Abstract
Uterine vascular anomalies (UVAs), while rare, can result in severe, life-threatening hemorrhage. An understanding of the presentation and management options for UVAs is important for interventional radiologists to appropriately evaluate and care for these patients. The authors propose a standardized terminology for UVAs to avoid confusion and conflating congenital from acquired vascular lesions, which have a different pathophysiology. Limited high-level evidence and no definitive guidelines for UVA management exist, although endovascular treatment with uterine artery embolization has generally become the first-line approach for symptomatic or persistent UVAs with high technical and clinical success rates. There is also no consensus on the optimal embolization technique; the authors propose an initial approach to first embolize the dominant uterine artery supplying the UVA with gelatin sponge, with the option to embolize the contralateral side at the time of initial embolization if there is persistent supply (avoiding bilateral empiric embolization). Repeat embolization is feasible and recommended in the setting of recurrence, and both clinical and imaging follow-up is important. Ultimately, a multidisciplinary approach with individualized patient management is needed, particularly in the face of a lack of consensus guidelines for the management of symptomatic UVAs.
Keywords: uterine arteriovenous anomaly, uterine arteriovenous fistula, uterine vascular malformation, uterine artery embolization, interventional radiology
Uterine vascular anomalies (UVAs), while rare, can result in severe, life-threatening hemorrhage, for which endovascular treatment is the first-line approach. An understanding of the presentation and management options for UVAs is important for interventional radiologists to appropriately evaluate and care for these patients. This review aims to provide a background on UVAs based on a review of the literature concentrating on more recent publications, including the appropriate definitions, presentation, and workup, followed by treatment approaches, focusing on uterine artery embolization (UAE). A “How We Do It” section focuses on the authors' approach to uterine artery embolization (UAE) in the setting of UVA.
Definitions
Uterine vascular anomaly is an umbrella term for hypervascular lesions in the uterus involving an abnormal connection between the uterine artery and venous plexus, almost always diagnosed during a woman's reproductive years. UVAs are rare, although the precise incidence is unknown due to heterogeneity in definitions and reporting bias in the literature, with the most recent systemic review by Ruiz Labarta et al identifying 371 cases of DSA-confirmed UVAs reported in the literature from 2000 to 2021. 1 2 3
UVAs can be categorized by general etiology of congenital versus acquired, with literature almost exclusively discussing the latter. 1 4 In practice and in the literature, there has been conflicting terminology, with “uterine arteriovenous malformation (AVM)” the prevailing, albeit technically inaccurate, term to describe all types of UVAs. 2 5 6 For this review, the term UVA is used to collectively describe hypervascular myometrial abnormalities, including true uterine AVMs, arteriovenous fistulas (AVFs), and non-AVM vascular anomalies, which Timmerman et al. described as low-flow lesions without early filling of a draining vein. 6 7
Congenital uterine AVMs are exceedingly rare but are considered “true” AVMs that arise from abnormal fetal angiogenesis resulting in an anomalous capillary plexus, often with associated genetic mutations and with more complex angioarchitecture including numerous intervening abnormal vessels. 5 6 8 Their treatment may be more difficult with a higher likelihood of persistence, may have extrauterine extension, and be associated with AVMs in other parts of the body. 3 9
Types of acquired UVAs have not been reliably differentiated in the literature, for example, having been referred to as an arteriovenous “fistula,” “shunting,” and “malformation” confined to the myometrium. 2 5 9 10 The higher-risk lesions typically exhibit at least one high-flow direct arteriovenous connection between intramural uterine arterial branches and the myometrial venous plexus. 8 Acquired UVAs are thought to develop in the setting of pregnancy or recent abortion, although their pathophysiology is not well understood and may be due to hormonal/placental changes promoting myometrial angiogenesis. 4 6 8 11 Furthermore, an AVF can result from direct arterial trauma, such as from caesarean section or dilation and curettage (D&C) with subsequent formation of an abnormal connection between a single artery and vein. In contrast, low-flow myometrial hypervascularity may be considered non-AVM UVAs, and have been attributed to trophoblastic changes during pregnancy, and are of unclear clinical significance, especially if asymptomatic 2 10 12 ( Fig. 1 ).
Fig. 1.
