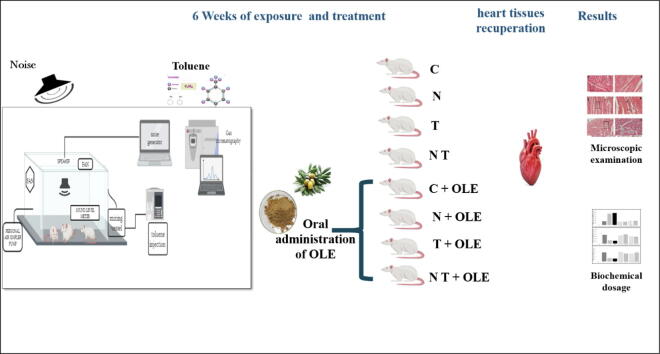
Graphical abstract
Keywords: Noise, Toluene, Heart tissue, Olea Europaea L. leaf extract, Rat, Antioxidant activity
Abstract
In many occupational settings, workers are frequently exposed to toluene and noise. However, the individual and combined effects of these exposures on the cardiovascular system have not been fully elucidated. Therefore, this study aimed to investigate the impact of simultaneous exposure to toluene and noise on the rat heart, while also evaluating the potential preventive effect of olive leaf extract (OLE). Forty-eight male Wistar rats were randomly assigned to eight groups (n = 6/group): control group (C), control group that received OLE (C + OLE), group exposed to noise (N), group exposed to noise and receiving OLE (N + OLE), group exposed to toluene (T), group exposed to toluene and receiving OLE (T + OLE), group co-exposed to noise and toluene (NT), and group co-exposed to noise and toluene and receiving OLE (NT + OLE). The rats in this study were subjected to simultaneous exposure to toluene and noise for a duration of six weeks, within a custom-built plexiglass chamber. Toluene was administered at a concentration of 300 ppm, while the noise level was set to 85 dB(A). The exposure chamber was equipped with a generation system, an exposure system, and a monitoring system, ensuring precise and accurate exposure conditions. After the six-week period, heart and blood samples were collected from the rats for subsequent analysis. Plasma levels of cholesterol (CHOL), triglycerides (TG), lactate dehydrogenase (LDH), and creatine kinase (CK) were measured, and histopathological investigation was conducted using HE staining. Additionally, superoxide dismutase (SOD) and catalase (CAT) activities, as well as malondialdehyde (MDA) levels in heart tissue were measured. Our results showed that simultaneous exposure to noise and toluene altered CHOL, TG, LDH, and CK levels, and also caused an increase in lipid peroxidation levels and superoxide dismutase activity, along with a decrease in catalase activity in the heart. A significant alteration in the myocardium was also observed. However, treatment with OLE was found to modulate these oxidative and histological changes, ultimately correcting the deleterious effects induced by the combined exposure to noise and toluene. Therefore, our study suggests that OLE could be a potential preventive measure for individuals exposed to toluene and noise in industrial settings.
1. Introduction
Toluene belongs to the category of volatile organic compounds, frequently used in the professional sector. This component is found in paints, inks, and thinners. Occupational exposure to toluene can be responsible for multiple and varied effects that can affect different functions and organs of the human body such as the kidney (Ballantyne, 2016), the liver (Ayan et al. 2013, Bingham et al., 2012, Tas et al., 2013) and the brain, inducing behavioral disorders (Benignus et al., 2005, Lee et al., 2020, Wayman and Woodward, 2018) and ventricular enlargement (Cruz et al., 2014).
Noise exposure is found in numerous professional sectors and is described to cause sensorineural hearing loss, even below legal thresholds. Noise has harmful extra-auditory consequences on the brain by inducing sleep disorders (Basner et al., 2014). It can also stimulate catecholamines and cortisol release, affecting the cardiovascular system. The effects of noise can also alter arterial hypertension and cause ischemic heart disease and strokes (Assunta et al., 2015, Skogstad et al., 2016, Sørensen, 2017).
Natural products, particularly those of vegetable origin, have been a historically significant source of therapeutic agents. Additionally, there is a well-established link between nutrition and health.
Several scientific teams have been exploring the potential of medicinal plants due to their high concentration of bioactive compounds and low toxicity. For thousands of years, medicinal plants have been utilized in traditional medicine systems. The Mediterranean diet, known for its wide-ranging consumption of plant-based products such as fruits, legumes, cereals, and herbs, is recognized for its health benefits. The olive tree (Olea europaea) is an iconic plant in the Mediterranean region. In Tunisia, the Oleaceae family, specifically olives (Olea europaea L.), are present in more than 50 different varieties (Trigui & Msallem, 2002) and have been extensively studied due to their numerous health benefits. Of particular interest are the olive leaves, which are rich in bioactive compounds such as oleuropein and hydroxytyrosol. These compounds have been associated with a range of therapeutic properties, including antioxidant, anti-inflammatory, antimicrobial, antiviral, and anti-tumor effects. (De Cicco et al., 2020, Şahin and Bilgin 2018, Romani et al., 2019). Hence, olive by-products, including olive leaves, are increasingly being explored for their potential medicinal benefits.
Given the growing concern about the adverse effects of exposure to harmful agents in the workplace, several epidemiological studies have investigated the effects of co-exposure to multiple harmful agents. Researchers such as Golmohammadi and Darvishi, 2019, Mohammadi et al., 2010 and Morata et al. (1993), have conducted studies in this area. In line with this research, our recent epidemiological investigation, conducted by Ben Attia et al. (2022), examined the combined exposure to solvents and noise in a composite product manufacturing company that specializes in sports field products. The study found that toluene was the most frequently encountered solvent, with a concentration of 300 ppm, and the sound intensity level of 85 dB(A) was consistently observed across different workstations (Ben Attia et al., 2022; Mhamdi et al., 2021).
Therefore, the aim of this study was to investigate the in vivo effects of simultaneous exposure to noise and toluene on biochemical and hematological parameters, oxidative stress biomarkers, and heart histology in male Wistar rats, as well as to evaluate the protective role of Olea europaea L. leaf extract against the harmful effects of co-exposure to noise and toluene.
2. Material and methods
2.1. Chemicals
Toluene, C7H8, is a colorless, flammable liquid with a sweet odor. It is commonly used as a solvent in various industrial processes. The toluene used in inhalation exposure was obtained from “SMSBio, Sud médical services, Tunisia.”.
2.2. Plant extract preparation
Olea europaea L. leaves were harvested at the beginning of November 2020 from El Mansoura, Siliana city, located in the northern region of Tunisia. To ensure the accurate identification of the plant species, a voucher specimen (TBA 001) was carefully prepared and deposited at the herbarium of the Botany department at Faculty of Sciences of Tunis. A taxonomist was consulted to ensure precise identification of the plant.
