PURPOSE
Enzalutamide and abiraterone both target androgen receptor signaling but via different mechanisms. The mechanism of action of one drug may counteract the resistance pathways of the other. We sought to determine whether the addition of abiraterone acetate and prednisone (AAP) to enzalutamide prolongs overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) in the first-line setting.
PATIENTS AND METHODS
Men with untreated mCRPC were randomly assigned (1:1) to receive first-line enzalutamide with or without AAP. The primary end point was OS. Toxicity, prostate-specific antigen declines, pharmacokinetics, and radiographic progression-free survival (rPFS) were also examined. Data were analyzed using an intent-to-treat approach. The Kaplan-Meier estimate and the stratified log-rank statistic were used to compare OS between treatments.
RESULTS
In total, 1,311 patients were randomly assigned: 657 to enzalutamide and 654 to enzalutamide plus AAP. OS was not statistically different between the two arms (median, 32.7 [95% CI, 30.5 to 35.4] months for enzalutamide v 34.2 [95% CI, 31.4 to 37.3] months for enzalutamide and AAP; hazard ratio [HR], 0.89; one-sided P = .03; boundary nominal significance level = .02). rPFS was longer in the combination arm (median rPFS, 21.3 [95% CI, 19.4 to 22.9] months for enzalutamide v 24.3 [95% CI, 22.3 to 26.7] months for enzalutamide and AAP; HR, 0.86; two-sided P = .02). However, pharmacokinetic clearance of abiraterone was 2.2- to 2.9-fold higher when administered with enzalutamide, compared with clearance values for abiraterone alone.
CONCLUSION
The addition of AAP to enzalutamide for first-line treatment of mCRPC was not associated with a statistically significant benefit in OS. Drug-drug interactions between the two agents resulting in increased abiraterone clearance may partly account for this result, although these interactions did not prevent the combination regimen from having more nonhematologic toxicity.
NCI study of enza/abi yields no advantage over enza for OS for mCRPC. Novel DDI findings might explain why.
INTRODUCTION
The discovery that the androgen receptor actively signals despite androgen-deprivation therapy transformed the treatment of what is now known as metastatic castration-resistant prostate cancer (mCRPC).1-3 This finding made the androgen receptor axis a prime therapeutic target even in a testosterone-depleted milieu. Enzalutamide is an oral agent that inhibits androgen receptor nuclear translocation, DNA binding, and coactivator peptide recruitment.4 As first-line treatment for mCRPC, enzalutamide prolongs radiographic progression-free survival (rPFS) by 81% and overall survival (OS) by 29% and delays time to chemotherapy, skeletal-related events, and other clinically relevant end points, compared with placebo.5 Enzalutamide is approved for the treatment of CRPC, including mCRPC, both before and after chemotherapy.
CONTEXT
Key Objective
Does the addition of abiraterone acetate and prednisone (AAP) to enzalutamide prolong overall survival in patients with metastatic castration-resistant prostate cancer in the first-line setting?
Knowledge Generated
In this phase III study, the addition of AAP to enzalutamide did not improve overall survival relative to enzalutamide alone, although radiographic progression-free survival was marginally improved. This negative result may, in part, relate to a potential drug-drug interaction that was newly detected in this trial, in which the clearance rate of AAP was increased 2- to 3-fold relative to the historic clearance rate of AAP alone. Although that PK effect might have blunted the clinical impact of the combination on survival, it did not prevent the combination from having more side effects than enzalutamide alone.
Relevance (M.A. Carducci)
-
This National Clinical Trials Network study led by Alliance has been long awaited and affirms current practice supporting single-agent androgen receptor signaling inhibitors as standard of care for metastatic castration-resistant prostate cancer. Combinations with other non–androgen receptor signaling inhibitors, such as poly(ADP-ribose) polymerase inhibitors and other agents, are key ongoing clinical investigations important in the field.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD, FACP, FASCO.
Abiraterone is a selective inhibitor of androgen biosynthesis that potently blocks CYP17, which is critical to testosterone synthesis by the adrenals and testes and within the prostate tumor.6,7 Like enzalutamide, abiraterone acetate and prednisone (AAP) also prolong rPFS, OS, and other clinically significant end points. In progression-free survival (PFS) analysis, AAP reduces the risk of radiographic progression or death by 57%.8 In OS analysis, AAP reduces the risk of death by 25% relative to prednisone alone in men with taxane-naive mCRPC.8 As a result of this and other trials,9 abiraterone was approved by the US Food and Drug Administration (FDA) for the treatment of mCRPC. AAP and enzalutamide have subsequently been shown to prolong survival in metastatic castration-sensitive disease, leading to FDA approval in that context as well.10,11 Although the efficacy of enzalutamide and abiraterone have not been directly compared, all data suggest equivalent efficacy.
The mechanisms of action of enzalutamide and abiraterone are different, and each is well suited to address the resistance mechanisms associated with the other. Resistance to enzalutamide is partly conferred by upregulation of androgen biosynthesis,12 which could be addressed by inhibiting biosynthesis. Resistance to abiraterone has been associated with upregulation of androgen receptor,13 for which direct inhibition of androgen receptor signaling with a drug such as enzalutamide could be effective. A phase I study demonstrated that the combination of abiraterone and enzalutamide was safe, did not appear to result in altered pharmacokinetics, and yielded favorable prostate-specific antigen (PSA) response rates and PFS.14 We hypothesized that the addition of abiraterone to enzalutamide for first-line treatment of mCRPC, compared with enzalutamide alone, would result in prolonged rPFS and OS. We further hypothesized that there would be no drug-drug interactions in accord with the previous phase I data. We sought to confirm the association of rPFS with OS as observed in the registration studies for abiraterone and enzalutamide15,16 and, as a post hoc analysis, examined whether expanding the definition of progression to include clinical progression and/or post-treatment radiographic progression would identify a stronger association between rPFS and OS.
PATIENTS AND METHODS
Alliance A031201 was a randomized phase III clinical trial conducted by the National Cancer Institute (NCI)-funded cooperative group Alliance for Clinical Trials in Oncology to compare the anticancer effects of first-line enzalutamide with or without AAP in men with mCRPC. The primary objective was to compare OS between the two treatment groups. Secondarily, toxicity, PSA declines, rPFS, and the association between rPFS and OS were also examined.
Inclusion criteria included histologically or cytologically confirmed adenocarcinoma of the prostate without neuroendocrine differentiation or small cell features. Progression was defined, in accordance with the recommendations of the Prostate Cancer Clinical Trials Working Group (PCWG) 2, as a minimum of two increasing PSA levels while on androgen-deprivation therapy (with an interval of ≥1 week between measurements), a minimum PSA level of 2 ng/mL, and a 4-week period of progression after withdrawal of first-generation antiandrogens if the patient received them. Alternatively, patients could be eligible by radiographic criteria, in accordance with modified PCWG23 recommendations, which later became codified in PCWG32 (and which, for ease of use, we will abbreviate simply as PCWG). At the time of this trial's design and activation, docetaxel was only known to benefit patients with mCRPC; all other contexts were investigational, including metastatic castration-sensitive prostate cancer (mCSPC). The protocol allowed patients to have received chemotherapy in the neoadjuvant or adjuvant settings but disallowed prior chemotherapy for any metastatic disease (whether mCRPC or mCSPC). Also excluded were prior treatment with androgen receptor signaling inhibitors, or with systemic steroids (≥10 mg of prednisone), and significant cancer-related pain (score of >3 on the Brief Pain Inventory [BPI] short form). Required laboratory values included platelets ≥100,000/µL, hemoglobin ≥9 g/dL, creatinine ≤2× upper limit of normal, bilirubin ≤1.5× upper limit of normal, AST and ALT ≤2× upper limit of normal, and testosterone ≤50 ng/dL. The trial was approved by the NCI Central Institutional Review Board. All patients signed written informed consent.
