Abstract
Background:
Periprosthetic joint infections (PJI) following joint arthroplasty are now the leading cause of reoperation and are associated with serious morbidity to the patient, often requiring several staged operations and a prolonged course of parenteral antibiotics. Prophylactic administration of intravenous antibiotics before skin incision is arguably the most important measure to prevent PJI; however, the dose effectiveness of cefazolin in target tissue is not well known. We aimed to identify parameters affecting local tissue concentration (LTC) of cefazolin.
Methods:
We performed a literature search using the following keywords: “orthopaedics,” “orthopedic,” “arthroplasty” and “cefazolin.” We included studies that measured LTC of cefazolin from samples obtained during either a total knee or total hip arthroplasty.
Results:
Of the 332 records screened, we included 10 studies that described LTC of cefazolin. The included studies evaluated dosing (n = 7), procedure type (n = 3), body mass index (n = 1) and tourniquet utilization (n = 1).
Conclusion:
Few studies have measured LTC levels of antibiotics (or levels of cefazolin) to validate current recommendations for antibiotic prophylaxis in orthopedic surgery. With infection as the leading reason for early reoperation or revision surgery, the parameters affecting LTC during orthopedic procedures need to be further assessed.
Abstract
Contexte:
Les infections de prothèse articulaire (IPA) suivant une arthroplastie de la hanche sont aujourd’hui la principale cause de réopération; elles sont associées à une grave morbidité chez le patient et nécessitent souvent plusieurs interventions en séquence et une antibiothérapie prolongée administrée par voie parentérale. L’administration prophylactique d’antibiotiques intraveineux avant l’incision cutanée est sans doute la mesure la plus importante pour prévenir les IPA; la dose efficace de céfazoline dans le tissu ciblé n’est toutefois pas connue avec précision. Nous avons voulu déterminer les paramètres modulant la concentration locale tissulaire de la céfazoline.
Méthodes:
Nous avons effectué une recherche documentaire à l’aide des mots-clés suivants : « orthopaedics » [orthopédie], « orthopedic » [orthopédique], « arthroplasty » [arthroplastie] et « cefazolin » [céfazoline]. Nous avons aussi inclus des études ayant mesuré la concentration locale de la céfazoline dans des échantillons obtenus dans le cadre d’arthroplasties totales du genou ou de la hanche.
Résultats;:
Après examen de 332 articles, nous avons inclus 10 études décrivant la concentration locale tissulaire de céfazoline, qui évaluaient la dose (n = 7), le type d’intervention (n = 3), l’indice de masse corporelle (n = 1) et l’utilisation d’un garrot (n = 1).
Conclusion:
Peu d’études ont mesuré les concentrations locales tissulaires d’antibiotiques (ou de céfazoline) afin de valider les recommandations actuelles sur l’antibioprophylaxie en chirurgie orthopédique. Les infections étant la principale cause de réopérations ou chirurgies de révision hâtives, les paramètres modulant la concentration locale tissulaire au cours des interventions orthopédiques doivent faire l’objet d’une évaluation plus poussée.
Despite numerous advancements in orthopedics, surgical site infection remains one of the most common and serious complications. Infection involving a joint arthroplasty portends a poor prognosis and usually requires revision surgery and prolonged parenteral antibiotics. Prosthetic joint infections (PJI) are serious complications of joint arthroplasties, with hip and knee prostheses most frequently affected. In Canada, the incidence of infections is 1.64% for hip arthroplasties and 1.52% for knee arthroplasties.1–4 Associated with serious morbidity, PJIs often require several reoperations and consume substantial health care resources. Treatment of a single PJI has been shown to cost about $50 000.3,4 Optimizing patient risk factors, managing the operating room environment, using proper skin preparation and using antibiotic prophylaxis effectively are all intended to prevent this devastating complication.5,6 Preoperative antibiotic prophylaxis is one of the most important strategies used to lower the risk of PJI.7,8 Cefazolin is the antibiotic of choice in arthroplasty procedures because of its broad-spectrum effect on methicillin-sensitive staphylococci and streptococci and its relatively low cost.7,9 However, there is limited modern evidence to support dosage recommendations for use of cefazolin in arthroplasty PJI prophylaxis. As stated in various international guidelines on surgical prophylaxis,10–12 patients undergoing implant surgery are often not included in research providing the basis for dosage recommendations.