The leaves were air-dried at room temperature in the absence of light and ground into a fine powder. Five grams of olive leaf powder were extracted with 200 mL of 70% ethanol and 30% water at 60 °C for 4 h, followed by filtration using a cellulosic plate filter. The resulting solution was concentrated at 65 °C under vacuum using a rotary evaporator and stored at 4 °C until further use. The extraction process was carried out at the Quality Control Laboratory of Herbes de Tunisie Company in El Mansoura, Siliana. (A. A., Hassan, 2022, Yateem et al., 2014).
2.2.1. Phytochemical analysis
2.2.1.1. Total phenol content (TPC)
Total phenolic compounds content was determined colorimetrically at 765 nm and expressed as gallic acid equivalent (GAE), according to the method described by Ben Hsouna et al., Ben Hsouna et al.. (2019). The samples were added to Folin-Ciocalteu reagent and Na2CO3 solution and placed in a water bath at 40◦C for 30 min before passing to the spectrophotometric analysis (spectrophotometer Genesys 10 UV screening, Thermo Electron Corporation, France). Total phenol content was expressed in g of gallic acid equivalent/ 100 g of dry matter (DW).
2.2.1.2. Total flavonoid contents (TFC)
Total flavonoid contents was determined following the modified procedure of Ben Hsouna et al., Ben Hsouna et al.. (2019). Briefly, 0.5 mL of aqueous extract was placed in a 5 mL volumetric flask, and then 2.5 mL of distilled water was added, followed by 0.15 mL of 5% NaNO2. After 5 min, 0.15 mL of 10% AlCl3 was added. 5 min later, 1 mL of 1 M NaOH was added, followed by distilled water until reaching the desired volume. The solution was mixed and absorbance was measured at 510 nm using a spectrophotometer (Genesys 10uv screening, Thermo Electron Corporation, France). Total flavonoid content was expressed as g quercetin / 100 g of DW.
2.2.1.3. Determination of radical scavenging activity by DPPH assay
The radical scavenging activity of the extract of O. europaea leaves was measured using a modified method from Ba et al. (2009), and the results were compared to the antioxidant activity of Trolox. The antioxidant capacity of the extract of O. europaea leaves is expressed as Trolox Equivalent/g of dry weight (DW) of the extract.
2.2.2. LC-MS analysis
HPLC analysis was performed using a reversed-phase column (Pursuit XRs ULTRA 2.8, C18, 100 × 2 mm, Agilent Technologies, UK). The injection volume for each sample was 20 µL, and the column temperature was maintained at 30 °C. The mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in MeOH (B). Separation was achieved using a gradient program at a flow rate of 1 mL/min, with an initial composition of 100% solvent A, followed by a gradient to 100% solvent B over 20 min, held at 100% solvent B for 5 min, and finally returned to 100% solvent A for 25 min. The drying gas flow rate was set to 1 mL/min at 320 °C. MS analysis was performed in the positive ion mode, with a mass range of m/z 100–2000. High-resolution mass spectral data was obtained using a Thermo Instruments ESI-MS system (LTQ XL/LTQ Orbitrap Discovery, UK) connected to a Thermo Instruments HPLC system (Accela PDA detector, Accela PDA autosampler, and Accela Pump).
2.3. Exposure protocols
To assess the potential synergistic effect of noise and toluene, a total of eight groups were used in this study. Two groups (Group N and Group N + OLE) were exposed to noise, while two other groups (Group T and Group T + OLE) were exposed to toluene. Two additional groups were co-exposed to both noise and toluene (Group NT and Group NT + OLE). Control groups (Group C and Group C + OLE) were kept in cages that were free from exposure to either noise or toluene.
2.3.1. Toluene exposure
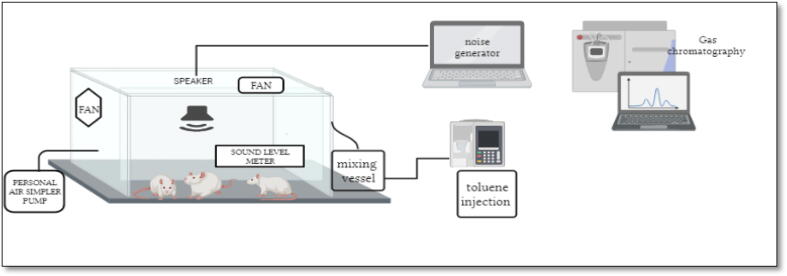
Two groups, Group T and Group T + OLE, were exposed to toluene for 6 h per day, 5 days per week, for a total of 6 weeks. The rats were exposed daily from 8:00 am to 2:00 pm in two identical inhalation chambers made of plexiglass. The chambers were designed to contain six rats at a time and were comprised of a generation system, an exposure system, and a monitoring system, following the guidelines proposed by Hinners et al. (1968).
To generate and maintain a toluene concentration of 300 ± 10 ppm for the 6-hour exposure period, liquid toluene (Loba Chemie Pvt Ltd) was injected into a mixing vessel using an isocratic pump, and the resulting mixture was then introduced into the exposure chamber. Toluene concentrations were monitored and analyzed by gas chromatography. The exposure chamber was equipped with an inlet fan to circulate the air and an outlet centrifugal fan to adjust the airflow. Circular openings were also created in the chamber to enable researchers to monitor and sample the toluene levels. The temperature in the chamber was maintained at 23 ± 1 °C and monitored using a digital thermometer.
2.3.2. Noise exposure
Two groups, Group N and Group N + OLE, were exposed to noise for 6 weeks. The acoustic exposure was achieved using Audacity 2.3.2 audio software, which generated an octave-band noise (8–16 kHz) at a level of 85 dB(A) through a sound speaker placed equidistant from all rats to ensure uniform noise intensity. The noise level was measured using a Class 1 accuracy Type 2238 Bruel and Kjaer integration sound level meter. The rats were exposed to the noise for 6 h per day, 5 days per week, automatically repeating after selecting the start and end times for each daily exposure period. Exposure occurred from 8:00 am to 2:00 pm each day, following the protocol established by Gannouni et al. (2013).
2.3.3. Toluene and noise co-exposure
To investigate the potential interaction between noise and toluene exposure, two groups, Group NT and Group NT + OLE, were co-exposed to both stressors for 6 weeks, 6 h per day and 5 days per week. The inhalation chambers used for this experiment were similar to those used for single exposure experiments with simultaneous generation of the stressors. A schematic of the experimental setup is presented in Fig. 1.
Fig. 1.
Schematic representation of the chamber used for exposure of rats to noise and toluene. The exposure chamber made of plexiglass with an internal volume of 0.43 m3. Six animals could be placed at the same time in this chamber daily for 6 h of exposure from 8: 00 am to 2:00 pm.
2.3.4. Sampling and analysis of toluene
To ensure accurate measurement of toluene concentration in the exposure chamber, pilot tests were conducted by generating the targeted concentration of 300 ppm. The active sampling method, consistent with Method 1501 developed by the U.S. National Institute for Occupational Safety and Health (NIOSH 2003), was employed using a personal sampling air pump (Casella CEL, Apex 182180B-K5) with activated charcoal as the adsorbent to sample the vapor. After extraction of samples from each section of the sampler's tubes using 1 mL of CS2, the mixture was shaken for 30 s to ensure maximum desorption. Finally, the samples were analyzed using gas chromatography-mass spectrometry (Agilent Technologies 6890 N Network GC System) to determine the toluene concentration.
