PURPOSE
To investigate the efficacy and safety of trastuzumab deruxtecan, an antibody-drug conjugate targeting human epidermal growth factor receptor 2 (HER2) with a topoisomerase I inhibitor payload, in patients with uterine carcinosarcoma (UCS) expressing HER2.
PATIENTS AND METHODS
Patients with recurrent UCS with HER2 immunohistochemistry scores ≥1+ previously treated with chemotherapy were included. Patients were assigned to the HER2-high (immunohistochemistry score ≥2+; n = 22) or low (immunohistochemistry score of 1+; n = 10) groups for primary and exploratory analyses, respectively. Trastuzumab deruxtecan 6.4 or 5.4 mg/kg was administered intravenously once every 3 weeks until unacceptable toxicity or disease progression. Dose modification was based on the updated recommended phase II dose for breast cancer to be 5.4 mg/kg. The primary end point was the objective response rate by central review in the HER2-high group. Secondary end points included the overall response rate (ORR) in the HER2-high group by investigator assessment, ORR in the HER2-low group, progression-free survival (PFS), overall survival (OS), and safety.
RESULTS
The ORR by central review in the HER2-high and HER2-low groups were 54.5% (95% CI, 32.2 to 75.6) and 70.0% (95% CI, 34.8 to 93.3) and those by investigator assessments were 68.2% and 60.0%, respectively. The median PFS and OS in the HER2-high and HER2-low groups were 6.2 and 13.3 months and 6.7 months and not reached, respectively. Grade ≥ 3 adverse events occurred in 20 patients (61%). Grades 1-2 and 3 pneumonitis/interstitial lung disease occurred in eight (24%) and one (3%) patient, respectively.
CONCLUSION
Trastuzumab deruxtecan has efficacy in patients with UCS, regardless of HER2 status. The safety profile was generally consistent with that previously reported. Toxicities were manageable with appropriate monitoring and treatment.
INTRODUCTION
Uterine carcinosarcoma (UCS), a rare high-grade endometrial malignancy, accounts for <5% of uterine cancers but causes a relatively high proportion of deaths that are attributable to uterine cancers.1,2 UCS was originally considered a mixed Mullerian tumor and treated as a sarcoma subtype; however, it is now recognized as a metaplastic carcinoma with a sarcoma component arising from dedifferentiation of the carcinoma component.3 Paclitaxel-carboplatin is considered the standard chemotherapy for UCS,4 but the prognosis of patients receiving paclitaxel-carboplatin remains poor (median survival, <2 years).1 Furthermore, evidence to recommend the standard chemotherapy as second- and later-line therapies is lacking.5-10 Therefore, identifying new treatments for advanced or recurrent disease is necessary.
CONTEXT
Key Objective
To investigate the efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients with advanced or recurrent uterine carcinosarcoma (UCS) who have low or high expression of human epidermal growth factor receptor 2 (HER2).
Knowledge Generated
T-DXd is the first anti-HER2 therapy that demonstrated clinical activity in previously treated patients with advanced or recurrent UCS regardless of HER2 status. The safety profile of T-DXd was generally consistent with that previously reported.
Relevance (G. Fleming)
UCSs are difficult to treat. A substantial number of them have some HER2 expression and these results open up a promising new therapeutic avenue.*
*Relevance section written by JCO Associate Editor Gini Fleming, MD.
HER2 is a promising therapeutic target in UCS.8 In several in vivo and in vitro studies, HER2-targeted therapies, including trastuzumab, trastuzumab emtansine, and EGFR and HER2 inhibitors, showed promising antitumor effects on HER2-expressing UCS cell lines.9,10 Moreover, HER2 amplification is reported in up to one third of UCS cases.11 HER2 immunohistochemistry (IHC) scores of 3+, 2+, and 1 + are noted in 5%-25%, 15%-35%, and 20%-40% of UCS cases, respectively.12-14 However, no clinical trials have investigated anti-HER2 therapies in UCS.
Trastuzumab deruxtecan (also DS-8201a or T-DXd) is an antibody-drug conjugate composed of a humanized monoclonal antibody that specifically targets HER2, a cleavable tetrapeptide-based linker and a potent topoisomerase I inhibitor as payload. T-DXd binds specifically to HER2 and induces antibody-dependent cellular cytotoxicity and cell death by topoisomerase I inhibition. T-DXd has a high antibody-drug ratio and a cleavable linker designed to be selectively cleaved by cathepsins and release the payload. The payload's high permeability allows it to exert its potent cytotoxic effect on adjacent cells, irrespective of HER2 expression. This cytotoxic effect, called the bystander killing effect, allows T-DXd to be effective even in tumors with heterogeneous HER2 expression.15 T-DXd has been approved for treating HER2-positive metastatic breast cancer and HER2-positive gastric cancer in various countries, including the United States and European countries.16,17 Additionally, T-DXd has demonstrated potent efficacy against HER2-low breast cancer,18,19 and several ongoing clinical trials are evaluating the efficacy and safety of T-DXd for HER2-low cancers.20,21
This multicenter phase II study aimed to evaluate the efficacy and safety of T-DXd in patients with advanced or recurrent HER2-expressing UCS, previously treated with chemotherapy.
