Abstract
Abdominal aortic aneurysm (AAA) is a severe vascular disease and a major public health issue with an unmet medical need for therapy. This disease is featured by a progressive dilation of the abdominal aorta, boosted by atherosclerosis, ageing, and smoking as major risk factors. Aneurysm growth increases the risk of aortic rupture, a life-threatening emergency with high mortality rates. Despite the increasing progress in our knowledge about the etiopathology of AAA, an effective pharmacological treatment against this disorder remains elusive and surgical repair is still the unique available therapeutic approach for high-risk patients. Meanwhile, there is no medical alternative for patients with small aneurysms but close surveillance. Clinical trials assessing the efficacy of antihypertensive agents, statins, doxycycline, or anti-platelet drugs, among others, failed to demonstrate a clear benefit limiting AAA growth, while data from ongoing clinical trials addressing the benefit of metformin on aneurysm progression are eagerly awaited. Recent preclinical studies have postulated new therapeutic targets and pharmacological strategies paving the way for the implementation of future clinical studies exploring these novel therapeutic strategies. This review summarises some of the most relevant clinical and preclinical studies in search of new therapeutic approaches for AAA.
Keywords: abdominal aortic aneurysm, clinical trials, therapeutic target
Introduction
Abdominal aortic aneurysm (AAA) is a progressive and life-threatening vascular degenerative disease affecting between 4% and 8% of men aged >65 years [1,2]. AAA consists in a focal dilation of the abdominal aorta exceeding 50% of its normal diameter (or >3 cm). AAA progresses insidiously, increasing the risk of aortic rupture, the more severe complication of this disease and a public health concern that often results in sudden death and accounts for 150,000–200,000 deaths each year globally.
Aneurysm growth is boosted by ageing, atherosclerosis and further by smoking, the major modifiable risk factor recognised for this disease. In the last two decades and specific countries, decreased prevalence and mortality from ruptured AAA have been attributed to the significant decline in smoking rates [2,3]. Additionally, the male sex is a major predisposing factor for this disease that exhibits a prominent sexual dimorphism. Indeed, AAA prevalence is 4-6 times higher in men than in women, although, the risk of aneurysm rupture is greater in women who also have worse outcomes after surgical repair. Notably, genetics is behind AAA aetiology with a double risk of aneurysm development in individuals with a family history of AAA in a first-degree relative [2,3].
The pathophysiology of AAA is complex and involves several interconnected and mutually regulated processes. Clues provided by human aneurysmal specimens corresponding to end-stage phases of the disease or by preclinical models indicate that AAA is a chronic inflammatory disease associated with the local upregulation of proteinases, responsible for the progressive destruction of the structural components of the vascular wall, elevated production of reactive oxygen species (ROS), and depletion of medial vascular smooth muscle cells (VSMC) (Figure 1) [2]. However, although these are the main mechanisms driving this chronic degenerative disease and considerable progress has been made in this field, a deep understanding of the intricate pathways involved in aneurysm progression and rupture is still lacking, which has hampered the development of effective pharmacological interventions for the treatment of AAA.
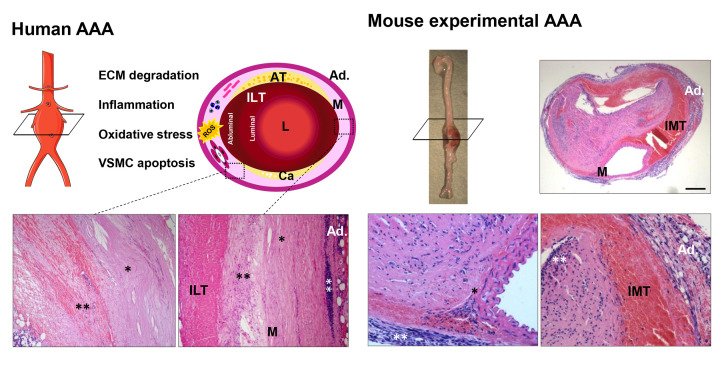
Figure 1. Pathophysiology of abdominal aortic aneurysm (AAA).
The scheme depicts the pathophysiological characteristics of human AAA, shows the main mechanisms involved in its development and evidences the common aspects, but also the differences between human and murine aneurysmal lesions. Human AAA is featured by an important inflammatory infiltrate that produces proteases, responsible for ECM (extracellular matrix) degradation, and releases cytokines and Reactive Oxygen Species (ROS) leading to the loss of VSMC due to apoptosis and necroptosis These processes favour the weakening of the arterial wall and elastin degradation. Commonly, a non-occlusive and multilayered intraluminal thrombus (ILT) covers the human aneurysmal sac, allowing blood to flow through a preserved lumen (L) and providing an additional source of ROS and proteolytic mediators. AAA often coexist with atherosclerosis (AT) and calcium deposits (Ca). The images below correspond to aortic sections from patients subjected to surgical repair. (*) Denote areas devoid of VSMC with a disorganised ECM, while (**) indicate areas enriched in inflammatory cells. Panel on the right side show an aneurysmal lesion from angiotensin II-infused ApoE−/− mice that, in conjunction with aneurysms induced by CaCl2 or elastase perfusion, well-established models of AAA, are only partially representative of the human disease. In the angiotensin II-infused ApoE−/− model, aneurysms show a suprarenal location with an intramural thrombus (IMT), but similar to human lesions, vascular inflammation, and ECM remodelling feature experimental AAA. Ad: Adventitia; M: media. The figure was partly generated using Servier Medical Art provided by Servier, licenced under a Creative Commons Attribution 3.0 unported license.
Certainly, despite AAA remains a major public health issue, there is a lack of pharmacological therapies limiting AAA progression, and surgical repair, by either open surgery or endovascular repair (EVAR), is the only therapeutic option. This intervention is exclusively indicated for asymptomatic patients at a high risk of aortic rupture or symptomatic or ruptured aneurysms, being aortic diameter the most reliable marker to assess aneurysm rupture risk. Specifically, surgical repair is recommended for male patients with AAA diameters equal to or larger than 5.5 cm or for women in whom aortic diameter reaches at least 5 cm. Unfortunately, clinical trials reported no benefit of surgical repair for AAA measuring less than 5.5 cm [3–7] and therefore, no therapeutic options are available for patients with small and asymptomatic aneurysms, who must be subjected to conservative management consisting of close monitoring with no other alternative. This follow-up involves imaging surveillance of disease progression until the aortic diameter reaches the surgical threshold size when the risk of rupture exceeds surgical risk [8]. The absence of medical therapies that halt or slow down AAA progression has encouraged an active search for identifying new therapeutic targets. In this context, several randomised clinical trials have evaluated the efficacy of different therapeutic approaches for the management of this disease, including statins, doxycycline, and anti-hypertensive drugs, among others; however, none of them has convincingly demonstrated a clear benefit on aneurysm expansion. This article provides an overview of previous and ongoing clinical trials in AAA and critically reviews current progress in preclinical research aiming at discovering novel candidate compounds or repurposing strategies for pharmacological intervention in this disease.
Clinical trials for testing pharmacotherapies limiting AAA progression
None of the tested drugs against AAA has conclusively evidenced a benefit on aneurysm progression or rupture, as summarised below (Figure 2). Major shortcomings that could underlie the failure of clinical trials evaluating pharmacological interventions in AAA might be related to the small cohort size and short follow-up, as well as the lack of reliable methods for determining aneurysm diameter, as discussed below.
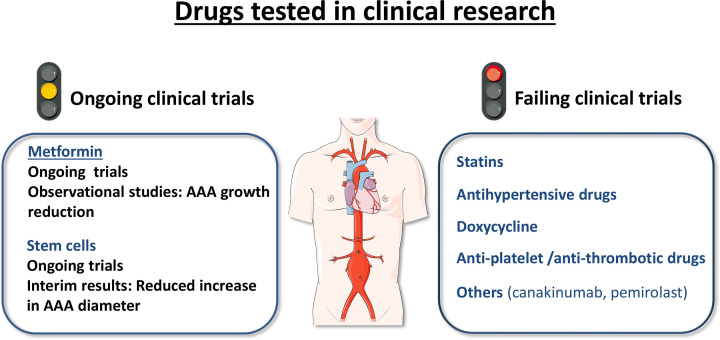
Figure 2. Clinical trials evaluating pharmacological interventions in abdominal aortic aneurysm (AAA).
Ongoing clinical trials are assessing the interest of metformin and stem cells as candidate therapeutic strategies for AAA. Clinical trials analysing the effectiveness of statins, antihypertensive drugs, doxycycline, anti-platelet and anti-thrombotic therapies and other drugs with anti-inflammatory activity failed to evidence a benefit on AAA progression. The figure was partly generated using Servier Medical Art provided by Servier, licenced under a Creative Commons Attribution 3.0 unported license.
Statins
Beyond the lipid-lowering activity of statins, it is now well recognised that some of their beneficial responses are derived from their pleiotropic effects, such as the improvement of endothelial function, the reduction of inflammation and oxidative stress and the attenuation of thrombosis [9–11]. Thus, several researchers have hypothesised that statins might limit AAA development through their anti-inflammatory and antioxidant properties, further supported by preclinical data in animal models [12]. Accordingly, two different studies addressing the impact of short-term preoperative treatment with statins in patients undergoing elective open repair for AAA revealed that this pharmacological intervention decreased vascular MMP9 levels [13], and inflammation, although did not significantly affect the progression of AAA [14]. Subsequently, several observational studies have evaluated the use of statins in the management of AAA, showing inconsistent data. Half of them argued in favour of a lower aneurysm growth rate in patients on statin therapy, while others, specifically the largest and most robust trials, reported no significant benefit on AAA progression [15]. Similarly, systematic reviews and meta-analyses of observational studies assessing the effect of statin therapy on growth outcomes led to discordant conclusions [16–18]. Consequently, the use of statins as a pharmacological approach to halt AAA growth remains uncertain. However, evidence that statins reduce long- and short-term mortality in patients after surgical AAA repair improving all-cause survival [16,19,20] sustains the prescription of statins for the management of this disease, in agreement with current guidelines. Prospective, and multicentre rigorous trials would be needed to clarify whether statins could limit aneurysm progression.
Antihypertensive drugs
Although preclinical studies reported convincing data about the effectiveness of antihypertensive drugs to ameliorate AAA formation [21–28], conclusive evidence supporting their clinical application is still lacking. Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II type 1 receptor blockers (ARB), and propranolol were the hypotensive agents more extensively studied. Two multicentre randomised trials tested the effectiveness of propranolol in limiting aneurysm growth. However, both of them reported no significant benefit of this drug on aneurysm expansion, while the low compliance with the treatment in the groups of propranolol-treated participants compromises the significance of these studies [29,30]. Likewise, the pharmacological manipulation of the renin–angiotensin system (RAS) by using ARBs has been proposed as a therapeutic option in patients with AAA, boosted by the benefit provided by this strategy in murine models of AAA [24–28]. Unfortunately, the first randomised, double-blind, placebo-controlled trial assessing the efficacy of telmisartan to slow the growth of small AAA (TEDY trial) has failed to demonstrate a benefit of this drug on aneurysm progression after 2 years of follow-up [31]. Interestingly, recent additional analysis in a subset of patients enrolled in this trial has revealed that telmisartan decreases peak wall stress (PWS) and peak wall rupture index (PWRI) in individuals with small AAA, arguing in favour of additional extended and more powered studies addressing this target [32]. Because of the essential role of Ang II signalling in AAA, special mention deserves the recently reported attenuation of experimental AAA by angiotensin-(1-7) [33], which is related to the stimulation of Mas and AT2 receptors, in agreement with previous studies in murine models supporting the benefit of AT2 agonism on aneurysm progression [34]; these data lay the foundations of future trials addressing the usefulness of AT2 agonists for this disorder. Finally, regarding ACE inhibitors it should be noted that the encouraging conclusions raised from a population-based case–control study reporting that ACE inhibition is related to a reduced risk of AAA rupture [35], were subsequently dismissed based on data from three independent trials [36–38], one of them even reporting a faster AAA growth in patients taking ACE inhibitors [37].
Targeting ECM remodelling: doxycycline
Doxycycline has become the pharmacological candidate for the treatment of AAA most extensively studied from the 90s, in both preclinical and clinical settings. Doxycycline is a tetracycline that besides its antibiotic activity behaves as a MMP inhibitor at sub-antimicrobial dose. This property aroused the interest of the scientific community in doxycycline and its relevance for the treatment of diseases in which enhanced MMP activity plays a critical pathophysiological role, such as AAA [39]. The earliest preclinical studies reported the ability of doxycycline to reduce the incidence of AAA, preserving elastin integrity and attenuating gelatinolytic activity in different animal models [40–43]. These data fostered the development of the first clinical trials, conducted in a limited number of patients, but evidencing that a short-term doxycycline treatment prior to elective AAA repair suppressed aortic MMP expression and activity [44,45] and attenuated vascular inflammation [46]. Interestingly, no benefit on the progression of already established AAA was found in animal studies [47] and this notion could be also extended to humans. In fact, a pilot trial in patients receiving doxycycline for three months evidenced no differences in aneurysm size or the rate of aneurysm growth after 18 months of surveillance [48], while results from a placebo-controlled randomised clinical trial published in 2013 even suggested that doxycycline aggravates aneurysm growth [49]. Concerns about the use of a dose too low to be effective have been raised in this last study, in which a significant number of treated patients had to be excluded from the final analysis [49]. Recently, the N-TA3CTrial that enrolled 254 patients with small infrarenal aneurysms tried to shed more light on this matter. This trial concluded that a 2-year doxycycline treatment does not attenuate the growth of AAA [50] consistent with a previous 6-month study conducted by the same researchers [51]. Of note, the AAA growth rate found in the N-TA3CTrial was lower than that initially expected, suggesting that a longer follow-up would have been necessary to appreciate any benefit. Although being disappointing, a reduction in serum C-reactive levels has been repeatedly found in this and previous trials [48,50] and therefore a favourable response to doxycycline in AAA patients could not be totally ruled out, warranting further research.
Anti-platelet and anti-thrombotic therapy
The presence of a non-occlusive intraluminal thrombus (ILT) covering the aneurysmal sac is a common feature in 70–80% of patients with AAA. The ILT seems to play a critical role in disease progression. Certainly, it is a biologically active and dynamic entity that entraps a myriad of cellular elements including erythrocytes, platelets, neutrophils, and macrophages, producing proteases, cytokines and ROS that favour the weakening of the arterial wall and promoting local hypoxia. This scenario has substantiated the potential of anti-thrombotic/anti-platelet therapies in the fight against AAA, supported by preclinical studies in animal models evidencing that this strategy limits aortic rupture or reduces aortic diameter [52–54]. However, although some studies showed that antiplatelet therapy with aspirin slows aneurysm growth in patients with middle-sized AAA [55], other clinical trials show inconsistent data [56,57], including a recent randomised clinical trial with the platelet inhibitor ticagrelor, which found no benefit on aneurysm growth in patients with small AAA [58]. Similarly, a retrospective analysis reported no benefit on patient outcomes with this strategy [59], while a recent systematic review and meta-analysis concludes that there is a lack of high-quality data to sustain the clinical benefit of antithrombotic therapy in patients with AAA [60]. Further, concerns have been raised about both the increased bleeding risk related to this therapy in the event of aneurysm rupture [56] and the association of long-term anticoagulation with type II endoleaks in patients subjected to EVAR [61,62], thus supporting that anticoagulation should be reconsidered. Novel well-designed and large clinical trials and more in-depth knowledge about the impact of ILT on individual biomechanics and aneurysm growth will be needed to draw clear conclusions.
Metformin
Diabetes is a major risk factor for cardiovascular diseases. Despite atherosclerosis and AAA sharing many of their risk factors [63], a paradoxical protective role of diabetes on the incidence, prevalence, growth rate and rupture of AAA has been repeatedly noticed in several study populations and meta-analysis [64–69]. The benefit associated with diabetes is gender-specific, exclusively circumscribed to males, while no difference in AAA incidence between diabetic and non-diabetic females has been detected [64]. These data raised the suspicion that the strong negative association between diabetes and AAA could rely on the medication used for the management of these patients, being metformin one of the most prescribed anti-diabetic drugs. Since then, it has become evident that metformin prescription is consistently associated with a significant reduction in aneurysm growth [70], but also in the need of AAA repair or aortic rupture-related deaths (Table 1) [70–77]. Besides its glucose‐lowering properties, positive vascular effects have been attributed to metformin, including, the attenuation of vascular inflammation, ROS production and neovascularization and the regulation of transduction pathways critical for AAA development such as AMP-activated protein kinase (AMPK) [78–81], mechanisms that could underlie the protection against AAA rendered by metformin. It should be noted, however, that although cohort studies and case-control studies support the ability of metformin to ameliorate AAA progression and aneurysm-related events, randomised controlled trials are needed to state this assumption more clearly. In the most recent years three trials have been launched addressing this issue: (i) the limiting AAA with metformin (LIMIT) trial (NCT04500756), (ii) the metformin therapy in non-diabetic AAA patients (MetAAA) trial (NCT03507413) and (iii) the abdominal aortic aneurysm growth inhibition (MAAAGI) trial (NCT04224051). These ongoing trials will bring new more rigorous evidence sustaining the favourable outcome afforded by metformin in AAA and its repurposing, which could provide a safe and cost-effective therapeutic strategy for this disorder.
Table 1. Cohort studies on metformin in patients with AAA.
| Ref. | AAA patient characteristics | Male sex (%) |
Average follow-up (years) | Diabetics (%) |
Metformin prescription (%) |
Outcome | Difference between using and not using metformin |
|---|---|---|---|---|---|---|---|
| [77] | Diabetics/untreated AAA (n=58) Age, years: 72.0 (1.0)§ |
82.7 | 2.6 | 100 | 25.8 | Maximum aortic diameter growth | −1.30 (--1.64, −0.96) ‡ |
| [75] |
Cohort 1 Patients with AAA (n=1357) Age, years: 73.9 (6.3)§ |
89.9 | 3.6 | 15.9 | 8.6 | Maximum aortic diameter growth | −0.59 (--1.09, −0.09)‡ |
|
Cohort 2 Patients with AAA (n=287) Age, years: 72.6 (7.6)§ |
81.8 | 2.9 | 24.0 | 13.5 | Maximum aortic diameter growth | --1.15 (−2.16, --0.14)‡ | |
|
Cohort 3 Patients with AAA (n=53) Age, years: 73.1 (7.4)§ |
84.9 | 1 | 35.8 | 30.1 | Maximum aortic diameter growth | −1.09 (--1.89, −0.29)‡ | |
| [78] | Diabetics/unruptured AAA (n=13,843) Age at AAA diagnosis, years: 69.8 (7.8)§ |
99.4 | 4.2 | 100 | 39.7 | Maximum aortic diameters growth | --0.30 (−0.37, --0.23)‡ |
| [79] |
Cohort 1 Asymptomatic/unrepaired AAA (n=1080) Age, years: 73.4 (7.2)§ |
81.5 | 3.2 | 21.6 | 11.9 | Incidence of AAA repair or death due to aneurysm rupture AND maximum aortic diameter growth | 0.46 (0.26, 0.80)¤ |
| --0.52 (−0.97, −0.07)‡ | |||||||
|
Cohort 2 Asymptomatic/unrepaired AAA (Ø ≤50 mm; n=763) Age, years: 73.5(7.3)§ |
79.8 | 3.6 | 76.2 | 13.8 | 0.45 (0.21, 0.96)¤ | ||
| −0.68 (--1.37, 0.01)‡ | |||||||
| [81] |
Cohort 1 – Diabetes/no metformin (n=43.073) Age, years: 72.27 (7.85)§ Cohort 2 – Diabetes/metformin (n=24.361) Age, years: 69.65 (7.15)§ |
100 | 5 | Cohort 1 and 2 100 | 36.1 | Surgery and/or death after AAA diagnosis | Cohort 1 0.88 (0.85, 0.91)¤ |
| Cohort 2 0.93 (0.84, 1.04)¤ |
|||||||
| [80] | Baseline Ø ≥30mm (n = 526) Age, years: 69.0 (5.7)§ |
94.2 | 3.2 | 18.6 | 12.3 | Maximum aortic diameter | −0.50 (--1.05, 0.05)‡ |
| [76] | Patients undergoing AAA repair (n = 306) Age, years: 71.6 (5.8)§ |
NM | 0.1 | 25.2 | 17.6 | Maximum aortic diameter growth | 0.09 (0.02, 0.47)‡ |
Ø: Aortic diameter; §: Mean (standard error);‡: Mean difference, 95% IC; ¤:Odds ratio, 95% IC; NM: Not mentioned.