2.4. Animals and treatments
Prior to the start of the experiments, male Wistar rats weighing between 250 and 300 g were housed in an animal facility for one week to allow for acclimation. To minimize stress, the animals were housed in pairs and provided with a commercial pellet diet (Industrial Society of Food, Sfax, Tunisia) and water ad libitum. The rats were maintained under controlled environmental conditions, with a 12-hour light/dark cycle and room temperature of 23 ± 1 °C. All experimental procedures were approved by the Ethics Committee of the National School of Veterinary Medicine of Sidi Thabet (Ref: 162020/FMT) and were conducted in accordance with the International Council for Laboratory Animal Science (ICLAS) guidelines.
2.4.1. Experimental design
Forty-eight male Wistar rats were divided randomly into eight groups:
Group C: Control rats, housed in normal conditions
Group C + OLE: Control rats treated only with Olea europaea L. leaf extract at 40 mg/kg/day for six weeks,
Group N: Rats exposed to noise
Group N + OLE: Rats exposed to noise and Olea europaea L. leaf extract at 40 mg/kg/day for six weeks
Group T: Rats exposed to toluene
Group T + OLE: Rats exposed to toluene and Olea europaea L. leaf extract at 40 mg/kg/day for six weeks
Group NT: Rats co-exposed to noise and toluene
Group NT + OLE: Rats co-exposed to noise and toluene and Olea europaea L. leaf extract at 40 mg/kg/day dose for six weeks.
The daily oral gavage administration of OLE extract was based on a preliminary study conducted by Bahri et al. (2020) at the Experimental Medicine Unit of the Faculty of Medicine in Tunis, where the dosage of 40 mg/kg/day was determined.
2.4.2. Sample collection
At the end of the experiment, rats were anesthetized via peritoneal injection of ketamine hydrochloride (50 mg/kg) and euthanized via decapitation. Blood was collected in EDTA-tubes for complete blood counts, and in heparinized tubes, centrifuged at 3,000 g for 10 min at 4 °C, and stored. The heart tissues were rapidly removed, washed with 0.1 M phosphate buffer saline (pH 6.8), and divided into two fragments. One fragment was fixed in 10% neutral formalin for subsequent histological analysis, while the other was stored at −80 °C for biochemical analysis.
2.4.2.1. Hematological parameters
Complete blood counts (red and white blood cells and platelets) were performed using I-sens automate (i-smart 30 pro electrolyte analyzer, UK).
2.4.2.2. Biochemical assays in plasma
Biochemical parameters, including cholesterol, triglycerides, lactate dehydrogenase, and creatine kinase, were measured in plasma using a COBAS INTEGRA 400 plus automated system (Switzerland) according to standardized methods.
2.4.3. Oxidative stress evaluation
Heart tissue samples (200 mg) were homogenized in PBS buffer (pH 7.2) using an ULTRA-TURRAX® IKA-WERK homogenizer (Germany). The homogenate was then centrifuged at 10,000 rpm for 20 min at 4 °C, and the resulting supernatant was collected, aliquoted, and stored at −80 °C for subsequent analysis of oxidative stress markers, including malondialdehyde (MDA), catalase (CAT), and superoxide dismutase (SOD).
2.4.3.1. Evaluation of lipid peroxidation
Lipid peroxidation was determined according to the method of Buege and Aust (1978). Samples were mixed with BHT-TCA solution (1%–20%, v/v) and centrifuged at 1,000 rpm for 10 min. The supernatant was mixed with TBA-Tris solution (120 mM- 26 mM, v/v) in an acidic environment (0.6 M HCl). The mixture was heated at 100 °C for 15 min then cooled at room temperature. The absorbance was measured at 532 nm using UV–vis spectrophotometer (Labomed, UV-2650, Inc, USA) and expressed in nmol of MDA/mg of protein.
2.4.3.2. Evaluation of catalase activity
Catalase (CAT) activity evaluation is based on the ability of the enzyme to degrade hydrogen peroxide (Aebi 1984). H2O2 degradation (30 mM) by CAT results in a decrease in the absorption of reaction mixture at 240 nm against a blank containing 50 mM phosphate buffer (pH = 7) and enzyme extract. The absorbance was recorded at regular time intervals (30 s) for 2 min and expressed as μmoles/min/mg of protein.
2.4.3.3. Evaluation of superoxide dismutase (SOD) activity
Superoxide dismutase (SOD) activity was evaluated as described by Beyer and Fridovich (1987) using epinephrine (50 mM carbonate buffer, pH 10.2) and bovine catalase (0.4 U/mL). This method is based on the inhibition of the auto-oxidation of epinephrine to adrenochrome in the presence of SOD. Absorbance was measured at 480 nm every minute for 5 min and was expressed in UI/min/mg of protein.
2.4.3.4. Evaluation of protein concentration
Protein content in supernatants was determined according to Bradford (1976) using bovine serum albumin as standard. Absorbance was recorded at 595 nm. The content was expressed as mg /mL.
2.4.4. Histological study
For the histological study, rat’s hearts were perfused and immersed in the fixative solution (10 % neutral-buffered formalin) for 48 h, dehydrated through graded series of ethanol, embedded in paraffin, and then sectioned with a microtome (Leica Biosystems RM2245, USA) to obtain 4–5 μm-thick paraffin sections. Dewaxed sections were then stained with hematoxylin and eosin (H&E).
2.4.5. Statistical analyses
Results were expressed as means ± SD. Statistical analyses were performed using the Biostat software for Windows. Significant differences between all groups (n = 6) were determined using the Kruskal-Wallis test followed by the U-Mann-Whitney test for multiple comparisons with statistical significance p < 0.05.
3. Results
3.1. Phytochemical characteristic of Olea europaea L. Leaf extract
Total polyphenols quantification was estimated by Folin-Ciocalteu test based on the colorimetric intensity of the extract obtained. Results showed in Table 1 indicate that OLE contain high amounts of total polyphenols (8524.44 mg GAE / 100 g), and high levels of flavonoids (7128.88 mg QU / 100 g). Additionally, the DPPH assay revealed a strong antioxidant activity of the OLE, with a Trolox equivalent of 9713.330 µM.
Table 1.
The content of total polyphenols (TPC) and flavonoids (TFC) in olive leaf.
| TPC (mg gallic acid equivalent / 100 g Olea europaea L. leaf powder) | 8524,44 |
| TFC (mg quercetin equivalent / 100 g Olea europaea L. leaf powder) | 7128,88 |
| DPPH (Trolox equivalent µM) | 9713,330 |
TPC is expressed in mg of gallic acid equivalent (mg GA/100 g leaf) and the content of total flavonoids TFC in g of quercetin equivalent (mg QU / 100 g leaf) and DPPH antioxidant activity (Trolox equivalent µM).
