Abstract
Background.
Acute rejection is still a major limitation for a successful outcome in lung transplantation. Since β-nicotinamide adenine dinucleotide (NAD+) has been shown to have various immunomodulatory properties on the innate and adaptive immune system, we evaluate here a potential protective effect of NAD+ against acute lung rejection.
Methods.
Rat single-lung transplantation was performed in 2 groups (n = 8 per group), using Brown-Norway donors and major histocompatibility complex–mismatched Lewis recipients. Recipients of the NAD+ group received 1000 mg/kg NAD+ intraperitoneally before transplantation and daily thereafter until euthanasia, whereas the control group received saline solution. At autopsy on day 5, blood samples were analyzed and the lung allograft was assessed by bronchioalveolar lavage, histology, and immunochemistry.
Results.
The NAD+ group maintained an intact compliant lung tissue, a strong trend of lower acute cellular rejection (A3 versus A3-A4) and significantly less lymphocytic bronchiolitis (B0-B2R versus B1R-Bx). In addition, a trend of fewer alveolar CD68+ macrophages and significantly fewer interstitial CD163+ macrophages was observed. Bronchoalveolar lavage in the NAD+ group showed significantly fewer proinflammatory cytokines interleukin (IL)-6, IL-13, TNFα, and a protective IL-6/IL-10-ratio. In blood samples, we observed significantly fewer neutrophils, and proinflammatory GRO/KC in the NAD+ group.
Conclusions.
NAD+ might be a promising substance in prevention of acute allograft rejection in lung transplantation.
Despite the many achievements in lung transplantation over the last decades, one of the major obstacles remaining is acute allograft rejection resulting in organ loss. According to the International Society of Heart and Lung Transplantation Registry, >30% of recipients experience at least 1 episode of acute rejection during the first year after transplant.1 Even a single episode of mild acute rejection has been shown to be one of the main risk factors for developing chronic lung allograft dysfunction.2 The development of safe and cost-effective pharmacologic treatment strategies to reduce the occurrence of acute rejection remains a fundamental goal in this research field.
Acute rejection results from a complex interaction of innate and adaptive immune responses. First foreign major histocompatibility complex (MHC) antigens are detected by professional antigen-presenting cells (APCs) like macrophages, dendritic cells, and nonprofessional APCs like mast cells and presented to T cells. T cells then undergo differentiation and trigger a specific response against antigens, synthesize key cytokines, and make cell–cell interactions that upregulate the innate and adaptive immune response. In addition, ischemia–reperfusion injury activates local macrophages and dendritic cells, which contribute to the infiltration of neutrophils into the graft stimulating acute rejection further.2,3
β-nicotinamide adenine dinucleotide (NAD+) is a natural coenzyme found in all living cells and plays a critical role in regulating metabolic processes. Evidence shows that exogenous NAD+ is able to regulate the homeostasis of the innate and adaptive immune system in different disease models.4-6 Exogenous NAD+ suppresses the dendritic cell pathway and promotes through mast cells the differentiation of CD4+T-cells that otherwise play a central role in the initiation of allograft rejection. NAD+ switches these T-cells into anti-inflammatory interferon gamma (IFNy)-producing T helper 1 (Th1)-cells, anti-inflammatory interleukin (IL)-4 producing Th2-cells, anti-inflammatory IL-10 and IFNy producing regulatory type 1 cells, and proinflammatory IL-17A producing Th17 cells.6 Furthermore, the importance of a stable NAD+ environment in maintaining an anti- inflammatory phenotype in resting macrophages has been reported.7
In a recent, fully MHC-mismatched tail skin transplant model in mice,5 daily intraperitoneal injections of NAD+ led to an impressive, prolonged allograft survival. The main reason for this allograft survival was a consequence of the robust IL-10 systemic production by CD4+T-cells.
From these very promising findings of NAD+ on skin allografts, we seek to evaluate the effect of NAD+ on acute rejection in lung allografts, which possess different immunological properties2 than other solid organs. We tested this effect in an established8-11 major MHC-mismatched rat left lung transplant model.
MATERIALS AND METHODS
Animals
Specific pathogen-free, male Brown-Norway inbred rats (Envigo, Horst, the Netherlands) and male Lewis inbred rats (Janvier Labs, Le Genest Saint-Isle, France), received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals.”12 The study protocol was approved by the Kanton Zurich Veterinarian committee (ZH222/18). The results of this study were presented in accordance with the ARRIVE guidelines.