In evidence-based and protocolized medicine, the trend is moving toward increased weight-based dosage of β-lactam drugs for surgical procedures. In addition, redosing during longer procedures is now considered paramount in surgical prophylaxis recommendations. However, these recommendations are often based on pharmacokinetic extrapolations and not on actual local tissue concentration (LTC) data. It is necessary to reach concentrations of prophylactic antibiotics that surpass the targeted pathogen’s minimum inhibitory concentration (MIC) of 1 μg/mL for at least the time between incision and wound closure for prophylactic antibiotics to prevent infections.13 Achieving four-fold (4 μg/mL) MIC in tissue is recommended for halting the specific pathogen.14 Cefazolin achieves its highest peak bone concentrations 40 minutes after parenteral application with a serum half-life of 108 minutes and bone half-life of 42 minutes.15,16 Since medications are not equally distributed throughout the body, it is critical to know that an antibiotic obtains adequate concentrations not only in serum, but also in the local tissues at the surgical site.17 Compared with conventional methods, modern techniques using liquid chromatography and mass spectrometry can accurately measure antibiotic concentration in tissues like fat and bone.18
We sought to provide a comprehensive summary of reported studies of LTC of cefazolin in total knee and total hip arthroplasty. We explored the parameters that influence LTC of cefazolin including route of administration, type of procedure, body mass index (BMI) and use of a limb tourniquet.
Methods
Search strategy and data collection
We conducted this review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.19 We searched the National Library of Medicine (PubMed) and MEDLINE (Ovid) using a combination of keywords including “orthopaedics,” “orthopedic,” “arthroplasty” and “cefazolin.” Studies retrieved from the searches were combined into a library on Endnote X9 which then underwent deduplication. Two authors (M.M. and J.M.) independently reviewed the search entries using strict inclusion and exclusion criteria. Studies were first screened by title and abstract. The remaining studies then underwent full text screening, and any discrepancies between reviewers were resolved through consensus. Eligible studies then underwent data extraction. No date restrictions were applied, and only studies written in English were included. To be eligible for inclusion, study participants had to have received cefazolin before skin incision for either total knee arthroplasty (TKA) or total hip arthroplasty (THA) and the LTC of cefazolin had to have been measured from samples obtained during the procedure. Data extracted from eligible studies included author, year, study title, study type, total number of participants, mean age, procedure, cefazolin dose, technique, infection rate and LTC.
Results
Included studies
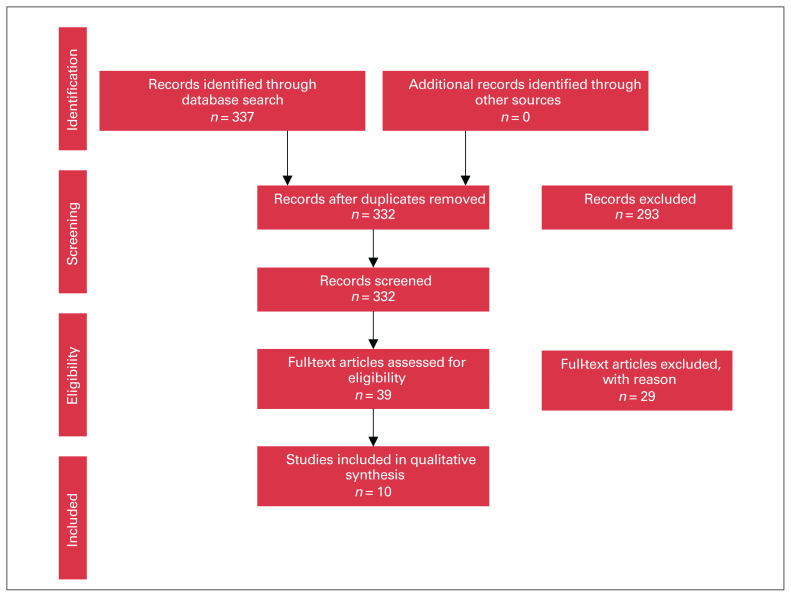
Our review included 10 studies that reported on the use of cefazolin in TKA and THA (Figure 1). These studies evaluated 4 different variables: dosing and route of administration, procedure type, BMI and tourniquet utilization. Variations in LTC of cefazolin were compared based on these factors. There were 7 studies that evaluated the change in LTC according to the dose and route of cefazolin administration. Three studies reported the effect of the type of procedure on LTC of cefazolin. One study assessed the effect of weight or BMI on LTC. One study reported the effect of timing of tourniquet inflation on LTC.