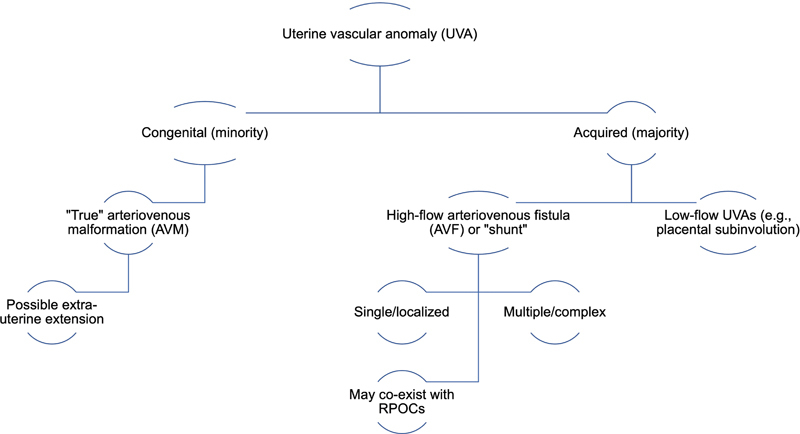
Proposed terminology for referring to uterine vascular anomalies (UVAs), the majority of which are acquired. Clinically relevant UVAs are believed to comprise both congenital AVMs and high-flow AVFs, generally defined as peak systolic velocity of >20 cm/s on spectral Doppler ultrasound evaluation. Both AVMs and AVF demonstrate early venous filling on digital subtraction angiography. RPOC, retained products of conception.
Of note, this article does not cover specific management of hypervascular uterine lesions of malignant (e.g., gestational trophoblastic disease) or infectious etiology, as this is beyond the scope of the review and the literature focuses primarily on treatment of acquired iatrogenic UVAs due to scarcity of other etiologies. 8
Presentation and Risk Factors
Women with UVAs most commonly present during their 30s, almost always with a history of recent delivery, abortion, or other uterine trauma, such as D&C or myomectomy. 1 3 8 13 The median reported time from the event to presentation is approximately 6 weeks, although can range from less than 1 day to years. 1 UVAs have varying presentations, from asymptomatic to mild pelvic pain and abnormal uterine bleeding to life-threatening hemorrhage. 8 Bleeding may have a gradual or sudden onset and may require a blood transfusion in up to 30 to 50% of cases. 1 3 Other presentations described include urinary incontinence and polyuria, dyspareunia, repeat miscarriages, or, rarely, high-out cardiac failure. 11 14
Evaluation and Diagnosis
Patients with severe hemorrhage and hemodynamic instability should be managed according to clinical protocols for hemorrhagic shock, including fluid and blood product resuscitation, as well as balloon tamponade. 5 In this scenario, the patient may proceed directly to UAE or other invasive therapy, depending on the clinical scenario, without further imaging workup. The recommended laboratory values to obtain are complete blood count, including hemoglobin/hematocrit and platelets (prior to an invasive procedure), coagulation panel, and serum beta human chorionic gonadotropin (b-hCG), which can suggest retained products of conception (RPOCs) or trophoblastic disease if persistently elevated. 1 8 11
However, in symptomatic patients who can undergo further imaging, transvaginal pelvic ultrasound (TVUS) is the initial imaging modality of choice, utilizing both color and spectral Doppler for vascular analysis, as it is readily available, cost-effective, and safe. 4 8 15 16 17 The classic finding of UVA on gray scale TVUS is anechoic tubular and tortuous structures in the myometrium. Color Doppler demonstrates dilated vascular structures with multidirectional, high velocity flow (defined as peak systolic velocity [PSV] of >20 cm/sec), usually with low resistance. 4 6 15 Timor-Tritsch et al argued that a diagnosis can reliably be established, but these findings are not pathognomonic, and it may be difficult to differentiate sonographically among hypervascular lesions, including subinvolution of the placenta, RPOCs, and AVF. 4 8 Some have adopted the blanket term of “enhanced myometrial vascularity” to describe these findings, possibly with increased detection in recent years due to more widespread use of TVUS as well as misdiagnosis as “AVMs” as explained previously. 2 4 12 16 In reality, clinically significant UVAs are still believed to be rare, found to be 0.1% incidence in the prospective study by Yazawa et al. 6 15
Several studies have suggested categorizing and managing UVAs by spectral Doppler parameters, such as PSV, with a step-up from expectant management to medical or invasive therapies, based on a higher PSV. 4 6 13 18 For example, a PSV exceeding 60 to 70 cm/s suggests the need for endovascular or surgical management. 4 While these findings may be adjunctive factors in determining therapy, management based on clinical parameters continues to be the primary approach until correlation with TVUS findings can be validated further.
The TVUS appearance of UVAs can be difficult to differentiate from, and may even coexist with, other vascularized lesions, especially RPOCs ( Fig. 2 ). 13 19 20 Therefore, secondary imaging with contrast-enhanced computed tomography angiography (CTA) or magnetic resonance (MR) angiography and dynamic contrast-enhanced MR imaging protocol can be obtained to better delineate the anatomy and extent of the UVA. 10 11 17 21 22 23 MR imaging is generally recommended over CTA due to superior soft-tissue resolution, such as to evaluate for RPOCs involving the endometrium, without ionizing radiation exposure, although with longer acquisition times and higher costs. MR findings of a uterine AVM or AVF include serpiginous signal voids on both T1- and T2-weighted images that rapidly enhance postcontrast, with identification of an early draining vein. 8 24 However, hypervascular lesions can remain difficult to differentiate even on MR imaging ( Fig. 3 ). CTA also provides pelvic and vascular anatomic details, including visualization of the direct arteriovenous communication, in a faster time than MR, although with slightly inferior soft-tissue contrast and with the use of ionizing radiation. 8 Correct diagnosis is important to avoid overtreatment of non-AVM UVAs as well as proceeding directly to D&C for presumed RPOCs, which can result in severe hemorrhage from disruption of the UVA. 8 19
Fig. 2.