Experimental Design
Orthotopic left lung transplantation was performed under aseptic conditions from Brown-Norway donor rats (231–271 g) to MHC–mismatched Lewis recipient rats (268–305 g). This established mismatch model of lung transplantation leads to irreversible acute total graft rejection within 6–7 d.9-11
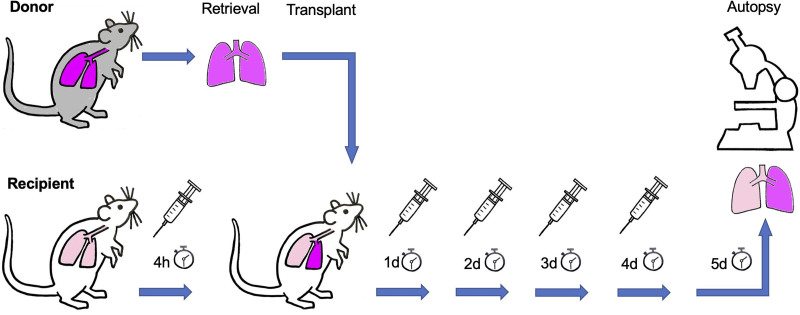
Figure 1 illustrates the study design. Recipients were randomly assigned into a treatment (NAD+) group and a control group (each group, n = 8). The NAD+ group received 1000 mg/kg NAD+ (β-nicotinamide adenine dinucleotide free acid, cat 124542, Merck KGaA, Darmstadt, Germany) intraperitoneally 4 h before the start of transplantation and then again every 24 h the following 4 d. The control group received instead of NAD+ 0.5 mL 0.9% NaCl solution as placebo. No immunosuppression was given. Euthanasia and autopsy were performed on day 5 posttransplant. At this point, biochemical and morphologic changes in lung allografts were compared between NAD+ and control groups. An additional comparison was performed with syngrafts of Lewis donors to Lewis recipients (n = 2 individuals) to show an ideal early postoperative transplant outcome.
FIGURE 1.
Experimental design. Orthotopic left lung transplantation was performed from Brown-Norway donor rats to major histocompatibility complex–mismatched Lewis recipient rats. The treatment NAD+ group (n = 8) received NAD+ intraperitoneally 4 h pretransplant and on days 1, 2, 3, and 4 after transplantation, whereas the control group (n = 8) received saline as placebo (symbolized by syringes). An additional syngraft group (n = 2) of Lewis recipients and Lewis donors was treated as the control group with placebo. NAD+, β-nicotinamide adenine dinucleotide.
Surgical Technique and Sampling
Donor Lung Procurement
According to our established protocol,13 donor rats were anaesthetized with a mixture of oxygen and isoflurane, underwent tracheotomy and mechanical ventilation with a rodent ventilator (Harvard Apparatus, Inc., Model Ventelite, Holliston, MA). A tidal volume of 10 mL/kg was applied for the volume-controlled mode with a respiratory rate of 75 breaths/min at a 50:50 inspiratory/expiratory ratio, and a positive end-expiratory pressure (PEEP) of 3 cmH2O with a fraction of inspired oxygen of 50. Following laparosternotomy, the rats were heparinized with 300 IU intravenous heparin via the inferior vena cava. The main pulmonary artery was cannulated via incision of the right ventricle and the left atrium cannulated via incision of the left ventricle and retrograde placement through the mitral valve. After incision of the inferior vena cava, the lung circulation was anterograde flushed with 10–20 mL ice cold Perfadex plus (XVIVO Perfusion, Uppsala, Sweden) at a perfusion pressure similar to the physiologic pulmonary artery systolic pressure of rats at 20 cmH2O. Next, the trachea was clamped and the lungs were inflated with a sustained airway pressure of 15 cmH2O. The isolated extracted heart-lung block was placed back table in a petri dish with gauze that was soaked with ice-cold Perfadex plus. Then the left lung was dissected, the left main bronchus clamped distally to ensure sustained airway pressure and a 16-gauge cuff placed in the pulmonary vein and the pulmonary artery as well as a 14-gauge cuff in the bronchus. They were fixed with 6-0 silk. The left lung was then stored in a Perfadex plus soaked petri dish on ice until transplantation to ensure an ongoing cold ischemic time.
Lung Transplantation Procedure
Recipient rats were anaesthetized with a mixture of oxygen and isoflurane, and intubated. Anesthesia was maintained with 2%–3% isoflurane during the operation and reperfusion period. Ventilation was performed with 65 breaths/min and pressure controlled mode at peak inspiratory pressure of 15 cmH2O, a PEEP of 3 cmH2O, and a 35:65 inspiratory/expiratory ratio. According to our established protocol,14 a left thoracotomy was performed through the fifth intercostal space and the left lung mobilized by dividing the pulmonary ligament. Following dissection of the hilum, the pulmonary artery, pulmonary vein, and the left main bronchus were isolated and transiently snared proximal by 5-0 silk. The 3 isolated structures were incised anteriorly, distal of their snaring and the cuffs of the donor lung placed into the equivalent recipient structures and fixed with a 7–0 polypropylene suture. All transient snaring was released and the transplanted lung inflated and perfused. In order to avoid tension pneumothorax we insert a 20-gauge polyethylene chest tube through the seventh intercostal space before we close the thoracotomy. This tube was removed when spontaneous breathing resumed. The thoracotomy and skin were closed in 3 layers. Then the animals were immediately weaned and extubated. After surgery, the animals were kept in designated rodent recovery cabinets for the first 24 h.
