Abstract
Objectives
The objective of this study was to report on the development of neuroinvasive West Nile virus (WNV) infection in the context of anti-CD20 monotherapy for multiple sclerosis (MS).
Methods
This is a case series study.
Results
In 2021–2022, we observed 4 cases of neuroinvasive WNV infection in our patient population of 2009 patients with MS on ocrelizumab, compared with a total of 46 cases of neuroinvasive WNV infection reported in Pennsylvania and 40 in New Jersey. Odds were 258 times that of the general population (95% confidence interval 97–691), χ2 p < 0.0001). All were women aged 41–61 years with variable disease duration, level of disability, and duration of anti-CD20 therapy. All presented in summer/early fall with fever, headache, and encephalopathy consistent with meningoencephalitis. Three patients had acute cerebellitis. Two had anterior nerve root involvement progressing to quadriparesis, and 1 developed refractory nonconvulsive status epilepticus. All required intubation and experienced significant morbidity. All had CSF pleocytosis. Two patients were WNV IgM positive in both the serum and CSF, 1 patient had positive serum IgM and CSF metagenomic next-generation sequencing (mNGS), while 1 had positive CSF mNGS with negative serum and CSF antibodies.
Discussion
Neuroinvasive WNV infection can develop with anti-CD20 monotherapy in the absence of additional immunosuppression. WNV serologies may be negative in the setting of anti-CD20 treatment; in the appropriate clinical context, one should consider direct detection methods such as PCR or mNGS-based testing.
Introduction
West Nile virus (WNV) is the most prevalent mosquito-borne arbovirus in the United States,1 with seasonal peaks between summer and fall. It is believed that less than 1% of individuals with WNV infection experience neuroinvasive disease,2 leading to significant morbidity or death. Neuroinvasive WNV in the setting of anti-CD20 therapies has been described in individuals with B-cell lymphoma,3-5 and there are reports of individuals with severe neuroinvasive WNV infection on rituximab for rheumatoid arthritis (RA)6,7 and systemic lupus erythematosus (SLE)5 who were on other concurrent immune-directed therapies. There are no prior reports of neuroinvasive WNV developing during anti-CD20 monotherapy in otherwise immunocompetent patients.
Methods
We reported demographic, clinical, serologic, and imaging findings of 4 cases of neuroinvasive WNV infection developing in patients with MS on anti-CD20 monotherapy.