Fig. 1.
Selection of studies for inclusion in our review, based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Dosing and route of administration
Of the 7 studies that reported on the doses or routes of cefazolin administration (Table 1), LTC measures of bone, serum or fat at 1 g or 2 g of cefazolin were compared. Williams and colleagues,20 Angthong and colleagues16 and Sharareh and colleagues21 compared 1 g of cefazolin with 2 g of cefazolin. In each study, there was a greater concentration of cefazolin in the bone samples from the group who received 2 g of cefazolin. Serum and bone LTC ranged from 17.3 to 210 μg/mL and 5.9 to 44.1 μg/g, respectively. Moreover, Williams and colleagues20 observed a greater concentration of cefazolin in the serum of the group who received 2 g when compared with the group who received 1 g. Angthong and colleagues16 found that there was a greater accumulation of cefazolin in the distal femur than in the proximal tibia in the group who received 2 g. Cunha and colleagues,22 Polk and colleagues23 and Rivera and colleagues,24 who studied the tissue concentration of only 1 dose of cefazolin, reported that the serum concentration of cefazolin was always greater than the bone concentration. Interestingly, Young and colleagues25 examined the difference in tissue accumulation when cefazolin was given through different routes of administration. When comparing intravenous (IV) with intraosseous (IO) administration of cefazolin, they found that in the fat and bone samples, IO administration yielded significantly greater LTC than IV administration.
Table 1.
Effect of cefazolin dosing and route of administration on target-site concentration
| Study (level of evidence) | Group | Tissue | Technique | Tissue concentration,* (SD) | Above MIC |
|---|---|---|---|---|---|
| Cunha et al.22 (prospective study) n = 71 | Disc diffusion | ||||
| 1 g | Serum (peak) | 210 (NR) | Yes | ||
| Bone (peak) | 30 (NR) | Yes | |||
| Polk et al.23 (prospective study) n = 20 | HPLC | ||||
| 1 g | Serum | 42 (5.0) | Yes | ||
| Bone | 7.7 (4.8) | Yes | |||
| Williams et al. 20 (prospective study) n = 23 | Disc diffusion | ||||
| 1 g | Serum | 51.7 (NR) | Yes | ||
| Bone | 5.9 (NR) | Yes | |||
| 2 g | Serum | 98.3 (NR) | Yes | ||
| Bone | 14.9 (NR) | Yes | |||
| Young et al. 25 (randomized control trial) n = 22 | HPLC | ||||
| 1 g IV | Fat | 10.6 (NR) | Yes | ||
| Bone | 11.4 (NR) | Yes | |||
| 1 g IO | Fat | 186 (NR) | Yes | ||
| Bone | 130 (NR) | Yes | |||
| Angthong et al. 16 (prospective study) n = 21 | HPLC | ||||
| 1 g | Bone femur | 22.6 (8.7) | Yes | ||
| Bone tibia | 21.4 (11.4) | Yes | |||
| 2 g | Bone femur | 44.1 (25.8) | Yes | ||
| Bone tibia | 35.5 (5.1) | Yes | |||
| Sharareh et al. 21 (prospective study) n = 34 | HPLC | ||||
| 1 g | Bone | 5.0 (3.1) | Yes | ||
| 2 g | Bone | 8.3 (5.6) | Yes | ||
| Rivera et al.24 (prospective study) n = 22 | HPLC | ||||
| 2 g | Serum | 17.3 (range 11.2–33.2) | Yes |
HPLC = high-pressure liquid chromatography; IO = intraosseous; IV = intravenous; MIC = minimum inhibitory concentration; NR = not reported; SD = standard deviation.
