Abstract
Many human viruses not only cause acute diseases but also establish persistent infections. Such persistent viruses can cause chronic diseases or can reactivate to cause acute diseases in AIDS patients or patients receiving immunosuppressive therapies. While the prevention of persistent infections is an important consideration in the design of modern vaccines, surprisingly little is known about this aspect of protection. In the current study, we tested the feasibility of vaccine prevention of retroviral persistence by using a Friend virus model that we recently developed. In this model, persistent virus can be detected at very low levels by immunosuppressing the host to reactivate virus or by transferring persistently infected spleen cells into highly susceptible mice. Two vaccines were analyzed, a recombinant vaccinia virus vector expressing Friend virus envelope protein and a live attenuated Friend virus. Both vaccines reduced pathogenic virus loads to levels undetectable by infectious center assays. However, only the live, attenuated vaccine prevented immunosuppression-induced reactivation of persistent virus. Thus, even very low levels of persistent Friend virus posed a significant threat during immunosuppression. Our results demonstrate that vaccine protection against establishment of retroviral persistence is attainable.
While currently used vaccines have been quite effective in reducing the incidence of many acute viral diseases, they do not necessarily function by total prevention of infection, and limited viral replication likely occurs. The replication of a pathogenic virus in an immunized host presents a potential medical problem if any virus is able to evade immunological destruction and establish a persistent infection. Such persistent infections can cause chronic diseases or may lead to severe acute diseases in cases where the host becomes immunocompromised due to chemotherapy for cancer or transplantations.
The propensity of retroviruses such as human immunodeficiency virus (HIV) to persist in the host, even under extremely adverse conditions, is exemplified by recent findings of persistent HIV in patients who have begun highly active antiretroviral therapy early in acute infection (5). Although plasma virus loads are often reduced to undetectable levels in such patients, low numbers of CD4+ T cells continue to harbor infectious virus (6, 13). The syncytium-inducing property of some of these persistent viruses indicates a high potential for pathogenesis. Thus, even very low levels of HIV are potentially quite dangerous, and an ideal retroviral vaccine should protect against such persistent viruses as well as against acute disease. However, there is currently little information about protection against persistent viral infections.
In the current studies, we have used Friend virus (FV) infection of mice to investigate the ability of two types of vaccines to prevent establishment of persistent FV infections. FV is a useful model to investigate basic aspects of retroviral vaccines since it is one of the few retroviruses that cause disease in immunocompetent adult mice. FV is a retroviral complex comprised of a replication-competent helper virus, Friend murine leukemia virus (F-MuLV), and a replication-defective spleen focus-forming virus (21). In susceptible adult animals, FV induces rapid polyclonal erythroblast proliferation (19, 23) followed within weeks by erythroleukemia (24, 25, 32). The present investigation utilized mice which recover from acute disease even when not vaccinated but which remain persistently infected for life. Approximately 95% of such persistently infected animals remain clinically normal for life (2, 4). However, depletion of CD4+ T cells in persistently infected mice induces relapse of splenomegaly and erythroleukemia in a high percentage (40 to 50%) of animals (16). In addition, persistent FV can be reactivated by transplantation of infected cells into highly susceptible mice. This bioassay is very sensitive in detecting persistence of pathogenic FV.
It was previously shown that the level of acutely infected spleen cells following challenge with pathogenic FV could be reduced by over 1,000-fold if mice were vaccinated with either (i) a recombinant vaccinia virus vector expressing the F-MuLV Env protein (vvF-MuLV Env) (12) or (ii) a live attenuated form of FV (FV-N) (9, 12). The attenuation of this vaccine virus was achieved by crossing a host genetic resistance barrier called Fv-1 (20, 30). Both types of vaccine have been shown to protect mice of susceptible strains against FV-induced erythroleukemia, although the vaccinia virus construct displayed strain-dependent protection while the FV-N vaccine was broadly protective (12). In the current experiments, we found that these vaccines also differed in their ability to protect against the establishment of persistent infections with pathogenic FV.