Adapted from [82].
Stem cells
Cell-based therapies have become a promising approach for cardiovascular disorders. Due to the regenerative properties of stem cells, cellular therapies have gained growing attention for instance, for patients with myocardial infarction or critical limb ischemia, but also preclinical data suggest their interest as a therapeutic option in AAA. Several cell types have been assessed in murine models of AAA, being mesenchymal stem cells (MSCs) the most extensively studied. However, dissimilar sources, doses and routes of administration have been assessed, which does not allow us to draw more clear conclusions [82]. Of note adipose-tissue derived MSCs from patients with AAA exhibit enhanced senescence [83], while safety issues regarding the risk of oncogenic transformation should be solved; thus a deep understanding of MSCs biology is urgently needed before considering MSCs-based therapeutic strategies.
A huge number of studies found that MSCs induce a vascular reparative response that leads to an attenuation of AAA development in murine models [82,84–87]. This effect seems to rely on paracrine responses mediated by the production of anti-inflammatory cytokines [85], the secretion of extracellular vesicles with paracrine immunomodulatory features [86], and the preservation of VSMC contractile phenotype [87]. Although an important number of these investigations reported the engraftment of MSCs into the arterial wall [85,88,89], evidence about their differentiation into VSMC or endothelial cells is limited [89], further supporting that paracrine-mediated effects underlie their benefit. Unfortunately, clinical trials assessing the benefit of MSCs-based therapies in AAA are scarce. The VIVAAA trial (NCT02846883) aiming to explore the safety and effectiveness of allogeneic MSCs in decreasing inflammation and aneurysm growth was prematurely interrupted due to slow enrolment and no results have been posted. In turn, the design of ARREST, a phase I, randomised controlled trial analysing the impact of MSCs infusion on aortic diameter and inflammatory parameters in patients with small AAA was initiated in 2018. Interim results in a small group of patients evidenced that MSCs infusion induces regulatory T cells (Tregs; 14 days post-infusion) and, after a 12-month follow-up, suppresses AAA inflammation (determined by 18-fluorodeoxyglucose uptake) associated with a reduced increase in aortic diameter [90]. Although the sample size was too small for statistical analysis, these promising results pave the way for larger trials exploring this novel therapeutic strategy in AAA, while data after the scheduled 5-year follow-up are eagerly awaited.
Other clinical trials
The inflammatory response is critical for the pathogenesis of AAA and mast cells are important players in the progression of this disorder. Mast cells release a large number of eicosanoids, cytokines and chemokines, which activate the renin–angiotensin system, contribute to neovascularization and VSMC apoptosis and promote the activation and release of proteolytic enzymes responsible for aortic wall weakening [91]. While compelling evidence supports the contribution of mast cells to experimental AAA [92,93] and a positive correlation between aortic diameter and the number of infiltrated mast cells has been detected in human aneurysmal samples [94], the AORTA trial, analysing the benefit provided by the mast cell inhibitor pemirolast, reported no significant impact on the growth of medium-sized AAAs [95]. Similarly, the study launched by Novartis focused on the anti-IL-1β antibody canakinumab in subjects with AAA showed a lack of efficacy in modifying aneurysmal size and was prematurely finished because the study did not meet expectations [96]. Finally, the benefit provided by cyclosporine A, on aneurysm expansion in two experimental models [97] and its ability to inactivate cyclophilin A, which could promote aneurysm development [98], added to its immunosuppressive properties encouraged the implementation of a clinical trial analysing the efficacy of this drug in patients with small AAA; this trial seems to be in progress and unfortunately, no results have been reported (NCT02225756).
The information compiled in this section is summarised in Table 2.
Table 2. Drugs against AAA tested in clinical trials.
| Drugs | Evidence from preclinical studies | Evidence from clinical studies | New clinical trials |
|---|---|---|---|
| Statins | Might limit AAA development through its anti-inflammatory and antioxidant properties [12]. | Decrease in vascular MMP9 [13]. Inconsistent data regarding AAA progression [16–18]. Statins improve survival after AAA repair [16,19,20]. |
Prospective and multicentre rigorous trials are needed to clarify the impact of statins on AAA growth. |
| Antihypertensive drugs | Antihypertensive drugs ameliorate AAA formation [21–28]. | Propranolol, telmisartan and ACEi failed to reduce AAA formation and growth [29,30]. Risk of faster AAA growth with ACEi [37]. |
Prospective trials in patients with small AAA will be required in view of reduced PWS and PWRI. |
| Doxycycline | Reduction of incidence of AAA, preserving elastin integrity and attenuating gelatinolytic activity [40–43]. | Doxycycline suppressed aortic expression of MMP [44–45]. Reduction in serum C-reactive levels [48–50]. Doxycycline does not attenuate aneurysm growth [50]. |
Clinical trials with longer follow-up. |
| Anti-platelet and anti-thrombotic therapy | Limitation of aortic rupture. Reduction of aneurysm diameter [52–54]. |
Inconsistent data regarding aneurysm growth [56–57]. Increased risk of bleeding in the event of AAA rupture [56]. Risk of type II endoleaks in patients subjected to EVAR [61–62]. |
New and high-quality clinical trials are essential. |
| Other drugs with anti-inflammatory properties | Cyclosporin A reduces aneurysm expansion [97]. | Pemirolast does not significantly affect AAA growth [95]. Lack of efficacy of canakinumab on AAA size [96]. |
Ongoing clinical trial testing cyclosporine A. |
| Metformin | Attenuation of vascular inflammation, ROS production, signalling and neovascularization [78–81]. | Observational studies support the ability of metformin to ameliorate AAA progression and aneurysm-related events [75–81]. | Randomised controlled clinical trials are needed to clarify this conclusion. |
| Stem cells | Lack of consistency among studies regarding cell types, sources, doses, and routes of administration [82]. | The VIVAAA trial was prematurely interrupted. ARREST trial interim results: suppression of inflammation and reduced aortic diameter growth [90]. | New clinical trials with a longer follow up. |
AAA, abdominal aortic aneurysm; ACEi, angiotensin-converting enzyme inhibitors; EVAR, endovascular aneurysm repair; MMP, matrix metalloproteinase; PWRI, aortic peak wall rupture index; PWS, aortic peak wall stress; ROS, reactive oxygen species.
Novel promising therapeutic targets
The absence of medical therapies for the management of AAA and the failure of clinical trials addressing this issue has encouraged an active investigation in search of new candidate strategies. Efforts have been directed at the mechanisms underlying the destructive vascular remodelling in AAA, which involves interrelated responses including, inflammation, ECM disruption, and medial VSMC paucity (by apoptosis, but also through necroptosis). Beyond these processes, recent insights, support the interest in new therapeutic targets, such as the sympathetic system, the crosstalk between ROS, mitochondrial dysfunction, and endoplasmic reticulum (ER) stress and the contribution of epigenetics to disease progression. Here we summarise some of the promising therapeutic approaches identified by targeting these processes (Figure 3).
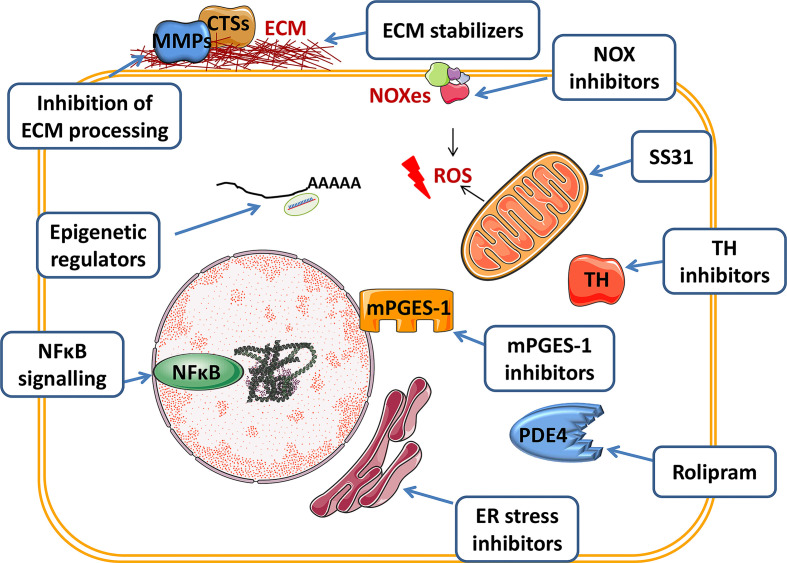
Figure 3. Promising therapeutic targets and medical interventions in abdominal aortic aneurysms (AAA).
The scheme summarises some of the candidate strategies identified in preclinical studies. The figure was partly generated using Servier Medical Art provided by Servier, licenced under a Creative Commons Attribution 3.0 unported license. CT, cathepsin; ECM, extracellular matrix; ER, endoplasmic reticulum; MMP, metalloproteinase; NFĸB, nuclear factor kappa B; NOX, NADPH oxidase; PDE4, phosphodiesterase 4; ROS, reactive oxygen species; TH, tyrosine hydroxylase.
The immune-inflammatory response as a therapeutic target for AAA
Vascular inflammatory cell infiltration is a consistent finding in human and experimental aneurysmal lesions. Inflammatory cells contribute to the destructive remodelling of the arterial wall and VSMC apoptosis through the release of cytokines and proteolytic enzymes and the increase in vascular oxidative stress. Although some studies suggest that immunosuppression could exacerbate aneurysm growth and promote its rupture questioning the safety of anti-inflammatory interventions in these patients [99], depletion of B cells, mast cells, neutrophils and natural killer T cells in rodents has been reported to ameliorate AAA development, while Tregs seems to play a protective role against this disease [2]. Therefore, it is tempting to speculate that targeting specific aspects of the inflammatory response might ameliorate aneurysm development, thereby representing a promising field of discovery, as discussed below.
mPGES-1 and EP4
In the synthesis of prostanoids, arachidonic acid is converted to prostaglandin H2 (PGH2) by cyclooxygenases (COXs) and further transformed into specific prostaglandins. Microsomal prostaglandin E2 synthase -1 (mPGES-1) catalyses the isomerization of PGH2 into PGE2, which is produced in great amounts under pro-inflammatory conditions [100] and recognised as a relevant player in AAA [101–103]. Certainly, the excessive production of PGE2 is a hallmark of human AAA. Studies in the late 1990s had already shown how PGE2 production in AAA specimens was extremely enhanced compared with control aortas [101,102]. This was associated with an upregulation of COX2 and mPGES-1 expression in human aneurysmal samples that seems to precede maximal leukocyte infiltration, both enzymes localising to vascular cells and leukocytes [104]. Previous reports showed that the treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) in a reduced number of patients with small aneurysms led to a decrease in AAA growth rates compared with those patients non-taking NSAIDs [102], in agreement with previous studies in animal models [105,106]. However, the use of NSAIDs in humans increases aortic stiffness [107], and in patients with AAA, it is a risk for type II endoleaks after endovascular repair, as described above for anticoagulation [108]. Added to this is the predisposition to myocardial infarction and stroke associated with COX-2-selective NSAIDs [109], thereby questioning their risk/benefit and discouraging the use of these drugs in AAA. Interestingly, mPGES-1 knockdown in mice protects against AngII-induced AAA formation [110]. Therefore, mPGES-1 emerges as an interesting alternative pharmacological target particularly relevant for the management of AAA. Additionally, E-prostanoid (EP) receptors, which mediate PGE2 biological actions, were also augmented in human AAA [104]. Among them, EP4 is strongly induced and localises with mPGES-1 in the microvasculature of human aneurysmal lesions where it seems to mediate the proangiogenic response induced by PGE2. Further, mPGES-1 and EP4 microvascular expression were enhanced by active smoking in patients with AAA [111]. Collectively, these data support the relevance of the COX-2/mPGES-1/EP4 axis in aneurysm neovascularisation, a process that could be involved in the progression and rupture of AAA. Certainly, EP4 seems to play a significant role in AAA, since its pharmacological inhibition in mice attenuates AAA incidence and severity, limiting vascular inflammation and ECM remodelling [112,113]. Of note EP4 inhibition not only prevents aneurysm formation but also attenuates the growth of established experimental aneurysms, more firmly supporting its translational relevance [112]. High expression of EP4 in VSMC from human AAA has been also reported and a recent study confirmed that the overexpression of this receptor in VSMC aggravates experimental AAA with enhanced infiltration of inflammatory macrophages and higher IL6 expression and MMP9 activity [114]. Further, EP4 stimulation in VSMC reduces protein levels of lysyl oxidase (LOX), a critical enzyme for the maintenance of matrix architecture [115,116], a mechanism that could contribute to aneurysm development by weakening the ECM (see below) [117]. Altogether these data support the interest of mPGES-1 and EP4 as promising targets in AAA; however, inconsistent data have been also reported since EP4 deletion on bone marrow cells fosters vascular inflammation, apoptosis and ECM remodelling and exacerbates AAA [118]. Therefore, a deeper understanding of cell-specific EP4-mediated responses will be needed to confirm whether systemic EP4 inhibition would confer protection against AAA.
PDE enzymes: PDE3 and PDE4 inhibitors
Cyclic nucleotide phosphodiesterases (PDEs) constitute a large superfamily of highly conserved enzymes (PDE1 to PDE11) that regulate cellular levels of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), second messengers that play a fundamental role in intracellular signalling. These enzymes are phosphohydrolases that selectively catalyse the hydrolysis and inactivation of cAMP and cGMP, thus regulating the magnitude and duration of their signalling, as well as, multiple metabolic and pathophysiological processes [119]. PDEs are grouped based on their selectivity for the substrate. While PDE3 hydrolyses cAMP and cGMP and PDE4 is the main cAMP-selective PDE, both enzymes are the major contributors to cAMP-hydrolysing activity in most cell types.
Although no data about PDE3 expression in human or experimental AAA has been provided, two independent studies demonstrated that the PDE3 inhibitor cilostazol, an antiplatelet agent used for the treatment of peripheral artery disease and stroke, suppresses AAA in preclinical models, attenuating vascular inflammation, MMP activity, and ROS production [120,121]. Unfortunately, the effectiveness of cilostazol against AAA has not been addressed in clinical trials.
Additionally, we have recently involved PDE4B in AAA [122]. This specific PDE4 isoform is significantly up-regulated in human and experimental aneurysmal lesions where localises to the inflammatory infiltrate. In fact, PDE4B is the main PDE4 subtype expressed in inflammatory cells and critically influences immune and inflammatory responses, thereby emerging as one of the main interesting targets against a wide range of disorders, specifically, inflammatory and autoimmune diseases [123,124]. Moreover, we found that PDE4D expression was similar in healthy aorta and AAA, both in humans and in the murine model, thus supporting that, while PDE4B could be involved in the pathophysiology of the aneurysm, the contribution of PDE4D would be secondary. However, a recent study reported no differences in vascular PDE4B expression in both patients with AAA and mouse aneurysmal lesions [125]. Surprisingly, this work detected significant up-regulation of PDE4D in aneurysmal VSMC and found that the cell-specific deletion of PDE4D abrogated the development of experimental AAA, further reducing blood pressure levels. The discrepancies between both reports would be related to the low number of human samples and the almost significant difference in the age between control and AAA groups in the study conducted by Gao et al., to the heterogeneity of human aneurysmal lesions, and, regarding preclinical studies, to differences in the mouse models of AAA [125]. Anyway, both studies demonstrated how PDE4 inhibition with rolipram significantly attenuated aneurysm formation, preserving elastin integrity and normalising MMP2 activity [122,125]. Further, Varona et al demonstrated that rolipram ameliorated vascular inflammatory infiltration and oxidative stress [122]. Therefore, and beyond the discrepancies between both studies, these data suggest that the selective inhibition of PDE4 activity could provide a benefit in vascular diseases such as AAA, as suggested by the low rate of cardiovascular events detected in patients with chronic obstructive pulmonary disease treated with roflumilast [126]. However, severe side effects are associated with non-isoform-specific PDE4 inhibitors, mainly related to the inhibition of the PDE4D subtype. In this context, it is tempting to speculate that the anti-inflammatory response provided by selective PDE4B inhibitors might benefit AAA patients [127,128]. Worthy of mention is the PDE5 inhibitor sildenafil, which inhibits cGMP hydrolysis and has been found to exacerbate the progression of experimental aneurysms [129]. Further, its abuse has been clinically related to aortic dissections [130]. Therefore, the prescription of sildenafil in patients with AAA should be considered carefully.
Inflammatory signalling
Accumulated evidence has highlighted the critical involvement of inflammatory signalling pathways in the control of vascular remodelling underlying AAA progression and their therapeutic potential. Nuclear factor (NF)κB, c-Jun N-terminal kinase (JNK), Janus kinase/signal transducers and activators of transcription (JAK/STAT) and WNT signalling are some of the key signal integrators controlling vascular inflammation that have been studied as novel targets for AAA therapy.
The activation of the NF-κB family and the consequent regulation of downstream targets triggers different processes such as innate and adaptive immunity, and cell survival, differentiation, and proliferation and regulates cellular responses to stress, hypoxia, stretch and ischaemia, being critical in vascular diseases such as atherosclerosis and AAA [131]. Studies developed in the first decade of this century focused on NFκB and ETS, transcription factors involved in the control of inflammation and MMP expression, and their role in AAA. Based on electrophoretic mobility shift assays, these authors reported the striking activation of both NFκB and ETS in the neck of human AAA, which is the most active region of aneurysmal lesions. Interestingly, decoy oligodeoxynucleotides (ODN), which competed with the binding of these transcription factors to their respective response elements and suppress their signalling, achieved a significant benefit on aneurysm progression in two experimental animal models. This strategy decreased aneurysm size, preserved elastic fibre integrity, reduced MMPs expression and limited macrophage infiltration, thus suggesting the potential of decoy-based therapies in AAA and the interest of targeting these signalling pathways. However, special consideration should be given to the divergent functions of NFkB in the cardiovascular system, playing both beneficial and detrimental roles depending on the cellular and physiological context. For example, NFκB activity in macrophages triggers anti-atherogenic responses whereas in endothelial cells is a pro-atherogenic factor [131]. Whether this dichotomy also operates in AAA is uncertain, but these data strongly support that approaches targeting NFκB therapeutically in human AAA should be carefully considered and would benefit from the development of cell-specific inhibitors.
