PURPOSE
We conducted a phase II trial evaluating the efficacy of VEGFR inhibitor axitinib and PD-L1 inhibitor avelumab in patients with recurrent/metastatic adenoid cystic carcinoma (R/M ACC).
PATIENTS AND METHODS
Eligible patients had R/M ACC with progression within 6 months before enrollment. Treatment consisted of axitinib and avelumab. The primary end point was objective response rate (ORR) per RECIST 1.1; secondary end points included progression-free survival (PFS), overall survival (OS), and toxicity. Simon's optimal two-stage design tested the null hypothesis of ORR ≤5% versus ORR ≥20% at 6 months; ≥4 responses in 29 patients would reject the null hypothesis.
RESULTS
Forty patients enrolled from July 2019 to June 2021; 28 were evaluable for efficacy (six screen failures; six evaluable for safety only). The confirmed ORR was 18% (95% CI, 6.1 to 36.9); there was one unconfirmed partial response (PR). Two patients achieved PR after 6 months; thus, the ORR at 6 months was 14%. The median follow-up time for surviving patients was 22 months (95% CI, 16.6 to 39.1 months). The median PFS was 7.3 months (95% CI, 3.7 to 11.2 months), 6-month PFS rate was 57% (95% CI, 41 to 78), and median OS was 16.6 months (95% CI, 12.4 to not reached months). Most common treatment-related adverse events (TRAEs) included fatigue (62%), hypertension (32%), and diarrhea (32%). Ten (29%) patients had serious TRAEs, all grade 3; four patients (12%) discontinued avelumab, and nine patients (26%) underwent axitinib dose reduction.
CONCLUSION
The study reached its primary end point with ≥4 PRs in 28 evaluable patients (confirmed ORR of 18%). The potential added benefit of avelumab to axitinib in ACC requires further investigation.
INTRODUCTION
Adenoid cystic carcinoma (ACC) is a malignancy of the secretory glands that usually arises in the head and neck. Despite aggressive local therapy, typically involving surgery and radiotherapy, most patients with ACC will develop recurrent or metastatic (R/M) disease.1,2 The course of R/M ACC can vary; although most patients have indolent disease, a subset experiences aggressive disease, frequently associated with the presence of NOTCH1 activating mutations.3
CONTEXT
Key Objective
To evaluate the efficacy of the VEGFR inhibitor axitinib combined with the PD-L1 inhibitor avelumab in patients with recurrent/metastatic adenoid cystic carcinoma (R/M ACC) with evidence of disease progression within 6 months.
Knowledge Generated
Axitinib plus avelumab demonstrated clinical activity in patients with progressive R/M ACC who had not been previously exposed to VEGFR or PD-L1/PD-1 inhibitors. The toxicity profile of the drug combination was similar to what has been reported with single-agent axitinib in ACC.
Relevance (R.G. Maki)
The combination of axitinib and avelumab provides a novel treatment option for this form of salivary gland cancer, which demonstrates an unusual biological pattern of slow growth yet early presentation with metastasis.*
*Relevance section written by JCO Associate Editor Robert G. Maki, MD, PhD, FACP, FASCO.
Currently, there is no standard-of-care palliative systemic therapy for patients with R/M ACC. The most commonly used regimens are multikinase inhibitors (MKIs) targeting VEGFR or platinum-based chemotherapy, both with modest activity.1,4-8
Axitinib is an oral MKI that targets VEGFR1-3, KIT, and PDGFR α/β. It has been studied as a single agent in R/M ACC in two phase II trials, rendering objective response rates (ORR) of 0%-11.5%, 6-month disease control rates of 39%-73%, and a median progression-free survival (PFS) of 5.7-10.8 months.5,9 These results led to the inclusion of axitinib as a therapeutic option for R/M ACC in the National Comprehensive Cancer Network guidelines.10
Antibodies that inhibit the PD-1/PD-L1 pathway have shown promising activity in many tumor types. However, PD-1/PD-L1 inhibitors as monotherapy or combined with a CTLA-4 inhibitor or histone deacetylases inhibitor have shown disappointing activity in ACC, with ORR ranging from 0% to 9% and median PFS < 5 months.11-15 The limited activity of PD-1/PD-L1 inhibitors in ACC is consistent with ACC's uninflamed and immunosuppressive tumor microenvironment and its low mutational burden, and both these characteristics are established biomarkers of lack of clinical benefit from single-agent PD-1/PD-L1 inhibitors.11,13,14,16
In addition to angiogenesis, VEGFR plays a critical role in promoting immune suppression.17-20 Antiangiogenic agents can increase the trafficking of immune effector cells into tumors and convert an immunosuppressive tumor microenvironment to an immunosupportive one.21 In mouse models, treatment with VEGFR inhibitors has yielded an increase in T-cell recruitment and infiltration into tumors and synergism with anti-PD1 antibodies.20,22 Consistently, VEGFR MKI combined with PD-1/PD-L1 inhibitors have shown higher efficacy than single-agent VEGFR MKI and have become the standard-of-care treatment for renal cell carcinoma and endometrial carcinoma.23,24
On the basis of the strong rationale of VEGFR and PD-L1 inhibitors' complementary mechanisms of action and their combination's promising efficacy in other solid tumors, we conducted a phase II trial evaluating the efficacy of axitinib plus avelumab in patients with progressive R/M ACC.
PATIENTS AND METHODS
Patients
Eligible patients were 18 years and older with a histologically confirmed diagnosis of R/M ACC, measurable disease per RECIST 1.1,25 Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function. Patients could have received any number of prior therapies except MKIs targeting VEGFR or PD-1/PD-L1 inhibitors. Progression of disease per RECIST 1.1 and/or new or worsening disease-related symptoms within 6 months before enrollment was required for study entry and evaluated prospectively. Complete eligibility criteria can be found in the study protocol (Online only). The study was approved by the institutional review board at MD Anderson Cancer Center (MDACC) and conducted according to the Declaration of Helsinki and Good Clinical Practice Guidelines. The cutoff date for analysis was November 11, 2022.
Study Procedures
This was a single-arm, open label, phase II, investigator-initiated trial designed to evaluate the efficacy and safety of axitinib plus avelumab in patients with R/M ACC and evidence of disease progression within 6 months before enrollment. Axitinib was administered orally at 5 mg twice a day, and avelumab was administered intravenously at 10 mg/kg on days 1 and 15 of 28-day cycles. Imaging for tumor assessment was performed at baseline and then every 8 weeks. Patients were treated until disease progression, death, unacceptable toxicity, consent withdrawal, or physician's discretion. Two axitinib dose reductions (3 and 2 mg twice a day) were allowed for patients who developed grade 3 or four adverse events (AEs) or intolerable grade 2 AEs attributed to axitinib. No avelumab dose reductions were allowed. For overlapping AEs (ie, toxicities that could be attributed to either axitinib or avelumab), decisions regarding which drug should have its dose reduced or be held were made according to the overall incidence of the AE for each individual drug if other factors (eg, onset of symptoms) could not help define the causative agent. In general, effort was made to continue axitinib.
