Abstract
Cisplatin is a bedrock of cancer management and one of the most used chemotherapeutic agents in the treatment of germ cell, lung, bladder, ovarian, and head and neck cancers. Approximately 500,000 patients diagnosed annually with these cancer types in the United States could be candidates for treatment with cisplatin. There is a 5-fold increase in the risk of hearing impairment or ototoxicity with cisplatin, which can manifest as ringing in the ear (tinnitus), high-frequency hearing loss, and at late stages, a decreased ability to hear normal conversation. More than half of adult and pediatric patients with cancer treated with cisplatin developed hearing impairment with major impact on patients' health-related quality of life. A considerable evidence gap persists regarding the burden and effective prevention and interception strategies for cisplatin-induced ototoxicity, especially in adult patients with cancer. We conducted a review of the published literature to provide an update on the status of this important clinical challenge. We also surveyed practicing oncologists within our network of academic and community practices to gain a better understanding of how the published literature compares with real-world practice. Our review of the literature showed a lack of standardized guidelines for monitoring and treatment of cisplatin-induced ototoxicity, especially in the adult cancer patient population. Our survey of practicing oncologists mirrored the findings from the published literature with a heterogeneity of practice, which highlights the need for standardization.
BACKGROUND AND BURDEN OF THE PROBLEM
Cisplatin and other platinum salt agents, including carboplatin and oxaliplatin, are widely used chemotherapy agents in patients with solid malignancies. These agents remain the backbone of treatment for ovarian, cervical, testicular, non–small-cell lung, bladder, and head and neck cancers. It is estimated that more than 500,000 patients diagnosed with these cancers annually in the United States could be candidates for treatment with cisplatin.1 However, adverse effects such as ototoxicity, neurotoxicity, and nephrotoxicity can sometimes limit their use. The incidence of ototoxicity induced by cisplatin has been estimated to be 36% of adult patients with cancer and 40%-60% of pediatric patients.2-4 Ototoxicity can be vestibular or cochlear toxicity or both, which can manifest as tinnitus (ringing in the ear), ear pain, and frank hearing loss.
The receipt of cisplatin is associated with a 5-fold increase in the risk of hearing impairment, and the incidence and severity are cumulative with exposure. Ototoxicity can manifest as tinnitus, hearing loss in the high-frequency range (4,000 to 8,000 Hz), or at late stages, a decreased ability to hear in the lower-frequency normal conversation range. It can occur during or after treatment and can be unilateral or bilateral affect both ears. Usually, hearing loss can start at higher frequencies in the beginning and can be permanent.5,6 In fact, severe ototoxicity with deafness has been reported even after a single cycle of cisplatin.7 Hence, monitoring and early identification of cisplatin-induced hearing loss are crucial to prevent detrimental impact on hearing and thereby the quality of life (QoL). Children affected by hearing loss have a poorer QoL as evident from their ability to communicate and interact with family and peers, their independence, and emotional well-being.8 The negative impact of hearing impairment on the patients' health-related QoL including social isolation, anxiety, and depression is well supported by a large body of evidence.9,10
There is no standard and commonly accepted definition of cisplatin-induced ototoxicity in the adult oncology field. This is because the assessment is conducted by various means and there is no standardized criterion. There is no reliable estimate of the prevalence of ototoxicity in adult patients with cancer because of lack of proper monitoring. However, in pediatric patients, where monitoring is better adopted, it is estimated that 40%-60% of patients treated with cisplatin will develop some degree of hearing impairment.3,4 Also, many strategies to minimize or treat cisplatin-induced ototoxicity have been postulated, but to date, to our knowledge, none have been approved in the adult population by the Food and Drug Administration (FDA). Increased knowledge and awareness about ototoxicity as a complication of treatment with cisplatin will lead to early detection and interception to prevent severe permanent damage.