MATERIALS AND METHODS
Mice.
Age- and sex-matched C57BL/10 × A.BY F1 mice of 3 to 6 months of age at experimental onset were used in all vaccine studies. Parental strains were obtained from the Jackson Laboratories, and breeding of F1 strains was done at Rocky Mountain Laboratories. All animals were treated in accordance with the regulations of the National Institutes of Health and the Animal Care and Use Committee of Rocky Mountain Laboratories. Relevant FV resistance genotypes in C57BL/10 × A.BY F1 mice are H-2b/H-2b, FV-1b/FV-1b, FV-2r/FV-2s, and Rfv-3r/Rfv-3s. BALB/c mice from Jackson Laboratories were used as recipients for spleen cell transplants to detect persistent virus.
FV and virus infections.
The FV-B (challenge virus) and FV-N (vaccine virus) used in these experiments were from uncloned virus stocks obtained from 10% spleen cell homogenates from BALB/c mice infected 9 days previously with polycythemia-inducing FV stocks (9, 12). The attenuation of the vaccine virus was achieved by crossing a host genetic resistance barrier called Fv-1 (20, 30). The N-tropic F-MuLV helper virus stock (stock no. 29-51N) (3) was a 24-h supernatant from infected Mus dunni cells (22). In virus challenge experiments, mice were injected intravenously with 0.5 ml of phosphate-buffered, balanced salt solution containing 2% fetal bovine serum and 1,500 spleen focus-forming units of FV complex. Disease was monitored by palpation for splenomegaly in a blinded fashion as described elsewhere (14).
T-cell depletions.
T-cell depletions were performed essentially as described elsewhere (7, 15, 26). Briefly, persistently infected mice were inoculated intraperitoneally with 0.5 ml of supernatant fluid obtained from artificial capillary cultures (Cellco, Germantown, Md.) for monoclonal antibody (MAb) 191.1 (anti-CD4). Mice were inoculated three times per week for 2 weeks. The MAb was rat anti-mouse immunoglobulin G2b (IgG2b) isotype. Blood samples from all mice were checked for T-cell depletion levels by flow cytometry at 7 to 10 days following the last injection of antibody. CD4+ T-cell levels in mononuclear blood cells from depleted mice ranged from <1 to 3% of the nucleated peripheral blood cells.
Infectious center (IC) assays.
Titrations of single cell suspensions from persistently infected mouse spleens were plated onto susceptible M. dunni cells, cocultivated for 5 days, fixed with ethanol, stained with F-MuLV envelope-specific MAb 720 (31), and developed with goat anti-mouse peroxidase conjugate (Cappel, West Chester, Pa.) to detect foci.
In vitro amplification of spleen virus.
For amplification of spleen virus, 10% homogenates of spleens from 10 individual FV-N-vaccinated, FV-B-challenged mice were made at 7 days postchallenge. One milliliter of this homogenate was added to cultures of 106 NIH 3T3 cells or 4 × 105 BALB/c 3T3 cells in 10 ml of RPMI with 10% fetal bovine serum and 4 mg of Polybrene per ml. After 2 weeks of culture, the cells were trypsinized, stained for viral antigen with antibody 720, developed with fluorescein isothiocyanate-labeled anti-mouse IgG, and analyzed by flow cytometry. As controls, the same cell lines were infected with FV-N or FV-B virus stocks obtained from acutely infected, Fv-1-matched mice.
Recombinant vaccinia virus constructs used in vaccines.
The vaccinia virus recombinants used for vaccinations in these studies, vvF-MuLV (12) and vvF-MCF (14, 17), were produced and used as previously described. The F-MuLV Env used in the vaccines was derived from clone 57 (29), and the negative control F-MCF Env was from clone 54B (28).
Spleen cell transfers.
Single cell suspensions from donor spleens were made in phosphate-buffered, balanced salt solution with 15 U of heparin per ml. Suspension (0.5 ml) containing 5 × 107 spleen cells was injected intravenously into the tail veins of BALB/c recipient mice.
MAb 48 passive transfers.
