Abstract
Previous investigations have shown that D. viscosa herbal extract is often used to treat a variety of diseases. Therefore, the purpose of this study was to investigate any additional potential impacts on rat liver and kidney damage induced by diabetes. Streptozotocin (STZ) (60 mg/kg/day) was given as a single dosage to cause type 1 diabetes. After then, diabetic rats received oral doses of D. viscosa for four weeks at 150 and 300 mg/kg/day. Blood, liver, and kidney tissues were collected at the end of the treatment and examined. Analysis was made of the serum lipid profile, liver, and kidney functions, as well as blood biochemistry. Moreover, the levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-1 beta (IL-1β), prostaglandin E-2 (PGE-2), and nitric oxide (NO) were estimated in serum. In liver and kidney samples, thiobarbituric acid reactive substances (TBARs) and reduced glutathione (GSH), as well as the pro-inflammatory cytokines and enzymatic activities of glutathione peroxidase (GPx), glutathione reeducates (GR), glutathione-S-transferase (GST), catalase (CAT), and superoxide dismutase (SOD) were analyzed. Histological changes in liver and kidney cross-sections were also observed. Our findings demonstrated that D. viscosa dramatically decreased pro-inflammatory indicators in blood, kidney, and liver tissues as well as blood glucose, and restored insulin levels, and lipid profiles. Additionally, it significantly raises the antioxidant enzyme activity SOD, CAT, GPx, and GST, while significantly lowering TBARs levels. The above-mentioned biochemical changes that took place in tissues were further supported by histological alterations. These findings imply that D. viscosa protects against STZ-induced hyperglycemia, aberrant lipid synthesis, and oxidative stress and that these benefits may be mediated by interacting with various targets to increase the levels of antioxidant enzymes in the liver and kidneys. Its mode of action and safety for use as medicine against various metabolic problems caused by diabetes require more research.
Keywords: Dodonaea viscosa, Diabetes, Streptozotocin, Oxidative stress, Inflammation
1. Introduction
Diabetes mellitus (DM) has emerged as a significant global challenge in healthcare delivery, particularly recently, with the worldwide incidence reaching epidemic proportions (Fouad, et al. 2023). It is reported to be associated with several serious complications that affect most bodily systems, including hepatotoxicity, neuropathy, and cardiovascular and renal disorders, because of hyperglycemia and glucose intolerance (Targher, 2020, Wang et al., 2020). During DM, deficiency of intracellular glucose in hepatic cells results in damaging and necrotic effects, which promote the apoptotic pathway (Francés, et al. 2013). Pro-inflammatory responses in hepatic cells during DM were reported (Lee, et al. 2019). Oxidative stress and reactive oxygen species (ROS) formation are considered essential factors in the pathogenesis of diabetic hepatotoxic injuries (Abulikemu, et al. 2023). During this event, the intracellular generation of free radicals exceeds the hepatocytes’ antioxidant capacity, leading to lipid peroxidation and oxidative necrosis. Moreover, the hepatocytes’ injury results in complex biological events, including neutrophil infiltration, and activation of Kupffer cells. Such a response induces apoptotic pathways and the release of inflammatory cytokines (Zeng, et al. 2019). In type 1 diabetes, the incidence of diabetic nephropathy (DN) was about 15%–25%, contributing to complications and a high mortality rate (Callaghan, et al. 2012). One vital pathophysiological feature in DN is oxidative stress, which can be attributable to hyperglycemia and involves the augmented production of reactive oxygen species (ROS) (Asbun and Francisco, 2006, Oluleye, 2010). Hyperglycemia intends to decrease antioxidative enzymatic activities and increase lipid peroxidation (LPO), and changes found in the glutathione redox state (Naoom, et al. 2023). TNF-α, IL-6, and IL1β are inflammatory cytokines that are linked to increased oxidative stress and aid in the development of diabetic nephropathy (Moin, et al. 2023). Basement membrane thickness loss, mesangial expansion, glomerular podocyte destruction, and microalbuminuria are all symptoms of DN (Fakhruddin, et al. 2017), as well as extracellular matrix (ECM) protein aggregation (Kolset, et al. 2012). This increase in inflammatory factors disrupts the systemic immunological processes in addition to causing inflammation of the kidneys. In addition, replication factors that promote apoptotic, fibrotic, and inflammatory processes that contribute to cell damage and other problems have been linked to excessive hyperglycemia and oxidative stress (Singh, et al. 2014).
Dodonaea viscosa (L.) Jacq (Sapindaceae) is a flowering evergreen shrub. Dodonaea is a genus of 60 species widely distributed in warmer parts of Australia, South Africa, North America, and South Asia (Muhammad et al., 2016b, Muhammad et al., 2016a). Herbal remedies using plant extracts were universally used before the development of current medicine (Hamza et al., 2022, SF et al., 2022), but they are still used in many countries to treat common ailments (Behrouj et al., 2015, Nasiri et al., 2014). Numerous phytochemicals, including flavonoids, terpenoids, saponins, sterols, phenolics, sugars, alkaloids, and tannins, had been reported to be present in Dodonaea viscosa (Al-Snafi, 2017, Khan et al., 2021, Shahzadi et al., 2012). Several particular phytochemicals, including C-alkylated flavonol derivatives, are present in D. viscosa, according to a literature review (Ali, et al. 2014) but hautriwaic acid is thought to be the main active ingredient responsible for the anti-inflammatory effect (Salinas-Sánchez, et al., 2012b, Salinas-Sánchez et al., 2012). Isoprenylated flavonol derivatives named dodoviscins are also reported (Gao et al., 2013, Zhang et al., 2012). Terpenoids were also obtained from D. viscosa, monoterpenoids such as β-pinene, limonene, myrcene, p-cymene, citronellal, linalool, linalyl acetate, γ-terpineol, and geraniol (Al-Snafi 2017). For centuries, D. viscosa has been used traditionally in the treatment of rheumatism, gout, diarrheas, fractures, snake bites, hepatic or splenic pain, smooth muscles disorders (uterine pain), skin infections (dermatitis), hemorrhoids, sore throat (Muhammad, et al. 2016a), as roundworms repellent (Lawal and Yunusa 2013) and anti-malarial drug (Mothana, et al. 2010). Additionally, researches on D. viscosa found it to have cytotoxic, anti-inflammatory, and antioxidant properties (Mothana, et al. 2010), antibacterial, antiviral (Asres, et al. 2001), hepato-protective (Ali, et al. 2014), anxiolytic, anticonvulsant (Karim, et al. 2015), gastro-protective (Arun and Asha 2008) and antidiabetic (Uddin, et al. 2018). With an emphasis on its antioxidant, anti-inflammatory, and anti-hyperlipidemic activities, this study aimed to investigate the possible protective effects of D. viscosa extract against STZ-induced hepatotoxicity and nephrotoxicity using an animal model of diabetes by focusing on oxidative stress and inflammatory markers.
2. Materials and methods
2.1. Animals:
Forty male albino Wistar rats weighing between 250 and 270 g (8–9 weeks old) were obtained from King Saud University's Pharmacy College Animal Care Center. Before beginning the trials, all received animals had a 10-day acclimatization period. The rats were kept under normal circumstances, including a constant 22 ± 1 °C temperature, 50–55% humidity, and 12-hour day/night cycles. The King Saud University Ethics Committee approved this animal study, permit number (SE-19–147), and all aspects of the experimental protocol, including the euthanasia technique, blood sample, and ultimate sacrifice, were done following the National Institutes of Health's guideline care policy (NIH, 1996).
