Abstract
Chronic pelvic pain (CPP) is a challenging condition affecting an estimated 15% of females in the United States. Multiorgan system dysfunction results in the complex clinical pain presentation. Similar to other chronic pain syndromes, CPP is influenced by biopsychosocial factors and requires a multimodal approach for optimal pain management. This review summarizes the clinical evaluation and medical management of CPP with a comprehensive approach.
Keywords: chronic pelvic pain, medical management, multimodal therapy for pain, pain management, interventional radiology, embolization, woman's health
Chronic pelvic pain (CPP) affects approximately 15% of women in the United States. 1 It is broadly defined as pain perceived from the pelvis, lasting more than 6 months, noncyclic or cyclic (related to menstruation), and/or pain with intercourse (dyspareunia). 2 Despite frequently assumed to be gynecologic in nature, 80% of the origins of CPP are not primarily caused by a gynecologic condition and, similarly, symptoms should not be presumed to be caused by a singular disease ( Table 1 ). The underlying mechanisms required for the initiation and persistence of CPP are complex and difficult to treat. Additionally, several chronic overlapping pain conditions (COPCs) 3 frequently coexist in the same patient ( Fig. 1 ), and an optimal treatment plan may benefit several cooccurring conditions and symptoms.
Table 1. System-based etiology of chronic pelvic pain.
| Systems | ||||||
|---|---|---|---|---|---|---|
| Psychological | Neurological | Musculoskeletal | Gastrointestinal | Genitourinary | Gynecologic | Integumentary |
| Anxiety Depression Trauma Somatization Catastrophization |
Vulvodynia Pudendal Neuralgia Peripheral neuropathy Small fiber neuropathy |
Myofascial pain Tender points Fibromyalgia Chronic low back pain Sacroiliac joint dysfunction Facet arthropathy Tendonitis Piriformis syndrome |
Irritable bowel syndrome Inflammatory bowel disease Chronic constipation/outlet constipation Diarrhea Chronic anal fissures |
Bladder pain syndrome/interstitial cystitis Overactive bladder Bladders spasms Recurrent urinary tract infections Nephrolithiasis |
Primary dysmenorrhea Endometriosis Ovarian cysts Adenomyosis Fibroids Genitourinary syndrome of menopause Vaginismus Vaginitis Pelvic venous disease Pelvic inflammatory disease |
Lichen sclerosis Lichen planus Herpes simplex Herpes zoster Lichen simplex chronicus |
Fig. 1.
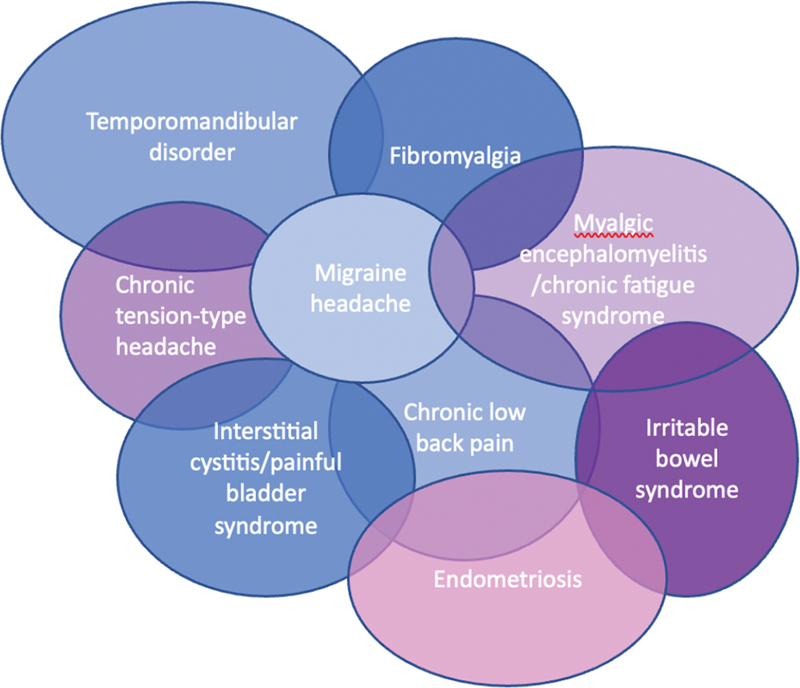
Chronic overlapping pain conditions.
