PURPOSE
Relapsed or refractory extranodal natural killer/T-cell lymphoma (R/R ENKTL) is a rare and aggressive type of non-Hodgkin lymphoma with limited treatment options. This phase II study evaluated the efficacy and safety of sugemalimab, an anti–PD-L1 monoclonal antibody, in R/R ENKTL.
METHODS
Eligible patients received sugemalimab 1,200 mg intravenously once every 3 weeks for up to 24 months or until progression, death, or study withdrawal. The primary end point was objective response rate (ORR) assessed by an independent radiologic review committee. Key secondary end points included ORR assessed by the investigators, complete response rate, duration of response, and safety.
RESULTS
At the data cutoff (February 23, 2022), 80 patients were enrolled and followed for a median of 18.7 months. At baseline, 54 (67.5%) had stage IV disease and 39 (48.8%) had received ≥2 lines of prior systemic therapy. Independent radiologic review committee–assessed ORR was 44.9% (95% CI, 33.6 to 56.6); 28 (35.9%) patients achieved a complete response and seven (9.0%) achieved a partial response, with a 12-month duration of response rate of 82.5% (95% CI, 62.0 to 92.6). Investigator-assessed ORR was 45.6% (95% CI, 34.3 to 57.2), and 24 (30.4%) patients achieved a complete response. Most treatment-emergent adverse events were grade 1-2 in severity, and grade ≥ 3 events were reported in 32 (40.0%) patients.
CONCLUSION
Sugemalimab showed robust and durable antitumor activity in R/R ENKTL. Treatment was well tolerated with expected safety profile for this drug class.
INTRODUCTION
Extranodal natural killer/T-cell lymphoma (ENKTL) is a rare, aggressive subtype of non-Hodgkin lymphoma derived from natural killer or cytotoxic T cells, and predominantly occurs in the nasal and paranasal areas.1 The distribution of ENKTL varies by geographical region and accounts for 3%-10% of patients with non-Hodgkin lymphoma in East Asia and Latin America and <1% in North America and Europe.1 Epstein-Barr virus (EBV) infection is found in the majority of ENKTL tumor cells and is thought to be implicated in its pathogenesis.1,2 High levels of circulating EBV-DNA copy numbers are strongly associated with tumor burden at diagnosis, disease progression, and poor prognosis.3
CONTEXT
Key Objective
The phase II registrational clinical study, GEMSTONE-201, is a single-arm, multicenter study investigating the efficacy and safety of sugemalimab, an anti–PD-L1 antibody, in patients with relapsed or refractory extranodal natural killer/T-cell lymphoma (R/R ENKTL) who have failed asparaginase-based chemotherapy. To our knowledge, this is the largest prospective study to date in R/R ENKTL, an area of significant unmet medical need.
Knowledge Generated
The data from GEMSTONE-201 indicate that sugemalimab offers a statistically significant and clinically meaningful benefit, with an acceptable safety profile in patients with R/R ENKTL. In the study, sugemalimab demonstrated statistically significant improvement in objective response rate compared with historical control, a numerically higher rate of complete response compared with known data, and also sustained duration of response among responders. The results assessed by the independent radiologic review committee and the investigator were highly concordant, indicating that the results of primary end point analyses were robust, and the results in each subgroup showed consistent clinical benefits.
Relevance (J.W. Friedberg)
Immune checkpoint blockade is active in R/R ENKTL; these promising results directly inform an ongoing larger prospective randomized trial in this space comparing chemotherapy alone to chemotherapy with sugemalimab.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
Treatment outcomes for ENKTL have improved with L-asparaginase–based regimens, especially against advanced disease.1,3 Despite improvement in overall survival, relapse rate has remained close to 50% at 5 years,4,5 and the median survival in the relapsed/refractory setting is only slightly more than 6 months.6 Currently, there is no standard treatment for relapsed/refractory disease, and the optimal strategy has yet to be determined. Although novel therapies in peripheral T-cell lymphoma such as chidamide, belinostat, and pralatrexate have shown antitumor activity in ENKTL, the limited number of patients with ENKTL enrolled in clinical trials and low response rates have highlighted the need for more effective therapies.7-9
Inhibition of PD-1/PD-L1 checkpoint signaling has been proposed as a therapeutic strategy for ENKTL as these tumor cells commonly express PD-L1.10,11 Moreover, EBV may induce immune tolerance of ENKTL by upregulating PD-L1 expression.11
Sugemalimab (previously CS1001) is a full-length, fully human immunoglobulin G4 (s228p) monoclonal anti–PD-L1 antibody. Unlike other antibodies that block Fc-null PD-L1, sugemalimab retains binding to FcγR I, inducing antibody-dependent cellular phagocytosis by crosslinking PD-L1–positive tumor cells with macrophages.12 Recently, sugemalimab regimens were approved by the National Medical Products Administration of China for stage III and IV non–small-cell lung cancer.13,14 Sugemalimab was also granted Breakthrough Therapy Designation by the United States Food and Drug Administration and China National Medical Products Administration for adult patients with relapsed/refractory ENKTL (R/R ENKTL) and was given the orphan drug designation for T-cell lymphoma in the United States.