The dysregulation of JNK signalling is another feature of human AAA [117]. Certainly, human AAA specimens showed high levels of phosphorylated JNK. Through several experimental approaches in two animal models, the authors showed that this route is essential for the development of AAA by activating a coordinated response that contributes to the destructive remodelling of the ECM, encompassing both the up-regulation of MMP9 activity and the suppression of LOX. More interestingly, the pharmacological inhibition of JNK with SP600125 abrogated aneurysm development and further regressed established AAA, thereby highlighting that targeting JNK could allow the repair and stabilization of the ECM in this disorder, being a solid candidate for pharmacologic intervention. Similarly, in a mouse model of AAA, higher JNK1 expression associated with enhanced MMP levels has been reported in males compared with females, suggesting that this might be partially responsible for the differential incidence of AAA between genders [132]. Although further research will be needed to confirm this hypothesis in humans, the higher JNK activity reported in aneurysms from males might be related to the lower vascular LOX expression in men with AAA compared with women that seem to underlie the sexual dimorphism of this disease [133]. Additionally, cJun/AP-1 and the C/EBP homologous protein (CHOP) that mediates cell apoptosis, were simultaneously induced in the aneurysmal lesions from Ang II-infused ApoE−/− mice. In fact, cJun/AP-1 regulates CHOP transcriptional activity, thus linking this signalling pathway not only to inflammation and ECM remodelling but also to the apoptosis-mediated loss of VSMC characterising this disease [134]. Interestingly, the inactivation of JNK activity is one of the common mechanisms underlying the attenuation of AAA achieved by either the genetic ablation of microRNA-33, a critical microRNA in the pathogenesis of this disease (see below) [135], an Ang II peptide vaccine [136] or by the biphosphonate zoledronate [137], supporting again the interest of this signalling cascade as a therapeutic option in AAA.
The JAK/signal transducer and activator of transcription (JAK/STAT) pathway also emerge as an appealing medical target for AAA. JAK/STAT signalling is overactivated in human AAA [138] and pSTAT3 levels are increased in human and murine aneurysmal lesions [139]. Accordingly, the benefit provided by several direct or indirect inhibitors of the STAT3 pathway has been reported in different animal models of AAA [139–142]. Conversely, STAT3 signalling has also been claimed as a critical factor for the maintenance of vascular integrity. In fact, deficiency of STAT3 in humans leads to vascular abnormalities and a high incidence of aneurysms [143] and the in vivo inhibition of this pathway by overexpressing SOCS3 increased the severity of aneurysms in a non-hyperlipidemic mouse model [143,144]. This effect seems to be cell-specific, linked to T-cells and the potential protective role of interleukin-17 on this disease [144]. Recent research highlights that STAT3 regulates the balance between the destruction and preservation of the aortic tissue and that depending on the cell type (macrophages vs VSMC) the inhibition of STAT3 results in an exacerbated incidence of aortic dissections or in a protective response that reinforces aortic biomechanics [145]. Therefore, there is already much to learn about this pathway to develop safe and effective therapeutic strategies targeting JAK/STAT signalling.
In the last years, Wnt signalling has aroused the interest of the scientific community. This pathway is aberrantly reactivated in several disorders, including cardiovascular diseases. Multiple elements of this pathway are dysregulated in human and experimental aneurysms, in which high levels of the transcriptionally active form of β-catenin have been found, pointing to an overactivation of the Wnt/β-catenin pathway in AAA [146]. This route has been involved in the control of monocyte migration and adhesion [147,148], but beyond its impact on inflammation, the Wnt/β-catenin cascade enhances MMP expression and influences VSMC homeostasis [149–152], which emphasises the potential of targeting this pathway as a therapeutic option for AAA. However, the effective blockade of the Wnt/β-catenin signalling pathway with either the porcupine inhibitor LGK974 or PRI-724, which disrupts the interaction between β-catenin and the (CREB)-binding protein, proved ineffective in limiting aneurysm formation induced by AngII-infusion in ApoE−/− mice. Although PRI-724, slightly slowed down the progress of AAA and improved the severity of aneurysmal lesions, no clear overall benefit on aortic diameter and AAA incidence were detected and, therefore, these data advocate for targeting specific elements of this signalling route as a possible strategy for the management of this disease. Previous studies reported that the Wnt/β-catenin inhibitor sclerostin limits aneurysm formation in an experimental model [153], while recently a comparable favourable outcome was obtained by the deletion of CNN4, a Wnt signalling target gene that exerts pro-inflammatory responses in different pathological scenarios [154]. Similarly, the benefit on AAA progression provided by miR-124a has been related at least in part to the regulation of Wnt/β-catenin signalling [155]. Multiple targetable candidates among Wnt pathway components could be potentially approached based on human data [146], supporting the rationale for further research in this field.
Finally, recent research strengthens the view that the engagement of immunoglobulin G (IgG) Fc receptors (FcγR) by immune complexes is relevant in AAA. In fact, FcγR expression was enhanced in human and mouse aneurysmal lesions, while FcγR knockdown decreased AAA incidence and aortic dilation, preserving elastin integrity and VSMC content and limiting vascular inflammation and oxidative stress, thus suggesting that the IgG-FcγR-axes might represent a therapeutic target for this disease [156,157].
Targeting ECM to preserve vascular integrity
ECM disruption contributes to the destructive remodelling of the aortic wall that features AAA. Thus, it has been speculated that limiting ECM degradation, for example targeting its proteolytic processing, might ameliorate disease progression. Further, strategies aiming at stabilising the ECM has been also proposed as an alternative to prevent aortic wall failure in AAA, as detailed below.
ECM Proteolytic Degradation
ECM remodelling in AAA involves the activation of several families of extracellular proteases such as MMPs but also of serine and cysteine proteases, all of them enzymes that degrade the main structural components of the arterial wall, namely collagen and elastin. The MMP-mediated proteolytic degradation has been extensively studied and identified as a critical step in the gradual weakening and instability of AAA, being MMP2 [158,159], MMP9 [159,160], and MMP12 [161], the main MMPs linked to this process. As it has already been discussed, tetracyclines and, in particular, doxycycline emerged as a non-selective MMP inhibitor that reduces the incidence of AAA in experimental models. However, studies in patients were inconclusive and questioned the usefulness of doxycycline as a therapeutic option for the management of AAA. Additionally, although more than 50 broadspectrum MMP inhibitors have been developed and tested in clinical trials for different disorders, all of them failed as therapeutic tools due to the lack of specificity and the occurrence of musculoskeletal side-effects, among others [162]. While doxycycline, formulated at a sub-antimicrobial dose, is the only MMP inhibitor approved by the FDA for periodontal disease, a new generation of MMP inhibitors more selective and with improved pharmacokinetic profiles have advanced to clinical trials for different diseases [163], and therefore the door is still open for new clinical trials testing MMP inhibitors in AAA.
Cysteine and serine proteases have also been involved in the pathogenesis of AAA. Early studies in human atherosclerotic and aneurysmal lesions revealed that the expression of elastolytic cysteine proteases, such as cathepsins L, S and K [164–166], is significantly increased, while that of cystatin C (a cysteine protease inhibitor) is markedly decreased. Similarly, enhanced levels of mast cell chymase and tryptase have been reported in human AAA [93,167].
The direct participation of these enzymes in AAA has been demonstrated by gene-knockdown in preclinical models [168–170], studies that linked the disturbed expression of these proteases not only to the ECM disarrangement but also to the inflammatory response, affecting chemotaxis and chemokine production, VSMC apoptosis, angiogenesis and protease activation. Interestingly, serum cathepsin S, chymase and tryptase levels correlate with the aneurysm growth rate in patients with AAA, while cystatin C levels seems to be inversely associated with abdominal aortic diameter [93,167,171,172], suggesting that they can serve as novel biomarkers for this disease. Altogether these data emphasised that the imbalance between vascular cysteine and serine proteases and cystatin C could promote proteolysis and favour aneurysm progression and that the maintenance of the equilibrium among these enzymes could be a therapeutic strategy. This research underscores the interest of chemical inhibitors of cathepsins as pharmacological tools for AAA, now being tested in experimental models with promising results [173–175].
Serine proteinases involved in fibrinolysis, specifically, tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA), have been also related to AAA due to their role in inflammation and proteolysis. Both uPA and tPA activate plasminogen to plasmin, which in turn initiates a proteolytic cascade leading to MMP activation and ECM degradation, which might contribute to AAA. Expression of uPA increases in human and experimental aneurysmal lesions [176,177], while uPA deficiency protects against aneurysm rupture in AngII-infused ApoE−/− mice, thus suggesting an active contribution of uPA to this disease [178]. Consistently, in this model, the local overexpression of the uPA inhibitor, plasminogen activator inhibitor-1 (PAI-1), limited aneurysm expansion [179]. However, conflicting results were subsequently published, showing that deficiencies of either uPA or its receptor (uPAR) did not influence the incidence or size of aneurysmal lesions in LDL receptor knockout mice challenged with Ang II nor enhanced the rate of AAA rupture [180]. Furthermore, bone marrow transplantation studies demonstrated that the increased AAA rupture could be attributed to the lack of uPA in hematopoietic cells and suggests that the absence of uPA affects the resolution of transmural thrombi while promoting aortic rupture, excluding an impact on MMP activity [180]. In view of these discrepancies, further research will be needed to clarify the contribution of the uPA–uPAR system to AAA.
Lysyl oxidases (LOXs) and other elastogenic proteins
The LOX family is comprised of five closely related ECM enzymes, involved in the covalent cross-linking of collagen and elastin, which conditions the tensile strength and elastic properties of connective tissues [115,116,181]. Early studies in the 1990's suggested that LOXs are critical to preserving the structure and mechanical properties of the aorta and that a decrease in LOX expression and activity is associated with the development of experimental aneurysms [117,182]. Subsequently, two independent groups reported that the genetic inactivation of the archetypal LOX form, but not that of other isoenzymes of this family, is lethal and associates with large aneurysmal lesions and aortic dissections [183,184]. In fact, the pharmacological inhibition of LOX activity induces AAA in different animal models [117,182,185,186], while interestingly, the local overexpression of LOX by periadventitial adenoviral delivery attenuates the progression of experimental aneurysms. Beyond EMC maturation, LOX also attenuates vascular inflammation through a reduction in MCP-1 secretion, limiting macrophage infiltration and JNK activation [187,188], further supporting the interest of LOX as a pharmacological target for this disease. Unfortunately, evidence about the contribution of downregulation of LOX to human disease is scarce, mainly due to the lack of human samples corresponding to the early stages of the aneurysmal disease, when surgery is not recommended. Nevertheless, and because AAA is considered a local manifestation of a general defect in the vasculature [1,189], it has been suggested that aneurysmal disease could be associated with changes in the expression pattern in other vascular beds. In particular, the proteomic profile of the internal mammary artery from patients with AAA reflects a significant upregulation of LOX and other elastin-related molecules, which was regarded as a compensatory response to protect vascular wall integrity [190]. More interestingly, recent findings have involved LOX in the sexual dimorphism of AAA [133]. The authors found lower vascular LOX expression and activity in males, both in human and animal models and determined that an androgen-mediated inhibition of LOX activity underlies the higher susceptibility to AAA exhibited by males, supporting that normalising LOX could abolish sexual dimorphism and limit AAA in males. Thus, LOX expression/activity could critically participate in AAA development which would support control of LOX activity as a strategy to preserve vascular integrity and curtail aneurysm progression. Further investigations will be needed to provide convincing proofs of the feasibility of this approach.
Besides LOXs, elastic fibre assembly is coordinated by fibulin-5 (FBLN5), an integrin-binding matricellular glycoprotein, which is critical for elastogenesis, but also participates in cell–cell and cell–matrix communication and controls vascular cells adhesion, proliferation, migration, and survival [191,192]. In the vascular wall, FBLN5 is responsible for the preservation of vascular integrity after injury avoiding anomalous remodelling [193,194]. Interestingly, in human aneurysmal lesions, the expression of FBLN5 is significantly decreased [195], while studies in AngII-infused C57BL/6J mice demonstrated the contribution of FBLN5 downregulation to aortic dilation. Certainly, vascular FBLN5 knockdown circumvented the intrinsic resistance of the C57BL/6J strain to AngII-induced dilation, increasing aortic diameter and the expression of inflammatory mediators [195]. As discussed below, the reduced expression of FBLN5 in AAA is related to the inflammatory component of the disease and epigenetic regulation mediated by the HDAC/SOX9 axis. The authors concluded that preserving normal FBLN5 vascular expression could prevent aortic wall failure and become a promising therapeutic strategy in AAA.
ECM stabilising agents
Agents that stabilise the ECM and prevent the progressive degradation of the vascular wall in AAA have been investigated in the last years with encouraging results. Among them, pentagalloyl glucose (PGG) stands out due to its safety. PGG is a tannic acid derivative that acts as an elastin-stabilising polyphenol [196]. The ability of PGG to stabilise elastin enables this compound to improve tissue biomechanical stability, a feature that has been exploited in tissue-engineering for the development of vascular grafts and that has prompted its application to restrict aortic aneurysm growth. The first evidence supporting PGG for the treatment of AAA was reported in 2007 when the benefit of this compound ameliorating CaCl2-induced aneurysms in rodents was demonstrated [197]. In this study PGG preserved the integrity of elastic lamellae in abdominal aorta despite no affected inflammation or MMP levels [197]. Since then, multiple investigations have confirmed the ability of PGG inhibiting AAA expansion in different experimental models [198,199] and have addressed strategies to specifically target PGG to aneurysmal lesions (including the use of PGG-loaded nanoparticles) [200]. The benefit conferred by PGG could also be due to the increase of LOX expression that would additionally contribute to ECM stabilisation [201]. Of note, PGG exhibits anti-inflammatory, vasodilatory and ROS scavenging properties and behaves as a non-competitive inhibitor of thrombin [196]. Whether these effects could also account for the favourable impact of PGG on experimental AAA and whether it could be useful in clinical practice deserves further research.
Loss of VSMC by Necroptosis
Depletion of VSMC is one of the main processes featuring AAA that contributes to aortic wall weakening and impairs ECM production and repair. VSMC loss has been classically attributed to apoptosis; however, in the last years, necroptosis (also known as programmed necrosis) has emerged as a critical phenomenon in VSMC paucity and as a new target for therapeutic intervention. This programmed cell-death pathway is orchestrated by the kinases RIP1 and RIP3 [202], both of them elevated in VSMC in human and experimental aneurysmal lesions. Noticeably, the deletion of RIP3 abrogated AAA formation and impaired the p65-dependent inflammatory response in the elastase-induced mouse model [203]. Mixed lineage kinase domain-like pseudokinase (MLKL) and calcium/calmodulin-dependent protein kinase II (CaMKII) are downstream effectors of RIP1-dependent necroptosis in AAA. Interestingly, pharmacological inhibition of necroptosis by targeting RIP1 or RIP3 ameliorates aneurysm expansion and even stabilises pre-existing aneurysms attenuating inflammation and inducing ECM repair [203,204], while, recently, the protective role of IL-37 on aneurysmal disease has been related to its ability to suppress RIP3-mediated necroptosis [205]. These findings have encouraged active research aiming at characterising the regulatory mechanisms underlying RIP3 upregulation in AAA [206], which will provide grounds for new therapeutic strategies for this disease.
Targeting epigenetic mechanisms in AAA
Epigenetics refers to those changes in the genome that do not involve modifications in the DNA sequence and that often occur in response to environmental cues or lifestyle factors. Epigenetic mechanisms of gene regulation encompass (i) histone modifications and chromatin remodelling, (ii) DNA methylation, and (iii) RNA-based mechanisms. Epigenetics drives the maladaptive tissue remodelling response underlying cardiovascular diseases. Accumulating evidence supports that epigenetics plays a central role in AAA and that its control may hold potential for therapeutic intervention [207,208]. In this section, we will review the most significant findings supporting the relevance of pharmacological interventions targeting the epigenetic landscape in AAA.
DNA methylation
DNA methylation involves the addition of a methyl group to the 5′ position of cytosine residues at cytosine preceding guanosine (CpG) islands. Most CpG islands are sites of transcription initiation and promoter methylation usually results in reduced accessibility of transcription factors to cis-DNA-binding elements, thereby suppressing transcription. Interestingly, the methylation pattern is dynamic and susceptible to changes in response to ageing and lifestyle factors such as smoking, both being major risk factors of AAA [209]. The DNA methylation status relies on the balance between DNA methyltransferases (DNMT), responsible for DNA methylation, and the activity of the ten–eleven translocation (TET) family of enzymes that demethylate CpG islands. Analysis in peripheral blood mononuclear cell DNA from healthy controls and patients with AAA, revealed significant differences in DNA methylation [210]. AAA is related to global hyper-methylation, while the methylation status positively correlates with aortic diameter [211]. Further, Tonghill et al identified gene-specific changes in DNA methylation in VSMC from human aneurysmal samples. Certainly, the decrease of both smooth muscle 22α (SM22α) and sclerostin, which are characteristic of human AAA and contribute to aneurysm development, is derived from their promoter hypermethylation [153,212]. Likewise, a cohort study from a population-based screening program in Sweden did observe higher global DNA methylation in patients with AAA and a linear association with baseline aortic diameter, although no significant association between DNA methylation and AAA growth was found during follow-up [213]. In turn, the DNA methylation rate and the expression of several DNMTs were increased in Tregs from patients with AAA [214]. Similarly, DNA methylation was markedly disturbed in aortas of patients with acute aortic dissection and associated with the altered gene expression pattern featuring this disorder [215]. Because DNA methylation is a dynamic process, therapeutic approaches aiming to attenuate the aberrant methylation status in AAA could be a useful strategy for this disease, however, no experimental data supporting this hypothesis are currently available, while the severe side effects of hypomethylating agents are a major pitfall for these drugs.
Histone post-translational modifications
Histone post-transcriptional modifications conditions the structure and packaging of chromatin, thereby controlling the accessibility of transcription factors to DNA. Acetylation and methylation are the most extensively studied histone modifications. Histone methylation, regulated through histone methyltransferases and histone demethylases, can lead to both transcriptional activation and repression, while promoter acetylation usually associates with transcriptional activation.
Histone methylation seems to play an essential role in macrophage-mediated inflammation in AAA. In particular, the histone demethylase Jumonji domain-containing protein D3 (JMJD3) is increased in infiltrating myeloid cells from human aneurysmal lesions resulting in an induction of the inflammatory immune response. Mechanistically, JMJD3 limits H3K27 trimethylation on pro-inflammatory cytokine promoters rendering them accessible to NFkB binding and thereby inducing the inflammatory response triggered by these cells [216].