The financial impact of CPP in the United States is significant, responsible for up to 10% of gynecologic office visits annually. 4 Women with CPP are also four times more likely to undergo gynecologic surgery and five times more likely to have a hysterectomy than women without CPP. 1 In 1996, CPP was conservatively estimated to cost $2.8 billion annually, 1 and when adjusted to 2020 dollars, costs were estimated at $5.8 billion. 5 As CPP is often shared with other commonly overlapping pain disorders, the total estimated annual U.S. costs for pain management surpassed $289 billion. 3
Pathophysiology of Chronic Pelvic Pain
Patients with CPP may additionally report symptoms consistent with bladder pain syndrome or functional bowel pain secondary to the neural cross-signaling between distinctive pelvic viscera. Known as viscero-viscero cross-sensitization, a pain activity in one organ or site sensitizes another organ to pain, in the absence of a separate pain stimulus. Distinctive characteristics of visceral pain includes its referral to distant sites secondary to the merging of visceral and somatic afferent pathways at the level of the spinal cord and higher centers within the central nervous system. This “neural convergence” in the pelvis results in visceral structures (reproductive organs, bladder, bowel) sharing neural pathways with somatic structures (skin, muscles, fascia, and bones). Uniquely, the parietal peritoneum is innervated by both somatic and visceral afferent nerves. Secondary to this, a persistent nociceptive visceral stimulus (i.e. uterine pain from adenomyosis or endometriosis) can trigger abdominopelvic myofascial pain through the viscero-somatic sensitization mechanism ( Fig. 2 ).
Fig. 2.
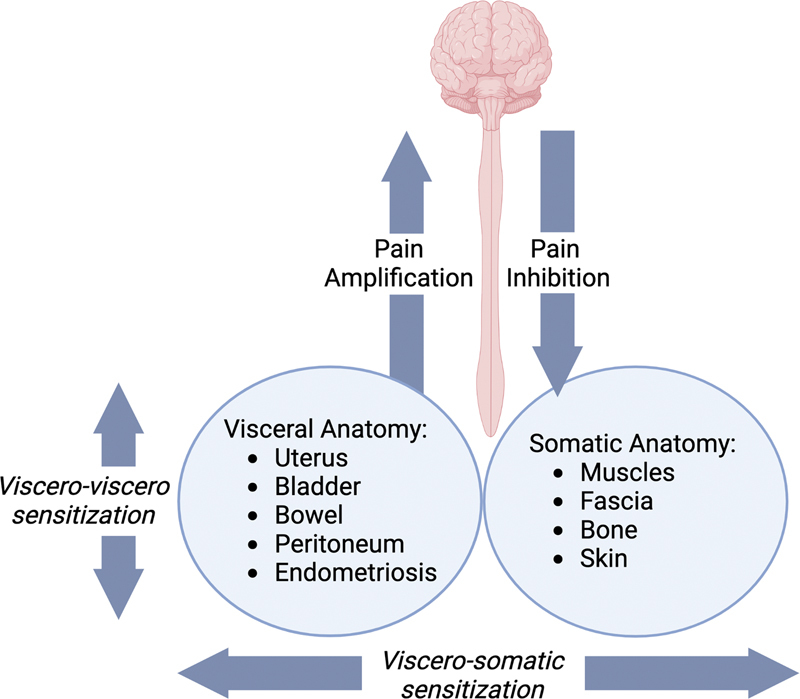
Viscero-viscero and viscero-somatic neural convergence pathways.
Clinical Assessment: The History and Physical Exam
Chronic pelvic pain conditions present with overlapping system symptoms and as such, the clinical evaluation may be a challenge. Patient assessment includes a detailed patient history and focused physical exam, both of which can better support clinicians in the primary diagnosis. The identification of coexisting pain conditions should also be considered during the evaluation, as this information completes the clinical picture and directs the initiation of timely and efficacious multimodal treatment.
Gathering the necessary information to guide care in a time-limited visit can be arduous. Apportioning adequate time may be necessary for complex pain patients with COPCs. This may be achieved by scheduling a longer clinic visit or having the patient return for planned follow-up visits at regular intervals, allowing time to identify a multifactorial approach needed for complex CPP patients. In addition to the direct clinical care component of the visits, time should be allotted for the necessary patient education essential for the development of a comprehensive patient-centered treatment plan.