There is a considerable evidence gap regarding appropriate monitoring for ototoxicity and effective strategies to prevent hearing impairment related to cisplatin in adult patients with cancer.3,11 This gap in knowledge affects accurate documentation/ awareness of the burden of this problem and thus the need to monitor and intercept it. As patients live longer because of advances in innovative therapies, it becomes important that particular attention be paid to the QoL of cancer survivors and the need to minimize the risk and severity of long-term toxicity of therapeutic interventions. We expect this review to serve as a timely reminder to sensitize the adult oncology community to the significant burden of ototoxicity and the need for monitoring and interception.
Mechanisms proposed to explain the development of ototoxicity include direct and indirect mechanisms such as cellular uptake of the platinum agent and resulting direct cytotoxicity. Also, there could be direct damage to mitochondrial and nuclear DNA leading to the death of cochlea cells.2,12 Cisplatin can also induce apoptosis by causing cell cycle arrest and activation of p53 in the hair cells.13 The proposed indirect mechanism involves the activation of NADPH oxidase 3 and xanthine oxidase by cisplatin, leading to the generation of reactive oxygen species (ROS).14,15 The redox reactions and the formation of ROS leads to inflammation and apoptotic cell death.16 Increased understanding of the mechanism of cisplatin-induced ototoxicity will facilitate the design and deployment of appropriate prevention and interception strategies in the future.
Monitoring, Prevention, and Interception Strategies
The advent of newer and better cancer treatment options, especially immunotherapy and targeted therapies, have led to improved patient prognosis and survival.1,17 As greater proportion of patients with cancer live longer because of improved management options, it has become important to not lose focus on QoL in survivorship. Cisplatin can cause hearing loss in up to 60% of the treated population, leading to significant impact on QoL. Data from a population-based longitudinal study of age-related hearing impairment (N = 2,688) showed that 28% of adults age 53-97 years had a mild hearing loss at 5-year follow-up and 24% had a moderate to severe hearing loss. Severity of hearing loss was significantly associated with decreased function in mental, physical, and communication abilities.9 In a different study involving a pediatric population treated for a range of solid tumors and leukemias, 41 of 78 children whose parents completed the Pediatric Audiology QoL questionnaire had sensorineural hearing loss as a result of the cancer treatment. Significant differences were found on all four scales of the questionnaire between children with and without hearing loss. Children with ototoxicity were rated as having poorer QoL in terms of their ability to communicate with family and peers, their independence, interactions with peers, and emotional well-being.8
The value of baseline audiometry has been established more than 2 decades ago in a study of 217 patients who completed an audiogram before receiving cisplatin-based treatment for cancers of esophagus, lung, or head and neck.3 Hearing abnormality in excess of the expected presbycusis was recorded in 57 (26%) patients. Postcisplatin audiograms demonstrated hearing loss in 19 of the 53 retested patients (36%) when compared with their baseline audiogram. As determined by tympanometry, none of these subjects had a conductive component to their hearing loss. This study showed that significant preexisting hearing abnormality is common in this patient population and that additional hearing loss occurs frequently even with low-dose cisplatin administration.3 It is also likely that the full extent of ototoxicity was not uncovered in this study since conventional audiograms may not be sensitive enough to detect inner hair cell damage in the cochlea, which may also be a mechanism through which platinum compounds cause ototoxicity.18 Moreover, there is a significant percentage of general adult population with mild (28%) to moderate/severe (24%) hearing loss, making it essential to conduct a detailed audiogram test at baseline before initiating treatment.
