PURPOSE
Venous thromboembolism (VTE) is a leading cause of death among patients with cancer. The Khorana score was developed for assessing the risk of VTE in outpatients with cancer receiving chemotherapy, but its accuracy in identifying patients at high risk has been questioned. The aim of this study was to develop and validate a clinical-genetic score that improves the assessment of VTE risk in oncology outpatients within 6 months of diagnosis.
METHODS
The new score was developed using the data of 364 outpatients belonging to the Spanish ONCOTHROMB 12-01 population. In this cohort, clinical data associated with the risk of VTE were collected at the time of diagnosis, including the Khorana score. These patients were also genotyped for the 51 genetic variants known to be associated with VTE. Multivariate logistic regression was performed to determine the weight of each genetic and clinical variable in relation to VTE risk, allowing a clinical-genetic risk score (the ONCOTHROMB score) to be developed. The Khorana and the ONCOTHROMB scores were then compared via the area under the receiver operating characteristic curve (AUC), calibration, and the number of patients needed to treat. The new score was then validated in a study of 263 patients in the Vienna Cancer and Thrombosis Study population.
RESULTS
Nine genetic variants, tumor site, TNM stage, and a body mass index of > 25 kg/m2 were found to be associated with VTE and were used to build the ONCOTHROMB score, which better predicted the overall risk of VTE than did the Khorana score (AUC, 0.781 v 0.580; P < .001). Similar AUC results were recorded in the validation study the Vienna Cancer and Thrombosis Study cohort involving patients with the same type of tumor (AUC for the ONCOTHROMB score v the Khorana score: 0.686 v 0.577; P < .001) and with all type of tumors (AUC for the ONCOTHROMB score v the Khorana score: 0.720 v 0.561; P < .0001).
CONCLUSION
The ONCOTHROMB score for VTE risk in outpatients with cancer, which takes into account both clinical and genetic variables, better identifies patients who might benefit from primary thromboprophylaxis than does the Khorana score.
INTRODUCTION
Venous thromboembolism (VTE) is a serious and common complication in patients with cancer; indeed, it is among the leading causes of death.1 The incidence of VTE in patients with cancer is higher than in the general population, especially during the first few months after diagnosis, and after starting chemotherapy.2,3 A VTE episode can have clinical and economic implications with a profound impact on patient mortality, an overall poorer prognosis,4 and quality of life.1
CONTEXT
Key Objective
Can we improve the ability to predict the risk of thrombosis in outpatients with cancer receiving chemotherapy to improve therapeutic decisions and reduce its associated mortality?
Knowledge Generated
We have developed and validated a combined score with clinical and genomic data that showed an excellent predictive capacity (on the basis of area under the receiver operating characteristic curve, sensitivity, specificity, positive predictive value, and negative predictive value) to suffer venous thromboembolic events in outpatients with cancer receiving chemotherapy.
Relevance (I.K. Mellinghoff)
The authors present a new scoring system to identify patients with cancer at risk of future venous thromboembolism. Prospective studies are needed to determine whether use of this new score leads to more effective prevention of thrombosis without the risk of more untoward bleeding.*
*Relevance section written by JCO Associate Editor Ingo K. Mellinghoff, MD.
Guidelines4-7 suggest that most hospitalized patients with active cancer should receive thromboprophylaxis. However, in the outpatient setting, thromboprophylaxis has only been suggested for patients at high risk of VTE.7-9 Outpatients with cancer can be assessed for VTE risk, for which the use of the Khorana score10 is suggested. However, it has been questioned whether the Khorana score identifies high-risk patients with sufficient accuracy,11-13 and attempts have therefore been made to develop better models.7,14-17 Certainly, there is a need to better stratify patients with cancer according to their risk of VTE; this would help identify those for whom thromboprophylaxis is most indicated.
In addition to clinical risk factors, prothrombotic genetic factors have been associated with an increased risk of VTE in patients with cancer.18,19 For instance, the Vienna Cancer and Thrombosis Study (Vienna-CATS) and the Tromsø cohort have reported that patients with cancer who are carriers of FS gene variants (rs6025 and rs4524) are at increased risk of VTE.19,20 In addition, the association of VEGFA-1154AA variant with the risk of chemotherapy-triggered VTE,21 and the interaction between MTHFR C677T and SERPINE1 4G/5G polymorphisms have also been published to be associated to increase the risk of VTE.22 In this context, our group provided proof-of-concept that a risk score incorporating clinical and genetic factors23 can return significantly better results than the Khorana score in terms of identifying patients with cancer at high risk of VTE.16 The aim of this study was to develop a new, clinical-genetic score that takes also into account the genetic variants identified with solid and strong association with VTE in genome-wide association studies.24,25 and that has greater discriminatory power than the Khorana score in terms of identifying patients at risk of VTE within 6 months of diagnosis. The new score was externally validated in a cohort of patients from the Vienna-CATS study.
METHODS
Study Subjects
The new ONCOTHROMB score was developed for predicting the risk of suffering a VTE within 6 months of a cancer diagnosis, using the data collected at diagnosis for patients in the ONCOTHROMB 12-01 study previously described.16 Of the 406 patients included in ONCOTHROMB 12-01 study, we could only include 364 patients because in 42 of the 406 patients, one or more of the data items to be included (mainly genetic variants) in the different analysis were missing. However, we did not find any significant differences between the populations included and not included in the study (Data Supplement, online only).
The new score was validated using the clinical and genetic data of 263 patients included in the Vienna-CATS study.26 These subjects had the same primary cancers as those seen in the ONCOTHROMB 12-01 cohort. Both the ONCOTHROMB 12-01 and Vienna-CATS studies were approved by the ethics committees of the participating hospitals. All patients provided written informed consent to be included.
The present report adheres to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis guidelines,27 and to the STARD2015 guidelines28 for reporting studies on diagnostic accuracy.