PATIENTS AND METHODS
Trial Design
NCCH1615/STATICE (DS-8201a in metastatic/recurrent UCS patients), a phase II, multicenter, single-arm, investigator-initiated trial, evaluated the efficacy and safety of T-DXd in patients with advanced or recurrent HER2-expressing UCS who received prior standard chemotherapy. The HER2 IHC score was centrally tested and evaluated. IHC was performed using a standard US Food and Drug Administration–approved in vitro diagnostic kit, the PATHWAY anti-HER2 (Clone 4B5, Ventana Medical Systems, Inc, Tucson, AZ). Because of the lateral/basolateral HER2 staining pattern of UCS cells, we adopted the ASCO/CAP HER2 testing guideline for gastroesophageal adenocarcinoma, which permits a similar staining pattern, rather than the ASCO/CAP HER2 testing guideline for breast cancer, which requires a circumferential membranous staining pattern.22-24 Fluorescence in situ hybridization (FISH) was not a criterion for inclusion in the trial because there was no correlation between IHC and FISH results in our previous study of UCS.23 The trial was conducted at seven sites in Japan from December 14, 2017, (first patient's consent) to December 8, 2020 (data cutoff). The main eligibility criteria were age 20 years and older, histologically confirmed UCS, HER2 IHC score ≥1+, prior standard chemotherapy, Eastern Cooperative Oncology Group performance status of 0-1, and ≥ 1 measurable tumor according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Patients with active secondary cancer, history of symptomatic congestive heart failure, or symptomatic brain metastases were excluded (Data Supplement [online only]).
Patients were classified into the HER2-high (IHC 2+ and 3+) or HER2-low (IHC 1+) group on the basis of the HER2 IHC score, regardless of the HER2 FISH status. Enrollment of 15-25 and 5-10 patients in the HER2-high and -low groups, respectively, was planned. At study onset, 6.4 mg/kg T-DXd was intravenously administered once every 3 weeks on the basis of one of the recommended doses for breast cancer. However, during the study, the recommended phase II dose for breast cancer was updated to 5.4 mg/kg. This initial dose modification aimed to reduce the risk of interstitial lung disease (ILD), including pneumonitis. Considering the risk-benefit balance of T-DXd, we modified the initial dose (in line with the decision by Daiichi Sankyo Ltd to change the recommended phase II dose) from 6.4 to 5.4 mg/kg, mid-study, after having enrolled nine and five patients in the HER2-high and HER2-low groups, respectively. Patients continued T-DXd until withdrawal of consent, unacceptable toxicity, or disease progression (Data Supplement).
Ethics
This trial was approved by the institutional review board at each participating institution and conducted according to the ethical principles of the Declaration of Helsinki. All patients provided written informed consent to participate in the trial.
End Points
The primary end point was overall response rate (ORR), and the responses were evaluated on the basis of RECIST version 1.1 every 6 weeks through 24 weeks, and every 12 weeks thereafter by central review in the HER2-high group. Complete response (CR) and partial response (PR) are assessed according to RECIST version 1.1. The confirmation of response was required at least 4 weeks after the initial evaluation. The secondary end points were the ORR by investigator assessment, progression-free survival (PFS), overall survival (OS), and safety. The disease control rate and duration of response (DoR) were also determined in both groups.
Safety
Adverse events (AEs) were evaluated and graded using the National Cancer Institute Common Terminology Criteria for AEs v4.0. For patients who developed pneumonitis or ILD grade ≥2 on the basis of institutional assessment, the treatment was immediately discontinued, and they were treated according to the protocol .
Statistical Analysis
The efficacy analysis included patients who received at least one dose of T-DXd, met the key eligibility criteria, and had at least one measurable lesion at baseline by central assessment. We initially planned to enroll 15-25 patients in the HER2-high group, which was determined on the basis of the Bayesian design.25 According to prior distributions on the basis of the Bayesian design, we assumed a mean of 5% for the no-effect response rate and used the Beta distribution with shape parameters of 10 and 190 (Beta [10, 190]) on the basis of investigator assessment. We also assumed a mean of 30% for the response rate of T-DXd on the basis of other clinical trial results in patients with UCS who previously received chemotherapy26,27 and then used Beta (0.6, 1.4). On the basis of these settings, three responders were required to achieve a posterior probability > 95% that the response rate of T-DXd was at least >5% for 15-21 patients (or four responders for 22–25 patients). This design controlled the type I error rate at the target level of <0.10. During the enrollment of patients into the HER2-high group, we enrolled up to 10 patients into the HER2-low group. The primary efficacy analysis was based on the HER2-high group. The analyses for HER2-low group were conducted in an exploratory fashion. Safety analyses included all treated patients who received at least one T-DXd dose.
The ORR and exact 95% CI on the basis of the Clopper-Pearson method were estimated in the HER2-high and HER2-low groups. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Selection and Baseline Characteristics
Of 84 patients screened between December 2017 and June 2020 at seven institutions in Japan (Fig 1), 50 were excluded. Among the remaining 34 enrolled patients, one did not receive T-DXd because of disease progression. Therefore, the safety population included 33 patients (14 and 19 patients received 6.4 and 5.4 mg/kg, respectively; Fig 1). One patient was excluded from the efficacy analysis because of a lack of measurable disease at baseline by central review. Finally, the efficacy population included 32 patients: 22 and 10 in the HER2-high and HER2-low groups, respectively. The median follow-up time in the efficacy population was 13.5 (range, 4.0-33.0) months.
FIG 1.
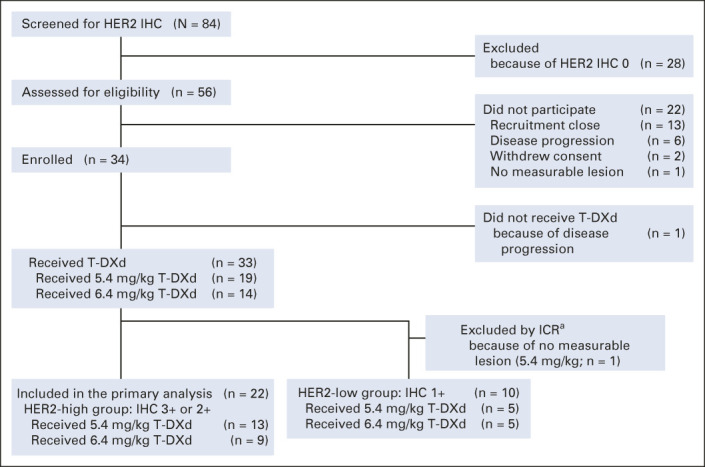
Flow diagram for the STATICE Trial. aOne patient was excluded from the efficacy analysis by ICR owing to no measurable lesion. HER2, human epidermal growth factor receptor2; ICR, independent central review; IHC, immunohistochemistry; T-DXd, trastuzumab deruxtecan.