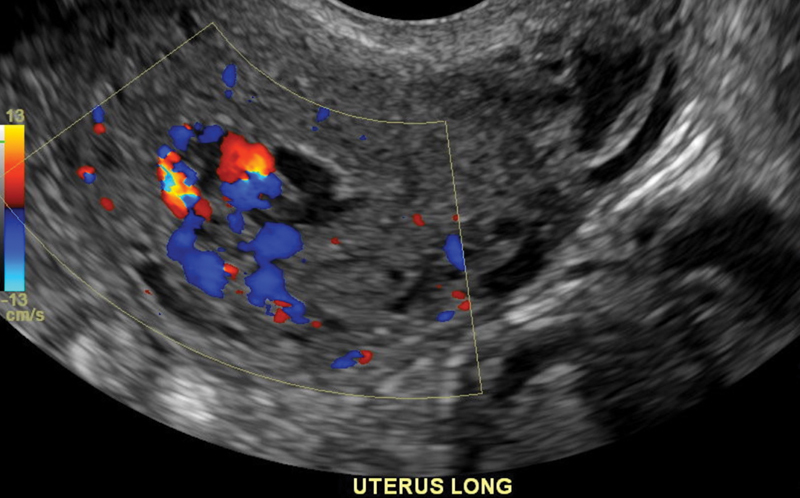
Transvaginal ultrasound demonstrates a tubular hypervascular structure in the submucosal region, consistent with an acquired uterine vascular anomaly based on the patient's history and clinical presentation.
Fig. 3.
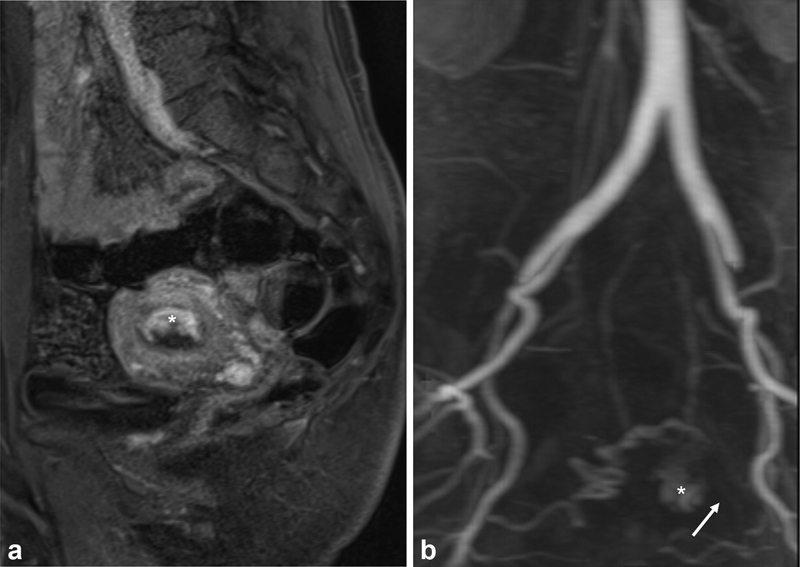
( a ) Post-contrast sagittal magnetic resonance (MR) image shows the submucosal hypervascular focus of a uterine vascular anomaly (*). ( b ) MR angiogram was thought to show a nidus (*) with arterial supply from the uterine artery and potential draining vein (arrow). Thus, the lesion was suspected to be an acquired arteriovenous fistula.
Technically, digital subtraction angiography (DSA) is the gold-standard for diagnosis, which will show anomalous vascularity with high arterial flow and early venous filling, either via a direct communication in the setting of an AVF or a more complex vascular network in the setting of an AVM. 8 However, in practice, noninvasive imaging, if available, is obtained first with DSA reserved for indeterminate cases or those planning for treatment with UAE ( Fig. 4 ).
Fig. 4.