Serum = μg/mL; bone = μg/g.
Procedure type
Three studies compared LTC in patients undergoing THA and TKA. All 3 studies observed a difference in bone LTC (Table 2). Concentrations observed were quite variable among studies, but the trend was similar. Bryan and colleagues,26 Yamada and colleagues27 and Sharareh and colleagues21 all found greater LTC of cefazolin in bone samples from patients who underwent THA than in those who underwent TKA. There were no observed differences in serum concentrations between procedures.
Table 2.
Effect of procedure type on target-site concentration
| Study (level of evidence) | Group | Tissue | Technique | Tissue concentration,* (SD) | Above MIC |
|---|---|---|---|---|---|
| Bryan et al.26 (prospective study) n = 48 | HPLC | ||||
| THA | Serum | 53.4 (18.9) | Yes | ||
| Bone | 1.6 (1.4) | Yes | |||
| TKA | Serum | 66.7 (35.2) | Yes | ||
| Bone | 0.6 (0.57) | No | |||
| Yamada et al.27 (prospective study) n = 43 | HPLC | ||||
| THA | Serum | 177.2 (68.1) | Yes | ||
| Bone | 32.3 (15.2) | Yes | |||
| TKA | Serum | 166 (38.5) | Yes | ||
| Bone | 16 (10.4) | Yes | |||
| Sharareh et al.21 (prospective study) n = 34 | HPLC | ||||
| THA | Bone | 11.5 (6.7) | Yes | ||
| TKA | Bone | 6.0 (3.6) | Yes |
HPLC = high-pressure liquid chromatography; MIC = minimum inhibitory concentration; SD = standard deviation; THA = total hip arthroplasty; TKA = total knee arthroplasty.
Serum = μg/mL; bone = μg/g.
Body mass index
Only 1 study evaluated the effect of BMI on LTC of cefazolin in patients undergoing arthroplasty (Table 3). Sharareh and colleagues21 gave 1 g of cefazolin if the patient weighed less than 70 kg and 2 g of cefazolin if the patient’s weight was greater than 70 kg. No notable difference was observed in LTC between BMI groups.
Table 3.
Effect of BMI on target-site concentration
| Study (level of evidence) | Group | Dosing | Technique | Tissue concentration,* (SD) | Above MIC |
|---|---|---|---|---|---|
| Sharareh et al.21 (prospective study) n = 34 | HPLC | ||||
| BMI < 25 | 1 g if < 70 kg | 8.05 (4.90) | Yes | ||
| BMI 25–30 | 6.7 (4.43) | Yes | |||
| BMI 31–35 | 2 g if > 70 kg | 8.4 (6.9) | Yes | ||
| BMI > 35 | 8.57 (3.4) | Yes |
BMI = body mass index; HPLC = high-pressure liquid chromatography; MIC = minimum inhibitory concentration; SD = standard deviation.
Serum = μg/mL; bone = μg/g.
Tourniquet utilization
Only 1 study assessed LTC based on the timing of tourniquet inflation (Table 4). Friedrich and colleagues28 compared the differences in LTC in soft tissue, bone and serum when waiting 1 minute, 2 minutes and 5 minutes to inflate the tourniquet after administration of cefazolin. Waiting 5 minutes to inflate the tourniquet had the greatest area under the concentration curve for soft tissue, bone and serum.
Table 4.
Effect of tourniquet utilization on target-site concentration
| Study (level of evidence) | Group | Tissue | Technique | Tissue concentration,* (AUC) | Above MIC |
|---|---|---|---|---|---|
| Friedrich et al.28 (prospective study) n = 24 |
HPLC | ||||
| 1 min | Soft tissues | 224 (21–408) | Yes | ||
| Bone | 200 (79–617) | Yes | |||
| Serum | 4699 (1328–8266) | Yes | |||
| 2 min | Soft tissues | 387 (133–533) | Yes | ||
| Bone | 182 (93–277) | Yes | |||
| Serum | 5170 (1301–26 272) | Yes | |||
| 5 min | Soft tissues | 671 (489–2395) | Yes | ||
| Bone | 235 (61–1804) | Yes | |||
| Serum | 6044 (2944–16 542) | Yes |
AUC = area under the serum concentration curve; HPLC = high-pressure liquid chromatography; MIC = minimum inhibitory concentration.