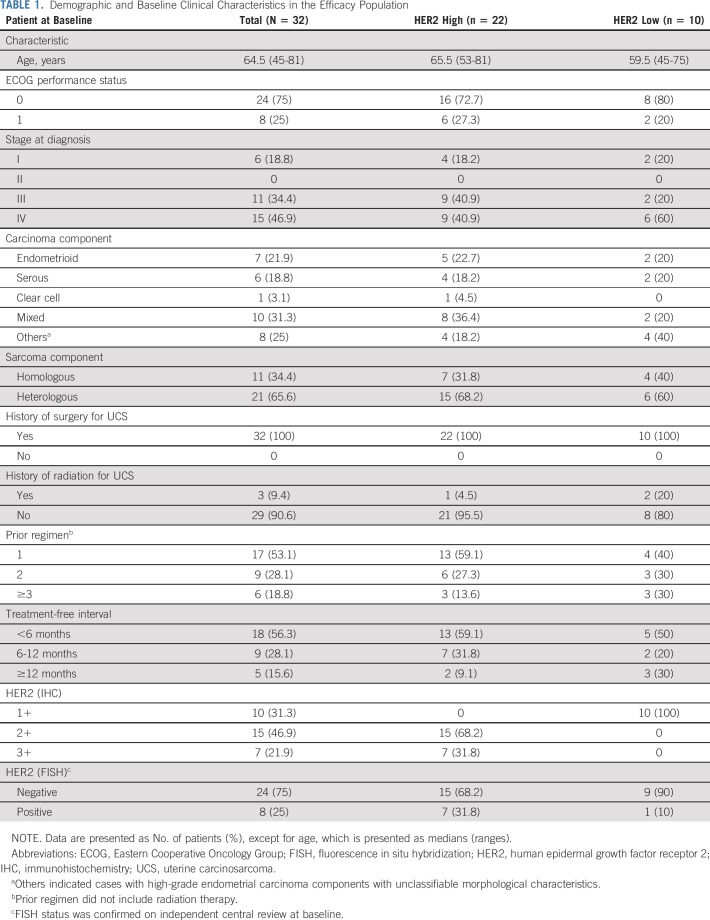
Table 1 shows the baseline patient demographics and clinical characteristics of the entire efficacy population (n = 32) and the population divided according to HER2 status (high v low). Data Supplement shows patient demographics and clinical characteristics of the entire enrolled population (n = 34) and the population divided according to HER2 status (high v low).
TABLE 1.
Demographic and Baseline Clinical Characteristics in the Efficacy Population
Treatment Characteristics
Approximately half (n = 17) of the patients who received either 6.4 mg/kg or 5.4 mg/kg initially, received up to seven doses, whereas five received ≥14 doses. In the safety population (n = 33), the median (range) cumulative doses were 40.8 (13-194) and 37.8 (11-113) mg/kg in the 6.4 and 5.4 mg/kg groups, respectively. Dose reduction was noted in six (43%) and four (21%) patients in the 6.4 and 5.4 mg/kg groups, respectively. The median (range) treatment durations were 143.0 (42-684) days and 167.0 (42-462) days in the 6.4 and 5.4 mg/kg groups, respectively.
Efficacy
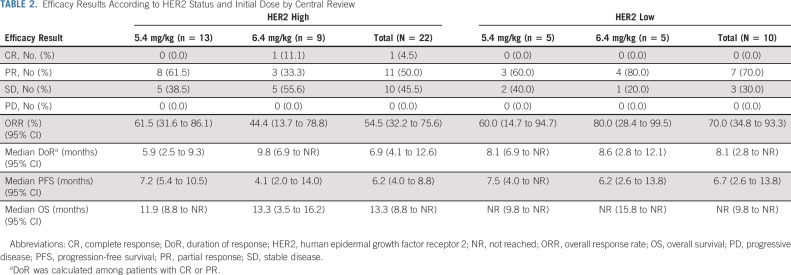
In the HER2-high group (n = 22), the ORR by central review (primary end point) was 54.5% (95% CI, 32.2 to 75.6). The best overall responses were CR, PR, and stable disease in one (4.5%), 11 (50.0%), and 10 patients (45.5%), respectively (Table 2). The number of required responders in the primary analysis exceeded the prespecified number.
TABLE 2.
Efficacy Results According to HER2 Status and Initial Dose by Central Review
The ORR was 68.2% (95% CI, 45.1 to 86.1) in the HER2-high group (n = 22) by investigator assessment. The ORRs in the HER2-low group in an exploratory analysis were 70.0% (95% CI, 34.8 to 93.3) by central review (n = 10) and 60.0% (95% CI, 26.2 to 87.8) by investigator assessment (n = 10). The median DoRs was 6.9 months (95% CI, 4.1 to 12.6) in the HER2-high group (n = 22) and 8.1 months (95% CI, 2.8 to not reached [NR]) in the HER2-low group (n = 10). The best overall responses, ORRs, and DoRs in the HER2-high and HER2-low groups by central review are summarized in Table 2. The disease control rate was 100.0% in the HER2-high and HER2-low groups. Similar results were obtained by investigator assessment (Data Supplement).