2.2. Chemicals and materials
STZ was purchased from Sigma-Aldrich, Inc., (St. Louis, MO, USA). All other chemicals, ELISA kits, and reagents were of the highest analytical grade commercially available.
2.3. Diabetes induction:
Streptozotocin (STZ) (65 mg/kg) dissolved in citrate buffer (pH 4.5) was injected intraperitoneally once to cause type 1 diabetes in rats. Control animals received the same injection volume of citrate buffer without STZ. Using an Accu Chek Compact Plus glucose meter system (Roche Diagnostics, Meylan, France), the blood glucose levels of the fasted rats were measured 48 h later. Animals with blood glucose levels>250 mg/dL were classified as diabetic models.
2.4. Plant collection and extraction:
3.0 kg of D. viscosa leaves were procured in January 2019 from the KSU College of Pharmacy Botanical Garden in Riyadh, Saudi Arabia. Dr. Mohammed Yusuf, a field taxonomist with the King Saud University College of Pharmacy's Department of Pharmacognosy, verified the sample's identity. A voucher specimen (voucher #15787) has previously been deposited at the herbarium at the pharmacy school at KSU in Riyadh, Saudi Arabia. The procedure for extracting and analyzing the components of D. viscosa leaves was done as previously described (Khan, et al. 2021).
2.5. Primary phytochemical screening:
To screen for phytochemicals, the procedures outlined by Kokate were used (Kokate 2014). In a nutshell, the phenolic chemicals were found using the ferric chloride test and the lead acetate test. By employing the Shinodas test and the alkaline reagent test, flavonoids in the extract were found. Dragendorff's test was used to identify alkaloids, and the Salkowsky test was used to identify terpenoids. The Libermann-Burchards test's procedures were used to identify the steroids, saponins, and anthraquinones. Molish's test was used to find the presence of carbohydrates. By employing a ferric chloride test to identify glycosides, and ninhydrin to identify the presence of amino acids. The essential oils in the D. viscosa extract were checked using a spot test, and the presence of gum and mucilage was determined using 100% methanol. The ferric chloride test was the last step in determining the presence of tannins.
2.6. Experimental design and procedures
Eight rats from each group of diabetic rats were randomly assigned to one of three groups. Rats were used in group 1 as controls, and they received vehicles (Control). Diabetic rats from group 2 were given a vehicle-based treatment known as STZ. Diabetic rats in group 3 were given D. viscosa 150 mg/kg/day orally under the name (Dv150). Diabetic rats were treated with D. viscosa 300 mg/kg/day orally in the final group (Dv 300). Following the introduction of diabetes, the extracts were initiated one week later and continued for four weeks. The CMC solution (0.25% carboxymethyl cellulose sodium, CMC) was used to suspend the D. viscosa extract. Animals in the control and STZ groups received the same amount of CMC solution during the whole treatment period. Blood samples were obtained via heart puncture and centrifuged at 4,000 rpm (1.8 g) for 10 min. under the anesthesia induced by intraperitoneal injection of ketamine (92 mg/kg, Hikma Pharmaceuticals, Amman, Jordan) and xylazine (10 mg/kg, Bayer, Turk). Each tube's serum supernatant was then kept for analysis at −20 °C. The animals were then euthanized, and liver and kidney tissues were removed from each animal and stored in 10% formalin for histopathology. A second, smaller cross-section of each tissue was placed in liquid nitrogen for a minute before being placed in a freezer at −80 °C.
2.7. Biochemical, oxidative stress, and inflammation assays
Using commercially available diagnostic kits, the levels of serum glucose, insulin, pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), creatinine, urea, total cholesterol (TC), total triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were measured (Human, Wiesbaden, Germany). Also, using rat ELISA kits, the levels of pro-inflammatory biomarkers such as TNF-α, IL-1β, IL-6, caspase-3, NO, and PGE-2 were determined according to the provided guidelines (R&D Systems Inc., USA). The physiological lysis buffer was used to homogenize the liver and kidney tissues (1:10, w/v). Diagnostic kits were used to measure the concentrations of TBARS and GSH (Cayman Chemical Co., USA). The enzymatic activity of SOD, CAT, GPx, GR, and GST in post-mitochondria supernatants of hepatic and renal tissues was assessed using assay kits (R&D Systems Inc., USA). TNF-α, IL-1β, and IL-6 levels were also assessed in hepatic and renal-prepared tissue samples using rat ELISA kits according to the provided protocol (R&D Systems Inc., USA).
2.8. Hepatic and renal histopathology analysis
After fixation in 10% neutral buffer formalin, liver, and kidney specimens were cut into segments, embedded in squares of paraffin wax, and then sections of thickness 5 µm were cut using a Leica CM3050 S Research Cryostat (Leica Bio-systems, USA). The liver and kidney slices were mounted, H&E stained, and subjected to a histopathologist's blind examination for histological alterations.
2.9. Statistical Analysis:
The current study results were presented as mean ± SE. One-way ANOVA was used for statistical analysis followed by the Newman-Keuls multiple comparison test by using Graph-Pad Prism version 5 software. a Control vs STZ group; bSTZ vs D. viscosa (1 5 0) or D. viscosa (3 0 0). P values consider significant when *P < 0.05, **P < 0.01 and ***P < 0.001.
3. Results:
Preliminary phytochemical screening of crude extract of D. viscosa leaves revealed the existence of a variety of phytochemicals such as phenolic compounds, flavonoids, triterpenoids, steroids, saponins, etc. However, the test for alkaloids and anthraquinones showed negative results (Table 1).
Table 1.
Phytochemical constituents present in the crude extract of D. viscosa.
| CLASS | Test | RESULT |
|---|---|---|
| Phenolic compounds |
Lead acetat test Ferric chloride test |
+ |
| Flavonoids |
Shinoda test Alkaline reagent test |
+ |
| Terpenoids | + | |
| Alkaloids | Dragendorff’s test | – |
| Steroids |
Libermann-Burchards test | + |
| Saponins | + | |
| Anthraquinones | _ | |
| Carbohydrates |
Molishs test |
+ |
| Glycosides |
Ferric chloride test | + |
| Amino Acid |
Ninhydrine test | + |
| Essential Oils | + | |
| Gum and Mucilages | + | |
| Tannins |
Ferric chloride test | + |
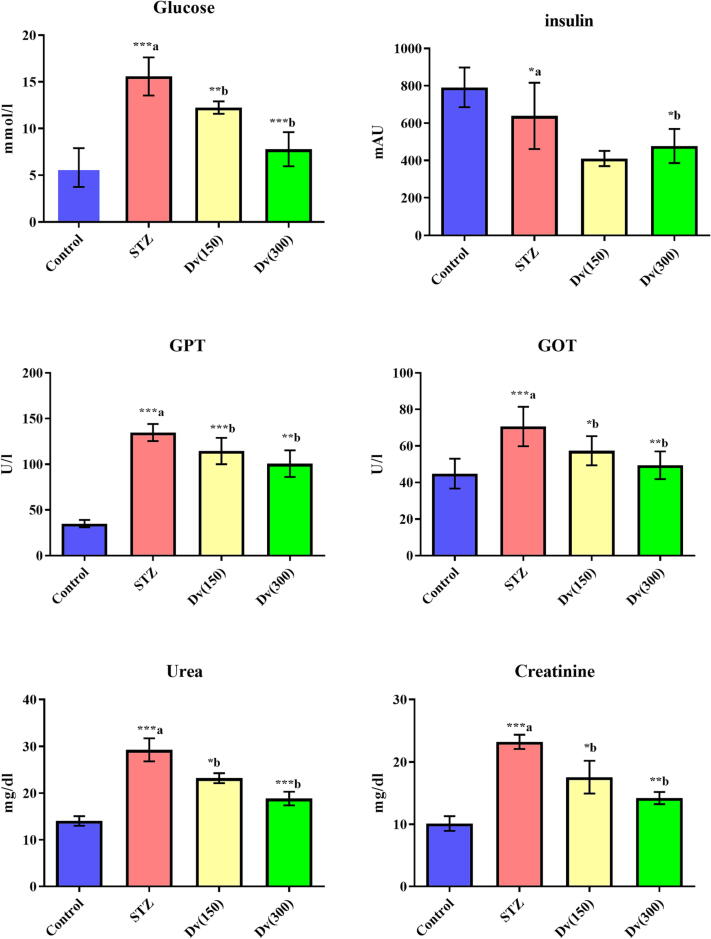
In diabetic rats, levels of blood glucose, GPT, GOT, urea, and creatinine were significantly (p < 0.001) increased while the insulin levels were decreased (p < 0.05) compared to control animals. Treatment of the diabetic rats with 150 and 300 mg/kg D. viscosa significantly reduced the blood glucose (p < 0.01 and p < 0.001; respectively), GPT (p < 0.001 and p < 0.01; respectively), GOT (p < 0.05 and p < 0.01; respectively), urea (p < 0.05 and p < 0.001; respectively) and creatinine (p < 0.05 and p < 0.01; respectively) levels as compared with STZ group. The decreased serum insulin levels in diabetic rats were significantly (p < 0.05) enhanced by the D. viscosa treatment in diabetic animals (Fig. 1).