Patients were randomly assigned (1:1) by the Alliance Statistics and Data Management Center to receive either enzalutamide alone (160 mg once daily by mouth) or enzalutamide plus AAP (abiraterone 1,000 mg once daily and prednisone 5 mg twice daily by mouth). Enzalutamide was provided by the study, as it was not approved for treatment of mCRPC before chemotherapy during the time this study was conducted, and therefore was provided to all patients (and served as the control arm of the trial as monotherapy). A stratified random block design was used, with randomization stratified by previous exposure to chemotherapy (yes or no) and prognostic risk groups according to the Halabi validated prognostic model of OS.17
AAP, however, had been approved for this indication, and patients were therefore required to obtain this drug on their own, in accordance with the standard of care. Patients were instructed to take enzalutamide in the morning, abiraterone in the evening on an empty stomach, and prednisone once in the morning and once in the evening. Dose reduction (enzalutamide to a minimum of 80 mg and abiraterone to a minimum of 500 mg) was permitted in the event of drug-related toxicities.
Treatment was terminated if clinical or radiographic progression occurred. On-treatment radiographic assessment was performed in accordance with PCWG. An increase in PSA was not grounds for termination of treatment.
Study Design and Data Analysis
The target number of events (deaths) was 616, and the sample size was 1,224 patients. The study was designed to detect a hazard ratio (HR) for OS of 0.77 in favor of combination therapy, using a one-sided type I error rate of 0.025 and 90% power, with an anticipated median OS of 35.3 months in the control arm. The following assumptions were made to achieve the target 616 deaths: a monthly accrual rate of 34 patients over a 36-month enrollment period, 14-24 months of follow-up after study closure, and OS time after an exponential distribution.
The trial was monitored semiannually by the Alliance Data and Safety Monitoring Board; five efficacy and futility interim analyses were planned. The Lan-DeMets spending function analog of a one-sided O'Brien-Fleming boundary was used to maintain the overall significance level of α = .025 while conducting interim analyses on OS. The efficacy and futility boundaries are provided in the study Protocol (online only) and Data Supplement (Table 1, online only).
The efficacy analyses included all randomly assigned patients, whereas the safety analyses consisted of patients who received treatment. The treatment comparisons were based on a stratified log-rank test for OS and rPFS. The Kaplan-Meier product-limit approach was used to estimate the OS and rPFS distributions. The P value for the primary OS treatment comparison was one-sided, and all other P values were two-sided. Exploratory analyses investigating the association between rPFS and OS were performed using three definitions of progression. An additional association analysis between time to progression and OS was also undertaken. These are described in detail in the Data Supplement, as are the statistical methods used.
The Alliance Data and Statistical Center performed registration, data collection, and statistical analyses. The date of the locked database for the final analysis was September 30, 2021.
Pharmacokinetic Studies
Venous blood samples were obtained before initiation of the treatment protocol and before administration of study medication at monthly clinic visits at the beginning of cycles 2-6. Plasma was separated by centrifugation, and plasma aliquots were stored at –20°C or below. These aliquots were shipped to the Alliance pharmacokinetic laboratory, where they were analyzed in batches for concentrations of enzalutamide, desmethyl enzalutamide, and abiraterone using a published liquid chromatography with tandem mass spectrometry assay.18 The plasma concentrations of enzalutamide and abiraterone were analyzed by nonlinear mixed-effect modeling using the Pumas AI program19 to estimate population apparent oral clearance.
Correlative Analyses
Specimens of baseline serum androgen, angiokine, micro-RNA, and pretreatment and post-treatment whole-blood RNA levels were collected for future analyses and correlation and cancer outcomes. These will be reported separately.
RESULTS
Patients
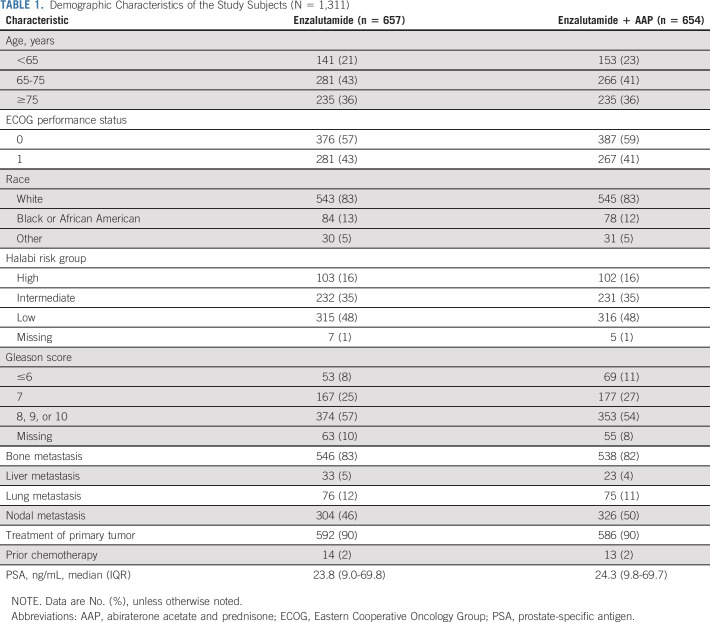
Between January 28, 2014, and August 31, 2016, 1,311 patients were randomly assigned: 657 in the enzalutamide arm and 654 in the combination arm (Fig 1). Patient accrual began on January 28, 2014, at 324 sites participating in the NCI-funded National Clinical Trials Network. The study was closed on November 2, 2018, by the Alliance Data and Safety Monitoring Board after the sixth and final planned analysis of the data, as part of the original statistical analysis plan. Patient characteristics were well balanced between the two treatment arms, including age, ECOG performance status, race, Gleason score, Halabi risk group, distribution of disease, PSA, and prior treatments (Table 1).
FIG 1.