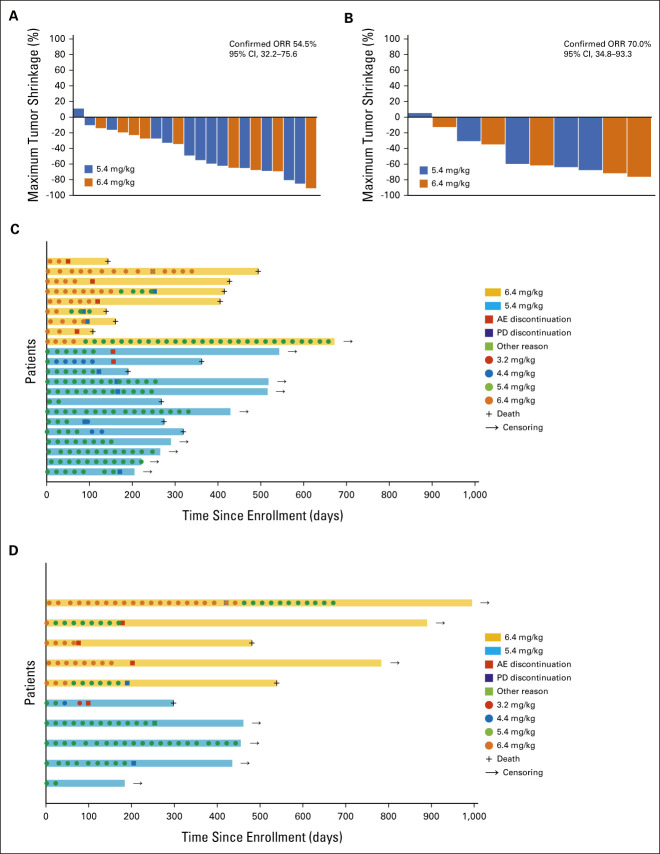
Antitumor activity in terms of maximum tumor shrinkage in the HER2-high and HER2-low groups is shown as waterfall plots in Figures 2A and 2B. Tumor shrinkage was evident in both groups, regardless of whether patients received 6.4 or 5.4 mg/kg as the initial dose. The individual timing of response and durability over time in the HER2-high and HER2-low groups are shown as swimmer plots in Figures 2C and 2D. Changes in tumor diameters from baseline, over time, are shown in the Data Supplement. Rapid tumor shrinkage was observed in patients, regardless of the initial dose.
FIG 2.
Individual antitumor activity, shown as waterfall plots, in the (A) HER2-high (n = 22) and (B) HER2-low (n = 10) groups and individual responses to treatment over time, shown as swimmer plots, in the (C) HER2-high (n = 22) and (D) HER2-low (n = 10) groups. (A) The overall response rate by central review is 54.5% (95% CI, 32.2 to 75.6%) in the HER2-high group. (B) The overall response rate by central review is 70.0% (95% CI, 34.8 to 93.3%) in the HER2-low group. AE, adverse event; HER2, human epidermal growth factor receptor 2; ORR, overall response rate; PD, progressive disease.
The median PFS was 6.7 months (95% CI, 5.4 to 8.8) in the entire efficacy cohort (n = 32), 6.2 months (95% CI, 4.0 to 8.8) in the HER2-high group (n = 22; Data Supplement), and 6.7 months (95% CI, 2.6 to 13.8) in the HER2-low group (n = 10) by central review (Data Supplement). Seventeen patients developed disease progression (77%), and the 6-month and 1-year PFS rates were 54.2% (95% CI, 31.6 to 72.2) and 19.9% (95% CI, 5.5 to 40.9) in the HER2-high group, respectively. The median OS was 15.8 months (95% CI, 10.5 to NR) in the entire efficacy cohort (n = 32), 13.3 months (95% CI, 8.8 to NR) in the HER2-high group (n = 22; Data Supplement), and NR (95% CI, 9.8 to NR) in the HER2-low group (n = 10; Data Supplement). Thirteen patients died (59.1%), and the 6-month and 1-year OS rates were 81.8% (95% CI, 58.5 to 92.8) and 54.2% (95% CI, 29.8 to 73.4) in the HER2-high group, respectively.
The results of the subgroup analyses of the ORR in the HER2-high group on the basis of central review are presented in the Data Supplement. Although these are difficult to interpret because of the small sample size, no major subgroup differences in the efficacy of T-DXd were noted.
Safety
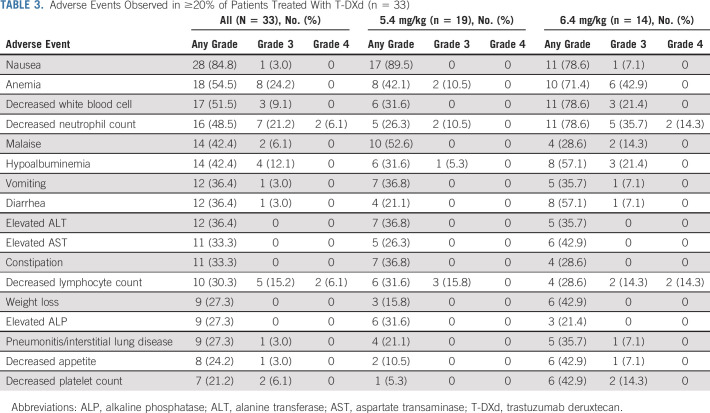
In the safety population, AEs, adverse drug reactions, and serious AEs occurred in all 33, 32 (97%), and 10 (30%) patients, respectively (Data Supplement). AEs that occurred in ≥ 20% of patients are listed in Table 3. Overall specific AEs and specific AEs by grade are listed in the Data Supplement. The most common AEs were nausea (28 [85%]), anemia (18 [55%]), and decreased white blood cell count (17 [52%] patients). Grade ≥ 3 AEs occurred in 20 patients (61%), with the most common being decreased neutrophil count (nine [27%]), anemia (eight [24%]), and decreased lymphocyte count (seven [21%] patients). Grade ≥ 3 AEs were more common in patients who received an initial dose of 6.4 mg/kg than in those who received an initial dose of 5.4 mg/kg (13 [93%] and seven [37%] patients, respectively). No deaths related to AEs were recorded.
TABLE 3.