Fig. 1.
Effect of D. viscosa on diabetic-induced changes in serum levels of glucose, insulin, GPT, GOT, urea and creatine.
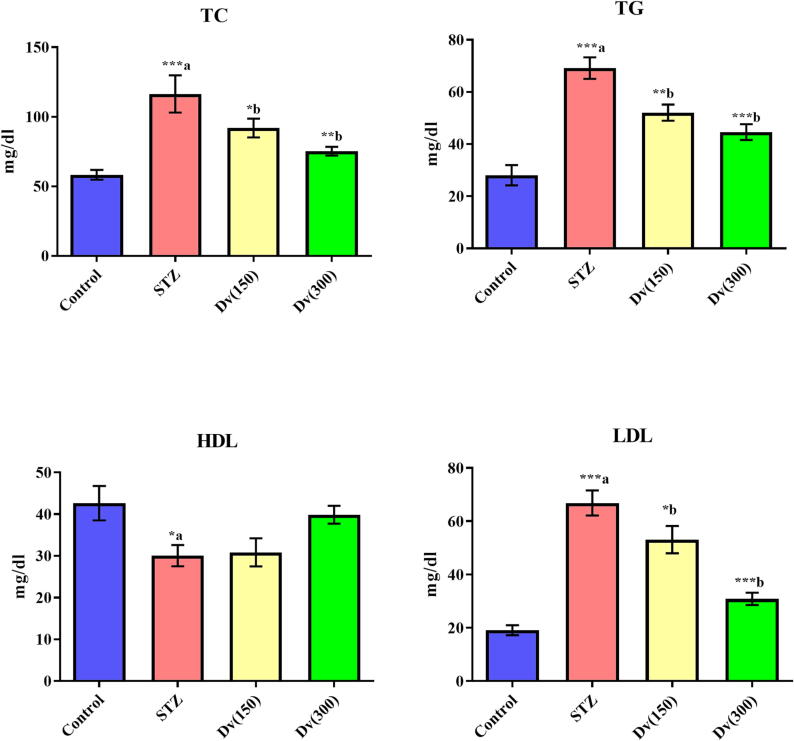
TC, TG, and LDL serum levels were markedly (p < 0.001) elevated in diabetic rats compared to controls, although HDL levels were significantly (p < 0.05) inhibited in the STZ group. Treatment with D. viscosa (150 and 300 mg/kg) produced a marked inhibition in serum levels of TC (p < 0.05 and p < 0.01; respectively), TG (p < 0.01 and p < 0.001; respectively), and LDL (p < 0.05 and p < 0.001; respectively) compared to STZ group. However, no statistically significant change was seen in HDL serum levels between rats treated with D. viscosa (150 and 300 mg/kg) and the STZ group (Fig. 2).
Fig. 2.
Effect of D. viscosa on diabetic-induced changes in serum levels of total cholesterol (TC), triglycerides (TG), high density lipoprotein-cholesterol (HDL-C) and low density lipoprotein-cholesterol (LDL-C).
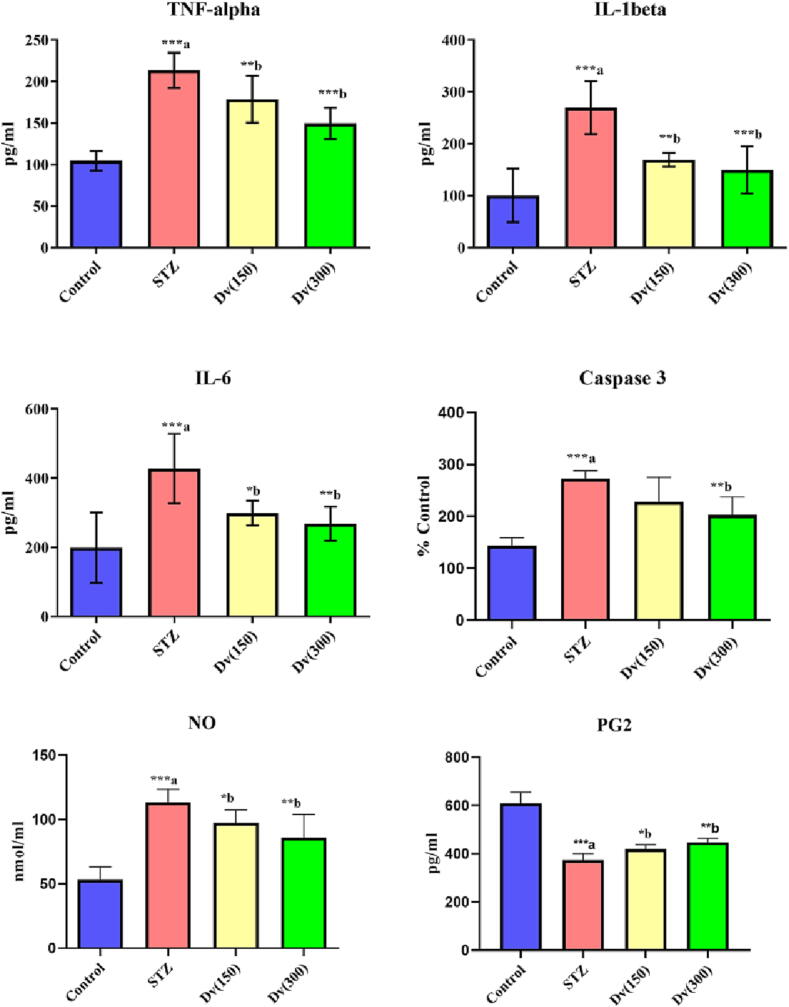
There was a substantial elevation (p < 0.001) in serum levels of cytokines such as TNF-α, IL-6, IL-1β, caspase-3, NO, and PG-2 in the diabetic rats compared to the control group of rats. Diabetic rats treated with both doses (150 and 300 mg/kg) of D. viscosa showed a marked diminishing in the serum expressions of TNF-α (p < 0.05 and p < 0.01; respectively), IL-1β (p < 0.01 and p < 0.001; respectively), caspase-3 (p < 0.05 and p < 0.01; respectively), NO (p < 0.05 and p < 0.01; respectively) and PG-2 (p < 0.05 and p < 0.01; respectively) compared to STZ group (Fig. 3).