Genetic Analysis
Both the ONCOTHROMB 12-01 (development cohort) and Vienna-CATS (validation cohort) patients were genotyped for the genetic variants mainly identified in genome-wide analysis as associated with VTE shown in the Data Supplement using DNA from blood extracted at the time of diagnosis. Genotyping was performed using TaqMan genotyping assays and the EP1 Fluidigm (an efficient end point polymerase chain reaction system for high-throughput SNP genotyping).
Diagnosis of VTE Events
VTE events were verified by objective imaging methods. Deep vein thrombosis in the lower limbs was diagnosed by ultrasound or ascending venography. Pulmonary embolism was diagnosed by ventilation-perfusion lung scanning, pulmonary angiography, or spiral computed tomography. Intracranial venous thrombosis was diagnosed by magnetic resonance imaging.
Development of the ONCOTHROMB Risk Score
The development of the ONCOTHROMB risk score has been done following the method previously described,16 and summarized in the Data Supplement.
Comparing the ONCOTHROMB and Khorana Scores
The Khorana score and the ONCOTHROMB score were calculated for each patient at the time of study inclusion, and their accuracy in terms of predicting observed VTE events compared. This was done by assessing the discrimination capability as the area under the receiver operating characteristic curve (AUC; larger values indicate better discrimination)29 and the calibration or the goodness to fit using the Hosmer-Lemeshow test.30 Sensitivity, specificity, positive predictive value and negative predictive value (PPV and NPV), and positive likelihood ratio (LR) and negative LR31 were determined for specific cutoff points. For the Khorana score, the cutoff defining high risk was set at three or higher.3 The ONCOTHROMB score cutoff point defining high risk was identified using the Youden J statistic as the point that maximizes the Youden index (defined as sensitivity + specificity – 1).32
The Khorana and ONCOTHROMB risk scores were then compared in both the ONCOTHROMB 12-01 and Vienna-CATS populations.
Validation of ONCOTHROMB Risk Score
As criteria for validating, we established that the AUCs calculated for Vienna-CATS population for ONCOTHROMB risk score were statistically superior to the Khorana score. In addition, we established that also in both populations (ONCOTHROMB 12-01 and Vienna-CATS), the AUCs were not statistically different.
We have also calculated the number of patients needed to treat (NNT) and we extended the validation including all the patients in the Vienna-CATS study and not only those with the same tumor as in ONCOTHROMB population (Data Supplement).
Statistical Analysis
Continuous variables were described as means ± standard deviation, and categorical variables as proportions. Continuous variables were compared using the Student t-test or Mann-Whitney U test as required. Categorical variables were compared using the χ2 or Fisher exact tests. The DeLong test was used to examine the difference between AUCs. All calculations were made using MedCalc Statistical Software v.18.11.3 (MedCalc Software bvba, Ostend, Belgium33; 2019).
RESULTS
Characteristics of the ONCOTHROMB 12-01 and Vienna-CATS Patients
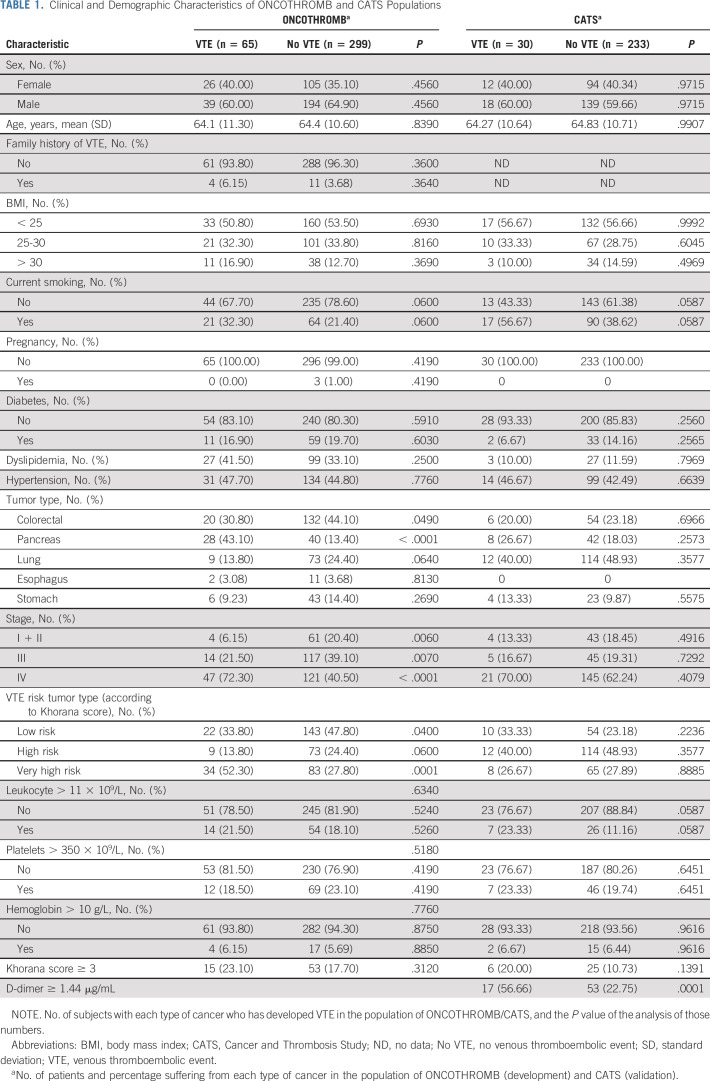
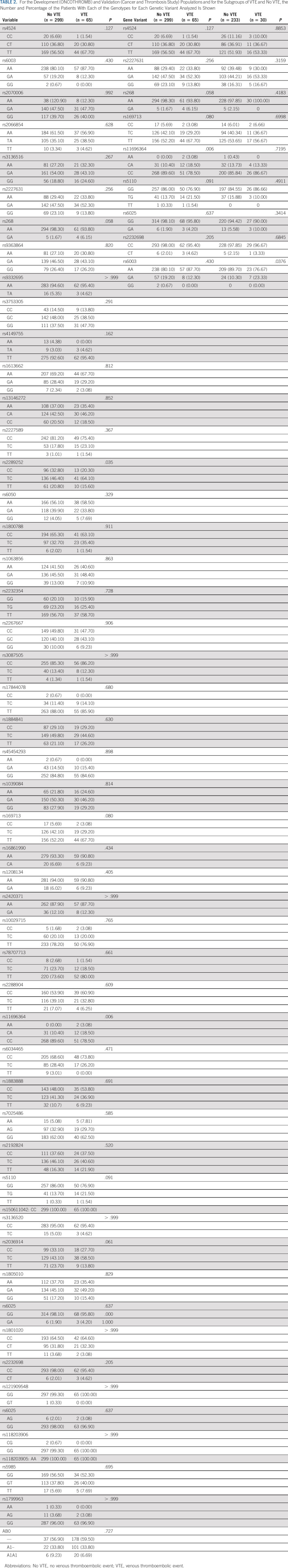
Table 1 shows the clinical and demographic characteristics, and Table 2 the distribution of genotypes, for the 364 ONCOTHROMB 12-01 and 263 Vienna-CATS patients. The overall incidence of VTE was 17.86%. Patients suffering from pancreatic cancer experienced VTEs at a significantly higher frequency (41.18%) than did those with other type of cancer (P < .001). The frequency of the genetic variants rs11696364 and rs2289252 was significantly higher in patients who experienced a VTE (Table 2).