Adverse Events Observed in ≥20% of Patients Treated With T-DXd (n = 33)
Adverse events leading to drug interruption, dose reduction, and drug withdrawal occurred in 13 (39%), three (9%), and 11 (33%) patients, respectively, are presented in the Data Supplement. The most common AE leading to drug withdrawal was pneumonitis/ILD (six [18%] patients), followed by malaise (two [6%] patients). Pneumonitis/ILD occurred in nine (27%) patients in the safety cohort; five (36%) and four (21%) patients received an initial dose of 6.4 mg/kg and 5.4 mg/kg, respectively. These events were mainly grade 1 or 2 (four patients each in the 6.4 mg/kg [29%] and 5.4 mg/kg [21%] groups); one patient (7%) in the 6.4 mg/kg group had grade 3 pneumonitis/ILD. No pneumonitis/ILD events were adjudicated by an independent committee.
DISCUSSION
T-DXd demonstrated promising antitumor activity in patients with both HER2-high and HER2-low UCS. The ORRs in the HER2-high and HER2-low groups were 54.5% (61.5% and 44.4% in the 5.4 and 6.4 mg/kg groups) and 70.0% (60.0% and 80.0% in the 5.4 and 6.4 mg/kg groups), respectively. The median DoRs in the HER2-high and HER2-low groups were 6.9 and 8.1 months, respectively. The median PFS and OS in the HER2-high group were 6.2 and 13.3 months while those in the HER2-low group were 6.7 months and NR, respectively. There seemed to be no apparent difference in the DoRs, PFS, and OS between the HER2-high and low groups.
These efficacies exceeded those of other agents examined in clinical trials for recurrent UCS. A recent systematic review of second- or later-line therapies for recurrent or treatment-refractory UCS, such as a dual PI3K/mTOR inhibitor, pazopanib, and imatinib, reported a median response rate of only 5.5%, a median PFS of only 2 months, and an OS ranging from 3.2 to 8.7 months.1 Additionally, a phase II study of topotecan, a topoisomerase I inhibitor, in the same class of drugs as the payload of T-DXd, showed a response rate of 10% but also strong toxicity in patients with UCS.28 Recent molecular studies have identified mutations or amplifications in multiple pathways in UCS, including mitogen-activated protein kinase, EGFR, and HER2 signaling. Results of these ongoing studies led to clinical trials for UCS, and emergence of new treatment for UCS is awaited.1
T-DXd has some efficacy in HER2-low breast cancer and other HER2-expressing cancers.16,29-31 The DESTINY-Breast04 and DAISY trials recently showed the efficacy of T-DXd in HER2-low breast cancer and HER2-low/undetected (IHC < 1%) breast cancer, respectively.19,32 Although we assessed efficacy in only 10 patients with HER2-low UCS, we observed a promising efficacy in the HER2-low and HER2-high groups. Unfortunately, we did not include patients without detectable HER2 expression. The reason for the efficacy of T-DXd for HER2-low UCS may be explained partially through a bystander effect of T-DXd on tumors with heterogeneous HER2 expression, which has been shown in in vitro and in vivo experiments.14,33 Furthermore, our coclinical study using patient-derived xenografts from patients with UCS with low HER2 expression who participated in this study demonstrated the antitumor activity of T-DXd.34 These findings suggest that T-DXd may have potential as treatment for UCS.
We evaluated HER2 IHC on the basis of the criteria for gastric cancer in this study.22 Rottmann et al recently proposed a new HER2 scoring criterion for UCS on the basis of the criteria for breast cancer14 but allowed for lateral/basolateral staining patterns rather than requiring circumferential membranous staining patterns. However, their criteria had not been published at trial initiation. The criteria we adopted are not notably different from those of Rottmann et al.14
Adverse events occurred in all patients, and serious AEs occurred in approximately one third of patients. However, there were no deaths due to AEs. Grade ≥ 3 AEs were more common in patients who received an initial dose of 6.4 mg/kg (13 [93%] patients) than in those who received an initial dose of 5.4 mg/kg (seven [37%] patients). New research focusing on the mechanism of AEs due to T-DXd is warranted. Pneumonitis/ILD is already known as an AE of T-DXd,35,36 and nine (27%) patients had pneumonitis/ILD, primarily grade 1 or 2 (24%). One patient (3%) who initially received 6.4 mg/kg T-DXd had grade 3 pneumonitis/ILD. Pneumonitis/ILD is considered clinically acceptable with appropriate monitoring and early intervention.36
This study showed a relatively higher incidence (27%) of pneumonitis/ILD compared with a previously reported pooled analysis of clinical trials for T-DXd (10%-15%).35 However, a similar high incidence (30%) of pneumonitis/ILD was also reported in Japanese patients with HER2-positive breast cancer treated with T-DXd.37 Ethnicity was discussed as one of the factors of interest potentially associated with ILD on the basis of a pooled analysis of clinical trials for T-DXd.35 Ethnicity, including Japanese or non-Japanese, should be discussed as factors potentially associated with ILD.38
In this study, T-DXd at doses of 5.4 and 6.4 mg/kg had promising efficacy in patients with HER2-expressing UCS. The efficacy result for each dose was based on a limited number of patients; however, the study results suggest that T-DXd is a potential treatment option for patients with UCS, for whom few treatment options are available. The safety results showed higher incidence of grades ≥ 3 AEs and pneumonitis/ILD in the 6.4 mg/kg group. Therefore, given the benefits and risks of treatment with T-DXd in this study, 5.4 mg/kg would be a preferred dose.
The key limitations of the present study are the open-label design, enrollment of only Japanese patients, and mixture of results based on the two initial doses. We evaluated a relatively small sample size; however, the predefined threshold of more than four responders among 22 treated patients was exceeded.
In conclusion, the results of this study suggest that T-DXd is effective in patients with both HER2-high and HER2-low advanced or recurrent UCS who were previously treated with chemotherapy. Therefore, T-DXd has the potential to become a novel treatment for UCS.
ACKNOWLEDGMENT
We thank all the patients, physicians, and clinical research coordinators especially Mari Takahashi who participated in this study. We thank Mark Snape, MBBS, CMPP of inScience Communications, Springer Healthcare, for writing the outline and the first draft of the manuscript.