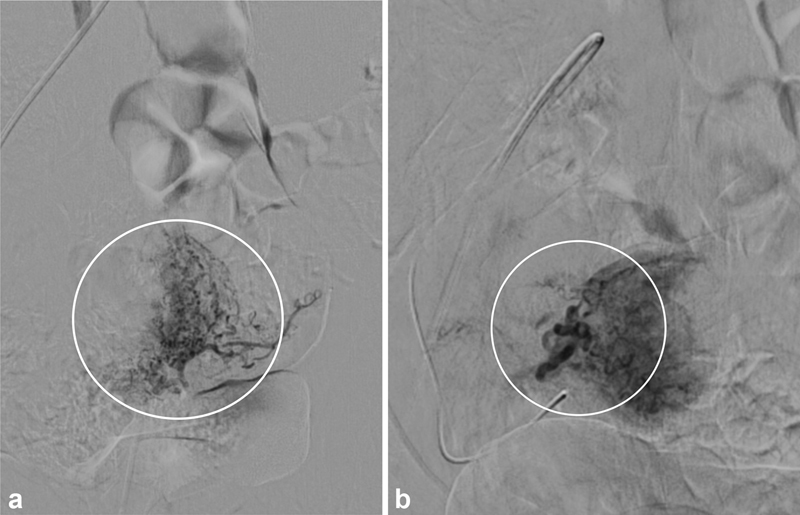
Digital subtraction arteriography was ultimately performed for the patient shown in Fig. 3 to determine the type of uterine vascular anomaly (UVA). ( a ) Injection of the left uterine artery demonstrates normal myometrial enhancement (circle). ( b ) Injection into the right uterine artery demonstrates an enlarged uterine artery with a hypervascular tuft (circle) without a draining vein; along with laboratory and clinical information, this UVA was ultimately determined to be retained products of conception (RPOC). Following discussion with gynecology, embolization was performed with gelatin sponge as the patient was symptomatic, and the RPOC subsequently shed without need for dilation and curettage.
Treatment Options
Limited high-level evidence and no definitive guidelines for UVA management exist. 1 16 Thus, treatment decisions for UVAs are generally based on clinical presentation, including volume of bleeding and hemodynamic status, and patient preference, supplemented by laboratory and imaging findings 1 19 25 ( Fig. 5 ). Several classification schemes have been described for vascular lesions, including the International Society for the Study of Vascular Anomalies nomenclature updated in 2018, although the practical utility has not yet been applied rigorously to the management of UVAs. 11 22 26
Fig. 5.
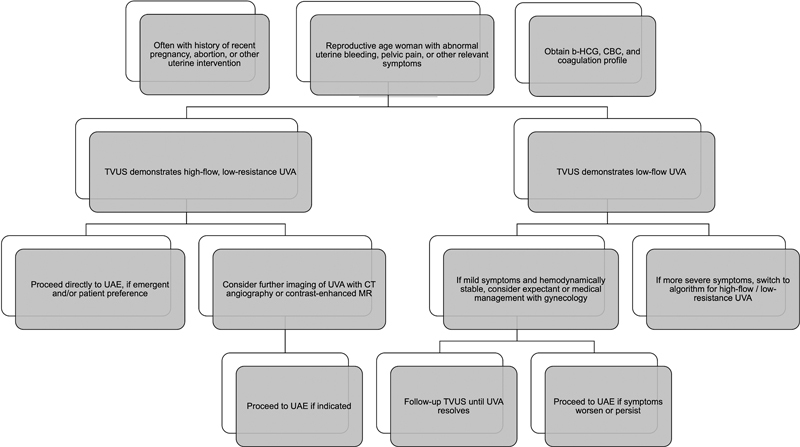
Suggested algorithm for the management and initial treatment of symptomatic uterine vascular anomalies (UVAs) based on clinical presentation, laboratory values, and transvaginal ultrasound (TVUS). Of note, an incidentally found asymptomatic UVA can likely be managed expectantly with close clinical and imaging follow-up. Patients presenting with severe bleeding and hemorrhagic shock may proceed directly to uterine artery embolization (UAE) for diagnosis and treatment, with appropriate resuscitation, and without further imaging. CT, computed tomography; MR, magnetic resonance imaging; b-HCG, beta human chorionic gonadotropin; CBC, complete blood cell count.
Expectant Management
Spontaneous regression of acquired UVAs has been described, generally with smaller or asymptomatic lesions. 6 11 21 Thus, expectant management, with serial TVUS (at 7–14-day intervals or more until resolution) and b-hCG, is a viable approach for this select population. Factors suggesting successful expectant management may include low-flow lesions, as identified on spectral Doppler and normal hemoglobin. 13 However, patients initially managed expectantly may require invasive interventions, sometimes emergently, if they progress to higher-flow lesions and/or become symptomatic. 4 13 15
Medical Therapy
Numerous medications have been reported for noninvasive management of UVAs in clinically stable patients, including progestin, GnRH agonists, methotrexate, combined hormonal contraceptives, uterotonics (methylergonovine), danazol, or a combination. 11 27 Progestins and GnRH agonists have the most safety and efficacy data. 27 Advantages include noninvasiveness and increased accessibility, although there is no consensus on optimal therapy, including agent and duration. As with expectant management, these patients may require future interventions if their symptoms persist or worsen. 18 27 Generally, the literature suggests that medical therapy can be considered for UVAs in symptomatic, hemodynamically stable patients who can have reliable clinical follow-up, although the exact inclusion criteria, including imaging findings, has not been fully delineated. 17 27
Uterine Artery Embolization: Technique/How We Do It
Indications/Contraindications
Endovascular treatment with arterial embolization of a UVA was first described in 1982 and is now considered the mainstay of management, with high technical and clinical success rates, generally >85%. 1 8 17 19 20 25 The following section is based on the authors' experiences and preferences.