MAb 48 is a mouse monoclonal IgG2a specific for F-MuLV gp70 envelope (3), and supernatants were prepared from a Cellmax artificial capillary system (Cellco, Georgetown, Md.). For experiments shown in Fig. 3, 0.5 ml of neat supernatant was injected intraperitoneally two times, on days −3 and −2 relative to virus challenge.
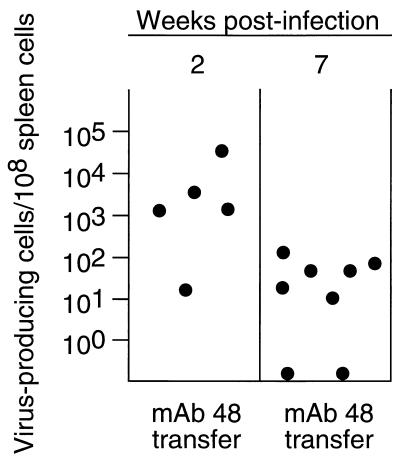
FIG. 3.
IC levels following passive antibody transfers. Following passive immunization with MAb 48 and infection with pathogenic FV-B, ICs from spleen cells were determined during acute infection (left column) and persistent infection (right column). Plasma titers of passively immunized mice were determined on the day of challenge. The mean geometric titer of virus-neutralizing antibodies in passively immunized mice was log2 8.4, standard deviation = 0.8, compared to log2 8.4, standard deviation = 1.1, in mice vaccinated with F-MuLV-N. The mean concentration of MAb 48 in the plasma of mice receiving passive immunization was 0.875 mg/ml at the time of challenge.
RESULTS
Detection of persistent FV by IC assays.
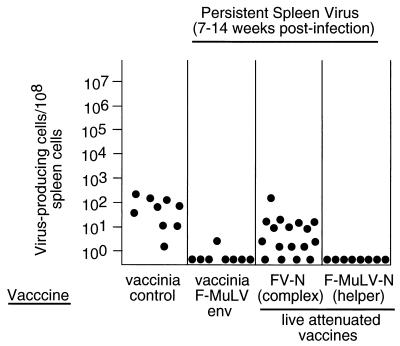
Initial experiments to test for protection against persistent FV were done by IC assays with spleen cells from mice that had been vaccinated and then challenged intravenously with pathogenic FV. Groups of mice were evaluated for levels of ICs at 7 to 14 weeks postchallenge when unvaccinated mice have recovered from acute infection but remain persistently infected. As expected, low levels of virus-producing spleen cells were found in all mice from the control group (Fig. 1). In contrast, only one of the eight mice vaccinated with vvF-MuLV Env had detectable ICs. However, most of the mice vaccinated with live attenuated FV-N complex had detectable ICs. Although this finding suggested a lack of protection by the live attenuated vaccine, interpretation of the result was confounded because the IC assay did not distinguish between persistence of the highly homologous vaccine virus and persistence of the challenge virus. We also tested vaccination with the F-MuLV helper component of FV-N, which is even more attenuated than FV-N but which retains immunological epitopes important in protection (9). In this case, no ICs were detected, suggesting that protection against persistence may have been achieved.
FIG. 1.
Effect of vaccination on cell-associated viral loads at 7 to 14 weeks post-FV-B challenge. Filled circles represent ICs from spleen cells of age- and sex-matched C57BL/10 × A.BY F1 mice vaccinated with different vaccines and challenged with 1,500 spleen focus-forming units of pathogenic FV-B at 30 days postvaccination. The negative control vaccine in column 1 was the recombinant vaccinia virus expressing a nonprotective F-MCF envelope protein. The lower limit of detection was one IC per 3 × 107 spleen cells.