Recovery of the Graft
At day 5, recipient animals were anesthetized, intubated, and ventilated according to the protocol of the donor rats. After median laparosternotomy, nonventilated lungs were treated with increased PEEP trials for recovery. To inflate collapsed or atelectatic lungs, the PEEP was increased from 3 to 6 cmH2O over 2–3 s. Four minutes after intubation and ventilation with a fraction of inspired oxygen of 100, arterial blood was aspirated from the ascending aorta with a heparinized syringe and blood gas analysis was performed (Epoc Blood Analysis System, Siemens Healthineers, Erlangen, Germany). Blood samples at autopsy were also estimated using a VetScan HM5 benchtop hematology analyzer (Abaxis, Inc., Union City, CA). Following cannulation of the pulmonary artery, the inferior vena cava was incised, the left atrial appendage was cut, and the lungs were flushed anterograde with 20 mL of 0.9% NaCl at 4 °C with 20 cmH2O pressure. The transplanted lung was recovered and rinsed in 0.9% NaCl solution to remove coagulated blood from the lung surface. The lower two-thirds of the transplanted lung was clamped and a bronchoalveolar lavage (BAL) of 0.5 mL 0.9% NaCl was taken from the upper one-third of the lung. The middle one-third of the transplanted lung was then put in RNAlater (Qiagen, Hilden, Germany) and the lowest one-third part of the graft was placed in 4% formaldehyde solution for histologic analysis. All samples were stored at −80 °C until further examination.
Histology and Immunohistochemistry
For histological assessment, specimens were formalin-fixed and paraffin-embedded. Cut sections were stained with hematoxylin and eosin. An experienced pathologist (M.H.) completely blinded to the study evaluated the grade of acute rejection (grade-A) and airway inflammation grade (grade-B) based on standard guidelines proposed by the International Society of Heart and Lung Transplantation.15
The CD68+ alveolar macrophages and CD163+ interstitial macrophages constitute important immune cells involved in allograft rejection. They were detected with rabbit primary antibodies purchased from Abcam (Abcam, Serotec, Cambridge, UK, anti-rat CD68 [ab 125212] or anti-rat CD163 [ab 182422] diluted at 1:200), and their signal detected by a biotinylated secondary antibody and a Vectastain peroxidase kit (ABC-Kit; Vector Laboratories, Burlingame, CA with 3,3-diaminobenzidine tetrahydrochloride). Counterstaining was performed with Mayer’s hematoxylin. The pathologist blinded to the study reflected semiquantitatively the graft tissue macrophage cell density. The presence of CD68-expressing macrophages was scored from 0 to 3 points (0 = absent, 1 = focal, 2 = moderate, 3 = frequent) and CD163-expressing macrophages were scored from 0 to 4 (1 = single positive cells, 2 = few, 3 = many and well ordered, 4 = many and diffuse).
BAL and Blood Plasma
We assayed 50 µL of BAL or plasma for levels of cytokines, chemokines, and mediators of wound healing and tissue repair using the 27-plex Discovery assay (rat Cytokine Array/Chemokine Array 27-Plex Panel; Cat no: RD27, Eve Technologies, Alberta, Canada). The whole blood samples were collected in ethylenediaminetetraacetic acid-treated tubes (BD Microtainer Cat. No 365975 Becton Dickinson, Franklin Lakes, NJ). We centrifuged blood samples for 10 min at 4 °C and 3300 rpm to separate blood cells from plasma. The supernatant was collected, transferred in a clean polypropylene tube and centrifuged for 10 min at 4 °C and 4000 rpm. The BAL samples were collected in ice-cold polypropylene tubes and centrifuged for 5 min at 4 °C at 1500 rpm. After centrifugation, only supernatant was collected and transferred into a clean polypropylene that was flash-frozen in liquid nitrogen and stored at –80 °C until use.
Statistical Analysis
Results are expressed as the standard error of the mean. Data were tested for normal distribution by the Shapiro–Wilk test. Differences between groups were analyzed by the Mann–Whitney U test and, when appropriate, by analysis of variance (ANOVA) with GraphPad PRISM Version 9.1.2. (GraphPad Software, Inc., La Jolla, CA). Significance was assumed for probability values <0.05.
RESULTS
Macroscopic and Physiological Assessment
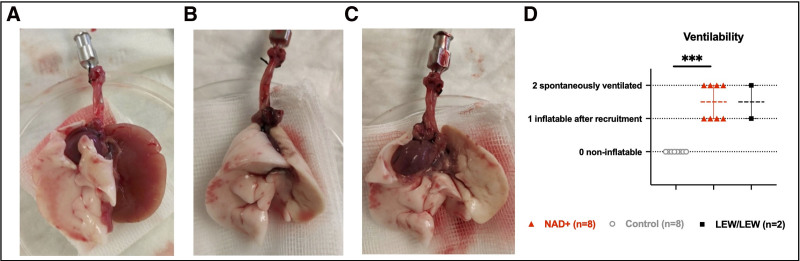
Five days after left lung transplantation all animals were alive with comparable well-being status and weight (P = 0.331). At autopsy at day 5, a spontaneous ventilation of the transplanted lung was visible in half of the NAD+ group, whereas the other half was inflatable and compliant after a short recruitment maneuver and looked macroscopically healthy (Figure 2). In contrast, all transplanted control lungs were stiff, macroscopically carnificated, and noninflatable, even after several recruitment maneuvers (P = 0.0002) (Figure 2). An isolated physiological assessment of the left transplanted lung was impossible because of the stiffness of the allograft in the control group.
FIGURE 2.
Macroscopic assessment at autopsy, 5 d posttransplant. A, Open flapped heart-lung bloc with typically carnificated left lung allograft as observed in the entire control group. B, Physiologically arranged and (C) open flapped heart-lung bloc of a normal-looking, ventilable, left lung allografts from the NAD+ group identical looking to syngraft (LEW/LEW) lungs. D, Comparison for the free ventilability of the lung grafts in the control, NAD+, and syngraft (LEW/LEW) groups. Note ***P ≤ 0.001. The stars are a significant P value vs the control. NAD+, β-nicotinamide adenine dinucleotide.