Fig. 3.
Effect of D. viscosa on diabetic-induced changes in serum inflammatory biomarkers including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), caspase-3, nitric oxide (NO) and prostaglandin-2 (PG2) levels.
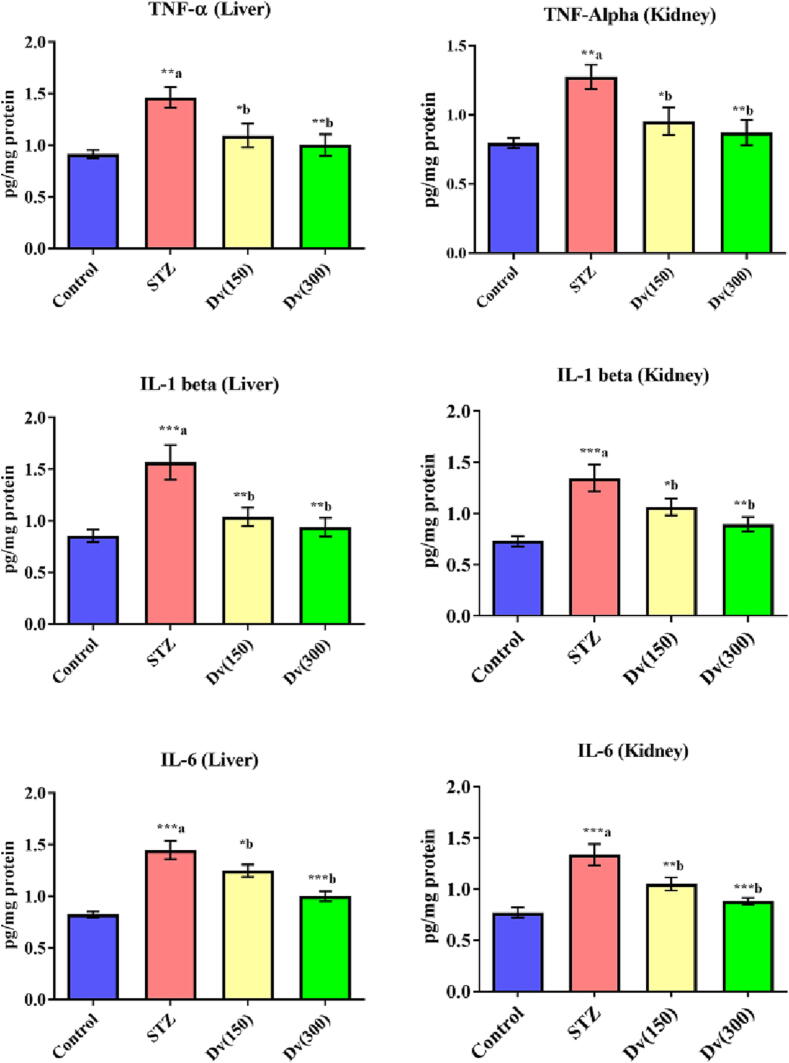
Homogenates of the liver and kidney of diabetic rats showed a notable boost in the levels of the pro-inflammatory cytokine, including TNF-α (p < 0.01), IL-6 (p < 0.001), and IL-1β (p < 0.001) as compared with a non-diabetic group (Fig. 4). Treatment of the diabetic rats with 150 and 300 mg/kg D. viscosa markedly repaired the hepatic expression of TNF-α (p < 0.05 and p < 0.01; respectively), IL-1β (p < 0.01 and p < 0.01; respectively), and IL-6 (p < 0.05 and p < 0.001; respectively) in diabetic animals as compared with the diabetic untreated group. A similar effect by D. viscosa treatment was seen on renal pro-inflammatory cytokine levels TNF-α (p < 0.05 and p < 0.01; respectively), IL-6 (p < 0.01 and p < 0.001; respectively), and IL-1β (p < 0.05 and p < 0.01; respectively) in diabetic rats when compared with controls (Fig. 4).
Fig. 4.
Effect of D. viscosa on diabetic-induced changes in hepatic and renal levels of tumor necrosis factor-alpha (TNF-α), interleukine-1beta (IL-1β) and interleukine-6 (IL-6).
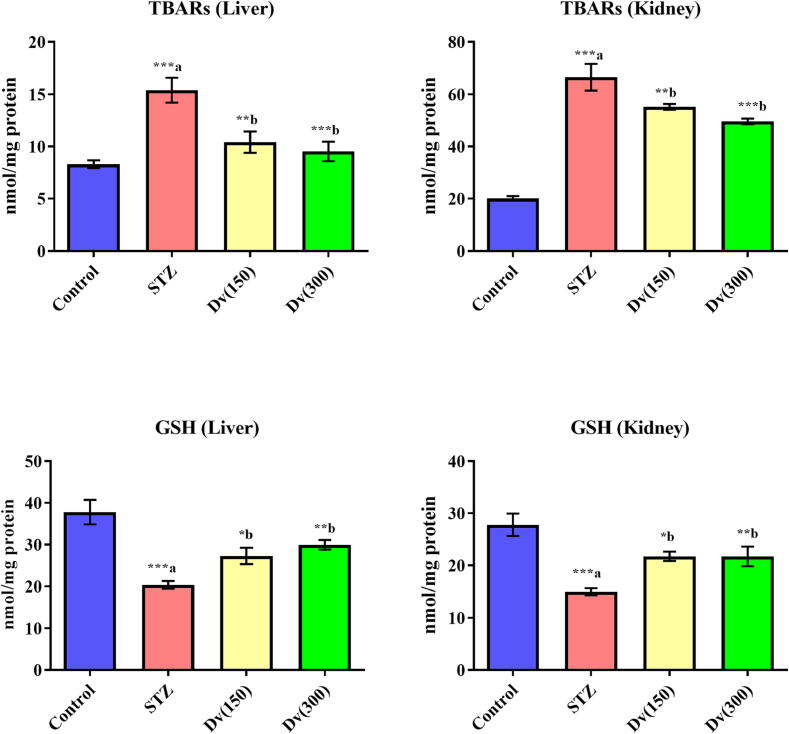
The lipid peroxidation biomarker TBARS levels were drastically (p < 0.001) enhanced, whereas the endogenous antioxidant known marker GSH levels were notably (p < 0.001) decreased as compared to the non-diabetic rats. The performed treatment with D. viscosa (150 and 300 mg/kg) in diabetic rats revealed significant inhibition in TBARS levels (p < 0.01 and p < 0.001; respectively) and increased GSH levels (p < 0.05 and p < 0.01; respectively) in hepatic and renal cells compared to the STZ group (Fig. 5).
Fig. 5.
Effect of D. viscosa on diabetic-induced changes in hepatic and renal levels of thiobarbituric acid reactive substances (TBARs) and glutathione (GSH).