Here, we report the results of a preplanned primary analysis of a multicenter, single-arm, phase II trial (GEMSTONE-201) of sugemalimab in patients with R/R ENKTL.
METHODS
Patients
Eligible patients were age 18-75 years and had histologically confirmed nasal and nonnasal ENKTL that was refractory to or relapsed after asparaginase-based chemotherapy or chemoradiotherapy. Enrolled patients had at least one measurable or evaluable lesion as per 2014 Lugano classification.15 Patients were required to provide immunohistochemically stained tumor tissue sections and corresponding pathologic reports or unstained tumor tissue sections (or tissue block) for central pathology review. Additional inclusion criteria were an Eastern Cooperative Oncology Group performance status score of 0 or 1, adequate organ function, and a life expectancy of ≥12 weeks.
Key exclusion criteria included the presence of aggressive natural killer–cell leukemia or hemophagocytic lymphohistiocytosis; primary or secondary central nervous system involvement; had prior anti–PD-1/anti–PD-L1 or anti–cytotoxic T-cell lymphocyte-4 treatment; had prior chemotherapy, immunotherapy, biological therapy, or underwent major surgery within 28 days, or radiotherapy within 90 days before the first dose of sugemalimab; had allogenic hematopoietic stem-cell transplantation ≤5 years, or autologous hematopoietic stem-cell transplantation within 90 days before the first dose of sugemalimab; had autoimmune disease that required systemic treatment in the past 2 years; and received systemic immunosuppressive agents within 14 days before the first dose of sugemalimab. The full list of eligibility criteria is provided in the trial Protocol (online only).
Study Design and Treatment
This single-arm, phase II trial was conducted at 16 centers across China. Patients received sugemalimab 1,200 mg intravenously once every 3 weeks (21-day cycle) for up to 2 years or until progression of disease, intolerable toxicity, withdrawal of consent, or death. All patients were followed for safety (90 days after the last dose of treatment or the start of a new anticancer treatment, whichever occurs earlier) and survival (every 12 weeks after the last dose of treatment). Patients could continue with treatment after initial disease progression at the discretion of the study investigator.
End Points and Assessments
The primary end point was objective response rate (ORR) assessed by an independent radiologic review committee (IRRC) on the basis of the Criteria for Response Assessment of Lymphoma in the 2014 Lugano classification.15 Key secondary end points included investigator-assessed ORR, IRRC- and investigator-assessed complete response rate and duration of response, and safety. The 6-month overall survival rate was also evaluated. Efficacy evaluation by imaging was performed at screening and every 12 weeks after the first dose of sugemalimab.
Safety was assessed on the basis of the frequency and severity of adverse events, coded according to the Medical Dictionary for Regulatory Activities, and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Adverse events of special interest were sponsor-assessed immune-related adverse events, defined using a list of preferred categories of terms specified by the sponsor. Adverse events were assessed throughout the treatment period, including the safety follow-up period.
Trial Oversight
CStone Pharmaceuticals funded the trial, provided the trial treatment, and collaborated with the authors on the design of the trial and the collection, analysis, and interpretation of the data. Tumor assessment was performed by both the IRRC and investigator. This study was conducted in accordance with the principles of the Declaration of Helsinki, the Committee for Proprietary Medicinal Products, International Council for Harmonisation Guidelines on Good Clinical Practice, and all other applicable regulations. The study protocol and all amendments were approved by the appropriate ethics committee at each study site. All patients provided written informed consent.
All authors had access to the data, were involved in the writing or critical review and editing of the manuscript, and vouch for the completeness and accuracy of data and for the fidelity of the trial protocol. A medical writer was employed by the sponsor to support the writing of the manuscript and provide editorial assistance.
Statistical Analysis
The null hypothesis was that ORR was 20% on the basis of historic control, compared with the estimated target of 40% in patients treated with sugemalimab. On the basis of an exact binomial test with a two-sided α of .05, a power of 97%, and a 5% dropout rate, a total of 80 patients were required to be enrolled in the study.
All patients who received at least one dose of sugemalimab were included in the safety analysis set. The efficacy analysis set consisted of all treated patients with confirmed diagnosis of ENKTL by central pathology.
CIs for the ORR were calculated using the exact binomial (Clopper-Pearson) method. The Kaplan-Meier method was used to analyze duration of response and overall survival. All statistical analyses were performed using SAS statistical software version 9.4 (or later).
RESULTS
Patient Characteristics
Between June 12, 2018, and May 26, 2021, 123 patients were screened; 80 patients were enrolled and treated with sugemalimab (Data Supplement, online only). Fifty-eight (72.5%) patients had discontinued treatment because of disease progression (41.3%), adverse events (13.8%), withdrawal by patient (10.0%), symptomatic deterioration without radiographic evidence of progression (6.3%), and death (1.3%). Twenty-two (27.5%) patients were still receiving treatment.