Tadaaki Nishikawa
Honoraria: AstraZeneca, Eisai, Chugai/Roche, Takeda, MSD, Taiho Pharmaceutical
Consulting or Advisory Role: Eisai
Speakers' Bureau: AstraZeneca, Eisai, Chugai/Roche, Takeda, MSD, Taiho Pharmaceutical
Research Funding: Daiichi Sankyo/Astra Zeneca (Inst)
Kosei Hasegawa
Honoraria: MSD K.K, Daiichi Sankyo, Chugai Pharma, AstraZeneca, Eisai, Kyowa Kirin, Takeda, Sanofi
Consulting or Advisory Role: MSD K.K, Kaken Pharmaceutical, Daiichi Sankyo, Roche, Genmab, Takeda, Sanofi
Research Funding: Ono Pharmaceutical, Daiichi Sankyo, Merck
Koji Matsumoto
Honoraria: Chugai Pharma, Kyowa Hakko Kirin, Eisai, MSD K.K, Lilly Japan, Taiho Oncology, Pfizer, Takeda, Bayer Yakuhin
Research Funding: Ono Pharmaceutical (Inst), MSD (Inst), AstraZeneca (Inst), Novartis (Inst), Chugai Pharma (Inst), Eisai (Inst), Daiichi Sankyo/UCB Japan, Lilly Japan
Yasuyuki Hirashima
Speakers' Bureau: AstraZeneca, Takeda, Intutive, Chugai Pharma, Eisai, MSD, TERUMO, Pfizer
Kazuhiro Takehara
Honoraria: Takeda, AstraZeneca, Eisai, Chugai Pharma, MSD, Mochida Pharmaceutical Co Ltd, Fuji Pharma, Taiho Pharmaceutical, AbbVie, Nippon Kayaku, Zeria Pharmaceutical
Research Funding: Chugai Pharma
Shigehiro Yagishita
Speakers' Bureau: LSI medience corp
Patents, Royalties, Other Intellectual Property: Glycosylation analysis of antibody drugs
Sawako Tomatsuri
Employment: AstraZeneca
Akinobu Hamada
Research Funding: CIMIC pharma Science, Konica Minolta, Tosoh Corporation, Chordia Therapeutics, Healios, LSI Medience, Lilly, Chugai Pharma, Eisai, Sysmex, Boehringer Ingelheim
Akihiro Hirakawa
Consulting or Advisory Role: Ono Pharmaceutical, Astellas Pharma, Kissei Pharmaceutical, Nippon Shinyaku, Kyowa Kirin International, Chugai Pharma, AbbVie, Novartis
Speakers' Bureau: Chugai Pharma
Kenichi Nakamura
Honoraria: Chugai Pharma, Taiho Pharmaceutical, IQvia, AstraZeneca, Lilly
Research Funding: Astellas Pharma (Inst), Eisai (Inst), Otsuka (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Chugai/Roche (Inst), Novartis (Inst), Pfizer (Inst), Bristol Myers Squibb Japan (Inst), Boehringer Ingelheim Seiyaku (Inst), SymBio Pharmaceuticals (Inst), Merck (Inst), SERVIER (Inst)
Kan Yonemori
Honoraria: Eisai, Pfizer, AstraZeneca, Novartis, Taiho Pharmaceutical, Lilly Japan, Daiichi Sankyo/Astra Zeneca, Takeda, Fujifilm, Ono Pharmaceutical, Chugai Pharma, MSD Oncology
Consulting or Advisory Role: Chugai Pharma, Ono Pharmaceutical, Novartis, Eisai, OncXerna Therapeutics
Research Funding: Ono Pharmaceutical (Inst), MSD (Inst), Daiichi Sankyo/Astra Zeneca (Inst), AstraZeneca/MedImmune (Inst), Taiho Pharmaceutical (Inst), Pfizer (Inst), Novartis (Inst), Takeda (Inst), Chugai Pharma (Inst), Sanofi (Inst), Seattle Genetics (Inst), Eisai (Inst), Lilly (Inst), Genmab (Inst), Boehringer Ingelheim (Inst), Kyowa Hakko Kirrin (Inst), Haihe Pharmaceutical (Inst), Nihonkayaku (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The National Cancer Center, Japan, had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
PRIOR PRESENTATION
Presented at the European Society for Medical Oncology (ESMO) Congress 2021, virtual, Paris, France, September 16-21, 2021.
SUPPORT
Supported by the Japan Agency for Medical Research and Development (AMED) under Grant No. JP16lk0201044 and then by Daiichi Sankyo Co, Ltd. Trastuzumab deruxtecan was provided by Daiichi Sankyo. Daiichi Sankyo had the opportunity to review the final manuscript but did not have the right to prevent publication.