Because of the limited objective data on real-world practice patterns for monitoring and reducing the risk of ototoxicity, we surveyed approximately 120 oncologists practicing within the UPMC Hillman Cancer Center network of academic and community practices as part of a quality-of-care assessment in December 2021 to obtain insight into practice patterns. The majority of survey responders (N = 35) identified lack of standardized monitoring algorithm and effective prevention strategies against hearing impairment as crucial factors that limit their ability to properly take care of patients. Despite this challenge, the majority of respondents (97%) indicated that they regularly discuss the risk of ototoxicity with all patients before receiving cisplatin. However, only 18% of the respondents obtain routine audiograms for patients before administering cisplatin (baseline), 69% order audiograms only if patients complain of hearing loss and/or tinnitus, while 35% of respondents do not perform regular monitoring for ototoxicity (Figs 1 and 2). Nearly half (47%) rated the impact of ototoxicity on patient's QoL and functioning as moderate to severe, and a solid majority (65%) felt that ototoxicity would have a moderate to major impact on their treatment decisions for the patient. Most oncologists participating in the survey also indicated that they will adopt any intervention and monitoring strategies shown to improve outcomes for patients. This heterogeneity of practice within a single network highlights the need for high-quality evidence to guide clinical practice and the urgent need to standardize the necessary diagnostic steps to monitor for ototoxicity and its effect on QoL in the adult oncology practice similar to the current practice in the pediatric patient population.19 It is worth highlighting that this survey is limited in scope to a single health care system and thus may not be generalizable to all settings without further validation.
FIG 1.
Prevalence of current ototoxicity monitoring practices by oncologists in our network (N = 35).
FIG 2.
Current audiogram ordering practices by oncologists in our network (N = 35).
American Speech-Language-Hearing Association and the American Academy of Audiology guidelines recommend the use of both behavioral and nonbehavioral (objective) measures of auditory function as part of the baseline evaluation since some patients may not be able to provide reliable behavioral thresholds during treatment.20 Indeed, it was previously reported that many patients (up to 30%) receiving chemotherapy may not be able to adequately follow through with ototoxicity screening or respond appropriately during testing because of ongoing disease/treatment effects.21 The strategy used in the Comprehensive Ototoxicity Monitoring Program for Veterans Affairs (COMP‐VA) study, which is a comprehensive ototoxicity monitoring program developed for VA patients receiving cisplatin, is very noteworthy in this regard. The program used an individualized pretreatment prediction model to estimate the likelihood of hearing shift in patients scheduled to receive cisplatin along with both manual and automated hearing testing using a portable audiometer appropriate for use on the chemotherapy unit during treatment. It also included objective methods for identifying outer hair cell changes and predicting audiogram changes using distortion-product otoacoustic emissions.21 On the basis of this model, they proposed time-efficient screening for sensitive range for ototoxicity (SRO) at baseline along with tympanometry and otoscopy. They also advocated for baseline otoacoustic emission testing especially if worsening hearing function is anticipated with chemotherapy on the basis of baseline testing. Their experience suggested auditory rehabilitation to be done at the end of treatment as it is unlikely to be effectively addressed amid ongoing treatment beyond an assistive device.
Steps used in the COMP-VA study21 included the following.
Pretreatment ototoxicity risk assessment.
Screening for early hearing changes (SRO).
Screening for outer hair cell dysfunction.
Screen failure follow-up testing.
Screening for tinnitus and appropriate referral for management.
Patient and provider education about ototoxicity and rehabilitative solutions.
On the basis of a similar model as above, a structured approach can be established perhaps at each institutional level to address cisplatin-induced ototoxicity in patients with cancer. A multidisciplinary action plan can then be developed involving the oncologist, the audiologist, and a pharmacist to plan chemotherapy induction with modifications as needed. It is vital to balance this risk versus benefit equation with regards to chemotherapy treatment in the setting of developing ototoxicity and the implication for patients vis-à-vis their individual goals of care. Fortunately, extended follow-up in cancer survivors who have received cisplatin has not shown major progression for frequencies 0.125-8 kHz beyond the first post-treatment decade.22 Hearing thresholds of patients approach those of general population probably because of a less-than-additive effect with age-related hearing in this population. Along with monitoring and managing ototoxicity with existing treatment options and symptom rehabilitation, many efforts are ongoing with several clinical trials for prevention and treatment of ototoxicity.