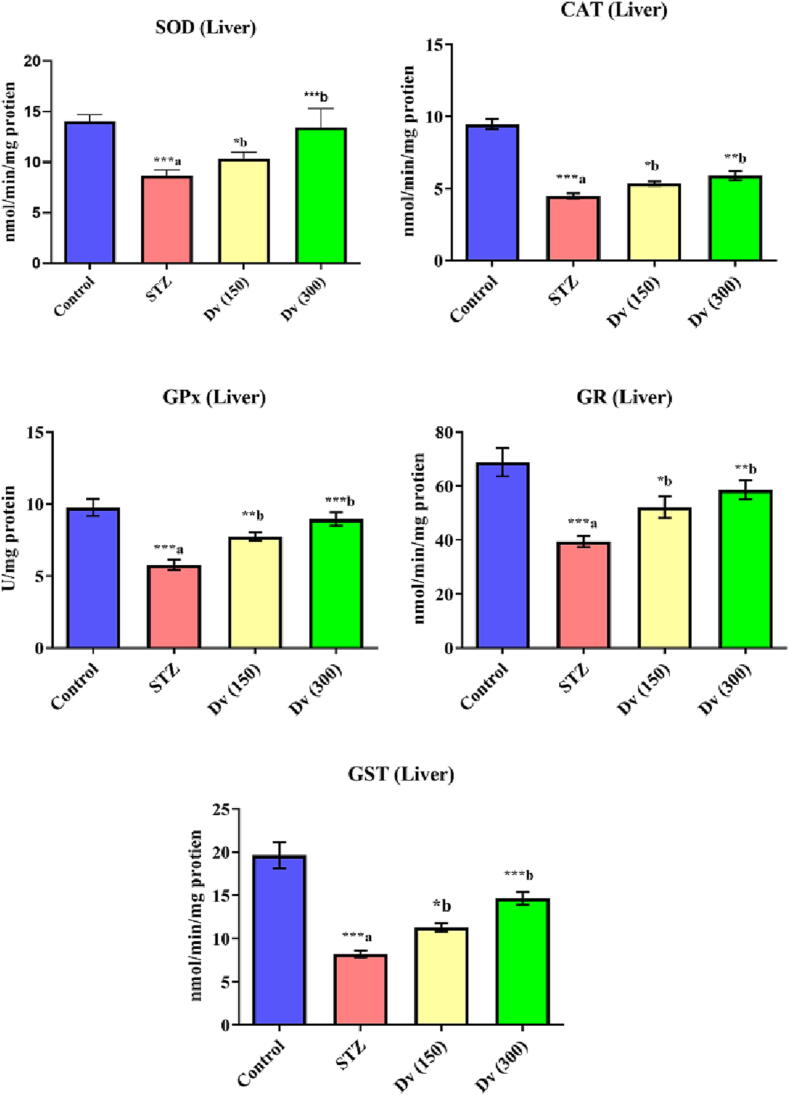
The enzymatic activities of SOD, CAT, GPx, GR, and GST in post mitochondria supernatant of diabetic rat’s hepatic cells showed a significant decrease (p < 0.001) compared to non-diabetic rats. Treatment of the diabetic rats with 150 and 300 mg/kg D. viscosa markedly increased the hepatic enzymatic activities of SOD (p < 0.05 and p < 0.001; respectively), CAT (p < 0.05 and p < 0.01; respectively), GPx (p < 0.01 and p < 0.001; respectively), GR (p < 0.05 and p < 0.01; respectively), and GST (p < 0.05 and p < 0.001; respectively) as compared with STZ group (Fig. 6).
Fig. 6.
Effect of D. viscosa on diabetic-induced changes in liver enzymatic activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione-S-transferase (GST).
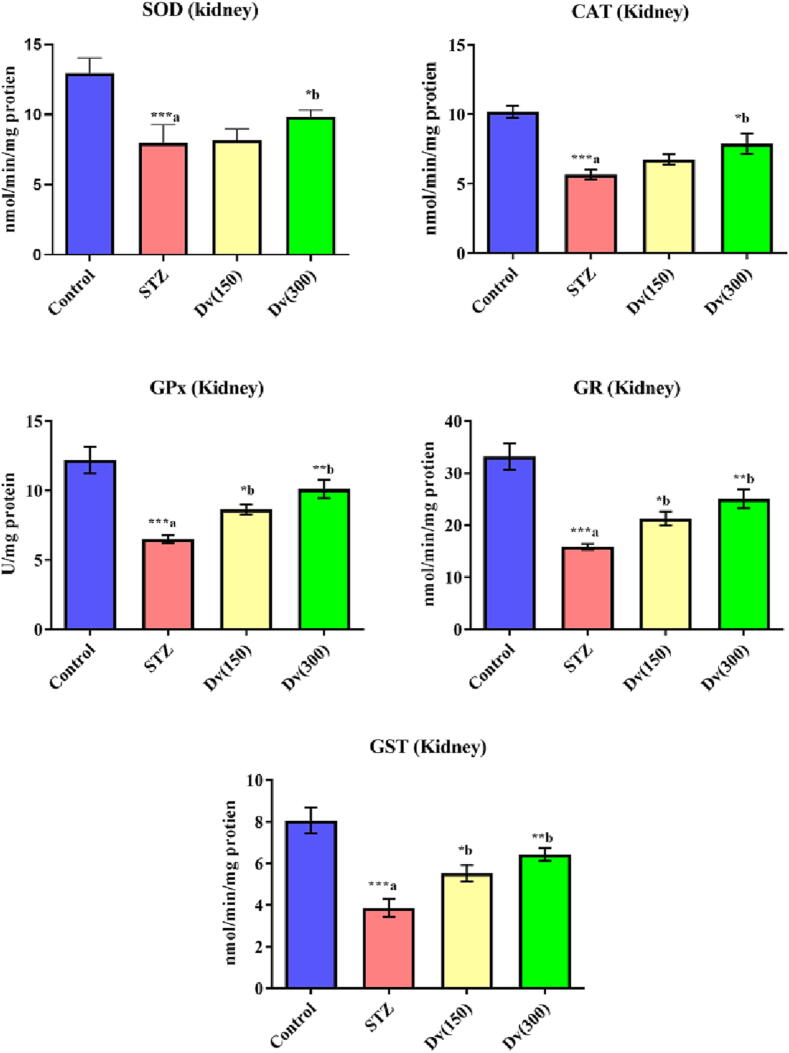
The renal enzymatic activities of SOD, CAT, GPx, GR, and GST in diabetic rats exhibited a substantial reduction (p < 0.001) compared to control animals. Treatment of the diabetic rats with 150 and 300 mg/kg D. viscosa markedly increased the hepatic enzymatic activities of SOD (p > 0.05 and p < 0.001; respectively), CAT (p > 0.05 and p < 0.01; respectively), GPx (p < 0.05 and p < 0.01; respectively), GR (p < 0.05 and p < 0.01; respectively), and GST (p < 0.05 and p < 0.001; respectively) as compared with STZ group (Fig. 7).
Fig. 7.
Effect of D. viscosa on diabetic-induced changes in renal enzymatic activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione-S-transferase (GST).
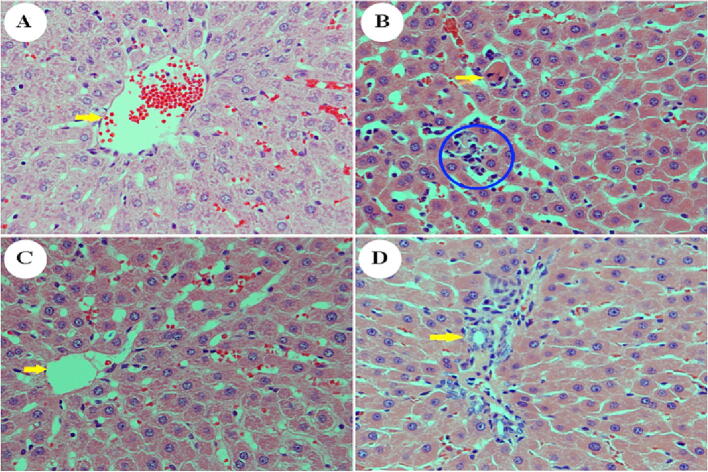
Histological analysis of the liver sections from non-diabetic animals exhibited normal architecture of hepatocytes cells with well-preserved cytoplasm, distinguished nucleus, nucleolus, and visible central veins (Fig. 8A). The liver sections of diabetic untreated animals revealed moderate steatosis, fatty degeneration, inflammatory infiltration, and necrotic hepatocytes (Fig. 8B). However, the histopathologic observations of the liver tissue section of animals treated with D. viscosa (150 mg/kg) showed regeneration of hepatocytes with a lack of fatty lobulation and hepatocyte necrosis (Fig. 8C). Moreover, the histological architecture of the liver section of the animals treated with D. viscosa (300 mg/kg) exhibited significant liver protection as evidenced by normal hepatocytes and the absence of necrosis and fatty infiltration (Fig. 8D).