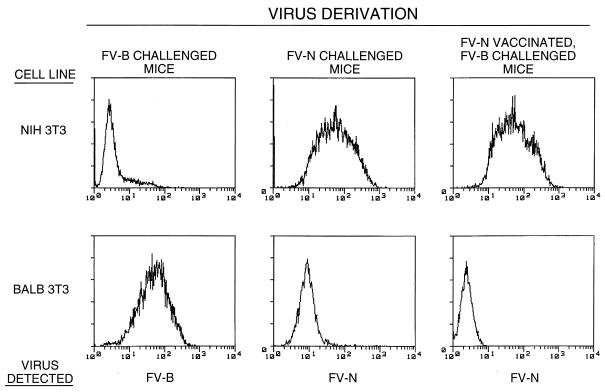
We wished to further investigate vaccination by FV-N complex and determine whether the vaccine or the challenge virus was being detected in the IC assays shown in Fig. 1. To this end, virus stocks derived from spleen cell homogenates from FV-N-vaccinated, FV-B-challenged mice were amplified in vitro on two indicator cell lines which were permissive for the replication of only FV-N or FV-B. Following amplification, the infected cells were tested for viral envelope antigen by flow cytometry. On NIH 3T3 cells, only FV-N vaccine virus replicated efficiently, whereas FV-B challenge virus replicated efficiently only on BALB 3T3 cells (Fig. 2). The virus isolated from FV-N-vaccinated, FV-B-challenged mice replicated only on NIH 3T3 cells, indicating that the persistent virus was of vaccine origin. Thus, the IC results suggested that all three test vaccines protected against persistent FV-B. However, IC assays are not highly sensitive, and we wished to confirm these findings with more sensitive bioassays.
FIG. 2.
In vitro amplification of spleen virus from FV-N-vaccinated, FV-B-challenged mice. Virus from 10% spleen homogenates was amplified as described in Materials and Methods and then stained with MAb 720 for viral antigen for analysis by flow cytometry. On BALB 3T3 cells, only FV-B replicated efficiently (left column), whereas FV-N showed optimal replication on NIH 3T3 cells (middle column). Some 7 of 10 FV-N-vaccinated, FV-B-challenged mice had spleen virus detectable by this assay, but only FV-N vaccine virus was detected in those mice (right column). The FV-N detection results for one representative animal are shown.
Protection against relapse of disease by a live attenuated virus vaccine.
Since we had recently found that about one-half of a given group of mice with persistent FV infections could be induced to relapse with splenomegaly if they were depleted of CD4+ T cells (16), we used this technique to assess the efficacy of vaccinations in protection against persistent FV infections. Adult mice were vaccinated, rested for 1 month, and then challenged with a high dose of FV. The mice were rested again for 6 to 8 weeks to allow resolution of acute infection and were then immunosuppressed by injections of CD4-depleting MAbs. Four of sixteen of the animals vaccinated with the vvF-MuLV Env developed splenomegaly and lethal erythroleukemia, indicating that this vaccine was only poorly protective against persistent FV-B infection (Table 1). The relapsed mice had high levels of FV in their spleens, indicating reactivation of persistent FV-B challenge virus (data not shown). In addition to the four leukemic mice, three other animals in this group developed a mild and transient splenomegaly, suggesting a loss but regain of immunological control over persistent FV. The negative control group of animals vaccinated with the nonprotective vaccinia vector (vvF-MCF Env) showed 40% relapse after CD4 depletion. In contrast, no splenomegaly was induced by CD4 depletion in the animals vaccinated with live attenuated FV-N (Table 1). This was an extremely significant level of protection compared to that for the controls (P < 0.0001, Fisher’s exact test) and included a large number of mice from several independent experiments. Similarly, vaccination with F-MuLV helper alone also protected against reactivation of virus following in vivo CD4 cell depletions (Table 1).
TABLE 1.
Effect of various vaccines on protection against FV persistence
| Immunization | No. of relapses follow-ing CD4 depletion/ total no. (%)a | No. of vaccinees that transferred Friend disease to BALB/c/ no. totald |
|---|---|---|
| None | 15/38 (39) | 9/9 |
| vvF-MCF | 6/15 (40) | NT |
| vvF-MuLV Env | 4/16 (25)bc | NT |
| FV-N (virus complex) | 0/38 (0) | 0/7 |
| F-MuLV-N (helper virus)e | 0/10 (0) | 0/4 |
| Postexposure FV-N | 3/8 (37.5) | NT |
Numbers are from at least two independent experiments with similar results. The percentage of residual CD4+ cells in mononuclear blood cells ranged from 1 to 3% after antibody (MAb 191.1) depletion.