Histology
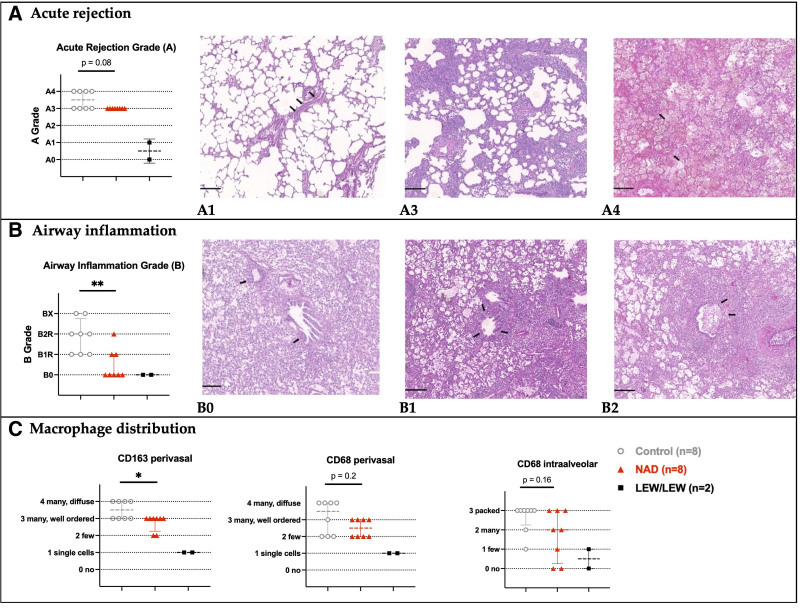
At day 5, the allografts of the NAD+ group showed a strong trend of lower acute cellular rejection compared with the control group (P = 0.08). Moderate rejection (A3) was observed in NAD+ group, whereas half of the control even showed a severe acute rejection (A4) with infarction and hemorrhage (Figure 3A). More importantly, lymphocytic bronchiolitis was significantly lower in the NAD+ group (B0-B2R) than in the control group (B1R-Bx) (Figure 3B, P = 0.008). Regarding macrophages (Figure 3C), the intraalveolar (P = 0.16) and the perivascular (P = 0.23) CD68+ macrophages were reduced in the NAD+ group, although not significantly when compared with the control group. Interstitial CD163+ macrophages expected to gather around vessels during the rejection process16 were significantly less present in the NAD+ group (P = 0.03).
FIGURE 3.
Histological assessment at autopsy, 5 d posttransplant. A, Acute cellular rejection grades in the control, NAD+, and syngraft (LEW/LEW) groups, according to standard ISHLT guidelines. A single layer of lymphocytes around blood vessels (arrows) not visible on low magnification (A1). Prominent and easily recognizable perivascular lymphocytic infiltrate (A2, not shown since not observed). Prominent perivascular lymphocytic infiltrates with infiltration into adjacent alveolar septa (A3). Diffuse perivascular, interstitial, and alveolar inflammation and necrotizing vasculitis (arrows) (A4). B, Airway inflammation grades in the control, NAD+, and syngraft groups (LEW/LEW), according to standard ISHLT guidelines. No evidence of bronchiolar inflammation (B0). Lymphocytes in the walls of small airways (arrows) without epithelial damage (B1), intraepithelial lymphocytosis, and ulceration (arrows) of small airways (B2). C, CD163+ and CD68+ macrophage distributions in control, NAD+, and syngraft (LEW/LEW) groups based on immunohistochemistry. Notes: Histological images in hematoxylin and eosin staining, scale bars = 200 μm. The stars are significant P values vs the control. *P ≤ 0.05; **P ≤ 0.01. ISHLT, International Society of Heart and Lung Transplantation; NAD+, β-nicotinamide adenine dinucleotide.
Cytokines and Chemokines
Bronchoalveolar Lavage
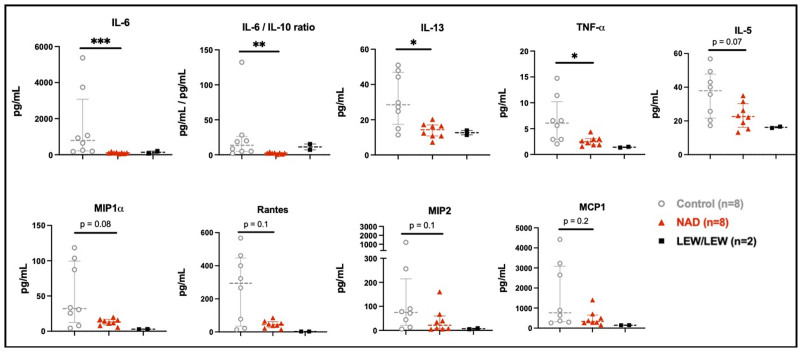
In the allograft BAL (Figure 4; Table 1), significantly fewer proinflammatory cytokines were observed in the NAD+ group such as IL-6 (P = 0.0003), IL-13 (P = 0.01), and TNFα (P = 0.03). Furthermore, the NAD+ group showed a trend toward fewer B-cell maturation-related proinflammatory IL-5 cytokines (P = 0.06) as well as less macrophage inflammatory protein (MIP)-1α (P = 0.08) and less MIP (P = 0.10). In the NAD+ group, Rantes, which attracts chemotactic cells (P = 0.10) and monocyte chemoattractant protein-1 (P = 0.16) was reduced but not significantly. No differences were recorded in proinflammatory IL-17A (P = 0.88) or anti-inflammatory cytokines IFNy (P = 0.57), IL-4 (P = 0.57), and IL-10 (P = 0.65). However, when the later was analyzed as a ration (IL-6/IL-10-ratio), a significantly increased anti-inflammatory effect emerged in the NAD+ group (P = 0.005).