Therapeutic Strategies to Reduce Ototoxicity
Many strategies to reduce or reverse the ototoxic effects of cisplatin have been evaluated in preclinical and clinical settings. However, none of these strategies have demonstrated to date the efficacy threshold required for regulatory approval, and many of them remain investigational in the adult cancer patient population. The most common strategies to reduce ototoxicity have been tailored toward the use of pharmacologic agents as a reversal agent for a specific mechanism proposed to underlie cisplatin-mediated ototoxicity. Since ROS is recognized as a key mediator of ototoxicity, the efficacy of antioxidants has been explored in preclinical studies using animal models. Similarly, strategies to counteract mediators of apoptosis and to reduce inflammatory cytokine levels have been tested. However, this strategy has been tempered by the concern that antiapoptotic agents might counteract the chemotherapeutic efficacy of cisplatin. Vitamin C, lactate, curcumin, mitoquinone, ginkgolide B, and N-acetylcysteine are some of the pharmacologic agents with antioxidant, anti-inflammatory, and antiapoptotic properties previously tested in preclinical studies.23
Another strategy to mitigate any negative impact of these therapeutic interventions on cisplatin efficacy is the route of delivery of the protective drugs. Both systemic administration and local administration have their own advantages and disadvantages. The main considerations with systemic administration of protective agents are ways to overcome biological barriers that could prevent the agent from getting into the inner ear, for example, the blood-perilymph, blood-endolymph, and perilymph-endolymph. Other considerations include the need to counter the side effects of systemic administration of the protective agent such as hematologic toxicity and compromised efficacy of the primary chemotherapy agent.24 Direct local delivery via intratympanic/cochlear route overcomes these disadvantages. However, they suffer the challenges of causing vestibular/cochlear toxicity with prolonged drug accumulation and can be very expensive for routine general use.23
The ‘clinical practice guideline’ published by Freyer et al for prevention of cisplatin-induced ototoxicity in children and adolescents recommended to not use amifostine, sodium diethyldithiocarbamate, intratympanic middle ear therapy, and to not alter cisplatin infusion duration. There is strong recommendation in support of sodium thiosulfate (STS) in nonion metastatic cancers (hepatoblastoma) and other solid tumor types (germ cell tumor, medulloblastoma or central nervous system primitive neuroectodermal tumor, neuroblastoma, osteosarcoma, or other cancer types treated with cisplatin) on the basis of two trials—one conducted by the International Childhood Liver Tumor Strategy Group (SIOPEL 6) and the other by the Children's Oncology Group (ACCL0431).24 They recommended against the use of STS in metastatic cancers.25 On the basis of the results of these two trials, on September 20, 2022, the US FDA approved STS (Pedmark, Fennec Pharmaceuticals Inc) to reduce the risk of ototoxicity associated with cisplatin in pediatric patients (1 month or older) with localized, nonmetastatic solid tumors.26
In the clinical trial by Duinkerken et al,27 they found that transtympanic injections of STS gel leads to prevention of cisplatin-induced ototoxicity. Of the 12 patients treated in this small trial, four did not develop hearing loss, and four of the eight that developed hearing impairment had improvement of hearing frequencies after being treated with STS. Similarly, Brock et al28 demonstrated in their clinical trial in patients with hepatoblastoma that intravenous STS administration 6 hours from the end of the cisplatin infusion achieved a 48% lower incidence of hearing loss than the cisplatin-alone group. There was no significant difference in 3-year event-free survival rates (82% v 79%) or overall survival rates (98% v 92%) between the two groups. In, ACCL0431, an open-label, phase III randomized trial, Freyer et al24 reported a significant reduction in the incidence of cisplatin-induced hearing loss in the STS treated group relative to the control group (28.6% v 56.4%) with no compromise of cisplatin efficacy.
