Abstract
Radiopharmaceutical therapy (RPT) is an invigorated form of cancer therapy that systemically delivers targeted radioactive drugs to cancer cells. Theranostics is a type of RPT that utilizes imaging, either of the RPT drug directly or a companion diagnostic, to inform whether a patient will benefit from the treatment. Given the ability to image the drug onboard theranostic treatments also lends itself readily to patient-specific dosimetry, which is a physics-based process that determines the overall absorbed dose burden to healthy organs and tissues and tumors in patients. While companion diagnostics identify who will benefit from RPT treatments, dosimetry determines how much activity these beneficiaries can receive to maximize therapeutic efficacy. Clinical data is starting to accrue suggesting tremendous benefits when dosimetry is performed for RPT patients. RPT dosimetry, which was once performed by florid and often inaccurate workflows, can now be performed more efficiently and accurately with FDA-cleared dosimetry software. Therefore, there is no better time for the field of oncology to adopt this form of personalize medicine to improve outcomes for cancer patients.
The concept of personalized medicine dates to the writings of Hippocrates around 400 BC. In the Corpus Hippocraticum the philosopher writes “Give different ones [therapeutic drinks] to different patients, for the sweet ones do not benefit everyone, nor do the astringent ones, nor are all patients able to drink the same things” (Peq9i mot9rxm C/De morbis III,17.6–8, translation by Potter, v. VI, p. 57). In this ancient passage the philosopher clearly warns against a “one size fits all” approach when caring for sick patients – some patients will benefit from a drug while some won’t and the amount of drug a patient can tolerate will vary between patients. But despite a palpable allegiance to Hippocratic thinking, it is time to ask if modern medicine has heeded to Hippocrates warnings particularly in the context of treating cancer patients two millennia following his observations. Until recently, one could make a solid case arguing that it has mostly not. The amount of drug delivered in chemotherapy, immunotherapy, and targeted therapies is often fixed between patients and this among is defined by achieving clinical trial endpoints in a population thereby neglecting interpatient and intrapatient variability. However, there is a growing interest in delivering “the right drug for the right patient at the right dose at the right time”. This is driven in large part by the renaissance occurring in the field of radiopharmaceutical therapy (RPT).
Radiopharmaceutical therapy is a type of radiation therapy that combines tumor-targeting vectors and radioisotopes to deliver cytotoxic payloads to cancers in the body(1–3). In contrast to more common radiotherapy delivery (e.g. external beam radiotherapy) where radiation is delivered from outside of the body, RPT delivers cytotoxic radiation directly to the cancers or their microenvironment following the systemic or localized delivery of the agent in the blood. Unlike EBRT, RPT is an attractive option for treating widespread metastatic disease. Notably, when used in combination with other treatment modalities, RPT has demonstrated efficacy with acceptable toxicity compared to nearly all other systemic treatment options(3,4).
RPT drugs have a unique property that makes them highly amenable to personized medicine which is that the spatio-temporal distribution of the radioactive drug in patients can be imaged after delivery or via surrogate imaging approaches prior to delivery. The portmanteau word ‘theranostics’ has been coined to describe this integration or targeted therapeutics (thera-) with analogue diagnostic (-nostics)(5). Theranostics has fueled the renaissance in RPT over the past two decades. Theranostic describes the use of a representative biomarker, typically imaged with PET/CT or SPECT/CT, to screen patients or to inform patient-specific delivery of RPT drugs. In the case of screening patients, the most apparent candidates for theranostic applications of RPT involves pairing biochemically identical isotopes for PET/CT imaging and therapy, such as 123I/124I/131I, 68Ga/177Lu, and 86Y/90Y(1). Another approach is to use a trace amount of the therapeutic drug that can be imaged with SPECT/CT. To provide patient-specific adjustment of the amount of drug delivered the biodistribution of the drug can be imaged using SPECT/CT at a single timepoint or multiple timepoints following the delivery of a treatment cycle and the subsequent cycle or cycle(s) can be adjusted accordingly. Patient-specific adjustments of drug amounts is primarily determined by the process of dosimetry. The ability to use patient-specific dosimetry as a predictive biomarker of treatment response has improved patient outcomes and revolutionized the field of personalized medicine.
Overview of Current RPT Treatments
There are several RPT drugs that have been FDA approved for a variety of different types of cancer. β-emitters are a common type of radionuclide used for RPT including 131I, 90Y, and 177Lu. Radioactive iodine (RAI) therapy has been used for nearly a century to treat thyroid cancer and other thyroid disorders(6). Radioactive iodine (RAI) therapy targets the sodium iodine symporter (NIS) which is highly expressed in differentiated thyroid cancers. The transmembrane sodium gradient across the NIS serves as the major driving force of sodium iodide uptake, which is produced and maintained by the sodium-potassium pump. The sodium gradient allows for remarkable uptake and localization of radioactive iodine into thyroid follicular cells and thyroid cancer cells. In fact, the iodine concentration gradient from the thyroid cell to extracellular fluid is greater than 20:1(7). In addition to Na131I for therapy, both Na123I and Na124I have been used for imaging via SPECT/CT or PET/CT, respectively(8)(9). More recently, newer radiopharmaceutical drugs have been introduced clinically including 177Lu-DOTATATE, 223RaCl2, 177Lu-PSMA, high specific activity (HSA) I131-MIBG. A summary of these four important radiopharmaceuticals is provided in Table 1. 223RaCl2 was approved by the FDA in 2013 for the treatment of castration-resistant prostate cancer (CRPC) and symptomatic bone metastases. 223RaCl2 is an alkaline earth metal that mimics calcium uptake in the bone. Patients with metastatic prostate cancer show increased levels of bone formation and resorption activity by osteoclasts making them good candidates for therapy with calcium mimetics such as 223RaCl2(10). 223Ra emits a total of four alpha particles over a six-stage decay process. These four alpha particles make up about 95% of the total amount of energy released during a 223Ra decay. The alpha particles emitted during the decay have a high linear energy transfer (LET) which means each alpha particle track deposits a significant amount of energy in small volumes making them an efficient generator of DNA double strand breaks. However, alpha particles have a very short range, on the order of 10–100 μm, which limits their damaging potential to 2 to 10 cells. The FDA approval process of 223RaCl2 primarily relied on results from the ALSYMPCA trial, which was a double-blind randomized phase III study for castrate-resistant prostate cancer(11). In this trial, the 223RaCl2 arm had an overall survival of 14.9 months compared to the placebo arm that had 11.3 months. 223RaCl2 was also associated with low myelosuppression rates and fewer adverse events versus the placebo and significant pain relief was documented up to 16 weeks after therapy. The amount of 223RaCl2 activity to prescribe to a patient is 50 kBq/kg body weight, given every 4 weeks for 6 administrations.