TABLE 1.
Clinical and Demographic Characteristics of ONCOTHROMB and CATS Populations
TABLE 2.
For the Development (ONCOTHROMB) and Validation (Cancer and Thrombosis Study) Populations and for the Subgroups of VTE and No VTE, the Number and Percentage of the Patients With Each of the Genotypes for Each Genetic Variant Analyzed Is Shown
Development of the ONCOTHROMB Score
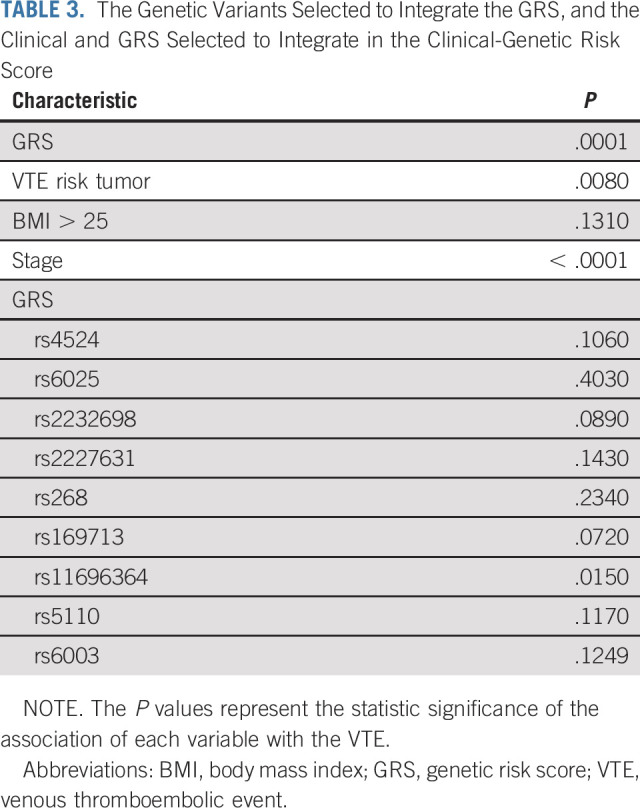
Table 3 shows the genetic and clinical factors that were associated with an increased risk of a VTE event (P ≤ .25) in multivariate analysis, and thus selected for inclusion in the development of the ONCOTHROMB score. Although rs6025 (Factor V Leiden) did not fulfill this inclusion criterion, it was included since there is strong evidence of its bearing on risk estimates for VTE in patients with cancer.18-20,34-37
TABLE 3.
The Genetic Variants Selected to Integrate the GRS, and the Clinical and GRS Selected to Integrate in the Clinical-Genetic Risk Score
Accuracy of the ONCOTHROMB and Khorana Scores
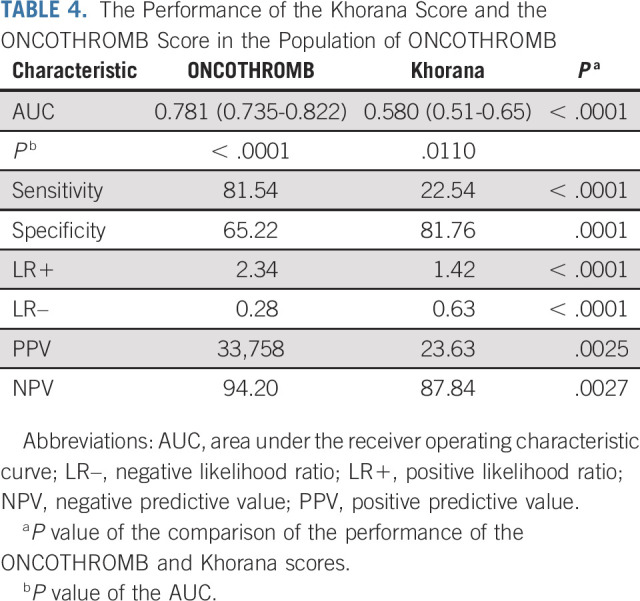
The ONCOTHROMB score showed significantly greater capacity than the Khorana score to distinguish between patients who would experience/not experience a VTE event (AUC, 0.781 v 0.580; P < .001; Table 4). The sensitivity of the ONCOTHROMB score was also significantly better than that of the Khorana (81.54% v 22.54%; P < .001), while the specificity of the Khorana score was higher (65.22% v 81.76%; P < .0001). The PPV and NPV values of the ONCOTHROMB score were significantly higher than those of the Khorana score (PPV, 33.758% v 23.63%; P = .0025, and NPV, 94.20% v 87.84%; P = .0027), as were the LRs (LR+, 2.34 v 1.42; P < .0001, and LR–, 0.28 v 0.63; P < .0001; for LR+, higher values are better than lower, and for LR–, lower values are better than higher; Table 4). LR values are useful measures of diagnostic accuracy.38 LR+ indicates how many times more likely a positive test result occurs in subjects with the disease than in those without the disease. LR– indicates how many times more likely a negative test result occurs in subjects with the disease than in those without the disease. Unlike PPV and NPV, neither LR+ nor LR– depends on disease prevalence. The ONCOTHROMB score calibrates well as the goodness to fit shows that the prediction does not significantly differs from the real data (P = .2693).