Fig. 8.
Effect of D. viscosa extract (150 mg/kg and 300 mg/kg) on STZ-induced pathological changes in the liver as visualized with H&E staining, 400X. Liver section from the control group (A). Note the presence of the central vein (arrowhead) and surrounding normal hepatocytes. Photomicrograph from a liver section of the STZ-treated group (B). Note the presence of lobular inflammation (blue circle) and scanty necrotic hepatocytes (arrowhead). A liver section was obtained from the group which received D. viscosa (150 mg/kg) (C). Note the absence of hepatocyte necrosis, inflammation, and fatty changes liver section obtained from the group of animals who received D. viscosa (300 mg/kg) (D). Note the presence of a portal tract surrounded by normal hepatocytes. The arrowhead points toward a bile duct.
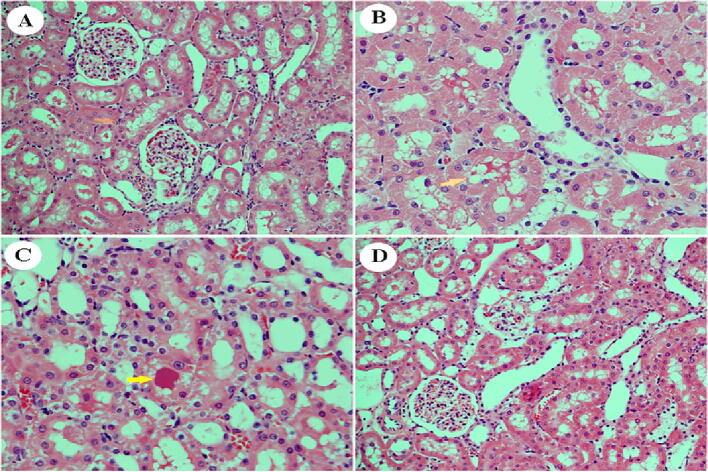
Section from the renal cortex of the non-diabetic group shows the normal architecture of the proximal convoluted tubules (PT), distal convoluted tubules (DT), Bowman's capsule, and glomerulus (G) (Fig. 9A). The kidney of untreated diabetic rats showed renal injury in the form of extreme vacuolation in the renal tubular cells and the loss of nuclei because of karyolysis (Fig. 9B). The renal cortex of diabetic animals treated with D. viscosa (150 mg/kg) showed partial protection. Note the absence of tubular necrosis but with some residual hyaline casts (Fig. 9C). Kidneys of diabetic animals treated with D. viscosa (300 mg/kg) showed that the harmful effects of STZ on kidneys in the glomeruli and renal tubules were amended (Fig. 9D).
Fig. 9.
Effect of D. viscosa extract (150 mg/kg and 300 mg/kg) on STZ-induced pathological changes in the kidney as visualized with H&E staining, 400X. Section from the renal cortex of the control group shows the normal architecture of the proximal convoluted tubules (PT), distal convoluted tubules (DT), Bowman's capsule, and glomerulus (G) (A). Kidneys of untreated diabetic rats showed renal injury in the form of extreme vacuolation in the renal tubular cells (arrowhead) and the loss of nuclei because of karyolysis (B). The renal cortex of diabetic animals co-treated with D. viscosa (150 mg/kg) showed partial protection. Note the absence of tubular necrosis but with some residual hyaline casts (arrowhead) (C). Kidneys of diabetic animals co-treated with D. viscosa (300 mg/kg showed that the adverse effects of STZ on kidneys in the glomeruli and renal tubules were amended (D).
4. Discussion
This study demonstrated that daily administration of D. viscosa herbal extract can reduce the diabetes complications of STZ-induced in rats. Presently used experimentally-induced diabetic model following a single STZ injection is more frequently used to evaluate the effect of new antidiabetic pure compounds and natural products in rats (Deeds et al., 2011, Wang-Fischer and Garyantes, 2018). In our study, rats that were daily treated with D. Viscosa showed potential beneficial effects by controlling hyperglycemia. DM is well documented for inducing complications in vital organs such as the liver and kidneys resulting in decreasing their metabolic functions. According to a previous study, ongoing hyperglycemia will result in oxidative stress and the production of free radicals, leading to impairment of intracellular integrity and cellular functions (Papachristoforou, et al. 2020). D. viscosa leaves contain a wide range of Phytochemical constituents which coincides with a previously published report by Venkatesh, et al (Sama, et al. 2008). Phenolic compounds and flavonoids possess a variety of pharmacological activities ranging from antifungal, antioxidant, and antibacterial (Teffo et al., 2010, Xu et al., 2018). In addition, Terpenoids exhibit antioxidant and anticholinesterase effects (Muhammad, et al. 2016a). In addition, the present plant content of steroid and saponins play an important role in biological activities like antityrosinase, antibacterial, antioxidant, and cytotoxicity (Akwu et al., 2020, Muhammad et al., 2017). Recently (Cheng, et al) have demonstrated the anticancer activity of these bioactive constituents (Cheng, et al. 2019).
Hepatotoxicity is known to raise the levels of liver enzymes including GPT and GOT. Similarly, renal markers including serum creatinine and urea (BUN) also increase due to renal toxicity or complications. Our results exhibited a significant increase in the GPT, GOT, urea, and creatinine levels in diabetic rats which is in agreement with previous studies (Al-Attar and Zari, 2010, Salahuddin et al., 2010). In addition, it has been shown that STZ-induced DM is associated with a significant decline in hepatic and renal functions that improved upon D. viscosa daily treatment (Yazdi, et al. 2019). This improvement in hepatic and renal parameters may refer to the active constituents like flavonoids and polyphenols in D. viscosa which enhance liver and kidney metabolic functions. The ethanolic extract of D. viscosa that we screened for phytochemical analysis produced flavonoids, phenolic compounds, terpenoids, steroids, and tannins. Disruption of the serum lipid profile is one of the frequent side effects of uncontrolled diabetes mellitus. It is commonly prevalent that diabetics patients would have an elevated level of cholesterols, lipoproteins, and triglycerides (Ginsberg, et al. 2005). In our study, STZ treatment induced serum dyslipidemia as evidenced by the increased levels of cholesterol, triglyceride, and LDL levels while the levels of HDL, which facilitate the transport of lipids from tissue to liver for disposal, were significantly lower compared to normal un-diabetic rats. Oral treatment with herbal extract D. viscosa counteracts the detrimental effects of uncontrolled glucose levels on lipid profile. In our study, D. visoca was able to restore the levels of insulin which are known to inhibit hormone-sensitive lipase (HSL) leading to a decrease in the release of free fatty acid from adipose tissue. This suggests that D. viscosa acts by controlling glucose levels in diabetic rats by restoring insulin secretion might keep the lipid profile within normal range.