Table 1:
OverView of clinical RPT drugs.
| Drug | Trade Name | Manufact urer | Dosing | Companion Diagnostic | Indication | Type of Emitter | Secondary Particles | Dose Limiting Organs/Tissues | SPECT/CT |
|---|---|---|---|---|---|---|---|---|---|
| Na131| | NA | Varies | Na123| | differentiated thyroid cancer, thyroid diseases | β- | γ (364 keV) | bone marrow, lungs | Yes | |
| 177Lu-DOTATATE | Lutathera® | Novartis | 7.4 MBq every 8 weeks x 4 administrations | 68Ga-DOTATATE (NETSPOT®) | GEP-NETs | β- | γ (208 keV) | bone marrow, kidneys | Yes |
| 223RaCl2 | Xofigo® | Bayer | 50 kBq/kg body weight every 4 weeks x 6 administrations | NA | CRPC | α | γ (82, 154 and 270 keV) | bone marrow | Yes |
| 177Lu-PSMA-617 | Pluvicto™ | Novartis | 7.4 MBq every 6 weeks x 6 administrations | 68Ga-gozetotide (LOCAMETZ®) | CRPC | β- | γ (208 keV) | bone marrow, kidneys, lacrimal glands, salivary glands | Yes |
| 131|-mibg Standard | 123|-mibg | Neuroblastoma, pheochromocytoma, paraganglioma, carcinoidtumors, medullarythyroid cancer | β- | γ (364 keV) | Bone marrow, kidneys, liver | Yes | |||
| 131|-MIBG High Specific Activity | Azedra® | Patients > 62.5 kg: 18,500 MBq Patients < 62.5: 296 MBq/kg * | Pheochromocytoma or paraganglioma | β- | γ (364 keV) | Bone marrow, kidneys, liver | Yes |
177Lu-DOTATATE received FDA-approval in 2018 to treat neuroendocrine tumors that affect the pancreas or gastrointestinal tract, which are known as GEP-NETs. GEP-NETs are the most common subtype of well-differentiated NETs, making up more than 70% of these cancers(12–15). 177Lu-DOTATATE targets somatostatin receptors in GEP-NETs. 177Lu-DOTATATE consists of a somatostatin agonist (SSA), a chelator DOTA, and the 177Lu isotope(16). The companion diagnostic to 177Lu-DOTATATE is 68Ga-DOTATATE that is used to screen for avid patient candidates for the treatment. The FDA approval of 177Lu-DOTATATE was based on findings from the randomized international phase III NETTER-1 trial as well as outcome data from a large European registry. The NETTER-1 trial showed progression-free survival at 20 months was 65.2% (95% confidence interval (CI), 50.0 to 76.8) in the 177Lu-DOTATATE group compared to 10.8% (95% CI, 3.5 to 23.0) in the control group and a response rate of 18% in the 177Lu-DOTATATE group compared to 3% in the control group (p < 0.001)(17). The most common adverse events from 177Lu-DOTATATE were nausea and vomiting. Grade 3/4 hematologic events including lymphopenia, thrombocytopenia, and anemia occurred in 9%, 2%, and 1% of patients, respectively. There was no evidence of renal toxic events during the 20-month observation period. The amount of 177Lu-DOTATATE activity to prescribe to a patient is 7.4 GBq given every 8 weeks for 4 administrations.
Meta-iodo-benzyl-guanidine (mIBG) is a norepinephrine analog. mIBG labelled with 131I has been used for decades to treat neuroblastoma and other pediatric tumors(18). Given its long history standard 131I-MIBG does not have FDA clearance and therefore is prescribed by physicians as an investigational drug for compassionate treatment (19). More recently, a new form of 131I-mIBG has been developed with a very high specific activity (i.e. the amount of activity per unit mass of the drug), HSA 131I-MIBG(20). In standard 131I-MIBG preparations only 1% of the mIBG molecules are labeled with 131I (123.3 MBq/mg) whereas in HSA 131I-MIBG nearly all of the mIBG is labeled with 131I (92,500 MBq/mg)(20). HSA 131I-MIBG received FDA approval in 2018 to treat patients with locally advanced or metastatic pheochromocytoma or paragangliomas who require systemic anticancer therapy. Bone marrow suppression and renal toxicities are a concern for patients undergoing either standard 131I-MIBG or HSA 131I-MIBG. For example, of 88 patients in the HSA 131I-MIBG clinical trial 33% experienced Grade 4 thrombocytopenia, 16% experienced Grade 4 neutropenia, and 7% experienced Grade 4 anemia(20). In the same patient population 7% developed renal failure or acute kidney injury(20). One should keep in mind that the prescribed activity for either type of 131I-MIBG is much higher than other RPT drugs since pediatric patients can better tolerate bone marrow suppression and can also receive stem cell support following treatment.