Biomarker Analysis
Targeted DNA sequencing was performed using either the MDACC platform (n = 21),26 FoundationOne (Cambridge, MA, n = 2),27 Tempus xT (Chicago, IL, n = 2),28 or MiSeq (Illumina, CA, n = 1).29 Immunohistochemistry staining for PD-L1 was performed using clone 22C3 (Agilent, Santa Clara, CA).
Statistical Analysis
The primary end point was ORR per RECIST 1.1. Secondary end points included ORR per iRECIST,30 duration of response (DOR), PFS, PFS rate at 6 months after treatment initiation, overall survival (OS), OS rate at 6 months after treatment initiation, safety, and toxicity.
All eligible patients who received at least one cycle of treatment and had at least one restaging image were considered evaluable for the primary end point. Safety analyses were performed on all patients who received any treatment dose. AEs were graded according to Common Terminology Criteria for AEs version 4.0.
This trial used a Simon optimal two-stage design with a null hypothesis of 5% ORR and the alternative hypothesis of 20% ORR with type I and type II errors set at 0.05 and 0.2, respectively. If ≥1 response(s) were observed in the 10 patients enrolled during the first stage, 19 additional patients would be entered in the second stage to reach a total of 29 evaluable patients. By the end of the study, the regimen would be accepted if the ORR at 6 months was ≥4 of 29 evaluable patients.
DOR was measured from the date response criteria were met until the first date that progression was documented. PFS was defined as the duration from start of treatment to date of progression or death, whichever occurred first. The PFS rate at 6 months was the percentage of patients without disease progression at 6 months. OS was defined as the duration from start of treatment to death. The OS rate at 6 months was the percentage of living patients at 6 months. The Kaplan-Meier approach was used to estimate the PFS and OS distributions, along with median estimates with 95% CI. Fisher's exact test was used to evaluate the association between objective response or clinical benefit rate and number of prior therapy lines. A two-sided α level of 0.05 was considered to indicate statistical significance for all analyses.
RESULTS
Patient and Disease Characteristics
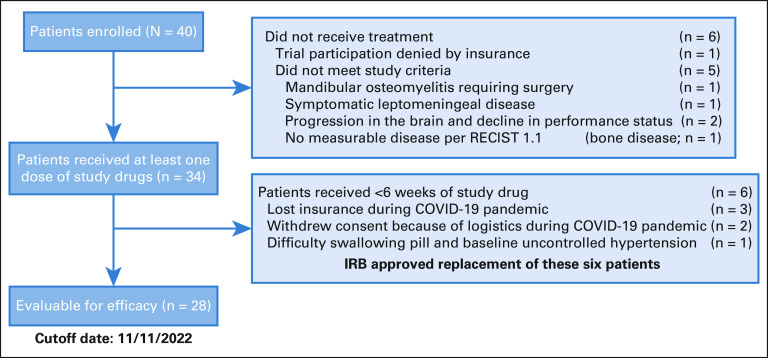
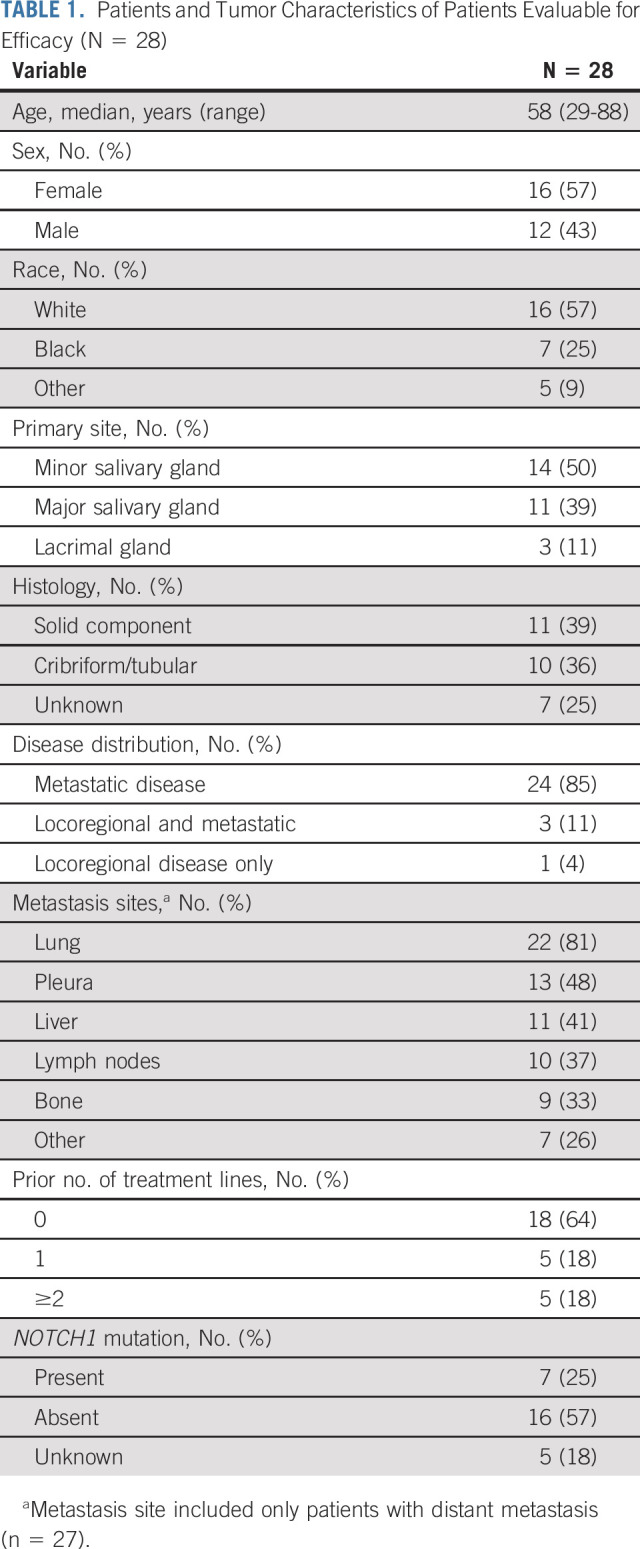
Between July 24, 2019, and June 29, 2021, 40 patients enrolled in this study; six did not meet screening criteria (Fig 1). Of 34 patients who received at least one dose of either study drug (Data Supplement [online only]), six received <6 weeks of axitinib and avelumab treatment and did not undergo the first restaging imaging assessment because of loss of insurance (n = 3), other logistic issues related to the COVID-19 pandemic (n = 2), or inability to swallow pills and uncontrolled preexisting hypertension (n = 1). None of these six patients had clinical signs of disease progression; thus, they were included only in the safety analysis. A total of 28 patients were included in the efficacy analysis. These patients' characteristics are summarized in Table 1. The median age was 58 (range, 29-88) years, 39% of patients had histology with a solid component, and 39% had received at least one prior line of palliative systemic therapy, detailed in the Data Supplement. Radiologic disease progression per RECIST 1.1 within 6 months of enrollment was confirmed by the radiologist in 23 of 28 (82%) evaluable patients; the remaining patients did not have prior imaging done within 6 months but had unequivocal clinical progression within this timeframe (n = 3) or had disease progression on the basis of the radiology report and principal investigator assessment, but the trial radiologist retrospectively recorded an 18% tumor growth (n = 1) or equivocal radiologic progression because of lack of contrast on prior scan (n = 1).