Beside the four leukemic mice, three additional animals developed a transient splenomegaly but later recovered.
Results from vvF-MuLV were not significantly different from those for vvF-MCF, the negative control vaccine (P = 0.365, Fisher’s exact test).
Results are the number of vaccinees which transferred Friend disease into BALB/c mice by transfer of 5 × 107 spleen cells. NT, not tested. Disease was monitored by palpation for splenomegaly in a blinded fashion as described elsewhere (14).
The N-tropic F-MuLV helper virus stock (3) was a 24-h supernatant from infected M. dunni cells.
To confirm that vaccination with live attenuated FV-N had prevented persistent infection with the pathogenic FV-B virus, we used transfer of spleen cells into naive mice of the highly susceptible BALB/c mouse strain. All of the BALB/c mice that received intravenous injections of 5 × 107 spleen cells from unvaccinated mice developed severe FV-induced splenomegaly and erythroleukemia (Table 1). However, when transfers were performed with donor mice that had been vaccinated with FV-N prior to challenge with pathogenic FV-B, there were no instances of splenomegaly (P < 0.0001 by Fisher’s exact test). Likewise, vaccination with F-MuLV helper virus alone was also sufficient to induce protection against persistent FV-B infection.
Finally, we tested whether postexposure vaccination with FV-N would stimulate the immune system and eliminate persistently infected cells. Five months after infection with FV-B, mice were vaccinated with FV-N. Following 1 month of rest, the mice were depleted of CD4 cells. Relapses of FV-induced splenomegaly in this group indicated the failure of postexposure vaccination to eliminate persistently infected cells (Table 1).
Passive transfer of virus-neutralizing antibodies.
One possible mechanism by which vaccination might have prevented establishment of persistence was by complete neutralization of the input virus through virus-neutralizing antibodies. In fact, FV-N vaccination induces such antibodies that are detectable before challenge (12). To investigate whether the presence of standing titers of antibody could completely prevent infection, we used passive transfers of an FV-neutralizing MAb known to completely neutralize the challenge virus in vitro and to mimic some of the effects of antiviral antisera in vivo (1, 15). Dosages were empirically determined such that plasma levels matched the titers found in vaccinated mice at the time point of challenge. At 2 weeks postinfection, all mice that received MAb 48 transfers had detectable ICs in their spleens (Fig. 3). By 7 weeks postinfection, six of eight mice had persistent FV detectable by the IC assay. Thus, the transfers of MAb 48 did not prevent initial infection or establishment of viral persistence.
DISCUSSION
The current results clearly show that vaccines which protect against acute disease do not necessarily protect against the establishment of a persistent infection with pathogenic retroviruses. Although vaccination with recombinant vvMuLV Env reduced the levels of persistent challenge virus, significant numbers of animals relapsed after immunosuppression. Thus, pathogenic retroviruses which evade vaccine-induced immune responses can become lifelong threats to the health of an infected host. We show here that, in principle, it is possible to protect against such threats by vaccination which appears to provide complete clearance of the virus.
Known differences between the immunological responses elicited by FV-N and those elicited by vvF-MuLV Env likely account for their differing abilities to protect against persistent virus. Vaccination by vvF-MuLV Env primes cytotoxic T-lymphocyte (CTL) and antibody responses, but only CD4+ T-cell responses are elicited and detectable prior to challenge with FV-B (12). In contrast, vaccination by FV-N elicits detectable CD8+ CTLs and neutralizing antibodies in addition to CD4+ T cells (12). The presence of a virus-neutralizing antibody titer at the time of FV challenge reduces the effective virus dose. However, our passive antibody transfer experiments show that the presence of a standing antibody titer alone does not totally prevent retroviral infection. Thus, the induction of CTLs by vaccination with FV-N is important because these effectors can kill cells infected by virus that escape antibody neutralization. It has been reported for lymphocytic choriomeningitis virus that only virus-specific CTLs are necessary to protect mice against persistent infection (27). A recent report suggests that this may be true for bovine leukemia virus as well (18). However, in the FV model we previously showed by adoptive transfer experiments that virus-specific CD4+ T cells and B cells were also needed in order to limit acute infection and prevent erythroleukemia (10). Since limiting the level of acute infection is probably a key element in preventing persistent infections, protection against FV persistence most likely requires equally complex immune responses.