3.2. Identification of active biomolecules
HPLC analysis of phenolic compounds in O. europaea leaf extract (Table 2) allowed the identification of seven compounds: Hydroxytyrosol glucoside, Hydroxytyrosol, Secologanoside, Oleuropein aglycon, Hydroxytyrosol acetate, Luteolin-7-O-β-D-glucopyranoside and Oleuropein.
Table 2.
Determination of Olea europaea L. leaf extracts bioactive compounds using liquid chromatography–mass spectrometry (LC-MS).
| N° Pic | Retention time (min) | Molecular formula | Compound |
|---|---|---|---|
| 1 | 12.1 | C14H20O8 | Hydroxytyrosol glucoside |
| 2 | 13.3 | C8H10O3 | Hydroxytyrosol |
| 3 | 20.1 | C16H22O11 | Secologanoside |
| 4 | 23.6 | C17H20O6 | Oleuropein aglycone |
| 5 | 33.1 | C10H12O4 | Hydroxytyrosol acetate |
| 6 | 42.8 | C21H20O11 | Luteolin-7-O-b-D-glucopyranoside |
| 7 | 44.3 | C25H32O13 | Oleuropein |
3.3. Identification of toluene by HPLC
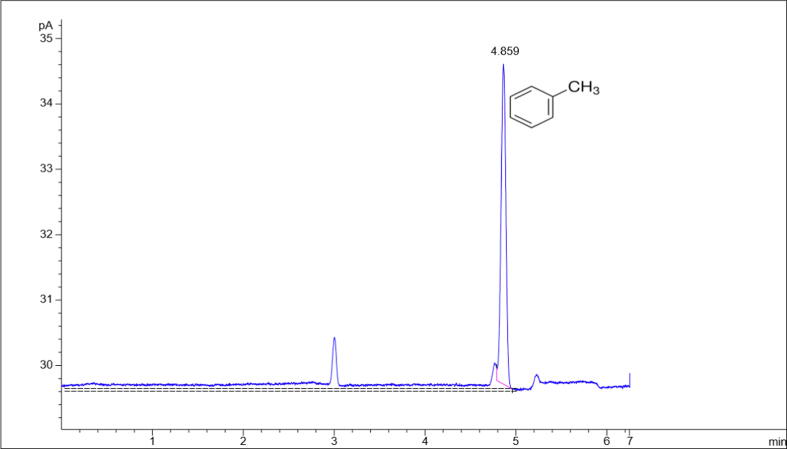
The chromatogram of the analysis of the air sample, collected by charcoal adsorption from the exposure chamber is shown in Fig. 2, presents the retention time of toluene at 4.859 min.
Fig. 2.
GC-FID chromatogram of the air sample collected by charcoal adsorption from the exposure chamber showing retention time of toluene. 4.859: Toluene retention time (min).
3.4. Evaluation of hematological parameters
Table 3 presents significant findings related to hematological parameters in rats exposed to toluene (T), noise and toluene co-exposure (NT), and control (C) groups. The results demonstrate a notable decrease in the red blood cell count in the T and NT groups compared to the C group (6.63 ± 0.62 (×106 µL) vs 8.42 ± 0.36 (×106 µL), p < 0.05 and 6.56 ± 0.80 (×106 µL) vs 8.42 ± 0.36 (×106 µL), p < 0.05; respectively). Additionally, there was a significant increase in white blood cells in the three exposure groups (N, T, and NT) compared to the C group (11.86 ± 1.14 (×103 µL) vs 8.20 ± 0.89 (×103 µL), p < 0.05 12.16 ± 1.41 (×103 µL) vs 8.20 ± 0.89 (×103 µL), p < 0.05 and 13.65 ± 2.38 (×103 µL) vs 8.20 ± 0.89 (×103 µL), p < 0.05; respectively). Notably, there was a significant increase in blood platelets in rats exposed to N, T, or NT compared to the C group (1217.33 ± 83.82 (×103 µL) vs 871.50 ± 53.37 (×103 µL), p < 0.05; 12.16 ± 1.41 871.50 ± 53.37 (×103 µL), p < 0.05 and 13.65 ± 2.38 871.50 ± 53.37 (×103 µL), p < 0.05; respectively). Interestingly, the administration of Olea Europaea L. leaf extract did not result in any significant changes in the hematological parameters as compared to the C group.
Table 3.
Complete blood counts.
| Red Blood Cells (×106 µL) |
White Blood Cells (×103 µL) |
Blood platelets (×103 µL) |
|
|---|---|---|---|
| C | 8.42 ± 0.36 | 8.20 ± 0.89 | 871.50 ± 53.37 |
| N | 8.41 ± 0.70 | 11.86 ± 1.14 * | 1217.33 ± 83.82 * |
| T | 6.63 ± 0.62 * | 12.16 ± 1.41 * | 987 ± 33.66 * |
| NT | 6.56 ± 0.80 * | 13.65 ± 2.38 * | 1407.17 ± 155.25 * |
| C + OLE | 8.60 ± 0.56 | 7.91 ± 0.66 | 866.833 ± 32.19 |
| N + OLE | 9.43 ± 0.78 ¥ | 9.58 ± 0.77¥ | 899.5 ± 57.93¥ |
| T + OLE | 7.77 ± 0.47 * $ | 9.75 ± 1.03$ | 889 ± 60.25 |
| NT + OLE | 8.15 ± 0.68 € | 9.63 ± 1.01€ | 913.83 ± 43.71€ |
The results are expressed as means ± SD of six animals. The different pairs of groups are compared using the Mann-Whitney U test.
* p < 0.05 vs control group, € p < 0.05 vs NT group, ¥ p < 0.05 vs N group, $ p < 0.05 vs T group.
3.5. Evaluation of noise and/or toluene effects on biochemical parameters
The Table 4 presents the plasma concentrations of cholesterol, triglycerides (TG), LDH, and creatine kinase (CK). In the control group, the mean total cholesterol level was 1.20 ± 0.08 mol/L, which showed a significant increase in the N and NT groups (1.31 ± 0.07 vs 1.20 ± 0.08 mol/L, p < 0.05, and 1.45 ± 0.05 vs 1.20 ± 0.08 mol/L, p < 0.05, respectively). Likewise, the average level of triglycerides in the control group was 0.9 ± 0.08 mol/L, which significantly increased in the N and NT groups (1.3 ± 0.07 vs 0.9 ± 0.08 mmol/L, p < 0.05; 1.4 ± 0.05 vs 0.9 ± 0.08 mmol/L, p < 0.05, respectively). The use of Olea Europaea L. leaf extract (OLE) during the exposure period maintained stable levels of both cholesterol and triglycerides. Furthermore, our findings revealed a significant increase in plasma LDH concentration in the T and NT groups compared to the control group (583 ± 54.01 vs 344.33 ± 50.53 (U/L), p < 0.05; 604.5 ± 112.65 vs 344.33 ± 50.53 (U/L), p < 0.05, respectively). Additionally, exposure to noise resulted in a significant increase in total CK concentration, from 368.5 ± 27.31 (U/L) in the control group to 1489.5 ± 139.21 (p < 0.05) in animals exposed to a noise level of 85 dB(A). Co-exposure to noise and toluene had an additive effect on the increase in total CK, which reached 1857.83 ± 142.39 (p ≤ 0.05). Treatment with OLE during the exposure period prevented the increase in LDH and CK levels compared to the control group. However, the concentration of CK decreased but remained higher than that of the control group.