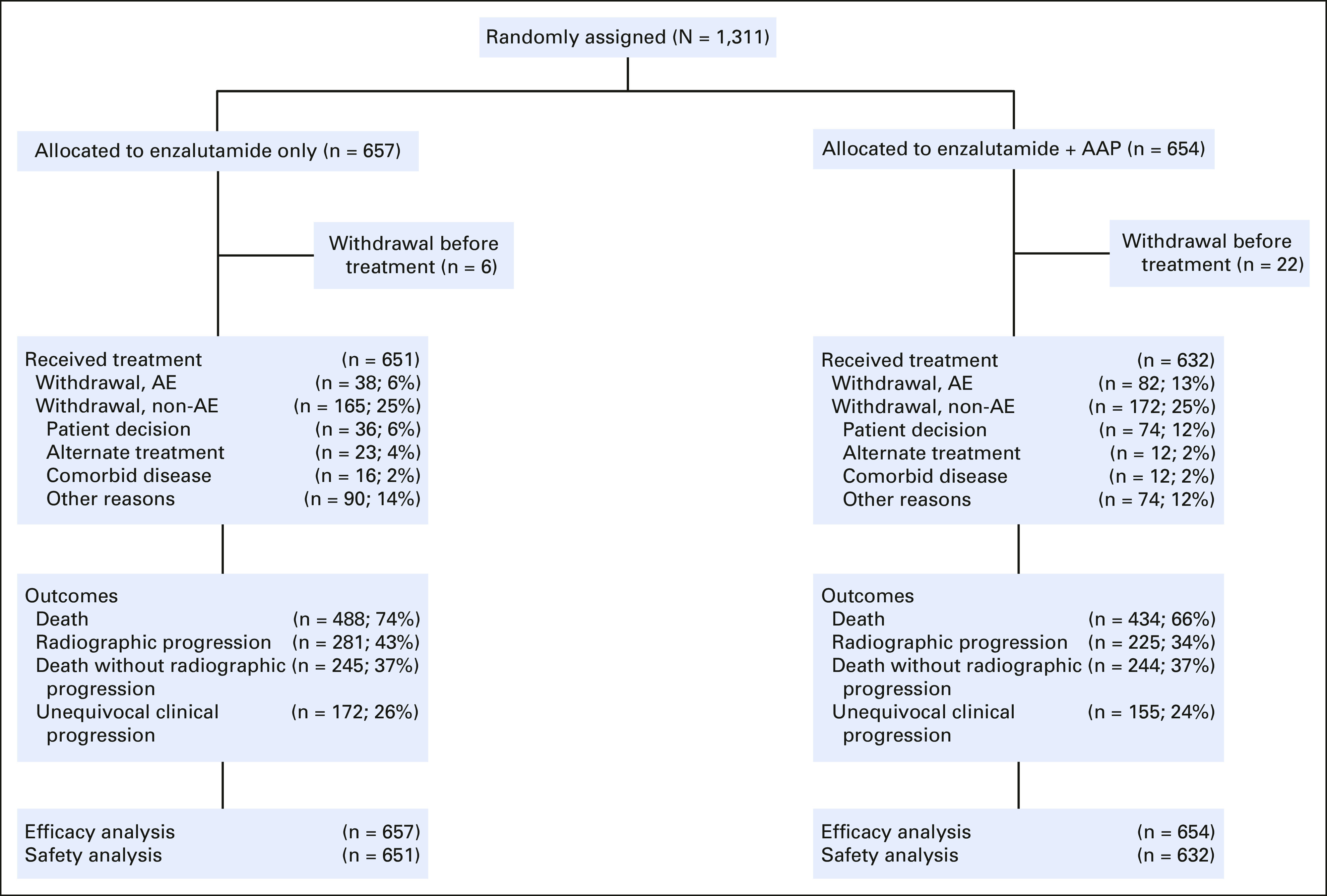
CONSORT diagram for A031201. AAP, abiraterone acetate and prednisone; AE, adverse event.
TABLE 1.
Demographic Characteristics of the Study Subjects (N = 1,311)
Treatment
The median exposure to treatment was 12.9 (IQR, 5.6-26.0) months overall, 13.7 (IQR, 6.4-27.6) months for the enzalutamide arm, and 12.3 (IQR, 5.5-25.3) months for the combination arm. Enzalutamide dose reductions were reported for 93 (14%) patients in the enzalutamide arm and for 168 (27%) patients in the combination arm. Within the combination arm, abiraterone and prednisone dose reductions were reported for 168 (27%) and 118 (19%) patients, respectively. Reasons for discontinuation of treatment included rPFS-defining criterion without clinical progression (24%), clinical progression without rPFS (13%), both rPFS and clinical progression event (10%), withdrawal after random assignment and before treatment (2%), adverse events (9%), and withdrawal of consent while receiving treatment (8%). Of note, more patients in the combination arm discontinued treatment for adverse events (13% v 6%) or withdrew consent before or during treatment (14% v 6%).
Efficacy
Primary end point.
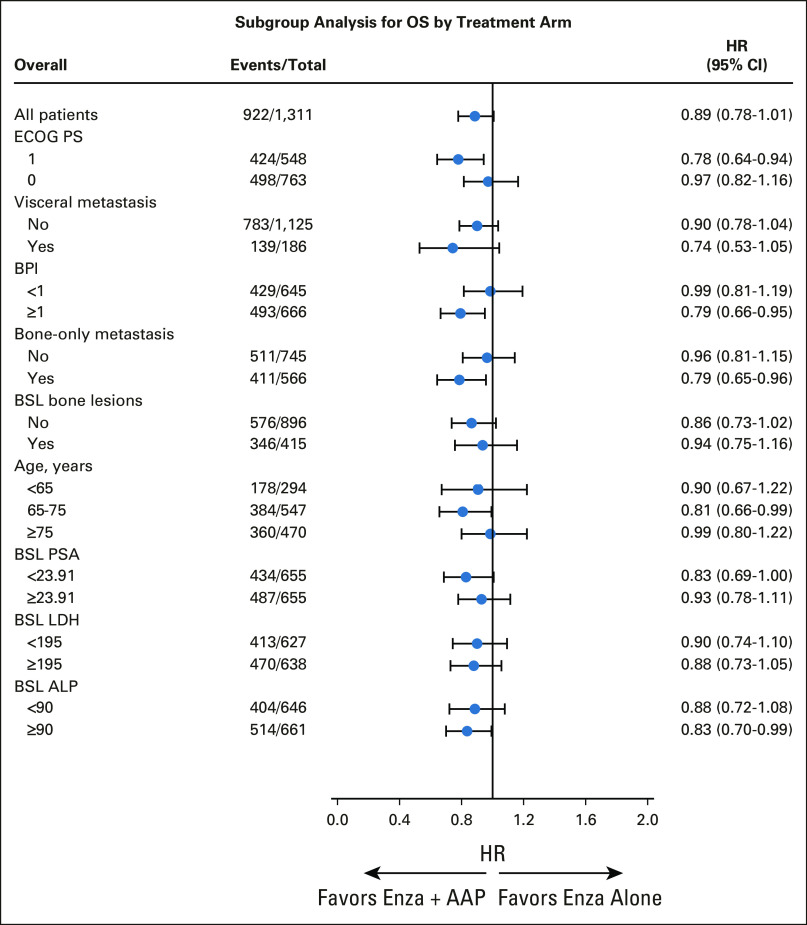
The median follow-up for the 389 surviving patients was 60.6 (IQR, 38.9-63.0) months; 488 patients (74%) in the enzalutamide arm and 434 patients (66%) in the combination arm died. OS was not meaningfully different between the two arms. The HR for OS was 0.89 (95% CI, 0.78 to 1.01; stratified log-rank test, one-sided P = .03; boundary significance level was .02). The Kaplan-Meier curves for OS are included in Figure 2A. Median OS for patients treated with enzalutamide alone was 32.7 (95% CI, 30.4 to 35.4) months, compared with 34.2 (95% CI, 31.3 to 37.3) months for patients treated with enzalutamide plus AAP. A forest plot of subgroups of patients and biomarkers of interest is presented in Figure 3. Patient subsets that appeared to have a small observed survival benefit from the combination regimen were those with an ECOG performance status of 1, those with a BPI score ≥1, and those with only bone metastases.
FIG 2.