FIGURE 4.
BAL cytokines and chemokines of the NAD+ control and syngraft (LEW/LEW) groups. The stars are significant P values vs the control. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. BAL, bronchoalveolar lavage; NAD+, β-nicotinamide adenine dinucleotide.
TABLE 1.
BAL and plasma cytokines, chemokines, and mediators of wound healing and tissue repair at 5 d posttransplantation in the study groups
| BAL | BAL | BAL | Plasma | Plasma | Plasma | |
|---|---|---|---|---|---|---|
| Control (N = 8) | NAD+ (N = 8) | Lew/Lew (N = 2) | Control (N = 8) | NAD+ (N = 8) | Lew/Lew (N = 2) | |
| EGF | 17.02 (21.66) | 14.38 (21.21) | 2.04 (1.29) | 84.62 (77.43) | 33.92 (35.35) | 0.14 (0.16) |
| Eotaxin | 5.199 (3.068) | 4.803 (3.661) | 1.474 (1.119) | 14.09 (11.39) | 12.08 (3.09) | 11.25 (4.05) |
| Fractalkine | 54.58 (42.09) | 94.33 (72.31) | 45.43 (48.52) | 132.5 (50.35) | 164.3 (41.81) | 100.5 (41.38) |
| G-CSF | 7.784 (7.852) | 5.862 (4.062) | 3.375 (0.697) | 32.76 (22.63) | 40.51 (34.49) | 26.43 (1.39) |
| GM-CSF | 20.40 (17.69) | 12.71 (9.61) | 8.02 (0.86) | 112.4 (25.8) | 129.8 (44.16) | 172.8 (2.02) |
| GRO/KC | 29.96 (41.33) | 62.61 (53.30) | 14.61 (6.80) | 863.3 (542.9) | 334.1 (203.2)* | 516.0 (74.51) |
| IFNγ | 497.9 (406.0) | 365.2 (305.8) | 103.0 (20.6) | 296.1 (116.8) | 443.0 (180.1) | 256.9 (76.0) |
| IL1-α | 67.81 (46.49) | 43.91 (28.49) | 22.58 (2.84) | 130.5 (222.7) | 88.6 (58.2) | 64.6 (11.8) |
| IL1-β | 268.6 (228.8) | 147.4 (85.7) | 35.3 (13.4) | 1201 (726) | 780 (288) | 1185 (1171) |
| IL-2 | 12.05 (6.66) | 17.03 (12.30) | 6.05 (0.98) | 114.3 (121.5) | 105.9 (105.7) | 62.0 (50.7) |
| IL-4 | 19.32 (9.18) | 17.98 (14.28) | 12 (0.08) | 34.42 (15.85) | 47.15 (32.40) | 30.29 (11.96) |
| IL-5 | 36.01 (14.04) | 23.18 (7.54) | 16.21 (0.56) | 53.17 (21.18) | 66.19 (40.01) | 64.28 (16.38) |
| IL-6 | 1546 (1937) | 106 (38)*** | 138 (94) | 2483 (1809) | 1354 (310) | 912 (92) |
| IL-10 | 107.8 (120.2) | 75.8 (82.2) | 11.7 (2.3) | 1044 (341) | 1022 (263) | 807 (510) |
| IL-12(p70) | 28.67 (11.65) | 26.32 (17.72) | 14.59 (5.71) | 96.7 (29.8) | 122.4 (90.7) | 124.8 (66.7) |
| IL-13 | 31.39 (13.93) | 13.95 (4.27)* | 12.62 (1.76) | 69.5 (104.8) | 45.4 (29.7) | 25.9 (0.5) |
| IL-17A | 58.01 (62.18) | 19.57 (16.94) | 5.97 (2.76) | 16.98 (8.03) | 31.81 (20.55) | 36.02 (19.62) |
| IL-18 | 1339 (1011) | 1305 (368) | 295 (155) | 1384(953) | 1385 (542) | 394 (273) |
| LIX | 534.6 (460.4) | 624.3 (763.0) | 71.1 (34.2) | 336.3 (226.1) | 630.8 (841.7) | 758.8 (480.0) |
| MCP-1 | 1593 (1612) | 499 (404) | 145 (2) | 785.9 (152.8) | 827.0 (183.0) | 742.2 (96.3) |
| MIP1-α | 51.41 (44.74) | 13.03 (4.72) | 2.89 (0.30) | 15.52 (10.20) | 15.18 (4.65) | 8.58 (3.35) |
| MIP-2 | 220.5 (405.5) | 41.2 (53.1) | 6.9 (2.8) | 86.44 (36.68) | 96.48 (33.35) | 141.30 (45.28) |
| Rantes | 266.3 (210.2) | 45.8 (22.6) | 2.87 (0.1) | 415.9 (109.5) | 466.2 (280.0) | 841.9 (477.5) |
| TNF-α | 6.596 (4.437) | 2.592 (0.906)* | 1.417 (0.103) | 8.725 (3.124) | 8.205 (4.710) | 7.915 (2.312) |
| VEGF | 312.9 (221.4) | 666.3 (1058.0) | 69.9 (1.5) | 184.8 (46.3) | 198.0 (36.1) | 107.3 (21.1) |
Summary of the means (M) and the SD for BAL and plasma in pg/mL. Note *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. The stars are significant P valuess vs the control.
