Abstract
Background
Convenient administration of coronavirus disease 2019 (COVID-19) treatment in community settings is desirable. Sotrovimab is a pan-sarbecovirus dual-action monoclonal antibody formulated for intravenous (IV) or intramuscular (IM) administration for early treatment of mild/moderate COVID-19.
Method
This multicenter phase 3 study based on a randomized open-label design tested the noninferiority of IM to IV administration according to an absolute noninferiority margin of 3.5%. From June to August 2021, patients aged ≥12 years with COVID-19, who were neither hospitalized nor receiving supplemental oxygen but were at high risk for progression, were randomized 1:1:1 to receive sotrovimab as a single 500-mg IV infusion or a 500- or 250-mg IM injection. The primary composite endpoint was progression to (1) all-cause hospitalization for >24 hours for acute management of illness or (2) all-cause death through day 29.
Results
Sotrovimab 500 mg IM was noninferior to 500 mg IV: 10 (2.7%) of 376 participants vs 5 (1.3%) of 378 met the primary endpoint, respectively (absolute adjusted risk difference, 1.06%; 95% CI, −1.15% to 3.26%). The 95% CI upper limit was lower than the prespecified noninferiority margin of 3.5%. The 250-mg IM group was discontinued early because of the greater proportion of hospitalizations vs the 500-mg groups. Serious adverse events occurred in <1% to 2% of participants across groups. Four participants experienced serious disease-related events and died (500 mg IM, 2/393, <1%; 250 mg IM, 2/195, 1%).
Conclusions
Sotrovimab 500-mg IM injection was well tolerated and noninferior to IV administration. IM administration could expand outpatient treatment access for COVID-19.
Clinical Trials Registration
ClinicalTrials.gov: NCT04913675.
Keywords: COMET-TAIL, COVID-19 treatment, intramuscular administration, neutralizing monoclonal antibody, sotrovimab
Sotrovimab administered intramuscularly at 500 mg was noninferior to 500 mg administered intravenously for treatment of mild/moderate COVID-19 in patients at high risk, as measured by all-cause hospitalization >24 hours or death through day 29, and it was well tolerated. Intramuscular sotrovimab should provide easier outpatient access to COVID-19 treatment.
Severe coronavirus disease 2019 (COVID-19) is associated with a substantial burden on health care resources, particularly among patients who are unvaccinated and patients with risk factors for progression to severe disease. To prevent disease progression and hospitalization in patients with mild to moderate COVID-19 who are at high risk, several monoclonal antibodies (mAbs) have been authorized for early treatment [1–6]. Sotrovimab targets a conserved epitope in the spike protein of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), demonstrating potent neutralizing activity against wild type and most SARS-CoV-2 variants, including B.1.617.2 (Delta) and B.1.1.529 (Omicron, BA.1, and BA1.1) [7–10]. Although 500-mg sotrovimab was not utilized in the United States with the emergence of Omicron BA.2 due to moderate decreases of in vitro neutralization activity [7, 11], sotrovimab continues to be included in the treatment guidelines for high-risk patients with COVID-19 in other countries, including the United Kingdom, Germany, Italy, Spain, Japan, and the United Arab Emirates [12–16]—an inclusion supported by recent observational clinical data suggesting continued clinical effectiveness through the Omicron BA.2 and BA.5 waves [17–20]. Monoclonal antibodies, including sotrovimab, continue to be recommended in guidelines for specific cases (eg, when remdesivir is not feasible) and for certain patients and high-risk groups (eg, those with contraindications to nirmatrelvir/ritonavir, those who are immunocompromised). Understanding the feasibility of an intramuscular (IM) route of administration for sotrovimab may inform development of future anti–SARS-CoV-2 mAbs, ensuring greater feasibility of dosing and broader access.
There remains a need for mAb treatments to prevent COVID-19 progression and decrease barriers for their administration, including infrastructure, staffing, isolation, and infection control associated with intravenous (IV) infusions [21]. Sotrovimab has been formulated for IV or IM administration. The simplicity of 1-time IM administration would allow for additional treatment capacity, reduce appointment times, and afford the ability to expand staffing resources to administer treatment, thereby decreasing health care burden. IM administration of mAbs could also prevent treatment delays, allowing provision of treatment earlier in the clinical course of disease, which has been associated with more favorable outcomes [4, 22].
From August 2020 to March 2021, the phase 3 COMET-ICE trial demonstrated the efficacy and safety of IV sotrovimab in patients with mild to moderate COVID-19 at high risk for disease progression [4, 5]. In the primary analysis (n = 1057), sotrovimab indicated a statistically significant reduction in hospitalization for >24 hours for acute management of any illness or death due to any cause through day 29 vs placebo (adjusted relative risk reduction, 79%; 95% CI, 50%–91%; P < .001) [5]. IV sotrovimab was well tolerated with no unanticipated safety signals.
The COMET-TAIL study evaluated the efficacy, safety, and tolerability of IM sotrovimab vs IV sotrovimab for the treatment of mild to moderate COVID-19 in patients at high risk.
METHODS
Study Design
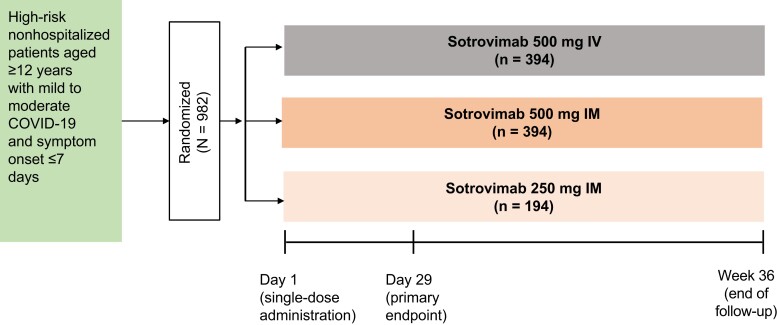
This multicenter phase 3 study based on a randomized open-label design was developed as a noninferiority trial and was not placebo controlled given the clinical efficacy of IV sotrovimab [4, 5] and the endorsement of mAbs in treatment guidelines at the time of the study [23, 24]. A 3.5% noninferiority margin was chosen on the basis of feedback and scientific reasoning in collaboration with the US Food and Drug Administration. IM doses of 250 and 500 mg were selected to ensure that sotrovimab concentrations in the lung were maintained at or above levels anticipated to be neutralizing for the duration of the treatment window.