There are few true contraindications to UAE, most of which have been developed from the literature on fibroid treatment. Current pregnancy is considered an absolute contraindication. 28 Active untreated pelvic infections have a risk of complications including abscess formation or sepsis, and should be treated first, if possible. Patients should be evaluated for coagulopathy, in accordance with Society of Interventional Radiology guidelines, noting that derangements should be corrected, and the decision to hold or reverse anticoagulation is based on the acuity of the situation. 29 An allergy to iodinated contrast is a relative contraindication, and these patients should be premedicated, or, in the case of anaphylaxis, consider anesthesia support or use of alternative contrast agents. Another relative contraindication is impaired renal function, for which preventive measures such as avoidance of other nephrotoxic drugs, minimizing contrast volume, and pre- and postprocedure isotonic saline infusion can be utilized.
Technique
Due to the rarity of UVAs and heterogeneity of techniques reported in the literature, there is no consensus protocol for UAE in this population. The goal of UAE is to occlude the fistulous connection or abnormal communicating vascular network, and has been performed both unilaterally and bilaterally, as well as from transfemoral and transradial access. 1 8 19 20 30 The authors prefer a transfemoral approach in this otherwise healthy, young patient population where additional risks can be avoided. A 5-Fr catheter is used to gain access to the anterior division of the internal iliac artery and a microcatheter is placed into the uterine artery. In general, the dominant side supplying the UVA should be embolized first. Embolization of the contralateral side may be performed depending on the angiographic appearance, clinical scenario, and provider preference. 30 31 There is no consensus on superiority of unilateral versus bilateral treatment or embolic agent, due to the heterogeneity of the existing data and relatively small sample sizes. 1 8 While some literature suggests empiric bilateral UAE due to cross-perfusion from experience in uterine fibroid embolization, 4 20 a more targeted approach can be considered for localized lesions, especially in women desiring future fertility as there continues to be limited quality data on UAE and impact on fertility.
Various embolic agents have been described, many of them used in combination, including gelatin sponge, coils, glue, polyvinyl alcohol particles, and microspheres. 1 8 17 30 When possible, unilateral embolization with gelatin sponge is the authors' preferred modality for acquired AVFs where patients almost always desire future fertility. However, initial coil embolization may be required for high-flow AVF/AVMs where particulates or liquids would move too quickly through the shunt. Embolization is performed to near stasis, generally defined as five heartbeats to avoid occlusion of normal myometrial branches, where possible. A potential downfall with coil embolization is that proximal occlusion may impair future access to the lesion if it were to recanalize from surrounding collaterals. Even if suspected to be noncontributory, the contralateral uterine artery should be examined with DSA after treatment unilaterally to ensure no contribution. Just as in UAE for other etiologies, collateral supply is possible and a completion aortogram with capture of the ovarian arteries is important, especially in complex UVAs.
Repeat treatments in the setting of recurrent uterine bleeding and/or AVM recanalization may be needed and can be performed safely and effectively. 1 8 11 19 UAE can also be performed prior to surgical management, such as D&C of RPOCs, to minimize blood loss. 19
Adverse Events
Reported adverse events from UAE for UVA treatment in the literature are sparse, with a 1.6% major adverse event rate in a recent systematic review. 1 Known risks include nontarget embolization, paradoxical embolus in the setting of migration into the venous system, and access site complications, 1 8 19 20 21 including a small risk of stroke via transradial access. 32 There is a theoretical risk of uterine necrosis based on studies of UAE for uterine myomas, although this complication has not been specifically documented in the setting of UVA. 1 11 The effects on fertility are also unknown, with numerous studies reporting successful and uncomplicated pregnancies post-UAE for UVA, although an actual fertility rate is difficult to ascertain due to small sample sizes. 1 21 25
Other Minimally Invasive Image-Guided Procedures