BAL, bronchoalveolar lavage; IFNγ, interferon gamma; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MIP, macrophage inflammatory protein; NAD+, β-nicotinamide adenine dinucleotide.
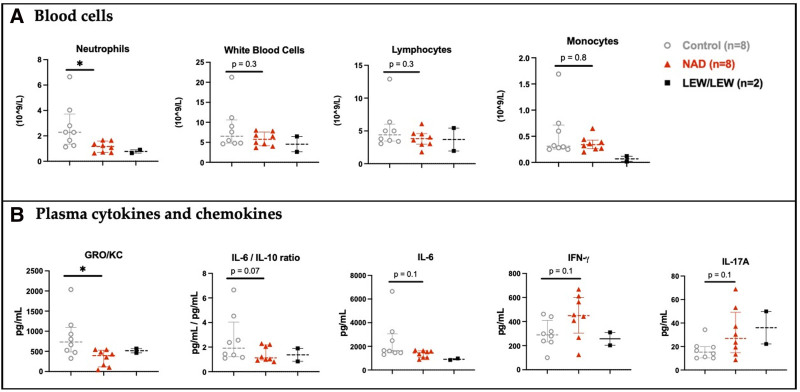
Blood Cells and Plasma
Significantly lower level of inflammation, based on a neutrophil count (P = 0.01) (Figure 5A), was observed in the NAD+ group compared with the control group. In plasma (Figure 5B; Table 1), significantly fewer proinflammatory GRO/KC (CXCL1) cytokines (P = 0.02) and a trend toward less proinflammatory IL-6 (P = 0.11) were observed. Concerning anti-inflammatory cytokines, a trend toward higher levels of IFNy (P = 0.10) was recorded for the NAD+ group, whereas the other anti-inflammatory cytokines IL-4 (P = 0.78) and IL-10 (P = 0.72) were comparable in the 2 groups. The IL-6/IL-10-ratio showed a trend toward less proinflammatory milieu in the NAD+ group (P = 0.07). In contrast to all these anti-inflammatory trends and as observed in the syngrafts, there was a trend toward higher proinflammatory IL-17A (P = 0.14) levels in the plasma of the NAD+ group.
FIGURE 5.
Blood cells and plasma cytokines and chemokines at 5 d posttransplantation in the study groups. A, Blood cells and (B) plasma cytokines and chemokines of the NAD+, control, and syngraft (LEW/LEW) groups. *P ≤ 0.05. The star represents a significant P value vs the control. IFNγ, interferon gamma; IL, interleukin; NAD+, β-nicotinamide adenine dinucleotide.
DISCUSSION
Acute rejection is still a major limitation for a successful outcome in lung transplantation. Compared to other solid organs, the lung possesses unique immunological properties and acute lung rejection is not dependent on secondary lymphoid organs as APCs can activate CD4+ and CD8+ T cells locally within the lung.17 NAD+, a coenzyme for over 500 enzymatic reactions, plays a critical role in regulating almost all major biological processes and participates in the homeostasis of the innate and adaptive immune system. Our study showed that NAD+ attenuated acute lung allograft rejection in a major MHC-mismatched rat left lung transplant model. Five days posttransplant, the rejection was lower as shown by a still compliant lung graft, less lymphocytic bronchiolitis, less alveolar and interstitial macrophages, a less proinflammatory cytokine milieu in the BAL and a lower systemic inflammation by neutrophils. Also, acute cellular rejection per se was reduced in this strong rejection model, although nonsignificant.
Previous studies derived from murine spleens,4-6 postulate that exogenous NAD+ suppresses the dendritic cell pathway and promotes through mast cells the differentiation of CD4+ T-cells into anti-inflammatory IFNγ-producing Th1-cells, anti-inflammatory IL-4 producing Th2-cells, anti-inflammatory IL-10 and IFNy producing regulatory type 1 cells, and proinflammatory IL-17A producing Th17-cells. In the BAL of this study, we did not observe local differences in cytokine expressions between NAD+-treated and control animals. In systemic blood we recorded trends of higher INFγ and IL17A in the NAD+ group, in line with the postulated mechanism. However, there was no difference in IL-10 and IL-4 expression. Sumitomo et al18 also did not detect a downregulation of IL-4 in the immunohistochemical analysis of allografts of nonimmunosuppressed animals 5 d after transplantation in a similar rat left-lung transplant model. This leads to the possible explanation that measuring IL-4 differences at 5 d posttransplant is not a relevant timing for comparison with this cytokine. The comparable IL-10 production in BAL and plasma for both our control and treated animals 5 d after transplantation remains, however, conflicting with the previous literature, raising the question if IL-10 is responsible for the major part of tolerance in our lung allografts.