The FDA approved 177Lu-PSMA-617 in 2022 for treatment of CRPCs in patients whose tumors overexpress the transmembrane protein prostate-specific membrane antigen (PSMA). PSMA’s location on the surface of cells makes it very accessible for targeted drugs. PSMA-617 is a PSMA inhibitor that targets the transmembrane protein on cancer cells(21). The FDA also approved the radiotracer 68Ga-gozetotide, which is the companion diagnostic to 177Lu-PSMA-617 and can detect if tumors overexpress the PSMA protein making them good candidates for 177Lu-PSMA-617 therapy. The landmark clinical trial demonstrating safety and efficacy of 177Lu-PSMA-617 in CRPC patients was the randomized phase 3 VISION trial(22). The trial demonstrated that patients who received 177Lu-PSMA-617 plus standard of care extended both overall survival (15.3 months vs 11.3 months) and progression free survival (8.7 months vs. 3.4 months) compared to standard of care alone. The incidence of grade 3/4 adverse events was higher in the 177Lu-PSMA-617 arm, but quality of life was not adversely affected. Interestingly, the amount of activity given to patients during each cycle is 7.4 GBq matching that of 177Lu-DOTATATE which is targeting an entirely different tumor. This activity is given every 6 weeks for up to 6 administrations, or until disease progression, or unacceptable toxicity.
There are a few takeaways from the preceding paragraphs. First, it is evident that RPT is becoming a viable treatment option for many different types of late-stage cancers. In some cases, RPT will also replace or be combined with other drugs to treat cancers earlier on during their progression. Second, except for HSA 131I-MIBG, the toxicity profiles measured in patients during RPT trials are much lower than other comparable therapies which indicates that a vast majority of patients clinically are being underdosed. Finally, all the approved RPT drugs can be imaged using SPECT/CT. As a result, these therapies are amenable to patient-specific dosimetry, which can and should be used to tailor the administered activities to each patient to improve clinical outcomes.
Dosimetry
Energy deposited from ionizing radiation to matter occurs through the action of individual charged particles that lose kinetic energy through the excitation or ionization of atoms in discrete and random absorption events. The energy deposition by particles in a volume is most accurately described as a stochastic distribution that fluctuates about a mean value. The specific energy imparted is the random variable which describes energy deposited in matter per unit mass. The absorbed dose is defined as the mean value of the specific energy distribution per unit mass of a specified volume,
where is the mean energy imparted in given mass .
The impact of the stochastic nature of energy deposition depends on the size of the irradiated volume and the particle depositing the energy. Take for example a hypothetical radioactive atom in the center of a cube of water with a volume of 1 cm3 (see Figure 1). If a photon is released during the decay, then it is impossible to predict with certainty that the photon will interact with an atom in the volume and deposit energy. If more radioactive atoms are allowed to decay in the volume one gains more confidence about the average behavior of energy deposition from these photons in the volume. Alternatively, at this scale, if a beta particle or alpha particle of a given energy is emitted one can all but guarantee that complete energy deposition will occur inside the volume. Adding additional radioactive atoms does little to diminish the confidence of knowing how much energy will be deposited from each decay because it is nearly always the same. However, if the irradiated volume in our example is allowed to get very small, the stochastic fluctuation of charged particle interactions and local energy deposition in the volume becomes relevant and the specific energy for given events begin to deviate substantially from the absorbed dose. Analogous to photons at larger scales, understanding the behavior of the individual particle tracks depositing energy at these small scales becomes important especially since these are scales at which biological damage occur. In fact, this behavior helps to explain why alpha particles cause more biological damage than beta particles for the same absorbed dose. Despite requiring less tracks through a volume to deposit the same absorbed dose the track structure of alpha particles makes it much more effective at causing biological damage. This behavior is exemplified in detail in Figure 2.
Figure 1:

Illustration that highlights the importance of physical scale when characterizing energy deposition of ionizing radiation. A radioactive source is located at the center of a 1 cm3 cube of water. (a) If the source emits a monoenergetic photon the photon could escape the volume without interacting (path 1) or it could interact in the volume before escaping (path 2). If the source is allowed to decay only a few times then it would be meaningless to determine the average energy deposited in the volume per decay because the sample size is insufficient to account for the average behavior of the particles emitted during a decay. As the number of particles emitted increases one becomes more confident about this average behavior (b) If the source emits a monoenergetic electron the electron could travel in a torturous trajectory and deposit most of its energy near its point of origin or it could travel in a straight trajectory spreading the energy it deposits more evenly throughout the volume (path 2). Regardless, one can all but guarantee that the average energy deposited in the volume per decay will be nearly constant and equal to the original energy of the electron. (c) If the source emits a monoenergetic alpha particle the particle will always deposit its energy very close to its origin and the average energy deposited in the volume per decay will always be the same. However, it is obvious that most of the volume does not experience energy deposition from the particle and the average behavior of the particle might not reflect the physical location and extent energy is deposited in the volume.
Figure 2:

Charge particles cause biological damage by ionizing molecules around or on DNA. Particle tracks that are more densely ionizing are more efficient at creating more damage.
It might be difficult to think of average behaviors described by stochastic processes when discrete physical quantities are measured. For example, when the temperature of air is measured with a thermometer one does not think of the discrete temperature reading as a measurement of the average behavior of the individual molecules striking the instrument even though that is precisely what is measured. In the case of absorbed dose each individual particle in a radiation field deposits energy in a random matter and we can only measure the average behavior of particles of the same type and energy interacting in the same medium. Our confidence in our measurement increases as the number of particles we measure increases. For a field that has particles of different energy and type the total absorbed dose is determined by summing the contributions of the average imparted by each particle and dividing by the mass. Therefore, it is natural and accurate to think of absorbed dose from a field of radiation as the collective sum of individual energy depositions in a volume divided by the mass of that volume.