TABLE 4.
The Performance of the Khorana Score and the ONCOTHROMB Score in the Population of ONCOTHROMB
The NNT values were (1) 12, if all patients included in the study had been treated; (2) 10, if only the patients with a Khorana score of ≥ 3 had been treated, and (3) six if only patients with a high ONCOTHROMB score (with the cutoff set at the maximum Youden index value) had been treated. Under the criteria of drug effectiveness used (ie, a reduction in cancer-associated VTE of 46%), the Khorana score would have helped prevent 19 of the 65 VTE detected, while the ONCOTHROMB score would have helped prevent 24. The administration of prophylactic treatment to all the patients included in the cohort would have helped prevent 30 VTE events with a nonsignificant higher risk of major bleeding events.39
Validation of the ONCOTHROMB Score
In the Vienna-CATS population, the overall incidence of VTE was significantly lower than that observed in the ONCOTHROMB 12-01 population (11.40% v 17.86%; P = .0134). Neither cancer type nor TNM stage was associated with a higher incidence of VTE in the Vienna-CATS cohort.
It is important to note that the proportions of patients with pancreatic and stomach cancer in both cohorts were similar, although colorectal and esophagus cancers were more prevalent in the ONCOTHROMB 12-01 than in the Vienna-CATS cohort, and lung cancer was more common in the latter than in the former (Table 1). The incidence of VTE in patients with pancreatic cancer was higher in the ONCOTHROMB 12-01 than in the Vienna-CATS cohort (41.18% v 16.00%; P = .0035), probably because of the more advanced tumor stage of the patients in the former. For all the other types of tumor, VTE incidence was similar in both cohorts.
Differences were seen between the cohorts in terms of the prevalence of genetic variants significantly associated with VTE (Table 2). In the ONCOTHROMB 12-01 cohort, rs11696364 was significantly more common in patients with VTE, while in the Vienna-CATS cohort, rs6003 was more common.
Accuracy of the ONCOTHROMB Score When Used With the Vienna-CATS Cohort
In the Vienna-CATS cohort, the ONCOTHROMB score returned an AUC of 0.686 (0.626-0.741; P < .0001), a sensitivity of 76.67%, and a specificity of 59.23%. Its PPV was 19.49%, NPV 95.17%, positive LR, 1.88, and negative LR, 0.39 (Table 5). The Khorana score failed to distinguish between patients who experienced/did not experience a VTE event (AUC, 0.577; P = .1627).
TABLE 5.
The Performance of the Khorana Score and the ONCOTHROMB Score in the Population of Vienna Cancer and Thrombosis Study
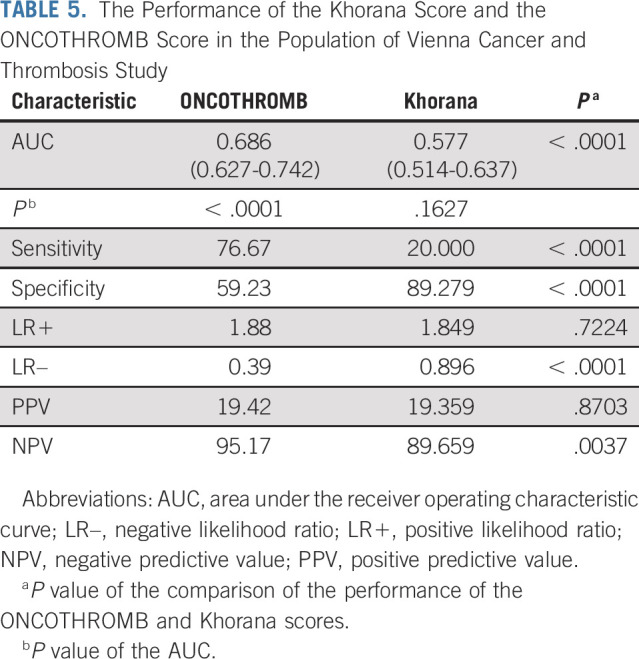
The sensitivity of the ONCOTHROMB score was significantly higher than that of the Khorana score (76.67% v 20.00%; P < .0001), while the specificity of the Khorana score was higher (59.23% v 89.27%; P < .0001). The NPV of the ONCOTHROMB score was significantly higher than that of the Khorana score (95.17% v 89.65%; P = .0169), and the LR– significantly better (0.394 v 0.896; P < .0001). No differences were seen for PPV and LR+ (PPV, 19.49 v 19.35; P = .9838, and LR+, 1.88 v 1.84, P = .7281).