Table 4.
Plasma biochemical parameters of the different groups.
| Cholesterol (mmol/L) | Triglyceride (mmol/L) |
Lactate dehydrogenase(U/L) | Creatine kinase (U/L) | |
|---|---|---|---|---|
| C | 1.20 ± 0.08 | 0.9 ± 0.08 | 344.33 ± 50.53 | 368.5 ± 27.31 |
| N | 1.31 ± 0.07 * | 1.3 ± 0.07 * | 365 ± 21.93 | 1489.5 ± 139.21* |
| T | 1.15 ± 0.08 € | 1.045 ± 0.08 | 583 ± 54.01 * | 1430 ± 169.68 * |
| NT | 1.45 ± 0.05 * | 1.4 ± 0.05 * | 604.5 ± 112.65 * | 1857.83 ± 142.39 * |
| C + OLE | 1.08 ± 0.04 ¥€ | 0.9 ± 0.04 ¥€ | 306.66 ± 28.30 | 336.16 ± 33.13 |
| N + OLE | 1.1 ± 0.08 ¥€ | 1.05 ± 0.08 ¥€ | 309.5 ± 11.50 ¥ | 562.83 ± 105.078 *¥ |
| T + OLE | 1.03 ± 0.08 ¥€ | 0.96 ± 0.08 ¥€ | 345 ± 26.45 $ | 549 ± 45.36 * $ |
| NT + OLE | 1.22 ± 0.07 € | 0.97 ± 0.07 ¥€ | 362.33 ± 22.69 € | 528.33 ± 124.52 * € |
The results were expressed as mean ± SD of six animals. The different pairs of groups were compared using the Mann-Whitney U test.
* p < 0.05 vs control group, € p < 0.05 vs NT group, ¥ p < 0.05 vs N group, $p < 0.05 vs T group.
3.6. Oxidative stress parameters
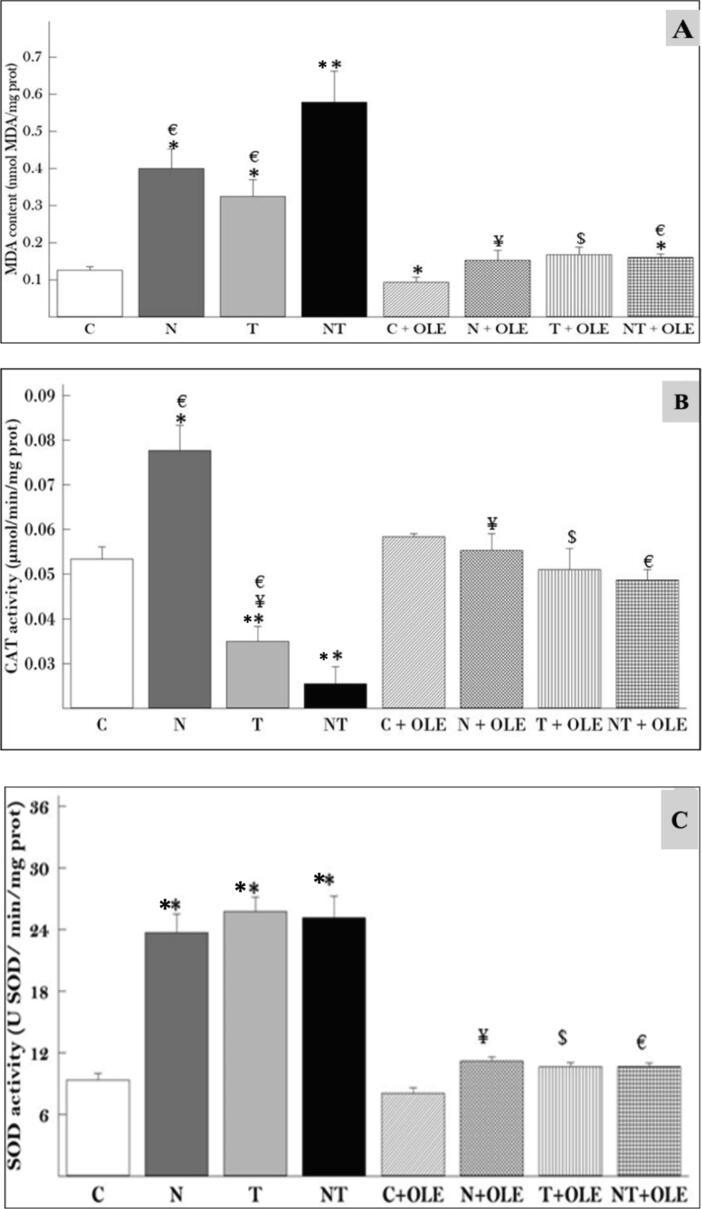
Figure Fig. 3 depicts the results for malondialdehyde (MDA) concentrations and catalase (CAT) and superoxide dismutase (SOD) activities in cardiac tissues.
Fig. 3.
Effect of exposure to noise (85 dB (A)) and/or toluene (300 ppm) and protection effect of Olive leaf extracts (40 mg/kg bw) on rat heart oxidative parameters. (A): Lipid peroxidation, (B): Catalase activity and (C): SOD activity. Results are expressed as means ± SD of six animals. Different pairs of groups were compared using the Mann-Whitney U test. * p < 0.05 vs control group, ** p ≤ 0.05 vs control group, € p < 0.05 vs NT group, ¥ p < 0.05 vs N group, $ p < 0.05 vs T group.
Exposure to 85 dB(A) noise and 300 ppm toluene inhalation resulted in a significant increase in MDA levels (P ≤ 0.05) compared to the control group (0.399 ± 0.061 vs. 0.125 ± 0.014n mol/mg protein and 0.324 ± 0.059 vs. 0.125 ± 0.014n mol/mg protein, respectively). Co-exposure to noise and toluene further elevated MDA levels (P ≤ 0.01) compared to the control group (0.578 ± 0.10 vs. 0.125 ± 0.014n mol/mg protein). However, treatment with Olea europaea L. leaf extract did not significantly alter MDA levels relative to the control group, suggesting the potential for the extract to protect against oxidative stress induced by noise and toluene exposure (Fig. 3A).