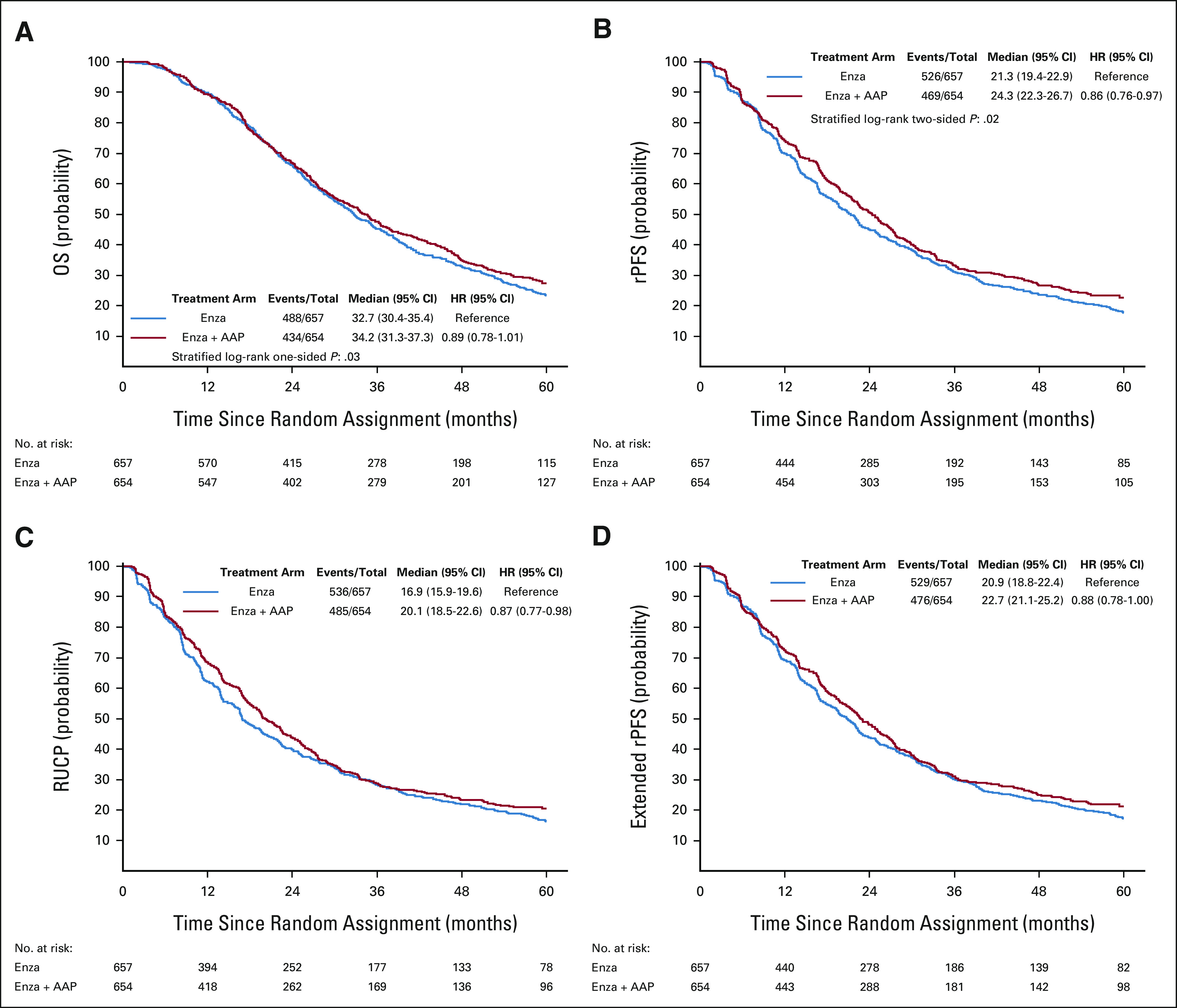
Kaplan-Meier curves of (A) OS and (B) rPFS according to the standard definition of progression (PCWG), (C) the PCWG definition with the addition of clinical progression, and (D) the PCWG definition with the addition of post-treatment radiographic progression of disease. AAP, abiraterone acetate and prednisone; enza, enzalutamide; HR, hazard ratio; OS, overall survival; PCWG, Prostate Cancer Clinical Trials Working Group; rPFS, radiographic progression-free survival; RUCP, radiographic and unequivocal clinical progression.
FIG 3.
Forest plot for OS in A031201. The cutoffs for BPI, PSA, LDH, and ALP were the median values. AAP, abiraterone acetate and prednisone; ALP, alkaline phosphatase; BPI, Brief Pain Inventory; BSL, baseline; ECOG, Eastern Cooperative Oncology Group; enza, enzalutamide; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival; PS, performance score; PSA, prostate-specific antigen.
Secondary end points.
rPFS.
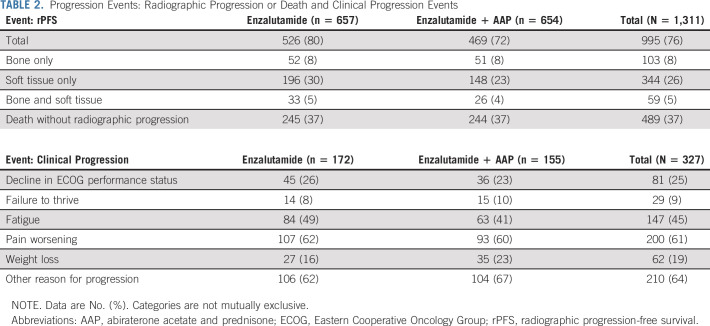
First-occurring rPFS events are listed in Table 2. Radiographic progression occurred in 506 patients (39%); death without radiographic progression occurred in 489 (37%). The most common type of radiographic progression was soft tissue progression (Table 2). The combination regimen (HR, 0.86 [95% CI, 0.76 to 0.97]; P = .02) was associated with superior rPFS (median rPFS, 21.3 [95% CI, 19.4 to 22.9] months for enzalutamide v 24.3 [95% CI, 22.3 to 26.7] months for enzalutamide plus AAP; Fig 2B). When the definition of progression was expanded to include clinical progression, the relative effect was not changed (median rPFS, 16.9 [95% CI, 15.9 to 19.6] months for enzalutamide v 20.1 [95% CI, 18.5 to 22.6] months for enzalutamide and AAP; HR, 0.87 [95% CI, 0.77 to 0.98]; Fig 2C). Expanding the definition of progression to include post-treatment radiographic progression marginally reduced the treatment effect (median rPFS, 20.9 [95% CI, 18.8 to 22.4] months for enzalutamide v 22.7 [95% CI, 21.1 to 25.2] months for enzalutamide and AAP; HR, 0.88 [95% CI, 0.78 to 1.00]; Fig 2D).
TABLE 2.
Progression Events: Radiographic Progression or Death and Clinical Progression Events
Association between rPFS and OS.
When the definition of progression was expanded to include clinical progression and post-treatment radiographic progression, the association between OS and rPFS was not found to be stronger than when the standard definition of progression (PCWG) was used (Data Supplement [Table 2]). Kendall's tau for the association between OS and rPFS for the PCWG definition of progression was 0.69 (95% CI, 0.66 to 0.71) versus 0.68 (95% CI, 0.65 to 0.70) for the expanded definition. An additional analysis assessing the association between OS and time to progression resulted in a Kendall's tau equal to 0.62 (95% CI, 0.58 to 0.65).