FIG 1.
Study flow chart diagram. IRB, Institutional Review Board
TABLE 1.
Patients and Tumor Characteristics of Patients Evaluable for Efficacy (N = 28)
Overall Response
Efficacy outcomes are summarized in Table 2. One patient had a partial response (PR) in the first stage of the study (n = 10), allowing continued enrollment for the second stage. The ORR was 21% (95% CI, 8.3 to 41; Fig 2A). Among the six responders, the median time to response was 5.1 months. One response was unconfirmed due to early progression in nontarget lesions, leaving a confirmed ORR of 18% (95% CI, 6.1 to 36.9). Of the six responses, two occurred after 6 months; thus the ORR at 6 months was 14% (95% CI, 4.0 to 32.7). The two patients who achieved a PR after 6 months remain on the study (Fig 2B). Representative radiologic images of two responders are shown in Figure 2C. Fourteen patients (50%) had stable disease (SD), and eight (29%) had progressive disease (PD) as the best overall response. Thus, the disease control rate (SD + PR) was 71% (95% CI, 51.3 to 86.8). The disease control rate at 6 months was 57% (95% CI, 37.2 to 75.5). The median DOR for the six responders was 5.5 months (95% CI, 3.7 to not reached [NR] months); the median DOR for patients who achieved a confirmed PR (n = 5) was 11 months (95% CI, 5.5 to NR months).
TABLE 2.
Efficacy Outcomes (N = 28)
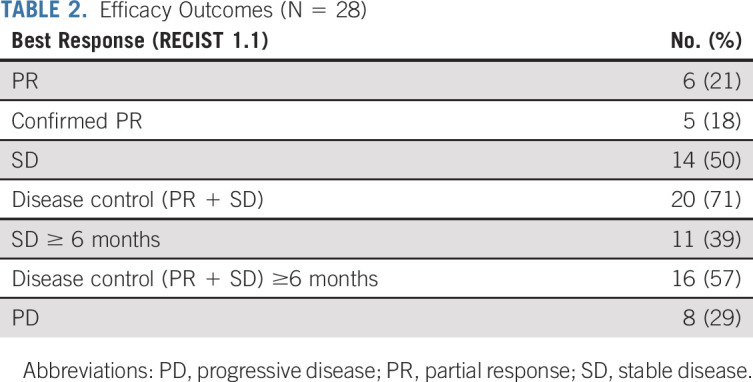
FIG 2.
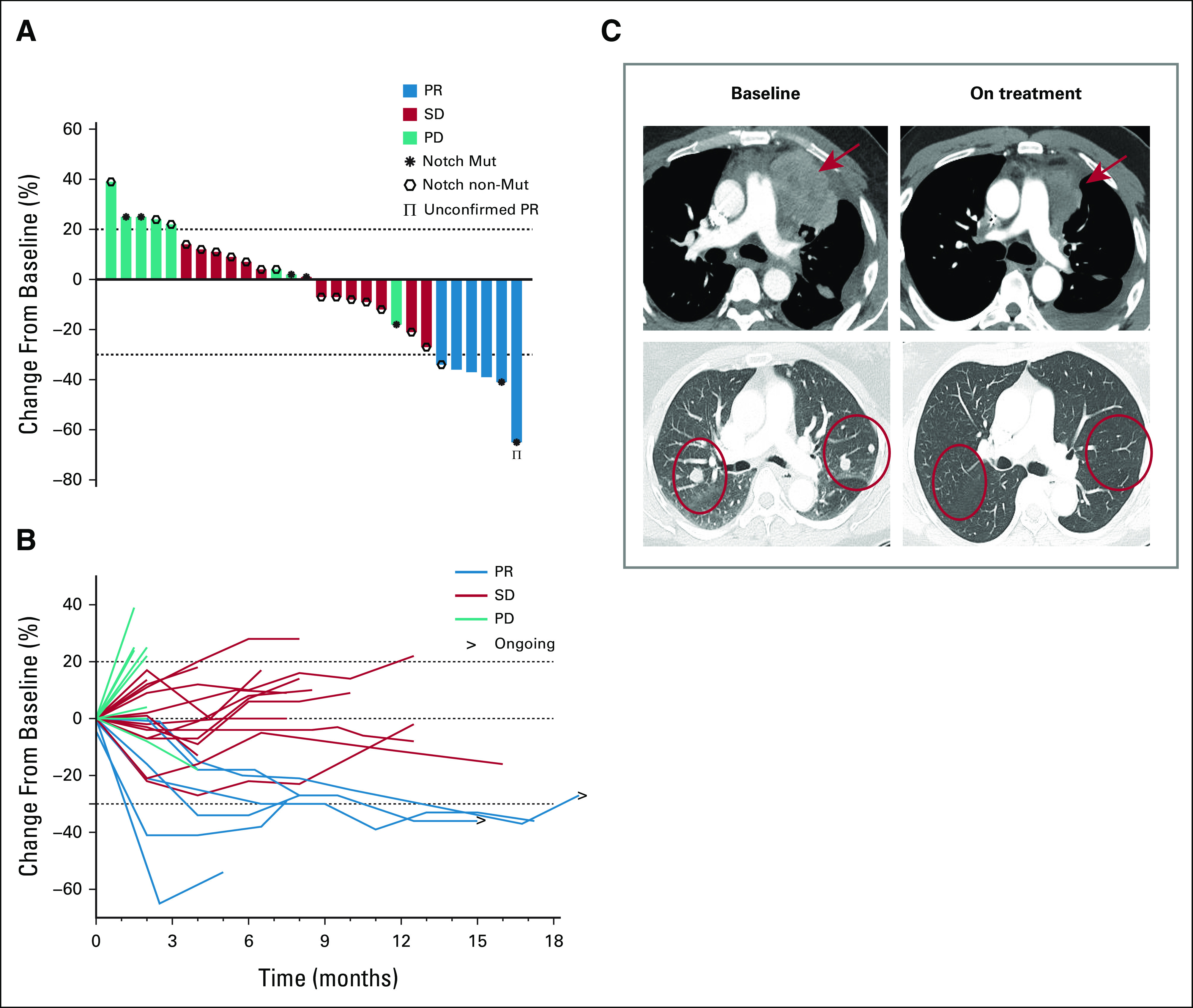
Efficacy outcomes. (A) Waterfall plot demonstrating maximum percent change in the sum of target lesions from baseline according to RECIST 1.1; each bar represents a patient. (B) Spider plot illustrating longitudinal percentage change in tumor size from baseline according to RECIST 1.1; each line represents a patient. (C) Illustrative computed tomography scans of two patients who achieved a partial response; red arrows and circles highlight metastatic tumor regions. PD, progressive disease; PR, partial response; SD, stable disease.