The NNT values were (1) 19, if all patients included in the study had been treated; (2) 10, if only the patients with a Khorana score of ≥ 3 had been treated, and (3) 10, if only patients with a high risk ONCOTHROMB score (with the cutoff set at the maximum Youden index value) had been treated. Under the criteria of drug effectiveness used (ie, a reduction in cancer-associated VTE of 46%), the Khorana score could have helped prevent three of the 30 VTE detected, while the ONCOTHROMB score could have helped prevent 12. The administration of the prophylactic treatment to all the patients included in the cohort could have prevented 14 VTE with a nonsignificant higher risk of major bleeding events.39
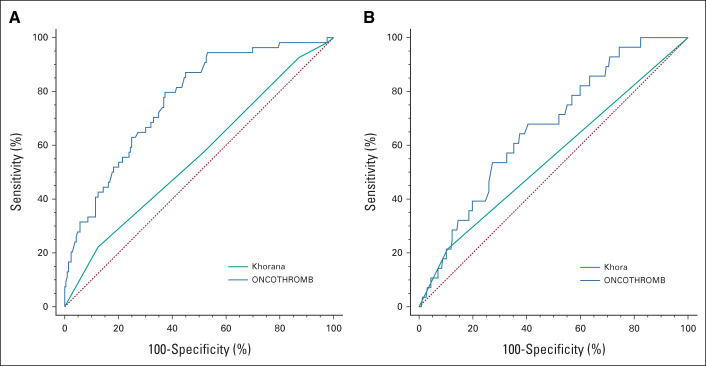
The difference in the AUC for the ONCOTHROMB score in the ONCOTHROMB 12-01 and Vienna-CATS populations was not significant (0.781 v 0.686; P = .070). Figure 1 shows the AUC for ONCOTHROMB score and for Khorana score both in ONCOTHROMB 12-01 and Vienna-CATS populations.
FIG 1.
Predictive capacity of the different models in the original and the validation studies. Receiver operating characteristic curves are shown for each model: (A) comparison in ONCOTHROMB (Khorana and ONCOTRHOMB scores) and (B) comparison in Vienna-CATS (Khorana and ONCOTRHOMB scores). AUC, area under the receiver operating characteristic curve; CATS, Cancer and Thrombosis Study.
In the ONCOTHROMB 12-01 cohort, the Khorana score declared 50 of the 65 patients who suffered a VTE as being at low risk. The ONCOTHROMB score, however, reclassified 41 of those 50 as being at high risk for VTE. In the Vienna-CATS cohort, the Khorana score declared 24 of the 30 patients who suffered a VTE as being at low risk for VTE. The ONCOTHROMB score, by contrast, reclassified 17 of those 24 as being at high risk for VTE.
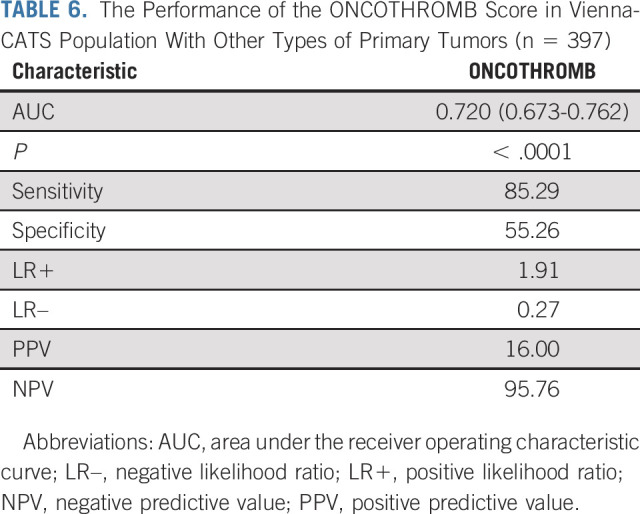
It is important to note that, when the validation was performed with all types of tumors in the Vienna-CATS cohort, the AUCs for the ONCOTHROMB score versus the Khorana score were 0.720 versus 0.561, respectively (P < .0001; Table 6).
TABLE 6.
The Performance of the ONCOTHROMB Score in Vienna-CATS Population With Other Types of Primary Tumors (n = 397)
DISCUSSION
When deciding whether to use primary thromboprophylaxis in outpatients with cancer who are candidates for chemotherapy, a clinician needs to determine the risk of VTE and weigh the potential benefit against the possibility of bleeding. Despite the increasing awareness of cancer-associated VTE, and the increasing evidence of the benefits of thromboprophylaxis (including from randomized clinical trials40-45 and the latest meta-analyses46-49), its use is limited among outpatients. This is probably because of the suboptimal capacity of the existing tools to predict the risk of experiencing a VTE event. In phase III randomized studies of primary thromboprophylaxis—the CASSINI and AVERT trials40,45—patients were selected on the basis of a Khorana score of ≥ 2, and the incidence of VTE in the placebo arms was 10.2% and 8.8% in these trials, respectively. The NNT for symptomatic VTE in these two trials combined was 40, only marginally more favorable than the NNT observed in low-molecular-weight heparin trials that involved the use of no risk assessment model50 (eg, in the SAVE ONCO trial,43 the NNT was 46). A risk assessment model with better predictive power might be used more often in clinical practice.
The present work presents a new predictive score, the ONCOTHROMB score, which shows significantly greater power to predict a VTE event than the Khorana score. In the development phase, nine genetic variants independently associated with VTE in outpatients with cancer were detected (Table 3). These were combined into a genetic risk score that showed a significant association with VTE. The ONCOTHROMB score combines this genetic risk score with three clinical variables also found to be independently associated with VTE in outpatients with cancer (Table 3).
From a clinical point of view, the ONCOTHROMB score identified patients at high risk significantly better than did the Khorana score (AUC, 0.781 v 0.592; P < .0001; Table 4). Among the patients identified at high risk by the ONCOTHROMB score, 34% eventually suffered a VTE event (Table 4); in comparison, 24% of the patients at high risk according to the Khorana score suffered a VTE (P = .025). Another demonstration of the clinical usefulness of the ONCOTHROMB score is its capacity to amend the misclassifications made by the Khorana score; the ONCOTHROMB score properly classified as being at high risk 82% of those patients who developed a VTE—patients the Khorana score identified as being at low risk.