Pain History
Routine information in a pain history including the onset, chronicity, relationship to menses, variations in severity, aggravating and alleviating factors, quality, and location are all pertinent. A detailed review of prior medications, procedures, and surgical interventions may be useful in evaluating contributing etiologies of pain. Obtaining a social history provides details on family dynamics, relationship, and employment status, offering insight into the influence of psychosocial factors on a patient's pain experience. A sexual history including information on dyspareunia if present (specifically details on discomfort with external touch, insertion, deep penetration, or with arousal/orgasm), onset of occurrence, and the impact of positional changes are relevant. A trauma-informed approach to gathering sensitive information is critical.
The information gathered guides individual care decisions in the multidisciplinary approach to the patient's pain. One may consider utilizing a pain-focused intake form or validated pain questionnaires for the assessment of complex pain patients in advance of their scheduled appointment.
Focused Physical Exam
Trauma may be experienced across a person's life, from a remote history to current, ongoing exposure. Patients with CPP have more than three times the odds of having a history of childhood abuse or witnessing domestic violence compared with patients without CPP. 6 A “routine” pelvic exam may be invasive and potentially triggering for patients with a trauma history. Given the high prevalence of trauma in patients with CPP, it should be assumed that all patients have experienced sexual trauma and the physical exam should be performed systematically, with a trauma-informed approach. The goal of the exam is to provide optimal support while avoiding trauma exacerbation using gender-neutral language, with specific attention to historical, cultural, and gender issues. The provider should fully explain what to expect both before and during the examination and the patient may choose the timing of exam (at the current visit or at a future visit). A safe and calm environment should be created, and the patient must be informed that the exam can and should be paused or terminated for any reason at their request. A medical chaperone should be present during sensitive parts of an exam and the patient may also prefer to have a support person present.
The comprehensive physical exam begins with an inspection of the patient's gait for significant asymmetry, followed by the back and abdomen. These components of the exam can be performed with the patient fully dressed, with careful draping if clothing is manipulated for skin or scar evaluation. When a pelvic exam is indicated, providers should leave the room when patients disrobe and dress. During the pelvic examination, the patient's body should be covered, exposing only those areas that are necessary to expose and for the shortest time required. For the pelvic exam, the patient is asked to move to the edge of the examination table and place their legs in the stirrups or in a frog-leg position. The provider should avoid triggering words and phrases such as “relax” or “take a deep breath.”
Careful visual inspection of the external genitalia is performed to evaluate for signs of atrophy, lesions, fissures, or abnormal discharge. For patients presenting with vulvar complaints, a single lubricated cotton swab is serially applied to the labia majora, prepuce, clitoris, interlabial sulci, labia minora, and the vulvar vestibule in a semicircle from lateral to the urethra to the posterior fourchette. With each pressure point, the patient is asked to grade their pain response using the 0 to 10 VAS pain scale. The urethra, bladder neck, and bladder are gently palpated separately. A single gloved digit is then inserted into the vagina. The urethra, bladder neck, and bladder are gently palpated, evaluating for pain or pressure.
The patient is asked to contract and release their pelvic floor muscles to assess for asymmetry, limited mobility, or even paradoxical relaxation. The superficial transverse perineal muscle is palpable at the vaginal opening under the hymenal remnant, followed by evaluation of the levator ani muscles (puborectalis and pubococcygeus) at the 5 and 7 o'clock positions approximately 3 to 5 cm inside the vaginal on the posterolateral vaginal walls. The obturator internus is also palpable, hooking the digit around the pubic rami and having the patient abduct their knee against the examiner's external hand, engaging the muscle. With each manipulation of the pelvic floor muscles, approximately 2 kg of pressure is gently applied. Pain or tenderness of the muscles is an abnormal finding.
A speculum exam may be performed when indicated, fully visualizing the vagina and cervix and using a cotton swab to examine for pressure point tenderness. The same exam is also recommended for vaginal apex pain in the setting of a prior hysterectomy. The bimanual exam is appropriate when deeper palpation of the pelvis is required. The uterine shape, mobility, and presence of tenderness can be assessed with this exam component, and the parametria and adnexa can be palpated for tenderness or masses. A rectovaginal exam may be performed to assess for deep infiltrating endometriosis and rectovaginal nodules or uterosacral ligament disease.