Elkal et al5 suggested that daily injection of NAD+ after transplant causes a systemic upregulation of IL-10 as the primary reason for the skin graft tolerance. They observed a robust systemic IL-10 production in about 50% of the systemic conventional CD4+ T cells, based on spleen cells assessment. The treatment of the recipient with IL-10 before transplantation enhanced graft survival of mouse heart allograft,19 rat liver allograft,20 and bone marrow transplantation21 and was effective in lung transplantation.22 However, a start of IL-10 administration at the time or after transplantation had little effect23 or was even detrimental in high dosages.24,25
The recipients in our study received NAD+ 4 h before transplantation. In a mouse model NAD+ administration induced IL-10 secretion by IFNγ producing Th1 cells within hours,4 letting us expect an increasing IL-10 production already at the point of implantation.
Reasons why we could not find a difference between treated and controls in regard of IL-10 levels could be the following: (1) it might be the relative scarcity of T cells in lung allografts leading to a lower local expression capacity of IL-10 compared with other solid organ grafts.16 (2) There was only transient upregulation of IL-10 after each administration of NAD+ that decreased again within 24 h and thus was undetectable at autopsy. (3) The amount of produced IL-10 varies strongly between individuals. For a better comparison of the pro- versus anti-inflammatory balance the ratio of IL-6 to IL-10 was suggested,26,27 which would prove a proportionally higher ratio of IL-10 in our NAD+-treated animals. (4) It might lay in the dosage and stability of circulating NAD+ used in this study, unable to cause a similar IL-10 production effect in rats as reported in mice. (5) A higher dosage of IL-10 might have been observed when IL-10 producing regulatory type 1-cells in spleens would have been examined, instead of BAL and serum. Or (6) the tolerance observed in the lung allograft was not predominantly induced by IL-10 but other still uncharacterized local and systemic mechanisms of action of NAD+.
Although NAD+ has a known powerful role on CD4+ T cells, mast cells, and dendritic cells, it may also have effects on other important immune cells such as B-cells, CD8+ cells, and innate lymphoid cells, which have caused in part the observed tolerance in our treated group. The robust systemic trend of higher levels of anti-inflammatory INFγ observed in our NAD+ group, is in line with previous studies of daily NAD+ administration in mice,4-6 and was postulated to originate from CD8+T cells or innate lymphoid cells.5
Moreover, there may be an effect of NAD+ on mitigating macrophages. Compared with other solid organs, macrophages accumulate more in lung tissue and are per se more common than T cells and will gather furthermore during acute rejection.16 Activated macrophages contribute to the infiltration of neutrophils into the graft. Minhas et al7 have shown that a stable NAD+ environment is vital in maintaining an anti-inflammatory phenotype in resting macrophages. In our study, interstitial and alveolar macrophages were reduced in the allografts of the NAD+ group. In parallel to this finding, we also recorded less MIP1α and MIP2 in the NAD+ group.
In addition, we observed significantly less proinflammatory GRO/KC (CXCL1) neutrophil chemoattractant plasma production and significantly less systemic neutrophil recruitment in the NAD+ group, which must have been reduced by a direct or indirect inhibitory effect of NAD+.
This study acknowledges several limitations. First, it was conducted with the least possible number of animals to reach a conclusion, which may have affected the statistical power of the findings. A larger sample size, a different dosage of NAD+, or a less acute rejection model might have shown a stronger effect. Our study did not evaluate the impact of NAD+ on primary graft dysfunction (PGD) as the first hit in the development of acute rejection. We have previously demonstrated the beneficial effect of NAD+ on ischemic lungs during ex vivo lung perfusion, which underlined that NAD+ starts to work already at the very beginning of reperfusion of the graft.13 Even though our model was designed and conducted avoiding any extra PGD, NAD+ might have helped to prevent PGD and consecutively might have showed better results in the treatment group.
In conclusion, NAD+ has the potential to attenuate acute allograft rejection in lung transplantation. According to these observations, NAD+ should be considered a promising substance that might have a role in strategies for preventing acute cellular rejection. As a next step, these findings should be tested in a larger animal model.
ACKNOWLEDGMENTS
The authors would like to thank Ines Kleiber-Schaaf for immunohistochemistry staining, and Dr Conny Waschkies for initial support in intubation and ventilation of the rats.
Footnotes
The authors declare no conflicts of interest.
Conceptualization, J.P.E., S.A., and I.I.; methodology, J.P.E., S.A., and I.I.; validation, J.P.E. and S.A.; formal analysis, J.P.E., S.A., and I.I.; investigation, J.P.E., J.C., S.A, M.H., and I.I.; resources, I.I.; data curation, J.P.E. and S.A.; writing—original draft preparation, J.P.E.; writing—review and editing, S.A., M.H., and I.I.; visualization, J.P.E. and M.H.; supervision, I.I.; project administration, J.P.E., S.A., and I.I.; funding acquisition, J.P.E. All authors have read and agreed to the published version of the manuscript.
This research was funded by a grant from the Theodor and Ida Herzog Egli foundation.
S.A. and I.I. contributed equally to this work.