There were no differences between ORR per RECIST versus iRECIST. In an exploratory analysis to evaluate the association between clinical benefit and number of prior lines of therapy, there was no association between objective response and therapy line (P = .63); however, patients who had received at least one prior systemic therapy had a tendency to lower rates of disease control (PR or SD) as compared with treatment-naïve patients (50% v 83.3%, respectively; P = .09).
Time to Event Outcomes
Of the 28 evaluable patients, four did not have a progression event; of these patients, two had a PR and continued on therapy and two were taken off study because of noncompliance (n = 1) and intolerable toxicity (fatigue) despite two axitinib dose reductions (n = 1). The median PFS was 7.3 months (95% CI, 3.7 to 11.2 months; Fig 3A), and the PFS rate at 6 months was 57% (95% CI, 41.5 to 78.8). The median PFS for patients treated in first-line was 7.4 months (95% CI, 3.9 to 14.3 months) versus 3 months (95% CI, 1.8 to NR) for those treated in second-line or beyond (P = .22).
FIG 3.
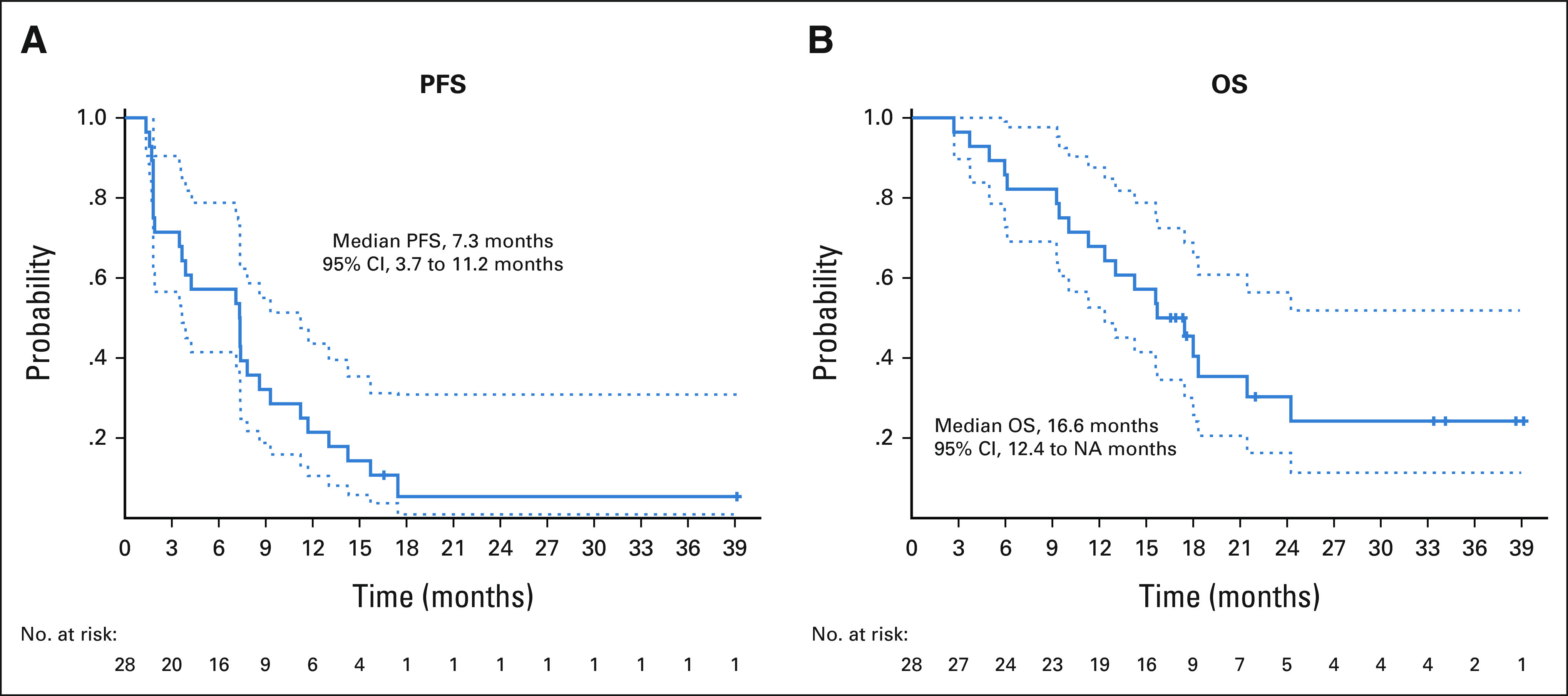
Kaplan-Meier curves for (A) PFS and (B) OS. OS, overall survival; PFS, progression-free survival.
During follow-up, 19 patients died. The median follow-up time for the nine surviving patients was 22 months (range, 13.7-36.2 months). The median OS was 16.6 months (95% CI, 12.4 to NR months; Fig 3B), and the OS rate at 6 months was 86% (95% CI, 73.7 to 99.7).
Toxicity, Dose Reductions, and Reasons for Discontinuation
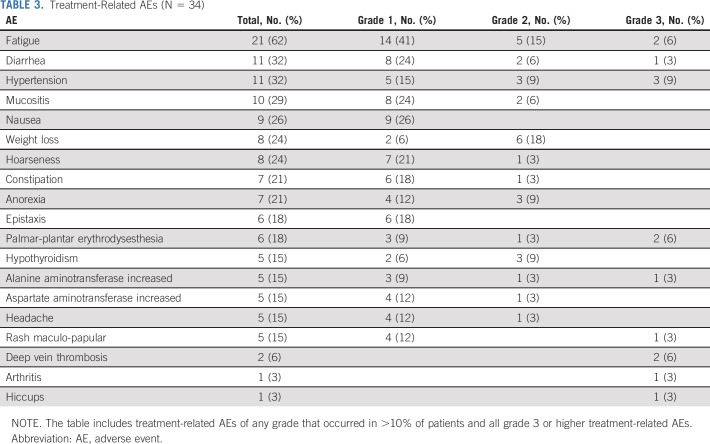
Thirty-four patients were included in the safety analysis. Treatment-related AEs (TRAEs) that occurred in more than 10% of patients and all serious AEs (grade 3 or higher) are listed in Table 3. The most common TRAEs were fatigue (62%), hypertension (32%), and diarrhea (32%). A total of 14 grade 3 TRAEs were reported in 10 patients (29%), with the most common being hypertension (9%), followed by fatigue (6%), deep vein thrombosis (6%), and palmar-plantar erythrodysesthesia (6%). No patients experienced TRAEs of grade 4 or 5.