Clinical progression.
Among the patients who underwent random assignment, 327 unequivocal clinical progression events (25% of the total cohort) were documented. The most common clinical progression event was increase in pain, which occurred in 16% of patients (107/657) in the enzalutamide arm and 14% of patients (93/654) in the combination arm. Weight loss was observed in 4% of patients (27/657) in the enzalutamide arm, compared with 5% of patients (35/654) in the combination arm, and fatigue was observed in 13% of patients (84/657) in the enzalutamide arm, compared with 10% of patients (63/654) in the combination arm. The most frequent unequivocal clinical progression event recorded was other reason, which reflected a variety of events (such as increase in PSA) that did not constitute progression as defined by the PCWG criteria.
PSA declines.
Rates of decrease in PSA were not different between the two arms: in the enzalutamide arm, 82% (528/645) of patients had a ≥50% decrease and 62% (403/645) had an ≥80% decrease, whereas in the enzalutamide plus AAP arm, 81% (512/632) had a 50% decrease and 64% (407/632) had an 80% decrease (Data Supplement [Fig 1]).
Safety.
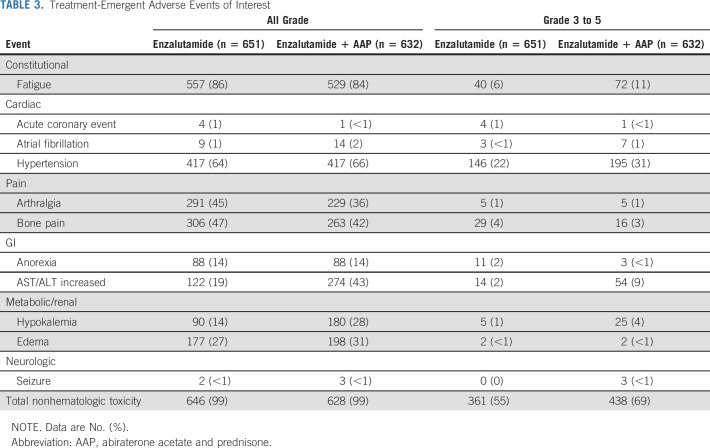
In both study arms, the treatment was well tolerated by most patients, given the low rate of grade 3 or higher toxicities (Table 3). However, the incidences of high-grade nonhematologic toxicity (69% v 55%), fatigue (11% v 6%), hypertension (31% v 22%), all-grade atrial fibrillation (2% v 1%), and transaminitis (43% v 19%) were higher in the combination arm, consistent with the known side-effect profile of abiraterone. By contrast, the incidence of all-grade arthralgia (45% v 36%) was higher in the enzalutamide arm, perhaps because of the use of prednisone with abiraterone. The incidence of seizure was similar between the arms: <1% in the enzalutamide arm and <1% in the combination arm.
TABLE 3.
Treatment-Emergent Adverse Events of Interest
Postprotocol treatment.
Postprotocol treatments were generally balanced between the two groups (Data Supplement [Table 3]); however, more patients in the enzalutamide arm received abiraterone (29% v 11%) and pembrolizumab (4% v 1%), and more patients in the combination arm received enzalutamide (12% v 9%) after completion of the protocol treatment. Use of subsequent docetaxel was balanced between the two arms, as were other known life-prolonging therapies.
Pharmacokinetics.
Pharmacokinetics were analyzed in 916 patients (464 enzalutamide arm and 452 combination arm). At the beginning of cycle 2, in the enzalutamide monotherapy arm, the estimated population apparent oral clearance of enzalutamide (n = 440) was 0.375 L/h (95% CI, 0.369 to 0.385); in the combination arm (n = 420), the estimated population apparent oral clearance of enzalutamide was 0.367 L/h (95% CI, 0.358 to 0.376) and that of abiraterone was 3,429 L/h (95% CI, 2,964 to 3,924).
DISCUSSION
This phase III randomized study of men with mCRPC aimed at determining the clinical benefit of targeting the androgen synthesis signaling axis with a combination of an androgen-deprivation therapy, an androgen biosynthesis inhibitor, and a next-generation antiandrogen. This was an adequately designed, NCI-funded, randomized phase III trial with the gold standard primary end point of OS. The results demonstrate that such a strategy does not significantly prolong OS. The results related to our secondary end points also do not support combination therapy. Although rPFS was 3 months longer in the combination arm, and although this difference was statistically significant, it is of limited clinical meaningfulness, given the added toxicities and costs. Rates of PSA declines were also not different between the two arms.
One possible reason for the lack of effect of the combination approach is the apparent effect of enzalutamide on hepatic clearance of abiraterone. Clearance of abiraterone in the combination arm was 2.2- to 2.9-fold higher than established values for abiraterone alone, which historically has been reported as 1,180-1,550 L/h.20-22 In the phase I trial of abiraterone plus enzalutamide, substantial variability was also observed: the Cmin concentration of abiraterone was 23% lower in the combination arm than in the abiraterone monotherapy arm; however, the cohort was small (16 patients), and the authors determined that the interaction between abiraterone and enzalutamide was not significant.14 The pharmacokinetic analysis in our study was more robust, as it represents data from 452 patients who received the combination treatment. Our results suggest that enzalutamide induces abiraterone metabolism, similar to other CYP3A4 inducers.20,23 Although this drug–drug interaction may have limited the efficacy of the combination, toxicities were still significantly higher, suggesting that further dose escalation of abiraterone may not be well tolerated or acceptable, and only a dedicated dose-escalation trial of higher doses of abiraterone given with enzalutamide could address this.
The data from A031201 are concordant with those from the recently published ACIS trial, in which 982 men were randomly assigned to AAP and apalutamide or AAP alone.24 In that study, the primary end point was rPFS, which was longer in the combination arm (24.0 [95% CI, 19.7 to 27.5] months) than in the monotherapy arm (16.6 [95% CI, 13.9 to 19.3] months; HR, 0.70 [95% CI, 0.60 to 0.83]; P < .0001); however, there was similarly no clinically significant benefit in OS, which was a secondary end point. ACIS did not include a pharmacokinetic component, although an earlier phase I study observed no significant pharmacokinetic interaction between abiraterone and apalutamide.25 Our data, however, raise the possibility that a pharmacokinetic effect may be at play, if examined in a larger and more robust study. The same might apply to the PLATO trial, in which men who had been treated with enzalutamide as first-line treatment for mCRPC were randomly assigned to receive either enzalutamide plus AAP or placebo and AAP in the second-line setting. In PLATO, rPFS, clinical progression, and mortality were not significantly different between the arms (HR, 0.83 [95% CI, 0.61 to 1.12]; P = .22).26 Finally, the pharmacokinetic phenomenon might also apply to the metastatic castration-sensitive context in the STAMPEDE trial, in which ADT and abiraterone seemed to have the same benefit as ADT, abiraterone, and enzalutamide. Taken together, these data demonstrate that combinations of biosynthesis inhibitors and androgen receptor modulators, whether in the first- or second-line setting, do not significantly improve patient outcomes relative to monotherapy.