Initial Diagnostic Testing
Laboratory Evaluation
An initial laboratory evaluation may include a pregnancy test (if patient is of reproductive age), screenings for abnormal vaginal discharge to rule out vaginitis or a sexually transmitted infection, and urinalysis for urinary symptoms. An endometrial biopsy may be considered for chronic abnormal uterine bleeding (especially if aged >45 years).
Imaging
Imaging is often used in the initial investigation or exacerbation of CPP. Pelvic ultrasonography is considered the primary modality of choice, as it can detect a multitude of gynecologic causes of pelvic pain (i.e., adenomyosis, endometriosis, fibroids, ovarian cysts, and pelvic venous disease). Transvaginal ultrasonography has the highest sensitivity and specificity in identifying certain causes of pain, such as ovarian endometriomas and cysts. A normal pelvic exam combined with a normal pelvic ultrasound is reassuring. Magnetic resonance imaging has also been found to have high diagnostic accuracy in the diagnosis of gynecologic causes of CPP, including deep infiltrating endometriosis, adenomyosis, abnormal pelvic varices, and uterine fibroids, but may have a limited benefit in the presence of a normal pelvic ultrasound.
Goals of Pain Management
A comprehensive discussion regarding individual pain and fertility goals commences the care plan foundation. Goal setting is necessary in the early planning of a pain management strategy, and clear objectives are associated with greater satisfaction for both the provider and patient. Rather than complete resolution of pain, breaking up the overarching goal into more achievable targets feels less impossible, with a focus on reducing symptom burden and improving quality of life. Conceivable goals include increasing length of pain-free or reduced-pain intervals, improved functionality for a specific activity, and incorporating behavioral tools for better pain coping. Regardless of the goals, a multidisciplinary, multimodal approach offers the highest likelihood of treatment success. Referral to appropriate services can be important partners in achieving these goals, including physical therapy, psychology/psychiatry, sexual therapy, pain management, general surgery, gastrointestinal medicine, urology, and interventional radiology services.
Nonpharmacologic Interventions
Cognitive Behavior Therapy
Psychological factors and psychiatric symptoms (depression, anxiety, somatization, catastrophization, high stress, and poor quality-of-life outcomes) frequently coexist in patients with CPP. 7 The bidirectional relationship of pain and emotion explains why a comprehensive treatment strategy addressing both the physical and psychological symptoms results in the best outcomes. 8 The health benefits of psychological and mind–body interventions, including mindfulness, can be implemented in patients suffering from CPP to improve pain outcomes. Cognitive behavior therapy (CBT), rooted in the belief that psychological problems are based on patterns of learned dysfunctional thinking and behaviors to a response, has been extensively studied in many chronic pain conditions. 9 10 CBT techniques for chronic pain include education about the influence of thoughts, emotions, and behaviors to the physical symptoms of pain as well as the emotional experience of chronic pain. Patients are taught cognitive restructuring and pain reframing, relaxation, meditation, activity pacing, sleep hygiene, and coping skills.
Nutrition
It is universally recognized that nutrition plays an important role in disease prevention and treatment. Despite the frequent associations of gastrointestinal symptoms and food intolerances in patients with CPP, there are limited data that dietary changes broadly result in significant symptom relief. Limiting intake of low fermentable oligosaccharides, disaccharides, and monosaccharides and polyols (FODMAPs)—carbohydrates found in a wide range of foods that are challenging to digest (e.g., garlic, onions, legumes, wheat, berries)—reduce symptoms in patients with irritable bowel syndrome. 11 In patients with chronic widespread musculoskeletal pain, there is an association with an altered gut microbiome. 12 Low vitamin D levels have been associated with higher pain scores in several pain conditions, 13 14 and supplementation of vitamin D3 (cholecalciferol) may improve pain outcomes in fibromyalgia 15 ; however, this has not been replicated in CPP secondary to endometriosis. 16
Physical Activity and Exercise
Skeletal muscle movement resulting in energy expenditure above resting levels has long been touted as globally beneficial for pain and overall health. Specific to patients with CPP, exercise has been associated with stress reduction and improvement in pain scores, though most studies lack the ability to draw strong conclusions from the existing data. 17
Physical Therapy
A multimodal approach including pelvic floor physical therapy has been proposed to improve CPP symptoms. 18 19 On top of promoting physical activity and exercise, physical therapy also includes relaxation, deep breathing, and meditation techniques, which are hypothesized to contribute to both physical and psychological benefits. Pelvic floor physical therapy programs typically include once weekly sessions for 8 to 12 weeks, followed by dismissal to a maintenance home exercise program.