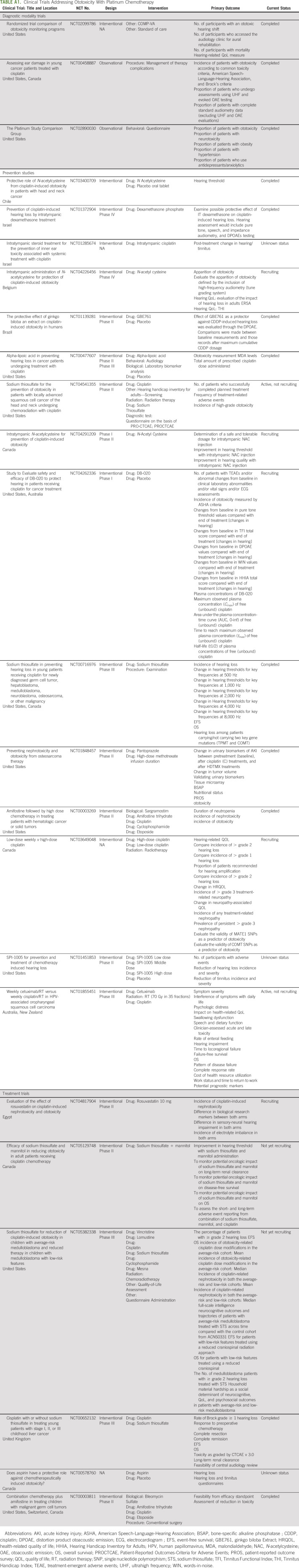
Intratympanic dexamethasone injection before cisplatin infusion showed attenuation of hearing loss and improvement of outer hair cell dysfunction in one randomized controlled trial and was a safe intervention that did not affect efficacy.29 Amifostine also reduced cisplatin-induced serious hearing loss in patients with average-risk but not in high-risk medulloblastoma.30 The role of statins as an otoprotective agent has also been explored in preclinical and clinical studies. Statins are HMG-CoA reductase inhibitors that also provide endothelial protection and antioxidant properties. Prior studies have shown that statins use can provide relief from tinnitus, reduce the intensity of tinnitus, and improve hearing at higher frequencies by lowering serum lipid levels.31,32 Lovastatin protected against cisplatin-induced hearing loss in mice models. It resulted in improved higher-frequency threshold hearing and reduced cisplatin-induced outer hair cell loss; however, it did not reduce platinum entry to the cochlea.33 In a recent retrospective and prospective data analysis by Fernandez et al,34 statin showed a beneficial role against cisplatin-induced ototoxicity in 277 adults treated with cisplatin for head and neck cancer. Patients on statins had a 9.7% incidence of hearing loss compared with 29.4% in the control group with an adjusted odds ratio of 0.47 showing that patients receiving atorvastatin were 53% less likely to develop cisplatin-induced hearing loss compared with controls. A list of ongoing clinical trials of strategies to mitigate ototoxicity including early diagnosis, monitoring, prevention, and treatment is provided in Appendix Table A1 (online only).
The recent approval of STS by the FDA to treat ototoxicity in pediatric patients with localized, nonmetastatic solid tumors greenlights the need for further testing of similar agents in adult patients. In the future, clinical trials of newer otoprotective agents will be expected, including agents against other platinum compounds beyond cisplatin and in patients with more advanced stages of disease. Although there remains a legitimate concern that pharmacologic agents for otoprotection might compromise therapeutic efficacy, available data to date have not borne this out. Nonetheless, when testing agents with the potential risk to interfere with cisplatin efficacy, an appropriate trial design that allows for efficacy preservation will be critical. As such, randomized trials limited to a single histologic type of cancer and standard chemotherapy regimen that allows for uniform inclusion criteria and response criteria would be optimal. Conversely, otoprotectants without any mechanistic rationale to compromise anticancer efficacy would be appropriate to be tested in a heterogeneous population of patients with cancer, as was the case in the ACCL0431 trial.35 Furthermore, improved drug delivery using microneedle technique could be used for localized administration beyond intratympanic delivery and possibly directly into the inner ear to increase potency of protective agents. Finally, there is also a need for standard assessment and measurement of severity of hearing loss. This will facilitate comparison of study end points and efficacy of various agents and strategies undergoing clinical investigation as otoprotective agents.