A secondary objective of the present trial was to test, and perhaps refine, the PCWG end point of rPFS and its relationship to OS. PREVAIL and COU-302 demonstrated that rPFS and OS (with progression defined according to the PCWG criteria) were closely associated, and this finding led to regulatory recognition of rPFS as a clinical trial end point for drug approval.15,16,27 The association had a Kendall's tau of 0.69 (95% CI, 0.66 to 0.71) when the standard PCWG criteria were used, which is similar to that of the treatment arms of COU-302 (0.63 [95% CI, 0.56 to 0.70])15 and PREVAIL (0.72 [95% CI, 0.68 to 0.77]).16 Although the findings of our trial were negative in that the investigational regimen did not meaningfully improve OS or rPFS, the trial did uphold the relationship between rPFS and OS—that is, patients with slower disease progression lived longer. Expanded definitions of disease progression did not improve this association.
In conclusion, the combination of enzalutamide plus AAP for first-line treatment of mCRPC offered no clinical advantage over enzalutamide alone. The combination was associated with an unfavorable drug-drug interaction that increased abiraterone clearance and with more adverse events. The combination of enzalutamide and abiraterone is currently not recommended in routine clinical practice.
Michael J. Morris
Stock and Other Ownership Interests: Doximity
Consulting or Advisory Role: NCCN, Exelixis, Lantheus Medical Imaging, AstraZeneca, Amgen, Daiichi, Convergent Therapeutics, Pfizer, ITM Isotope Technologies Munich, Clarity Pharmaceuticals
Research Funding: Bayer (Inst), Progenics (Inst), Corcept Therapeutics (Inst), Roche/Genentech (Inst), Janssen (Inst), Celgene (Inst), Novartis (Inst)
Uncompensated Relationships: Bayer, Janssen Oncology, Novartis
Glenn Heller
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Olivia Bobek
Employment: Epic
Stock and Other Ownership Interests: Inovio Pharmaceuticals, Johnson & Johnson/Janssen, Procter & Gamble, Pfizer, Merck
Charles Ryan
Honoraria: Janssen Oncology, Bayer
Consulting or Advisory Role: Bayer, Dendreon, Advanced Accelerator Applications, Myovant Sciences, Clovis Oncology (Inst), Roivant
Research Funding: Clovis Oncology (Inst), Genzyme (Inst)
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology, Amgen, Bayer, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Celgene, Constellation Pharmaceuticals, Curium Pharma, Lilly, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Tempus, Orion, AIkido Pharma
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer, Amgen, Astellas Pharma, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Constellation Pharmaceuticals, Curium Pharma, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Medivation, Tempus, Orion, AIkido Pharma
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), Constellation Pharmaceuticals (Inst), Celgene
Patents, Royalties, Other Intellectual Property: Co-inventor of a biomarker technology that has been licensed to Qiagen
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
Alan H. Bryce
Honoraria: Astellas Pharma, Bayer, Pfizer, Verity Pharmaceuticals, Myovant Sciences, Research to Practice, AstraZeneca, Advanced Accelerator Applications/Novartis, Castle Biosciences, Horizon CME
Research Funding: Janssen Oncology (Inst)
Travel, Accommodations, Expenses: Clovis Oncology (Inst), Phosplatin Therapeutics (Inst)
Olwen Hahn
Employment: Solaris Health
Leadership: Via Oncology
Stock and Other Ownership Interests: Teleflex Medical, Novavax
Honoraria: Cardinal Health
Consulting or Advisory Role: Pfizer, HMP
Travel, Accommodations, Expenses: Cardinal Health
Himisha Beltran
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Pfizer, Blue Earth Diagnostics, Foundation Medicine, Amgen, Loxo/Lilly, Daicchi Sankyo, Bayer, Sanofi, Curie Therapeutics, Merck, Novartis
Research Funding: Janssen (Inst), AbbVie/Stemcentrx (Inst), Bristol Myers Squibb Foundation (Inst), Daiichi Sankyo (Inst)
Travel, Accommodations, Expenses: Janssen Oncology
Andrew J. Armstrong
Consulting or Advisory Role: Bayer, Pfizer, Astellas Scientific and Medical Affairs, Inc, AstraZeneca, Merck, Bristol Myers Squibb, Janssen, FORMA Therapeutics, Novartis, Exelixis, Myovant Sciences, GoodRx, Epic Sciences, IDEAYA Biosciences
Research Funding: Dendreon (Inst), Bayer (Inst), Pfizer (Inst), Novartis (Inst), Janssen Oncology (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Constellation Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), BeiGene (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), FORMA Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture technology (Inst)
Travel, Accommodations, Expenses: Astellas Scientific and Medical Affairs, Inc
Lawrence Schwartz
Consulting or Advisory Role: Novartis, Regeneron, Bristol Myers Squibb/Celgene
Research Funding: Merck Sharp & Dohme (Inst), Boehringer Ingelheim (Inst)
Patents, Royalties, Other Intellectual Property: Varian Medical Systems
Lionel D. Lewis
Consulting or Advisory Role: G1 Therapeutics, 7 Hills Pharma
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst), AbbVie (Inst), Bayer (Inst), Curis (Inst)
Expert Testimony: Amgen
Travel, Accommodations, Expenses: 7 Hills Pharma
Jan H. Beumer
Employment: Voisin Consulting
Stock and Other Ownership Interests: GlaxoSmithKline
Consulting or Advisory Role: Genentech
Research Funding: AbbVie (Inst), TriSalus Life Sciences (Inst)
Patents, Royalties, Other Intellectual Property: Sulphoraphane for melanoma chemoprevention (Inst)
Expert Testimony: Pfizer, Spectrum Pharmaceuticals, AstraZeneca/Merck, Astellas Pharma, Taiho Pharmaceutical
Uncompensated Relationships: Qrono, Applied Isotope Technologies
Amir Goldkorn
Research Funding: Thermo Fisher Scientific, RareCyte, Menarini Silicon Biosystems
Patents, Royalties, Other Intellectual Property: I am listed as a co-inventor on a patent held jointly by USC and Caltech for a microfilter we developed for capturing live circulating tumor cells from blood
Bruce J. Roth
Consulting or Advisory Role: Seattle Genetics, Merck, Secura Bio
Research Funding: Medivation (Inst)
Mary-Ellen Taplin
Honoraria: Janssen-Ortho, Clovis Oncology, UpToDate, Research to Practice, Pfizer, AstraZeneca, Roivant, AbbVie, Arcus Biosciences, Constellation Pharmaceuticals, Epizyme, Targeted Oncology, Arvinas, Blue Earth Diagnostics, Hengrui Therapeutics, Propella Therapeutics
Consulting or Advisory Role: Janssen-Ortho, Bayer, Best Doctors, Inc, UpToDate, Clovis Oncology, Research to Practice, Myovant Sciences, Pfizer, AstraZeneca, Arcus Ventures
Research Funding: Janssen-Ortho (Inst)
Travel, Accommodations, Expenses: Advanced Prostate Cancer Society
Susan Halabi
Employment: ASCO
Honoraria: Sanofi, AVEO, Bristol Myers Squibb
Eric J. Small
Stock and Other Ownership Interests: Fortis, Harpoon Therapeutics, Teon Therapeutics
Honoraria: Janssen, Johnson and Johnson
Consulting or Advisory Role: Janssen Oncology, Teon Therapeutics, Fortis
Open Payments Link: https://openpaymentsdata.cms.gov/physician/660367
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented, in part, as an oral presentation at the 2019 ASCO Annual Scientific Meeting, Chicago, IL, May 31-June 4, 2019 (Abstract #5008).