The absorbed dose is a physical quantity that has been used for several decades in radiation protection, radiation therapy, and nuclear medicine to predict the biological impact of an exposure. The process in which absorbed dose is calculated or measured is known as dosimetry. The field dedicated to characterizing energy deposition at very small scales is called microdosimetry. There is an exhaustive amount of clinical data suggesting that the absorbed dose is the exemplar biomarker for predicting treatment outcomes following radiation therapy and data is emerging suggesting that absorbed dose is similarly predictive for RPT. Dosimetry is used for planning and optimizing every patient treatment in external beam radiation therapy and brachytherapy. Dosimetry will likely soon become an important clinical tool used for delivering personalized RPT(23). As highlighted in Figure 3 dosimetry is a more complex in RPT compared to external beam radiation therapy (EBRT) and brachytherapy due to the variation of particle types seen in RPT and the scales that should be considered to ensure that the dosimetry is performed correctly. However, when certain conditions are met, dosimetry can play an important role in personalizing RPT. A very important condition is that the resolution at which the activity can be imaged in the patient should match the geometrical extent to which the particle can deposit energy. For photons it is their mean free path (i.e. average distance a photon travels before its first collision) and in the case of for beta and alpha particles it is their range. As such, voxel-level dosimetry works well for beta-emitters but might not be as accurate for alpha particles due to their very short ranges.
Figure 3:

The scales of RPT dosimetry. Several ROIs are larger organs that have dimensions in the centimeter range. The activity distributions in these organs are often very heterogenous. Activity can be imaged in patients with ‘voxel’ resolutions in the millimeter range. It is assumed that the activity in these ‘voxels’ is homogeneous and that each cell in the voxel experiences the same burden from particles emitted during radioactive decay. Depending on the distribution of the drug and the type of particle emitted during radioactive decay this assumption may or may not be accurate. Biological effects from particles emitted during radioactive decay happen in the nanometer range.
Evidence that Dosimetry Improves Clinical Outcomes
So far most of the evidence supporting the use of dosimetry in the clinic has come from retrospective analysis of patient data. A seminal study by Wahl demonstrated a large variance in the predicted activity needed to deliver 75 cGy to the whole body from 131I-tositumab in 634 patients after they were imaged using whole body scintigraphy(24). Wahl also showed large variance in activity needed to deliver 10 Gy to the liver in 18 131I-tositumab patients imaged with both scintigraphy and SPECT/CT(25). Note that the bone marrow and liver were the dose limiting organs for this therapy. More recently, Sandstrom evaluated kidney doses in 777 177Lu-DOTATATE patients(26)(27). These patients were imaged with SPECT/CT scans at 1-, 4-, and 7-days post administration. Like previous studies, significant variability in kidney doses was shown even though patients were administered the same activity. In fact, if one used the common EBRT kidney dose limit as a conservative limit for RPT, then 80% of these 177Lu-DOTATATE patients were underdosed.
Recent clinical trials have demonstrated efficacy and safety when patient-specific dosimetry is used for RPT. The prospective P-PRRT Trial used patient-specific dosimetry to limit the absorbed dose to the kidneys from 77Lu-DOTATATE(28). For most of the patients the amount of administered activity was larger than what was used as standard of care allowing increased absorbed doses to the tumors. Also, no severe renal toxicities were reported, and Grade 3 or 4 toxicities occurred in less than 10% of patients apart from lymphocytopenia which occurred in 51% of patients but without clinical consequences. The ILUMINET trial was a single arm phase II trial that was designed to evaluate the safety and efficacy of patient-specific dosimetry(29). Similar to the P-PRRT trial, kidney dosimetry was performed on all patients. All patients were planned for treatment up to a cumulative kidney biologically effective dose (BED) of 27 ± 2 Gy. Patients that met the inclusion criteria were administered enough activity to deliver a renal BED of 40 ± 2 Gy. The trial demonstrated that patient-specific dosimetry is clearly feasible with low toxicity and promising efficacy.
Patient-Specific Dosimetry in RPT
The general procedure for voxel-level patient-specific dosimetry is highlighted in Figure 4. This section covers three dimensional patient specific dosimetry. Other techniques can that used two dimensional planar images or point-wise measurements such as blood draws or whole-body survey meter readings can also been used but are not covered here. The first step in the workflow is to administer the radiopharmaceutical that is going to be imaged. This is often the drug that is used for therapy which is imaged after a treatment cycle is administered. This approach towards patient-specific dosimetry is called peri-treatment dosimetry. However, other approaches include imaging a trace amount of the therapeutic drug or to image the companion diagnostic to the therapeutic drug which is called pre-treatment dosimetry.
Figure 4:

Voxel-level patient-specific dosimetry workflow.
Imaging is performed using nuclear medicine scanners. In the case of peri-treatment dosimetry a SPECT/CT scanner is used to acquire three dimensional images of the activity of the drug in the body. In the case of pre-treatment dosimetry either SPECT/CT or PET/CT imaging can be used depending on the tracer or companion diagnostic being imaged. To better quantify the pharmacokinetic distribution of the drug patients are generally imaged at multiple timepoints that span multiple days post injection. The ideal number of timepoints depends on the physical and biological properties of the drug, but most studies have used 3–4 timepoints as a compromise between accuracy and logistical challenges related to patient scheduling. There has been a recent push to continue to reduce the number of imaging timepoints used in the dosimetry workflow - even down to a single timepoint. These approaches replace patient-specific imaging data with population data to model the pharmacokinetics of the drug. Reducing imaging timepoints can impact the overall accuracy of the dose calculation and make dosimetry less amenable to identifying outliers in the patient population.