ACKNOWLEDGMENT
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
APPENDIX
TABLE A1.
Clinical Trials Addressing Ototoxicity With Platinum Chemotherapy
Taofeek Owonikoko
Stock and Other Ownership Interests: CAMBIUM MEDICAL TECHNOLOGIES, Taobob LLC, GenCart, Coherus Biosciences
Consulting or Advisory Role: Novartis, Celgene, AbbVie, Eisai, G1 Therapeutics, Takeda, Bristol Myers Squibb, MedImmune, BerGenBio, Lilly, Amgen, AstraZeneca, PharmaMar, Boehringer Ingelheim, EMD Serono, Xcovery, Bayer, Merck, Jazz Pharmaceuticals, Zentalis, Wells Fargo, Ipsen, Eisai, Roche/Genentech, Janssen, Exelixis, BeiGene, Triptych Health Partners, Daichi, Xcovery, Coherus Biosciences
Speakers' Bureau: AbbVie
Research Funding: Novartis (Inst), Astellas Pharma (Inst), Bayer (Inst), Regeneron (Inst), AstraZeneca/MedImmune (Inst), AbbVie (Inst), G1 Therapeutics (Inst), Bristol Myers Squibb (Inst), Corvus Pharmaceuticals, United Therapeutics (Inst), Amgen (Inst), Loxo/Lilly (Inst), Fujifilm (Inst), Pfizer (Inst), Aeglea Biotherapeutics (Inst), Incyte (Inst), Merck (Inst), Oncorus (Inst), Ipsen (Inst), GlaxoSmithKline (Inst), Calithera Biosciences (Inst), Eisai (Inst), WindMIL (Inst), Turning Point Therapeutics (Inst), Roche/Genentech (Inst), Mersana (Inst), Meryx Pharmaceuticals (Inst), Boehringer Ingelheim (Inst), Boehringer Ingelheim (Inst)
Patents, Royalties, Other Intellectual Property: OVERCOMING ACQUIRED RESISTANCE TO CHEMOTHERAPY TREATMENTS THROUGH SUPPRESSION OF STAT3 (Inst), SELECTIVE CHEMOTHERAPY TREATMENTS AND DIAGNOSTIC METHODS RELATED THERETO (Inst), DR4 Modulation and its Implications in EGFR-Target Cancer Therapy Ref:18,089 PROV (CSP) United States Patent Application No. 62/670,210 June 26, 2018 (Co-Inventor) (Inst), Soluble FAS ligand as a biomarker of recurrence in thyroid cancer; provisional patent 61/727,519 (Inventor) (Inst)
Other Relationship: Roche/Genentech, EMD Serono, Novartis
Uncompensated Relationships: Reflexion Medical
Open Payments Link: https://openpaymentsdata.cms.gov/physician/253457
No other potential conflicts of interest were reported.
SUPPORT
T.K.O. was supported by grant awards (1R01CA273216 and P30CA047904) from the National Institutes of Health.