Patient Consent Statement
The study was conducted in accordance with the consensus ethical principles derived from the Declaration of Helsinki and the International Ethical Guidelines of the Council for International Organizations of Medical Sciences, applicable guidelines of the International Council for Harmonisation Good Clinical Practice, and applicable laws and regulations. Ethics approval was obtained from the following institutional review boards and ethics committees: Advarra Institutional Review Board and CEQ at Medical Center of Limited Liability Company Harmoniya krasy. The protocol was also approved by CPP Sud-Est II—Groupement Hospitalier Est, but no participants were enrolled in France. Written informed consent/assent was provided by all participants.
Participants
Eligible patients were aged ≥12 years at the time of consent and were at high risk for progression of COVID-19, including age ≥55 years and the presence of comorbidities (eg, diabetes, obesity, chronic kidney disease, congenital heart disease, congestive heart failure, chronic lung disease, sickle cell disease, neurodevelopmental disorders, immunosuppression, or chronic liver disease). Initially, eligibility was considered independent of vaccination status. The protocol was amended on 29 June 2021 to exclude fully vaccinated immunocompetent participants (defined as those with at least 14 days since receiving the final dose in a COVID-19 vaccine series) because they may have reduced rates of progression. There were no eligibility restrictions for history of SARS-CoV-2 infection.
Participants had a positive SARS-CoV-2 test result by any validated diagnostic test (eg, reverse transcriptase–polymerase chain reaction [RT-PCR], antigen-based testing on any specimen type), oxygen saturation ≥94% while breathing room air, and COVID-19 symptoms. Eligible participants received sotrovimab ≤7 days from onset of symptoms.
Exclusions included individuals who were hospitalized or likely to require hospitalization within 24 hours (as assessed by the investigator) and those with severe COVID-19 (ie, shortness of breath at rest, respiratory distress, or requiring supplemental oxygen for COVID-19).
Randomization and Intervention
Participants were randomly assigned 1:1:1 to receive a single 500-mg IV infusion or 500- or 250-mg IM injection of sotrovimab, with stratification based on age (12–17, 18–64, and ≥65 years), COVID-19 vaccination history (receipt of any COVID-19 vaccine dose), and geographic region.
After IM injection or 15-minute IV infusion, participants were monitored for 30 minutes, during which time vital signs were measured at 15 and 30 minutes; for IM injection, solicited assessment of injection-site reactions also occurred at 15 and 30 minutes. Participants were monitored for 36 weeks on an outpatient basis with collection of nasopharyngeal swabs for virology, blood draws for pharmacokinetic (PK) sampling, and safety laboratory tests (Figure 1). Sparse PK samples were collected through week 24, and sotrovimab serum concentrations were determined via an electrochemiluminescent method validated on the Meso Scale Discovery platform. This analysis includes efficacy data through day 29, safety data through week 36, and PK data through week 24.
Figure 1.
Study design. IM, intramuscular; IV, intravenous.
Outcomes
The primary endpoint was a composite of progression to (1) hospitalization for >24 hours for acute management of any illness or (2) death due to any cause through day 29. Secondary efficacy endpoints were as follows: SARS-CoV-2 viral load in nasal secretions measured by quantitative RT-PCR (key secondary endpoint 1); a composite of emergency department visit for management of any illness, hospitalization for acute management of any illness for any duration, or death due to any cause (key secondary endpoint 2); and development of severe and/or critical respiratory COVID-19 as manifested by requirement for respiratory support (including oxygen).
Adverse events (AEs), AEs of special interest, and disease-related events (DREs) were assessed through week 12. Serious AEs were assessed through week 36. AEs of special interest were systemic and local infusion/injection-related reactions and local tolerability (injection-site reactions). Injection-site reactions in the IM groups were solicited at days 1, 3, 5, and 8 postinjection and reported separate from AEs. DREs were defined as AEs related to expected COVID-19 progression, signs, or symptoms, unless they were more severe than expected or the investigator considered them related to the study drug.
Statistical Analysis
A sample size of 340 participants per treatment group was expected to provide approximately 90% power to demonstrate that IM injection of sotrovimab was noninferior to IV infusion of sotrovimab for the primary endpoint. Analysis was based on a 1-sided 2.5% type I error rate, with the assumption of a COVID-19 progression rate of 2% in the sotrovimab IM and IV groups and a 3.5% noninferiority margin on the risk difference scale.
The intent-to-treat (ITT) population included all randomized participants, excluding those who were immunocompetent and fully vaccinated under the original protocol. The primary analysis population was based on the ITT population but excluded participants not meeting key eligibility criteria (eg, those without a positive baseline SARS-CoV-2 test result). Safety was assessed in all randomized participants exposed to the study treatment (as-treated population).