The balance between histone acetyltransferases (HATs) and histone deacetylases (HDACs) controls the degree of histone acetylation. Notably, in the vasculature, HDACs strongly influence gene transcription and affect the expression of genes critically involved in essential processes underlying AAA development, such as ECM remodelling, inflammation and VSMC differentiation and function. Increased mRNA levels of specific HDACs have been reported in Tregs from patients with AAA [214]. Similarly, the expression of class I and II HDACs (HDACs 1, 2, 4 and 7) was significantly augmented in aneurysmal lesions from AAA patients, where they localize to both VSMC and infiltrated inflammatory cells and associated with a consequent decrease of vascular histone H3 acetylation (H3K18) [217]. Interestingly, in a mouse model of AAA that also exhibits reduced histone acetylation and higher HDAC expression resembling human disease [217,218], the administration of MS-275 (a class I HDAC inhibitor) or MC-1568 (that inhibits class II HDACs), attenuated the incidence and severity of AAA, normalised aortic diameter and reduced AngII-induced mortality, limiting vascular and systemic inflammation and also ECM destructive remodelling [217]. Among other effects, HDAC inhibitors prevented the cytokine-mediated reduction of FBLN5, an essential protein for elastic fibre formation and one of the most downregulated proteins in human AAA [195,219]. By in vivo approaches, Orriols et al. demonstrated the critical contribution of FBLN5 knockdown to AngII-induced aortic dilation and vascular inflammation identifying the involvement of a SOX9/HDAC-dependent mechanism in the downregulation of FBLN5 by inflammation. Thus, preserving FBLN5 could prevent the disorganization of ECM induced by inflammation in AAA [195]. Further, and considering that class I and II HDAC inhibitors have been proven effective in several types of cancer, these studies suggest that HDAC inhibitors could be repurposed for the treatment of AAA. However, caution should be adopted, since inconsistent data have been also reported in human aneurysmal disease, with higher acetylation of the histone substrates H3K9, H3K18 and H3K14 associated with enhanced expression of vascular HATs [220]. Further, it is interesting to note that sirtuin 1 (Sirt1) activity is decreased in aortic specimens from patients with AAA [221]. Sirt1 is an HDAC class III member that participates in the suppression of inflammation. The study by Horimatsu et al. found that the hypolipidaemic agent niacin protects against AAA formation through the increase in Sirt1 activity, in agreement with previous studies reporting that the macrophage-specific deletion of Sirt1 promotes aneurysm formation [222], thus suggesting that Sirt1 agonism could become a promising therapeutic strategy for AAA.
Noncoding RNAs
Noncoding RNAs do not influence chromatin architecture but participate in post-transcriptional regulation of gene expression. Noncoding RNAs encompass small RNAs, mainly microRNAs (miRNAs) and long noncoding RNAs (lncRNAs).
The tissue- and plasma-specific miRNA signature in human AAA has been characterised by high-throughput approaches. Two independent studies [223,224], revealed the significant upregulation in aneurysmal lesions of microRNAs related to fibrosis (miR-29), inflammation (miR-124a, miR-146a, miR-155, and miR-223) and endothelium (miR-126, let-7 family members, and miR-21). Further, miR-30c2, miR-204, miR-133a, and miR-133b were downregulated associated with a consequent increase in their target genes involved in apoptosis and inflammation, fundamental processes in AAA pathogenesis [223]. The increase of miR-155 in human AAA was confirmed by subsequent studies [225] was associated with the down-regulation of its target genes CTLA4 and SMAD2, critically involved in the immune response. In turn, miR-21, which influences VSMC proliferation and apoptosis, is also increased in both human and experimental aneurysmal lesions. Probably, this is a compensatory response to limit aneurysm expansion, since in murine models miR-21 overexpression prevented AAA progression, while its blockade augmented aortic diameter [226]. Interestingly, miR-33, which is highly expressed in central zones of human AAA compared with marginal areas, participates in the control of vascular inflammation inducing a proinflammatory M1 macrophage phenotype, while it is knockdown ameliorates experimental AAA [135]. Regarding miR-29, increased aortic expression of miR-29 family members, along with decreased mRNA levels for its targets genes, encoding for ECM proteins, have been reported in two murine models [227], in which the in vivo blockade of this miRNA reduced aneurysm growth [227,228]. Because miR-29 inhibition associates with an enhanced expression of ECM components the risk of fibrosis by targeting this miRNA cannot be overlooked.
Progression of experimental AAA is also related to a downregulation of the miR-23b-24-27b cluster, being miR-24 significantly inhibited in human AAA, where its level inversely correlates with aneurysm size. miR-24 was identified as a critical regulator of vascular inflammation in AAA by targeting chitinase 3-like 1 (Chi3l1) in macrophages, VSMC and endothelial cells, suggesting that the decrease of miR-24 contributes to aneurysm growth and that both miR-24 and CHI3L1 could become novel plasma biomarkers of AAA disease progression and therapeutic targets for this disease [229].
Of note, this is only a small sample, likely the most translationally relevant, of the huge amount of information about miRNAs and their involvement in the pathogenesis of AAA, indicative of the complexity of this aspect of the disease [230,231]. There is an evident need for broader knowledge to more clearly uncover how miRNAs influence vascular remodelling in AAA and their real therapeutic value. miRNAs have emerged as highly interesting pharmacological tools to restore cell homeostasis, but an additional challenge for therapeutics is how to interfere with miRNA function in an effective and safe manner. There are still important technical concerns to overcome in this field, however, clinical trials assessing miRNA-based interventions have been launched for different disorders, and some of them have been approved by FDA, including two therapies against cardiovascular diseases [232,233].
In turn, LncRNAs comprise a more heterogenous group of RNA molecules, larger than 200 nucleotides, that modulate gene transcription through a variety of ways still incompletely characterised, [230]. Concerning AAA, numerous studies have shown the crucial role of lncRNAs in different processes underlying aortic growth, but only a few of them have been functionally characterised. Results from in vivo studies in animal models have analysed the participation of H19, GAS5, PVT1, XIST, SENCR, MYOSLID, SMILR, and NEAT1 in AAA pathophysiology [230,234,235]. In fact, silencing these lncRNAs reduces AAA formation and attenuates aortic diameter by different mechanisms, like limiting the MMP-dependent proteolytic degradation of the aortic wall, reducing the expression of inflammatory mediators and attenuating apoptosis [236–239]. Although these data suggest the utility of lncRNAs as therapeutic targets, similar to miRNAs there are several concerns to translate them to clinical research. However, there is currently a huge interest in targeting these molecules in cardiovascular diseases with some trials testing their utility as biomarkers and some noncoding RNA-based therapies being analysed in preclinical trials. These studies encourage future research in the field of AAA [240,241].
Other epigenetic mechanisms
Beyond these aspects related to histone post-translational modifications, DNA methylation, and noncoding RNAs there is much to be learned about epigenomics in AAA. Intensive research on this issue is being undertaken with appealing results. The bromodomain extra-terminal (BET)-containing a family of epigenetic reader proteins emerges as a novel epigenetic player orchestrating complex transcriptional programs in cardiovascular homeostasis and more specifically in AAA, for which targeting BET could become an attractive pharmacological strategy [242,243]. More interestingly, recent research identified ATP-dependent chromatin remodelling enzyme complexes as critical players in AAA. Endothelial-specific deletion of Brahma related gene 1 (Brg1), a chromatin remodelling protein, attenuates AAA [244]. Similarly, BAF60c and BAF60a, unique subunits of the SWI/SNF (switch/sucrose nonfermentable) chromatin remodelling complex were identified as important regulators of VSMC homeostasis with opposite functions, underscoring their critical contribution to the control of vascular inflammation and ECM remodelling in AAA and the potential of targeting BAF60-mediated epigenetic modifications for this disease [245,246]. Further research is obviously guaranteed.
The sympathetic system as a new target in AAA
The vascular and nervous systems are in close connection and emerge as critical communication systems in mammals, existing in coordinated mutual cross-talk with important pathophysiological repercussions [247]. The disturbance in the sympathetic nervous system has a fundamental role in the development of highly prevalent cardiovascular diseases including hypertension, heart failure and myocardial infarction [248,249]. The contribution of the sympathetic system to aortopathies such as AAA is less characterised, although progress has been made in the last few years, highlighting its interest as a new pharmacological target for these patients. Certainly, the occurrence of human thoracic aortic dissections has been related to an increase in sympathetic activity, while sympathetic hyperactivity and aortic sympathetic nerve sprouting have been reported in these patients, suggesting that the sympathetic nervous system may modulate both functional and structural properties of the aorta [250,251]. In fact, in aortic aneurysms, catecholamines control TGFβ signalling, attenuating VSMC proliferation and promoting apoptosis and thereby affecting vascular integrity. Further, it has been recently reported that local sympathetic denervation ameliorates experimental aneurysms [252]. In agreement, high-throughput studies in a new mouse model that overexpresses the neuron-derived orphan receptor-1 (NOR-1) in the vascular wall and reproduces the main features of human AAA, suggested the upregulation of genes involved in the tyrosine hydroxylase (TH) pathway that leads to the synthesis of catecholamines [253]. By a thorough analysis of human and murine aneurysmal lesions, we uncovered the up-regulation of several components of this signalling route in AAA and evidenced that TH localises to vascular innervations, inflammatory cells and some scattered VSMC in these samples. More interestingly, these studies provided sound evidence for the benefit of TH inhibition attenuating aneurysm formation [254,255]. The administration of α-methyl-p-tyrosine (AMPT), an orally available competitive inhibitor of TH, which decreases the endogenous levels of catecholamines, showed that the blockade of this pathway protected against AngII-induced AAA in two experimental models, normalising MMP expression and activity, preserving elastin integrity and limiting vascular inflammation and oxidative stress. Although it was developed as an antihypertensive drug, AMPT is ineffective against essential hypertension, and it is exclusively indicated for the treatment of pheochromocytoma and other rare tumours that produce excessive amounts of catecholamines (DEMSER). Of note, the association of pheochromocytoma with AAA has been reported, thus supporting again that catecholamine overproduction might promote aneurysm development and growth [256]. Therefore, these data support that TH inhibition could become an interesting approach for the treatment of AAA. Because of the short half-life and the central-adverse effects of AMPT, further research will be needed for the development of a new generation of TH inhibitors with improved pharmacokinetics and safety for the management of AAA.
Reactive oxygen species (ROS), endoplasmic reticulum (ER) and mitochondrial stress: potential targets in AAA
The implication of vascular oxidative stress in the pathogenesis of AAA has been extensively documented [257,258]. The vascular inflammatory environment critically contributes to the augmented ROS production, which, in turn, exacerbates the recruitment of inflammatory cells in a reciprocal feedback loop, thereby contributing to AAA progression [259]. Human studies have found a negative correlation between antioxidant consumption and the size of AAA, while a positive relationship was reported between aneurysm diameter and markers of lipid peroxidation, suggesting that antioxidant therapy might be beneficial for these patients [260]. NADPH oxidase is the major source of vascular ROS and increased O2− levels coupled with higher NADPH oxidase activity, and enhanced expression of the p47phox and p22phox subunits have been described in human AAA [257,261]. Accordingly, p47phox knockdown attenuates experimental aneurysms [262], and subsequent studies also supported the contribution of NOX2-derived ROS to this disease [84]. Therefore, NADPH oxidases arise as promising therapeutic targets for limiting aneurysm progression and rupture through the reduction of oxidative stress [263]. However, ROS also play essential functions in cellular signalling and homeostasis, which explains the failure of clinical studies testing the potential benefit of antioxidant supplements for cardiovascular diseases [264]. In this scenario, and to avoid serious undesired effects, huge efforts should be made for the development of isoform- and cell-specific NADPH oxidase inhibitors, whose impact on aneurysm growth will surely be addressed in the future.
Oxidative stress is closely related to the induction of endoplasmic reticulum (ER) stress, which is involved in the pathogenesis of several cardiovascular diseases [265–267], including human AAA and other aortopathies [261,268]. Certainly, analysis in a large cohort of patients with AAA reported enhanced vascular expression of activating transcription factor 6 (ATF6), IRE-1, active X-binding protein 1 (XBP-1) and the pro-apoptotic factor CHOP, while no differences for elements of the ATF4 axis of the ER stress response were detected at the end stage of AAA [261]. In agreement, classic ER stress inhibitors reduced aortic diameter and the incidence and severity of experimental aneurysms, attenuating inflammation and vascular remodelling [139,269,270]. Further, it should be highlighted that under pathological conditions the cross-talk between mitochondria and the ER promotes mitochondrial ROS generation and mitochondrial dysfunction [271,272]. It has been described that the expression of transcription factors that activate mitochondrial biogenesis and regulate mitochondrial function (PGC1α, NRF1 and TFAM), as well as that of Cyt B and Cyt C oxidase, are decreased in human aneurysmal specimens, accompanied by a reduction in the mitochondrial mass [261]. Consistent with these data, another study reported a decrease in PGC1α in the medial wall of the human aneurysmal aorta [273]. Additionally, mitochondria-dependent ROS/apoptosis activation plays a critical role in the progression of AAA in animal models of the disease [269,274]. Interestingly, the inhibition of mitochondrial stress by the mitochondria-targeted tetrapeptide SS31 prevented aneurysm formation in AngII-infused ApoE−/− mice. Reduced ROS production and vascular inflammation, but also an attenuation of ER stress accounted for the benefit provided by SS31. Altogether, these results reinforce the concept that the maintenance of mitochondrial homeostasis could be an interesting therapeutic strategy in AAA and support that pharmacological strategies aiming at alleviating both mitochondrial and ER stress may hold a promise to halt aneurysm progression and prevent its rupture.
Concluding remarks
Multiple potential medical targets for AAA have been identified in preclinical approaches mainly in rodents. The studies included in this revision are only a small part of the titanic efforts made in this field. However, the translation of these experimental insights into the clinics have utterly failed and the main reason for this failure might lie in the critical divergencies between humans and murine models regarding inflammatory/immune and metabolic responses [275,276]. Being a chronic disorder that progresses gradually and silently for decades, experimental models reproduce an accelerated form of the disease that is not fully representative of this condition. Since human surgical specimens are scarce, experimental data are valuable but we must be aware of the drawbacks of experimental approaches [277]. In this context, there is an active search and progress in the development of preclinical models for this disease in order to improve their translational relevance. Further, approaching pharmacological studies in (i) aged animals, (ii) more than one experimental model representing distinct mechanisms of AAA and (iii) in the presence of comorbidities would increase the pathophysiological significance of the results. Of note, very few studies have addressed the impact of therapeutic strategies limiting the growth of already established aneurysms; in fact, the low number of AAA patients suitable for clinical trials enrolment remains one of the main obstacles to explore the effectiveness of any therapeutic strategy. Hence, to draw appropriate conclusions with translational value, the design of future preclinical investigations addressing novel medical therapies in AAA should consider all these aspects.
Acknowledgements
We apologize to authors whose relevant work could not be cited due to space constraints.
Abbreviations
- AAA
abdominal aortic aneurysm
- AMPT
α-methyl-p-tyrosine
- ATF6
activating transcription factor 6
- BET
bromodomain extra-terminal
- ECM
extracellular matrix
- ER
endoplasmic reticulum
- MMP
metalloproteinase
- NFĸB
nuclear factor kappa B
- NOX
NADPH oxidase
- PDE4
phosphodiesterase 4
- ROS
reactive oxygen species
- TH
tyrosine hydroxylase
- VSMC
vascular smooth muscle cell
- XBP-1
X-binding protein 1
Contributor Information
José Martínez-González, Email: jose.martinez@iibb.csic.es.
Cristina Rodríguez, Email: crodriguezs@santpau.cat.
Data Availability
Not applicable.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This research was funded by the Spanish Ministerio de Ciencia e Innovación (PID2021-122509OB-I00) [grant number MCIN/ AEI/10.13039/501100011033]; “ERDF A way of making Europe”; Instituto de Salud Carlos III (ISCIII) [grants numbers PI21/01048 and PI20/01649]; Fundación Española de Arteriosclerosis-Sociedad Española de Arteriosclerosis (Beca de Investigación Básica 2020); and Fondo Europeo de Desarrollo Regional (FEDER), a way to make Europe. L.P-U. and R.A.-P are supported by a PFIS fellowship (ISCIII).
CRediT Author Contribution
Lídia Puertas-Umbert: Writing—original draft, Writing—review & editing. Rafael Almendra-Pegueros: Writing—original draft, Writing—review & editing. Francesc Jiménez-Altayó: Writing—original draft, Writing—review & editing. Marc Sirvent: Writing—original draft, Writing—review & editing. María Galán: Writing—original draft, Writing—review & editing. José Martínez-González: Conceptualization, Resources, Funding acquisition, Writing—original draft, Writing—review & editing. Cristina Rodríguez: Conceptualization, Resources, Funding acquisition, Writing—original draft, Writing—review & editing.
