Pharma, Lilly Japan, Ono Pharmaceutical, MSD K.K, Pfizer, Boehringer Ingelheim, Roche, Takeda, Yakult Honsha
Research Funding: AstraZeneca Japan (Inst), Lilly Japan (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), MSD K.K (Inst), Pfizer (Inst), Amgen (Inst), Boehringer Ingelheim (Inst), Merck (Inst)
Suresh S. Ramalingam
Consulting or Advisory Role: GlaxoSmithKline
Research Funding: AbbVie (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Vertex (Inst), Takeda (Inst), EMD Serono (Inst), Genmab (Inst), Advaxis (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Other Relationship: American Cancer Society
Dong-Wan Kim
Research Funding: Alpha Biopharma (Inst), AstraZeneca/MedImmune (Inst), Hanmi (Inst), Janssen (Inst), Merus (Inst), Mirati Therapeutics (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Roche/Genentech (Inst), Takeda (Inst), TP Therapeutics (Inst), Xcovery (Inst), Yuhan (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Chong Kun Dang Pharmaceutical (Inst), BridgeBio Pharma (Inst), GlaxoSmithKline (Inst), Merck (Inst), inno.N (Inst)
Alfredo Addeo
Consulting or Advisory Role: Roche, AstraZeneca/MedImmune, Bristol Myers Squibb Foundation, MSD Oncology, Pfizer, Novartis, Astellas Pharma, Amgen, Lilly
Speakers' Bureau: Lilly, AstraZeneca/MedImmune
Travel, Accommodations, Expenses: Roche, Takeda
Jayesh Desai
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: BeiGene, Amgen (Inst), Pierre Fabre, Bayer, GlaxoSmithKline, Merck KGaA, Boehringer Ingelheim, Roche/Genentech, Daiichi Sankyo Europe GmbH, Novartis, Pfizer
Research Funding: Roche (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Bionomics (Inst), BeiGene (Inst), Lilly (Inst), Bristol Myers Squibb (Inst), AstraZeneca/MedImmune (Inst)
Martin Schuler
Honoraria: Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Amgen, Janssen-Cilag
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Roche, Takeda, Amgen, GlaxoSmithKline, Merck Serono, Sanofi, Janssen Oncology
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Highly sensitive method for mutation detection by PCR (Inst)
Pascale Tomasini
Consulting or Advisory Role: AstraZeneca, Roche, Bristol Myers Squibb Foundation, Takeda, Amgen, Janssen
Travel, Accommodations, Expenses: Bristol Myers Squibb/Pfizer, AstraZeneca, Takeda
David S. Hong
Stock and Other Ownership Interests: OncoResponse, Telperian, MolecularMatch
Consulting or Advisory Role: Bayer, Guidepoint Global, Gerson Lehrman Group, Alphasights, Axiom Biotechnologies, Medscape, Numab, Pfizer, Takeda, Trieza Therapeutics, WebMD, Infinity Pharmaceuticals, Amgen, Adaptimmune, Boxer Capital, EcoR1 Capital, Tavistock Life Sciences, Baxter, COG, Genentech, GroupH, Janssen, Acuta, HCW Precision, Prime Oncology, ST Cube, Alkermes, AUM Biosciences, Bridgebio, Cor2Ed, Gilead Sciences, Immunogen, Liberum, Oncologia Brasil, Pharma Intelligence, Precision Oncology Experimental Therapeutics, Turning Point Therapeutics, ZIOPHARM Oncology, Cowen, Gennao Bio, MedaCorp, YingLing Pharma, RAIN
Research Funding: Genentech (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Adaptimmune (Inst), AbbVie (Inst), Bayer (Inst), Infinity Pharmaceuticals (Inst), Kite, a Gilead Company (Inst), MedImmune (Inst), National Cancer Institute (Inst), Fate Therapeutics (Inst), Pfizer (Inst), Novartis (Inst), Numab (Inst), Turning Point Therapeutics (Inst), Kyowa (Inst), Loxo (Inst), Merck (Inst), Eisai (Inst), Genmab (Inst), Mirati Therapeutics (Inst), Mologen (Inst), Takeda (Inst), AstraZeneca (Inst), Navire (Inst), VM Pharma (Inst), Erasca, Inc (Inst), Bristol Myers Squibb (Inst), Adlai Nortye (Inst), Seagen (Inst), Deciphera (Inst), Pyramid Biosciences (Inst), Lilly (Inst), Endeavor BioMedicines (Inst), F. Hoffmann LaRoche (Inst), Ignyta (Inst), Teckro (Inst), TCR2 Therapeutics (Inst)