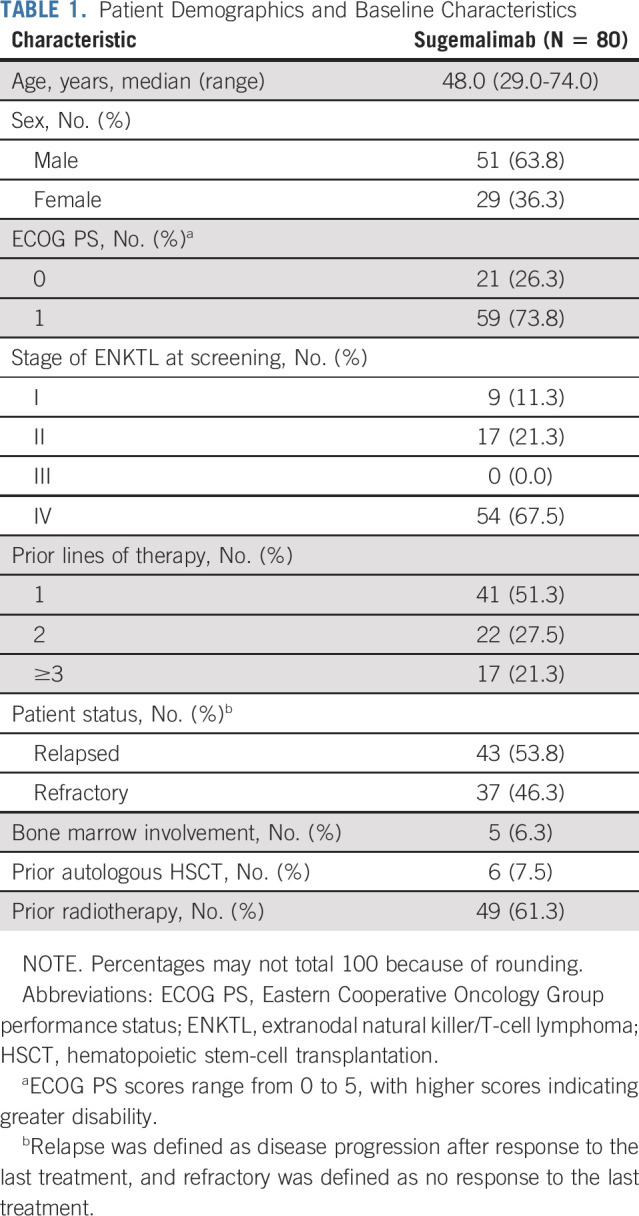
Patient demographic and baseline characteristics are presented in Table 1. Comparative patient representation of the study is presented in the Data Supplement. The median age was 48.0 (range, 29.0-74.0) years, and 59 (73.8%) patients had an Eastern Cooperative Oncology Group performance status score of 1. At screening, 54 (67.5%) patients had stage IV disease, and 39 (48.8%) patients had previously received ≥2 lines of systemic treatment. A total of 80 patients were included in the safety analysis set. One patient could not be confirmed as ENKTL by central pathology and was excluded from the efficacy analysis; one patient was considered not to have measurable or evaluable lesions at baseline as per the IRRC's retrospective assessment and was excluded from the IRRC's efficacy analysis. Therefore, a total of 79 and 78 patients were included in the investigator's and IRRC's efficacy analysis sets, respectively.
TABLE 1.
Patient Demographics and Baseline Characteristics
Efficacy
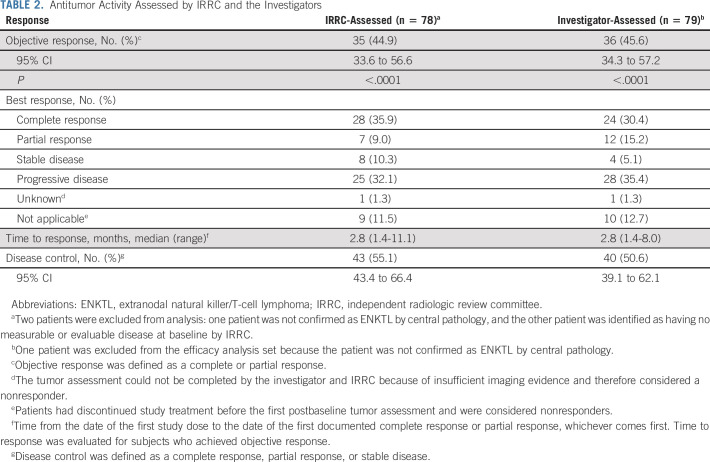
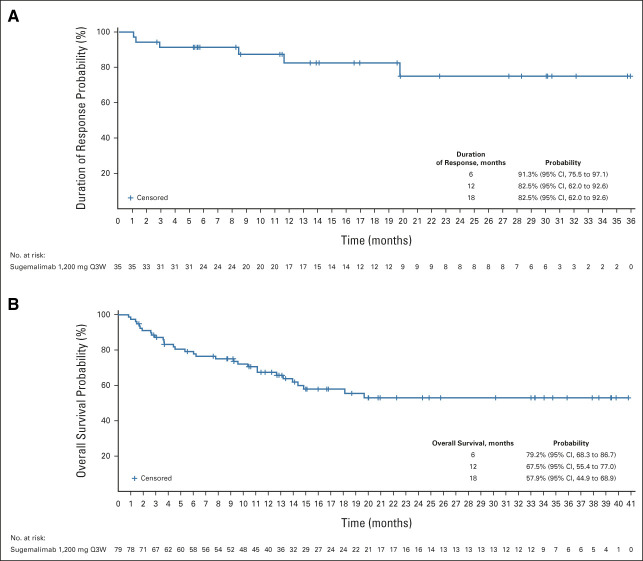
At the data cutoff date (February 23, 2022), the median follow-up time was 18.7 months. The IRRC-assessed ORR was 44.9% (95% CI, 33.6 to 56.6; Table 2); 28 (35.9%) patients achieved a complete response and seven (9.0%) achieved a partial response. Median duration of response was not reached (95% CI, 19.7 months to not reached; Fig 1A); 6-, 12-, and 18-month duration of response rates were 91.3% (95% CI, 75.5 to 97.1), 82.5% (95% CI, 62.0 to 92.6), and 82.5% (95% CI, 62.0 to 92.6), respectively.