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under the Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA189960, UG1CA232760, UG1CA233180, UG1CA233184, UG1CA233253, UG1CA233290, and P30 CA008748 (to Memorial Sloan Kettering Cancer Center), UG1CA233327, UG1CA233339, U10CA180888, and UG1CA180830 (SWOG), P30CA047904 and R50CA211241 (to University of Pittsburgh), https://acknowledgments.alliancefound.org. Also supported in part by funds from Astellas.
CLINICAL TRIAL INFORMATION
NCT01949337 (Alliance A031201)
S.H. and E.J.S. contributed equally to this work.
DATA SHARING STATEMENT
The authors agree to adhere to NCI and Alliance policies on data sharing. This includes timely dissemination of research findings and facilitating the sharing of data and other research resources. Per National Cancer Institute's National Clinical Trials Network (NCTN) guidelines, any investigator may submit a request for data from published Alliance or legacy American College of Surgeons Oncology Group (ACOSOG), Cancer and Leukemia Group B (CALGB), or North Central Cancer Treatment Group (NCCTG) trials. To submit a data request, the investigator should complete an Alliance Data Sharing Request Form and send it by e-mail to concepts@allianceNCTN.org. Further information on Alliance/NCTN policies on data sharing may be found at https://urldefense.com/v3/__https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=*2FPublic*2FDatasharing__;JSU!!N11eV2iwtfs!p0El52scvL37ZS9J-Fn3zB1EQLjfZnmh0J0_kkn34FMs90dYma-A5cLOq2V38krHl82hRPnjQAfBxDVD$.
AUTHOR CONTRIBUTIONS
Conception and design: Michael J. Morris, Charles Ryan, Olwen Hahn, Andrew J. Armstrong, Lawrence Schwartz, Lionel D. Lewis, Brooke Langevin, Colleen Watt, Mary-Ellen Taplin, Susan Halabi, Eric J. Small
Provision of study materials or patients: Michael J. Morris, Charles Ryan, Andrew J. Armstrong, Lionel D. Lewis, Eric C. McGary, Bruce J. Roth
Collection and assembly of data: Michael J. Morris, David W. Hillman, Charles Ryan, Emmanuel S. Antonarakis, Olwen Hahn, Andrew J. Armstrong, Lawrence Schwartz, Eric C. McGary, Paul T. Mehan, Amir Goldkorn, Bruce J. Roth, Han Xiao, Mary-Ellen Taplin
Data analysis and interpretation: Michael J. Morris, Glenn Heller, David W. Hillman, Olivia Bobek, Charles Ryan, Emmanuel S. Antonarakis, Alan H. Bryce, Himisha Beltran, Andrew J. Armstrong, Lionel D. Lewis, Jan H. Beumer, Brooke Langevin, Bruce J. Roth, Mary-Ellen Taplin, Susan Halabi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase III Study of Enzalutamide Compared With Enzalutamide Plus Abiraterone for Metastatic Castration-Resistant Prostate Cancer (Alliance A031201 Trial)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael J. Morris
Stock and Other Ownership Interests: Doximity
Consulting or Advisory Role: NCCN, Exelixis, Lantheus Medical Imaging, AstraZeneca, Amgen, Daiichi, Convergent Therapeutics, Pfizer, ITM Isotope Technologies Munich, Clarity Pharmaceuticals
Research Funding: Bayer (Inst), Progenics (Inst), Corcept Therapeutics (Inst), Roche/Genentech (Inst), Janssen (Inst), Celgene (Inst), Novartis (Inst)
Uncompensated Relationships: Bayer, Janssen Oncology, Novartis
Glenn Heller
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Olivia Bobek
Employment: Epic
Stock and Other Ownership Interests: Inovio Pharmaceuticals, Johnson & Johnson/Janssen, Procter & Gamble, Pfizer, Merck
Charles Ryan
Honoraria: Janssen Oncology, Bayer
Consulting or Advisory Role: Bayer, Dendreon, Advanced Accelerator Applications, Myovant Sciences, Clovis Oncology (Inst), Roivant
Research Funding: Clovis Oncology (Inst), Genzyme (Inst)
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology, Amgen, Bayer, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Celgene, Constellation Pharmaceuticals, Curium Pharma, Lilly, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Tempus, Orion, AIkido Pharma
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer, Amgen, Astellas Pharma, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Constellation Pharmaceuticals, Curium Pharma, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, ISMAR Health Care, Medivation, Tempus, Orion, AIkido Pharma
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), Constellation Pharmaceuticals (Inst), Celgene
Patents, Royalties, Other Intellectual Property: Co-inventor of a biomarker technology that has been licensed to Qiagen
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
Alan H. Bryce
Honoraria: Astellas Pharma, Bayer, Pfizer, Verity Pharmaceuticals, Myovant Sciences, Research to Practice, AstraZeneca, Advanced Accelerator Applications/Novartis, Castle Biosciences, Horizon CME
Research Funding: Janssen Oncology (Inst)
Travel, Accommodations, Expenses: Clovis Oncology (Inst), Phosplatin Therapeutics (Inst)
Olwen Hahn
Employment: Solaris Health
Leadership: Via Oncology
Stock and Other Ownership Interests: Teleflex Medical, Novavax
Honoraria: Cardinal Health
Consulting or Advisory Role: Pfizer, HMP
Travel, Accommodations, Expenses: Cardinal Health
Himisha Beltran
Consulting or Advisory Role: Janssen Oncology, AstraZeneca, Pfizer, Blue Earth Diagnostics, Foundation Medicine, Amgen, Loxo/Lilly, Daicchi Sankyo, Bayer, Sanofi, Curie Therapeutics, Merck, Novartis
Research Funding: Janssen (Inst), AbbVie/Stemcentrx (Inst), Bristol Myers Squibb Foundation (Inst), Daiichi Sankyo (Inst)
Travel, Accommodations, Expenses: Janssen Oncology
Andrew J. Armstrong
Consulting or Advisory Role: Bayer, Pfizer, Astellas Scientific and Medical Affairs, Inc, AstraZeneca, Merck, Bristol Myers Squibb, Janssen, FORMA Therapeutics, Novartis, Exelixis, Myovant Sciences, GoodRx, Epic Sciences, IDEAYA Biosciences
Research Funding: Dendreon (Inst), Bayer (Inst), Pfizer (Inst), Novartis (Inst), Janssen Oncology (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Constellation Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), BeiGene (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), FORMA Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture technology (Inst)
Travel, Accommodations, Expenses: Astellas Scientific and Medical Affairs, Inc
Lawrence Schwartz
Consulting or Advisory Role: Novartis, Regeneron, Bristol Myers Squibb/Celgene
Research Funding: Merck Sharp & Dohme (Inst), Boehringer Ingelheim (Inst)
Patents, Royalties, Other Intellectual Property: Varian Medical Systems
Lionel D. Lewis
Consulting or Advisory Role: G1 Therapeutics, 7 Hills Pharma
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst), AbbVie (Inst), Bayer (Inst), Curis (Inst)
Expert Testimony: Amgen
Travel, Accommodations, Expenses: 7 Hills Pharma
Jan H. Beumer
Employment: Voisin Consulting
Stock and Other Ownership Interests: GlaxoSmithKline
Consulting or Advisory Role: Genentech
Research Funding: AbbVie (Inst), TriSalus Life Sciences (Inst)
Patents, Royalties, Other Intellectual Property: Sulphoraphane for melanoma chemoprevention (Inst)
Expert Testimony: Pfizer, Spectrum Pharmaceuticals, AstraZeneca/Merck, Astellas Pharma, Taiho Pharmaceutical
Uncompensated Relationships: Qrono, Applied Isotope Technologies
Amir Goldkorn
Research Funding: Thermo Fisher Scientific, RareCyte, Menarini Silicon Biosystems
Patents, Royalties, Other Intellectual Property: I am listed as a co-inventor on a patent held jointly by USC and Caltech for a microfilter we developed for capturing live circulating tumor cells from blood
Bruce J. Roth
Consulting or Advisory Role: Seattle Genetics, Merck, Secura Bio
Research Funding: Medivation (Inst)
Mary-Ellen Taplin
Honoraria: Janssen-Ortho, Clovis Oncology, UpToDate, Research to Practice, Pfizer, AstraZeneca, Roivant, AbbVie, Arcus Biosciences, Constellation Pharmaceuticals, Epizyme, Targeted Oncology, Arvinas, Blue Earth Diagnostics, Hengrui Therapeutics, Propella Therapeutics
Consulting or Advisory Role: Janssen-Ortho, Bayer, Best Doctors, Inc, UpToDate, Clovis Oncology, Research to Practice, Myovant Sciences, Pfizer, AstraZeneca, Arcus Ventures
Research Funding: Janssen-Ortho (Inst)
Travel, Accommodations, Expenses: Advanced Prostate Cancer Society
Susan Halabi
Employment: ASCO
Honoraria: Sanofi, AVEO, Bristol Myers Squibb
Eric J. Small
Stock and Other Ownership Interests: Fortis, Harpoon Therapeutics, Teon Therapeutics
Honoraria: Janssen, Johnson and Johnson
Consulting or Advisory Role: Janssen Oncology, Teon Therapeutics, Fortis
Open Payments Link: https://openpaymentsdata.cms.gov/physician/660367
No other potential conflicts of interest were reported.