Sleep
Sleep is a key potential modifiable target across all pain disorders including pelvic pain. Healthy, uninterrupted, and restful sleep is essential for overall brain health and cognition. Sleep dysfunction is associated with increased pain sensitivity; however, better understanding of this as a causal relationship or association requires further investigation. 20 Given the opportunity for direct modification, sleep hygiene should be reviewed with all patients who report sleep disruptions for overall health and mood. 17
Acupuncture
Acupuncture is an effective and safe method for treating a multitude of pain disorders, including pregnancy-related and endometriosis-related pain. 21 22 A systematic review and meta-analysis of four randomized controlled trials in women with CPP concluded that the addition of acupuncture may benefit routine pain treatment, though the study quality was mostly interpreted as low. 23 Despite this, focused studies evaluating acupuncture intervention for patients with CPP are needed.
Pharmacologic Interventions
Analgesics
Acetaminophen, recognized for its potentiating properties, increases the effectiveness of other analgesic medications via central prostaglandin synthesis inhibition. Uncommonly an effective pain reliever on its own, the pairing with other analgesics may result in better pain outcomes.
Nonsteroidal anti-inflammatory drugs (NSAIDs) act through the nonselective inhibition of the cyclooxygenase (COX) enzyme preventing the production of prostaglandins and thromboxane. NSAIDs decrease the prostaglandin levels in the mucosa (primarily driven by inhibition of COX1), correlating with gastric toxicities. As COX2 is not expressed in the gastrointestinal tract, COX2 selective inhibitors are perceived as safer for the GI system than conventional NSAIDs. 24 In small quantities, NSAIDs have minimal risk (high-volume use is associated with gastrointestinal toxicities, cardiovascular risks, renal injuries, and hepatotoxicity and hypertension), and are accessible and reasonable first-line treatment. There is limited evidence for the use of opioids for CPP which should be considered only in refractory cases.
Hormonal Medications
For patients with gynecologic pain as the primary or secondary cause, their CPP may benefit from menstrual suppression. Combined low-estrogenic, oral contraceptives (COCs) are routinely used as first-line hormonal treatment in the pain management of dysmenorrhea, adenomyosis, endometriosis. 25 26 Continuous use of monophasic pills rather than cyclic administration appears to be more effective in reducing the recurrence of dysmenorrhea but is not effective in treating noncyclic pelvic pain and dyspareunia. There are rising data on the role of oral progestin-only treatment as primary medical treatment for pelvic pain associated with endometriosis and lesion quiescence, but not necessarily lesion elimination. 27 Emerging data also raise considerations of a GnRH antagonist as a reasonable second-line treatment for endometriosis pain.
Adenomyosis and endometriosis share several clinical features and notably both have a negative impact on women's quality of life secondary to abnormal uterine bleeding and pain. While adenomyosis may be surgically approached with hysteroscopic or laparoscopic resection, high-intensity focused ultrasonography, uterine artery embolization, or hysterectomy, medical management of symptoms is led by progestin therapy, either local with a levonorgestrel intrauterine device or systemic progestin therapy. 28 First-line medical therapy for pelvic congestion syndrome is also hormonal suppression, with both progestins and gonadotropin-releasing hormone agonists effective in decreasing pain symptoms. 29
Antidepressants
Antidepressants, specifically tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors, are frequently incorporated into the treatment for patients with neuropathic pain. There is scarce evidence on the pain benefits of these drugs in patients with visceral pain syndromes or patients with CPP. 30 The analgesic effect is thought to be from the inhibition of the reuptake of serotonin and noradrenaline, increasing neurotransmitter availability, and subsequently increasing inhibitory pain control. CPP, while not considered exclusively a neuropathic pain disorder, may benefit from the neuromodulatory effect of these medications in addition to its effect on comorbid psychiatric diseases when present.