Similar observations have been made by Ahmad et al. who explored the anti-hyperlipidemic and hepato-protective activity of D. viscosa leaves extracts in alloxan-induced diabetic rabbits (Ahmad, et al. 2012). However, the reno-protective effect of D. viscosa has not been studied yet, the current study has shown the protective activity of D. viscosa ethanoic extract against STZ-induced renal damage as indicated by improvement in renal functions and kidney histopathology. It was documented that D. viscosa protects the systemic, hepatic, and renal tissues from the harmful effects of hyperglycemia in diabetic rats. Treatment with D. viscosa in diabetic rats significantly decreased liver and kidney inflammatory markers such as TNF-α, IL-6, and IL-1 β and restored the integrity of particular construction and nephropathy growth. Our data emphasize that STZ induce diabetes was associated with elevated levels of TNF-a IL-1β and IL-6 in the liver and kidney. These results are consistent with earlier publications. (Alqahtani et al., 2020, Mohany et al., 2020). These vital organs are primarily susceptible to the effects of hyperglycemia-induced inflammation leading to tissue damage. It has been proven that TNF-α is crucial for the development of diabetic-induced kidney damage. (Navarro and Mora-Fernández 2006). Daily administration of D. viscosa for 30 days significantly lowered pro-inflammatory markers in treated rats. D. viscosa revealed abrogation of diabetic-induced proinflammatory markers, potentially as a consequence of D. viscosa role in adjusting blood glucose levels and restoring insulin secretion. The reported favorable effects of D. viscosa could be mediated by the anti-inflammatory and antioxidant properties of the active constituents present in D. viscosa. These results agree with previous findings that showed the anti-inflammatory activity of D. viscosa extract (Getie et al., 2003, Khalil et al., 2006; Salinas-Sánchez, et al. 2012a). In addition, caspase-3 levels were upregulated in diabetic animals suggesting the involvement of apoptotic pathways. The anti-inflammatory effect of D. viscosa extract may be related to the inhibition of the synthesis of prostaglandin-E2 and caspase-3 levels that we found in the present study. An earlier study showed that the levels of caspase-3 were elevated in diabetic rats, indicating that apoptotic pathways may be involved. Histological evidence shows that these inflammatory responses combined have detrimental consequences on liver tissues. The same STZ animal model of DM revealed fatty degeneration and inflammatory cell infiltrates in diabetic mice that were not treated (Alotaibi, et al. 2019). Our findings also showed that giving D. viscosa to STZ-induced diabetic rats drastically enhanced the levels of important antioxidant enzymes like GPx, CAT, SOD, GR, and GST.
Overall, our research has shown that D. viscosa has protective benefits against the pathogenesis of altered glucose metabolism brought on by STZ-induced diabetes by reducing oxidative tissue injury and inflammation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation,"Ministry of Education" in Saudi Arabia for funding this research work through the project no. (IFKSUOR3-346-1).
Authors’ contributions
All authors participated in the design, interpretation, analysis, and reviewing the manuscript. Ali Alqahtani and Perwez Alam arranged the plant material, authenticated it, and made phytochemical analysis and extraction. Ahmed Alanazi, Mohamed Mohany, Khalid Alhazzani, Khaled Alhosaini, Homood Sobeai, Omer Almarfadi, Mohammed Ahmed, Sary Alsanea, Mohammed Alqinyah, Hussain Alhamami, and Salim Al-Rejaie have made substantial contributions in animal treatment, biochemical analysis and interpretation of the data and writing the manuscript. Mohamed Mohany and Mohammed Almutery also contributed to histopathological preparations and slide screening.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
References
- Abulikemu A., et al. Silica nanoparticles aggravated the metabolic associated fatty liver disease through disturbed amino acid and lipid metabolisms-mediated oxidative stress. Redox Biol. 2023;59 doi: 10.1016/j.redox.2022.102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M., et al. Antihyperlipidaemic and hepatoprotective activity of Dodonaea viscosa leaves extracts in alloxan-induced diabetic rabbits (Oryctolagus cuniculus) Pak Vet J. 2012;32(1):50–54. [Google Scholar]
- Akwu, Nneka, et al. 2020 Isolation of lupeol from Grewia lasiocarpa stem bark: Antibacterial, antioxidant, and cytotoxicity activities. 21(12).
- Al-Attar A.M., Zari T.A. Influences of crude extract of tea leaves, Camellia sinensis, on streptozotocin diabetic male albino mice. Saudi J Biol Sci. 2010;17(4):295–301. doi: 10.1016/j.sjbs.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H., et al. Hautriwaic acid as one of the hepatoprotective constituent of Dodonaea viscosa. Phytomedicine. 2014;21(2):131–140. doi: 10.1016/j.phymed.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Alotaibi, Moureq R, et al. 2019 In vivo assessment of combined effects of glibenclamide and losartan in diabetic rats. 28(2):178-185. [DOI] [PMC free article] [PubMed]
- Alqahtani F., et al. Angiotensin II receptor Neprilysin inhibitor (LCZ696) compared to Valsartan attenuates Hepatotoxicity in STZ-induced hyperglycemic rats. Int. J. Med. Sci. 2020;17(18):3098. doi: 10.7150/ijms.49373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Snafi A.E. A review on Dodonaea viscosa: A potential medicinal plant. IOSR Journal of Pharmacy. 2017;7(2):10–21. [Google Scholar]
- Arun M., Asha V.V. Gastroprotective effect of Dodonaea viscosa on various experimental ulcer models. J. Ethnopharmacol. 2008;118(3):460–465. doi: 10.1016/j.jep.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Asbun, Juan, and Francisco J %J Journal of the American College of Cardiology Villarreal 2006 The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. 47(4):693-700. [DOI] [PubMed]
- Asres K., et al. Antiviral activity against human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) of ethnobotanically selected Ethiopian medicinal plants. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2001;15(1):62–69. doi: 10.1002/1099-1573(200102)15:1<62::aid-ptr956>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Behrouj, Hamid, et al. 2015 Therapeutic effect of Silybum marianum plant extract on tamoxifen-induced fatty liver in rats. 3(1):5-27160.
- Callaghan, Brian C, et al. 2012 Enhanced glucose control for preventing and treating diabetic neuropathy. (6). [DOI] [PMC free article] [PubMed]
- Cheng, Chien-shan, et al. 2019 New therapeutic aspects of steroidal cardiac glycosides: the anticancer properties of Huachansu and its main active constituent Bufalin. 19(1), 1-27. [DOI] [PMC free article] [PubMed]
- Deeds M.C., et al. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45(3):131–140. doi: 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhruddin, Selim, Wael Alanazi, and Keith E %J Journal of diabetes research Jackson 2017 Diabetes-induced reactive oxygen species: mechanism of their generation and role in renal injury. 2017. [DOI] [PMC free article] [PubMed]
- Fouad S.A., et al. Formulation of novel niosomal repaglinide chewable tablets using coprocessed excipients: in vitro characterization, optimization and enhanced hypoglycemic activity in rats. Drug Deliv. 2023;30(1):2181747. doi: 10.1080/10717544.2023.2181747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francés, Daniel EA, et al. 2013 Cyclooxygenase‐2 over‐expression inhibits liver apoptosis induced by hyperglycemia. 114(3):669-680. [DOI] [PubMed]
- Gao, Yuan, et al. 2013 Isoprenylated flavonoids and clerodane diterpenoids from Dodonaea viscosa. 3(5):250-255.