TABLE 2.
Antitumor Activity Assessed by IRRC and the Investigators
FIG 1.
Kaplan-Meier plots of the (A) duration of response and (B) overall survival among patients with extranodal natural killer/T-cell lymphoma treated with sugemalimab. The duration of response was determined by the independent radiology review committee for 35 patients who had a response. The Kaplan-Meier curve of overall survival for 79 patients included in the efficacy analysis set. Tick marks indicate censored data. Q3W, once every 3 weeks.
Investigator-assessed ORR was 45.6% (95% CI, 34.3 to 57.2); 24 (30.4%) patients achieved a complete response, and 12 (15.2%) achieved a partial response (Table 2). Median duration of response was not reached (95% CI, 13.9 months to not reached; Data Supplement); 6-, 12-, and 18-month duration of response rates were 76.9% (95% CI, 59.0 to 87.8), 72.7% (95% CI, 53.5 to 84.9), and 67.5% (95% CI, 46.8 to 81.6), respectively.
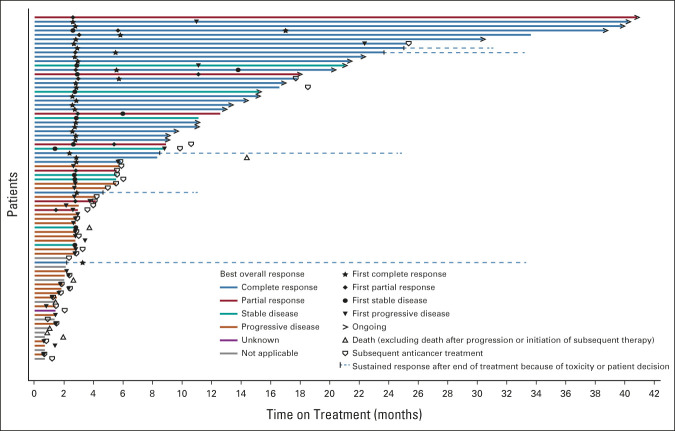
The concordance rate between IRRC- and investigator-assessed ORR was 95.7%. Swimmer plots for the best overall response assessed by IRRC and the investigators are shown in Figure 2 and the Data Supplement, respectively. In total, five patients changed from stable disease/partial response to complete response in their best response by IRRC assessment. In general, IRRC- and investigator-assessed ORRs were consistent across prespecified patient subgroups (Data Supplement).
FIG 2.
Swimmer plot of the treatment duration and best overall response assessed by IRRC as of the data cutoff date for all 78 patients. Arrow indicates ongoing treatment at cutoff date. Two patients were excluded from analysis for reasons that one patient was not confirmed as extranodal natural killer/T-cell lymphoma by central pathology, and the other patient was identified as no measurable or evaluable disease at baseline by IRRC. IRRC, independent radiologic review committee.
Median overall survival was not reached (95% CI, 14.0 months to not reached; Fig 1B); 6-, 12-, and 18-month overall survival rates were 79.2% (95% CI, 68.3 to 86.7), 67.5% (95% CI, 55.4 to 77.0), and 57.9% (95% CI, 44.9 to 68.9), respectively.