Results
The Table summarizes pertinent details of the 4 cases.
Table.
Demographic and Clinical Summary of 4 Patients With MS Receiving Ocrelizumab Who Developed Neuroinvasive WNV
Case 1
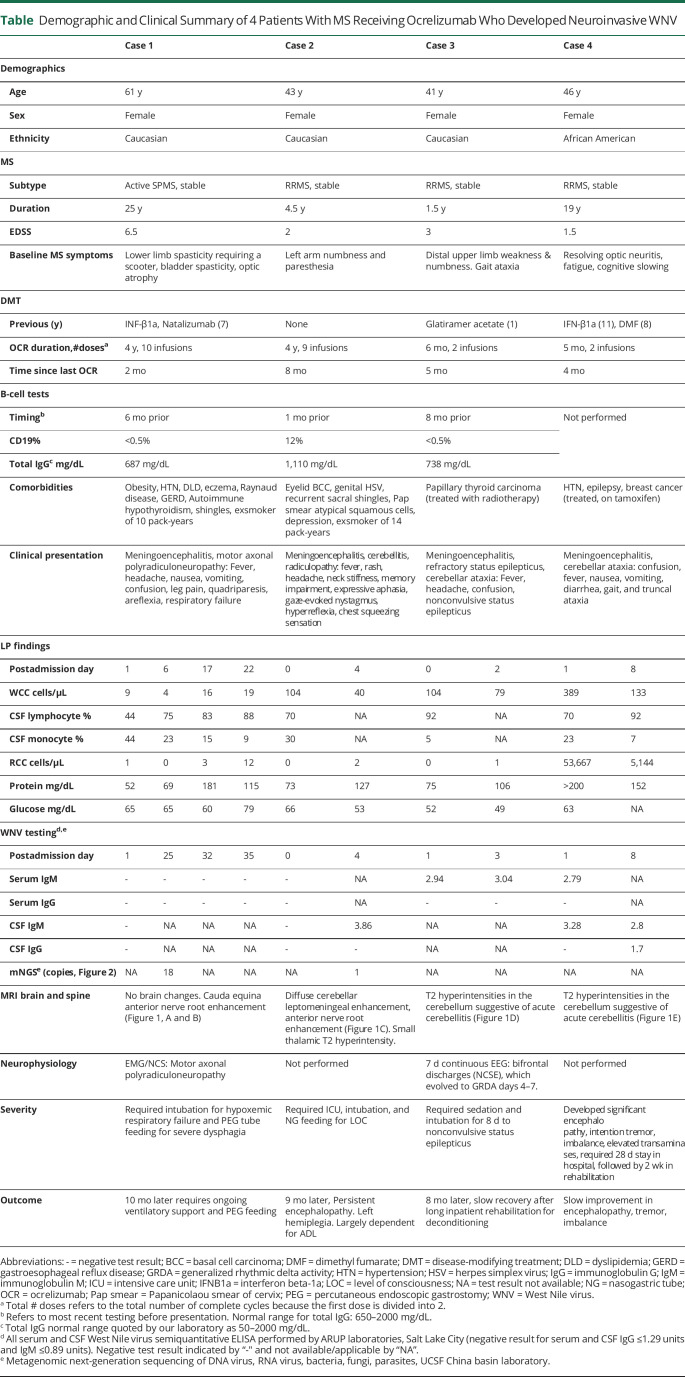
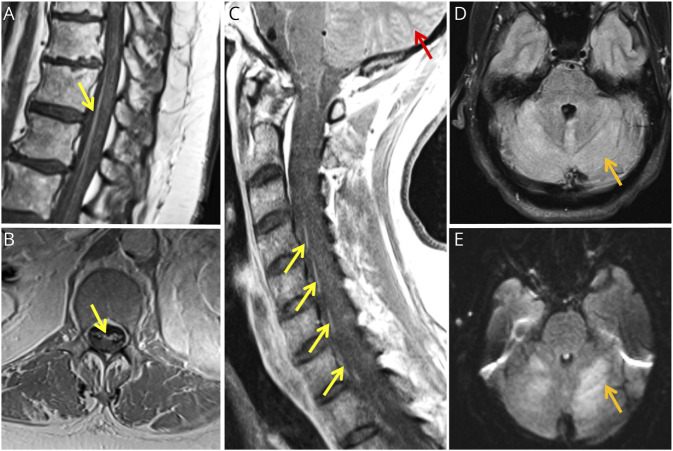
A 61-year-old woman with a 25-year history of MS received 4 years of ocrelizumab monotherapy after prior therapy with IFN-β1a and natalizumab. On arriving in San Francisco from Philadelphia for vacation in late summer, she developed fever, encephalopathy, progressive quadriparesis, and diminished reflexes. Brain MRI demonstrated no evidence of new MS disease activity. Lumbar puncture (LP) revealed a mild pleocytosis and negative infectious encephalitis testing including WNV IgM and IgG. She ultimately required endotracheal ventilation for respiratory failure. Repeat brain MRI was unchanged, and spine MRIs revealed prominent lumbosacral anterior nerve root enhancement (Figure 1, A and B). EMG demonstrated severe motor axonal polyradiculoneuropathy. Repeat CSF analysis revealed 4 white blood cells/μL, elevated protein (69 mg/dL), negative test for Lyme disease, and antiganglioside antibodies. CSF and serial tests for IgM and IgG WNV serum antibodies remained negative. CSF metagenomic next-generation sequencing (mNGS) testing returned positive for WNV with the detection of 18 reads (Figure 2A). Reads mapped to the WNV genome with 97.1%–100% nucleotide identity (Figure 2B). False positivity and cross-contamination were excluded as likely possibilities (Figure 2C). For WNV sequences, see eAppendix 1 (links.lww.com/NXI/A884). Ten months later, she requires intermittent ventilatory support through a tracheostomy and is slowly regaining limited strength in her upper extremities.
Figure 1. MR Images of Neuroinvasive WNV Manifestations in 4 Patients With MS Treated With Ocrelizumab.
MRI of the lumbosacral spine T1-weighted sequences postgadolinium infusion in case 1 in sagittal (A) and axial (B) views showing anterior nerve root enhancement (yellow arrows). (C) MRI of the cervical and thoracic spine T1-weighted sequences postgadolinium infusion in case 2 in sagittal view showing anterior nerve root enhancement (yellow arrows) and diffuse leptomeningeal enhancement of the cerebellar folia (red arrow). MRI of the brain T2-weighted fluid-attenuated inversion recovery (FLAIR) sequences in axial views in case 3 (D) and case 4 (E) showing signal hyperintensity of the cerebellum (orange arrows).
Figure 2. Detection of WNV in Case 1 Patient by CSF mNGS.