A.C. and M.P.S. contributed equally to this work and should be considered co‐first authors.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Masood Pasha Syed
Provision of study materials or patients: Masood Pasha Syed
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cisplatin-Induced Ototoxicity: A Concise Review of the Burden, Prevention, and Interception Strategies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Taofeek Owonikoko
Stock and Other Ownership Interests: CAMBIUM MEDICAL TECHNOLOGIES, Taobob LLC, GenCart, Coherus Biosciences
Consulting or Advisory Role: Novartis, Celgene, AbbVie, Eisai, G1 Therapeutics, Takeda, Bristol Myers Squibb, MedImmune, BerGenBio, Lilly, Amgen, AstraZeneca, PharmaMar, Boehringer Ingelheim, EMD Serono, Xcovery, Bayer, Merck, Jazz Pharmaceuticals, Zentalis, Wells Fargo, Ipsen, Eisai, Roche/Genentech, Janssen, Exelixis, BeiGene, Triptych Health Partners, Daichi, Xcovery, Coherus Biosciences
Speakers' Bureau: AbbVie
Research Funding: Novartis (Inst), Astellas Pharma (Inst), Bayer (Inst), Regeneron (Inst), AstraZeneca/MedImmune (Inst), AbbVie (Inst), G1 Therapeutics (Inst), Bristol Myers Squibb (Inst), Corvus Pharmaceuticals, United Therapeutics (Inst), Amgen (Inst), Loxo/Lilly (Inst), Fujifilm (Inst), Pfizer (Inst), Aeglea Biotherapeutics (Inst), Incyte (Inst), Merck (Inst), Oncorus (Inst), Ipsen (Inst), GlaxoSmithKline (Inst), Calithera Biosciences (Inst), Eisai (Inst), WindMIL (Inst), Turning Point Therapeutics (Inst), Roche/Genentech (Inst), Mersana (Inst), Meryx Pharmaceuticals (Inst), Boehringer Ingelheim (Inst), Boehringer Ingelheim (Inst)
Patents, Royalties, Other Intellectual Property: OVERCOMING ACQUIRED RESISTANCE TO CHEMOTHERAPY TREATMENTS THROUGH SUPPRESSION OF STAT3 (Inst), SELECTIVE CHEMOTHERAPY TREATMENTS AND DIAGNOSTIC METHODS RELATED THERETO (Inst), DR4 Modulation and its Implications in EGFR-Target Cancer Therapy Ref:18,089 PROV (CSP) United States Patent Application No. 62/670,210 June 26, 2018 (Co-Inventor) (Inst), Soluble FAS ligand as a biomarker of recurrence in thyroid cancer; provisional patent 61/727,519 (Inventor) (Inst)
Other Relationship: Roche/Genentech, EMD Serono, Novartis
Uncompensated Relationships: Reflexion Medical
Open Payments Link: https://openpaymentsdata.cms.gov/physician/253457
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics. CA: A Cancer J Clinicians 72:7-33, 2022 [DOI] [PubMed] [Google Scholar]
- 2.Karasawa T, Steyger PS: An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett 237:219-227, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy JL, Adelstein DJ, Newman CW, et al. : Cisplatin ototoxicity: The importance of baseline audiometry. Am J Clin Oncol 22:305-308, 1999 [DOI] [PubMed] [Google Scholar]
- 4.FDA : Prescribing Information for Cisplatin. FDA, Silver Springs, Maryland, 1978. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf [Google Scholar]
- 5.Esfahani Monfared Z, Khosravi A, Safavi Naini A, et al. : Analysis of cisplatin-induced ototoxicity risk factors in Iranian patients with solid tumors: A cohort, prospective and single Institute study. Asian Pac J Cancer Prev 18:753-758, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rybak LP: Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg 15:364-369, 2007 [DOI] [PubMed] [Google Scholar]
- 7.McDonald ME, Mattson J, Hill E: Profound sensorineural hearing loss after one cycle of intraperitoneal cisplatin in treatment of advanced ovarian cancer. Gynecol Oncol Rep 20:103-104, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajput K, Edwards L, Brock P, et al. : Ototoxicity-induced hearing loss and quality of life in survivors of paediatric cancer. Int J Pediatr Otorhinolaryngol 138:110401, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Dalton DS, Cruickshanks KJ, Klein BEK, et al. : The impact of hearing loss on quality of life in older adults. Gerontologist 43:661-668, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Mulrow CD, Aguilar C, Endicott JE, et al. : Association between hearing impairment and the quality of life of elderly individuals. J Am Geriatr Soc 38:45-50, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Konrad-Martin D, Poling GL, Garinis AC, et al. : Applying U.S. National guidelines for ototoxicity monitoring in adult patients: Perspectives on patient populations, service gaps, barriers and solutions. Int J Audiol 57:S3-s18, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marullo R, Werner E, Degtyareva N, et al. : Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One 8:e81162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devarajan P, Savoca M, Castaneda MP, et al. : Cisplatin-induced