Safety
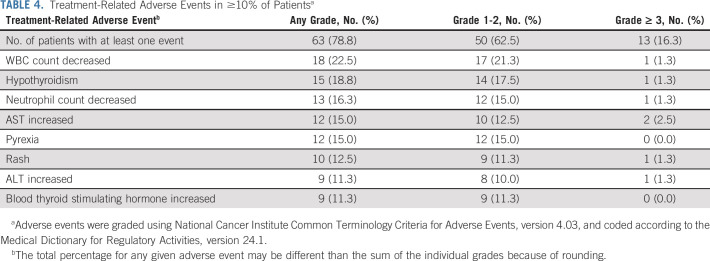
Seventy-seven (96.3%) patients experienced at least one treatment-emergent adverse event during the study (Table 3). Grade ≥3 treatment-emergent adverse events were reported in 32 (40.0%) patients. The most common (≥10%) treatment-emergent adverse events are presented in the Data Supplement. The majority of treatment-related adverse events were grade 1 or 2; grade 3 or 4 events were reported in 13 (16.3%) patients, with increased aspartate aminotransferase and anemia occurring in two (2.5%) patients each, and the other events occurred in one (1.3%) patient each (Table 4). Serious adverse events were reported in 19 (23.8%) patients; six (7.5%) were considered related to treatment and all except one event of sinus node dysfunction resolved at the data cutoff date without sequelae. Adverse events of special interest occurred in 25 (31.3%; Data Supplement) patients with two (2.5%) patients reporting grade ≥ 3 severity. Discontinuation of the study drug because of treatment-emergent adverse events occurred in 11 (13.8%) patients; five (6.3%) were considered related to treatment, including increased blood bilirubin in two (2.5%) patients, and cellulitis orbital, pyrexia, and facial nerve disorder in one (1.3%) patient each, which were all grade 1 or 2 events. Five (6.3%) patients died due to adverse events, which were not attributed to sugemalimab, as assessed by the investigator.
TABLE 3.
Adverse Events
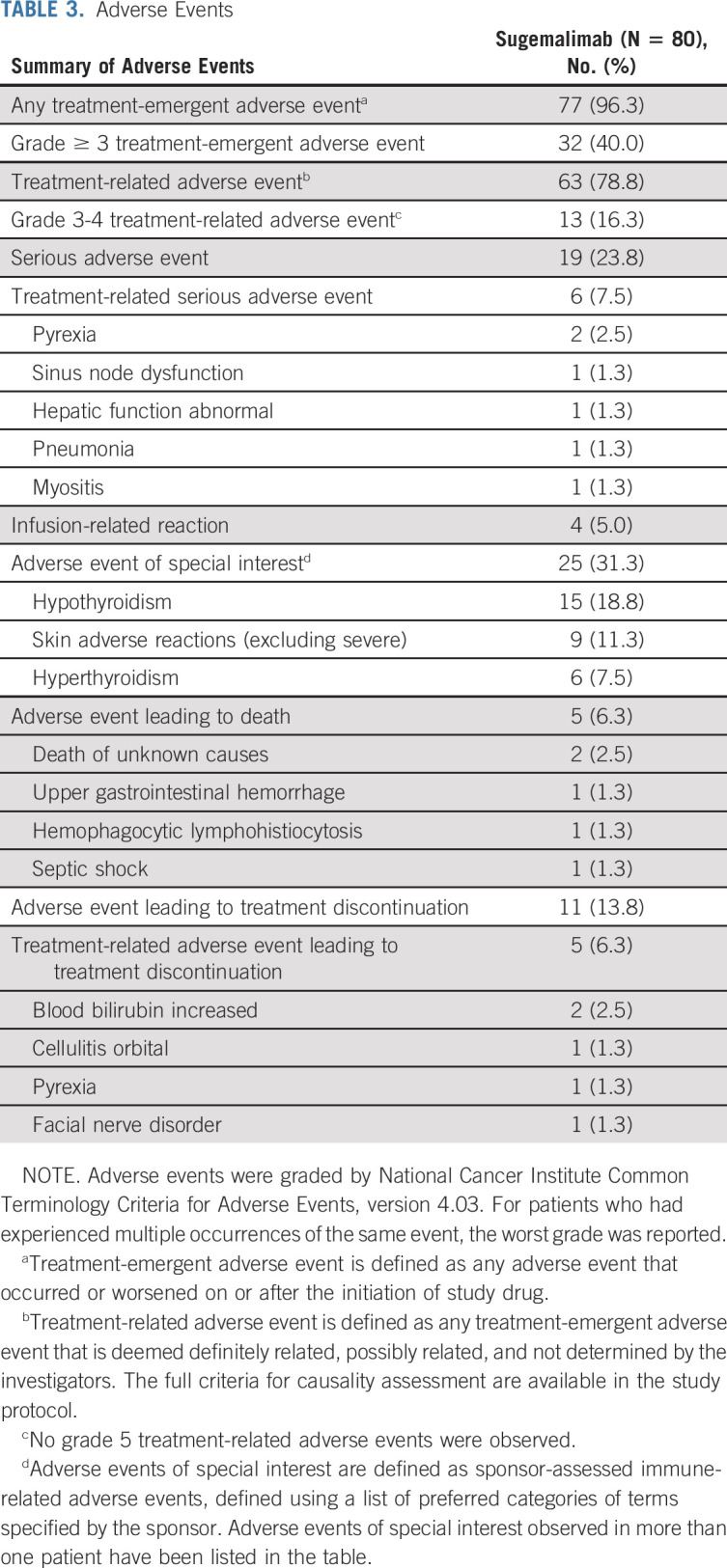
TABLE 4.
Treatment-Related Adverse Events in ≥10% of Patientsa
Antidrug antibody was detected in two (2.5%) and four (5.0%) patients at baseline and postbaseline, respectively. However, neutralizing antibodies were only detected in one (1.3%) patient after baseline.