References
- 1.Nordon I.M., Hinchliffe R.J., Loftus I.M. and Thompson M.M. (2011) Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 8, 92–102 10.1038/nrcardio.2010.180 [DOI] [PubMed] [Google Scholar]
- 2.Golledge J. (2019) Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 16, 225–242 10.1038/s41569-018-0114-9 [DOI] [PubMed] [Google Scholar]
- 3.Lindeman J.H.N. (2015) The pathophysiologic basis of abdominal aortic aneurysm progression: a critical appraisal. Expert Rev. Cardiovasc. Ther. 13, 839–851 10.1586/14779072.2015.1052408 [DOI] [PubMed] [Google Scholar]
- 4.Powell J.T., Brown L.C., Forbes J.F., Fowkes F.G.R., Greenhalgh R.M., Ruckley C.V.et al. (2007) Final 12-year follow-up of surgery versus surveillance in the U.K. Small Aneurysm Trial. Br. J. Surg. 94, 702–708 10.1002/bjs.5778 [DOI] [PubMed] [Google Scholar]
- 5.Lederle F.A., Wilson S.E., Johnson G.R., Reinke D.B., Littooy F.N., Acher C.W.et al. (2002) Immediate repair compared with surveillance of small abdominal aortic aneurysms. N. Engl. J. Med. 346, 1437–1444 10.1056/NEJMoa012573 [DOI] [PubMed] [Google Scholar]
- 6.Ouriel K., Clair D.G., Kent K.C., Zarins C.K.and Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators (2010) Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J. Vasc. Surg. 51, 1081–1087 10.1016/j.jvs.2009.10.113 [DOI] [PubMed] [Google Scholar]
- 7.Cao P., De Rango P., Verzini F., Parlani G., Romano L., Cieri E.et al. (2011) Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 41, 13–25 10.1016/j.ejvs.2010.08.026 [DOI] [PubMed] [Google Scholar]
- 8.Schanzer A. and Oderich G.S. (2021) Management of abdominal aortic aneurysms. N. Engl. J. Med. 385, 1690–1698 10.1056/NEJMcp2108504 [DOI] [PubMed] [Google Scholar]
- 9.Martínez-González J. and Badimon L. (2007) Influence of statin use on endothelial function: from bench to clinics. Curr. Pharm. Des. 13, 1771–1786 10.2174/138161207780831220 [DOI] [PubMed] [Google Scholar]
- 10.Babelova A., Sedding D.G. and Brandes R.P. (2013) Anti-atherosclerotic mechanisms of statin therapy. Curr. Opin. Pharmacol. 13, 260–264 10.1016/j.coph.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 11.Mihos C.G., Pineda A.M. and Santana O. (2014) Cardiovascular effects of statins, beyond lipid-lowering properties. Pharmacol. Res. 88, 12–19 10.1016/j.phrs.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 12.Golledge J., Cullen B., Moran C. and Rush C. (2010) Efficacy of simvastatin in reducing aortic dilatation in mouse models of abdominal aortic aneurysm. Cardiovasc. Drugs Ther. 24, 373–378 10.1007/s10557-010-6262-8 [DOI] [PubMed] [Google Scholar]
- 13.Evans J., Powell J.T., Schwalbe E., Loftus I.M. and Thompson M.M. (2007) Simvastatin attenuates the activity of matrix metalloprotease-9 in aneurysmal aortic tissue. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 34, 302–303 10.1016/j.ejvs.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 14.van der Meij E., Koning G.G., Vriens P.W., Peeters M.F., Meijer C.A., Kortekaas K.E.et al. (2013) A clinical evaluation of statin pleiotropy: statins selectively and dose-dependently reduce vascular inflammation. PLoS One 8, e53882 10.1371/journal.pone.0053882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li R., Liu Y. and Jiang J. (2023) Research advances in drug therapy for abdominal aortic aneurysms over the past five years: An updated narrative review. Int. J. Cardiol. 372, 93–100 10.1016/j.ijcard.2022.11.058 [DOI] [PubMed] [Google Scholar]
- 16.Salata K., Syed M., Hussain M.A., de Mestral C., Greco E., Mamdani M.et al. (2018) Statins reduce abdominal aortic aneurysm growth, rupture, and perioperative mortality: A systematic review and meta-analysis. J. Am. Heart Assoc. 7, e008657 10.1161/JAHA.118.008657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su Z., Guo J. and Gu Y. (2022) Pharmacotherapy in clinical trials for abdominal aortic aneurysms: A systematic review and meta-analysis. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 28, 10760296221120424 10.1177/10760296221120423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takagi H., Yamamoto H., Iwata K., Goto S., Umemoto T.and ALICE (All-Literature Investigation of Cardiovascular Evidence) Group (2012) Effects of statin therapy on abdominal aortic aneurysm growth: a meta-analysis and meta-regression of observational comparative studies. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 44, 287–292 10.1016/j.ejvs.2012.06.021 [DOI] [PubMed] [Google Scholar]
- 19.Twine C.P. and Williams I.M. (2011) Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br. J. Surg. 98, 346–353 10.1002/bjs.7343 [DOI] [PubMed] [Google Scholar]
- 20.Xiong X., Wu Z., Qin X., Huang Q., Wang X., Qin J.et al. (2022) Meta-analysis suggests statins reduce mortality after abdominal aortic aneurysm repair. J. Vasc. Surg. 75, 356.e4–362.e4 10.1016/j.jvs.2021.06.033 [DOI] [PubMed] [Google Scholar]
- 21.Mieth A., Revermann M., Babelova A., Weigert A., Schermuly R.T. and Brandes R.P. (2013) L-type calcium channel inhibitor diltiazem prevents aneurysm formation by blood pressure-independent anti-inflammatory effects. Hypertension 62, 1098–1104 10.1161/HYPERTENSIONAHA.113.01986 [DOI] [PubMed] [Google Scholar]
- 22.Miao X.N., Siu K.L. and Cai H. (2015) Nifedipine attenuation of abdominal aortic aneurysm in hypertensive and non-hypertensive mice: Mechanisms and implications. J. Mol. Cell Cardiol. 87, 152–159 10.1016/j.yjmcc.2015.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seto S.-W., Krishna S.M., Moran C.S., Liu D. and Golledge J. (2014) Aliskiren limits abdominal aortic aneurysm, ventricular hypertrophy and atherosclerosis in an apolipoprotein-E-deficient mouse model. Clin. Sci. 127, 123–134 10.1042/CS20130382 [DOI] [PubMed] [Google Scholar]
- 24.Sakaue T., Suzuki J., Hamaguchi M., Suehiro C., Tanino A., Nagao T.et al. (2017) Perivascular adipose tissue angiotensin II type 1 receptor promotes vascular inflammation and aneurysm formation. Hypertens. Dallas Tex 1979 70, 780–789 10.1161/HYPERTENSIONAHA.117.09512 [DOI] [PubMed] [Google Scholar]
- 25.Kaschina E., Schrader F., Sommerfeld M., Kemnitz U.R., Grzesiak A., Krikov M.et al. (2008) Telmisartan prevents aneurysm progression in the rat by inhibiting proteolysis, apoptosis and inflammation. J. Hypertens. 26, 2361–2373 10.1097/HJH.0b013e328313e547 [DOI] [PubMed] [Google Scholar]
- 26.Krueger F., Kappert K., Foryst-Ludwig A., Kramer F., Clemenz M., Grzesiak A.et al. (2017) AT1-receptor blockade attenuates outward aortic remodeling associated with diet-induced obesity in mice. Clin. Sci. 131, 1989–2005 10.1042/CS20170131 [DOI] [PubMed] [Google Scholar]
- 27.Iida Y., Xu B., Schultz G.M., Chow V., White J.J., Sulaimon S.et al. (2012) Efficacy and mechanism of angiotensin II receptor blocker treatment in experimental abdominal aortic aneurysms. PloS ONE 7, e49642 10.1371/journal.pone.0049642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xuan H., Xu B., Wang W., Tanaka H., Fujimura N., Miyata M.et al. (2018) Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms. J. Vasc. Surg. 67, 573.e2–584.e2 10.1016/j.jvs.2016.12.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Propanolol Aneurysm Trial Investigators (2002) Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J. Vasc. Surg. 35, 72–79 10.1067/mva.2002.121308 [DOI] [PubMed] [Google Scholar]
- 30.Lindholt J.S., Henneberg E.W., Juul S. and Fasting H. (1999) Impaired results of a randomised double blinded clinical trial of propranolol versus placebo on the expansion rate of small abdominal aortic aneurysms. Int. Angiol. J. Int. Union Angiol. 18, 52–57 [PubMed] [Google Scholar]
- 31.Golledge J., Pinchbeck J., Tomee S.M., Rowbotham S.E., Singh T.P., Moxon J.V.et al. (2020) Efficacy of telmisartan to slow growth of small abdominal aortic aneurysms: A randomized clinical trial. JAMA Cardiol. 5, 1374–1381 10.1001/jamacardio.2020.3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh T.P., Moxon J.V., Gasser T.C., Dalman R.L., Bourke M., Bourke B.et al. (2022) Effect of telmisartan on the peak wall stress and peak wall rupture index of small abdominal aortic aneurysms: An exploratory analysis of the TEDY trial. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 64, 396–404 10.1016/j.ejvs.2022.07.042 [DOI] [PubMed] [Google Scholar]
- 33.Xue F., Yang J., Cheng J., Sui W., Cheng C., Li H.et al. (2020) Angiotensin-(1-7) mitigated angiotensin II-induced abdominal aortic aneurysms in apolipoprotein E-knockout mice. Br. J. Pharmacol. 177, 1719–1734 10.1111/bph.14906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange C., Sommerfeld M., Namsolleck P., Kintscher U., Unger T. and Kaschina E. (2018) AT2R (angiotensin AT2 receptor) agonist, compound 21, prevents abdominal aortic aneurysm progression in the rat. Hypertens. Dallas Tex 1979 72, e20–e29 [DOI] [PubMed] [Google Scholar]
- 35.Hackam D.G., Thiruchelvam D. and Redelmeier D.A. (2006) Angiotensin-converting enzyme inhibitors and aortic rupture: a population-based case-control study. Lancet Lond. Engl. 368, 659–665 10.1016/S0140-6736(06)69250-7 [DOI] [PubMed] [Google Scholar]
- 36.Kortekaas K.E., Meijer C.A., Hinnen J.W., Dalman R.L., Xu B., Hamming J.F.et al. (2014) ACE inhibitors potently reduce vascular inflammation, results of an open proof-of-concept study in the abdominal aortic aneurysm. PLoS ONE 9, e111952 10.1371/journal.pone.0111952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweeting M.J., Thompson S.G., Brown L.C., Greenhalgh R.M. and Powell J.T. (2010) Use of angiotensin converting enzyme inhibitors is associated with increased growth rate of abdominal aortic aneurysms. J. Vasc. Surg. 52, 1–4 10.1016/j.jvs.2010.02.264 [DOI] [PubMed] [Google Scholar]
- 38.Bicknell C.D., Kiru G., Falaschetti E., Powell J.T., Poulter N.R.and AARDVARK Collaborators (2016) An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomized placebo-controlled trial (AARDVARK). Eur. Heart J. 37, 3213–3221 10.1093/eurheartj/ehw257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sapadin A.N. and Fleischmajer R. (2006) Tetracyclines: nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 54, 258–265 10.1016/j.jaad.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 40.Petrinec D., Liao S., Holmes D.R., Reilly J.M., Parks W.C. and Thompson R.W. (1996) Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. J. Vasc. Surg. 23, 336–346 10.1016/S0741-5214(96)70279-3 [DOI] [PubMed] [Google Scholar]
- 41.Boyle J.R., McDermott E., Crowther M., Wills A.D., Bell P.R. and Thompson M.M. (1998) Doxycycline inhibits elastin degradation and reduces metalloproteinase activity in a model of aneurysmal disease. J. Vasc. Surg. 27, 354–361 10.1016/S0741-5214(98)70367-2 [DOI] [PubMed] [Google Scholar]
- 42.Pyo R., Lee J.K., Shipley J.M., Curci J.A., Mao D., Ziporin S.J.et al. (2000) Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Invest. 105, 1641–1649 10.1172/JCI8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manning M.W., Cassis L.A. and Daugherty A. (2003) Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 23, 483–488 10.1161/01.ATV.0000058404.92759.32 [DOI] [PubMed] [Google Scholar]
- 44.Thompson R.W. and Baxter B.T. (1999) MMP inhibition in abdominal aortic aneurysms. Rationale for a prospective randomized clinical trial. Ann. N. Y. Acad. Sci. 878, 159–178 10.1111/j.1749-6632.1999.tb07682.x [DOI] [PubMed] [Google Scholar]
- 45.Curci J.A., Mao D., Bohner D.G., Allen B.T., Rubin B.G., Reilly J.M.et al. (2000) Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J. Vasc. Surg. 31, 325–342 10.1016/S0741-5214(00)90163-0 [DOI] [PubMed] [Google Scholar]
- 46.Lindeman J.H.N., Abdul-Hussien H., van Bockel J.H., Wolterbeek R. and Kleemann R. (2009) Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 119, 2209–2216 10.1161/CIRCULATIONAHA.108.806505 [DOI] [PubMed] [Google Scholar]
- 47.Xie X., Lu H., Moorleghen J.J., Howatt D.A., Rateri D.L., Cassis L.A.et al. (2012) Doxycycline does not influence established abdominal aortic aneurysms in angiotensin II-infused mice. PLoS ONE 7, e46411 10.1371/journal.pone.0046411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosorin M., Juvonen J., Biancari F., Satta J., Surcel H.M., Leinonen M.et al. (2001) Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J. Vasc. Surg. 34, 606–610 10.1067/mva.2001.117891 [DOI] [PubMed] [Google Scholar]
- 49.Meijer C.A., Stijnen T., Wasser M.N.J.M., Hamming J.F., van Bockel J.H., Lindeman J.H.N.et al. (2013) Doxycycline for stabilization of abdominal aortic aneurysms: a randomized trial. Ann. Intern. Med. 159, 815–823 10.7326/0003-4819-159-12-201312170-00007 [DOI] [PubMed] [Google Scholar]
- 50.Baxter B.T., Matsumura J., Curci J.A., McBride R., Larson L., Blackwelder W.et al. (2020) Effect of doxycycline on aneurysm growth among patients with small infrarenal abdominal aortic aneurysms: A randomized clinical trial. JAMA 323, 2029–2038 10.1001/jama.2020.5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baxter B.T., Pearce W.H., Waltke E.A., Littooy F.N., Hallett J.W., Kent K.C.et al. (2002) Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J. Vasc. Surg. 36, 1–12 10.1067/mva.2002.125018 [DOI] [PubMed] [Google Scholar]
- 52.Owens A.P., Edwards T.L., Antoniak S., Geddings J.E., Jahangir E., Wei W.-Q.et al. (2015) Platelet inhibitors reduce rupture in a mouse model of established abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 35, 2032–2041 10.1161/ATVBAHA.115.305537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dai J., Louedec L., Philippe M., Michel J.-B. and Houard X. (2009) Effect of blocking platelet activation with AZD6140 on development of abdominal aortic aneurysm in a rat aneurysmal model. J. Vasc. Surg. 49, 719–727 10.1016/j.jvs.2008.09.057 [DOI] [PubMed] [Google Scholar]
- 54.Liu O., Jia L., Liu X., Wang Y., Wang X., Qin Y.et al. (2012) Clopidogrel, a platelet P2Y12 receptor inhibitor, reduces vascular inflammation and angiotensin II induced-abdominal aortic aneurysm progression. PLoS ONE 7, e51707 10.1371/journal.pone.0051707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lindholt J.S., Björck M. and Michel J.B. (2013) Anti-platelet treatment of middle-sized abdominal aortic aneurysms. Curr. Vasc. Pharmacol. 11, 305–313 10.2174/1570161111311030005 [DOI] [PubMed] [Google Scholar]
- 56.Wemmelund H., Jørgensen T.M.M., Høgh A., Behr-Rasmussen C., Johnsen S.P. and Lindholt J.S. (2017) Low-dose aspirin and rupture of abdominal aortic aneurysm. J. Vasc. Surg. 65, 616.e4–625.e4 10.1016/j.jvs.2016.04.061 [DOI] [PubMed] [Google Scholar]
- 57.Cameron S.J., Russell H.M. and Owens A.P. (2018) Antithrombotic therapy in abdominal aortic aneurysm: beneficial or detrimental? Blood 132, 2619–2628 10.1182/blood-2017-08-743237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wanhainen A., Mani K., Kullberg J., Svensjö S., Bersztel A., Karlsson L.et al. (2020) The effect of ticagrelor on growth of small abdominal aortic aneurysms-a randomized controlled trial. Cardiovasc. Res. 116, 450–456 [DOI] [PubMed] [Google Scholar]
- 59.Thompson A., Cooper J.A., Fabricius M., Humphries S.E., Ashton H.A. and Hafez H. (2010) An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J. Vasc. Surg. 52, 55.e2–61.e2 10.1016/j.jvs.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 60.Wong K.H.F., Zlatanovic P., Bosanquet D.C., Saratzis A., Kakkos S.K., Aboyans V.et al. (2022) Antithrombotic therapy for aortic aneurysms: A systematic review and meta-analysis. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 64, 544–556 10.1016/j.ejvs.2022.07.008 [DOI] [PubMed] [Google Scholar]
- 61.Kong D.S., Balceniuk M.D., Mix D., Ellis J.L., Doyle A.J., Glocker R.J.et al. (2022) Long-term anticoagulation is associated with type II endoleaks and failure of sac regression after endovascular aneurysm repair. J. Vasc. Surg. 76, 437.e2–444.e2 10.1016/j.jvs.2022.01.144 [DOI] [PubMed] [Google Scholar]
- 62.Bobadilla J.L., Hoch J.R., Leverson G.E. and Tefera G. (2010) The effect of warfarin therapy on endoleak development after endovascular aneurysm repair (EVAR) of the abdominal aorta. J. Vasc. Surg. 52, 267–271 10.1016/j.jvs.2010.02.290 [DOI] [PubMed] [Google Scholar]
- 63.Golledge J. and Norman P.E. (2010) Atherosclerosis and abdominal aortic aneurysm: cause, response, or common risk factors? Arterioscler. Thromb. Vasc. Biol. 30, 1075–1077 10.1161/ATVBAHA.110.206573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai C.-L., Lin C.-L., Wu Y.-Y., Shieh D.-C., Sung F.-C. and Kao C.-H. (2015) Advanced complicated diabetes mellitus is associated with a reduced risk of thoracic and abdominal aortic aneurysm rupture: a population-based cohort study. Diabetes Metab. Res. Rev. 31, 190–197 10.1002/dmrr.2585 [DOI] [PubMed] [Google Scholar]
- 65.De Rango P., Farchioni L., Fiorucci B. and Lenti M. (2014) Diabetes and abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 47, 243–261 10.1016/j.ejvs.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 66.Golledge J., Karan M., Moran C.S., Muller J., Clancy P., Dear A.E.et al. (2008) Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions. Eur. Heart J. 29, 665–672 10.1093/eurheartj/ehm557 [DOI] [PubMed] [Google Scholar]
- 67.Huang Z., Su H., Zhang T. and Li Y. (2022) Double-edged sword of diabetes mellitus for abdominal aortic aneurysm. Front. Endocrinol. 13, 1095608 10.3389/fendo.2022.1095608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kristensen K.L., Dahl M., Rasmussen L.M. and Lindholt J.S. (2017) Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms: A substudy from the VIVA (Viborg Vascular) randomized screening trial. Arterioscler. Thromb. Vasc. Biol. 37, 730–736 10.1161/ATVBAHA.116.308874 [DOI] [PubMed] [Google Scholar]
- 69.Raffort J., Lareyre F., Clément M., Hassen-Khodja R., Chinetti G. and Mallat Z. (2018) Diabetes and aortic aneurysm: current state of the art. Cardiovasc. Res. 114, 1702–1713 10.1093/cvr/cvy174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golledge J., Moxon J., Pinchbeck J., Anderson G., Rowbotham S., Jenkins J.et al. (2017) Association between metformin prescription and growth rates of abdominal aortic aneurysms. Br. J. Surg. 104, 1486–1493 10.1002/bjs.10587 [DOI] [PubMed] [Google Scholar]
- 71.Turowicz A., Kobecki J., Laskowska A., Wojciechowski J., Świątkowski F. and Chabowski M. (2021) Association of metformin and abdominal aortic aneurysm repair outcomes. Ann. Vasc. Surg. 75, 390–396 10.1016/j.avsg.2021.02.048 [DOI] [PubMed] [Google Scholar]