apoptosis in auditory cells: Role of death receptor and mitochondrial pathways. Hearing Res 174:45-54, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Mukherjea D, Whitworth CA, Nandish S, et al. : Expression of the kidney injury molecule 1 in the rat cochlea and induction by cisplatin. Neuroscience 139:733-740, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Lynch ED, Gu R, Pierce C, et al. : Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hearing Res 201:81-89, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Fetoni AR, Paciello F, Troiani D: Cisplatin chemotherapy and cochlear damage: Otoprotective and chemosensitization properties of polyphenols. Antioxid Redox Signaling 36:1229-1245, 2022 [DOI] [PubMed] [Google Scholar]
- 17.Howlader N, Forjaz G, Mooradian MJ, et al. : The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 383:640-649, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobarinas E, Salvi R, Ding D: Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hearing Res 302:113-120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson SE, Taylor J, Patel P, et al. : Cancer survivors treated with platinum-based chemotherapy affected by ototoxicity and the impact on quality of life: A narrative synthesis systematic review. Int J Audiol 58:685-695, 2019 [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Audiology : American Academy of Audiology Position Statement and Clinical Practice Guidelines: Ototoxicity Monitoring American Academy of Audiology. American Academy of Audiology, 2013 [Google Scholar]
- 21.Konrad-Martin D, Reavis KM, McMillan G, et al. : Proposed comprehensive ototoxicity monitoring program for VA healthcare (COMP-VA). J Rehabil Res Dev 51:81-100, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skalleberg J, Småstuen MC, Oldenburg J, et al. : The relationship between cisplatin-related and age-related hearing loss during an extended follow-up. Laryngoscope 130:E515-e521, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Yu D, Gu J, Chen Y, et al. : Current strategies to combat cisplatin-induced ototoxicity. Front Pharmacol 11:999, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freyer DR, Chen L, Krailo MD, et al. : Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 18:63-74, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freyer DR, Brock PR, Chang KW, et al. : Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: A clinical practice guideline. Lancet Child Adolesc Health 4:141-150, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA Approves Sodium Thiosulfate to Reduce the Risk of Ototoxicity Associated with Cisplatin in Pediatric Patients with Localized, Non-metastatic Solid Tumors. FDA, Silver Springs, MD, 2022
- 27.Duinkerken CW, de Weger VA, Dreschler WA, et al. : Transtympanic sodium thiosulfate for prevention of cisplatin-induced ototoxicity: A randomized clinical trial. Otol Neurotol 42:678-685, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Brock PR, Maibach R, Childs M, et al. : Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 378:2376-2385, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshak T, Steiner M, Kaminer M, et al. : Prevention of cisplatin-induced hearing loss by intratympanic dexamethasone: A randomized controlled study. Otolaryngol Head Neck Surg 150:983-990, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Gurney JG, Bass JK, Onar-Thomas A, et al. : Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma. Neuro-Oncology 16:848-855, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olzowy B, Canis M, Hempel JM, et al. : Effect of atorvastatin on progression of sensorineural hearing loss and tinnitus in the elderly: Results of a prospective, randomized, double-blind clinical trial. Otol Neurotol 28:455-458, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Sutbas A, Yetiser S, Satar B, et al. : Low-cholesterol diet and antilipid therapy in managing tinnitus and hearing loss in patients with noise-induced hearing loss and hyperlipidemia. Int Tinnitus J 13:143-149, 2007 [PubMed] [Google Scholar]
- 33.Fernandez K, Spielbauer KK, Rusheen A, et al. : Lovastatin protects against cisplatin-induced hearing loss in mice. Hearing Res 389:107905, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez KA, Allen P, Campbell M, et al. : Atorvastatin is associated with reduced cisplatin-induced hearing loss. J Clin Invest 131:e142616, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freyer DR, Brock P, Knight K, et al. : Interventions for cisplatin-induced hearing loss in children and adolescents with cancer. Lancet Child Adolesc Health 3:578-584, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]