Anticonvulsants
Gabapentin and pregabalin bind to calcium channels on primary afferent nociceptors, reducing the release of neurotransmitters. Both drugs have been broadly studied in peripheral pain syndromes. A recent systematic review evaluated the effect of neuromodulatory drugs on CPP pain intensity, and while most studies reported some improvement in pain, the highest quality study showed no differences compared to placebo. 30 This study, known as the GaPP2 trial (Gabapentin for Chronic Pelvic Pain in Women), is a multicenter, randomized, double-blind, placebo-controlled trial comparing high systemic doses of gabapentin in women with CPP, specifically excluding women with known causes of pain, such as endometriosis. Despite this, gabapentinoids are frequently used clinically, have a good safety profile, and few drug interactions. In patients with multiple comorbid pain disorders, they may be considered to reduce comorbid etiologies of body pain.
Skeletal Muscle Relaxants
Skeletal muscle relaxants (SMRs) are among the most prescribed medications in the United States. An assessment of prescribing patterns from 2005 to 2016 highlighted use. While new SMR prescriptions remained stable over time, office visits where the SMR was renewed tripled from 2005 to 2016 (8.5–24.7 million visits). 31 SMRs are not recommended for use beyond the acute treatment of muscle spasm and are not considered first-line treatment for myofascial pelvic pain. In acute low back pain, SMRs provide clinically significant short-term pain relief. 32 Myofascial pelvic pain and CPP often coexist. Pelvic floor physical therapy remains the first-line treatment for myofascial pelvic pain. 25 There are two types of SMRs—antispastic and antispasmodics. Antispasticity agents act in upper motor syndromes (e.g., multiple sclerosis, cerebral palsy, traumatic brain injury, spinal cord injury, and post-stroke syndrome) and should not be prescribed for musculoskeletal conditions due to lack of evidence. Antispasmodics are intended for use for peripheral muscle spasm. Cyclobenzaprine is the most heavily studied SMR and has been shown to be effective for various musculoskeletal conditions, including fibromyalgia. 31 There are no available data specifically evaluating SMR in CPP and if clinically indicated, the authors recommend weighing individual factors including symptom severity, desired benefits, and duration of treatment with potential side effects.
Cannabinoids
Cannabis has been used medicinally for thousands of years and cannabis-based products are frequently used by patients who suffer from chronic pain. 33 Cannabis contains delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), both cannabinoids that bind to endocannabinoid system receptors. The analgesic effect of cannabinoids is multifactorial and primarily neuropathic in nature, including the inhibition of neurotransmitter and neuropeptide release from presynaptic nerve endings, altering postsynaptic neuron excitability, activating the inhibitory pain pathways, and reducing neural inflammation. A cross-sectional survey of 119 women with pelvic and perineal pain, dyspareunia, or endometriosis identified almost 24% of patients with CPP regularly used cannabis as an adjunct to their pain therapy. 34 Despite their popularity, there are limited high-quality data on pain outcomes in all pain conditions, including gynecologic disorders and CPP.
Conclusion
Given the growing prevalence of CPP, the gynecology provider will be increasingly relied on to care for this patient population. Provider comfort with diagnosis, treatment, and collaboration with a multidisciplinary team is essential in managing CPP. A systematic and nuanced approach to history and exam can help identify the numerous and overlapping gynecologic and nongynecologic conditions that may be present in the CPP patient. Given the often-multifactorial etiology, a multimodal and multidisciplinary treatment plan tailored to the overlapping conditions is critical in a successful treatment regimen. Goal setting and anticipation of slow and measured progress is helpful to build patient and provider trust as the treatment regimen unfolds. Close collaboration with colleagues partnering in the treatment of CPP optimizes titration of overlapping medications or therapies and development of improved systems to facilitate this collaboration will be key.