- Getie M., et al. Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa. Rumex nervosus and Rumex abyssinicus. Fitoterapia. 2003;74(1–2):139–143. doi: 10.1016/s0367-326x(02)00315-5. [DOI] [PubMed] [Google Scholar]
- Ginsberg H.N., Zhang Y.L., Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005;36(3):232–240. doi: 10.1016/j.arcmed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Hamza A.A., et al. Salvadora persica attenuates DMBA-induced mammary cancer through downregulation oxidative stress, estrogen receptor expression and proliferation and augmenting apoptosis. Biomed Pharmacother. 2022;147 doi: 10.1016/j.biopha.2022.112666. [DOI] [PubMed] [Google Scholar]
- Karim N., et al. GABAA receptor modulation and neuropharmacological activities of viscosine isolated from Dodonaea viscosa (Linn) Pharmacol. Biochem. Behav. 2015;136:64–72. doi: 10.1016/j.pbb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Khalil N.M., Sperotto J.S., Manfron M.P. Antiinflammatory activity and acute toxicity of Dodonaea viscosa. Fitoterapia. 2006;77(6):478–480. doi: 10.1016/j.fitote.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Khan M.F., et al. The Reproductive Toxicity Associated with Dodonaea viscosa, a Folk Medicinal Plant in Saudi Arabia. Evid Based Complement Alternat Med. 2021;2021:6689110. doi: 10.1155/2021/6689110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate, CK %J Vallabh Prakashan, New Delhi, pp viii 2014 Practical Pharmacognosy. 5th Editon. 222.
- Kolset S.O., et al. Diabetic nephropathy and extracellular matrix. 2012;60(12):976–986. doi: 10.1369/0022155412465073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal D., Yunusa I. Dodonea Viscosa Linn: its medicinal, pharmacological and phytochemical properties. Int. J. Innov. Appl. Stud. 2013;2(4):476–482. [Google Scholar]
- Lee, Heaji, Yunsook %J Nutrition research Lim, and practice 2019 Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice. 13(5), 377-383. [DOI] [PMC free article] [PubMed]
- Mohany M., et al. LCZ696 mitigates diabetic-induced nephropathy through inhibiting oxidative stress, NF-κB mediated inflammation and glomerulosclerosis in rats. PeerJ. 2020;8:e9196. doi: 10.7717/peerj.9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moin H., et al. Effectiveness of analog of Humanin in ameliorating streptozotocin-induced diabetic nephropathy in Sprague Dawley rats. Peptides. 2023;165 doi: 10.1016/j.peptides.2023.171014. [DOI] [PubMed] [Google Scholar]
- Mothana R.AA., et al. Antimicrobial, antioxidant and cytotoxic activities and phytochemical screening of some yemeni medicinal plants. Evid. Based Complement. Alternat. Med. 2010;7(3):323–330. doi: 10.1093/ecam/nen004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A., et al. Phytochemicals from Dodonaea viscosa and their antioxidant and anticholinesterase activities with structure–activity relationships. Pharm. Biol. 2016;54(9):1649–1655. doi: 10.3109/13880209.2015.1113992. [DOI] [PubMed] [Google Scholar]
- Muhammad, Akhtar, et al. 2016b Phytochemicals from Dodonaea viscosa and their antioxidant and anticholinesterase activities with structure–activity relationships. 54(9):1649-1655. [DOI] [PubMed]
- Muhammad, Dima, et al. 2017 Triterpenoid saponins and other glycosides from the stems and bark of Jaffrea xerocarpa and their biological activity. 141:121-130. [DOI] [PubMed]
- Naoom A.Y., et al. Actinidia deliciosa as a complemental therapy against nephropathy and oxidative stress in diabetic rats. Food Sci. Human Wellness. 2023;12(6):1981–1990. [Google Scholar]
- Nasiri, Abolfazl, et al. 2014 The effects of aqueous extract of chicory root on steatosis, lipid profile and liver damage enzyme markers in tamoxifen-treated rats. 1(3):185-194.
- Navarro J.F., Mora-Fernández C. The role of TNF-alpha in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine Growth Factor Rev. 2006;17(6):441–450. doi: 10.1016/j.cytogfr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Oluleye, TS %J African Journal of Medicine, and Medical Sciences 2010 Diabetic retinopathy: Current developments in pathogenesis and management. 39(3):199-206. [PubMed]
- Papachristoforou E., et al. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J Diabetes Res. 2020;2020:7489795. doi: 10.1155/2020/7489795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin M., Jalalpure S.S., Gadge N.B. Antidiabetic activity of aqueous bark extract of Cassia glauca in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 2010;88(2):153–160. doi: 10.1139/Y09-121. [DOI] [PubMed] [Google Scholar]
- Salinas-Sánchez D.O., et al. Anti-inflammatory activity of hautriwaic acid isolated from Dodonaea viscosa leaves. Molecules. 2012;17(4):4292–4299. doi: 10.3390/molecules17044292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas-Sánchez, David Osvaldo, et al. 2012b Anti-inflammatory activity of hautriwaic acid isolated from Dodonaea viscosa leaves. 17(4):4292-4299. [DOI] [PMC free article] [PubMed]
- Sama V., et al. Pharmacognostical Studies on Dodonaea viscosa leaves. 2008;2(4) [Google Scholar]
- SF, E. Lhabal, et al. 2022 Biosynthesis and Characterization of Gold and Copper Nanoparticles from Salvadora persica Fruit Extracts and Their Biological Properties. Int J Nanomedicine 17:6095-6112. [DOI] [PMC free article] [PubMed]
- Shahzadi, Tayyaba, Muhammad Ajaib, and Khalid Mohammed Khan, 2012 Phytochemical screening, free radical scavenging, antioxidant activity and phenolic content of Dodonaea viscose Jacq. Electronic 77(4):423.
- Singh V.P., et al. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18(1):1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targher, Giovanni %J Hepatobiliary Surgery, and Nutrition 2020 Is it time for non-alcoholic fatty liver disease screening in patients with type 2 diabetes mellitus? 9(2), 239. [DOI] [PMC free article] [PubMed]
- Teffo, Leah Snow, MA Aderogba, and Jacobus Nicolaas %J South African Journal of Botany Eloff 2010 Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from Dodonaea viscosa Jacq. var. angustifolia leaf extracts. 76(1), 25-29.
- Uddin Z., et al. Isolation and characterization of protein tyrosine phosphatase 1B (PTP1B) inhibitory polyphenolic compounds from Dodonaea viscosa and their kinetic analysis. Front. Chem. 2018;6:40. doi: 10.3389/fchem.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Qi, et al. 2020 Hyperglycemia aggravates acute liver injury by promoting liver‐resident macrophage NLRP 3 inflammasome activation via the inhibition of AMPK/mTOR‐mediated autophagy induction. 98(1):54-66. [DOI] [PMC free article] [PubMed]
- Wang-Fischer Y., Garyantes T. Improving the Reliability and Utility of Streptozotocin-Induced Rat Diabetic Model. J Diabetes Res. 2018;2018:8054073. doi: 10.1155/2018/8054073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Dandan, et al. 2018. In vitro and in vivo effectiveness of phenolic compounds for the control of postharvest gray mold of table grapes. 139:106-114.
- Yazdi H.B., et al. Liver Dysfunction and Oxidative Stress in Streptozotocin-Induced Diabetic Rats: Protective Role of Artemisia Turanica. J Pharmacopuncture. 2019;22(2):109–114. doi: 10.3831/KPI.2019.22.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Hanqing, Zhongtao %J Medical science monitor: international medical journal of experimental Liu, and clinical research 2019 Atorvastatin induces hepatotoxicity in diabetic rats via oxidative stress, inflammation, and anti-apoptotic pathway. 25, 6165. [DOI] [PMC free article] [PubMed]
- Zhang L.-B., et al. Isoprenylated flavonoid and adipogenesis-promoting constituents of Dodonaea viscosa. 2012;75(4):699–706. doi: 10.1021/np2009797. [DOI] [PubMed] [Google Scholar]