REFERENCES
- 1.Chambers DC, Cherikh WS, Harhay MO, et al. ; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult lung and heart-lung transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1042–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsiao HM, Scozzi D, Gauthier JM, et al. Mechanisms of graft rejection after lung transplantation. Curr Opin Organ Transplant. 2017;22:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benzimra M, Calligaro GL, Glanville AR. Acute rejection. J Thorac Dis. 2017;9:5440–5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tullius SG, Biefer HR, Li S, et al. NAD+ protects against EAE by regulating CD4+ T-cell differentiation. Nat Commun. 2014;5:5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkhal A, Rodriguez Cetina Biefer H, Heinbokel T, et al. NAD(+) regulates Treg cell fate and promotes allograft survival via a systemic IL-10 production that is CD4(+) CD25(+) Foxp3(+) T cells independent. Sci Rep. 2016;6:22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez Cetina Biefer H, Heinbokel T, Uehara H, et al. Mast cells regulate CD4(+) T-cell differentiation in the absence of antigen presentation. J Allergy Clin Immunol. 2018;142:1894–1908.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minhas PS, Liu L, Moon PK, et al. Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019;20:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi A, Hamakawa H, Sakai H, et al. Noninvasive assessment for acute allograft rejection in a rat lung transplantation model. Physiol Rep. 2014;2:e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erne BV, Jungraithmayr W, Buschmann J, et al. Effect of N-acetylcysteine on acute allograft rejection after rat lung transplantation. Ann Thorac Surg. 2013;95:1021–1027. [DOI] [PubMed] [Google Scholar]
- 10.Oishi H, Okada Y, Kikuchi T, et al. Transbronchial human interleukin-10 gene transfer reduces acute inflammation associated with allograft rejection and intragraft interleukin-2 and tumor necrosis factor-alpha gene expression in a rat model of lung transplantation. J Heart Lung Transplant. 2010;29:360–367. [DOI] [PubMed] [Google Scholar]
- 11.Okada Y, Zuo XJ, Toyoda M, et al. Adenovirus mediated IL-10 gene transfer to the airway of the rat lung for prevention of lung allograft rejection. Transpl Immunol. 2006;16:95–98. [DOI] [PubMed] [Google Scholar]
- 12.Council NR. Guide for the Care and Use of Laboratory Animals: Eighth Edition. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 13.Ehrsam JP, Chen J, Rodriguez Cetina Biefer H, et al. Ex vivo lung perfusion with β-nicotinamide adenine dinucleotide (NAD+) improves ischemic lung function. Antioxidants (Basel). 2022;11:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inci I, Zhai W, Arni S, et al. N-Acetylcysteine attenuates lung ischemia-reperfusion injury after lung transplantation. Ann Thorac Surg. 2007;84:240–246. Discussion 246. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. [DOI] [PubMed] [Google Scholar]
- 16.Jungraithmayr W, Inci I, Bain M, et al. Distribution of macrophages and T cells in syngrafts and allografts after experimental rat lung transplantation. Immunobiology. 2010;215:206–214. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier JM, Li W, Hsiao HM, et al. Mechanisms of graft rejection and immune regulation after lung transplant. Ann Am Thorac Soc. 2017;14:S216–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumitomo M, Sakiyama S, Tanida N, et al. Difference in cytokine production in acute and chronic rejection of rat lung allografts. Transpl Int. 1996;9(Suppl 1):S223–S225. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Fu F, Lu L, et al. Recipient pretreatment with mammalian IL-10 prolongs mouse cardiac allograft survival by inhibition of anti-donor T cell responses. Transplant Proc. 1999;31:115. [DOI] [PubMed] [Google Scholar]
- 20.Zou XM, Yagihashi A, Hirata K, et al. Downregulation of cytokine-induced neutrophil chemoattractant and prolongation of rat liver allograft survival by interleukin-10. Surg Today. 1998;28:184–191. [DOI] [PubMed] [Google Scholar]
- 21.Holler E, Roncarolo MG, Hintermeier-Knabe R, et al. Prognostic significance of increased IL-10 production in patients prior to allogeneic bone marrow transplantation. Bone Marrow Transplant. 2000;25:237–241. [DOI] [PubMed] [Google Scholar]
- 22.Pierog J, Gazdhar A, Stammberger U, et al. Synergistic effect of low dose cyclosporine A and human interleukin 10 overexpression on acute rejection in rat lung allotransplantation. Eur J Cardiothorac Surg. 2005;27:1030–1035. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Fu F, Lu L, et al. Differential effects of exogenous interleukin-10 on cardiac allograft survival: inhibition of rejection by recipient pretreatment reflects impaired host accessory cell function. Transplantation. 1999;68:1402–1409. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Lu L, Li Y, et al. High-dose cellular IL-10 exacerbates rejection and reverses effects of cyclosporine and tacrolimus in Mouse cardiac transplantation. Transplant Proc. 1997;29:1081–1082. [DOI] [PubMed] [Google Scholar]
- 25.Qian S, Li W, Li Y, et al. Systemic administration of cellular interleukin-10 can exacerbate cardiac allograft rejection in mice. Transplantation. 1996;62:1709–1714. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Su J, Xie Y, et al. Plasma IL-6/IL-10 ratio and IL-8, LDH, and HBDH level predict the severity and the risk of death in AIDS patients with pneumocystis pneumonia. J Immunol Res. 2016;2016:1583951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneda H, Waddell TK, De Perrot M, et al. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant. 2006;6:544–551. [DOI] [PubMed] [Google Scholar]