Following the accepted recommendations for developing and validating the new score,51,52 external validation was performed in an independent cohort—that of the well-known Vienna-CATS study. It is important to highlight that, in this validation cohort, the AUC for the ONCOTHROMB score was significantly higher than that for the Khorana score (0.686 v 0.577; P < .0001) and not significantly different to that obtained in the development (ONCOTHROMB 12-01) cohort, neither were differences seen in the sensitivity, specificity, or NPV. However, the PPV was significantly lower than in the ONCOTHROMB 12-01 cohort (19.42 v 33.375; P = .0001). Similarly, the AUC and the sensitivity for the Khorana score in the validation cohort were not significantly different to those obtained in the development cohort. However, the specificity of the Khorana score in the validation cohort was significantly higher (89.27 v 81.76; P < .004).
It is important to note that for the validation of the ONCOTHROMB score, patients in the Vienna-CATS study with the same type of primary tumors as those in the ONCOTHROMB 12-01 cohort were selected. However, it is very likely that the different distribution of patients in accordance with the tumor type in both populations is responsible for the difference (nonsignificant) observed between the AUC in both populations. An additional validation was performed with all the patients from the Vienna-CATS population with other types of primary tumors and similarly satisfactory results were obtained, with an AUC for the ONCOTHROMB score versus the Khorana score of 0.720 versus 0.561 (P < .0001; Table 6).
In could be argued that the use of a GRS might not always be generalizable across populations, given differences in allelic frequencies. However, we have validated ONCOTHROMB risk score in a different country and we have previously demonstrated the validity of the GRSs in different populations both in VTE and cardiovascular event predictions.23,53,54
In 2019, a systematic review and a meta-analysis55 concluded that the Khorana score can select high-risk patients for thromboprophylaxis overall, which is in support of the suggestions presented in some guidelines. However, several limitations of the Khorana score need to be taken into account, including the different in predicted performance across cancer types and the modest proportion of patients with VTE assigned to the high-risk group. In that meta-analysis, the estimated risk of VTE on the basis of Khorana score was considerably lower for patients with lung cancer and hematologic malignancies than for those with other cancer types. Probably, the relative high number of patients with lung cancer in the populations included in our study could explain in part the low AUC obtained with the Khorana score. This, at the same time, provides extra value to ONCOTHROMB score demonstrating high predictive value even in lung cancer and a derived score of ONCOTHROMB score (TiC-LYMPHO) in some hematologic malignancies.56 The great contribution of our approach is the inclusion of genetic variants together with clinical risk factors, because the relevance of the genetic background in the risk of developing VTE is considered very high since the heritability of VTE has been estimated at about 60%.57
We acknowledge that our results should be further validated. However, considering our previous experience with clinical-genetic risk scores, we expect to obtain similar results worldwide.23,53,54
The message for the clinical oncologist is that, from the time of diagnosis until 6 months later, the new ONCOTHROMB score for VTE risk in outpatients with cancer (1) better identifies those at high risk of suffering VTE from those at low risk (the ONCOTHROMB score has a higher AUC value than the Khorana score); (2) identifies a larger number of patients likely to suffer a VTE (the ONCOTHROMB score is more sensitive than the Khorana score); (3) provides a more reliable classification of low risk (the NPV of the ONCOTHROMB score is higher than that of the Khorana score); and (4) the ONCOTHROMB score can amend most (82%) of the misclassifications made by the Khorana score in patients with VTE. On the basis of the improvement in VTE predictive capacities, further clinical trials to evaluate our score in prophylaxis efficiency for prevention of VTE are warranted.
In summary, this paper reports a validated clinical-genetic risk score that is significantly better than the Khorana score at identifying outpatients with cancer at high risk of experiencing a VTE event, who would likely benefit from thromboprophylaxis despite the risk of hemorrhage. As the peak incidence of cancer-associated VTE is from 0 to 6 months after diagnosis,2,3 it is recommended that the ONCOTHROMB score be calculated at the moment cancer is suspected.4,20 This new score could improve the prediction, prevention, and treatment of VTE in oncology outpatients, providing for more efficient and safer thromboprophylaxis in those at high risk. The results could change clinical practice and have an important impact in national health systems.
Andrés Muñoz
Consulting or Advisory Role: Celgene, Sanofi, Bristol Myers Squibb/Pfizer, LEO Pharma, Daiichi Sankyo, Incyte, AstraZeneca, MSD Oncology, Lilly, Roche, Servier
Speakers' Bureau: Rovi, Bayer, Servier, Menarini, STADA
Research Funding: Sanofi, LEO Pharma, Celgene, Rovi
Patents, Royalties, Other Intellectual Property: Risk assessment model in venous thromboembolism in patients with cancer
Travel, Accommodations, Expenses: Celgene, Roche, Merck Serono, Amgen, Pfizer, Servier
Mercedes Salgado
Consulting or Advisory Role: Amgen, Servier, Sanofi
Speakers' Bureau: Shenzen Techdow Pharma, Sanofi, Lilly, Rovi, Eisai Farmacéutica, Pierre Fabre, Servier
Travel, Accommodations, Expenses: Servier, LEO Pharma, Rovi
Laura Ortega
Consulting or Advisory Role: Sanofi
Speakers' Bureau: LEO Pharma, Sanofi
Travel, Accommodations, Expenses: Pierre Fabre, Amgen
Ana Rupérez
Honoraria: Servier, Merck Serono, Bayer Health
Eduardo Salas
Stock and Other Ownership Interests: Gendiag
Jose Manuel Soria
Stock and Other Ownership Interests: Exheus
Consulting or Advisory Role: Exheus
Research Funding: Rovi
Patents, Royalties, Other Intellectual Property: Patents with my institution, Patents with Exheus
No other potential conflicts of interest were reported.