DISCUSSION
To our knowledge, this is the largest registrational trial reported to date evaluating the efficacy and safety of an immune checkpoint inhibitor in R/R ENKTL. At the data cutoff, the IRRC-assessed ORR was 44.9%, with 28 (35.9%) patients achieving a complete response. Treatment responses were durable, with an 18-month duration of response rate of 82.5%. There was a high concordance rate between the IRRC- and investigator-assessed ORRs, indicating consistency and robustness of the response analyses. Although the median overall survival was not reached, 18-month overall survival rate was 57.9%. Subgroup analyses of IRRC-assessed ORR indicated that sugemalimab is likely to be efficacious across a broad range of patients with ENKTL, including those who were heavily pretreated. Our safety findings were consistent with the expected safety profile of this drug class and with previous reports of sugemalimab in advanced non–small-cell lung cancer and other solid tumors.13,14,16,17
In this study, the majority of responding patients achieved complete response, and although cross-trial comparison is difficult and should be interpreted with caution, this was numerically higher than the reported data from other PD-1/PD-L1 inhibitors.18-20 Moreover, achieving complete response, instead of partial response, has been closely correlated with longer survival in ENKTL.21-24 Although such survival benefit among complete responders could not be verified by this single-arm study, there was a trend toward and potential for an overall survival benefit with sugemalimab, with a 12-month overall survival rate of 67.5% (95% CI, 55.4 to 77.0), given that the historical survival in this setting was approximately 20%-30%.6,25 A key strength of our study is the large number of patients enrolled, ensuring that the results are robust. High concordance rate between IRRC and investigator response assessment minimizes evaluation bias. Although the absence of a control arm is a limitation of the study, ENKTL is a rare malignancy, making patient enrollment in a large study difficult. Although ENKTL is more prevalent in East Asia and Latin America, previous studies indicated broad similarity in clinical presentation and treatment outcomes in the Western and Asian populations for ENKTL.26,27 As such, the clinical benefit of sugemalimab could potentially be extended to non-Asian populations.
To date, the standard treatment for R/R ENKTL has not been established. The US National Comprehensive Cancer Network and the Chinese Society of Clinical Oncology guidelines for lymphomas recommend clinical trials in this setting.28,29 Common salvage chemotherapies after relapse include L-asparaginase–based and gemcitabine-containing regimens, which fail to confer a long-term survival.6 Another cytotoxic therapy, mitoxantrone hydrochloride liposome, recently approved in China for relapsed/refractory peripheral T-cell lymphoma, was associated with an ORR of 52.4% in 21 patients with ENKTL, six of whom achieved a complete response.30 However, grade ≥ 3 hematologic toxicities such as leukocytopenia (50.0%) and neutropenia (45.4%) were common.
Targeted therapies are emerging as promising treatment options for T-cell lymphomas. Histone deacetylase inhibitors, such as chidamide and belinostat, have been shown to improve outcomes in patients with relapsed/refractory peripheral T-cell lymphoma, but their clinical activity in ENKTL was either modest or uncertain. Chidamide, approved in China for relapsed/refractory peripheral T-cell lymphoma, showed an ORR of 19% among 16 patients with ENKTL, with one patient achieving a complete response.9 Belinostat is known to exhibit antitumor activity in peripheral T-cell lymphoma. However, in the phase II BELIEF study, only two patients had ENKTL, which was too few to make any meaningful clinical conclusions.8 Anti-CD38 has also emerged as a promising target in R/R ENKTL. In a phase II study, daratumumab yielded an ORR of 25.0%; however, none of the 32 patients had a complete response.31
Our study design was informed by the antitumor activity of immune checkpoint inhibitors shown in earlier case series of R/R ENKTL with small sample sizes. In one study by Kwong et al,32 pembrolizumab resulted in a complete response in five of seven patients with R/R ENKTL after a median of seven cycles. Another case series with pembrolizumab reported an ORR of 57.1% in seven patients with relapsed/refractory NKTL; two achieved a complete response after a median of four treatment cycles.33 Nivolumab treatment yielded responses in all three patients with very poor prognoses, and one remained in complete response after nine cycles.34 More recently, a phase II study conducted in China reported an ORR of 75.0% and a complete response rate of 21.4% in 28 patients with R/R ENKTL who received anti–PD-1 sintilimab.18 However, the outcomes in this study were assessed by investigators only, which potentially biased the evaluation as pointed out by the authors.18 Five patients who experienced pseudoprogression were also considered as responders. In a phase II relapsed/refractory peripheral T-cell lymphoma study, geptanolimab, an anti–PD-1 antibody, reported an ORR of 63.2% among 19 patients with ENKTL.20 Kim et al also demonstrated the efficacy of avelumab, an anti–PD-L1 antibody, in 21 patients with ENKTL achieving an ORR of 38% and a complete response rate of 24%.19 Although the variation in ORR in these studies may be attributed to the small sample sizes and patient heterogeneity, the results showed that PD-1/PD-L1 blockade was effective in R/R ENKTL, in line with the results of our study.