Serum = mg*min/L; bone = μg × min/g.
Discussion
Despite routine use of prophylactic antibiotics, PJI remains a serious issue in arthroplasty. Cefazolin is well suited for the most common PJI pathogens, having bactericidal activity against most Gram-positive organisms, aerobic Gram-negative bacilli and anerobic Gram-positive organisms.29 Despite many institutional differences worldwide, the standard preoperative dose is 1 g, and the dose increases to 2 g for patients weighing more than 80 kg and for procedures with tourniquet inflation.30,31 However, dosage recommendations and corresponding efficacy remain unclear, partly because of the unknown distribution of drugs throughout the body and actual LTC. Sufficient LTC of an antibiotic is required for optimal infection prevention by achieving MIC of 1 μg/mL. The LTC can vary owing to several parameters such as BMI, dose, route of administration, procedure type and tourniquet inflation. Knowing the LTC of cefazolin during TKA or THA will facilitate optimization of antibiotic prophylaxis and help reduce the incidence of PJI.
Of the more than 300 studies screened, only 10 reported the LTC of cefazolin in TKA and THA. Most of the study groups used mass spectrometry to quantify LTC, with some variations in their extraction protocol. Data from the studies were retrieved and used to evaluate the target parameters: dosing and route of administration, procedure type, BMI and tourniquet usage. Of the 7 studies that observed the effect of dosing on LTC, it was found that 2 g of IV cefazolin yielded a greater LTC in the bone and in the serum than a 1 g dose. Young and colleagues25 reported greater LTC with an IO administration protocol, but clinical reduction of PJI has yet to be proven to outbalance the inconvenience and applicability of such a protocol. Among the studies that evaluated the difference in LTC when comparing THA and TKA, LTC sampling tended to achieve greater bone concentration of cefazolin in THA than TKA, while maintaining similar serum concentration. We observed a twofold increase in proximal femur (THA) bone LTC compared with THA sampling across all 3 studies. Other than the effect of tourniquet inflation to explain such a trend, research groups should consider evaluating if vascularity of different bony regions affects LTC during surgery. In addition, Sharareh and colleagues21 evaluated the effect of BMI on bone LTC, showing there was no statistical difference in concentrations of cefazolin in bone between the different BMI groups. The average bone concentration was consistent with other studies, ranging between 6.7 and 8.6 μg/mL. Although morbid obesity is considered a modifiable risk factor for PJI, there is presently a lack of consensus regarding a BMI threshold above which the high risk of PJI outweighs the benefits of surgery. More research should focus solely on particularities of antibiotic prophylaxis in the context of obesity to optimize LTC and hopefully reduce infection rates in these patients. Finally, there was only 1 study that assessed the effect of a tourniquet on LTC. Waiting 5 minutes before inflating the tourniquet after cefazolin infusion had the greatest tissue penetration in soft tissue, bone and serum compared with waiting 1 or 2 minutes before inflating the tourniquet; however, longer wait times were not evaluated. To our knowledge, no studies to date have assessed LTC during a TKA with and without a tourniquet.
Limitations
Our review had several limitations that could be addressed in future research. A substantial range and variations in LTC were observed across all 10 studies. These discrepancies could be explained by evolution of extraction techniques over the last 30 years. Standardizing techniques, parameters and patient population was not possible here. Another limitation is that infection rate was not considered as a primary outcome. Having LTC as our main outcome limited the number of included studies in our review. Our primary search was intended to observe LTC in all orthopedic procedures, but no studies outside of arthroplasty procedures fit our inclusion criteria. This led us to narrow our search and focus solely on TKA and THA. Furthermore, PJI occurs in the context of a complex combination of preoperative, operative and postoperative factors. Although extensive research is conducted on PJI prevention, this review focused on and highlighted the paucity of studies showing levels of LTC and on whether usual antibiotic prophylaxis practices in orthopedics achieve required MIC for common microorganisms.