Future Directions
Significant work remains to understand the role of nutrition, exercise, and sleep in treating the diverse and varied conditions that contribute to CPP. The data supporting pharmacologic treatment of CPP with antidepressants, anticonvulsants, skeletal muscle relaxers, and cannabinoids have lagged behind clinical need, and tailored robust studies evaluating the effectiveness of these interventions remain an unmet need. While the currently identified conditions encompassed within CPP are numerous ( Table 1 ), many remain to be identified or well understood. Pelvic venous disease, for example, is increasingly considered to be a underrecognized etiology of CPP, and a multimodal approach to treatment of this condition including collaboration with interventional radiology will be critical to improving patient outcomes. Our current efforts to better understand and provide optimal treatment for the growing and diverse CPP patient population are just the beginning.
Funding Statement
Funding E.T.C. provides expert witness testimony.
Footnotes
Conflict of Interest A.B.M. reports no conflict.
References
- 1.Mathias S D, Kuppermann M, Liberman R F, Lipschutz R C, Steege J F. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87(03):321–327. doi: 10.1016/0029-7844(95)00458-0. [DOI] [PubMed] [Google Scholar]
- 2.Howard F M. Chronic pelvic pain. Obstet Gynecol. 2003;101(03):594–611. doi: 10.1016/s0029-7844(02)02723-0. [DOI] [PubMed] [Google Scholar]
- 3.Maixner W, Fillingim R B, Williams D A, Smith S B, Slade G D. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain. 2016;17(09):T93–T107. doi: 10.1016/j.jpain.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard F M. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv. 1993;48(06):357–387. doi: 10.1097/00006254-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Lamvu G, Carrillo J, Ouyang C, Rapkin A. Chronic pelvic pain in women: a review. JAMA. 2021;325(23):2381–2391. doi: 10.1001/jama.2021.2631. [DOI] [PubMed] [Google Scholar]
- 6.Krantz T E, Andrews N, Petersen T R et al. Adverse childhood experiences among gynecology patients with chronic pelvic pain. Obstet Gynecol. 2019;134(05):1087–1095. doi: 10.1097/AOG.0000000000003533. [DOI] [PubMed] [Google Scholar]
- 7.Till S R, As-Sanie S, Schrepf A, Arbor A, Arbor A. Psychology of chronic pelvic pain: prevalence, neurobiological vulnerabilities, and treatment. Clin Obstet Gynecol. 2019;62(01):22–36. doi: 10.1097/GRF.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell L C, Clauw D J, Keefe F J. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003;54(03):399–409. doi: 10.1016/s0006-3223(03)00545-6. [DOI] [PubMed] [Google Scholar]
- 9.Hilton L, Hempel S, Ewing B A et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51(02):199–213. doi: 10.1007/s12160-016-9844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fordham B, Sugavanam T, Edwards K et al. Cognitive-behavioural therapy for a variety of conditions: an overview of systematic reviews and panoramic meta-analysis. Health Technol Assess. 2021;25(09):1–378. doi: 10.3310/hta25090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black C J, Staudacher H MFA, Ford A C. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2022;71(06):1117–1126. doi: 10.1136/gutjnl-2021-325214. [DOI] [PubMed] [Google Scholar]
- 12.Freidin M B, Stalteri M A, Wells P M et al. An association between chronic widespread pain and the gut microbiome. Rheumatology (Oxford) 2021;60(08):3727–3737. doi: 10.1093/rheumatology/keaa847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin O A, Abouzeid S M, Ali S A, Amin B A, Alswat K A. Clinical association of vitamin D and serotonin levels among patients with fibromyalgia syndrome. Neuropsychiatr Dis Treat. 2019;15:1421–1426. doi: 10.2147/NDT.S198434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darling A M, Chavarro J E, Malspeis S, Harris H R, Missmer S A. A prospective cohort study of vitamins B, C, E, and multivitamin intake and endometriosis. J Endometr. 2013;5(01):17–26. doi: 10.5301/je.5000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wepner F, Scheuer R, Schuetz-Wieser B et al. Effects of vitamin D on patients with fibromyalgia syndrome: a randomized placebo-controlled trial. Pain. 2014;155(02):261–268. doi: 10.1016/j.pain.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Nodler J L, DiVasta A D, Vitonis A F et al. Supplementation with vitamin D or ω-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2020;112(01):229–236. doi: 10.1093/ajcn/nqaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutke A, Sundfeldt K, De Baets L. Lifestyle and chronic pain in the pelvis: state of the art and future directions. J Clin Med. 2021;10(22):5397. doi: 10.3390/jcm10225397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bittelbrunn C C, de Fraga R, Martins C et al. Pelvic floor physical therapy and mindfulness: approaches for chronic pelvic pain in women-a systematic review and meta-analysis. Arch Gynecol Obstet. 2023;307(03):663–672. doi: 10.1007/s00404-022-06514-3. [DOI] [PubMed] [Google Scholar]