Segmentation and registration are important components of the dosimetry workflow. The dose distribution in RPT is dependent on the biological and physical properties of a drug in an ROI so the ROI contours serve to quantify the existing dose distribution. In general, two main segmentation techniques have been used for defining ROIs. Manual anatomical or functional segmentation using either the CT image or the SPECT image is typically done for segmenting normal organ/tissue ROIs. Segmentation using universal or regional thresholding techniques is often used to define tumor ROIs. In the case of segmentation, delineation of an ROI can impact the quantification accuracy of SPECT/CT or PET/CT significantly(30,31). Several variables can impact the accuracy of ROI segmentation and ultimately the predicted dose in the ROI including: (1.) inter-observer variability, (2.) temporal variability over a series of images, (3.) and the choice of a segmentation threshold in the case of segmenting using SPECT thresholding techniques.
RPT dosimetry calculations are often performed by utilizing serial measurements of SPECT/CT or PET/CT images taken days apart to adequately characterize the pharmacokinetic behavior of the RPT drug in patients. Patient motion, inconsistent patient positioning on the imaging couch, and organ deformation will likely occur between or during imaging sessions causing misalignment in the image data sets between timepoints. Correct registration of these serial images is important to support accurate and precise dosimetry calculations in tumors and organs of interest. Misalignment of the serial SPECT images with a translational error of less than 9.5 mm and rotation error of less than 6° could lead to absorbed dose errors up to 90%(32). Multi-timepoint dosimetry should utilize a combination of either whole-body or piece-wise rigid registration followed by deformable registration. Most commercial dosimetry products have the capabilities to perform these operations.
The cumulative activity in an ROI or voxels within an ROI is calculated by fitting either the activity concentration over the multiple timepoints and then integrating the fit over the time post-administration. Different fitting methods can be used depending on the behavior of the drug, and can be utilized to calculate absorbed dose distributions. In most cases the pharmacokinetic behavior of an RPT drug in an organ or tumor can be modelled by the combination of exponential functions representing the uptake or elimination of the drug over time. Several different exponential models can be used to fit activity concentrations or dose rates as a function of time. Another approach is to use the trapezoidal rule for all but the last timepoint and adjusting the integration to reflect radiological decay following the last timepoint. It is also possible to calculate the dose rates to an ROI or voxels within an ROI and then integrate these rates to get the total absorbed dose. This approach has been used in order to calculate radiobiological parameters such as biological effective dose (BED)(33,34).
The final step in the dosimetry workflow is the dose calculation step. Historically, dose calculation was performed at the organ level using 3D imaging data and pre-calculated dose factors. These dose factors, which are known as S-values are derived from Monte Carlo simulations in computational reference phantoms. They are calculated assuming homogeneous activity in each source organ and the dosimetric contribution from these source organs is determined to itself (i.e. self-dose) or to neighboring organs (i.e. cross-fire). The absorbed dose to a target organ from a source organ is calculated by taking the product of the S-value and the cumulative activity in the source organ determined by methods described previously. The total absorbed dose to each target organ is the sum of the absorbed dose contributions resulting from all source organs.
There are two main limitations of organ-level approaches. First, the anatomical specificity of the patient is not considered since S-values are calculated in computational phantoms. While S-values can be scaled by patient-specific organ masses to improve dosimetric accuracy, the relative positions of the organs cannot be adjusted. In addition, tumors are not present in these phantoms. Therefore, tumor dosimetry is often done by using specific absorbed fractions calculated in spheres which often misrepresents the actual shape of the tumor and neglects crossfire dose contributions from source organs adjacent to and at larger distances from the tumor. Second, heterogeneous uptake of activity in the source organ cannot be modelled. Uniform activity is assumed in all regions or organs and tumors, which will often misrepresent the heterogeneous activity distribution in relevant organ and tumor ROIs.
A more accurate alternative to organ level dosimetry is to perform voxel level dosimetry. Voxel-level dosimetry methods utilize patient-specific 3D anatomical information acquired from CT or MR scans to model the patient anatomy and 3D SPECT or PET scans to determine the cumulative activity in each voxel. The absorbed dose is predicted at the voxel level by calculating the dosimetric impact of the cumulative activity to each source voxel and its neighboring voxels by simulating radiation transport in the patient anatomy.
Currently three main approaches have been used to model radiation transport at the voxel-level: local energy deposition, dose point kernel (DPK) convolution and dose voxel kernel (DVK) convolution which uses voxel-level S-values (VSVs) based on the MIRD formalism, and direct Monte Carlo (MC) radiation transport(2). The local energy deposition method assumes all of the energy released during radioactive decay in a voxel is deposited locally in that voxel. Therefore, this method neglects radiation transport of the particles released during decay. This method is accurate when the decay releases short ranged particles (e.g. alpha particles), but fails to account for the wash out of dose to surrounding voxels when longer ranged particles are emitted during the decay (e.g. betas and gammas). Dose point kernels and dose voxel kernels are pre-calculated kernels from radioactive decay. These 3D kernels are convolved with the cumulative activity in each voxel to determine the absorbed dose in the voxel. The kernel convolution accounts for dose washout that occurs between the voxels. However, since dose kernels are often calculated in water the kernel convolutions neglect the impact of tissue heterogeneity in the patient. Correction techniques have been developed to help account for tissue heterogeneities.
The most accurate and versatile approach for performing voxel-level dose calculations is the direct MC method(2). The direct MC method models each physical process that impacts transport of radiation in a medium. Individual particles are tracked from creation until the particle is absorbed, escapes the region of interest or reaches an energy that is below a user-defined threshold value(35). The history of the particle is determined by randomly sampling well-known probability distributions that govern the physical processes that occur during its transport. Once a desired number of particles is simulated, characteristics of the radiation field in the physical system, such as absorbed dose, can be inferred from the mean behavior of the simulated particles.