This systematic review did highlight several gaps in our understanding of LTC of cefazolin that would help us optimize antibiotic prophylaxis. Although all included studies used the premise that prophylactic antibiotics should be completely infused within 60 minutes before the surgical incision, no study looked at the effect of different preoperative time intervals on LTC throughout the surgery. Cefazolin achieves highest peak bone concentrations 40 minutes after parenteral application with a serum half-life of 108 minutes and a bone half-life of 42 minutes.20 By using the given pharmacokinetics of cefazolin and a mathematical model, Bicanic and colleagues31 inferred that parenteral application of cefazolin should be no longer than 30 minutes before incision (tourniquet inflation) and no less than 10 minutes before tourniquet inflation, if given in bolus to achieve maximal blood and bone LTC. This must be further studied with dedicated LTC measurements based on different administration courses. Furthermore, the duration of LTC is not well studied and could help guide the timing for redosing. Another parameter that should be further assessed is BMI as it is considered a modifiable risk factor for PJI in the arthroplasty literature, with the risk for infection increasing gradually as BMI increases. In this review, only 1 study assessed the change in LTC with BMI. As obesity rates continue to rise worldwide, it is paramount to understand the change in LTC to optimize antibiotic prophylaxis and hopefully prevent adverse outcomes.
We found a single study analyzing the effect of timing of tourniquet inflation on LTC. No studies assessed the effect of performing a TKA with or without a tourniquet on LTC. Tourniquet inflation during TKA is commonly used to reduce bleeding in the surgical field, thereby facilitating exposure and cementation. However, this reduced circulation in the limb may also reduce antibiotic distribution and clearance in the periincisional tissues. Recently, improved immediate pain and functional outcomes of TKA without a tourniquet have been suggested.32 The effect of TKA surgery without a tourniquet on LTC should be explored.
Conclusion
We highlighted a paucity of studies that measured levels of LTC to validate the current recommendations for antibiotic prophylaxis for arthroplasty surgery. Future studies evaluating the parameters highlighted in our review are essential considering that PJI will remain the leading source of early reoperation or revision surgery in arthroplasty and that prophylactic antibiotics are the single most effective preventative measure.
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study. M. Mannarino and J. Montreuil acquired and analyzed the data. M. Mannarino and J. Montreuil wrote the article, which M. Tanzer and A. Hart reviewed. All authors approved the final version to be published.
References
- 1.Agodi A, Auxilia F, Barchitta M, et al. Risk of surgical site infections following hip and knee arthroplasty: results of the ISChIA-GISIO study. Ann Ig 2017;29:422–30. [DOI] [PubMed] [Google Scholar]
- 2.de Jong L, Klem T, Kuijper TM, et al. Factors affecting the rate of surgical site infection in patients after hemiarthroplasty of the hip following a fracture of the neck of the femur. Bone Joint J 2017;99-B:1088–94. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz SM, Lau E, Watson H, et al. Economic burden of peri-prosthetic joint infection in the United States. J Arthroplasty 2012;27:61–5.e1. [DOI] [PubMed] [Google Scholar]
- 4.Parvizi J, Pawasarat IM, Azzam KA, et al. Periprosthetic joint infection: the economic impact of methicillin-resistant infections. J Arthroplasty 2010;25:103–7. [DOI] [PubMed] [Google Scholar]
- 5.Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am 2013;95:e50. [DOI] [PubMed] [Google Scholar]
- 6.Prokuski L. Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg 2008;16:283–93. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher N, Sofianos D, Berkes MB, et al. Prevention of perioperative infection. J Bone Joint Surg Am 2007;89:1605–18. [DOI] [PubMed] [Google Scholar]
- 8.Aslam S, Darouiche RO. Prosthetic joint infections. Curr Infect Dis Rep 2012;14:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bratzler DW, Houck PM, et al. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis 2004;38:1706–15. [DOI] [PubMed] [Google Scholar]
- 10.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013;70:195–283. [DOI] [PubMed] [Google Scholar]