Exposure to noise led to a significant increase in CAT activity compared to the control group (0.077 ± 0.006 vs. 0.053 ± 0.035 µmol/min/g protein, P ≤ 0.05). Conversely, exposure to toluene alone and co-exposure to noise and toluene resulted in a highly significant reduction in CAT activity (0.035 ± 0.004 vs. 0.053 ± 0.035 µmol/min/g protein, P = 0.01 and 0.0255 ± 0.004 vs. 0.053 ± 0.035 µmol/min/g protein, P = 0.01, respectively). Notably, in the N + OLE, T + OLE, and NT + OLE groups, CAT activity was significantly restored compared to the control group (0.060 ± 0.002 vs. 0.053 ± 0.035 µmol/min/g protein, P = 0.02; 0.051 ± 0.01 vs. 0.053 ± 0.035 µmol/min/g protein; and 0.046 ± 0.005 vs. 0.053 ± 0.035 µmol/min/g protein, P = 0.002, respectively), indicating the potential protective effect of Olea europaea L. leaf extract against oxidative stress induced by noise and toluene exposure (Fig. 3B).
Exposure to noise and toluene, either alone or in combination, induced a significant increase in SOD activity compared to the control group (p ≤ 0.01). Specifically, the SOD activity levels were 23.672 ± 5.86 USOD/min/mg prot in rats exposed to noise, 25.758 ± 3.98 USOD/min/mg prot in rats exposed to toluene, and 25.121 ± 6.51 USOD/min/mg prot in rats exposed to both noise and toluene, relative to the control rats (9.366 ± 2.03 USOD/min/mg prot).
Remarkably, a significant recovery of SOD activity was observed in rats administered Olea europaea L. leaf extract when compared to rats exposed to physicochemical stressors, resulting in a significant decrease in SOD activity (p ≤ 0.01) (Fig. 3C).
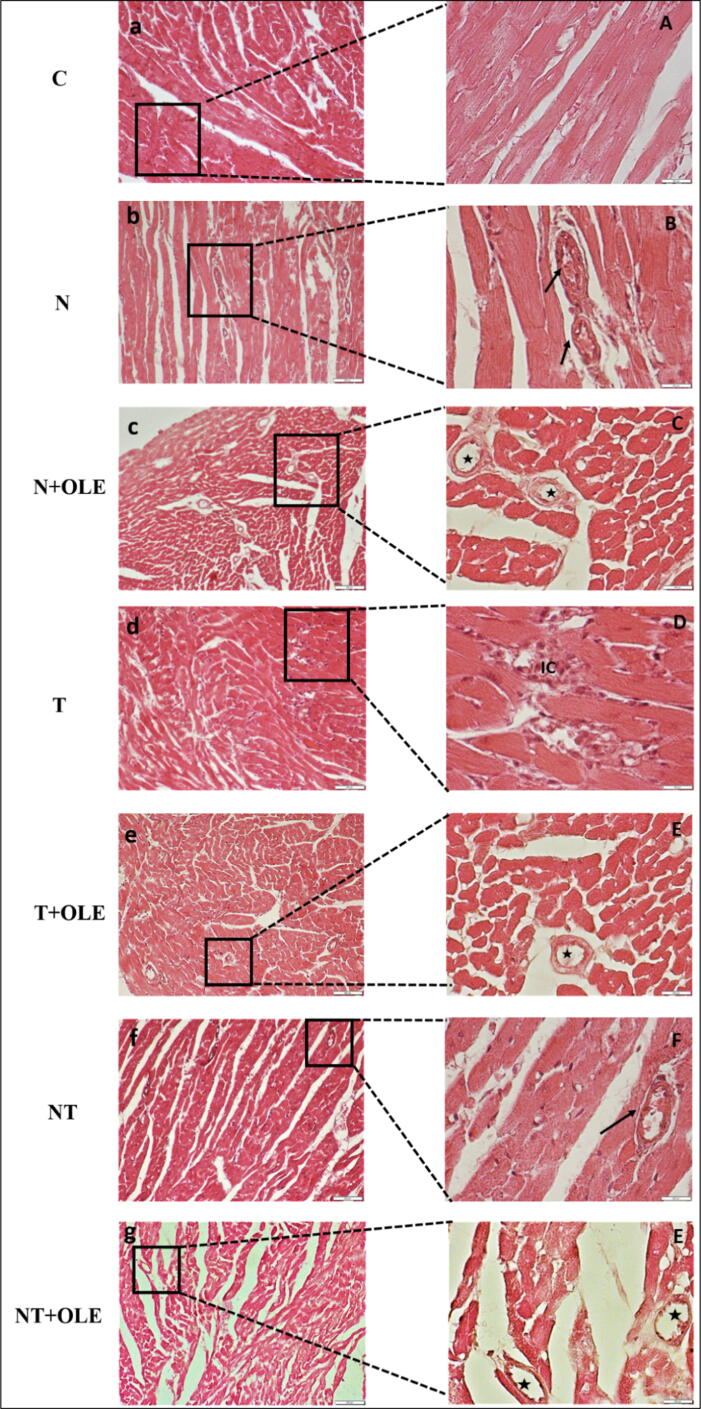
3.7. Heart histological analysis
A histological analysis revealed normal myocardial histology in the control group (Fig. 4a, A). However, architectural alterations in cardiac tissue were observed in rats exposed to 85 dB(A) noise and 300 ppm toluene (Fig. 4f, F).
Fig. 4.
Microscopic observation (×100 on the left, ×400 on the right) in the heart tissue (H&E) of control (A-a) and exposed rat to noise (85 dB(A)) and/or toluene (300 ppm) and protected with olive leaf extracts (40 mg/kg bw). (→) blocked arteries. ( ) unblocked arteries. (IC) inflammatory cells.
) unblocked arteries. (IC) inflammatory cells.
In the group exposed to noise, numerous arteries were obstructed by blood clots (Fig. 4b, B). Conversely, no changes in cardiac tissue were observed in the group exposed to noise and OLE treatment, with no identifiable clots in arteries (Fig. 4c, C).
In rats exposed to toluene, an abundance of inflammatory cells was observed, although arteries and veins remained intact (Fig. 4d, D). However, no changes were recorded in heart histology of the group exposed to toluene and OLE compared to the control group (Fig. 4e, E).
The co-exposure group of rats to noise and toluene showed the most severe tissue damage with the presence of scattered inflammatory cells, dilated veins, and obstructed arteries by blood clots (Fig. 4f, F). Treatment with OLE in the NT + OLE group exhibited no significant difference in heart histology compared to the sham group.
4. Discussion
Toluene is a volatile solvent widely used in the industrial sector worldwide, and it is also one of the most commonly abused inhalants (ATSDR, 2000). Occupational diseases are a prevalent issue among industrial employees exposed to chemical and/or physical hazards, such as noise. Furthermore, workers are often exposed to multiple risk factors simultaneously in the workplace. The interaction of physical agents and chemical substances, even at low doses, can have additive or synergistic effects on health. The aim of this study was to comprehensively evaluate the potential cardiotoxic effects of exposure to toluene and/or noise on rat hematological and biochemical parameters, and to investigate the potential protective effect of O. europaea leaf extract against such effects.