Finally, information regarding the patient-specific dose distribution in ROIs is often calculated. Dose statistics can be determined in ROIs in the form of the mean dose, maximum/minimum dose, or any other quantity of interest. If more granularity is desired then the dose-volume histogram in ROIs can be calculated.
Currently, there are four FDA cleared products for RPT dosimetry. More details regarding these products are provided in Table 2. It should be noted that there are no FDA cleared products to perform patient-specific treatment planning akin to EBRT. The intended use of these products is for retrospective dose calculations in patients undergoing RPT procedures.
Table 2:
List of FDA cleared dosimetry software products.
| Software | Company | Isotopes | Dose Calculation Method |
|---|---|---|---|
| SurePlan™ MRT | MIM Software Inc. | 177Lu, 131| | VSV |
| Torch™ | Voximetry Inc. | 177Lu, 131|, 90Y | Direct MC |
| Voxel Dosimetry™ | Hermes Medical Solutions | 68Ga, 123|, 131|, 111|n, 131|, 177Lu, 99mTc, 90Y | Semi-MC |
| Planet® Dose | Dosisoft | 177Lu, 131| | VSV/LDM |
Clinical Dosimetry Infrastructure
Performing patient-specific dosimetry is a complex process that requires multidisciplinary expertise and adequate coordination. A possible dosimetry infrastructure is outlined in Figure 5. Currently, most clinics that have dosimetry resources and expertise do not perform dosimetry on every patient. Often times it is a team of experts (in this example the oncologist and/or radiation oncologist, nuclear medicine physician, and medical physicist) that identifies patients that might benefit from dosimetry. This could be due to high disease burden and concern about residual damage to surrounding healthy tissue or it could be due to patients with impaired organ function that could impact the expected clearance of the drug (e.g. patient on dialysis). It is plausible that dosimetry becomes an integral part of patient management for all RPT patients in the not so distant future. If it is decided that dosimetry is not necessary, then the patient would receive standard activity administration. Otherwise, the dosimetry workflow is initiated and the nuclear medicine manager coordinates scanning times for patient imaging sessions and calibration scans if needed. If calibration is needed, it is important that a medical physicist is available to oversee and approve the calibration process. Following patient imaging, the imaging data is imported into an FDA cleared dosimetry software (see Table 2) and the dose calculation is performed. The dosimetry is reported back to the dosimetry team and is used to decide if adjusting the amount of activity administered to the patient is warranted, but always following guidelines on the drug label. It is important to note that clinics may decide to image after every cycle as the pharmacokinetic behavior of the drug can change due to changes in patient physiology including more or less viable tumor burden.
Figure 5:
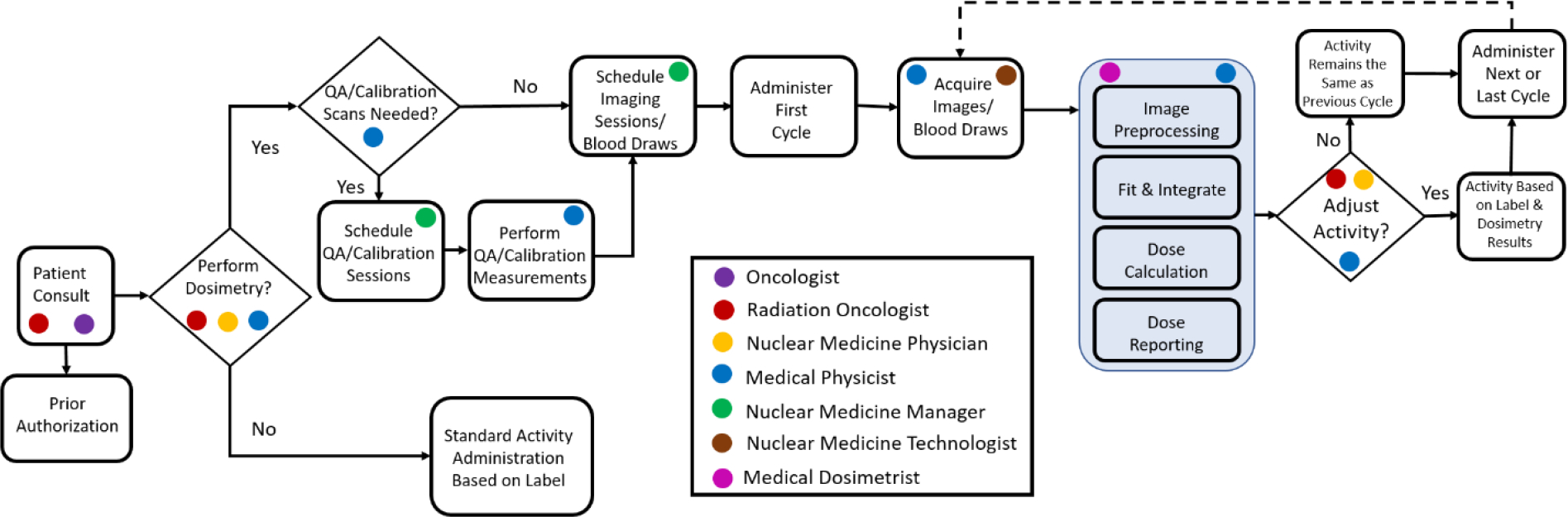
Example of a clinical dosimetry infrastructure. Roles of important stakeholders in the dosimetry infrastructure are indicated. The patient-specific dose calculation process is highlighted in blue.