- 11.Moine P, Fish DN. Pharmacodynamic modelling of intravenous antibiotic prophylaxis in elective colorectal surgery. Int J Antimicrob Agents 2013;41:167–73. [DOI] [PubMed] [Google Scholar]
- 12.Brill MJ, Houwink API, Schmidt S, et al. Reduced subcutaneous tissue distribution of cefazolin in morbidly obese versus non-obese patients determined using clinical microdialysis. J Antimicrob Chemother 2014;69:715–23. [DOI] [PubMed] [Google Scholar]
- 13.Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery 1961;50:161–8. [PubMed] [Google Scholar]
- 14.Quintiliani R, Nightingale C. Principles of antibiotic usage. Clin Orthop Relat Res 1984;31–5. [PubMed] [Google Scholar]
- 15.Steinberg JP, et al. Timing of antimicrobial prophylaxis and the risk of surgical site infections: results from the Trial to Reduce Anti-microbial Prophylaxis Errors. Ann Surg 2009;250:10–6. [DOI] [PubMed] [Google Scholar]
- 16.Angthong C, Krajubngern P, Tiyapongpattana W, et al. Intraosseous concentration and inhibitory effect of different intravenous cefazolin doses used in preoperative prophylaxis of total knee arthroplasty. J Orthop Traumatol 2015;16:331–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller M, dela Pena A, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob Agents Chemother 2004;48:1441–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osorio C, Garzon L, Jaimes D, et al. Impact on antibiotic resistance, therapeutic success, and control of side effects in therapeutic drug monitoring (TDM) of daptomycin: a scoping review. Antibiotics (Basel) 2021;10:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DN, Gustilo RB, Beverly R, et al. Bone and serum concentrations of five cephalosporin drugs. Relevance to prophylaxis and treatment in orthopedic surgery. Clin Orthop Relat Res 1983;253–65. [PubMed] [Google Scholar]
- 21.Sharareh B, Sutherland C, Pourmand D, et al. Effect of body weight on cefazolin and vancomycin trabecular bone concentrations in patients undergoing total joint arthroplasty. Surg Infect 2016;17:71–7. [DOI] [PubMed] [Google Scholar]
- 22.Cunha BA, Gossling HR, Pasternak HS, et al. The penetration characteristics of cefazolin, cephalothin, and cephradine into bone in patients undergoing total hip replacement. J Bone Joint Surg Am 1977;59:856–9. [PubMed] [Google Scholar]
- 23.Polk R, Hume A, Kline BJ, et al. Penetration of moxalactam and cefazolin into bone following simultaneous bolus or infusion. Clin Orthop Relat Res 1983;177:216–21. [PubMed] [Google Scholar]
- 24.Rivera A, Sánchez A, Luque S, et al. Intraoperative bacterial contamination and activity of different antimicrobial prophylaxis regimens in primary knee and hip replacement. Antibiotics (Basel) 2020;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young SW, Zhang M, Freeman JT, et al. Higher cefazolin concentrations with intraosseous regional prophylaxis in TKA. Clin Orthop Relat Res 2013;471:244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryan CS, Morgan SL, Caton RJ, et al. Cefazolin versus cefamandole for prophylaxis during total joint arthroplasty. Clin Orthop Relat Res 1988;228:117–22. [PubMed] [Google Scholar]
- 27.Yamada K, Matsumoto K, Tokimura F, et al. Are bone and serum cefazolin concentrations adequate for antimicrobial prophylaxis? Clin Orthop Relat Res 2011;469:3486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedrich LV, White RL, Brundage DM, et al. The effect of tourniquet inflation on cefazolin tissue penetration during total knee arthroplasty. Pharmacotherapy 1990;10:373–7. [PubMed] [Google Scholar]
- 29.Li ZL, Hou YF, Zhang BQ, et al. Identifying common pathogens in periprosthetic joint infection and testing drug-resistance rate for different antibiotics: a prospective, single center study in Beijing. Orthop Surg 2018;10:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho VP, Nicolau DP, Dakin GF, et al. Cefazolin dosing for surgical prophylaxis in morbidly obese patients. Surg Infect (Larchmt) 2012;13:33–7. [DOI] [PubMed] [Google Scholar]
- 31.Bicanic G, Crnogaca K, Barbaric K, et al. Cefazolin should be administered maximum 30 min before incision in total knee arthroplasty when tourniquet is used. Med Hypotheses 2014;82: 766–8. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Graham D, Gillies K, et al. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res 2014;26:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]