The primary efficacy estimand was based on a hypothetical strategy to account for all intercurrent events (ie, not receiving randomized treatment, discontinuation of study treatment, and use of medication not permitted during the study). Data observed after an intercurrent event were set to missing. Missing data were imputed under a missing-at-random assumption based on multiple imputation—specifically, the probability that a participant with missing data would go on to be a progressor is the same as a participant with complete data when covariates are adjusted for the missing data. This method was chosen as a moderately conservative means of handling missing data. A post hoc change was made to the multiple imputation algorithm from daily to weekly imputation due to the bias that was observed in the imputed progression rates (see Supplementary Data: Statistical Technical Appendix). A supplementary estimand was conducted in the efficacy population by handling all intercurrent events with a treatment policy strategy (ie, regardless of the intercurrent events). A tipping point analysis for the primary endpoint was conducted to determine the impact of imputing missing data as progressions.
The proportion of participants meeting the primary endpoint and key secondary endpoint 2 were compared between treatments by a binomial regression model with an identity link function and adjusted for treatment group, age (<65, ≥65 years), and sex as covariates. The adjusted risk difference and associated 95% CI were computed to test the noninferiority of IM vs IV sotrovimab, which was declared if the upper bound of the 2-sided 95% CI for the adjusted risk difference was <3.5%. Participants in the ITT population with a laboratory-confirmed quantifiable baseline nasopharyngeal swab at day 1 (virology population) were evaluated for mean area under the curve of SARS-CoV-2 viral load in nasal secretions as measured by quantitative RT-PCR from day 1 to day 8 (AUCd1-8). Viral loads from IM and IV doses were compared for equivalence based on the 2-sided 90% CI for the treatment ratio falling within equivalence bounds of 0.5 to 2.0.
A gatekeeping hierarchical testing procedure was used for testing the key secondary efficacy endpoints (Supplementary Figure 1). All statistical analyses were conducted with SAS version 9.4 (SAS Institute).
During the study, a discrepancy was noted in the rate of progression in the 250-mg IM group vs the 500-mg IM and IV groups. An ad hoc interim data set was reviewed by an independent data monitoring committee, and enrollment into the 250-mg IM group was subsequently discontinued. The study changed to a 2-group design with 1:1 randomization up to ∼340 participants per group (500 mg IM and IV). The 250-mg IM group was removed from the testing hierarchy, and data are summarized descriptively.
RESULTS
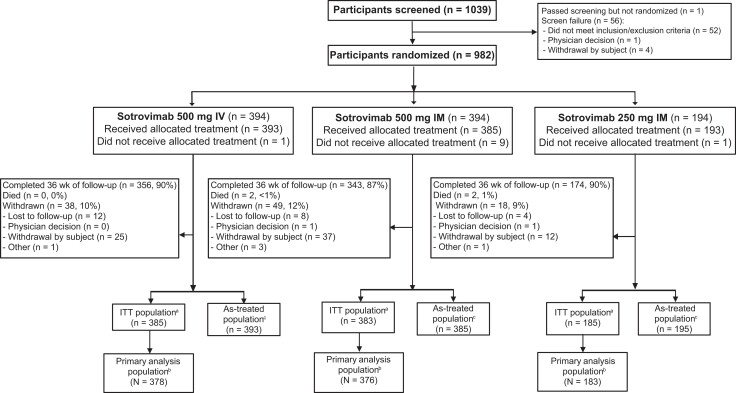
Between 10 June and 19 August 2021, 1039 participants were screened, and 982 were randomized to sotrovimab 500 mg IV (n = 394), 500 mg IM (n = 394), and 250 mg IM (n = 194; Figure 2). Of the 982 participants, 29 were randomized under the original protocol as immunocompetent and fully vaccinated and thus excluded. An additional 16 participants were excluded: 2 without a positive SARS-CoV-2 test result and 14 who were immunocompetent and fully vaccinated and inadvertently enrolled under protocol amendment 2. Thus, the primary analysis population consisted of 937 patients (500 mg IV, n = 378; 500 mg IM, n = 376; 250 mg IM, n = 183).
Figure 2.
Patient enrollment and treatment assignment (sotrovimab). aITT population includes all randomly assigned participants, excluding those who were immunocompetent and fully vaccinated under the original protocol. bPrimary analysis population is defined as the ITT population minus those who violated key inclusion/exclusion criteria. cThe as-treated (safety) population includes all participants who received the study treatment. Two participants who were randomized to sotrovimab 500 mg IM received 250 mg IM and are included in the latter as-treated population. IM, intramuscular; IV, intravenous; ITT, intent to treat.
Participants were enrolled in the Ukraine (<1%) and the United States (>99%), predominantly in Florida (Supplementary Table 1). Demographic and baseline characteristics were balanced across the sotrovimab groups, except for sex (Table 1). Approximately 23% were aged ≥65 years; most were Hispanic or Latino. The most common risk factors for COVID-19 progression were obesity, age ≥55 years, chronic lung disease, and diabetes; 3% of participants in each group had an immunosuppressive disease. Nearly one-third of participants had ≥2 risk factors for COVID-19 progression. Most participants (86%–88%) had a symptom duration ≤5 days at baseline.
Table 1.