The ability to bill for the tasks and services outline in Figure 5 will be vital to support the dosimetry efforts in the clinical setting. Fortunately, there are multiple radiation therapy billing codes that can be adopted for patient-specific RPT dosimetry. A detailed overview of possible codes to use for RPT dosimetry was recently provided by Graves et al(36).
Discussion
The present excitement pertaining to RPT is palpable. RPT drugs are being introduced into the clinic at an unprecedented rate. Centers devoted to RPT are springing up across the US. There are well over 200 clinical trials in the US alone that involve RPT agents(1). A significant amount of money is being invested in development and translation of novel RPT agents by companies that is fueling the RPT renaissance. It is not too farfetched to imagine a time in the future when RPT replaces chemotherapy to treat both early and advanced staged cancers.
As discussed previously, RPT and theranostics are not necessarily one of the same, despite often being mislabeled as such. The concept of theranostics may or may not accompany the renaissance in RPT. Drug companies need to continue to see the value in developing a companion diagnostic with each therapy agent, even if the diagnostic could approximately double the development and regulatory costs of the theranostic pair. There is also a sizable risk that a non-radioactive drug eventually outperforms its radioactive counterpart in clinical trials. Likely drug companies will need to integrate patient-specific dosimetry into theranostic clinical trials in order to continue to demonstrate improved safety and efficacy compared to other competitive radioactive or non-radioactive drugs.
There is no question that the field of medicine is undergoing a paradigm shift in how we provide care for patients and the timing of this shift is ideal for RPT and theranostics. Fee-for-service medicine is becoming a thing of the past as it is starting to be replaced by value-based care. With rising drug costs and only modest gains in treatment outcomes this change could significantly disrupt modern oncologic practices. The field of oncology is at risk of remaining on the sidelines while losing out on all of the benefits of value-based care to patients, providers, payers and society. These changes will come in different forms and will require different responses, but one exciting outcome of value-based care is the adoption of new technologies in the clinic that fee-for-service based practices could not justify. Theranostics and patient-specific dosimetry has to play a central role in this transformation.
Despite increasing evidence demonstrating the benefit of patient-specific dosimetry, there remains some skepticism about the feasibility of implementing dosimetry on a routine basis in the clinic. One concern is that adding extra clinical visits for dosimetry scans increases the burden on the patient. While there are certainly exceptions, it is often the case that most patients will agree and comply with the treatment plan that their physicians prescribe to them (37). Compliance can also improve when the benefits of a treatment or aspect of a treatment are articulated clearly to the patient. This communication is especially important since theranostic patients will likely interact with several physicians that are part of the theranostic team including the oncologist, radiation oncologist, and nuclear medicine physician. Fortunately, the benefits from dosimetry are now quantifiable making it easier to articulate these benefits to the patient, which can sometimes be difficult to do (38).
A second concern is related to the cost of implementing dosimetry in the clinic on providers, payers, and patients. As previously mentioned, billing codes that currently exists for radiation oncology might help ease the cost burden for providers (36). However, it is not completely clear if payers will accept these codes for RPT dosimetry. Likely, a clinic will have to “test the waters” with payers on a dosimetry case or a handful of cases during prior authorization to see what is acceptable and what is not. In addition, radiation oncology, nuclear medicine, and medical physics professional organizations need to lobby for more appropriately defined billing codes for RPT patient-specific dosimetry. It is worth noting that the cost for patient-specific dosimetry is a small fraction of the cost of an actual RPT treatment, which should place some financial responsibility for dosimetry on the drug provider especially since it is becoming clear that dosimetry improves clinical outcomes.
A final concern is the limited amount of activity (of a given isotope) that is available in the global supply chain at any point in time. Even without routine dosimetry, physicians are currently struggling to secure RPT drugs that they would like to administer to patients without significant delays. Data suggests that patient-specific dosimetry could drastically increase the global demand for RPT drugs because most RPT patients can tolerate more drug. As a result, the government and industry need to work together to address this current shortcoming that might only worsen with time.
The good news is these concerns are being addressed and patient-specific dosimetry is starting to be used in the clinical setting. It is only a matter of time when oncology leverages theranostics to its fullest extent and patient-specific dosimetry is performed for most if not all RPT patients. And it is only then that Hippocrates vision for patient care will be realized for perhaps the first time in oncology.