The treatment-emergent adverse events reported in this study were consistent with the safety profile of sugemalimab13,14,16,17 and other immune checkpoint inhibitors in similar patients.18-20 Treatment-related adverse events were manageable as most of them were grade 1-2 in severity. Hematologic disorders including leukopenia, thrombocytopenia, neutropenia, and anemia were common.18-20 There were no deaths related to the study drug. Most adverse events of special interest were grade 1-2 in severity, and none were fatal. In general, sugemalimab appeared to be better tolerated than chemotherapy, as grade 3-4 adverse events were commonly observed in the latter.35,36
In conclusion, sugemalimab showed potent, durable antitumor activity and a manageable safety profile in the largest study of an immune checkpoint inhibitor in patients with R/R ENKTL, representing a promising treatment option for patients with this rare and aggressive disease. A randomized, phase III study (ClinicalTrials.gov identifier: NCT05700448) is being planned to further assess the efficacy and safety of sugemalimab combined with chemotherapy versus chemotherapy alone in patients with R/R ENKTL.
ACKNOWLEDGMENT
The authors thank all the patients and their families for participating in the study. We also thank the investigators who participated in the study, Muge Qile of CStone Pharmaceuticals, and Lawrence Law of Parexel for medical writing support.
Ye Guo
Honoraria: Merck Serono, Roche, MSD, BMS
Consulting or Advisory Role: Merck Serono, MSD, Bayer, Roche
Qinzhou Qi
Employment: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Xiaoli Zhu
Employment: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Dan Zhu
Employment: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Siyuan Wang
Employment: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Teng Fang
Employment: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Hangjun Dai
Employment: Cstone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Qingmei Shi
Employment: Cstone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Jason Yang
Employment: CStone Pharmaceuticals
Leadership: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2022 American Society of Clinical Oncology Annual Meeting, June 3-7, 2022, Chicago, IL, and Virtual (abstr 7501).
SUPPORT
Supported by CStone Pharmaceuticals (Suzhou) Co Ltd, Suzhou, China.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Study data may be shared upon submission of a request to CStone Pharmaceuticals. The data request will be reviewed, and if agreed, the requestors will need to sign a data sharing agreement.
AUTHOR CONTRIBUTIONS
Conception and design: Huiqiang Huang, Rong Tao, Qinzhou Qi, Xiaoli Zhu, Dan Zhu, Siyuan Wang, Teng Fang, Hangjun Dai, Qingmei Shi, Jason Yang
Provision of study materials or patients: Huiqiang Huang, Rong Tao, Siguo Hao, Yu Yang, Hong Cen, Hui Zhou, Ye Guo, Liqun Zou, Yunhong Huang, Jie Jin, Liling Zhang, Haiyan Yang, Xiaojing Xing, Huilai Zhang, Yanyan Liu, Kaiyang Ding
Collection and assembly of data: All authors
Data analysis and interpretation: Huiqiang Huang, Qinzhou Qi, Xiaoli Zhu, Dan Zhu, Siyuan Wang, Teng Fang, Hangjun Dai, Qingmei Shi, Jason Yang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sugemalimab Monotherapy for Patients With Relapsed or Refractory Extranodal Natural Killer/T-Cell Lymphoma (GEMSTONE-201): Results From a Single-Arm, Multicenter, Phase II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ye Guo
Honoraria: Merck Serono, Roche, MSD, BMS
Consulting or Advisory Role: Merck Serono, MSD, Bayer, Roche
Qinzhou Qi
Employment: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Xiaoli Zhu
Employment: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Dan Zhu
Employment: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Siyuan Wang
Employment: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Teng Fang
Employment: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Hangjun Dai
Employment: Cstone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Qingmei Shi
Employment: Cstone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
Jason Yang
Employment: CStone Pharmaceuticals
Leadership: CStone Pharmaceuticals
Stock and Other Ownership Interests: CStone Pharmaceuticals
Travel, Accommodations, Expenses: CStone Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Harabuchi Y, Takahara M, Kishibe K, et al. : Extranodal natural killer/T-cell lymphoma, nasal type: Basic science and clinical progress. Front Pediatr 7:141, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleem A, Natkunam Y: Extranodal NK/T-cell lymphomas: The role of natural killer cells and EBV in lymphomagenesis. Int J Mol Sci 21:1501, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Q, Chen K, Young KH: Epstein-Barr virus-positive T/NK-cell lymphoproliferative disorders. Exp Mol Med 47:e133, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Mel S, Hue SSS, Jeyasekharan AD, et al. : Molecular pathogenic pathways in extranodal NK/T cell lymphoma. J Hematol Oncol 12:33, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox CP, Civallero M, Ko YH, et al. : Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: A prospective cohort study from the International T-Cell Project. Lancet Haematol 7:e284-e294, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Lim SH, Hong JY, Lim ST, et al. : Beyond first-line non-anthracycline-based chemotherapy for extranodal NK/T-cell lymphoma: Clinical outcome and current perspectives on salvage therapy for patients after first relapse and progression of disease. Ann Oncol 28:2199-2205, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Hong X, Song Y, Huang H, et al. : Pralatrexate in Chinese patients with relapsed or refractory peripheral T-cell lymphoma: A single-arm, multicenter study. Target Oncol 14:149-158, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connor OA, Horwitz S, Masszi T, et al. : Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: Results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol 33:2492-2499, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Dong M, Hong X, et al. : Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol 26:1766-1771, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Nagato T, Ohkuri T, Ohara K, et al. : Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: A potential rationale for immunotherapy. Cancer Immunol Immunother 66:877-890, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jo JC, Kim M, Choi Y, et al. : Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol 96:25-31, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Li Z, Tang L, et al. : Abstract 3260: The preclinical characterization of CS1001, an anti-PD-L1 IgG4 monoclonal antibody and its activity beyond T cell regulation. Cancer Res 80, 2020. (16 suppl; abstr 3260) [Google Scholar]