- 72.Fujimura N., Xiong J., Kettler E.B., Xuan H., Glover K.J., Mell M.W.et al. (2016) Metformin treatment status and abdominal aortic aneurysm disease progression. J. Vasc. Surg. 64, 46.e8–54.e8 10.1016/j.jvs.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Itoga N.K., Rothenberg K.A., Suarez P., Ho T.-V., Mell M.W., Xu B.et al. (2019) Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J. Vasc. Surg. 69, 710.e3–716.e3 10.1016/j.jvs.2018.06.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Golledge J., Morris D.R., Pinchbeck J., Rowbotham S., Jenkins J., Bourke M.et al. (2019) Editor's Choice - Metformin prescription is associated with a reduction in the combined incidence of surgical repair and rupture related mortality in patients with abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 57, 94–101 10.1016/j.ejvs.2018.07.035 [DOI] [PubMed] [Google Scholar]
- 75.Unosson J., Wågsäter D., Bjarnegård N., Basso R.D., Welander M., Mani K.et al. (2021) Metformin prescription associated with reduced abdominal aortic aneurysm growth rate and Reduced chemokine expression in a swedish cohort. Ann. Vasc. Surg. 70, 425–433 10.1016/j.avsg.2020.06.039 [DOI] [PubMed] [Google Scholar]
- 76.Sutton S.S., Magagnoli J., Cummings T.H. and Hardin J.W. (2020) Association between metformin and abdominal aortic aneurysm in diabetic and non-diabetic US veterans. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 68, 1015–1018 10.1136/jim-2019-001177 [DOI] [PubMed] [Google Scholar]
- 77.Niu W., Shao J., Yu B., Liu G., Wang R., Dong H.et al. (2022) Association between metformin and abdominal aortic aneurysm: A meta-analysis. Front. Cardiovasc. Med. 9, 908747 10.3389/fcvm.2022.908747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diaz-Morales N., Rovira-Llopis S., Bañuls C., Lopez-Domenech S., Escribano-Lopez I., Veses S.et al. (2017) Does metformin protect diabetic patients from oxidative stress and leukocyte-endothelium interactions? Antioxid. Redox Signal. 27, 1439–1445 10.1089/ars.2017.7122 [DOI] [PubMed] [Google Scholar]
- 79.Vasamsetti S.B., Karnewar S., Kanugula A.K., Thatipalli A.R., Kumar J.M. and Kotamraju S. (2015) Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes 64, 2028–2041 10.2337/db14-1225 [DOI] [PubMed] [Google Scholar]
- 80.Kim S.A. and Choi H.C. (2012) Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 425, 866–872 10.1016/j.bbrc.2012.07.165 [DOI] [PubMed] [Google Scholar]
- 81.Isoda K., Young J.L., Zirlik A., MacFarlane L.A., Tsuboi N., Gerdes N.et al. (2006) Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler. Thromb. Vasc. Biol. 26, 611–617 10.1161/01.ATV.0000201938.78044.75 [DOI] [PubMed] [Google Scholar]
- 82.Li X., Wen H., Lv J., Luan B., Meng J., Gong S.et al. (2022) Therapeutic efficacy of mesenchymal stem cells for abdominal aortic aneurysm: a meta-analysis of preclinical studies. Stem Cell Res. Ther. 13, 81 10.1186/s13287-022-02755-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang X., Zhang H., Liang X., Hong Y., Mao M., Han Q.et al. (2019) Adipose-derived mesenchymal stem cells isolated from patients with abdominal aortic aneurysm exhibit senescence phenomena. Oxid. Med. Cell. Longev. 2019, 1305049 10.1155/2019/1305049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma A.K., Salmon M.D., Lu G., Su G., Pope N.H., Smith J.R.et al. (2016) Mesenchymal stem cells attenuate NADPH oxidase-dependent high mobility group box 1 production and inhibit abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 36, 908–918 10.1161/ATVBAHA.116.307373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie J., Jones T.J., Feng D., Cook T.G., Jester A.A., Yi R.et al. (2017) Human adipose-derived stem cells suppress elastase-induced murine abdominal aortic inflammation and aneurysm expansion through paracrine factors. Cell Transplant. 26, 173–189 10.3727/096368916X692212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spinosa M., Lu G., Su G., Bontha S.V., Gehrau R., Salmon M.D.et al. (2018) Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 32, fj201701138RR 10.1096/fj.201701138RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wen H., Wang M., Gong S., Li X., Meng J., Wen J.et al. (2020) Human umbilical cord mesenchymal stem cells attenuate abdominal aortic aneurysm progression in sprague-dawley rats: Implication of vascular smooth muscle cell phenotypic modulation. Stem Cells Dev. 29, 981–993 10.1089/scd.2020.0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu X., Yamawaki-Ogata A., Oshima H., Ueda Y., Usui A. and Narita Y. (2013) Intravenous administration of mesenchymal stem cells prevents angiotensin II-induced aortic aneurysm formation in apolipoprotein E-deficient mouse. J. Transl. Med. 11, 175 10.1186/1479-5876-11-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hosoyama K., Wakao S., Kushida Y., Ogura F., Maeda K., Adachi O.et al. (2018) Intravenously injected human multilineage-differentiating stress-enduring cells selectively engraft into mouse aortic aneurysms and attenuate dilatation by differentiating into multiple cell types. J. Thorac. Cardiovasc. Surg. 155, 2301.e4–2313.e4 10.1016/j.jtcvs.2018.01.098 [DOI] [PubMed] [Google Scholar]
- 90.Leckie K., Green L.L., King J.R., Wang K.S., Motaganahalli R.L., Fajardo A.et al. (2019) Allogeneic mesenchymal stem cells induce regulatory T cells and suppress aneurysm inflammation: Interim results of the phase 1 ARREST trial. J. Vasc. Surg. 70, e62–e63 10.1016/j.jvs.2019.06.138 [DOI] [Google Scholar]
- 91.Swedenborg J., Mäyränpää M.I. and Kovanen P.T. (2011) Mast cells: important players in the orchestrated pathogenesis of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 31, 734–740 10.1161/ATVBAHA.110.213157 [DOI] [PubMed] [Google Scholar]
- 92.Sun J., Sukhova G.K., Yang M., Wolters P.J., MacFarlane L.A., Libby P.et al. (2007) Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J. Clin. Invest. 117, 3359–3368 10.1172/JCI31311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun J., Zhang J., Lindholt J.S., Sukhova G.K., Liu J., He A.et al. (2009) Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation 120, 973–982 10.1161/CIRCULATIONAHA.109.849679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaartinen M., Penttilä A. and Kovanen P.T. (1994) Mast cells of two types differing in neutral protease composition in the human aortic intima. Demonstration of tryptase- and tryptase/chymase-containing mast cells in normal intimas, fatty streaks, and the shoulder region of atheromas. Arterioscler. Thromb. J. Vasc. Biol. 14, 966–972 10.1161/01.ATV.14.6.966 [DOI] [PubMed] [Google Scholar]
- 95.Sillesen H., Eldrup N., Hultgren R., Lindeman J., Bredahl K., Thompson M.et al. (2015) Randomized clinical trial of mast cell inhibition in patients with a medium-sized abdominal aortic aneurysm. Br. J. Surg. 102, 894–901 10.1002/bjs.9824 [DOI] [PubMed] [Google Scholar]
- 96.Novartis Pharmaceuticals (2020) A randomized, double-blind, placebo-controlled, multiple dose study of subcutaneous ACZ885 for the treatment of abdominal aortic aneurysm. Clinicaltrials.gov 2020 [cited 2023 Apr 27] https://clinicaltrials.gov/ct2/show/NCT02007252 [Google Scholar]
- 97.Dai J., Michineau S., Franck G., Desgranges P., Becquemin J.-P., Gervais M.et al. (2011) Long term stabilization of expanding aortic aneurysms by a short course of cyclosporine A through transforming growth factor-beta induction. PLoS One 6, e28903 10.1371/journal.pone.0028903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Satoh K., Nigro P., Matoba T., O'Dell M.R., Cui Z., Shi X.et al. (2009) Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat. Med. 15, 649–656 10.1038/nm.1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lindeman J.H.N., Rabelink T.J. and van Bockel J.H. (2011) Immunosuppression and the abdominal aortic aneurysm: Doctor Jekyll or Mister Hyde? Circulation 124, e463–e465 10.1161/CIRCULATIONAHA.110.008573 [DOI] [PubMed] [Google Scholar]
- 100.Samuelsson B., Morgenstern R. and Jakobsson P.-J. (2007) Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol. Rev. 59, 207–224 10.1124/pr.59.3.1 [DOI] [PubMed] [Google Scholar]
- 101.Holmes D.R., Wester W., Thompson R.W. and Reilly J.M. (1997) Prostaglandin E2 synthesis and cyclooxygenase expression in abdominal aortic aneurysms. J. Vasc. Surg. 25, 810–815 10.1016/S0741-5214(97)70210-6 [DOI] [PubMed] [Google Scholar]
- 102.Walton L.J., Franklin I.J., Bayston T., Brown L.C., Greenhalgh R.M., Taylor G.W.et al. (1999) Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation 100, 48–54 10.1161/01.CIR.100.1.48 [DOI] [PubMed] [Google Scholar]
- 103.Wang M., Ihida-Stansbury K., Kothapalli D., Tamby M.C., Yu Z., Chen L.et al. (2011) Microsomal prostaglandin e2 synthase-1 modulates the response to vascular injury. Circulation 123, 631–639 10.1161/CIRCULATIONAHA.110.973685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Camacho M., Dilmé J., Solà-Villà D., Rodríguez C., Bellmunt S., Siguero L.et al. (2013) Microvascular COX-2/mPGES-1/EP-4 axis in human abdominal aortic aneurysm. J. Lipid Res. 54, 3506–3515 10.1194/jlr.M042481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miralles M., Wester W., Sicard G.A., Thompson R. and Reilly J.M. (1999) Indomethacin inhibits expansion of experimental aortic aneurysms via inhibition of the cox2 isoform of cyclooxygenase. J. Vasc. Surg. 29, 884–892, discussion 892-893 10.1016/S0741-5214(99)70216-8 [DOI] [PubMed] [Google Scholar]
- 106.King V.L., Trivedi D.B., Gitlin J.M. and Loftin C.D. (2006) Selective cyclooxygenase-2 inhibition with celecoxib decreases angiotensin II-induced abdominal aortic aneurysm formation in mice. Arterioscler. Thromb. Vasc. Biol. 26, 1137–1143 10.1161/01.ATV.0000216119.79008.ac [DOI] [PubMed] [Google Scholar]
- 107.Claridge M., Hobbs S., Quick C., Day N., Bradbury A. and Wilmink T. (2005) Nonsteroidal antiinflammatory drugs are associated with increased aortic stiffness. Vasc. Health Risk Manag. 1, 149–153 10.2147/vhrm.1.2.149.64082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matsubara Y., Inoue K., Mori K., Morita M., Takebayashi S. and Kume M. (2020) Nonsteroidal anti-inflammatory drug use is a risk factor for early Type II endoleak after endovascular abdominal aortic repair. Vascular 28, 53–58 10.1177/1708538119866603 [DOI] [PubMed] [Google Scholar]
- 109.Bresalier R.S., Sandler R.S., Quan H., Bolognese J.A., Oxenius B., Horgan K.et al. (2005) Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 352, 1092–1102 10.1056/NEJMoa050493 [DOI] [PubMed] [Google Scholar]
- 110.Wang M., Lee E., Song W., Ricciotti E., Rader D.J., Lawson J.A.et al. (2008) Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation 117, 1302–1309 10.1161/CIRCULATIONAHA.107.731398 [DOI] [PubMed] [Google Scholar]
- 111.Dilmé J.-F., Solà-Villà D., Bellmunt S., Romero J.-M., Escudero J.-R., Camacho M.et al. (2014) Active smoking increases microsomal PGE2-synthase-1/PGE-receptor-4 axis in human abdominal aortic aneurysms. Mediators Inflamm. 2014, 316150 10.1155/2014/316150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mamun A., Yokoyama U., Saito J., Ito S., Hiromi T., Umemura M.et al. (2018) A selective antagonist of prostaglandin E receptor subtype 4 attenuates abdominal aortic aneurysm. Physiol. Rep. 6, 10.14814/phy2.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cao R.Y., St Amand T., Li X., Yoon S.-H., Wang C.P., Song H.et al. (2012) Prostaglandin receptor EP4 in abdominal aortic aneurysms. Am. J. Pathol. 181, 313–321 10.1016/j.ajpath.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 114.Hiromi T., Yokoyama U., Kurotaki D., Mamun A., Ishiwata R., Ichikawa Y.et al. (2020) Excessive EP4 signaling in smooth muscle cells induces abdominal aortic aneurysm by amplifying inflammation. Arterioscler. Thromb. Vasc. Biol. 40, 1559–1573 10.1161/ATVBAHA.120.314297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rodríguez C., Rodríguez-Sinovas A. and Martínez-González J. (2008) Lysyl oxidase as a potential therapeutic target. Drug News Perspect. 21, 218–224 10.1358/dnp.2008.21.4.1213351 [DOI] [PubMed] [Google Scholar]
- 116.Rodríguez C., Martínez-González J., Raposo B., Alcudia J.F., Guadall A. and Badimon L. (2008) Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc. Res. 79, 7–13 10.1093/cvr/cvn102 [DOI] [PubMed] [Google Scholar]
- 117.Yoshimura K., Aoki H., Ikeda Y., Fujii K., Akiyama N., Furutani A.et al. (2005) Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat. Med. 11, 1330–1338 10.1038/nm1335 [DOI] [PubMed] [Google Scholar]
- 118.Tang E.H.C., Shvartz E., Shimizu K., Rocha V.Z., Zheng C., Fukuda D.et al. (2011) Deletion of EP4 on bone marrow-derived cells enhances inflammation and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 31, 261–269 10.1161/ATVBAHA.110.216580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peng T., Qi B., He J., Ke H. and Shi J. (2020) Advances in the development of phosphodiesterase-4 Inhibitors. J. Med. Chem. 63, 10594–10617 10.1021/acs.jmedchem.9b02170 [DOI] [PubMed] [Google Scholar]
- 120.Zhang Q., Huang J.-H., Xia R.-P., Duan X.-H., Jiang Y.-B., Jiang Q.et al. (2011) Suppression of experimental abdominal aortic aneurysm in a rat model by the phosphodiesterase 3 inhibitor cilostazol. J. Surg. Res. 167, e385–e393 10.1016/j.jss.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 121.Umebayashi R., Uchida H.A., Kakio Y., Subramanian V., Daugherty A. and Wada J. (2018) Cilostazol Attenuates Angiotensin II-Induced Abdominal Aortic Aneurysms but Not Atherosclerosis in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 38, 903–912 10.1161/ATVBAHA.117.309707 [DOI] [PubMed] [Google Scholar]
- 122.Varona S., Puertas L., Galán M., Orriols M., Cañes L., Aguiló S.et al. (2021) Rolipram prevents the formation of abdominal aortic aneurysm (AAA) in mice: PDE4B as a target in AAA. Antioxid. Basel Switz. 10, 460 10.3390/antiox10030460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Azam M.A. and Tripuraneni N.S. (2014) Selective phosphodiesterase 4B inhibitors: A review. Sci. Pharm. 82, 453–481 10.3797/scipharm.1404-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang P., Wu P., Ohleth K.M., Egan R.W. and Billah M.M. (1999) Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol. Pharmacol. 56, 170–174 10.1124/mol.56.1.170 [DOI] [PubMed] [Google Scholar]
- 125.Gao R., Guo W., Fan T., Pang J., Hou Y., Feng X.et al. (2022) Phosphodiesterase 4D contributes to angiotensin II-induced abdominal aortic aneurysm through smooth muscle cell apoptosis. Exp. Mol. Med. 54, 1201–1213 10.1038/s12276-022-00815-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.White W.B., Cooke G.E., Kowey P.R., Calverley P.M.A., Bredenbröker D., Goehring U.-M.et al. (2013) Cardiovascular safety in patients receiving roflumilast for the treatment of COPD. Chest 144, 758–765 10.1378/chest.12-2332 [DOI] [PubMed] [Google Scholar]
- 127.Keravis T. and Lugnier C. (2012) Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 165, 1288–1305 10.1111/j.1476-5381.2011.01729.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schick M.A. and Schlegel N. (2022) Clinical implication of phosphodiesterase-4-inhibition. Int. J. Mol. Sci. 23, 1209 10.3390/ijms23031209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang C., Mohan A., Shi H. and Yan C. (2022) Sildenafil (Viagra) aggravates the development of experimental abdominal aortic aneurysm. J. Am. Heart Assoc. 11, e023053 10.1161/JAHA.121.023053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tiryakioglu S.K., Tiryakioglu O., Turan T. and Kumbay E. (2009) Aortic dissection due to sildenafil abuse. Interact. Cardiovasc. Thorac. Surg. 9, 141–143 10.1510/icvts.2009.205849 [DOI] [PubMed] [Google Scholar]
- 131.Van der Heiden K., Cuhlmann S., Luong L.A., Zakkar M. and Evans P.C. (2010) Role of nuclear factor kappaB in cardiovascular health and disease. Clin. Sci. Lond. Engl. 1979 118, 593–605 10.1042/CS20090557 [DOI] [PubMed] [Google Scholar]
- 132.DiMusto P.D., Lu G., Ghosh A., Roelofs K.J., Sadiq O., McEvoy B.et al. (2012) Increased JNK in males compared with females in a rodent model of abdominal aortic aneurysm. J. Surg. Res. 176, 687–695 10.1016/j.jss.2011.11.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Okuyama M., Jiang W., Javidan A., Chen J.Z., Howatt D.A., Yang L.et al. (2020) Lysyl oxidase inhibition ablates sexual dimorphism of abdominal aortic aneurysm formation in mice. Circulation 142, 1993–1995 10.1161/CIRCULATIONAHA.119.044986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun D., Zhang M., Li Y., Mei S., Qin J. and Yan J. (2019) c-Jun/Ap-1 is upregulated in an Ang II-induced abdominal aortic aneurysm formation model and mediates chop expression in mouse aortic smooth muscle cells. Mol. Med. Rep. 19, 3459–3468 10.3892/mmr.2019.10017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nakao T., Horie T., Baba O., Nishiga M., Nishino T., Izuhara M.et al. (2017) Genetic ablation of microRNA-33 attenuates inflammation and abdominal aortic aneurysm formation via several anti-inflammatory pathways. Arterioscler. Thromb. Vasc. Biol. 37, 2161–2170 10.1161/ATVBAHA.117.309768 [DOI] [PubMed] [Google Scholar]
- 136.Kurashiki T., Miyake T., Nakagami H., Nishimura M. and Morishita R. (2020) Prevention of progression of aortic aneurysm by peptide vaccine against ang II (angiotensin II) in a rat model. Hypertens. Dallas Tex 1979 76, 1879–1888 10.1161/HYPERTENSIONAHA.119.14442 [DOI] [PubMed] [Google Scholar]
- 137.Tsai S.-H., Huang P.-H., Peng Y.-J., Chang W.-C., Tsai H.-Y., Leu H.-B.et al. (2013) Zoledronate attenuates angiotensin II-induced abdominal aortic aneurysm through inactivation of Rho/ROCK-dependent JNK and NF-κB pathway. Cardiovasc. Res. 100, 501–510 10.1093/cvr/cvt230 [DOI] [PubMed] [Google Scholar]
- 138.Ohno T., Aoki H., Ohno S., Nishihara M., Furusho A., Hiromatsu S.et al. (2018) Cytokine profile of human abdominal aortic aneurysm: involvement of JAK/STAT pathway. Ann. Vasc. Dis. 11, 84–90 10.3400/avd.oa.17-00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qin Z., Bagley J., Sukhova G., Baur W.E., Park H.-J., Beasley D.et al. (2015) Angiotensin II-induced TLR4 mediated abdominal aortic aneurysm in apolipoprotein E knockout mice is dependent on STAT3. J. Mol. Cell Cardiol. 87, 160–170 10.1016/j.yjmcc.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 140.Wu Q.-Y., Cheng Z., Zhou Y.-Z., Zhao Y., Li J.-M., Zhou X.-M.et al. (2020) A novel STAT3 inhibitor attenuates angiotensin II-induced abdominal aortic aneurysm progression in mice through modulating vascular inflammation and autophagy. Cell Death Dis. 11, 131 10.1038/s41419-020-2326-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xiao J., Wei Z., Chen X., Chen W., Zhang H., Yang C.et al. (2020) Experimental abdominal aortic aneurysm growth is inhibited by blocking the JAK2/STAT3 pathway. Int. J. Cardiol. 312, 100–106 10.1016/j.ijcard.2020.03.072 [DOI] [PubMed] [Google Scholar]