CLINICAL TRIAL INFORMATION
umin.ac.jp/ctr Identifier: UMIN000029506
T.N. and K.H. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Tadaaki Nishikawa, Kosei Hasegawa, Yasuyuki Hirashima, Tomoyasu Kato, Shigehiro Yagishita, Akinobu Hamada, Mamiko Kawasaki, Hiroshi Yoshida, Akihiro Hirakawa, Kenichi Nakamura, Kan Yonemori
Administrative support: Sawako Tomatsuri
Provision of study materials or patients: Koji Matsumoto, Masahiko Mori, Kazuhiro Takehara
Collection and assembly of data: Kosei Hasegawa, Koji Matsumoto, Masahiko Mori, Yasuyuki Hirashima, Kazuhiro Takehara, Kazuya Ariyoshi, Tomoyasu Kato, Shigehiro Yagishita, Akinobu Hamada, Mamiko Kawasaki, Sawako Tomatsuri, Yukari Nagasaka, Hiroshi Yoshida, Akihiro Hirakawa
Data analysis and interpretation: Tadaaki Nishikawa, Kosei Hasegawa, Koji Matsumoto, Yasuyuki Hirashima, Tomoyasu Kato, Shigehiro Yagishita, Akinobu Hamada, Mamiko Kawasaki, Satoshi Kawashima, Hiroshi Yoshida, Ryunosuke Machida, Akihiro Hirakawa, Kenichi Nakamura, Kan Yonemori
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2–Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tadaaki Nishikawa
Honoraria: AstraZeneca, Eisai, Chugai/Roche, Takeda, MSD, Taiho Pharmaceutical
Consulting or Advisory Role: Eisai
Speakers' Bureau: AstraZeneca, Eisai, Chugai/Roche, Takeda, MSD, Taiho Pharmaceutical
Research Funding: Daiichi Sankyo/Astra Zeneca (Inst)
Kosei Hasegawa
Honoraria: MSD K.K, Daiichi Sankyo, Chugai Pharma, AstraZeneca, Eisai, Kyowa Kirin, Takeda, Sanofi
Consulting or Advisory Role: MSD K.K, Kaken Pharmaceutical, Daiichi Sankyo, Roche, Genmab, Takeda, Sanofi
Research Funding: Ono Pharmaceutical, Daiichi Sankyo, Merck
Koji Matsumoto
Honoraria: Chugai Pharma, Kyowa Hakko Kirin, Eisai, MSD K.K, Lilly Japan, Taiho Oncology, Pfizer, Takeda, Bayer Yakuhin
Research Funding: Ono Pharmaceutical (Inst), MSD (Inst), AstraZeneca (Inst), Novartis (Inst), Chugai Pharma (Inst), Eisai (Inst), Daiichi Sankyo/UCB Japan, Lilly Japan
Yasuyuki Hirashima
Speakers' Bureau: AstraZeneca, Takeda, Intutive, Chugai Pharma, Eisai, MSD, TERUMO, Pfizer
Kazuhiro Takehara
Honoraria: Takeda, AstraZeneca, Eisai, Chugai Pharma, MSD, Mochida Pharmaceutical Co Ltd, Fuji Pharma, Taiho Pharmaceutical, AbbVie, Nippon Kayaku, Zeria Pharmaceutical
Research Funding: Chugai Pharma
Shigehiro Yagishita
Speakers' Bureau: LSI medience corp
Patents, Royalties, Other Intellectual Property: Glycosylation analysis of antibody drugs
Sawako Tomatsuri
Employment: AstraZeneca
Akinobu Hamada
Research Funding: CIMIC pharma Science, Konica Minolta, Tosoh Corporation, Chordia Therapeutics, Healios, LSI Medience, Lilly, Chugai Pharma, Eisai, Sysmex, Boehringer Ingelheim
Akihiro Hirakawa
Consulting or Advisory Role: Ono Pharmaceutical, Astellas Pharma, Kissei Pharmaceutical, Nippon Shinyaku, Kyowa Kirin International, Chugai Pharma, AbbVie, Novartis
Speakers' Bureau: Chugai Pharma
Kenichi Nakamura
Honoraria: Chugai Pharma, Taiho Pharmaceutical, IQvia, AstraZeneca, Lilly
Research Funding: Astellas Pharma (Inst), Eisai (Inst), Otsuka (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Chugai/Roche (Inst), Novartis (Inst), Pfizer (Inst), Bristol Myers Squibb Japan (Inst), Boehringer Ingelheim Seiyaku (Inst), SymBio Pharmaceuticals (Inst), Merck (Inst), SERVIER (Inst)
Kan Yonemori
Honoraria: Eisai, Pfizer, AstraZeneca, Novartis, Taiho Pharmaceutical, Lilly Japan, Daiichi Sankyo/Astra Zeneca, Takeda, Fujifilm, Ono Pharmaceutical, Chugai Pharma, MSD Oncology
Consulting or Advisory Role: Chugai Pharma, Ono Pharmaceutical, Novartis, Eisai, OncXerna Therapeutics
Research Funding: Ono Pharmaceutical (Inst), MSD (Inst), Daiichi Sankyo/Astra Zeneca (Inst), AstraZeneca/MedImmune (Inst), Taiho Pharmaceutical (Inst), Pfizer (Inst), Novartis (Inst), Takeda (Inst), Chugai Pharma (Inst), Sanofi (Inst), Seattle Genetics (Inst), Eisai (Inst), Lilly (Inst), Genmab (Inst), Boehringer Ingelheim (Inst), Kyowa Hakko Kirrin (Inst), Haihe Pharmaceutical (Inst), Nihonkayaku (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Matsuzaki S, Klar M, Matsuzaki S, et al. : Uterine carcinosarcoma: Contemporary clinical summary, molecular updates, and future research opportunity. Gynecol Oncol 160:586-601, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Jonson AL, Bliss RL, Truskinovsky A, et al. : Clinical features and outcomes of uterine and ovarian carcinosarcoma. Gynecol Oncol 100:561-564, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Zhao S, Bellone S, Lopez S, et al. : Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition. Proc Natl Acad Sci USA 113:12238-12243, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell MA, Filiaci VL, Hensley ML, et al. : Randomized phase III trial of paclitaxel and carboplatin versus paclitaxel and ifosfamide in patients with carcinosarcoma of the uterus or ovary: An NRG oncology trial. J Clin Oncol 40:968-977, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos SM, Brady WE, Moxley KM, et al. : A phase II evaluation of pazopanib in the treatment of recurrent or persistent carcinosarcoma of the uterus: A gynecologic oncology group study. Gynecol Oncol 133:537-541, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebata T, Yonemori K, Nishikawa T, et al. : Treatment outcome of second-line chemotherapy for gynecologic carcinosarcoma. Oncology 98:699-705, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Huh WK, Sill MW, Darcy KM, et al. : Efficacy and safety of imatinib mesylate (Gleevec) and immunohistochemical expression of c-Kit and PDGFR-beta in a Gynecologic Oncology Group Phase Il Trial in women with recurrent or persistent carcinosarcomas of the uterus. Gynecol Oncol 117:248-254, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Guzzo F, Bellone S, Buza N, et al. : HER2/neu as a potential target for immunotherapy in gynecologic carcinosarcomas. Int J Gynecol Pathol 31:211-221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicoletti R, Lopez S, Bellone S, et al. : T-DM1, a novel antibody-drug conjugate, is highly effective against uterine and ovarian carcinosarcomas