The findings of this study reveal that OLE contains high levels of polyphenols and flavonoids and exhibits strong antioxidant activity as determined by the DPPH assay. Polyphenols are well-known for their biological activity and have been demonstrated to possess various health benefits, including anti-inflammatory, anti-cancer, and anti-oxidant properties (Rudrapal et al., 2022, Bié et al., 2023). Flavonoids, a subclass of polyphenols, are highly abundant in olive leaves and have been found to exert a range of biological effects, such as anti-inflammatory, anti-microbial, and anti-cancer activity. Therefore, the high levels of polyphenols and flavonoids observed in OLE may contribute to its potential health benefits (Bucciantini et al., 2021, Chagas et al., 2022).
The DPPH assay is a widely used method to evaluate the antioxidant activity of natural compounds, and the strong antioxidant activity of OLE observed in this study suggests that OLE may have the potential to scavenge free radicals and protect against oxidative stress-related diseases. As a result, the findings of this study suggest that OLE may serve as a promising source of natural antioxidants with potential health benefits, especially in the context of oxidative stress-related diseases.
The antioxidant capacity of olive leaves is primarily ascribed to the existence of phenolic compounds (Goulas et al., 2010, Xie et al., 2015, Rahmania et al., 2015). The evaluation of individual phenolic compounds has linked their antioxidant effects to specific functional groups, quantities, and hydroxyl positions within their structures, which contribute to their redox properties (Goulas et al. 2010). Moreover, research findings suggest that phenolic compounds exhibit a synergistic effect on antioxidant capacity when present together, such as in OLE, which is greater than the sum of their individual effects (Xie et al., 2015, Lee and Lee, 2010).
To fully understand the potential implications of our phytochemical results, it is imperative to evaluate the oxidative status.
Our findings demonstrate a significant increase in lipid peroxidation in rats exposed to noise, which can be attributed to the augmented production of reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxyl radicals caused by the noise exposure. (Ben Hsouna et al., Ben Hsouna et al.., 2022; Hahad et al., 2019). Notably, our study also reveals a noteworthy rise in catalase and SOD activities in rats exposed to an 85 dB(A) sound level, which aligns with earlier investigations by Samson et al. (2007) and Gannouni et al. (2013).
Moreover, our investigation demonstrated a noteworthy surge in lipid peroxidation levels in rats that were exposed to toluene and exposed to both toluene and noise. These results are consistent with previous studies conducted by El-Nabi Kamel and Shehata (2008), indicating the presence of oxidative stress. Lipid peroxidation is a commonly used biomarker to assess oxidative stress in humans after exposure to harmful substances (Baydas, 2005). Toluene is known to stimulate the generation of reactive oxygen species during its metabolic process (Murata et al., 1999), which may account for the observed increase in lipid peroxidation. Likewise, a human study reported heightened levels of malondialdehyde, a lipid peroxidation byproduct, in the serum of individuals who were exposed to paint thinner (Halifeoglu et al., 2000).
Our study revealed that exposure to toluene or co-exposure to noise and toluene resulted in a significant decrease in catalase activity and an increase in superoxide dismutase (SOD) activity in rat hearts. This may be due to the excess production of superoxide anions, which can stimulate SOD activity leading to an accumulation of H2O2 that may exceed catalase's ability to detoxify it. In addition, catalase plays a crucial role in H2O2 detoxification by converting it into water and oxygen. Therefore, the interaction between SOD and catalase is essential to maintain cellular redox balance. When H2O2 levels exceed catalase's antioxidant capacity, it could result in a reduction of its activity.
These findings are consistent with previous studies that reported an increase in SOD activity and a decrease in catalase activity in rats exposed to toluene (Mehri et al., 2015). Volatile organic compounds have been identified to induce oxidative stress as a key mechanism of toxicity, primarily through the production of reactive oxygen species (ROS), which can disrupt the balance between ROS formation and the antioxidant defense system. Toluene exposure has been found to elevate the levels of malondialdehyde (MDA) in blood and tissues, while also reducing the activities of antioxidant enzymes (Coskun et al., 2005). Montes et al. (2019) similarly reported that sub-chronic toluene exposure in rats led to increased ROS production and altered antioxidant levels in the hippocampus and prefrontal cortex. These findings suggest that modulating antioxidant defenses could be a potential strategy for mitigating the adverse effects of toxicity caused by volatile organic compounds.
Interestingly, our results suggest that exposure to noise may exacerbate these effects, as we observed a greater decrease in catalase activity in rats co-exposed to toluene and noise compared to those exposed to toluene alone. These findings highlight the importance of considering the combined effects of multiple environmental stressors on oxidative stress markers.
In contrast, according to our findings, treatment with O. europaea leaf extract (N + OLE, T + OLE, NT + OLE) did not result in significant differences in antioxidant enzyme levels and lipid peroxidation in rat heart tissue compared to the control group. However, we did find a decrease in lipid peroxidation and an improvement in enzymatic antioxidant activities in rats treated with the extract. These effects may be attributed to the presence of oleuropein and hydroxytyrosol, which act as potent antioxidants by scavenging reactive oxygen species (ROS) and trapping free radicals to prevent cell damage.
The beneficial effects of Olea europea L. leaf extract are closely related to the abundance of bioactive compounds present in the extract. Previous research has demonstrated the antioxidant activity of O. europaea in rats, which is consistent with our findings (Turkez et al., 2012, Olmez et al., 2015; Elgebaly et al., 2018). These results suggest that O. europaea leaf extract may be a promising natural antioxidant therapy for oxidative stress-related diseases.
A noteworthy point is that oxidative stress has been suggested to contribute to the adverse health effects of noise exposure, including the promotion of blood coagulation and thrombus formation (Miller et al., 2012).
Significantly increased platelet counts were observed in rats exposed to noise (N) and co-exposed to noise and toluene (NT), while no significant changes were found in rats treated with O. europaea leaf extract. The increase in platelet count due to noise exposure resulted in the formation of platelet aggregates, which in turn led to obstruction of blood vessels in the heart, as observed in histological examination. Toukh et al. (2014) reported a correlation between hypercoagulability and elevated levels of cortisol and corticosterone in plasma due to exposure to construction noise. Noise-induced stress causes an increase in blood catecholamines, which subsequently stimulates thrombus formation and raises the risk of myocardial infarction (Haft & Fani 1973).
O. europaea leaf extract, which is rich in oleuropein and other phenolic compounds, has been shown to have a significant impact on maintaining platelet count. Numerous in vivo and in vitro studies have suggested that the phenolic compounds present in O. europaea leaf and oil extract may have anti-platelet effects, potentially protecting against platelet aggregation and its complications (De Roos et al., 2011; Rubio-sent et al., 2015; Tsantila et al., 2007, Turner et al., 2005).
In addition, exposure to noise (N), toluene (T), and co-exposure to noise and toluene (NT) resulted in an increase in the number of white blood cells and a decrease in red blood cell count, which was normalized in the groups of rats treated with O. europaea.