Footnotes
COI Statement: Bryan Bednarz is Chairman and CSO of Voximetry, Inc a nuclear medicine dosimetry company located in Middleton, WI and has financial interest in the company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sgouros G, Bodei L, McDevitt MR, Nedrow JR. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St. James S, Bednarz B, Benedict S, et al. Current Status of Radiopharmaceutical Therapy. Int J Radiat Oncol Biol Phys. 2021;109:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodei L, Herrmann K, Schöder H, Scott AM, Lewis JS. Radiotheranostics in oncology: current challenges and emerging opportunities. Nat Rev Clin Oncol 2022 198. 2022;19:534–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sgouros G, Dewaraja YK, Escorcia F, et al. Tumor Response to Radiopharmaceutical Therapies: The Knowns and the Unknowns. J Nucl Med. 2021;62:12S–22S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Idée J-M, Louguet S, Ballet S, Corot C. Theranostics and contrast-agents for medical imaging: a pharmaceutical company viewpoint. Quant Imaging Med Surg. 2013;3:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hertz S, Roberts A. Radioactive iodine in the study of thyroid physiology; the use of radioactive iodine therapy in hyperthyroidism. J Am Med Assoc. 1946;131:81–86. [DOI] [PubMed] [Google Scholar]
- 7.BERSON SA, YALOW RS. THE IODIDE TRAPPING AND BINDING FUNCTIONS OF THE THYROID. J Clin Invest. 1955;34:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durski JM, Hruska CB, Bogsrud TV., Ryder M, Johnson GB. 123I Scan with Whole-Body Retention Measurement at 48 Hours for Simplified Dosimetry before 131I Treatment of Metastatic Thyroid Cancer. Clin Nucl Med. 2021;46:e151–e153. [DOI] [PubMed] [Google Scholar]
- 9.Phan HTT, Jager PL, Paans AMJ, et al. The diagnostic value of 124I-PET in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirley M, McCormack PL. Radium-223 dichloride: a review of its use in patients with castration-resistant prostate cancer with symptomatic bone metastases. Drugs. 2014;74:579–586. [DOI] [PubMed] [Google Scholar]
- 11.Parker C, Nilsson S, Heinrich D, et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N Engl J Med. 2013;369:213–223. [DOI] [PubMed] [Google Scholar]
- 12.Kulke MH, Mayer RJ. Carcinoid Tumors. https://doi.org/101056/NEJM199903183401107. 1999;340:858–868. [DOI] [PubMed] [Google Scholar]
- 13.Strosberg JR, Weber JM, Feldman M, Coppola D, Meredith K, Kvols LK. Prognostic validity of the American joint committee on cancer staging classification for midgut neuroendocrine tumors. J Clin Oncol. 2013;31:420–425. [DOI] [PubMed] [Google Scholar]
- 14.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 15.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. [DOI] [PubMed] [Google Scholar]
- 16.Kwekkeboom DJ, De Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177Lu- DOTA0,Tyr3]octreotate: Toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. [DOI] [PubMed] [Google Scholar]
- 17.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177 Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agrawal A, Rangarajan V, Shah S, Puranik A, Purandare N. MIBG (metaiodobenzylguanidine) theranostics in pediatric and adult malignancies. Br J Radiol. 2018;91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wieland DM, Mangner TJ, Inbasekaran MN, Brown LE, Wu JL. Adrenal Medulla Imaging Agents: A Structure-Distribution Relationship Study of Radiolabeled Aralkylguanidines1. J Med Chem. 1984;27:149–155. [DOI] [PubMed] [Google Scholar]
- 20.Pryma DA, Chin BB, Noto RB, et al. Efficacy and Safety of High-Specific-Activity 131I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J Nucl Med. 2019;60:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients - PubMed. https://pubmed.ncbi.nlm.nih.gov/2449118/. [PubMed]
- 22.Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ICRU Report 96, Dosimetry-Guided Radiopharmaceutical Therapy – ICRU. https://www.icru.org/report/icru-report-96-dosimetry-guided-radiopharmaceutical-therapy/.
- 24.Wahl RL. The clinical importance of dosimetry in radioimmunotherapy with tositumomab and iodine I 131 tositumomab. Semin Oncol. 2003;30:31–38. [DOI] [PubMed] [Google Scholar]
- 25.Wahl RL, Frey EC, Jacene HA, et al. Prospective SPECT-CT Organ Dosimetry-Driven Radiation-Absorbed Dose Escalation Using the In-111 (111In)/Yttrium 90 (90Y) Ibritumomab Tiuxetan (Zevalin®) Theranostic Pair in Patients with Lymphoma at Myeloablative Dose Levels. Cancers 2021, Vol 13, Page 2828. 2021;13:2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilan E, Sandstrom M, Wassberg C, et al. Dose Response of Pancreatic Neuroendocrine Tumors Treated with Peptide Receptor Radionuclide Therapy Using 177Lu-DOTATATE. J Nucl Med. 2015;56:177–182. [DOI] [PubMed] [Google Scholar]
- 27.Sandström M, Freedman N, Fröss-Baron K, Kahn T, Sundin A. Kidney dosimetry in 777 patients during 177Lu-DOTATATE therapy: aspects on extrapolations and measurement time points. EJNMMI Phys. 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Prete M, Buteau F-A, Arsenault F, et al. Personalized 177 Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging. 2019;46:728–742. [DOI] [PubMed] [Google Scholar]
- 29.Sundlöv A, Katarina ·, Gleisner S, et al. Phase II trial demonstrates the efficacy and safety of individualized, dosimetry-based. Eur J Nucl Med Mol Imaging.:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besemer AE, Titz B, Grudzinski JJ, et al. Impact of PET and MRI threshold-based tumor volume segmentation on patient-specific targeted radionuclide therapy dosimetry using CLR1404. Phys Med Biol. 2017;62:6008–6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uribe CF, Esquinas PL, Tanguay J, et al. Accuracy of 177Lu activity quantification in SPECT imaging: a phantom study. EJNMMI Phys. 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papavasileiou P, Divoli A, Hatziioannou K, Flux GD. The importance of the accuracy of image registration of SPECT images for 3D targeted radionuclide therapy dosimetry. Phys Med Biol. 2007;52. [DOI] [PubMed] [Google Scholar]
- 33.Hobbs RF, Sgouros G. Calculation of the biological effective dose for piecewise defined dose-rate fits. Med Phys. 2009;36:904–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Besemer AE, Yang YM, Grudzinski JJ, Hall LT, Bednarz BP. Development and Validation of RAPID: A Patient-Specific Monte Carlo Three-Dimensional Internal Dosimetry Platform. Cancer Biother Radiopharm. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graves SA, Bageac A, Crowley JR, Merlino DAM. Reimbursement Approaches for Radiopharmaceutical Dosimetry: Current Status and Future Opportunities. J Nucl Med. 2021;62:48S–59S. [DOI] [PubMed] [Google Scholar]
- 37.Bar-Ad V, Wilson KW, Munro S, et al. Increasing on-treatment visit compliance for radiation therapy cancer patients. https://doi.org/101200/jco20133131_suppl175. 2013;31:175–175. [Google Scholar]
- 38.Dobler CC. Treatment burden is important to patients but often overlooked by clinicians. Breathe. 2021;17:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