overexpressing HER2. Clin Exp Metastasis 32:29-38, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwab CL, English DP, Black J, et al. : Neratinib shows efficacy in the treatment of HER2 amplified carcinosarcoma in vitro and in vivo. Gynecol Oncol 139:112-117, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diver EJ, Foster R, Rueda BR, et al. : The therapeutic challenge of targeting HER2 in endometrial cancer. Oncologist 20:1058-1068, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amant F, Vloeberghs V, Woestenborghs H, et al. : ERBB-2 gene overexpression and amplification in uterine sarcomas. Gynecol Oncol 95:583-587, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Livasy CA, Reading FC, Moore DT, et al. : EGFR expression and HER2/neu overexpression/amplification in endometrial carcinosarcoma. Gynecol Oncol 100:101-106, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Rottmann D, Snir OL, Wu X, et al. : HER2 testing of gynecologic carcinosarcomas: Tumor stratification for potential targeted therapy. Mod Pathol 33:118-127, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Ogitani Y, Hagihara K, Oitate M, et al. : Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 107:1039-1046, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modi S, Saura C, Yamashita T, et al. : Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382:610-621, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shitara K, Bang YJ, Iwasa S, et al. : Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 382:2419-2430, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Modi S, Park H, Murthy RK, et al. : Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: Results from a phase Ib study. J Clin Oncol 38:1887-1896, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modi S, Jacot W, Yamashita T, et al. : Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387:9-20, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.gov Clinicaltrials : Study of Trastuzumab Deruxtecan (T-DXd) vs Investigator's Choice Chemotherapy in HER2-low, Hormone Receptor positive, Metastatic Breast Cancer (DB-06), 2022. https://clinicaltrials.gov/ct2/show/NCT04494425 [Google Scholar]
- 21.gov Clinicaltrials : Trastuzumab Deruxtecan Alone or in Combination with Anastrozole for the Treatment of Early Stage HER2 Low, Hormone Receptor Positive Breast Cancer, 2022. https://clinicaltrials.gov/ct2/show/NCT04553770 [Google Scholar]
- 22.Bartley AN, Washington MK, Colasacco C, et al. : HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: Guideline from the College of American Pathologists, American Society for clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol 35:446-464, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Yoshida H, Nishikawa T, Matsumoto K, et al. : Histopathological features of HER2 overexpression in uterine carcinosarcoma: Proposal for requirements in HER2 testing for targeted therapy. Virchows Arch 478:1161-1171, 2021 [DOI] [PubMed] [Google Scholar]
- 24.Wolff AC, Hammond MEH, Hicks DG, et al. : Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of clinical oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997-4013, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Thall PF, Simon R: Practical Bayesian guidelines for phase IIB clinical trials. Biometrics 50:337-349, 1994 [PubMed] [Google Scholar]
- 26.McMeekin DS, Sill MW, Darcy KM, et al. : A phase II trial of thalidomide in patients with refractory uterine carcinosarcoma and correlation with biomarkers of angiogenesis: A Gynecologic Oncology Group Study. Gynecol Oncol 127:356-361, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Miller BE, Blessing JA, Stehman FB, et al. : A phase II evaluation of weekly gemcitabine and docetaxel for second-line treatment of recurrent carcinosarcoma of the uterus: A gynecologic oncology group study. Gynecol Oncol 118:139-144, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Miller DS, Blessing JA, Schilder J, et al. : Phase II evaluation of topotecan in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecol Oncol 98:217-221, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Tsurutani J, Iwata H, Krop I, et al. : Targeting HER2 with trastuzumab deruxtecan: A dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov 10:688-701, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa K, Nagasaka M, Felip E, et al. : OA04.05 Trastuzumab deruxtecan in HER2-overexpressing metastatic non-small cell lung cancer: Interim results of DESTINY-Lung01. J Thorac Oncol 16:S109-S110, 2021 [Google Scholar]
- 31.Siena S, Di Bartolomeo M, Raghav K, et al. : Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol 22:779-789, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Diéras V, Deluche E, Lusque A, et al. : Abstract PD8-02: Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY). Cancer Res 82:PD8-02, 2022 [Google Scholar]
- 33.Suzuki M, Yagishita S, Sugihara K, et al. : Visualization of intratumor pharmacokinetics of [fam-] trastuzumab deruxtecan (DS-8201a) in HER2 heterogeneous model using phosphor-integrated dots imaging analysis. Clin Cancer Res 27:3970-3979, 2021 [DOI] [PubMed] [Google Scholar]
- 34.Yagishita S, Nishikawa T, Yoshida H, et al. : 1767P Co-clinical PDX study of trastuzumab deruxtecan in HER2-positive uterine carcinosarcoma (STATICE trial, NCCH1615). Ann Oncol 32:S1213-S1214, 2021 [Google Scholar]
- 35.Swain SM, Nishino M, Lancaster LH, et al. : Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-Focus on proactive monitoring, diagnosis, and management. Cancer Treat Rev 106:102378, 2022 [DOI] [PubMed] [Google Scholar]
- 36.Powell CA, Modi S, Iwata H, et al. : Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 7:100554, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ministry of Health, Labour and Welfare : Report on the Deliberation Results https://www.pmda.go.jp/files/000238706.pdf [Google Scholar]
- 38.Saito S, Lasky JA, Hagiwara K, et al. : Ethnic differences in idiopathic pulmonary fibrosis: The Japanese perspective. Respir Invest 56:375-383, 2018 [DOI] [PubMed] [Google Scholar]