There are also no guidelines in the setting of UVA/bleeding recurrence after UAE, although other minimally invasive imaged-guided techniques have been described, often if the UVA is refractory to prior UAE attempts. A retrograde transvenous approach has been described in settings where a transarterial approach is not deemed safe or feasible, with or without a staged UAE to follow. 11 33 34 More specifically, Kishino et al reported a balloon-occluded retrograde obliteration with sclerosant, 33 while Morita et al performed superselective transvenous coil and glue embolization. 34 Several cases of direct percutaneous and transvaginal embolization of the UVA under ultrasound and/or fluoroscopic guidance have been described, whether after failure of initial medical management or UAE. 35 36 Varying embolics were reported, including glue, coils, and gelatin sponge. Another technique described in case reports has been use of ultrasound-guided high-intensity focused ultrasound (HIFU) as initial, noninvasive treatment of a symptomatic UVA due to the patient's desire to retain fertility. 37 38 HIFU has been utilized in the treatment of uterine fibroids, as well as other vascular anomalies and solid tumors. 39 40
Surgical Interventions
Hysterectomy is now reserved as a primary treatment option for women based on individual preference, including no desire for future fertility, lack of access to transcatheter therapies, or after failure of other less-invasive methods, including medical and endovascular management. 8 17 Other less invasive surgical techniques, such as laparoscopic occlusion of the internal iliac and/or uterine arteries, have been described, and are generally secondary to transcatheter techniques. 8 Operative hysteroscopy and D&C may be considered in the scenario of combined UVA-RPOCs, with specific techniques to minimize the risk of UVA disruption. 19 23
Post-UAE Procedure and Follow-up Care
Postembolization syndrome is well-characterized following UAE for fibroids and has been described after embolization of UVAs. 1 It is characterized by self-limited postprocedure pain, nausea, fever, and fatigue, generally occurring with 24 to 48 hours postprocedure and managed with oral nonsteroid anti-inflammatory agents and antiemetics. 8
No guidelines exist on UVA treatment follow-up regimen. However, patients should be followed up by gynecology and interventional radiology to evaluate for resolution of both symptoms and imaging findings of UVA. Again, there is no consensus for imaging surveillance, but the authors suggest TVUS with color Doppler should be performed approximately 1 week postembolization and then every 2 weeks, as needed, until UVA resolution has been confirmed. 4 Further evaluation with CTA or MR imaging can be done if there is concern for recurrence.
Asymptomatic, incidentally found UVAs may require a more individualized approach to determine if or when treatment should be performed. Management posthysterectomy or other surgical intervention is beyond the scope of this review.
Conclusion
In summary, interventional radiologists should have knowledge of the presentation and management of UVAs, most of which are acquired and are rare, but may result in life-threatening hemorrhage. Literature on UVAs is sparse, primarily relying on small case series and case reports, thus limiting the conclusions that can be drawn. 1 Diagnostic imaging, especially TVUS, has grown in importance, but management ultimately relies on the patient's clinical status and preference, in collaboration with obstetrics/gynecology ( Fig. 5 ). UAE is the initial line of treatment in symptomatic patients, especially in those who wish to avoid surgical procedures and desire future fertility. However, in patients who are hemodynamically stable, expectant or medical management may be considered in consultation with a gynecologist, as some acquired UVAs have been shown to resolve spontaneously. There is no consensus in the literature on the optimal embolization technique, whether unilateral or bilateral, or embolic agent, and is thus largely dependent on the preference of the interventional radiologist and clinical scenario. The authors propose an initial approach to embolize the dominant uterine artery supplying the UVA with gelatin sponge, with the option to embolize the contralateral side if there is persistent supply. Clinical and imaging follow-up is important to ensure UVA resolution. Repeat embolization is feasible and recommended in the setting of recurrence, whether transarterial or through other minimally invasive image-guided techniques that have been recently reported. Ultimately, surgical management may be necessary in refractory cases.
Moving forward, the terminology for UVAs should be standardized to avoid confusion and conflating congenital from acquired vascular lesions, which have a different pathophysiology and likely require distinctive management. Further studies are needed to validate the sole use of TVUS in diagnosing UVAs and for triaging higher-risk lesions to immediate UAE. A multidisciplinary approach with individualized patient management is needed, particularly in the face of a lack of consensus guidelines for the management of symptomatic UVAs.
Acknowledgements
None.
Conflict of Interest None declared.
Disclosures
M.M.M. has no disclosures. T.M.C. serves as medical director of Varian Interventional Solutions.