TABLE 3.
Treatment-Related AEs (N = 34)
Four (12%) patients discontinued avelumab because of fatigue (n = 3, all grade 2) or hepatitis (n = 1, grade 3); only hepatitis was considered an immune-related AE. Nine patients (26%) had their axitinib dose reduced; the main reasons for dose reductions were fatigue (n = 4), palmar-plantar erythrodysesthesia (n = 3), hypertension (n = 1), and weight loss (n = 1). Three patients had two axitinib dose reductions because of diarrhea (n = 1), weight loss (n = 1), and fatigue (n = 1). One patient (3.5%) discontinued axitinib because of persistent fatigue (grade 3) despite two dose reductions. Further details on AEs leading to axitinib drug reduction are available in the Data Supplement.
Genomic Sequencing and Efficacy According to NOTCH1 Mutational Status
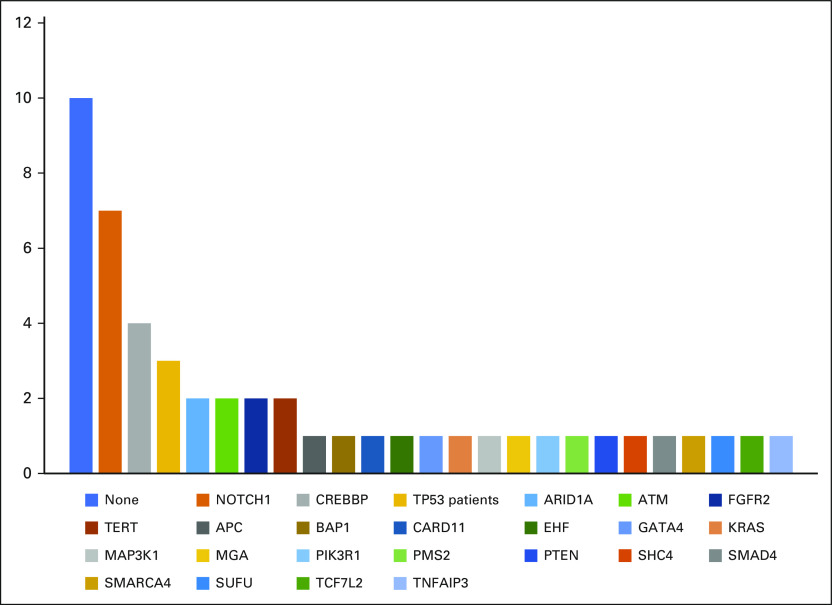
Twenty-five (89%) patients had available DNA targeted sequencing (Appendix Fig A1, online only). The most common somatic mutation was NOTCH1, which occurred in seven patients (28%), followed by CREBBP in four (16%) and TP53 in three (13%). The tumor mutational burden overall was low (median 2.6 mutations/megabase [range, 1.0-5.0]; n = 5). Only seven patients were tested for PD-L1 by immunohistochemistry, all with negative expression (CPS < 1). There was no significant association between objective response (PR) or disease control (PR + SD) and NOTCH1 mutational status (P > .1, Fisher exact test).
DISCUSSION
Patients with R/M ACC have few therapeutic options. VEGFR inhibitors have modest activity in ACC, mostly rendering disease stabilization.5-8 The premise of this study was that the VEGFR inhibitor axitinib would increase T-cell tumor infiltration and that combination treatment with avelumab would improve the efficacy over what has been reported with axitinib alone.
The results reported herein—with axitinib plus avelumab conferring a confirmed ORR of 18%—compare favorably with response rates reported for axitinib or PD-1 inhibitors as monotherapy in ACC.5,7,9,11,13 In a single-arm phase II study of axitinib, the reported ORR was 9% while in a randomized phase II study of axitinib versus observation, the ORR was 0% in the axitinib arm, and 11.5% in the observational arm after the patients crossed over to receive axitinib.9 Taking into consideration all the caveats of a small single-arm phase II study and comparisons between trials, the potential improvement from an ORR of 0% to 11.5% with axitinib monotherapy to 18% with axitinib plus avelumab would favor a potential additive effect between the two drugs in patients with ACC.
Notably, the median time to response in our trial was 5.1 months, with two patients achieving a PR after 1 year of therapy. Both VEGFR inhibitors and PD-1/PD-L1 inhibitors can lead to delayed tumor responses31,32; however, tumor shrinkage was noted in the first assessment (8 weeks) of all patients who eventually achieved a PR. Overall, the confirmed responses were durable, with a median DOR of 11 months; three patients had responses lasting more than 15 months, two of them have an ongoing response.
The median PFS of 7.3 months with a 6-month PFS rate of 57% is encouraging, particularly considering that a radiologist confirmed disease progression per RECIST within 6 months in 23 of 28 (82%) evaluable patients. Of the five patients in which PD per RECIST was not confirmed, three had no available scans within 6 months but unequivocal clinical progression, and two had RECIST progression per radiology report and principal investigator assessment, but equivocal PD by the trial radiologist review of the scans (18% tumor growth in one patient and equivocal new kidney lesion because of lack of contrast in the previous scan in the other patient). Of note, the patient with a 18% tumor growth in 6 months achieved a PR (−36%) and remains on study for more than 16 months; detailed information on the disease course for these five patients is available in the Data Supplement. To put the PFS results of our study into perspective, the median PFS in the axitinib single-arm study was 5.7 months with a 6-month PFS rate of 39%, and although clinical or radiological PD within 6 months was required, the percentage of patients who had PD per RECIST in that study was not reported.5 Notably, in the Korean randomized trial of axitinib versus observation, the median PFS of 10.8 months and the 6-month PFS rate of 73% were higher than what we report with axitinib plus avelumab; however, the patients enrolled in the randomized study likely had a more indolent disease. The inclusion criteria allowed for progression of disease within 9 months instead of 6 months; the limited genomic data (available in 28 of 60 patients) revealed a lower proportion of patients with NOTCH1 mutations, a predictor of poor prognosis in ACC (17% v 28% in our study); the disease control rate was of 100%, with no PD at the first assessment, and the median OS was approximately 27.2 months versus 16.6 months in our trial.3,9 Another possibility for the differences in outcomes is interethnic differences in pharmacokinetics, which has not been explored.