Travel, Accommodations, Expenses: Genmab, Society for Immunotherapy of Cancer, Bayer Schering Pharma, ASCO, AACR, Telperian
Piro Lito
Leadership: Frontier Medicines
Consulting or Advisory Role: Black Diamond Therapeutics, Repare Therapeutics, AmMax Bio, Revolution Medicines
Speakers' Bureau: Boehringer Ingelheim
Research Funding: Mirati Therapeutics (Inst), Revolution Medicines (Inst), Amgen (Inst), Boehringer Ingelheim (Inst), Virtec Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: I am listed as an inventor on a patent application filed by MSKCC that describes an approach to treat BRAF mutant cancers (Inst), I am listed as an inventor on a patent application filed by MSKCC that describes an approach to treat KRAS mutant cancers (Inst)
Qui Tran
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Simon Jones
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Travel, Accommodations, Expenses: Amgen
Abraham Anderson
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Patents, Royalties, Other Intellectual Property: I am listed as an inventor on several Amgen patents. I do not receive royalties on these patents
Travel, Accommodations, Expenses: Amgen
Antreas Hindoyan
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Wendy Snyder
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Travel, Accommodations, Expenses: Amgen
Ferdinandos Skoulidis
Stock and Other Ownership Interests: Moderna Therapeutics, BioNTech
Honoraria: McGill Universite de Montreal, ESMO, RV Mais, Moving Innovation and Technology, Physicans' Education Resource
Consulting or Advisory Role: Amgen, Intellisphere, Navire, BeiGene, Medscape, Calithera Biosciences, Tango Therapeutics, Guardant Health, Novartis
Research Funding: Amgen (Inst), AIMM Therapeutics (I), Mirati Therapeutics (Inst), Boehringer Ingelheim (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst)
Bob T. Li
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst), Hengrui Therapeutics (Inst), Amgen (Inst), Lilly (Inst), MORE Health (Inst), Bolt Biotherapeutics (Inst), Ambrx (Inst)
Patents, Royalties, Other Intellectual Property: US62/514,661 (Inst), US62/685,057 (Inst), Karger Publishers—Book royalty, Shanghai Jiao Tong University Press—Book royalty
Travel, Accommodations, Expenses: MORE Health, Jiangsu Hengrui Medicine
Uncompensated Relationships: Amgen, AstraZeneca, Genentech, Lilly, Boehringer Ingelheim, Daiichi Sankyo
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the American Association of Cancer Research Annual Meeting, New Orleans, LA, April 8-13, 2022.
SUPPORT
Supported by Amgen Inc.
CLINICAL TRIAL INFORMATION
NCT03600883 (CodeBreaK100)
G.K.D., F.S., and B.T.L. contributed equally to this work.
DATA SHARING STATEMENT
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Outcomes and Molecular Correlates of Sotorasib Efficacy in Patients With Pretreated KRAS G12C-Mutated Non–Small-Cell Lung Cancer: 2-Year Analysis of CodeBreaK 100
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Grace K. Dy
Consulting or Advisory Role: AstraZeneca, Mirati Therapeutics, Lilly, Amgen
Research Funding: Amgen (Inst), AstraZeneca (Inst), Mirati Therapeutics (Inst), Lilly (Inst), Sanofi (Inst), Bioatla (Inst), Regeneron (Inst), Iovance Biotherapeutics (Inst), Revolution Medicines (Inst)