- 142.Bernal S., Lopez-Sanz L., Jimenez-Castilla L., Prieto I., Melgar A., La Manna S.et al. (2021) Protective effect of suppressor of cytokine signalling 1-based therapy in experimental abdominal aortic aneurysm. Br. J. Pharmacol. 178, 564–581 10.1111/bph.15330 [DOI] [PubMed] [Google Scholar]
- 143.Chandesris M.-O., Azarine A., Ong K.-T., Taleb S., Boutouyrie P., Mousseaux E.et al. (2012) Frequent and widespread vascular abnormalities in human signal transducer and activator of transcription 3 deficiency. Circ. Cardiovasc. Genet. 5, 25–34 10.1161/CIRCGENETICS.111.961235 [DOI] [PubMed] [Google Scholar]
- 144.Romain M., Taleb S., Dalloz M., Ponnuswamy P., Esposito B., Pérez N.et al. (2013) Overexpression of SOCS3 in T lymphocytes leads to impaired interleukin-17 production and severe aortic aneurysm formation in mice–brief report. Arterioscler. Thromb. Vasc. Biol. 33, 581–584 10.1161/ATVBAHA.112.300516 [DOI] [PubMed] [Google Scholar]
- 145.Aoki H., Majima R., Hashimoto Y., Hirakata S. and Ohno-Urabe S. (2021) Ying and Yang of Stat3 in pathogenesis of aortic dissection. J. Cardiol. 77, 471–474 10.1016/j.jjcc.2020.10.010 [DOI] [PubMed] [Google Scholar]
- 146.Puertas-Umbert L., Varona S., Ballester-Servera C., Alonso J., Aguiló S., Orriols M.et al. (2022) Activation of Wnt/β-catenin signaling in abdominal aortic aneurysm: A potential therapeutic opportunity? Genes Dis. 103639–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Amini-Nik S., Cambridge E., Yu W., Guo A., Whetstone H., Nadesan P.et al. (2014) β-Catenin-regulated myeloid cell adhesion and migration determine wound healing. J. Clin. Invest. 124, 2599–2610 10.1172/JCI62059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee D.K., Nathan Grantham R., Trachte A.L., Mannion J.D. and Wilson C.L. (2006) Activation of the canonical Wnt/beta-catenin pathway enhances monocyte adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 347, 109–116 10.1016/j.bbrc.2006.06.082 [DOI] [PubMed] [Google Scholar]
- 149.Quasnichka H., Slater S.C., Beeching C.A., Boehm M., Sala-Newby G.B. and George S.J. (2006) Regulation of smooth muscle cell proliferation by beta-catenin/T-cell factor signaling involves modulation of cyclin D1 and p21 expression. Circ. Res. 99, 1329–1337 10.1161/01.RES.0000253533.65446.33 [DOI] [PubMed] [Google Scholar]
- 150.Bedel A., Nègre-Salvayre A., Heeneman S., Grazide M.-H., Thiers J.-C., Salvayre R.et al. (2008) E-cadherin/beta-catenin/T-cell factor pathway is involved in smooth muscle cell proliferation elicited by oxidized low-density lipoprotein. Circ. Res. 103, 694–701 10.1161/CIRCRESAHA.107.166405 [DOI] [PubMed] [Google Scholar]
- 151.Mill C. and George S.J. (2012) Wnt signalling in smooth muscle cells and its role in cardiovascular disorders. Cardiovasc. Res. 95, 233–240 10.1093/cvr/cvs141 [DOI] [PubMed] [Google Scholar]
- 152.Wu B., Crampton S.P. and Hughes C.C.W. (2007) Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity 26, 227–239 10.1016/j.immuni.2006.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Krishna S.M., Seto S.-W., Jose R.J., Li J., Morton S.K., Biros E.et al. (2017) Wnt Signaling Pathway Inhibitor Sclerostin Inhibits Angiotensin II-Induced Aortic Aneurysm and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 37, 553–566 10.1161/ATVBAHA.116.308723 [DOI] [PubMed] [Google Scholar]
- 154.Williams H., Wadey K.S., Frankow A., Blythe H.C., Forbes T., Johnson J.L.et al. (2021) Aneurysm severity is suppressed by deletion of CCN4. J. Cell Commun. Signal. 15, 421–432 10.1007/s12079-021-00623-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li Y., Lv M., Lu M. and Guan H. (2022) miR-124a involves in the regulation of Wnt/β-Catenin and P53 pathways to inhibit abdominal aortic aneurysm via targeting BRD4. Comput. Math. Methods Med. 2022, 9241959 10.1155/2022/9241959 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 156.Lopez-Sanz L., Bernal S., Jimenez-Castilla L., Prieto I., La Manna S., Gomez-Lopez S.et al. (2021) Fcγ receptor activation mediates vascular inflammation and abdominal aortic aneurysm development. Clin. Transl. Med. 11, e463 10.1002/ctm2.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.López-Sanz L., Bernal S., Jiménez-Castilla L., Pardines M., Hernández-García A., Blanco-Colio L.et al. (2023) The presence of activating IgG Fc receptors in macrophages aggravates the development of experimental abdominal aortic aneurysm. Clin. E Investig. En Arterioscler. Publ. Soc. Espanola Arterioscler. 354185–194S0214-9168(23)00001–3 10.1016/j.arteri.2022.12.004 [DOI] [PubMed] [Google Scholar]
- 158.Davis V., Persidskaia R., Baca-Regen L., Itoh Y., Nagase H., Persidsky Y.et al. (1998) Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 18, 1625–1633 10.1161/01.ATV.18.10.1625 [DOI] [PubMed] [Google Scholar]
- 159.Longo G.M., Xiong W., Greiner T.C., Zhao Y., Fiotti N. and Baxter B.T. (2002) Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Invest. 110, 625–632 10.1172/JCI0215334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Thompson R.W., Holmes D.R., Mertens R.A., Liao S., Botney M.D., Mecham R.P.et al. (1995) Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J. Clin. Invest. 96, 318–326 10.1172/JCI118037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Curci J.A., Liao S., Huffman M.D., Shapiro S.D. and Thompson R.W. (1998) Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J. Clin. Invest. 102, 1900–1910 10.1172/JCI2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vandenbroucke R.E. and Libert C. (2014) Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 13, 904–927 10.1038/nrd4390 [DOI] [PubMed] [Google Scholar]
- 163.Fields G.B. (2019) The rebirth of matrix metalloproteinase inhibitors: moving beyond the dogma. Cells 8, 984 10.3390/cells8090984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abdul-Hussien H., Soekhoe R.G.V., Weber E., von der Thüsen J.H., Kleemann R., Mulder A.et al. (2007) Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am. J. Pathol. 170, 809–817 10.2353/ajpath.2007.060522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sukhova G.K. and Shi G.-P. (2006) Do cathepsins play a role in abdominal aortic aneurysm pathogenesis? Ann. N. Y. Acad. Sci. 1085, 161–169 10.1196/annals.1383.028 [DOI] [PubMed] [Google Scholar]
- 166.Liu J., Sukhova G.K., Yang J.-T., Sun J., Ma L., Ren A.et al. (2006) Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis 184, 302–311 10.1016/j.atherosclerosis.2005.05.012 [DOI] [PubMed] [Google Scholar]
- 167.Zhang J., Sun J., Lindholt J.S., Sukhova G.K., Sinnamon M., Stevens R.L.et al. (2011) Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ. Res. 108, 1316–1327 10.1161/CIRCRESAHA.111.243758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sun J., Sukhova G.K., Zhang J., Chen H., Sjöberg S., Libby P.et al. (2012) Cathepsin K deficiency reduces elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol. 32, 15–23 10.1161/ATVBAHA.111.235002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sun J., Sukhova G.K., Zhang J., Chen H., Sjöberg S., Libby P.et al. (2011) Cathepsin L activity is essential to elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler. Thromb. Vasc. Biol. 31, 2500–2508 10.1161/ATVBAHA.111.230201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qin Y., Cao X., Guo J., Zhang Y., Pan L., Zhang H.et al. (2012) Deficiency of cathepsin S attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Cardiovasc. Res. 96, 401–410 10.1093/cvr/cvs263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shi G.P., Sukhova G.K., Grubb A., Ducharme A., Rhode L.H., Lee R.T.et al. (1999) Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J. Clin. Invest. 104, 1191–1197 10.1172/JCI7709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lv B.-J., Lindholt J.S., Cheng X., Wang J. and Shi G.-P. (2012) Plasma cathepsin S and cystatin C levels and risk of abdominal aortic aneurysm: a randomized population-based study. PloS ONE 7, e41813 10.1371/journal.pone.0041813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lai C.-H., Chang J.-Y., Wang K.-C., Lee F.-T., Wu H.-L. and Cheng T.-L. (2020) Pharmacological inhibition of cathepsin S suppresses abdominal aortic aneurysm in mice. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 59, 990–999 10.1016/j.ejvs.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 174.Jadhav P.K., Schiffler M.A., Gavardinas K., Kim E.J., Matthews D.P., Staszak M.A.et al. (2014) Discovery of cathepsin S inhibitor LY3000328 for the treatment of abdominal aortic aneurysm. ACS Med. Chem. Lett. 5, 1138–1142 10.1021/ml500283g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Camardo A., Carney S. and Ramamurthi A. (2020) Assessing the targeting and fate of cathepsin k antibody-modified nanoparticles in a rat abdominal aortic aneurysm model. Acta Biomater. 112, 225–233 10.1016/j.actbio.2020.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shireman P.K., McCarthy W.J., Pearce W.H., Shively V.P., Cipollone M. and Kwaan H.C. (1997) Elevations of tissue-type plasminogen activator and differential expression of urokinase-type plasminogen activator in diseased aorta. J. Vasc. Surg. 25, 157–164 10.1016/S0741-5214(97)70333-1 [DOI] [PubMed] [Google Scholar]
- 177.Wang Y.X., Martin-McNulty B., Freay A.D., Sukovich D.A., Halks-Miller M., Li W.W.et al. (2001) Angiotensin II increases urokinase-type plasminogen activator expression and induces aneurysm in the abdominal aorta of apolipoprotein E-deficient mice. Am. J. Pathol. 159, 1455–1464 10.1016/S0002-9440(10)62532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Deng G.G., Martin-McNulty B., Sukovich D.A., Freay A., Halks-Miller M., Thinnes T.et al. (2003) Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ. Res. 92, 510–517 10.1161/01.RES.0000061571.49375.E1 [DOI] [PubMed] [Google Scholar]
- 179.Qian H.S., Gu J.-M., Liu P., Kauser K., Halks-Miller M., Vergona R.et al. (2008) Overexpression of PAI-1 prevents the development of abdominal aortic aneurysm in mice. Gene Ther. 15, 224–232 10.1038/sj.gt.3303069 [DOI] [PubMed] [Google Scholar]
- 180.Uchida H.A., Poduri A., Subramanian V., Cassis L.A. and Daugherty A. (2011) Urokinase-type plasminogen activator deficiency in bone marrow-derived cells augments rupture of angiotensin II-induced abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 31, 2845–2852 10.1161/ATVBAHA.111.234997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Martínez-González J., Varona S., Cañes L., Galán M., Briones A.M., Cachofeiro V.et al. (2019) Emerging roles of lysyl oxidases in the cardiovascular system: New concepts and therapeutic challenges. Biomolecules 9, 610 10.3390/biom9100610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huffman M.D., Curci J.A., Moore G., Kerns D.B., Starcher B.C. and Thompson R.W. (2000) Functional importance of connective tissue repair during the development of experimental abdominal aortic aneurysms. Surgery 128, 429–438 10.1067/msy.2000.107379 [DOI] [PubMed] [Google Scholar]
- 183.Mäki J.M., Räsänen J., Tikkanen H., Sormunen R., Mäkikallio K., Kivirikko K.I.et al. (2002) Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106, 2503–2509 10.1161/01.CIR.0000038109.84500.1E [DOI] [PubMed] [Google Scholar]
- 184.Hornstra I.K., Birge S., Starcher B., Bailey A.J., Mecham R.P. and Shapiro S.D. (2003) Lysyl oxidase is required for vascular and diaphragmatic development in mice. J. Biol. Chem. 278, 14387–14393 10.1074/jbc.M210144200 [DOI] [PubMed] [Google Scholar]
- 185.Brüel A., Ortoft G. and Oxlund H. (1998) Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis 140, 135–145 10.1016/S0021-9150(98)00130-0 [DOI] [PubMed] [Google Scholar]
- 186.Remus E.W., O'Donnell R.E., Rafferty K., Weiss D., Joseph G., Csiszar K.et al. (2012) The role of lysyl oxidase family members in the stabilization of abdominal aortic aneurysms. Am. J. Physiol. Heart Circ. Physiol. 303, H1067–H1075 10.1152/ajpheart.00217.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Orriols M., Guadall A., Galán M., Martí-Pàmies I., Varona S., Rodríguez-Calvo R.et al. (2014) Lysyl oxidase (LOX) in vascular remodelling. Insight from a new animal model. Thromb. Haemost. 112, 812–824 10.1160/TH14-01-0024 [DOI] [PubMed] [Google Scholar]
- 188.Onoda M., Yoshimura K., Aoki H., Ikeda Y., Morikage N., Furutani A.et al. (2010) Lysyl oxidase resolves inflammation by reducing monocyte chemoattractant protein-1 in abdominal aortic aneurysm. Atherosclerosis 208, 366–369 10.1016/j.atherosclerosis.2009.07.036 [DOI] [PubMed] [Google Scholar]
- 189.Sonesson B., Hansen F. and Länne T. (1997) Abdominal aortic aneurysm: a general defect in the vasculature with focal manifestations in the abdominal aorta? J. Vasc. Surg. 26, 247–254 10.1016/S0741-5214(97)70185-X [DOI] [PubMed] [Google Scholar]
- 190.Kidholm C.L., Beck H.C., Madsen J.B., Palstrøm N.B., Lindholt J.S. and Rasmussen L.M. (2018) Preliminary analysis of proteome alterations in non-aneurysmal, internal mammary artery tissue from patients with abdominal aortic aneurysms. PLoS ONE 13, e0192957 10.1371/journal.pone.0192957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Orriols M., Varona S., Aguiló S., Galán M., Martínez González J. and Rodríguez C. (2016) Inflammation inhibits vascular fibulin-5 expression: Involvement of transcription factor SOX9. Clin. E Investig. En Arterioscler. Publ. Soc. Espanola Arterioscler. 28, 271–280 10.1016/j.arteri.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 192.Guadall A., Orriols M., Rodríguez-Calvo R., Calvayrac O., Crespo J., Aledo R.et al. (2011) Fibulin-5 is up-regulated by hypoxia in endothelial cells through a hypoxia-inducible factor-1 (HIF-1α)-dependent mechanism. J. Biol. Chem. 286, 7093–7103 10.1074/jbc.M110.162917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chapman S.L., Sicot F.-X., Davis E.C., Huang J., Sasaki T., Chu M.-L.et al. (2010) Fibulin-2 and fibulin-5 cooperatively function to form the internal elastic lamina and protect from vascular injury. Arterioscler. Thromb. Vasc. Biol. 30, 68–74 10.1161/ATVBAHA.109.196725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Spencer J.A., Hacker S.L., Davis E.C., Mecham R.P., Knutsen R.H., Li D.Y.et al. (2005) Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc. Natl. Acad. Sci. U. S. A. 102, 2946–2951 10.1073/pnas.0500058102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Orriols M., Varona S., Martí-Pàmies I., Galán M., Guadall A., Escudero J.R.et al. (2016) Down-regulation of Fibulin-5 is associated with aortic dilation: role of inflammation and epigenetics. Cardiovasc. Res. 110, 431–442 10.1093/cvr/cvw082 [DOI] [PubMed] [Google Scholar]
- 196.Patnaik S.S., Simionescu D.T., Goergen C.J., Hoyt K., Sirsi S. and Finol E.A. (2019) Pentagalloyl glucose and Its functional role in vascular health: biomechanics and drug-delivery characteristics. Ann. Biomed. Eng. 47, 39–59 10.1007/s10439-018-02145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Isenburg J.C., Simionescu D.T., Starcher B.C. and Vyavahare N.R. (2007) Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation 115, 1729–1737 10.1161/CIRCULATIONAHA.106.672873 [DOI] [PubMed] [Google Scholar]
- 198.Schack A.S., Stubbe J., Steffensen L.B., Mahmoud H., Laursen M.S. and Lindholt J.S. (2020) Intraluminal infusion of Penta-Galloyl Glucose reduces abdominal aortic aneurysm development in the elastase rat model. PLoS ONE 15, e0234409 10.1371/journal.pone.0234409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Simionescu D., Casco M., Turner J., Rierson N., Yue J. and Ning K. (2020) Chemical stabilization of the extracellular matrix attenuates growth of experimentally induced abdominal aorta aneurysms in a large animal model. JVS-Vasc. Sci. 1, 69–80 10.1016/j.jvssci.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nosoudi N., Chowdhury A., Siclari S., Parasaram V., Karamched S. and Vyavahare N. (2016) Systemic delivery of nanoparticles loaded with pentagalloyl glucose protects elastic lamina and prevents abdominal aortic aneurysm in rats. J Cardiovasc. Transl. Res. 9, 445–455 10.1007/s12265-016-9709-x [DOI] [PubMed] [Google Scholar]
- 201.Sinha A., Nosoudi N. and Vyavahare N. (2014) Elasto-regenerative properties of polyphenols. Biochem. Biophys. Res. Commun. 444, 205–211 10.1016/j.bbrc.2014.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Linkermann A. and Green D.R. (2014) Necroptosis. N. Engl. J. Med. 370, 455–465 10.1056/NEJMra1310050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang Q., Liu Z., Ren J., Morgan S., Assa C. and Liu B. (2015) Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ. Res. 116, 600–611 10.1161/CIRCRESAHA.116.304899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhou T., Wang Q., Phan N., Ren J., Yang H., Feldman C.C.et al. (2019) Identification of a novel class of RIP1/RIP3 dual inhibitors that impede cell death and inflammation in mouse abdominal aortic aneurysm models. Cell Death Dis. 10, 226 10.1038/s41419-019-1468-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ding Y., Wang Y., Cai Y., Pan C., Yang C., Wang M.et al. (2022) IL-37 Expression in patients with abdominal aortic aneurysm and its role in the necroptosis of vascular smooth muscle cells. Oxid. Med. Cell. Longev. 2022, 1806513 10.1155/2022/1806513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li K., Zhang D., Zhai S., Wu H. and Liu H. (2023) METTL3-METTL14 complex induces necroptosis and inflammation of vascular smooth muscle cells via promoting N6 methyladenosine mRNA methylation of receptor-interacting protein 3 in abdominal aortic aneurysms. J. Cell Commun. Signal. 173897–914 10.1007/s12079-023-00737-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zarzour A., Kim H.W. and Weintraub N.L. (2019) Epigenetic regulation of vascular diseases. Arterioscler. Thromb. Vasc. Biol. 39, 984–990 10.1161/ATVBAHA.119.312193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rosa-Garrido M., Chapski D.J. and Vondriska T.M. (2018) Epigenomes in cardiovascular disease. Circ. Res. 122, 1586–1607 10.1161/CIRCRESAHA.118.311597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yousefi P.D., Suderman M., Langdon R., Whitehurst O., Davey Smith G. and Relton C.L. (2022) DNA methylation-based predictors of health: applications and statistical considerations. Nat. Rev. Genet. 23, 369–383 10.1038/s41576-022-00465-w [DOI] [PubMed] [Google Scholar]