In our study, the frequency and severity of AEs observed with axitinib plus avelumab were consistent with the known safety profiles of axitinib and avelumab when administered as monotherapy or in combination.33 A serious AE occurred in 29% of our patients, each grade 3 and manageable; avelumab was discontinued in four (12%) patients, because of a grade 3 AE (immune-related hepatitis) in only one, and the axitinib dose was reduced in 26% of patients, with one patient (3.5%) discontinuing axitinib because of poor tolerance. Notably, the toxicity profile of axitinib plus avelumab was similar to what has been reported with axitinib single agent in ACC and renal cell carcinoma (Data Supplement) and compares favorably with the toxicity reported with single-agent lenvatinib in ACC. In a phase II study of lenvatinib in R/M ACC, 62.5% of patients had at least one grade 3 or 4 AE, 72% required at least one lenvatinib dose reduction, and 56% discontinued lenvatinib because of drug toxicity.6 Although lenvatinib rendered numerically higher ORR and PFS rates in ACC compared with single-agent axitinib on the basis of cross-study comparisons (ORR to lenvatinib 12%-16%; median event-free or PFS of 8.2 to 9.1 months), lenvatinib's toxicity frequently limits its use.6,34
In our study, targeted DNA sequencing was available for most patients (89%). NOTCH1 was the most commonly mutated gene (28%). Given the known prognostic effect of NOTCH1 mutations in ACC,3 we compared PR and disease-control rates in NOTCH1-mutant versus wild-type ACC and found no correlation between NOTCH1 mutational status and response outcomes; however, as expected, the median PFS and OS was significantly shorter for patients with NOTCH1 mutations (Appendix Fig A2, online only). Owing to ACC's significant biologic variability, biomarkers predictive of benefit from axitinib plus avelumab would be of great interest for further development of the combination. Interestingly, correlative studies from specimens of patients with renal cell carcinoma treated with axitinib plus avelumab in a phase III trial revealed that the biomarkers of response to PD-1/PD-L1 inhibitors, such as tumor mutational burden and PD-L1 expression, did not predict benefit from the drug combination. Instead, HLA type, high expression levels of UTS2 (a potent vasoconstrictor associated with inflammatory responses), and specific gene mutations such as DNMT1 and MYH7B were associated with prolonged PFS with axitinib plus avelumab.35 Baseline tumor samples for most patients with ACC who enrolled in our study are available, and correlative studies are planned.
Some limitations of our study include its relatively small sample size, although the number of evaluable patients enrolled is consistent with most phase II trials in this rare disease. Our study was conducted during the COVID-19 pandemic, which led to a larger than expected number of nonevaluable patients and issues with avelumab compliance/delays, particularly during the lockdown. Strengths of this study are that most patients had confirmation of PD per RECIST within 6 months before enrollment, allowing better interpretation of the PFS data (which can be challenging in the context of malignancies with heterogeneous and often indolent behavior) and the maturity of the data, with a long follow-up time (median 22 months) for the surviving patients and with most patients having had an event (disease progression or death).
In summary, axitinib plus avelumab showed activity in patients with incurable ACC and had a manageable toxicity profile. The potential added benefit of avelumab to axitinib should be further validated. Results of an additional study investigating a VEGFR inhibitor combined with an anti–PD-1/PD-L1 agent in ACC are awaited (ClinicalTrials.gov identifier: NCT04209660). Future analysis of pretreatment tumor samples may reveal biomarkers of benefit from axitinib plus avelumab and will be pursued.
ACKNOWLEDGMENT
Editorial support was provided by Bryan Tutt, Scientific Editor, Research Medical Library.
APPENDIX
FIG A1.
Genomic analysis of evaluable patients (N = 23).
FIG A2.
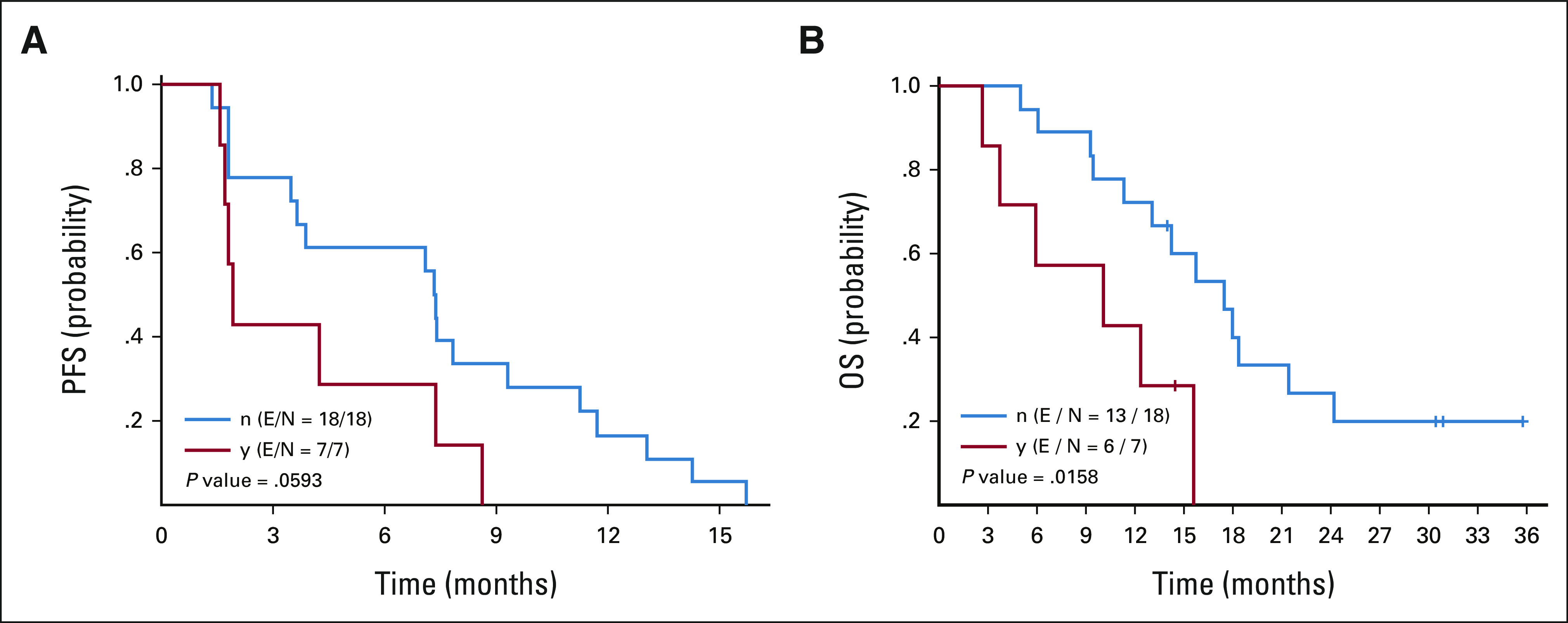
Kaplan-Meier curves for (A) PFS and (B) OS according to NOTCH1 mutational status (y = NOTCH1 mutant; n = NOTCH1 wild type; E = event, progression or death; N = number of patients at risk). OS, overall survival; PFS, progression‐free survival.