Ramaswamy Govindan
Consulting or Advisory Role: Merck, Jacobio, Precisca
Vamsidhar Velcheti
Honoraria: ITeos Therapeutics
Consulting or Advisory Role: Bristol Myers Squibb, Merck, AstraZeneca/MedImmune, GlaxoSmithKline, Amgen, Elevation Oncology, Merus, Taiho Oncology
Research Funding: Genentech (Inst), Trovagene (Inst), Eisai (Inst), OncoPlex Diagnostics (Inst), Alkermes (Inst), NantWorks (Inst), Genoptix (Inst), Altor BioScience (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Atreca (Inst), Heat Biologics (Inst), Leap Therapeutics (Inst), RSIP Vision (Inst), GlaxoSmithKline (Inst)
Gerald S. Falchook
Employment: Sarah Cannon Research Institute, HealthONE
Honoraria: Rocky Mountain Oncology Society
Consulting or Advisory Role: Fujifilm (Inst), EMD Serono, Silicon Therapeutics (Inst), Navire (Inst), Turning Point Therapeutics (Inst), Silicon Therapeutics (Inst), Predicine (Inst), Inspirna (Inst), Regeneron (Inst), Jubilant Pharmaceuticals (Inst), BostonGene (Inst), BostonGene (Inst), AbbVie (Inst), Teon Therapeutics (Inst), Merck (Inst)
Speakers' Bureau: Total Health Conferencing
Research Funding: Millennium (Inst), EMD Serono (Inst), Celgene (Inst), MedImmune (Inst), Genmab (Inst), Vegenics (Inst), Novartis (Inst), AstraZeneca (Inst), Incyte (Inst), ARMO BioSciences (Inst), Kolltan Pharmaceuticals (Inst), 3-V Biosciences (Inst), AbbVie (Inst), Aileron Therapeutics (Inst), DelMar Pharmaceuticals (Inst), eFFECTOR Therapeutics (Inst), Strategia Therapeutics (Inst), Fujifilm (Inst), Hutchison MediPharma (Inst), Regeneron (Inst), Biothera (Inst), Curegenix (Inst), Curis (Inst), Lilly (Inst), Jounce Therapeutics (Inst), OncoMed (Inst), Precision Oncology (Inst), Syndax (Inst), Taiho Pharmaceutical (Inst), Tesaro (Inst), Takeda (Inst), BeiGene (Inst), Ignyta (Inst), Merck (Inst), Rgenix (Inst), Tarveda Therapeutics (Inst), Tocagen (Inst), Loxo (Inst), Jacobio (Inst), Ciclomed (Inst), miRNA Therapeutics (Inst), Celldex (Inst), ADC Therapeutics (Inst), Amgen (Inst), Exelixis (Inst), Bioatla (Inst), Turning Point Therapeutics (Inst), Ribon Therapeutics (Inst), Cyteir (Inst), Xencor (Inst), Daiichi (Inst), Epizyme (Inst), Abbisko (Inst), Prelude Therapeutics (Inst), Poseida (Inst), Oncorus (Inst), Synthorx (Inst), BioInvent (Inst), Sapience Therapeutics (Inst), Bicycle Therapeutics (Inst), Silicon Therapeutics (Inst), PureTech (Inst), Immunogen/MacroGenics (Inst), IgM Biosciences (Inst), Navire (Inst), TeneoBio (Inst), Erasca, Inc (Inst), RasCal (Inst), Boehringer Ingelheim (Inst), Pyramid Biosciences (Inst), Samumed (Inst), ABL Bio (Inst), Freenome (Inst), Artios (Inst), NiKang Therapeutics (Inst), Molecular Templates (Inst), Sirnaomics (Inst), Accutar Biotech (Inst), Relay Therapeutics (Inst), Simcha Therapeutics (Inst), Black Diamond Therapeutics (Inst), Seagen (Inst), Jubilant Pharmaceuticals (Inst), Metabomed (Inst), Agenus (Inst), Tallac Therapeutics (Inst), Zhuhai Yufan Biotechnologies (Inst), Mirati Therapeutics (Inst), Immunitas (Inst), Jazz Pharmaceuticals (Inst), Bayer (Inst)
Patents, Royalties, Other Intellectual Property: Handbook of Targeted Cancer Therapy
Travel, Accommodations, Expenses: Millennium, Sarah Cannon Research Institute, EMD Serono, Bristol Myers Squibb, Fujifilm, Amgen, Synthorx/Sanofi
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, Ipsen
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: Bristol Myers Squibb
Jürgen Wolf