See accompanying editorial on page 2881
SUPPORT
Supported by the SETH, SEOM, Asociación ActivaTT por la Salud and FIS (PI/20/00325).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Andrés Muñoz, Eduardo Salas, Ingrid Pabinger, Jose Manuel Soria
Financial support: Jose Manuel Soria
Provision of study materials or patients: Andres Muñoz, Cihan Ay, Carme Font, Vanesa Pachón, Victoria Castellón, Virginia Martínez-Marín, Mercedes Salgado, Eva Martínez, Julia Calzas, Laura Ortega, Ana Rupérez, Ingrid Pabinger
Collection and assembly of data: Andrés Muñoz, Cihan Ay, Ella Grilz, Sonia López, Carme Font, Vanesa Pachón, Victoria Castellón, Virginia Martínez-Marín, Mercedes Salgado, Eva Martínez, Julia Calzas, Laura Ortega, Ana Rupérez, Ingrid Pabinger, Jose Manuel Soria
Data analysis and interpretation: Andrés Muñoz, Cihan Ay, Vanesa Pachón, Mercedes Salgado, Julia Calzas, Eduardo Salas, Ingrid Pabinger, Jose Manuel Soria
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
A Clinical-Genetic Risk Score for Predicting Cancer-Associated Venous Thromboembolism: A Development and Validation Study Involving Two Independent Prospective Cohorts
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Andrés Muñoz
Consulting or Advisory Role: Celgene, Sanofi, Bristol Myers Squibb/Pfizer, LEO Pharma, Daiichi Sankyo, Incyte, AstraZeneca, MSD Oncology, Lilly, Roche, Servier
Speakers' Bureau: Rovi, Bayer, Servier, Menarini, STADA
Research Funding: Sanofi, LEO Pharma, Celgene, Rovi
Patents, Royalties, Other Intellectual Property: Risk assessment model in venous thromboembolism in patients with cancer
Travel, Accommodations, Expenses: Celgene, Roche, Merck Serono, Amgen, Pfizer, Servier
Mercedes Salgado
Consulting or Advisory Role: Amgen, Servier, Sanofi
Speakers' Bureau: Shenzen Techdow Pharma, Sanofi, Lilly, Rovi, Eisai Farmacéutica, Pierre Fabre, Servier
Travel, Accommodations, Expenses: Servier, LEO Pharma, Rovi
Laura Ortega
Consulting or Advisory Role: Sanofi
Speakers' Bureau: LEO Pharma, Sanofi
Travel, Accommodations, Expenses: Pierre Fabre, Amgen
Ana Rupérez
Honoraria: Servier, Merck Serono, Bayer Health
Eduardo Salas
Stock and Other Ownership Interests: Gendiag
Jose Manuel Soria
Stock and Other Ownership Interests: Exheus
Consulting or Advisory Role: Exheus
Research Funding: Rovi
Patents, Royalties, Other Intellectual Property: Patents with my institution, Patents with Exheus
No other potential conflicts of interest were reported.
REFERENCES
- 1.Noble S, Pasi J: Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer 102:S2-S9, 2010. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blom JW, Doggen CJM, Osanto S, et al. : Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 293:715-722, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Dalal M, Lin J, et al. : Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 119:648-655, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Lyman GH, Bohlke K, Falanga A, et al. : Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract 11:e442-e444, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Munoz Martin AJ, Font Puig C, Navarro Martin LM, et al. : Clinical guide SEOM on venous thromboembolism in cancer patients. Clin Transl Oncol 16:1079-1090, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandalà M, Falanga A, Roila F: Management of venous thromboembolism (VTE) in cancer patients: ESMO clinical practice guidelines. Ann Oncol 2222:85-92, 2011. (suppl 6) [DOI] [PubMed] [Google Scholar]
- 7.Farge D, Frere C, Connors JM, et al. : 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 20:e566-e581, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Key NS, Khorana AA, Kuderer NM, et al. : Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 38:496-520, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Wang T, Zwicker JI, Ay C, et al. : The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: Guidance from the SSC of the ISTH. J Thromb Haemost 17:1772-1778, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khorana AA, Kuderer NM, Culakova E, et al. : Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111:4902-4907, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Es N, Franke VF, Middeldorp S, et al. : The Khorana score for the prediction of venous thromboembolism in patients with pancreatic cancer. Thromb Res 150:30-32, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Tafur AJ, Caprini JA, Cote L, et al. : Predictors of active cancer thromboembolic outcomes: RIETE experience of the Khorana score in cancer-associated thrombosis. Thromb Haemost 117:1192-1198, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Muñoz Martín AJ, García Alfonso P, Rupérez Blanco AB, et al. : Incidence of venous thromboembolism (VTE) in ambulatory pancreatic cancer patients receiving chemotherapy and analysis of Khorana's predictive model. Clin Transl Oncol 16:927-930, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Pabinger I, van Es N, Heinze G, et al. : A clinical prediction model for cancer-associated venous thromboembolism: A development and validation study in two independent prospective cohorts. Lancet Haematol 5:e289-e298, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerotziafas GT, Taher A, Abdel-Razeq H, et al. : A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer: The prospective COMPASS–cancer-associated thrombosis study. Oncologist 22:1222-1231, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muñoz Martín AJ, Ortega I, Font C, et al. : Multivariable clinical-genetic risk model for predicting venous thromboembolic events in patients with cancer. Br J Cancer 118:1056-1061, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cella CA, Di Minno G, Carlomagno C, et al. : Preventing venous thromboembolism in ambulatory cancer patients: The ONKOTEV study. Oncologist 22:601-608, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gran OV, Brækkan SK, Hansen J-B: Prothrombotic genotypes and risk of venous thromboembolism in cancer. Thromb Res 164:S12-S18, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Pabinger I, Ay C, Dunkler D, et al. : Factor V Leiden mutation increases the risk for venous thromboembolism in cancer patients—Results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost 13:17-22, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Gran OV, Smith EN, Brækkan SK, et al. : Joint effects of cancer and variants in the factor 5 gene on the risk of venous thromboembolism. Haematologica 101:1046-1053, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchis MLD, Palmirotta R, Riondino S, et al. : VEGF gene promoter polymorphisms and risk of VTE in chemotherapy-treated cancer patients. Thromb Haemost 115:143-151, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Xu P, Shu Q, et al. : Combined effect of MTHFR C677T and PAI-1 4G/5G polymorphisms on the risk of venous thromboembolism in Chinese lung