Demographic and Baseline Characteristics: Primary Analysis Population
| Sotrovimab, No. (%) or Median (IQR) | |||
|---|---|---|---|
| 500 mg IV (n = 378) | 500 mg IM (n = 376) | 250 mg IM (n = 183) | |
| Sex | |||
| Female | 218 (58) | 187 (50) | 106 (58) |
| Male | 160 (42) | 189 (50) | 77 (42) |
| Age, y | 51.0 (38.0–65.0) | 52.0 (37.5–64.5) | 48.0 (37.0–57.0) |
| 12–17 | 2 (<1) | 0 (0) | 1 (<1) |
| 18–64 | 281 (74) | 282 (75) | 156 (85) |
| ≥65 | 95 (25) | 94 (25) | 26 (14) |
| 65–74 | 65 (17) | 56 (15) | 17 (9) |
| 75–84 | 24 (6) | 32 (9) | 8 (4) |
| ≥85 | 6 (2) | 6 (2) | 1 (<1) |
| Ethnicity | |||
| Hispanic or Latino | 312 (83) | 320 (85) | 157 (86) |
| Not Hispanic or Latino | 66 (17) | 56 (15) | 26 (14) |
| Race | |||
| Asian | 0 (0) | 2 (<1) | 0 (0) |
| Black or African American | 14 (4) | 17 (5) | 8 (4) |
| Mixed race | 1 (<1) | 1 (<1) | 1 (<1) |
| Native Hawaiian or other Pacific Islander | 1 (<1) | 0 (0) | 0 (0) |
| White | 360 (96) | 352 (94) | 172 (95) |
| Other | 0 (0) | 3 (<1) | 1 (<1) |
| BMI, kg/m2 | 30.97 (27.54–33.15) | 30.83 (27.75–32.70) | 31.23 (27.43–33.15) |
| Conditions as risk factor for COVID-19 progression | |||
| Any condition | 376 (>99) | 374 (>99) | 181 (99) |
| Obesitya | 237 (63) | 233 (62) | 115 (63) |
| Age ≥55 y | 158 (42) | 163 (43) | 62 (34) |
| Chronic lung diseases | 59 (16) | 69 (18) | 43 (23) |
| Diabetes | 48 (13) | 46 (12) | 22 (12) |
| Immunosuppressive disease or medications | 11 (3) | 12 (3) | 5 (3) |
| Chronic liver disease | 4 (1) | 4 (1) | 4 (2) |
| Congestive heart failure: NYHA class II or more | 4 (1) | 3 (<1) | 1 (<1) |
| Chronic kidney disease: eGFR <60 by MDRD equation | 0 (0) | 2 (<1) | 0 (0) |
| Congenital heart disease | 1 (<1) | 0 (0) | 1 (<1) |
| Sickle cell disease | 1 (<1) | 0 (0) | 0 (0) |
| No. of conditions met | |||
| 0 | 2 (<1) | 2 (<1) | 2 (1) |
| 1 | 266 (70) | 253 (67) | 126 (69) |
| 2 | 79 (21) | 87 (23) | 39 (21) |
| 3 | 26 (7) | 31 (8) | 15 (8) |
| >3 | 5 (1) | 3 (<1) | 1 (<1) |
| COVID-19 vaccination historyb | 17 (4) | 18 (5) | 11 (6) |
| Symptom duration, d | |||
| ≤5 | 324 (86) | 332 (88) | 160 (87) |
| 5–7 | 54 (14) | 44 (12) | 23 (13) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; IM, intramuscular; IV, intravenous; MDRD, Modification of Diet in Renal Disease; NYHA, New York Heart Association.
Obesity was defined as having a BMI ≥85th percentile for age/gender based on growth charts per the Centers for Disease Control and Prevention.
COVID-19 vaccination history was defined as receipt of at least 1 dose of any COVID-19 vaccination prior to randomization.
Among participants receiving 500 mg of sotrovimab, 10 of 376 (2.7%) in the IM group vs 5 of 378 (1.3%) in the IV group met progression criteria for the primary endpoint (adjusted absolute risk difference, 1.06%; 95% CI, −1.15% to 3.26%; Table 2). The upper limit of the CI was lower than the prespecified noninferiority margin of 3.5%, indicating that 500 mg IM is noninferior to 500 mg IV for treatment of mild/moderate COVID-19. Among the 5 participants in the 500-mg IV group who were hospitalized >24 hours, 2 events were reported as COVID-19 related and 3 were due to other causes (acute renal failure of donor kidney, appendicitis, and elevated glucose level). For the 10 participants in the 500-mg IM group who were hospitalized >24 hours, 6 events were COVID-19 related and 4 were due to other causes (worsening bacterial pneumonia, acute appendicitis, shingles, and decompensated heart failure). Two participants in the 500-mg IM group who were hospitalized for COVID-19–related events died. Ten participants (5.5%) receiving 250 mg IM progressed, with 9 events related to COVID-19 and 1 due to other causes (exacerbation of chronic obstructive pulmonary disease). Two participants in the 250-mg IM group progressed to hospitalization for COVID-19–related events and died after day 29.
Table 2.
Primary and Secondary Efficacy Outcomes Through Day 29
| Sotrovimab, No. (%)a | |||
|---|---|---|---|
| 500 mg IV | 500 mg IM | 250 mg IM | |
| Primary outcomeb | 378 | 376 | 183 |
| Hospitalized >24 h or death: due to any cause | 5 (1.3) | 10 (2.7) | 10 (5.5) |
| Hospitalized >24 h | 5 (1.3) | 10 (2.7) | 10 (5.5) |
| Death | 0 (0) | 2 (0.5) | 0 (0) |
| Alive and not hospitalized | 364 (96.3) | 351 (93.4) | 170 (92.9) |
| Missing | 9 (2.4) | 15 (4.0) | 3 (1.6) |
| 500 mg IV vs IMc | 1.06 (−1.15 to 3.26) | … | … |
| Secondary outcomes | |||
| Mean AUCd1-8 SARS-CoV-2 viral load, log10 copies/mL (virology population) | 287 | 278 | 136 |
| Geometric mean (% CV) | 25.42 (34.14) | 25.56 (36.94) | 25.46 (37.90) |
| Adjusted LS geometric mean | 25.03 | 25.96 | … |
| Ratio (90% CI) | 1.04 (1.00-1.07) | … | … |
| Hospitalization, ED visit, or death: due to any causeb | 378 | 376 | 183 |
| Hospitalization, ED visit, or death | 9 (2.4) | 12 (3.2) | 11 (6.0) |
| Hospitalized | 5 (1.3) | 10 (2.7) | 10 (5.5) |
| ED visit | 5 (1.3) | 3 (0.8) | 3 (1.6) |
| Death | 0 (0) | 2 (0.5) | 0 (0) |
| Alive and not hospitalized and no ED visit | 360 (95.2) | 349 (92.8) | 169 (92.3) |
| Missing | 9 (2.4) | 15 (4.0) | 3 (1.6) |
| 500 mg IV vs IMd | 0.86 (−1.56 to 3.28) | … | … |
| Progression to severe/critical respiratory COVID-19 (intent-to-treat population) | 385 | 383 | 185 |
| Progression to severe/critical respiratory COVID-19e | 1 (0.3) | 6 (1.6) | 8 (4.3) |
| Low-flow nasal cannula/face mask (severe) | 1 (0.3) | 2 (0.5) | 4 (2.2) |
| Non-rebreather mask or high-flow nasal cannula/noninvasive ventilation (including continuous positive airway pressure support) | 0 (0) | 2 (0.5) | 2 (1.1) |
| Mechanical ventilation/extracorporeal membrane oxygenation | 0 (0) | 0 (0) | 2 (1.1) |
| Death | 0 (0) | 2 (0.5) | 0 (0) |
| No progression to severe/critical respiratory COVID-19 | 375 (97.4) | 362 (94.5) | 174 (94.1) |
| Missing | 9 (2.3) | 15 (3.9) | 3 (1.6) |
Abbreviations: AUCd1-8, area under the curve (day 1 to day 8); CV, coefficient of variation; ED, emergency department; IM, intramuscular; IV, intravenous; LS, least squares; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are presented as No. (%) unless noted otherwise.