Honoraria: AbbVie, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, MSD, Novartis, Roche, Amgen, Bayer, Blueprint Medicines, Chugai Pharma Europe, Daiichi Sankyo Europe GmbH, Ignyta, Janssen, Lilly, Loxo, Loxo/Lilly, Pfizer, Seagen, Takeda, Nuvalent, Inc
Consulting or Advisory Role: AbbVie, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Chugai Pharma, Ignyta, Lilly, MSD Oncology, Novartis, Pfizer, Roche, Janssen, Loxo/Lilly, Blueprint Medicines, Amgen, Takeda, Amgen, Bayer, Blueprint Medicines, Daiichi Sankyo Europe GmbH, Seagen, Nuvalent, Inc
Research Funding: Bristol Myers Squibb, Novartis, Pfizer, Janssen
Travel, Accommodations, Expenses: Bristol Myers Squibb, Janssen, Novartis, Pfizer
Adrian G. Sacher
Research Funding: AstraZeneca, Genentech/Roche, Bristol Myers Squibb
Uncompensated Relationships: Genentech/Roche, AstraZeneca
Toshiaki Takahashi
Honoraria: AstraZeneca Japan, Chugai Pharma, Lilly Japan, Ono Pharmaceutical, MSD K.K, Pfizer, Boehringer Ingelheim, Roche, Takeda, Yakult Honsha
Research Funding: AstraZeneca Japan (Inst), Lilly Japan (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), MSD K.K (Inst), Pfizer (Inst), Amgen (Inst), Boehringer Ingelheim (Inst), Merck (Inst)
Suresh S. Ramalingam
Consulting or Advisory Role: GlaxoSmithKline
Research Funding: AbbVie (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Vertex (Inst), Takeda (Inst), EMD Serono (Inst), Genmab (Inst), Advaxis (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Other Relationship: American Cancer Society
Dong-Wan Kim
Research Funding: Alpha Biopharma (Inst), AstraZeneca/MedImmune (Inst), Hanmi (Inst), Janssen (Inst), Merus (Inst), Mirati Therapeutics (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Roche/Genentech (Inst), Takeda (Inst), TP Therapeutics (Inst), Xcovery (Inst), Yuhan (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Chong Kun Dang Pharmaceutical (Inst), BridgeBio Pharma (Inst), GlaxoSmithKline (Inst), Merck (Inst), inno.N (Inst)
Alfredo Addeo
Consulting or Advisory Role: Roche, AstraZeneca/MedImmune, Bristol Myers Squibb Foundation, MSD Oncology, Pfizer, Novartis, Astellas Pharma, Amgen, Lilly
Speakers' Bureau: Lilly, AstraZeneca/MedImmune
Travel, Accommodations, Expenses: Roche, Takeda
Jayesh Desai
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: BeiGene, Amgen (Inst), Pierre Fabre, Bayer, GlaxoSmithKline, Merck KGaA, Boehringer Ingelheim, Roche/Genentech, Daiichi Sankyo Europe GmbH, Novartis, Pfizer
Research Funding: Roche (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Bionomics (Inst), BeiGene (Inst), Lilly (Inst), Bristol Myers Squibb (Inst), AstraZeneca/MedImmune (Inst)
Martin Schuler
Honoraria: Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Amgen, Janssen-Cilag
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Roche, Takeda, Amgen, GlaxoSmithKline, Merck Serono, Sanofi, Janssen Oncology
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Highly sensitive method for mutation detection by PCR (Inst)
Pascale Tomasini
Consulting or Advisory Role: AstraZeneca, Roche, Bristol Myers Squibb Foundation, Takeda, Amgen, Janssen
Travel, Accommodations, Expenses: Bristol Myers Squibb/Pfizer, AstraZeneca, Takeda
David S. Hong
Stock and Other Ownership Interests: OncoResponse, Telperian, MolecularMatch
Consulting or Advisory Role: Bayer, Guidepoint Global, Gerson Lehrman Group, Alphasights, Axiom Biotechnologies, Medscape, Numab, Pfizer, Takeda, Trieza Therapeutics, WebMD, Infinity Pharmaceuticals, Amgen, Adaptimmune, Boxer Capital, EcoR1 Capital, Tavistock Life Sciences, Baxter, COG, Genentech, GroupH, Janssen, Acuta, HCW Precision, Prime Oncology, ST Cube, Alkermes, AUM Biosciences, Bridgebio, Cor2Ed, Gilead Sciences, Immunogen, Liberum, Oncologia Brasil, Pharma Intelligence, Precision Oncology Experimental Therapeutics, Turning Point Therapeutics, ZIOPHARM Oncology, Cowen, Gennao Bio, MedaCorp, YingLing Pharma, RAIN