Our current results may have relevance to vaccination against persistent human retroviruses such as HIV. However, little is known about the role of persistent HIV in disease pathogenesis, and it is possible that persistent HIV would pose no greater threat to its hosts than persistent FV does to immunocompetent mice. If so, vaccines which limit acute retrovirus replication might be protective in most cases. However, unlike FV, a major target for HIV infection is CD4+ T cells, and it is known that persistent infections can interfere with cellular functions (8). Thus, persistent HIV itself could be immunosuppressive and allow self-reactivations. Additionally, the well-established capacity of HIV to mutate and escape immunological control could result in the production of virulent substrains over time. As a caution against the safety of persistent HIV infections, it was recently reported that some patients in the Sydney Blood Bank Cohort infected long-term with an attenuated form of HIV have begun to show slow disease progression (11). Therefore, protection against persistence is likely to be a key element in a successful vaccination against retroviruses such as HIV.
ACKNOWLEDGMENTS
We are grateful to Bruce Chesebro, John Portis, and Byron Caughey for critical reading of the manuscript.
U.D. is supported by a fellowship from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Britt W J, Chesebro B. Use of monoclonal anti-gp70 antibodies to mimic the effects of the Rfv-3 gene in mice with Friend virus-induced leukemia. J Immunol. 1983;130:2363–2367. [PubMed] [Google Scholar]
- 2.Chesebro B, Bloom M, Wehrly K, Nishio J. Persistence of infectious Friend virus in spleens of mice after spontaneous recovery from virus-induced erythroleukemia. J Virol. 1979;32:832–837. doi: 10.1128/jvi.32.3.832-837.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesebro B, Britt W, Evans L, Wehrly K, Nishio J, Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of Friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983;127:134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- 4.Chesebro B, Wehrly K, Stimpfling J. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly. Mapping of a gene within the major histocompatibility complex. J Exp Med. 1974;140:1457–1467. doi: 10.1084/jem.140.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun T W, Engel D, Berrey M M, Shea T, Corey L, Fauci A S. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature (London) 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 8.de la Torre J C, Oldstone M B. Anatomy of viral persistence: mechanisms of persistence and associated disease. Adv Virus Res. 1996;46:311–343. doi: 10.1016/s0065-3527(08)60075-5. [DOI] [PubMed] [Google Scholar]
- 9.Dittmer U, Brooks D M, Hasenkrug K J. Characterization of a live-attenuated retroviral vaccine demonstrates protection via immune mechanisms. J Virol. 1998;72:6554–6558. doi: 10.1128/jvi.72.8.6554-6558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dittmer U, Brooks D M, Hasenkrug K J. Requirement for multiple lymphocyte subsets in protection against retroviral infection by a live attenuated vaccine. Nat Med. 1998;5:189–193. doi: 10.1038/5550. [DOI] [PubMed] [Google Scholar]
- 11.Dyer W B, Ogg G S, Demoitie M A, Jin X, Geczy A F, Rowland-Jones S L, McMichael A J, Nixon D F, Sullivan J S. Strong human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte activity in Sydney Blood Bank Cohort patients infected with nef-defective HIV type 1. J Virol. 1999;73:436–443. doi: 10.1128/jvi.73.1.436-443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earl P L, Moss B, Morrison R P, Wehrly K, Nishio J, Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986;234:728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- 13.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 14.Hasenkrug K J, Brooks D M, Robertson M N, Srinivas R V, Chesebro B. Immunoprotective determinants in Friend murine leukemia virus envelope protein. Virology. 1998;248:66–73. doi: 10.1006/viro.1998.9264. [DOI] [PubMed] [Google Scholar]