Primary analysis population, hypothetical estimand: patients are counted in each subcategory of progression experienced up to the time point in question and so may be included in >1 category.
Absolute adjusted risk difference (95% CI). In percentages.
Adjusted risk difference (95% CI). In percentages.
Severe respiratory COVID-19 was defined as a requirement for supplemental oxygen by nasal cannula, face mask, high-flow oxygen devices, or noninvasive ventilation. Critical respiratory COVID-19 was defined as a requirement for invasive mechanical ventilation or extracorporeal membrane oxygenation. A patient's worst respiratory status over day 1 to day 29 is reported, with death as the maximal value.
For key secondary endpoint 1 (SARS-CoV-2 viral load in nasal secretions in the virology population, n = 757), the adjusted mean viral load AUCd1-8 was equivalent between sotrovimab 500 mg IV (25.03 log10 copies/mL) and 500 mg IM (25.96 log10 copies/mL; 90% CI, 1.00–1.07), falling within the bounds of 0.5 to 2.0. The unadjusted mean AUCd1-8 for sotrovimab 250 mg IM was 25.46 log10 copies/mL (Table 2). Absolute viral load over time is shown in Supplementary Figure 2. Overall, there was no clear difference in the change in viral load over time across treatment arms.
For key secondary endpoint 2 (COVID-19 progression to emergency department visit, hospitalization for any duration, or death), 9 (2.4%) participants in the 500-mg IV group and 12 (3.2%) in the 500-mg IM group met the progression criteria, with an adjusted risk difference of 0.86% (95% CI, −1.56% to 3.28%); the upper limit of the CI was lower than the prespecified noninferiority margin of 3.5% (Table 2). Progression to severe and/or critical respiratory COVID-19 occurred in 1 (0.3%) and 6 (1.6%) participants in the sotrovimab 500-mg IV and IM groups, respectively (Table 2). The participant who progressed after sotrovimab 500 mg IV required low-flow oxygen by nasal cannula or face mask. In the sotrovimab 500-mg IM group, 2 participants required low-flow oxygen, and 2 required a non-rebreather mask or high-flow oxygen. Another 2 required supplemental oxygen via bilevel positive airway pressure and invasive mechanical ventilation; both subsequently died. Eleven participants (6.0%) receiving sotrovimab 250 mg IM met the criteria for key secondary endpoint 2, and 8 (4.3%) progressed to severe and/or critical respiratory COVID-19 (Table 2).
Based on tipping point analyses, the outcome for the primary endpoint would switch from noninferior to not noninferior if the underlying progression rate in the missing data was ≥9% (or approximately 2 of 13 participants in the 500-mg IM group and 1 of 9 in the 500-mg IV group; Supplementary Figure 3). Results based on the treatment policy estimand were consistent with those of the hypothetical estimand (Supplementary Table 2).
Overall, the incidence of AEs was low and similar between the IV and IM treatment groups through week 12 (Table 3). Grade 3 or 4 AEs occurred in 2% of participants in each treatment group, and none of these AEs were considered related to sotrovimab. Serious AEs were reported in 3 (<1%), 7 (2%), and 3 (2%) participants in the 500-mg IV, 500-mg IM, and 250-mg IM groups, respectively. No serious AEs were considered related to treatment. All serious AEs are listed in the Supplementary Results. AEs related to expected progression, signs, or symptoms of COVID-19 were reported separately as DREs. The most frequent DREs were COVID-19 pneumonia and pneumonia (Table 3). Two participants in the 500-mg IM group and 2 in the 250-mg IM group experienced serious DREs and died (Supplementary Data). No one in the 500-mg IV group died.
Table 3.