Research Funding: Genentech (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Adaptimmune (Inst), AbbVie (Inst), Bayer (Inst), Infinity Pharmaceuticals (Inst), Kite, a Gilead Company (Inst), MedImmune (Inst), National Cancer Institute (Inst), Fate Therapeutics (Inst), Pfizer (Inst), Novartis (Inst), Numab (Inst), Turning Point Therapeutics (Inst), Kyowa (Inst), Loxo (Inst), Merck (Inst), Eisai (Inst), Genmab (Inst), Mirati Therapeutics (Inst), Mologen (Inst), Takeda (Inst), AstraZeneca (Inst), Navire (Inst), VM Pharma (Inst), Erasca, Inc (Inst), Bristol Myers Squibb (Inst), Adlai Nortye (Inst), Seagen (Inst), Deciphera (Inst), Pyramid Biosciences (Inst), Lilly (Inst), Endeavor BioMedicines (Inst), F. Hoffmann LaRoche (Inst), Ignyta (Inst), Teckro (Inst), TCR2 Therapeutics (Inst)
Travel, Accommodations, Expenses: Genmab, Society for Immunotherapy of Cancer, Bayer Schering Pharma, ASCO, AACR, Telperian
Piro Lito
Leadership: Frontier Medicines
Consulting or Advisory Role: Black Diamond Therapeutics, Repare Therapeutics, AmMax Bio, Revolution Medicines
Speakers' Bureau: Boehringer Ingelheim
Research Funding: Mirati Therapeutics (Inst), Revolution Medicines (Inst), Amgen (Inst), Boehringer Ingelheim (Inst), Virtec Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: I am listed as an inventor on a patent application filed by MSKCC that describes an approach to treat BRAF mutant cancers (Inst), I am listed as an inventor on a patent application filed by MSKCC that describes an approach to treat KRAS mutant cancers (Inst)
Qui Tran
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Simon Jones
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Travel, Accommodations, Expenses: Amgen
Abraham Anderson
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Patents, Royalties, Other Intellectual Property: I am listed as an inventor on several Amgen patents. I do not receive royalties on these patents
Travel, Accommodations, Expenses: Amgen
Antreas Hindoyan
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Wendy Snyder
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Travel, Accommodations, Expenses: Amgen
Ferdinandos Skoulidis
Stock and Other Ownership Interests: Moderna Therapeutics, BioNTech
Honoraria: McGill Universite de Montreal, ESMO, RV Mais, Moving Innovation and Technology, Physicans' Education Resource
Consulting or Advisory Role: Amgen, Intellisphere, Navire, BeiGene, Medscape, Calithera Biosciences, Tango Therapeutics, Guardant Health, Novartis
Research Funding: Amgen (Inst), AIMM Therapeutics (I), Mirati Therapeutics (Inst), Boehringer Ingelheim (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst)
Bob T. Li
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst), Hengrui Therapeutics (Inst), Amgen (Inst), Lilly (Inst), MORE Health (Inst), Bolt Biotherapeutics (Inst), Ambrx (Inst)
Patents, Royalties, Other Intellectual Property: US62/514,661 (Inst), US62/685,057 (Inst), Karger Publishers—Book royalty, Shanghai Jiao Tong University Press—Book royalty
Travel, Accommodations, Expenses: MORE Health, Jiangsu Hengrui Medicine
Uncompensated Relationships: Amgen, AstraZeneca, Genentech, Lilly, Boehringer Ingelheim, Daiichi Sankyo
No other potential conflicts of interest were reported.