(A) Heat map of the mNGS run generated using the UCSF SURPI+ pipeline.15 Each column represents a CSF sample or control and each row detection of a virus at the family, genus, and species taxonomic level. Asterisks denote reads that are not specific at a given level and thus are “declassified” to a higher taxonomic rank. Cells are color coded with gray denoting 0 reads and increasing read counts shown as a gradient from green to yellow to red. Eighteen WNV reads are detected in case 1 patient CSF (“case 1”); 11,420 reads in a different WNV case from an OSH (“OSH WNV”). Note that the RNA virus positive control for the assay is HIV 1 (“PC”).15 (B) Coverage map generated using the UCSF SURPI+ pipeline. The 18 reads from the case 1 patient are automatically mapped to the most closely matched reference genome in the NCBI GenBank database. The coverage is shown in green and the percent identity in purple. (C) Comparison of WNV genomes from case 1 and OSH patient. The complete WNV genome from the OSH patient was assembled using Geneious version 11.1.5,16 and then the 18 WNV reads from the case 1 patient (gray) were mapped to the genome. Single-nucleotide differences are shown as vertical lines, with 3 differences identified in the mapped consensus regions (“Consensus”) corresponding to the WNV prM and envelope genes. NC, negative control; NCBI, National Center for Biotechnology Information; NS, nonstructural; OSH, outside hospital; PC, positive control; prM, premembrane; SURPI, sequence-based ultrarapid pathogen identification.
Case 2
A 43-year-old woman with a 4.5-year history of RRMS received 4 years of ocrelizumab treatment as first-line therapy. Two weeks before her early fall presentation, she was hiking in the Poconos and reported multiple insect bites. She developed sore throat, rash, chills, chest tightness, severe meningismus, and confusion. Evaluation revealed a maculopapular rash, neck stiffness, impaired recall, naming, and fluency, papilledema, bilateral gaze-evoked nystagmus, and pathologically brisk reflexes. MRI showed no new MS lesions. LP revealed a lymphocytic pleocytosis (104 cells/μL, 70% lymphocytes) and elevated protein (73 mg/dL). Extensive CSF and serum infectious screening including WNV IgM and IgG was negative. Her mental status deteriorated, and she required endotracheal ventilation. On day 5, brain MRI revealed diffuse cerebellar leptomeningeal enhancement (Figure 1C). Spine MRI revealed enhancing ventral nerve roots (Figure 1C). On day 6, repeat serum WNV IgM was strongly positive, remaining positive 2 weeks later. A single WNV read from patient CSF was detected by mNGS (eAppendix 2, links.lww.com/NXI/A884. No WNV reads were detected in any other sample on the run. Nine months later, she is functionally dependent for activities of daily living, follows simple commands, and answers in 1-word responses. She is hemiplegic on the left, has urinary retention, and experiences severe radicular pain.
Case 3
A 41-year-old woman with an 18-month history of RRMS received her first 2 infusions of ocrelizumab after prior treatment failure with glatiramer acetate. Five months later, after an early fall hike in the Philadelphia region, she developed fever and headache followed by 2 days of progressive confusion. On admission, she was febrile and encephalopathic. LP revealed marked lymphocytic pleocytosis (104 cells/μL, 92% lymphocytes) and elevated protein (75 mg/dL). MRI demonstrated cerebellar FLAIR hyperintensities suggestive of acute cerebellitis (Figure 1D). After 2 days of empiric antibiotics and endotracheal ventilation, an EEG revealed nonconvulsive status epilepticus, requiring propofol and 3 antiepileptic medications. Repeat LP showed a lymphocytic pleocytosis (WBC 79 cells/μL) and elevated protein (106 mg/dL). Serum ELISA and CSF WNV IgM were positive on 2 tests separated by 1 week (2.94 and 3.04 IV). She was extubated 2 weeks later and had residual moderate quadriparesis and ataxia. Eight months later, she requires a walker to ambulate due to gait ataxia and lower limb weakness.
Case 4
A 46-year-old woman with a 19-year history of RRMS received her first 2 infusions of ocrelizumab after prior treatment failures with IFNβ-1a and dimethyl fumarate. In early fall, 3 months after infusion, she was admitted to the hospital with fever, nausea, emesis, and diarrhea, followed by encephalopathy, intention tremor, and weakness. Reflexes remained intact. Initial LP revealed significant lymphocytic pleocytosis (389 cells/μL, 92% lymphocytes) and highly elevated protein (>200 mg/dL). MRI demonstrated patchy FLAIR hyperintensities in bilateral cerebral and cerebellar hemispheres. Serum and CSF WNV IgM were positive on initial and repeat testing. Mental status slowly improved during the 28-day hospitalization followed by 2 weeks in rehabilitation to regain walking ability. She improved gradually over 18 months, specifically in cognition, strength, and gait. She is left with minimal leg weakness, gait and balance difficulties, memory problems, and word-finding difficulties.