AEs Through Week 12: As-treated Population
| Sotrovimab, No. (%) | |||
|---|---|---|---|
| 500 mg IV (n = 393) | 500 mg IM (n = 385) | 250 mg IM (n = 195) | |
| Any AE | 39 (10) | 41 (11) | 26 (13) |
| Related to study treatmenta | 7 (2) | 4 (1) | 3 (2) |
| Leading to permanent discontinuation of study treatment | 0 (0) | 0 (0) | 0 (0) |
| Leading to dose interruption/delay | 1 (<1) | 0 (0) | 0 (0) |
| Any grade 3 or 4 AE | 8 (2) | 8 (2) | 4 (2) |
| Related to study treatmenta | 0 (0) | 0 (0) | 0 (0) |
| Any serious AEb | 3 (<1) | 7 (2) | 3 (2) |
| Related to study treatmenta | 0 (0) | 0 (0) | 0 (0) |
| Fatal | 0 (0) | 0 (0) | 0 (0) |
| Any injection/infusion-related reaction | 2 (<1) | 1 (<1) | 1 (<1) |
| Chills | 1 (<1) | 0 (0) | 0 (0) |
| Hypersensitivity | 1 (<1) | 0 (0) | 0 (0) |
| Pyrexia | 1 (<1) | 0 (0) | 0 (0) |
| Pruritus | 0 (0) | 1 (<1) | 0 (0) |
| Asthma | 0 (0) | 0 (0) | 1 (<1) |
| Any DRE | 18 (5) | 16 (4) | 20 (10) |
| Leading to study discontinuation | 0 (0) | 2 (<1) | 2 (1) |
| Any grade 3 or 4 DRE | 4 (1) | 4 (1) | 6 (3) |
| Leading to study discontinuation | 0 (0) | 0 (0) | 0 (0) |
| Any serious DRE | 3 (<1) | 6 (2) | 10 (5) |
| Leading to study discontinuation | 0 (0) | 2 (<1) | 2 (1) |
| Fatal | 0 (0) | 2 (<1) | 2 (1) |
| DREs in ≥2 patients across treatment groups | |||
| COVID-19 pneumonia | 2 (<1) | 4 (1) | 4 (2) |
| Pneumonia | 1 (<1) | 2 (<1) | 5 (3) |
| Increased lipase level | 1 (<1) | 4 (1) | 2 (1) |
| Cough | 3 (<1) | 0 (0) | 2 (1) |
| Acidosis | 2 (<1) | 2 (<1) | 0 (0) |
| Dyspnea | 1 (<1) | 2 (<1) | 1 (<1) |
| Pharyngeal erythema | 1 (<1) | 2 (<1) | 1 (<1) |
| Bronchitis | 1 (<1) | 0 (0) | 2 (1) |
| Back pain | 0 (0) | 1 (<1) | 1 (<1) |
| COVID-19 | 0 (0) | 2 (<1) | 0 (0) |
| Dehydration | 1 (<1) | 0 (0) | 1 (<1) |
| Headache | 1 (<1) | 1 (<1) | 0 (0) |
| Pyrexia | 0 (0) | 0 (0) | 2 (1) |
| Thrombocytopenia | 1 (<1) | 0 (0) | 1 (<1) |
| Thrombocytosis | 1 (<1) | 1 (<1) | 0 (0) |
The as-treated population includes all patients who received the study intervention and are analyzed according to the treatment received.
Abbreviations: AE, adverse event; DRE, disease-related event; IM, intramuscular; IV, intravenous.
Relatedness was determined by individual study investigators.
Serious AEs were collected through week 36; no serious AEs occurred after week 12.
Four participants had injection/infusion-related reactions (500 mg IV, n = 2; 500 mg IM, n = 1; 250 mg IM, n = 1; Table 3). Solicited injection-site reactions following sotrovimab 500 mg IM and 250 mg IM were grade 1 in 39 (10%) and 22 (11%) participants, grade 2 in 7 (2%) and 2 (1%), and grade 3 in 1 (<1%) and 0 participants, respectively. Grade 1 pain and tenderness at 15 to 30 minutes postdose were the most common injection-site reactions. Few events occurred at day 3 and beyond.
In the 500-mg IV and IM groups, median serum concentrations of sotrovimab in participants with progression of COVID-19 were comparable to those who did not progress (Supplementary Figure 4 and Supplementary Table 3). However, in the 250-mg IM treatment group, median serum sotrovimab concentrations trended lower in participants who experienced progression (8.7 μg/mL on day 8) as compared with those without progression (14.0 μg/mL on day 8), suggesting that interparticipant variability might explain clinical outcomes at this lower IM dose.
DISCUSSION
Access to treatment with mAbs for SARS-CoV-2 can be limited due to logistical challenges of IV administration. An IM route could improve patient access, allowing delivery in most clinical settings. In this study, sotrovimab 500 mg IM was noninferior to 500 mg IV for the early treatment of mild to moderate COVID-19 in nonhospitalized participants at high risk. No clinically meaningful safety or tolerability issues were observed over 36 weeks, and data support postdose monitoring for 30 minutes, which may further reduce health care burden. Infusion-related reactions were rare, and nearly all injection-site reactions were mild and resolved quickly. These findings support the use of an IM route of administration of a 500-mg dose of sotrovimab for susceptible variants.
Notably, 2 participants died in the 500-mg IM group vs no deaths in the 500-mg IV group. The increased rate of progression in the 250-mg IM group as compared with either of the 500-mg groups cannot be explained by differences in viral load, as AUCd1-8 values were similar across groups, consistent with findings that upper airway viral load is not an optimal biomarker for efficacy of SARS-CoV-2 treatments [1, 25, 26]. However, an exposure-response analysis suggested that serum concentrations on day 5 and day 8 were significant predictors of response. Reductions in day 5 and day 8 serum levels were associated with higher model-predicted occurrence of progression [27]. PK analyses found that IM administration was associated with lower mean exposures and increased variability as compared with IV infusion, which may have played a role in the higher number of participants who progressed in the 500-mg IM group vs the 500-mg IV group [27]. Given the PK differences and the potential for less optimal efficacy between the IM and IV routes of administration, consideration should be given to weighing the risks and benefits of expanding access to patients who may otherwise not be able to receive treatment. Alternative injection sites are being explored in healthy volunteers to investigate their role on the relative bioavailability and PK variability of sotrovimab 500 mg IM [28].