REFERENCES
- 1.Isaacs JT, Coffey DS: Adaptation versus selection as the mechanism responsible for the relapse of prostatic cancer to androgen ablation as studied in the Dunning R-3327 H adenocarcinoma. Cancer Res 41:5070-5074, 1981 [PubMed] [Google Scholar]
- 2.Scher HI, Morris MJ, Stadler WM, et al. : Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 34:1402-1418, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, Halabi S, Tannock I, et al. : Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26:1148-1159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran C, Ouk S, Clegg NJ, et al. : Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324:787-790, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer TM, Armstrong AJ, Rathkopf DE, et al. : Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424-433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potter GA, Barrie SE, Jarman M, et al. : Novel steroidal inhibitors of human cytochrome P45017 (17 alpha-hydroxylase-C17,20-lyase): Potential agents for the treatment of prostatic cancer. J Med Chem 38:2463-2471, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Attard G, Reid AH, A'Hern R, et al. : Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol 27:3742-3748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CJ, Smith MR, de Bono JS, et al. : Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138-148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bono JS, Logothetis CJ, Molina A, et al. : Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995-2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fizazi K, Tran N, Fein L, et al. : Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377:352-360, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. : ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 37:2974-2986, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efstathiou E, Titus M, Wen S, et al. : Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol 67:53-60, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efstathiou E, Titus M, Tsavachidou D, et al. : Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol 30:637-643, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efstathiou E, Titus M, Wen S, et al. : Enzalutamide in combination with abiraterone acetate in bone metastatic castration-resistant prostate cancer patients. Eur Urol Oncol 3:119-127, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris MJ, Molina A, Small EJ, et al. : Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol 33:1356-1363, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathkopf DE, Beer TM, Loriot Y, et al. : Radiographic progression-free survival as a clinically meaningful end point in metastatic castration-resistant prostate cancer: The PREVAIL randomized clinical trial. JAMA Oncol 4:694-701, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halabi S, Lin CY, Kelly WK, et al. : Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 32:671-677, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K-P, Parise RA, Holleran JL, et al. : Simultaneous quantitation of abiraterone, enzalutamide, N-desmethyl enzalutamide, and bicalutamide in human plasma by LC-MS/MS. J Pharm Biomed Anal 138:197-205, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rackauckas C, Ma Y, Noack A, et al. : Accelerated predictive healthcare analytics with Pumas, a high performance pharmaceutical modeling and simulation platform. bioRxiv 10.1101/2020.11.28.402297 [Google Scholar]
- 20.Stuyckens K, Saad F, Xu XS, et al. : Population pharmacokinetic analysis of abiraterone in chemotherapy-naïve and docetaxel-treated patients with metastatic castration-resistant prostate cancer. Clin Pharmacokinet 53:1149-1160, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Bernard A, Vaccaro N, Acharya M, et al. : Impact on abiraterone pharmacokinetics and safety: Open-label drug–drug interaction studies with ketoconazole and rifampicin. Clin Pharmacol Drug Dev 4:63-73, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Benoist GE, Hendriks RJ, Mulders PFA, et al. : Pharmacokinetic aspects of the two novel oral drugs used for metastatic castration-resistant prostate cancer: Abiraterone acetate and enzalutamide. Clin Pharmacokinet 55:1369-1380, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benoist GE, van der Doelen MJ, Ter Heine R, et al. : A clinically relevant decrease in abiraterone exposure associated with carbamazepine use in a patient with castration-resistant metastatic prostate cancer. Br J Clin Pharmacol 84:1064-1067, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saad F, Efstathiou E, Attard G, et al. : Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): A randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol 22:1541-1559, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posadas EM, Chi KN, de Wit R, et al. : Pharmacokinetics, safety, and antitumor effect of apalutamide with abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: Phase Ib study. Clin Cancer Res 26:3517-3524, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Attard G, Borre M, Gurney H, et al. : Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J Clin Oncol 36:2639-2646, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kluetz PG, Ning YM, Maher VE, et al. : Abiraterone acetate in combination with prednisone for the treatment of patients with metastatic castration-resistant prostate cancer: US Food and Drug Administration drug approval summary. Clin Cancer Res 19:6650-6656, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors agree to adhere to NCI and Alliance policies on data sharing. This includes timely dissemination of research findings and facilitating the sharing of data and other research resources. Per National Cancer Institute's National Clinical Trials Network (NCTN) guidelines, any investigator may submit a request for data from published Alliance or legacy American College of Surgeons Oncology Group (ACOSOG), Cancer and Leukemia Group B (CALGB), or North Central Cancer Treatment Group (NCCTG) trials. To submit a data request, the investigator should complete an Alliance Data Sharing Request Form and send it by e-mail to concepts@allianceNCTN.org. Further information on Alliance/NCTN policies on data sharing may be found at https://urldefense.com/v3/__https://www.allianceforclinicaltrialsinoncology.org/main/public/standard.xhtml?path=*2FPublic*2FDatasharing__;JSU!!N11eV2iwtfs!p0El52scvL37ZS9J-Fn3zB1EQLjfZnmh0J0_kkn34FMs90dYma-A5cLOq2V38krHl82hRPnjQAfBxDVD$.