- 13.Zhou C, Wang Z, Sun Y, et al. : Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): Interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol 23:220-233, 2022 [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Chen M, Jiang O, et al. : Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): Interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 23:209-219, 2022 [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059-3068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen L, Li J, Miao Z, et al. : 1445P CS1001, an anti-PD-L1 antibody, combined with standard of care (SOC) chemotherapy for first line (1L) advanced GC/GEJ and ESCC: Preliminary results from 2 phase Ib cohorts of CS1001-101 study. Ann Oncol 31:S909, 2020 [Google Scholar]
- 17.Shen L, Li J, Xu N, et al. : A phase Ia/Ib trial of the anti-programmed death-ligand 1 (PD-L1) human monoclonal antibody (mAb), CS1001, in patients (pts) with advanced solid tumours or lymphomas. Ann Oncol 30:v516, 2019 [Google Scholar]
- 18.Tao R, Fan L, Song Y, et al. : Sintilimab for relapsed/refractory extranodal NK/T cell lymphoma: A multicenter, single-arm, phase 2 trial (ORIENT-4). Signal Transduct Target Ther 6:365, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SJ, Lim JQ, Laurensia Y, et al. : Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: An open-label phase 2 study. Blood 136:2754-2763, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, Wu J, Wang Z, et al. : Efficacy and safety of geptanolimab (GB226) for relapsed or refractory peripheral T cell lymphoma: An open-label phase 2 study (Gxplore-002). J Hematol Oncol 14:12, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li CC, Tien HF, Tang JL, et al. : Treatment outcome and pattern of failure in 77 patients with sinonasal natural killer/T-cell or T-cell lymphoma. Cancer 100:366-375, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Dong M, He X, et al. : Efficacy and safety of gemcitabine-based combination regimens in patients with extranodal natural killer/T cell lymphoma, nasal type. Blood 124:3071, 2014 [Google Scholar]
- 23.Zhang L, Jiang M, Xie L, et al. : Five-year analysis from phase 2 trial of “sandwich” chemoradiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer Med 5:33-40, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi M, Suzuki R: JSH practical guidelines for hematological malignancies, 2018: II. Lymphoma-9. Extranodal NK/T-cell lymphoma, nasal type (ENKL). Int J Hematol 109:371-376, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Bellei M, Foss FM, Shustov AR, et al. : The outcome of peripheral T-cell lymphoma patients failing first-line therapy: A report from the prospective, International T-Cell Project. Haematologica 103:1191-1197, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haverkos BM, Pan Z, Gru AA, et al. : Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT): An update on epidemiology, clinical presentation, and natural history in North American and European cases. Curr Hematol Malig Rep 11:514-527, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi S, Yahalom J, Hsu M, et al. : Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk Lymphoma 57:2575-2583, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J Ma J; Union for China Lymphoma Investigators of Chinese Society of Clinical Oncology : Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for malignant lymphoma 2021 (English version). Chin J Cancer Res 33:289-301, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NCCN Guidelines T-Cell Lymphomas Version 2.2022. https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf [Google Scholar]
- 30.Gao Y, Huang H, Wang X, et al. : Safety and efficacy of mitoxantrone hydrochloride liposome in patients with relapsed or refractory peripheral T-cell lymphoma and extranodal NK/T-cell lymphoma: A prospective, single-arm, open-label, multi-center, phase II clinical trial. Blood 136:36-37, 2020. (suppl 1)32430502 [Google Scholar]
- 31.Huang H, Zhu J, Yao M, et al. : Daratumumab monotherapy for patients with relapsed or refractory natural killer/T-cell lymphoma, nasal type: An open-label, single-arm, multicenter, phase 2 study. J Hematol Oncol 14:25, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong YL, Chan TSY, Tan D, et al. : PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 129:2437-2442, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Li X, Cheng Y, Zhang M, et al. : Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol 11:15, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan TSY, Li J, Loong F, et al. : PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: Efficacy and safety. Ann Hematol 97:193-196, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi M, Kwong YL, Kim WS, et al. : Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: The NK-Cell Tumor Study Group study. J Clin Oncol 29:4410-4416, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Jaccard A, Gachard N, Marin B, et al. : Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 117:1834-1839, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study data may be shared upon submission of a request to CStone Pharmaceuticals. The data request will be reviewed, and if agreed, the requestors will need to sign a data sharing agreement.