Enrollment predominantly occurred in the state of Florida and coincided with a surge in the SARS-CoV-2 Delta variant. Of 764 participants with sequencing results available, 674 (88.2%) were infected with the Delta variant [29]. Epidemiologic data in Florida showed an average weekly case hospitalization rate of 11.5% during the same period as this study’s enrollment [30]. The enrolled population was mostly Hispanic/Latino, one often underrepresented in clinical trials, but there was limited racial diversity in the current trial. In addition, only 3% of patients were immunocompromised. Another potential limitation is that the amount of missing data exceeds the number of primary outcome events, meaning that the conclusion is sensitive to the method of handling missing data. Under the assumption that the underlying progression rate for the missing data in the 500-mg IV group is 1.2% (ie, similar to its nonmissing data), the underlying progression rate for the missing data in the 500-mg IM group would have to be at least 7.2% (or just <1 additional participant having a progression) for the primary endpoint outcome to have exceeded the noninferiority margin.
The global population continues building immunity, and the proportion of infected patients progressing to severe disease is decreasing; however, current global estimates indicate that several thousand people continue to die every week due to COVID-19 (>6000–41 000 per week in 2023) [31]. As the pandemic evolves to an endemic phase, it will continue to be important to understand which patients benefit most from treatments to prevent severe disease. Although oral antivirals are available, anti–SARS-CoV-2 mAbs such as sotrovimab continue to fulfill an unmet need by providing a safe, tolerable, single-dose treatment option without drug-drug interactions. The availability of a therapy that can be administered by the IM route will be critical for underserved populations and those without access to IV infusion centers. With data from the TACKLE study, which demonstrated that a 600-mg IM dose of tixagevimab-cilgavimab reduced the risk of progression to hospitalization or death by 50.5% vs placebo [22], this study provides proof of concept that IM administration of mAbs is a feasible alternative to IV administration and is a valuable treatment option for select patient groups. The knowledge gained from this study will support development of more feasible options for anti–SARS-CoV2 mAbs, which are critical to expand access to underserved populations, decrease burden on the health care system, and protect vulnerable patient populations.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Adrienne E Shapiro, Departments of Global Health and Medicine, University of Washington, Seattle, Washington, USA; Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Elias Sarkis, Sarkis Clinical Trials, Gainesville, Florida, USA.
Jude Acloque, BioClinical Research Alliance, Miami, Florida, USA.
Almena Free, Pinnacle Research Group, Anniston, Alabama, USA.
Yaneicy Gonzalez-Rojas, Optimus U Corporation, Miami, Florida, USA.
Rubaba Hussain, RH Medical Urgent Care, New York, New York, USA.
Erick Juarez, Florida International Medical Research, Miami, Florida, USA.
Jaynier Moya, Pines Care Research Center, Pembroke Pines, Florida, USA.
Naval Parikh, Napa Research, Pompano Beach, Florida, USA.
David Inman, GSK, Stevenage, UK.
Deborah Cebrik, Vir Biotechnology, Inc., San Francisco, California, USA.
Ahmed Nader, GSK, Upper Providence, Pennsylvania, USA.
Nadia Noormohamed, GSK, Upper Providence, Pennsylvania, USA.
Qianwen Wang, GSK, Stevenage, UK.
Andrew Skingsley, GSK, Brentford, UK.
Daren Austin, GSK, Brentford, UK.
Amanda Peppercorn, GSK, Cambridge, Massachusetts, USA.
Maria L Agostini, Vir Biotechnology, Inc., San Francisco, California, USA.
Sergio Parra, Vir Biotechnology, Inc., San Francisco, California, USA.
Sophia Chow, Vir Biotechnology, Inc., San Francisco, California, USA.
Erik Mogalian, Vir Biotechnology, Inc., San Francisco, California, USA.
Phillip S Pang, Vir Biotechnology, Inc., San Francisco, California, USA.
David K Hong, Vir Biotechnology, Inc., San Francisco, California, USA.
Jennifer E Sager, Vir Biotechnology, Inc., San Francisco, California, USA.
Wendy W Yeh, Vir Biotechnology, Inc., San Francisco, California, USA.
Elizabeth L Alexander, Vir Biotechnology, Inc., San Francisco, California, USA.
Leah A Gaffney, Vir Biotechnology, Inc., San Francisco, California, USA.
Anita Kohli, Arizona Liver Health, Chandler, Arizona, USA; Arizona Clinical Trials, Tucson, Arizona, USA.
Notes
Author contributions. P. S. P., D. K. H., E. L. A., W. W. Y., E. M., J. E. S., D. A., S. C., and A. P. conceptualized and designed the study. All authors acquired, analyzed, and/or interpreted the data. D. I. and D. C. conducted the statistical analyses. A. K., L. A. G., and D. I. accessed and verified the data. All authors drafted the manuscript and critically reviewed and revised the manuscript for important intellectual content. All authors had full access to all the data in the study, take responsibility for the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication.
Acknowledgments. The authors thank Courtney St Amour, PhD, of Lumanity Scientific Inc. for medical writing support, which was funded by Vir Biotechnology and GSK, and Joseph Hogan, MS, Emma Gierman, MPH, and Katrina Wheeler, BS, of Vir Biotechnology for clinical operations support.
Data sharing statement. The data collected for this study will not be made available to others. The study protocol and statistical analysis plan are included in the Supplementary Data.
Financial support. The study was supported by Vir Biotechnology in collaboration with GSK.