Discussion
These are the first reported cases of neuroinvasive WNV infection in individuals (whether with MS or other conditions) on anti-CD20 monotherapy who are otherwise immunocompetent. While patients with lymphoma and patients with RA and SLE on additional concurrent immune therapy are known to be at risk of immune suppression, none of our patients had other compelling explanations for immunosuppression besides anti-CD20 therapy. Furthermore, 1 patient with multiple negative CSF and serum WNV serologies was only diagnosed after a convincingly positive WNV result by mNGS. Anti-CD20 therapy is known to blunt serologic responses, and this case suggests that serologic testing may be insufficiently sensitive for diagnostic purposes in this context.
In 2021 and 2022, the Centers for Disease Control and Prevention (CDC) reported 46 cases of neuroinvasive WNV infection in Pennsylvania and 40 in New Jersey, less than the US country-wide 2-year incidence of 0.77 cases per 100,000.8 In the same period, we observed 4 cases in our population of 2009 patients with MS on ocrelizumab (projected incidence 201/100,000). The likelihood that we would see this discrepancy by chance is less than 1 in a million (1-sided binomial test). The odds of a patient on ocrelizumab in our population being diagnosed with neuroinvasive WNV were 258 times that of the general population reported to the CDC (95% CI 97–688, χ2 p value < 0.0001). Notably, the CDC figures are based on nonmandatory reporting and therefore may underestimate the incidence. On review of the FDA Adverse Event Reporting System (FAERS),9 there were 17 reports of serious WNV in ocrelizumab-treated patients with MS 2018–2022 (9 female, 3 male, 5 unspecified; ages 34–68 years), including 2 deaths. There were 65 reports of WNV in rituximab-treated individuals; none were treated for MS, although 24 were treated for other autoimmune indications. To date, there are no reports of WNV in ofatumumab-treated patients. In our practice, ocrelizumab constitutes 28% of patients receiving DMT; by comparison, during this time, there were no cases of neuroinvasive WNV infection in the other 72% of our patients on alternative DMTs. Together, our cases and the FAERS data indicate a concerning signal of the increased risk of neuroinvasive WNV infection in patients with MS treated with ocrelizumab.
We found no particular clinical features associated with the development or severity of neuroinvasive WNV: neither duration of anti-CD20 treatment (ranging from 5 months to 4 years), patient age (41–61 years), nor existing MS-related disability (EDSS 1.5–6.5) seemed to influence risk. None of our patients were hypogammaglobulinemic before WNV infection, and none had received immune-depleting therapies before ocrelizumab. WNV infection was not associated with clinical or imaging evidence of new MS disease activity.
Our series also highlights a potential challenge to diagnosing suspected arbovirus infections in patients on anti-CD20 therapy by serologic testing alone. One 61-year-old female patient on ocrelizumab for 4 years with normal total IgG had serially negative WNV serologies but positive CSF mNGS, findings indicative of active CNS viral replication. The possibility of mNGS false positivity or cross-contamination was explored in depth and determined to be highly unlikely. A recent report describes an analogous case of seronegative arboviral encephalitis in an immunosuppressed patient who was also only successfully diagnosed after CSF mNGS.10 Elsewhere, an immunosuppressed renal transplant recipient with negative serologies was diagnosed with neuroinvasive WNV by CSF mNGS, followed by delayed seroconversion.11 In our cases, we note that mNGS is not the only technique that could have led us to the appropriate diagnosis and that the sensitivity of targeted virus-specific PCR is highly concordant.12 Clinical CSF mNGS testing for broad-spectrum pathogen detection is only available at present from the UCSF Clinical Microbiology Laboratory,13 although WNV PCR testing is widely available from commercial reference laboratories including Eurofins Viracor, ARUP, and Quest Diagnostics.