References
- 1.Ruiz Labarta F J, Pintado Recarte M P, González Leyte M et al. Uterine artery embolization of uterine arteriovenous malformation: a systematic review of success rate, complications, and posterior pregnancy outcomes. J Pers Med. 2022;12(07):1098. doi: 10.3390/jpm12071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müngen E. Vascular abnormalities of the uterus: have we recently over-diagnosed them? Ultrasound Obstet Gynecol. 2003;21(06):529–531. doi: 10.1002/uog.163. [DOI] [PubMed] [Google Scholar]
- 3.Peitsidis P, Manolakos E, Tsekoura V, Kreienberg R, Schwentner L. Uterine arteriovenous malformations induced after diagnostic curettage: a systematic review. Arch Gynecol Obstet. 2011;284(05):1137–1151. doi: 10.1007/s00404-011-2067-7. [DOI] [PubMed] [Google Scholar]
- 4.Timor-Tritsch I E, Haynes M C, Monteagudo A, Khatib N, Kovács S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity/arteriovenous malformations. Am J Obstet Gynecol. 2016;214(06):7310–7.31E12. doi: 10.1016/j.ajog.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Polat P, Suma S, Kantarcý M, Alper F, Levent A. Color Doppler US in the evaluation of uterine vascular abnormalities. Radiographics. 2002;22(01):47–53. doi: 10.1148/radiographics.22.1.g02ja0947. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman D, Wauters J, Van Calenbergh S et al. Color Doppler imaging is a valuable tool for the diagnosis and management of uterine vascular malformations. Ultrasound Obstet Gynecol. 2003;21(06):570–577. doi: 10.1002/uog.159. [DOI] [PubMed] [Google Scholar]
- 7.Lowe L H, Marchant T C, Rivard D C, Scherbel A J. Vascular malformations: classification and terminology the radiologist needs to know. Semin Roentgenol. 2012;47(02):106–117. doi: 10.1053/j.ro.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Yoon D J, Jones M, Taani J A, Buhimschi C, Dowell J D. A systematic review of acquired uterine arteriovenous malformations: pathophysiology, diagnosis, and transcatheter treatment. AJP Rep. 2016;6(01):e6–e14. doi: 10.1055/s-0035-1563721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molvi S NM, Dash K, Rastogi H, Khanna S B. Transcatheter embolization of uterine arteriovenous malformation: report of 2 cases and review of literature. J Minim Invasive Gynecol. 2011;18(06):812–819. doi: 10.1016/j.jmig.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Iraha Y, Okada M, Iraha R et al. CT and MR imaging of gynecologic emergencies. Radiographics. 2017;37(05):1569–1586. doi: 10.1148/rg.2017160170. [DOI] [PubMed] [Google Scholar]
- 11.Giurazza F, Corvino F, Silvestre M et al. Uterine arteriovenous malformations. Semin Ultrasound CT MR. 2021;42(01):37–45. doi: 10.1053/j.sult.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Grewal K, Al-Memar M, Fourie H, Stalder C, Timmerman D, Bourne T. Natural history of pregnancy-related enhanced myometrial vascularity following miscarriage. Ultrasound Obstet Gynecol. 2020;55(05):676–682. doi: 10.1002/uog.21872. [DOI] [PubMed] [Google Scholar]
- 13.Lee T Y, Kim S H, Lee H J et al. Ultrasonographic indications for conservative treatment in pregnancy-related uterine arteriovenous malformations. Acta Radiol. 2014;55(09):1145–1152. doi: 10.1177/0284185113514222. [DOI] [PubMed] [Google Scholar]
- 14.Abdullah H MA, Oliver T, Lamfers R, Narayana Gowda S. High output heart failure in a young woman secondary to massive arteriovenous malformations from a uterine tumor. BMJ Case Rep. 2020;13(02):e233887. doi: 10.1136/bcr-2019-233887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazawa H, Soeda S, Hiraiwa T et al. Prospective evaluation of the incidence of uterine vascular malformations developing after abortion or delivery. J Minim Invasive Gynecol. 2013;20(03):360–367. doi: 10.1016/j.jmig.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 16.O'Rourke-Suchoff D, Benitez S, Higgins M CCS, Stier E A. Diagnosis and treatment of women with radiologic findings suspicious for uterine arteriovenous malformations. J Obstet Gynaecol. 2021;41(05):769–773. doi: 10.1080/01443615.2020.1798905. [DOI] [PubMed] [Google Scholar]
- 17.Salmeri N, Papale M, Montresor C, Candiani M, Garavaglia E. Uterine arteriovenous malformation (UAVM) as a rare cause of postpartum hemorrhage (PPH): a literature review. Arch Gynecol Obstet. 2022;306(06):1873–1884. doi: 10.1007/s00404-022-06498-0. [DOI] [PubMed] [Google Scholar]
- 18.Taneja A, Chopra I, Kaur H et al. Successful management of abnormal uterine bleeding from uterine arteriovenous malformations with progesterone in postabortal patients. J Obstet Gynaecol Res. 2019;45(06):1114–1117. doi: 10.1111/jog.13939. [DOI] [PubMed] [Google Scholar]