References
- 1. Chen P, Nirula A, Heller B, et al. . SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med 2021; 384:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dougan M, Nirula A, Azizad M, et al. . Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med 2021; 385:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinreich DM, Sivapalasingam S, Norton T, et al. . REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med 2021; 385:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. . Early COVID-19 treatment with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385:1941–50. [DOI] [PubMed] [Google Scholar]
- 5. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. . Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2022; 327:1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dougan M, Azizad M, Chen P, et al. . Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. medRxiv [preprint]. 12 March 2022. Available at: 10.1101/2022.03.10.22272100 [DOI]
- 7. Cathcart AL, Havenar-Daughton C, Lempp FA, et al. . The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv [preprint]. 1 April 2022. Available at: 10.1101/2021.03.09.434607 [DOI]
- 8. National Institutes of Health . NIH OpenData Portal SARS-CoV-2 variants and therapeutics. Available at:https://opendata.ncats.nih.gov/variant/activity. Accessed 10 February 2022.
- 9. Cameroni E, Bowen JE, Rosen LE, et al. . Broadly neutralizing antibodies overcome SARS-CoV-2 omicron antigenic shift. Nature 2022; 602:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. VanBlargan LA, Errico JM, Halfmann PJ, et al. . An infectious SARS-CoV-2 B.1.1.529 omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med 2022; 28:490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park YJ, Pinto D, Walls AC, et al. . Imprinted antibody responses against SARS-CoV-2 omicron sublineages. Science 2022; 378:619–27. [DOI] [PubMed] [Google Scholar]
- 12. National Institute for Health and Care Excellence . Casirivimab plus imdevimab, nirmatrelvir plus ritonavir, sotrovimab and tocilizumab for treating COVID-19. Available at:https://www.nice.org.uk/guidance/TA878/history. Accessed 10 March 2023.
- 13. STAKOB (Robert Koch-Institut) . Hinweise zu erkennung, diagnostik und therapie von patienten mit COVID-19. Available at:https://www.rki.de/DE/Content/Kommissionen/Stakob/Stellungnahmen/Stellungnahme-Covid-19_Therapie_Diagnose.pdf?__blob=publicationFile. Accessed 10 March 2023.
- 14. Spanish Agency of Medicines and Medical Products . Criteria for assessing the administration of new antiviral therapeutic alternatives against SARS-CoV-2 infection. Available at:https://www.aemps.gob.es/medicamentos-de-uso-humano/acceso-a-medicamentos-en-situaciones-especiales/criterios-para-valorar-la-administracion-de-las-nuevas-alternativas-terapeuticas-antivirales-frente-a-la-infeccion-por-sars-cov-2/?lang=en. Accessed 10 March 2023.
- 15. Italian Medicines Agency . Medicines usable for treatment of COVID-19 disease. Available at:https://www.aifa.gov.it/en/web/guest/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento-della-malattia-covid19. Accessed 10 March 2023.
- 16. National guidelines for clinical management and treatment of COVID-19. Version 9.0. United Arab Emirates Ministry of Health and Prevention; 2023.
- 17. Zheng B, Green ACA, Tazare J, et al. . Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform. BMJ 2022; 379:e071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin-Blondel G, Marcelin AG, Soulie C, et al. . Sotrovimab to prevent severe COVID-19 in high-risk patients infected with omicron BA.2. J Infect 2022; 85:e104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harman K, Nash SG, Webster HH, et al. . Comparison of the risk of hospitalization among BA.1 and BA.2 COVID-19 cases treated with sotrovimab in the community in England. Influenza Other Respir Viruses 2023; 17:e13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng B, Tazare J, Nab L, et al. . Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform. medRxiv [preprint]. 22 January 2023. Available at: 10.1101/2022.05.22.22275417. [DOI] [PMC free article] [PubMed]
- 21. Goldstein RH, Walensky RP. The challenges ahead with monoclonal antibodies: from authorization to access. JAMA 2020; 324:2151–2. [DOI] [PubMed] [Google Scholar]
- 22. Montgomery H, Hobbs FDR, Padilla F, et al. . Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2022; 10:985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. COVID-19 Treatment Guidelines Panel, National Institutes of Health . Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at:https://www.covid19treatmentguidelines.nih.gov/. Accessed 13 January 2022. [PubMed]
- 24. Infectious Diseases Society of America . Overview of IDSA COVID-19 treatment guidelines, version 5.5.0. In: IDSA guidelines on the treatment and management of patients with COVID-19. October 27, 2021. Available at:https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 3 November 2021.
- 25. Atique M, Ghafoor A, Javed R, Fatima N, Yousaf A, Zahra S. Correlation of viral load with the clinical and biochemical profiles of COVID-19 patients. Cureus 2021; 13:e16655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gottlieb RL, Nirula A, Chen P, et al. . Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021; 325:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sager JE, El-Zailik A, Passarell J, et al. . Population pharmacokinetics and exposure-response analysis of sotrovimab in the early treatment of COVID-19. CPT Pharmacometrics Syst Pharmacol 2023; 12:853–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ClinicalTrials.gov. Relative bioavailability, safety, and tolerability of single-dose sotrovimab injection in adults (COSMIC). NCT05280717. Available at: https://clinicaltrials.gov/ct2/show/NCT05280717. Accessed 10 March 2023.
- 29. Agostini ML, Schnell G, di Iulio J, et al. . Resistance analysis in the COMET-TAIL study: participants with mild-to-moderate COVID-19 treated with intramuscular or intravenous sotrovimab. Open Forum Infect Dis 2022; 9:ofac492.988. [Google Scholar]
- 30. HealthData.gov . COVID-19 state profile report—Florida—ht94-9tjc—archive repository. Available at:https://healthdata.gov/dataset/COVID-19-State-Profile-Report-Florida-ht94-9tjc-Ar/rm7g-8483. Accessed 10 March 2023.
- 31. World Health Organization . Weekly epidemiological update on COVID-19: 20 July 2022. Available at:https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---20-july-2022. Accessed 25 April 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.