We postulate that anti-CD20 treatment may blunt the development of diagnostic WNV IgM and IgG antibodies, rendering testing less sensitive in this population. This is not unique to infectious exposures; studies on novel vaccine response have similarly demonstrated the potential for a blunted antibody response in patients with MS on anti-CD20 therapy, though their T-cell responses remain robust.14 Thus, an inability to mount antiviral antibody responses may contribute both to increased risk of neuroinvasive infection and diminished ability to establish the diagnosis using only serologic testing.3,5
In conclusion, WNV diagnosis requires a high index of suspicion for patients receiving immunosuppressive therapies presenting in the summer and fall with encephalopathy, motor neuronopathy, and CSF pleocytosis. Anti-CD20 therapies may simultaneously increase the risk of developing neuroinvasive disease while reducing the sensitivity of diagnostic serologic testing; direct detection approaches such as mNGS or virus-specific PCR should be favored in this patient population.
Acknowledgment
The authors thank Dr. Andrew Cucciara for his assistance with the statistics presented in this article.
Appendix. Authors

Study Funding
The authors report no targeted funding.
Disclosure
C.Y.C. is a founder and is on the scientific advisory board for Delve Bio. C.Y.C. is also an inventor on US patent 11380421, “Pathogen Detection using Next Generation Sequencing,” under which algorithms for taxonomic classification, filtering, and pathogen detection are used by SURPI+ software. M.R.W. is a founder and is on the scientific advisory board for Delve Bio. C.Y.C. The other authors declare no competing interests. Go to Neurology.org/NN for full disclosures.
References
- 1.Centers for Disease Control and Prevention. West Nile Virus [online], 2022. Accessed December 10, 2022. cdc.gov/westnile/index.html.
- 2.DeBiasi RL, Tyler KL. West Nile Virus meningoencephalitis. Nat Clin Pract Neurol. 2006;2(5):264-275. doi: 10.1038/ncpneuro0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morjaria S, Arguello E, Taur Y, et al. West Nile Virus Central Nervous System Infection in patients treated with rituximab: implications for diagnosis and prognosis, with a review of literature. Open Forum Infect Dis. 2015;2(4):ofv136. doi: 10.1093/ofid/ofv136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens M, Choe L, Rivera JE, Avila JD. West Nile Virus neuroinvasive disease associated with rituximab therapy. J Neurovirol. 2020;26(4):611-614. doi: 10.1007/s13365-020-00854-z [DOI] [PubMed] [Google Scholar]
- 5.Kapadia RK, Staples JE, Gill CM, et al. Severe arboviral neuroinvasive disease in patients on rituximab therapy: a review. Clin Infect Dis. 2023;76(6):1142–1148. doi: 10.1093/cid/ciac766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdalla AA, Fanciullo J, Ateeli H. Delayed diagnosis of West Nile meningoencephalitis in a patient receiving rituximab for rheumatoid arthritis. Cureus. 2022;14(10):e30221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goates C, Tsuha S, Working S, Carey J, Spivak ES. Seronegative West Nile Virus infection in a patient treated with rituximab for rheumatoid arthritis. Am J Med. 2017;130(6):e257-e258. doi: 10.1016/j.amjmed.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. West Nile Virus Disease Cases by State 2022 [online], 2022. Accessed December 10, 2022. cdc.gov/westnile/statsmaps/preliminarymapsdata2022/disease-cases-state-2022.html. [Google Scholar]
- 9.Food and Drug Administration. FDA Adverse Event Reporting System (FAERS) Public Dashboard [online]. Accessed March 24, 2023. fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard. [Google Scholar]
- 10.Shoskes A, Hassett C, Dani D, Majeed A. Pearls & Oysters: seronegative eastern equine encephalitis in an immunocompromised stem-cell transplant recipient. Neurology. 2022;99(22):1004-1007. doi: 10.1212/WNL.0000000000201334 [DOI] [PubMed] [Google Scholar]
- 11.Wilson MR, Zimmermann LL, Crawford ED, et al. Acute West Nile Virus meningoencephalitis diagnosed via metagenomic deep sequencing of cerebrospinal fluid in a renal transplant patient. Am J Transplant. 2017;17(3):803-808. doi: 10.1111/ajt.14058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babiker A, Bradley HL, Stittleburg VD, et al. Metagenomic sequencing to detect respiratory viruses in persons under investigation for COVID-19. J Clin Microbiol. 2020;59(1):e02142-20. doi: 10.1128/jcm.02142-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UCSF Center for Next-Gen Precision Diagnostics [online]. 2022. Accessed May 8, 2023. nextgendiagnostics.ucsf.edu. [Google Scholar]
- 14.Apostolidis S, Kakara M, Painter M, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990-2001. doi. 10.1038/s41591-021-01507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller S, Naccache SN, Samayoa E, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. 2019;29(5):831-842. doi: 10.1101/gr.238170.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearse M, Moir R, Wilson A, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647-1649. doi: 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]