- 210.Ryer E.J., Ronning K.E., Erdman R., Schworer C.M., Elmore J.R., Peeler T.C.et al. (2015) The potential role of DNA methylation in abdominal aortic aneurysms. Int. J. Mol. Sci. 16, 11259–11275 10.3390/ijms160511259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Toghill B.J., Saratzis A., Freeman P.J., Sylvius N., Bown M.J.and UKAGS collaborators (2018) SMYD2 promoter DNA methylation is associated with abdominal aortic aneurysm (AAA) and SMYD2 expression in vascular smooth muscle cells. Clin. Epigenetics 10, 29 10.1186/s13148-018-0460-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhong L., He X., Si X., Wang H., Li B., Hu Y.et al. (2019) SM22α (Smooth muscle 22α) prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching through suppressing reactive oxygen species/NF-κB (Nuclear Factor-κB). Arterioscler. Thromb. Vasc. Biol. 39, e10–e25 10.1161/ATVBAHA.118.311917 [DOI] [PubMed] [Google Scholar]
- 213.Vats S., Sundquist K., Wang X., Zarrouk M., Ågren-Witteschus S., Sundquist J.et al. (2020) Associations of global DNA methylation and homocysteine levels with abdominal aortic aneurysm: A cohort study from a population-based screening program in Sweden. Int. J. Cardiol. 321, 137–142 10.1016/j.ijcard.2020.06.022 [DOI] [PubMed] [Google Scholar]
- 214.Xia Q., Zhang J., Han Y., Zhang X., Jiang H., Lun Y.et al. (2019) Epigenetic regulation of regulatory T cells in patients with abdominal aortic aneurysm. FEBS Open Bio 9, 1137–1143 10.1002/2211-5463.12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen Y., Xu X., Chen Z., Huang B., Wang X. and Fan X. (2022) DNA methylation alternation in Stanford- A acute aortic dissection. BMC Cardiovasc. Disord. 22, 455 10.1186/s12872-022-02882-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Davis F.M., Tsoi L.C., Melvin W.J., denDekker A., Wasikowski R., Joshi A.D.et al. (2021) Inhibition of macrophage histone demethylase JMJD3 protects against abdominal aortic aneurysms. J. Exp. Med. 218, e20201839 10.1084/jem.20201839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Galán M., Varona S., Orriols M., Rodríguez J.A., Aguiló S., Dilmé J.et al. (2016) Induction of histone deacetylases (HDACs) in human abdominal aortic aneurysm: therapeutic potential of HDAC inhibitors. Dis. Model. Mech. 9, 541–552 10.1242/dmm.024513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Greenway J., Gilreath N., Patel S., Horimatsu T., Moses M., Kim D.et al. (2020) Profiling of histone modifications reveals epigenomic dynamics during abdominal aortic aneurysm formation in mouse models. Front. Cardiovasc. Med. 7, 595011 10.3389/fcvm.2020.595011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xu X., Zhang F., Lu Y., Yu S., Sun W., Sun S.et al. (2019) Silencing of NONO inhibits abdominal aortic aneurysm in apolipoprotein E-knockout mice via collagen deposition and inflammatory inhibition. J. Cell. Mol. Med. 23, 7449–7461 10.1111/jcmm.14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Han Y., Tanios F., Reeps C., Zhang J., Schwamborn K., Eckstein H.-H.et al. (2016) Histone acetylation and histone acetyltransferases show significant alterations in human abdominal aortic aneurysm. Clin. Epigenetics 8, 3 10.1186/s13148-016-0169-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Horimatsu T., Blomkalns A.L., Ogbi M., Moses M., Kim D., Patel S.et al. (2020) Niacin protects against abdominal aortic aneurysm formation via GPR109A independent mechanisms: role of NAD+/nicotinamide. Cardiovasc. Res. 116, 2226–2238 10.1093/cvr/cvz303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang Z., Xu J., Liu Y., Wang T., Pei J., Cheng L.et al. (2018) Mouse macrophage specific knockout of SIRT1 influences macrophage polarization and promotes angiotensin II-induced abdominal aortic aneurysm formation. J. Genet. Genomics Yi Chuan Xue Bao 45, 25–32 10.1016/j.jgg.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 223.Pahl M.C., Derr K., Gäbel G., Hinterseher I., Elmore J.R., Schworer C.M.et al. (2012) MicroRNA expression signature in human abdominal aortic aneurysms. BMC Med. Genomics 5, 25 10.1186/1755-8794-5-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kin K., Miyagawa S., Fukushima S., Shirakawa Y., Torikai K., Shimamura K.et al. (2012) Tissue- and plasma-specific MicroRNA signatures for atherosclerotic abdominal aortic aneurysm. J. Am. Heart Assoc. 1, e000745 10.1161/JAHA.112.000745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Biros E., Moran C.S., Wang Y., Walker P.J., Cardinal J. and Golledge J. (2014) microRNA profiling in patients with abdominal aortic aneurysms: the significance of miR-155. Clin. Sci. Lond. Engl. 1979 126, 795–803 10.1042/CS20130599 [DOI] [PubMed] [Google Scholar]
- 226.Maegdefessel L., Azuma J., Toh R., Deng A., Merk D.R., Raiesdana A.et al. (2012) MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci. Transl. Med. 4, 122ra22 10.1126/scitranslmed.3003441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Boon R.A., Seeger T., Heydt S., Fischer A., Hergenreider E., Horrevoets A.J.G.et al. (2011) MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ. Res. 109, 1115–1119 10.1161/CIRCRESAHA.111.255737 [DOI] [PubMed] [Google Scholar]
- 228.Maegdefessel L., Azuma J., Toh R., Merk D.R., Deng A., Chin J.T.et al. (2012) Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J. Clin. Invest. 122, 497–506 10.1172/JCI61598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Maegdefessel L., Spin J.M., Raaz U., Eken S.M., Toh R., Azuma J.et al. (2014) miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat. Commun. 5, 5214 10.1038/ncomms6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kumar S., Boon R.A., Maegdefessel L., Dimmeler S. and Jo H. (2019) Role of noncoding RNAs in the pathogenesis of abdominal aortic aneurysm. Circ. Res. 124, 619–630 10.1161/CIRCRESAHA.118.312438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Iyer V., Rowbotham S., Biros E., Bingley J. and Golledge J. (2017) A systematic review investigating the association of microRNAs with human abdominal aortic aneurysms. Atherosclerosis 261, 78–89 10.1016/j.atherosclerosis.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 232.Diener C., Keller A. and Meese E. (2022) Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet. TIG 38, 613–626 10.1016/j.tig.2022.02.006 [DOI] [PubMed] [Google Scholar]
- 233.Schellinger I.N., Dannert A.R., Mattern K., Raaz U. and Tsao P.S. (2021) Unresolved issues in RNA therapeutics in vascular diseases with a focus on aneurysm disease. Front. Cardiovasc. Med. 8, 571076 10.3389/fcvm.2021.571076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xu Y., Yang S. and Xue G. (2023) The role of long non-coding RNA in abdominal aortic aneurysm. Front. Genet. 14, 1153899 10.3389/fgene.2023.1153899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wu Z.-Y., Trenner M., Boon R.A., Spin J.M. and Maegdefessel L. (2020) Long noncoding RNAs in key cellular processes involved in aortic aneurysms. Atherosclerosis 292, 112–118 10.1016/j.atherosclerosis.2019.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li D.Y., Busch A., Jin H., Chernogubova E., Pelisek J., Karlsson J.et al. (2018) H19 induces abdominal aortic aneurysm development and progression. Circulation 138, 1551–1568 10.1161/CIRCULATIONAHA.117.032184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sun Y., Zhong L., He X., Wang S., Lai Y., Wu W.et al. (2019) LncRNA H19 promotes vascular inflammation and abdominal aortic aneurysm formation by functioning as a competing endogenous RNA. J. Mol. Cell Cardiol. 131, 66–81 10.1016/j.yjmcc.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 238.Le T., He X., Huang J., Liu S., Bai Y. and Wu K. (2021) Knockdown of long noncoding RNA GAS5 reduces vascular smooth muscle cell apoptosis by inactivating EZH2-mediated RIG-I signaling pathway in abdominal aortic aneurysm. J. Transl. Med. 19, 466 10.1186/s12967-021-03023-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Huang Y., Ren L., Li J. and Zou H. (2021) Long non-coding RNA PVT1/microRNA miR-3127-5p/NCK-associated protein 1-like axis participates in the pathogenesis of abdominal aortic aneurysm by regulating vascular smooth muscle cells. Bioengineered 12, 12583–12596 10.1080/21655979.2021.2010384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kok F.O. and Baker A.H. (2019) The function of long non-coding RNAs in vascular biology and disease. Vascul. Pharmacol. 114, 23–30 10.1016/j.vph.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 241.Huang C.-K., Kafert-Kasting S. and Thum T. (2020) Preclinical and clinical development of noncoding RNA therapeutics for cardiovascular disease. Circ. Res. 126, 663–678 10.1161/CIRCRESAHA.119.315856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Duan Q., Mao X., Liao C., Zhou H., Sun Z., Deng X.et al. (2016) Inhibition of BET bromodomain attenuates angiotensin II induced abdominal aortic aneurysm in ApoE-/- mice. Int. J. Cardiol. 223, 428–432 10.1016/j.ijcard.2016.08.238 [DOI] [PubMed] [Google Scholar]
- 243.Borck P.C., Guo L.-W. and Plutzky J. (2020) BET epigenetic reader proteins in cardiovascular transcriptional programs. Circ. Res. 126, 1190–1208 10.1161/CIRCRESAHA.120.315929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang X., Liu S., Weng X., Wu T., Yu L., Xu Y.et al. (2018) Brg1 trans-activates endothelium-derived colony stimulating factor to promote calcium chloride induced abdominal aortic aneurysm in mice. J. Mol. Cell Cardiol. 125, 6–17 10.1016/j.yjmcc.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 245.Zhao G., Zhao Y., Lu H., Chang Z., Liu H., Wang H.et al. (2022) BAF60c prevents abdominal aortic aneurysm formation through epigenetic control of vascular smooth muscle cell homeostasis. J. Clin. Invest. 132, e158309 10.1172/JCI158309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chang Z., Zhao G., Zhao Y., Lu H., Xiong W., Liang W.et al. (2020) BAF60a deficiency in vascular smooth muscle cells prevents abdominal aortic aneurysm by reducing inflammation and extracellular matrix degradation. Arterioscler. Thromb. Vasc. Biol. 40, 2494–2507 10.1161/ATVBAHA.120.314955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wälchli T., Wacker A., Frei K., Regli L., Schwab M.E., Hoerstrup S.P.et al. (2015) Wiring the vascular network with neural cues: A CNS perspective. Neuron 87, 271–296 10.1016/j.neuron.2015.06.038 [DOI] [PubMed] [Google Scholar]
- 248.Parati G. and Esler M. (2012) The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur. Heart J. 33, 1058–1066 10.1093/eurheartj/ehs041 [DOI] [PubMed] [Google Scholar]
- 249.Grassi G., Seravalle G. and Mancia G. (2015) Sympathetic activation in cardiovascular disease: evidence, clinical impact and therapeutic implications. Eur. J. Clin. Invest. 45, 1367–1375 10.1111/eci.12553 [DOI] [PubMed] [Google Scholar]
- 250.Zhipeng H., Zhiwei W., Lilei Y., Hao Z., Hongbing W., Zongli R.et al. (2014) Sympathetic hyperactivity and aortic sympathetic nerve sprouting in patients with thoracic aortic dissection. Ann. Vasc. Surg. 28, 1243–1248 10.1016/j.avsg.2013.11.016 [DOI] [PubMed] [Google Scholar]
- 251.Hu R., Wang Z., Ren Z. and Liu M. (2016) Autonomic remodeling may be responsible for decreased incidence of aortic dissection in STZ-induced diabetic rats via down-regulation of matrix metalloprotease 2. BMC Cardiovasc. Disord. 16, 200 10.1186/s12872-016-0375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hu Z., Li B., Wang Z., Hu X., Zhang M., Chen R.et al. (2020) The sympathetic transmitter norepinephrine inhibits VSMC proliferation induced by TGFβ by suppressing the expression of the TGFβ receptor ALK5 in aorta remodeling. Mol. Med. Rep. 22, 387–397 10.3892/mmr.2020.11088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Cañes L., Martí-Pàmies I., Ballester-Servera C., Alonso J., Serrano E., Briones A.M.et al. (2021) High NOR-1 (Neuron-derived orphan receptor 1) expression strengthens the vascular wall response to sngiotensin II leading to aneurysm formation in mice. Hypertens. Dallas Tex 1979 77, 557–570 10.1161/HYPERTENSIONAHA.120.16078 [DOI] [PubMed] [Google Scholar]
- 254.Cañes L., Alonso J., Ballester-Servera C., Varona S., Escudero J.R., Andrés V.et al. (2021) Targeting tyrosine hydroxylase for abdominal aortic aneurysm: impact on inflammation, oxidative stress, and vascular remodeling. Hypertens. Dallas Tex 1979 78, 681–692 10.1161/HYPERTENSIONAHA.121.17517 [DOI] [PubMed] [Google Scholar]
- 255.Martínez-González J., Cañes L., Alonso J., Ballester-Servera C., Rodríguez-Sinovas A., Corrales I.et al. (2021) NR4A3: A key nuclear receptor in vascular biology, cardiovascular remodeling, and beyond. Int. J. Mol. Sci. 22, 11371 10.3390/ijms222111371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kota S., Kota S., Meher L., Jammula S., Mohapatra S. and Modi K. (2013) Coexistence of pheochromocytoma with abdominal aortic aneurysm: an untold association. Ann. Med. Health Sci. Res. 3, 258–261 10.4103/2141-9248.113672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Miller F.J., Sharp W.J., Fang X., Oberley L.W., Oberley T.D. and Weintraub N.L. (2002) Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler. Thromb. Vasc. Biol. 22, 560–565 10.1161/01.ATV.0000013778.72404.30 [DOI] [PubMed] [Google Scholar]
- 258.McCormick M.L., Gavrila D. and Weintraub N.L. (2007) Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 27, 461–469 10.1161/01.ATV.0000257552.94483.14 [DOI] [PubMed] [Google Scholar]
- 259.Zhang L. and Wang Y. (2015) B lymphocytes in abdominal aortic aneurysms. Atherosclerosis 242, 311–317 10.1016/j.atherosclerosis.2015.07.036 [DOI] [PubMed] [Google Scholar]
- 260.Pincemail J., Defraigne J.O., Cheramy-Bien J.P., Dardenne N., Donneau A.F., Albert A.et al. (2012) On the potential increase of the oxidative stress status in patients with abdominal aortic aneurysm. Redox Rep. Commun. Free Radic. Res. 17, 139–144 10.1179/1351000212Y.0000000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Navas-Madroñal M., Rodriguez C., Kassan M., Fité J., Escudero J.R., Cañes L.et al. (2019) Enhanced endoplasmic reticulum and mitochondrial stress in abdominal aortic aneurysm. Clin. Sci. 133, 1421–1438 10.1042/CS20190399 [DOI] [PubMed] [Google Scholar]
- 262.Thomas M., Gavrila D., McCormick M.L., Miller F.J., Daugherty A., Cassis L.A.et al. (2006) Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation 114, 404–413 10.1161/CIRCULATIONAHA.105.607168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Salmon M. (2022) NADPH oxidases in aortic aneurysms. Antioxid. Basel Switz. 11, 1830 10.3390/antiox11091830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G. and Gluud C. (2007) Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297, 842–857 10.1001/jama.297.8.842 [DOI] [PubMed] [Google Scholar]
- 265.Galán M., Kassan M., Kadowitz P.J., Trebak M., Belmadani S. and Matrougui K. (2014) Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim. Biophys. Acta 1843, 1063–1075 10.1016/j.bbamcr.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kassan M., Galán M., Partyka M., Saifudeen Z., Henrion D., Trebak M.et al. (2012) Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler. Thromb. Vasc. Biol. 32, 1652–1661 10.1161/ATVBAHA.112.249318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hotamisligil G.S. (2010) Endoplasmic reticulum stress and atherosclerosis. Nat. Med. 16, 396–399 10.1038/nm0410-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Siegert A.-M., García Díaz-Barriga G., Esteve-Codina A., Navas-Madroñal M., Gorbenko Del Blanco D., Alberch J.et al. (2019) A FBN1 3′UTR mutation variant is associated with endoplasmic reticulum stress in aortic aneurysm in Marfan syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 107–114 10.1016/j.bbadis.2018.10.029 [DOI] [PubMed] [Google Scholar]
- 269.Navas-Madroñal M., Almendra-Pegueros R., Puertas-Umbert L., Jiménez-Altayó F., Julve J., Pérez B.et al. (2023) Targeting mitochondrial stress with Szeto-Schiller 31 prevents experimental abdominal aortic aneurysm: Crosstalk with endoplasmic reticulum stress. Br. J. Pharmacol. 180172230–2249 10.1111/bph.16077 [DOI] [PubMed] [Google Scholar]
- 270.Ni X.-Q., Lu W.-W., Zhang J.-S., Zhu Q., Ren J.-L., Yu Y.-R.et al. (2018) Inhibition of endoplasmic reticulum stress by intermedin1-53 attenuates angiotensin II-induced abdominal aortic aneurysm in ApoE KO Mice. Endocrine 62, 90–106 10.1007/s12020-018-1657-6 [DOI] [PubMed] [Google Scholar]
- 271.Timmins J.M., Ozcan L., Seimon T.A., Li G., Malagelada C., Backs J.et al. (2009) Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J. Clin. Invest. 119, 2925–2941 10.1172/JCI38857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Guarente L. (2008) Mitochondria–a nexus for aging, calorie restriction, and sirtuins? Cell 132, 171–176 10.1016/j.cell.2008.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gabrielson M., Vorkapic E., Folkesson M., Welander M., Matussek A., Dimberg J.et al. (2016) Altered PPARγ coactivator-1 alpha expression in abdominal aortic aneurysm: possible effects on mitochondrial biogenesis. J. Vasc. Res. 53, 17–26 10.1159/000446653 [DOI] [PubMed] [Google Scholar]
- 274.Sinha I., Sinha-Hikim A.P., Hannawa K.K., Henke P.K., Eagleton M.J., Stanley J.C.et al. (2005) Mitochondrial-dependent apoptosis in experimental rodent abdominal aortic aneurysms. Surgery 138, 806–811 10.1016/j.surg.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 275.Lin S., Lin Y., Nery J.R., Urich M.A., Breschi A., Davis C.A.et al. (2014) Comparison of the transcriptional landscapes between human and mouse tissues. Proc. Natl. Acad. Sci. U. S. A. 111, 17224–17229 10.1073/pnas.1413624111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mestas J. and Hughes C.C.W. (2004) Of mice and not men: differences between mouse and human immunology. J. Immunol. Baltim. Md 1950 172, 2731–2738 [DOI] [PubMed] [Google Scholar]
- 277.Sénémaud J., Caligiuri G., Etienne H., Delbosc S., Michel J.-B. and Coscas R. (2017) Translational relevance and recent advances of animal models of abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 37, 401–410 10.1161/ATVBAHA.116.308534 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.