- 19.van Reijn-Baggen D A, Han-Geurts I JM, Voorham-van der Zalm P J, Pelger R CM, Hagenaars-van Miert C HAC, Laan E TM. Pelvic floor physical therapy for pelvic floor hypertonicity: a systematic review of treatment efficacy. Sex Med Rev. 2022;10(02):209–230. doi: 10.1016/j.sxmr.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Sivertsen B, Lallukka T, Petrie K J, Steingrímsdóttir ÓA, Stubhaug A, Nielsen C S. Sleep and pain sensitivity in adults. Pain. 2015;156(08):1433–1439. doi: 10.1097/j.pain.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Wang Y, Xu J et al. Acupuncture for low back and/or pelvic pain during pregnancy: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2022;12(12):e056878. doi: 10.1136/bmjopen-2021-056878. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Xu Y, Zhao W, Li T, Zhao Y, Bu H, Song S. Effects of acupuncture for the treatment of endometriosis-related pain: a systematic review and meta-analysis. PLoS One. 2017;12(10):e0186616. doi: 10.1371/journal.pone.0186616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung S H, Sung A D, Sung H K, An T E, Kim K H, Park J K. Acupuncture treatment for chronic pelvic pain in women: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:9.415897E6. doi: 10.1155/2018/9415897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rostom A, Muir K, Dubé Cet al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane collaboration systematic review Clin Gastroenterol Hepatol 2007507818–828., 828.e1–828.e5, quiz 768 [DOI] [PubMed] [Google Scholar]
- 25.Brown J, Farquhar C. Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;2014(03):CD009590. doi: 10.1002/14651858.CD009590.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pain C P. Clinical management guidelines for obstetrician – gynecologists. Obstet Gynecol. 2020;135(03):98–109. [Google Scholar]
- 27.Liang B, Wu L, Xu H et al. Efficacy, safety and recurrence of new progestins and selective progesterone receptor modulator for the treatment of endometriosis: a comparison study in mice. Reprod Biol Endocrinol. 2018;16(01):32. doi: 10.1186/s12958-018-0347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dueholm M. Minimally invasive treatment of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2018;51:119–137. doi: 10.1016/j.bpobgyn.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Tu F F, Hahn D, Steege J F. Pelvic congestion syndrome-associated pelvic pain: a systematic review of diagnosis and management. Obstet Gynecol Surv. 2010;65(05):332–340. doi: 10.1097/OGX.0b013e3181e0976f. [DOI] [PubMed] [Google Scholar]
- 30.Andrade M A, Soares L C, Oliveira M AP, De Oliveira P. The effect of neuromodulatory drugs on the intensity of chronic pelvic pain in women: a systematic review. Rev Bras Ginecol Obstet. 2022;44(09):891–898. doi: 10.1055/s-0042-1755459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soprano S E, Hennessy S, Bilker W B, Leonard C E. Assessment of physician prescribing of muscle relaxants in the United States, 2005-2016. JAMA Netw Open. 2020;3(06):e207664. doi: 10.1001/jamanetworkopen.2020.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel Shaheed C, Maher C G, Williams K A, McLachlan A J. Efficacy and tolerability of muscle relaxants for low back pain: systematic review and meta-analysis. Eur J Pain. 2017;21(02):228–237. doi: 10.1002/ejp.907. [DOI] [PubMed] [Google Scholar]
- 33.Busse J W, Wang L, Kamaleldin M et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320(23):2448–2460. doi: 10.1001/jama.2018.18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrubba A R, Ebbert J O, Spaulding A C, DeStephano D, DeStephano C C. Use of cannabis for self-management of chronic pelvic pain. J Womens Health (Larchmt) 2021;30(09):1344–1351. doi: 10.1089/jwh.2020.8737. [DOI] [PubMed] [Google Scholar]