REFERENCES
- 1.Garon EB, Hellmann MD, Rizvi NA, et al. : Five-year overall survival for patients with advanced non–small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J Clin Oncol 37:2518-2527, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H, Gettinger S, Vokes EE, et al. : Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: Nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol 39:723-733, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. : Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627-1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Baas P, Kim DW, et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387:1540-1550, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Rittmeyer A, Barlesi F, Waterkamp D, et al. : Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255-265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M, Kaiser R, Mellemgaard A, et al. : Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol 15:143-155, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Canon J, Rex K, Saiki AY, et al. : The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 575:217-223, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Lito P, Solomon M, Li LS, et al. : Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science 351:604-608, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue JY, Zhao Y, Aronowitz J, et al. : Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature 577:421-425, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrem JM, Peters U, Sos ML, et al. : K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503:548-551, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LUMYKRAS (Sotorasib). Summary of Product Characteristics. Cambridge, United Kingdom, Amgen Ltd, 2021. https://www.medicines.org.uk/emc/product/12871/smpc#gref [Google Scholar]
- 12.LUMYKRAS (Sotorasib). Summary of Product Characteristics. Breda, the Netherlands, Amgen Europe BV , 2022. https://www.ema.europa.eu/en/documents/product-information/lumykras-epar-product-information_en.pdf [Google Scholar]
- 13.BioSpace : LUMAKRAS® (Sotorasib) Receives Approval in Japan for Patients With KRAS G12C-Mutated Advanced Non-small Cell Lung Cancer. 2022. https://www.biospace.com/article/releases/lumakras-sotorasib-receives-approval-in-japan-for-patients-with-kras-g12c-mutated-advanced-non-small-cell-lung-cancer/ [Google Scholar]
- 14.LUMAKRAS (Sotorasib) Tablets, for Oral Use. Prescribing Information. Thousand Oaks, CA, Amgen, 2021. https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Lumakras/lumakras_pi_hcp_english.pdf [Google Scholar]
- 15.ClinicalTrials.gov : A Phase 1/2, Study Evaluating the Safety, Tolerability, PK, and Efficacy of Sotorasib (AMG 510) in Subjects With Solid Tumors With a Specific KRAS Mutation (CodeBreaK 100). 2022. https://www.clinicaltrials.gov/ct2/show/NCT03600883 [Google Scholar]
- 16.Skoulidis F, Li BT, Dy GK, et al. : Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med 384:2371-2381, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong DS, Fakih MG, Strickler JH, et al. : KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med 383:1207-1217, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jänne PA, Riely GJ, Gadgeel SM, et al. : Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med 387:120-131, 2022 [DOI] [PubMed] [Google Scholar]
- 19.Garon EB, Ciuleanu TE, Arrieta O, et al. : Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 384:665-673, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Hanna N, Shepherd FA, Fossella FV, et al. : Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589-1597, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Jänne PA, van den Heuvel MM, Barlesi F, et al. : Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer: The SELECT-1 randomized clinical trial. JAMA 317:1844-1853, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh A, Daemen A, Nickles D, et al. : NRF2 activation promotes aggressive lung cancer and associates with poor clinical outcomes. Clin Cancer Res 27:877-888, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reck M, Brahmer J, Bennett B, et al. : Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer 102:23-30, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Barlesi F, Garon EB, Kim DW, et al. : Health-related quality of life in KEYNOTE-010: A phase II/III study of pembrolizumab versus docetaxel in patients with previously treated advanced, programmed death ligand 1-expressing NSCLC. J Thorac Oncol 14:793-801, 2019 [DOI] [PubMed] [Google Scholar]
- 25.de Langen AJ, Johnson ML, Mazieres J, et al. : Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: A randomised, open-label, phase 3 trial. Lancet, 401:733-746, 2023 [DOI] [PubMed] [Google Scholar]
- 26.Avanzini S, Kurtz DM, Chabon JJ, et al. : A mathematical model of ctDNA shedding predicts tumor detection size. Sci Adv 6:eabc4308, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razavi P, Li BT, Brown DN, et al. : High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat Med 25:1928-1937, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jee J, Lebow ES, Yeh R, et al. : Overall survival with circulating tumor DNA-guided therapy in advanced non-small-cell lung cancer. Nat Med 28:2353-2363, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West HJ, McCleland M, Cappuzzo F, et al. : Clinical efficacy of atezolizumab plus bevacizumab and chemotherapy in KRAS-mutated non-small cell lung cancer with STK11, KEAP1, or TP53 comutations: Subgroup results from the phase III IMpower150 trial. J Immunother Cancer 10:e003027, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricciuti B, Arbour KC, Lin JJ, et al. : Diminished efficacy of programmed death-(ligand)1 inhibition in STK11- and KEAP1-mutant lung adenocarcinoma is affected by KRAS mutation status. J Thorac Oncol 17:399-410, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awad MM, Liu S, Rybkin II, et al. : Acquired resistance to KRASG12C inhibition in cancer. N Engl J Med 384:2382-2393, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Murciano-Goroff YR, Xue JY, et al. : Diverse alterations associated with resistance to KRAS(G12C) inhibition. Nature 599:679-683, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li BT, Daly B, Gospodarowicz M, et al. : Reimagining patient-centric cancer clinical trials: A multi-stakeholder international coalition. Nat Med 28:620-626, 2022 [DOI] [PubMed] [Google Scholar]