Renata Ferrarotto
Consulting or Advisory Role: Bicara Therapeutics, Prelude Therapeutics, Regeneron, Intellisphere, Merck Serono, G1 Therapeutics, Ayala Pharmaceuticals, Guidepoint Global, Elevar Therapeutics
Research Funding: G1 Therapeutics (Inst), AstraZeneca/MedImmune (Inst), EMD Serono (Inst), Genentech/Roche (Inst), Merck Serono (Inst), Pfizer/EMD Serono (Inst), Ayala Pharmaceuticals (Inst), Prelude Therapeutics (Inst)
George Blumenschein
Employment: Janssen, Johnon & Johnson
Stock and Other Ownership Interests: Virogin Biotech
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Celgene, Clovis Oncology, AbbVie, ARIAD, Merck, Genentech, Novartis, Xcovery, Adicet Bio, Amgen, AstraZeneca, Roche, MedImmune, Maverick Therapeutics, Johnson & Johnson, Virogin Biotech, Gilead Sciences, Daiichi Sankyo, INC, Novartis, Tyme, Janssen Oncology, Lilly, Instil Bio, BeiGene, CytomX Therapeutics, InterVenn Biosciences, Onconova Therapeutics, Regeneron, Sanofi, Seattle Genetics, Genzyme
Research Funding: Merck, Celgene, Genentech, Xcovery, Novartis, Bristol Myers Squibb, GlaxoSmithKline, Adaptimmune, Macrogenics, Kite, a Gilead company, Immatics, Torque, Incyte, MedImmune, Exelixis, Immunocore, Roche, AstraZeneca, Bayer, Tmunity Therapeutics, Inc, Regeneron, BeiGene, Repertoire Immune Medicines, Daiichi Sankyo INC, Verastem, Amgen, CytomX Therapeutics, Duality Biologics, Mythic Therapeutics
Mehmet Altan
Consulting or Advisory Role: BMS, GlaxoSmithKline, AstraZeneca
Speakers' Bureau: Nektar
Research Funding: Lilly (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Jounce Therapeutics (Inst), Adaptimmune (Inst), Merck (Inst), Genentech (Inst), Nektar (Inst), Shattuck Labs (Inst)
Travel, Accommodations, Expenses: Nektar
Other Relationship: Hengenix
Jason J. Johnson
Consulting or Advisory Role: BioClinica, Kura Oncology, InformAI
Research Funding: Blue Earth Diagnostics
Myrna Godoy
Consulting or Advisory Role: Siemens Healthineers
Research Funding: Siemens Healthineers (Inst)
Michael Kupferman
Stock and Other Ownership Interests: AbbVie, Intersect ENT, Abbott Laboratories, Arlo
Bonnie S. Glisson
Consulting or Advisory Role: Regeneron
Research Funding: Medimmune (Inst), ISA Pharmaceuticals (Inst), CUE Biopharma (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AbbVie/Stemcentrx, ISA Pharmaceuticals
Yasir Elamin
Consulting or Advisory Role: Lilly, AstraZeneca, Turning Point Therapeutics, Takeda, Sanofi, Spectrum Pharmaceuticals, Bristol Myers Squibb/Medarex
Research Funding: Spectrum Pharmaceuticals, AstraZeneca, Takeda, Xcovery, Lilly, Elevation Oncology, Turning Point Therapeutics, Blueprint Medicines, Forward, Precision Therapeutics
Travel, Accommodations, Expenses: Lilly
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2022 ASCO Annual Meeting, Chicago, IL, June 6, 2022, as a poster discussion.
SUPPORT
Supported by Pfizer, as part of an alliance between Pfizer and the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945), by NCI Grant P30 CA016672l, and by donor funds.
CLINICAL TRIAL INFORMATION
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Clinical Trial of Axitinib and Avelumab in Patients With Recurrent/Metastatic Adenoid Cystic Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Renata Ferrarotto
Consulting or Advisory Role: Bicara Therapeutics, Prelude Therapeutics, Regeneron, Intellisphere, Merck Serono, G1 Therapeutics, Ayala Pharmaceuticals, Guidepoint Global, Elevar Therapeutics
Research Funding: G1 Therapeutics (Inst), AstraZeneca/MedImmune (Inst), EMD Serono (Inst), Genentech/Roche (Inst), Merck Serono (Inst), Pfizer/EMD Serono (Inst), Ayala Pharmaceuticals (Inst), Prelude Therapeutics (Inst)
George Blumenschein
Employment: Janssen, Johnon & Johnson
Stock and Other Ownership Interests: Virogin Biotech
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Celgene, Clovis Oncology, AbbVie, ARIAD, Merck, Genentech, Novartis, Xcovery, Adicet Bio, Amgen, AstraZeneca, Roche, MedImmune, Maverick Therapeutics, Johnson & Johnson, Virogin Biotech, Gilead Sciences, Daiichi Sankyo, INC, Novartis, Tyme, Janssen Oncology, Lilly, Instil Bio, BeiGene, CytomX Therapeutics, InterVenn Biosciences, Onconova Therapeutics, Regeneron, Sanofi, Seattle Genetics, Genzyme
Research Funding: Merck, Celgene, Genentech, Xcovery, Novartis, Bristol Myers Squibb, GlaxoSmithKline, Adaptimmune, Macrogenics, Kite, a Gilead company, Immatics, Torque, Incyte, MedImmune, Exelixis, Immunocore, Roche, AstraZeneca, Bayer, Tmunity Therapeutics, Inc, Regeneron, BeiGene, Repertoire Immune Medicines, Daiichi Sankyo INC, Verastem, Amgen, CytomX Therapeutics, Duality Biologics, Mythic Therapeutics
Mehmet Altan
Consulting or Advisory Role: BMS, GlaxoSmithKline, AstraZeneca
Speakers' Bureau: Nektar
Research Funding: Lilly (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Jounce Therapeutics (Inst), Adaptimmune (Inst), Merck (Inst), Genentech (Inst), Nektar (Inst), Shattuck Labs (Inst)
Travel, Accommodations, Expenses: Nektar
Other Relationship: Hengenix
Jason J. Johnson
Consulting or Advisory Role: BioClinica, Kura Oncology, InformAI
Research Funding: Blue Earth Diagnostics
Myrna Godoy
Consulting or Advisory Role: Siemens Healthineers
Research Funding: Siemens Healthineers (Inst)
Michael Kupferman
Stock and Other Ownership Interests: AbbVie, Intersect ENT, Abbott Laboratories, Arlo
Bonnie S. Glisson
Consulting or Advisory Role: Regeneron
Research Funding: Medimmune (Inst), ISA Pharmaceuticals (Inst), CUE Biopharma (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AbbVie/Stemcentrx, ISA Pharmaceuticals
Yasir Elamin
Consulting or Advisory Role: Lilly, AstraZeneca, Turning Point Therapeutics, Takeda, Sanofi, Spectrum Pharmaceuticals, Bristol Myers Squibb/Medarex
Research Funding: Spectrum Pharmaceuticals, AstraZeneca, Takeda, Xcovery, Lilly, Elevation Oncology, Turning Point Therapeutics, Blueprint Medicines, Forward, Precision Therapeutics
Travel, Accommodations, Expenses: Lilly
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Renata Ferrarotto, Diana Bell, Jason J. Johnson, Michael Kupferman, Ehab Hanna, Bonnie S. Glisson, Yasir Elamin
Financial support: Michael Kupferman
Administrative support: Michael Kupferman
Provision of study materials or patients: Renata Ferrarotto, Mehmet Altan, Kaiyi Li, Bonnie S. Glisson, Adel El-Naggar
Collection and assembly of data: Renata Ferrarotto, Luana G. Sousa, Mehmet Altan, Diana Bell, Frank Mott, Flavia Bonini, Kaiyi Li, Jason J. Johnson, Yoshitsugu Mitani, Myrna Godoy, Yasir Elamin
Data analysis and interpretation: Renata Ferrarotto, Luana G. Sousa, Lei Feng, George Blumenschein, Mehmet Altan, Diana Bell, Eduardo A. Dal Lago, Mario L. Marques-Piubelli, Jason J. Johnson, Anna Lee, Michael Kupferman, Bonnie S. Glisson, Yasir Elamin, Adel El-Naggar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