- 15.Hasenkrug K J, Brooks D M, Chesebro B. Passive immunotherapy for retroviral disease: influence of major histocompatibility complex type and T-cell responsiveness. Proc Natl Acad Sci USA. 1995;92:10492–10495. doi: 10.1073/pnas.92.23.10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasenkrug K J, Brooks D M, Dittmer U. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J Virol. 1998;72:6559–6564. doi: 10.1128/jvi.72.8.6559-6564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasenkrug K J, Brooks D M, Nishio J, Chesebro B. Differing T-cell requirements for recombinant retrovirus vaccines. J Virol. 1996;70:368–372. doi: 10.1128/jvi.70.1.368-372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hislop A D, Good M F, Mateo L, Gardner J, Gatei M H, Daniel R C, Meyers B V, Lavin M F, Suhrbier A. Vaccine-induced cytotoxic T lymphocytes protect against retroviral challenge. Nat Med. 1998;4:1193–1196. doi: 10.1038/2690. [DOI] [PubMed] [Google Scholar]
- 19.Hoatlin M E, Kabat D. Host-range control of a retroviral disease: Friend erythroleukemia. Trends Microbiol. 1995;3:51–57. doi: 10.1016/s0966-842x(00)88875-7. [DOI] [PubMed] [Google Scholar]
- 20.Jolicoeur P. The Fv-1 gene of the mouse and its control of murine leukemia virus replication. Curr Top Microbiol Immunol. 1979;86:67–122. doi: 10.1007/978-3-642-67341-2_3. [DOI] [PubMed] [Google Scholar]
- 21.Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- 22.Lander M R, Chattopadhyay S K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984;52:695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J-P, D’Andrea A D, Lodish H F, Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature (London) 1990;343:762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- 24.Moreau-Gachelin F, Tavitian A, Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukemia. Nature (London) 1988;331:277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- 25.Munroe D G, Peacock J W, Benchimol S. Inactivation of the cellular p53 gene is a common feature of Friend virus-induced erythroleukemia: relationship of inactivation to dominant transforming alleles. Mol Cell Biol. 1990;10:3307–3313. doi: 10.1128/mcb.10.7.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nash A A, Jayasuriya A, Phelan J, Cobbold S P, Waldmann H, Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987;68:825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- 27.Oldstone M B, Tishon A, Eddleston M, de la Torre J C, McKee T, Whitton J L. Vaccination to prevent persistent viral infection. J Virol. 1993;67:4372–4378. doi: 10.1128/jvi.67.7.4372-4378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliff A, Collins L, Mirenda C. Molecular cloning of Friend mink cell focus-inducing virus: identification of mink cell focus-inducing virus-like messages in normal and transformed cells. J Virol. 1983;48:542–546. doi: 10.1128/jvi.48.2.542-546.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliff A I, Linemeyer D, Ruscetti S, Lowe R, Lowy D R, Scolnick E M. Subgenomic fragment of molecularly cloned Friend murine leukemia virus DNA contains the gene(s) responsible for Friend murine leukemia virus-induced disease. J Virol. 1980;35:924–936. doi: 10.1128/jvi.35.3.924-936.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pincus T, Rowe W P, Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to Friend murine leukemia virus. J Exp Med. 1971;133:1234–1241. doi: 10.1084/jem.133.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson M N, Miyazawa M, Mori S, Caughey B, Evans L H, Hayes S F, Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and western blotting. J Virol Methods. 1991;34:255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- 32.Wendling F, Tambourin P E. Oncogenicity of Friend-virus-infected cells: determination of origin of spleen colonies by the H-2 antigens as genetic markers. Int J Cancer. 1978;22:479–486. doi: 10.1002/ijc.2910220418. [DOI] [PubMed] [Google Scholar]