- 19.Gao F, Ma X, Xu Y, Fu L, Guo X. Management of acquired uterine arteriovenous malformations associated with retained products of conception. J Vasc Interv Radiol. 2022;33(05):547–553. doi: 10.1016/j.jvir.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Camacho A, Ahn E H, Appel E et al. Uterine artery embolization with Gelfoam for acquired symptomatic uterine arteriovenous shunting. J Vasc Interv Radiol. 2019;30(11):1750–1758. doi: 10.1016/j.jvir.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Delplanque S, Le Lous M, Proisy M et al. Fertility, pregnancy, and clinical outcomes after uterine arteriovenous malformation management. J Minim Invasive Gynecol. 2019;26(01):153–161. doi: 10.1016/j.jmig.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Grill N, Struebing F, Krebs L, Sadick M.Diagnostic radiology findings and spectrum of therapeutic interventions in gynaecological and urogenital vascular anomalies Br J Radiol 202194(1124):2.0210246E7–2.0210246E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groszmann Y S, Healy Murphy A L, Benacerraf B R. Diagnosis and management of patients with enhanced myometrial vascularity associated with retained products of conception. Ultrasound Obstet Gynecol. 2018;52(03):396–399. doi: 10.1002/uog.18954. [DOI] [PubMed] [Google Scholar]
- 24.Chen H Z, Zhao F M, Liu L J et al. Accuracy of flow-void diameters on MR images in diagnosing uterine arteriovenous malformations in patients with pregnancy-related diseases. Sci Rep. 2021;11(01):19806. doi: 10.1038/s41598-021-99209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghanaati H, Firouznia K, Moradi B, Behestani S. Fertility outcomes after uterine artery embolization for symptomatic uterine arteriovenous malformations: a single-center retrospective study in 33 women. Cardiovasc Intervent Radiol. 2022;45(07):983–991. doi: 10.1007/s00270-022-03105-2. [DOI] [PubMed] [Google Scholar]
- 26.ISSVA classification for vascular anomalies. International Society for the Study of Vascular AnomaliesAccessed March 13, 2023 at:https://www.issva.org/classification
- 27.Rosen A, Chan W V, Matelski J, Walsh C, Murji A. Medical treatment of uterine arteriovenous malformation: a systematic review and meta-analysis. Fertil Steril. 2021;116(04):1107–1116. doi: 10.1016/j.fertnstert.2021.05.095. [DOI] [PubMed] [Google Scholar]
- 28.Task Force on Uterine Artery Embolization and the Standards Division of the Society of Interventional Radiology . Andrews R T, Spies J B, Sacks D et al. Patient care and uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2009;20(07):S307–S311. doi: 10.1016/j.jvir.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Patel I J, Rahim S, Davidson J C et al. Society of Interventional Radiology Consensus Guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions - Part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(08):1168–11840. doi: 10.1016/j.jvir.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Picel A C, Koo S J, Roberts A C. Transcatheter arterial embolization with n-butyl cyanoacrylate for the treatment of acquired uterine vascular malformations. Cardiovasc Intervent Radiol. 2016;39(08):1170–1176. doi: 10.1007/s00270-016-1328-z. [DOI] [PubMed] [Google Scholar]
- 31.Castillo M S, Borge M A, Pierce K L. Embolization of a traumatic uterine arteriovenous malformation. Semin Intervent Radiol. 2007;24(03):296–299. doi: 10.1055/s-2007-985737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu R, Peters G L, Charles H, Kokabi N, Bercu Z L, Majdalany B S. Transradial uterine artery embolization complicated by stroke. Semin Intervent Radiol. 2022;39(06):591–595. doi: 10.1055/s-0042-1759700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishino M, Miyasaka N, Takeguchi Y, Ohashi I.Retrograde transvenous obliteration for uterine arteriovenous malformation Obstet Gynecol 2014123(2, Pt 2, Suppl 2):427–430. [DOI] [PubMed] [Google Scholar]
- 34.Morita R, Abo D, Kinota N et al. Successful transvenous embolization for type II uterine arteriovenous malformation: a case report. Radiol Case Rep. 2021;16(08):2007–2011. doi: 10.1016/j.radcr.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuc N, Gans J H, Wattamwar K, Hirschl D, Golowa Y, Cynamon J. Direct percutaneous embolization of uterine arteriovenous malformations. J Vasc Interv Radiol. 2022;33(05):607–609. doi: 10.1016/j.jvir.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Lehrman E D, Heller M, Poder L, Kerlan R, Huddleston H G, Kohi M P. Transvaginal obliteration of a complex uterine arteriovenous fistula using ethylene vinyl alcohol copolymer. J Vasc Interv Radiol. 2017;28(06):842–843. doi: 10.1016/j.jvir.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Yan X, Zhao C, Tian C, Wen S, He X, Zhou Y. Ultrasound-guided high-intensity focused ultrasound ablation for treating uterine arteriovenous malformation. BJOG. 2017;124 03:93–96. doi: 10.1111/1471-0528.14749. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Tan X, Xiong W, Wang X. High-intensity-focused ultrasound for uterine arteriovenous malformation associated with retained products of conception: a case report. Asian J Surg. 2023;46(01):653–654. doi: 10.1016/j.asjsur.2022.07.026. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Wang T, Lei B. High-intensity focused ultrasound (HIFU) ablation versus surgical interventions for the treatment of symptomatic uterine fibroids: a meta-analysis. Eur Radiol. 2022;32(02):1195–1204. doi: 10.1007/s00330-021-08156-6. [DOI] [PubMed] [Google Scholar]
- 40.Izadifar Z, Izadifar Z, Chapman D, Babyn P. An introduction to high intensity focused ultrasound: systematic review on principles, devices, and clinical applications. J Clin Med. 2020;9(02):460. doi: 10.3390/jcm9020460. [DOI] [PMC free article] [PubMed] [Google Scholar]