REFERENCES
- 1.Ferrarotto R, Heymach JV, Glisson BS: MYB-fusions and other potential actionable targets in adenoid cystic carcinoma. Curr Opin Oncol 28:195-200, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Stephens PJ, Davies HR, Mitani Y, et al. : Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest 123:2965-2968, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrarotto R, Mitani Y, Diao L, et al. : Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to NOTCH1 Inhibitors. J Clin Oncol 35:352-360, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurie SA, Ho AL, Fury MG, et al. : Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: A systematic review. Lancet Oncol 12:815-824, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Ho AL, Dunn L, Sherman EJ, et al. : A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann Oncol 27:1902-1908, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchekmedyian V, Sherman EJ, Dunn L, et al. : Phase II study of lenvatinib in patients with progressive, recurrent or metastatic adenoid cystic carcinoma. J Clin Oncol 37:1529-1537, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keam B, Kang EJ, Ahn M-J, et al. : Randomized phase II study of axitinib versus observation in patients with recurred or metastatic adenoid cystic carcinoma. J Clin Oncol 38:6503, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Locati LD, Cavalieri S, Bergamini C, et al. : Phase II trial with axitinib in recurrent and/or metastatic salivary gland cancers of the upper aerodigestive tract. Head & Neck 41:3670-3676, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Kang EJ, Ahn MJ, Ock CY, et al. : Randomized phase II study of axitinib versus observation in patients with recurred or metastatic adenoid cystic carcinoma. Clin Cancer Res 27:5272-5279, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Pfister DG, Spencer S, Adelstein D, et al. : Head and neck cancers, version 2.2020, NCCN clinical Practice guidelines in oncology. J Natl Compr Canc Netw 18:873-898, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Cohen RB, Delord J-P, Doi T, et al. : Pembrolizumab for the treatment of advanced salivary gland carcinoma: Findings of the phase 1b KEYNOTE-028 study. Am J Clin Oncol 41:1083-1088, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmood U, Bang A, Chen YH, et al. : A Randomized phase 2 study of pembrolizumab with or without radiation in patients with recurrent or metastatic adenoid cystic carcinoma. Int J Radiat Oncol*Biol*Phys 109:134-144, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tchekmedyian V, Sherman EJ, Dunn L, et al. : A phase II trial cohort of nivolumab plus ipilimumab in patients (Pts) with recurrent/metastatic adenoid cystic carcinoma (R/M ACC). J Clin Oncol 37:6084, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fayette J, Even C, Digue L, et al. : NISCAHN: A phase II, multicenter nonrandomized trial aiming at evaluating nivolumab (N) in two cohorts of patients (pts) with recurrent/metastatic (R/M) salivary gland carcinoma of the head and neck (SGCHN), on behalf of the unicancer head & neck Group. J Clin Oncol 37:6083, 2019 [Google Scholar]
- 15.Rodriguez CP, Wu QV, Voutsinas J, et al. : A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin Cancer Res 26:837-845, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Darabi S, Braxton DR, Eisenberg BL, et al. : Predictive biomarkers for immunotherapy response beyond PD-1/PD-L1. Oncology (Williston Park) 34:321-327, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Griffioen AW, Damen CA, Blijham GH, et al. : Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 88:667-673, 1996 [PubMed] [Google Scholar]
- 18.Peske JD, Woods AB, Engelhard VH: Control of CD8 T-Cell infiltration into tumors by vasculature and microenvironment. Adv Cancer Res 128:263-307, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voron T, Colussi O, Marcheteau E, et al. : VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 212:139-148, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee WS, Yang H, Chon HJ, et al. : Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 52:1475-1485, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukumura D, Kloepper J, Amoozgar Z, et al. : Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol 15:325-340, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes PE, Caenepeel S, Wu LC: Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol 37:462-476, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Rini BI, Plimack ER, Stus V, et al. : Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Makker V, Rasco D, Vogelzang NJ, et al. : Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 20:711-718, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Meric-Bernstam F, Brusco L, Shaw K, et al. : Feasibility of large-scale genomic testing to facilitate enrollment onto genomically matched clinical trials. J Clin Oncol 33:2753-2762, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frampton GM, Fichtenholtz A, Otto GA, et al. : Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 31:1023-1031, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaubier N, Tell R, Lau D, et al. : Clinical validation of the tempus xT next-generation targeted oncology sequencing assay. Oncotarget 10:2384-2396, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravi RK, Walton K, Khosroheidari M: MiSeq: A next generation sequencing platform for genomic analysis. Methods Mol Biol 1706:223-232, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Seymour L, Bogaerts J, Perrone A, et al. : iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143-e152, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borcoman E, Nandikolla A, Long G, et al. : Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Educ Book 38:169-178, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Lee C-H, Shah AY, Rasco D, et al. : Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (study 111/KEYNOTE-146): A phase 1b/2 study. Lancet Oncol 22:946-958, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motzer RJ, Penkov K, Haanen J, et al. : Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1103-1115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locati LD, Galbiati D, Calareso G, et al. : Patients with adenoid cystic carcinomas of the salivary glands treated with lenvatinib: Activity and quality of life. Cancer 126:1888-1894, 2020 [DOI] [PubMed] [Google Scholar]
- 35.Motzer RJ, Robbins PB, Powles T, et al. : Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: Biomarker analysis of the phase 3 JAVELIN renal 101 trial. Nat Med 26:1733-1741, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]