cancer patients. Clin Appl Thrombosis/Hemostasis 27:107602962110312, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soria JM, Morange P-E, Vila J, et al. : Multilocus genetic risk scores for venous thromboembolism risk assessment. J Am Heart Assoc 3:e001060, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinds DA, Buil A, Ziemek D, et al. : Genome-wide association analysis of self-reported events in 6135 individuals and 252 827 controls identifies 8 loci associated with thrombosis. Hum Mol Genet 25:1867-1874, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang W, Teichert M, Chasman DI, et al. : A genome-wide association study for venous thromboembolism: The extended cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Genet Epidemiol 37:512-521, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ay C, Simanek R, Vormittag R, et al. : High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). Blood 112:2703-2708, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Collins GS, Reitsma JB, Altman DG, et al. : Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. Eur Urol 67:1142-1151, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Bossuyt PM, Reitsma JB, Bruns DE, et al. : STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. Clin Chem 61:1446-1452, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Hanley JA, Hajian-Tilaki KO: Sampling variability of nonparametric estimates of the areas under receiver operating characteristic curves: An update. Acad Radiol 4:49-58, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Hosmer DW, Hosmer T, Lemeshow S: A goodness-of-fit tests for the multiple logistic regression model. Commun Stat 10:1043-1069, 1980 [Google Scholar]
- 31.Attia J: Diagostic tests: Moving beyond sensitivity and specificity: Using likelihood ratios to help interpret diagnostic tests. Aust Prescr 26:111-113, 2003 [Google Scholar]
- 32.Schisterman EF, Perkins NJ, Liu A, et al. : Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 16:73-81, 2005 [DOI] [PubMed] [Google Scholar]
- 33.MedCalc. Easy-to-use statistical software. https://www.medcalc.org
- 34.Decousus H, Moulin N, Quenet S, et al. : Thrombophilia and risk of venous thrombosis in patients with cancer. Thromb Res 120:S51-S61, 2007. (suppl) [DOI] [PubMed] [Google Scholar]
- 35.Heraudeau A, Delluc A, Le Henaff M, et al. : Risk of venous thromboembolism in association with factor V leiden in cancer patients—The EDITH case-control studyde Frutos P. PLoS One 13:e0194973, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy M, Andreescu ACM, Greenblatt MS, et al. : Factor V Leiden, prothrombin 20210A and the risk of venous thrombosis among cancer patients. Br J Haematol 128:386-388, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Montagnana M, Lippi G, Danese E. An Overview of Thrombophilia and Associated Laboratory Testing. Methods Mol Biol. 1646:113-135, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Eusebi P: Diagnostic accuracy measures. Cerebrovasc Dis 36:267-272, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Di Nisio M, Porreca E, Otten H-M, et al. : Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 8:CD008500, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Khorana AA, Soff GA, Kakkar AK, et al. : Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med 380:720-728, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Agnelli G, Gussoni G, Bianchini C, et al. : Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study. Lancet Oncol 10:943-949, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Maraveyas A, Waters J, Roy R, et al. : Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer 48:1283-1292, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Agnelli G, George DJ, Kakkar AK, et al. : Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 366:601-609, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Pelzer U, Opitz B, Deutschinoff G, et al. : Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: Outcomes from the CONKO-004 trial. J Clin Oncol 33:2028-2034, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Carrier M, Abou-Nassar K, Mallick R, et al. : Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 380:711-719, 2019 [DOI] [PubMed] [Google Scholar]
- 46.Becattini C, Verso M, Muňoz A, et al. : Updated meta-analysis on prevention of venous thromboembolism in ambulatory cancer patients. Haematologica 105:838-848, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Aharon I, Stemmer SM, Leibovici L, et al. : Low molecular weight heparin (LMWH) for primary thrombo-prophylaxis in patients with solid malignancies—systematic review and meta-analysis. Acta Oncol 53:1230-1237, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Di Nisio M, Porreca E, Ferrante N, et al. : Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 2:CD008500, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Tun NM, Guevara E, Oo TH: Benefit and risk of primary thromboprophylaxis in ambulatory patients with advanced pancreatic cancer receiving chemotherapy: A systematic review and meta-analysis of randomized controlled trials. Blood Coagul Fibrinolysis 27:270-274, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Agnelli G: Direct oral anticoagulants for thromboprophylaxis in ambulatory patients with cancer. N Engl J Med 380:781-783, 2019 [DOI] [PubMed] [Google Scholar]
- 51.Altman DG, Royston P: Statistics in medical journals: Some recent trends. Stat Med 19:3275-3289, 2000 [DOI] [PubMed] [Google Scholar]
- 52.McGinn TG, Guyatt GH, Wyer PC, et al. : Users' guides to the medical literature XXII: How to use articles about clinical decision rules. JAMA 284:79-84, 2000 [DOI] [PubMed] [Google Scholar]
- 53.McInnes G, Daneshjou R, Katsonis P, et al. : Predicting venous thromboembolism risk from exomes in the Critical Assessment of Genome Interpretation (CAGI) challenges. Hum Mutat 40:1314-1320, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salas E, Farm M, Pich S, et al. : Predictive ability of a clinical-genetic risk score for venous thromboembolism in Northern and Southern European populations. TH Open 05:e303-e311, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mulder FI, Candeloro M, Kamphuisen PW, et al. : The Khorana score for prediction of venous thromboembolism in cancer patients: A systematic review and meta-analysis. Haematologica 104:1277-1287, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bastos-Oreiro M, Ortiz J, Pradillo V, et al. : Incorporating genetic and clinical data into the prediction of thromboembolism risk in patients with lymphoma. Cancer Med 10:7585-7592, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Souto JC, Almasy L, Borrell M, et al. : Genetic susceptibility to thrombosis and its relationship to physiological risk factors: The GAIT study. Am J Hum Genet 67:1452-1459, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]