CK is an enzyme predominantly located in muscles and plays a crucial role in energy storage through creatine phosphorylation. Conversely, LDH is primarily present in plasma and is an indicator of cellular energy imbalance, leading to LDH accumulation and consequent cell death (Grub et al., 2000). In our study, we observed a significant rise in CK and LDH activities in the group of rats exposed to toluene, noise, and their combination. These findings suggest the possibility of cardiomyopathy in these rats, as increased CK and LDH activities are commonly associated with cardiac damage.
Moreover, we noted a significant increase in the levels of triglycerides and cholesterol in the groups of rats exposed to toluene, noise, and their combination. These results suggest that both isolated and combined exposure to toluene and noise can lead to disruptions in lipid metabolism and hypercholesterolemia. These lipid disturbances may contribute to the development of atherosclerosis, a condition characterized by the accumulation of lipids in the arterial walls, leading to vascular obstruction and an increased risk of cardiovascular diseases. The observed lipid disturbances in our study, combined with the CK and LDH elevation, highlight the potential for cardiovascular complications in rats exposed to toluene and noise.
However, treatment with Olea europaea L. leaf extract did not induce any significant changes in the aforementioned biochemical parameters. This finding suggests that Olea europaea L. extract may have a protective effect against the negative impacts of exposure to noise and toluene on the heart tissue. This is in line with previous studies that have reported the beneficial effects of Olea europaea L. extract on obesity and vascular dysfunction. For instance, Vezza et al. (2019) found that Olea europaea L. extract improved plasma and tissue metabolic profiles, as well as vascular function, in mice fed a high-fat diet. The extract's antioxidant and anti-platelet aggregation properties were found to reduce oxidative stress and improve endothelial function in the vascular wall. Thus, our results suggest that Olea europaea L. extract may have potential as a protective agent against the negative effects of environmental pollutants on cardiovascular health.
The histological examination of the myocardium provided valuable insights and a detailed investigation of the results obtained in our study. We observed structural and functional alterations in the rat myocardium due to noise exposure. In our study, noise exposure induced artery obstruction and blood clots in the myocardium of rats, which was absent in animals protected with O. europaea leaf extract. Additionally, rats exposed to 300 ppm toluene showed numerous inflammatory cells, which were corrected after treatment with O. europaea leaf extract. However, the myocardium of rats co-exposed to noise and toluene presented blocked arteries, blood clots, and numerous inflammatory cells. Interestingly, we observed no histological modifications in the group of rats receiving O. europaea extract compared to the sham group.
Previous research has also reported the beneficial effects of this extract on cardiovascular function, both in endothelial cells cultured in vitro (Burja et al., 2019) and in an experimental model of hypertension (Romero et al., 2016). These observations were consistent with the results of our hematological and antioxidant analyses.
Several studies have investigated the correlation between noise exposure, blood pressure, and myocardial damage, and our findings are consistent with those of previous research. Münzel et al. (2018) established a link between environmental noise exposure and adverse cardiovascular effects, and a longitudinal study by Eriksson et al. (2018) found that occupational noise exposure increases the risk of coronary artery disease. Gan et al. (2016) confirmed that intense occupational noise exposure is associated with the presence of coronary heart disease, and Banerjee et al. (2014) reported an association between mean noise levels exceeding 65 dB(A) and the occurrence of coronary heart disease.
5. Conclusion
To summarize, the study suggests that toluene and noise exposure can have cardiotoxic effects, as shown by changes in hematological and oxidative stress parameters and myocardial histopathology. The Olea Europaea L. (OLE) leaf extract demonstrated protective effects against these effects, indicating its potential as a safe and natural therapeutic option to counteract the adverse impact of environmental toxicants on cardiovascular health.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
We gratefully acknowledge support from the quality control laboratory of the Herbs of Tunisia Company in El Mansoura, Siliana, and Dr. Sana BAHRI, for providing the Olea europea L. leaf extract.
We are grateful to the Department of Pathology, Charles Nicolle Hospital, Tunis, Tunisia.
We would also like to acknowledge the support and cooperation of the experimental medicine unit’s team of the faculty of Medicine of Tunis in carrying out this work.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
References
- Aebi H. Catalase in vitro. Oxygen Radicals in Biological Systems. 1984:121–126. doi: 10.1007/978-1-4684-4705-6_13. [DOI] [Google Scholar]
- Assunta C., Ilaria S., Simone S., Gianfranco T., Teodorico C., Carmina S., Anastasia S., Roberto G., Francesco T., Valeria R.M. Noise and cardiovascular effects in workers of the sanitary fixtures industry. Int. J. Hyg. Environ. Health. 2015;218(1):163–168. doi: 10.1016/j.ijheh.2014.09.007. [DOI] [PubMed] [Google Scholar]
- ATSDR: Agence for Toxic Substances and Disease Registry. Toxicological profile for toluene. Department of Health and Human Services, Public Health Services. Atlanta, GA: U.S. 2000.
- Ayan, M., Tas, U., Sogut, E., Kuloglu, T., Cayli, S., Kocaman, N., Karaca, Z.I., & Sahin, M. The apoptotic effect of a high dose of toluene on liver tissue during the acute phase: an experimental study. Toxicology and Industrial Health, 29(8), 728–736. https://doi.org/10.1177/0748233712442731. [DOI] [PubMed]
- Bahri S., Abdennabi R., Nahdi A., Ali R., Mlika M., Jameleddine S. Effect of Tunisian Olive Leaf Extract on oxidative stress and lung fibrosis in rats. Eur. Respir. J. 2020;56:3382. congress-2020.3382. https://erj.ersjournals.com/content/56/suppl_64/3382. [Google Scholar]
- Ballantyne, B. Perspectives in basic and applied toxicology. Elsevier Science, Wiltshire, England.
- Banerjee, D., Das, P.P., & Foujdar, A. Association between road traffic noise and prevalence of coronary heart disease. Environmental monitoring and assessment, 186(5), 2885–2893. https://doi.org/10.1007/s10661-013-3587-3. [DOI] [PubMed]
- Basner, M., Babisch, W., Davis, A., Brink, M., Clark, C., Janssen, S., & Stansfeld, S. Auditory and non-auditory effects of noise on health. Lancet (London, England), 383(9925), 1325–1332. https://doi.org/10.1016/S0140-6736(13)61613-X. [DOI] [PMC free article] [PubMed]
- Baydas, G., Ozveren, F., Tuzcu, M., & Yasar, A. Effects of thinner exposure on the expression pattern of neural cell adhesion molecules, level of lipid peroxidation in the brain and cognitive function in rats. European Journal of Pharmacology, 2005 512(2-3), 181–187. https://doi.org/10.1016/j.ejphar.2005.02.038. [DOI] [PubMed]
- Ben Attia T., Gannouni N., Mhamdi F., Elj A., Magroun I., Mhamdi A. Effet de la co-exposition aux bruit-solvants sur la pression artérielle : à propos d’une enquête médicale et environnementale dans une entreprise de fabrication de produits composites. Revue Prévention & Ergonomie. 2022;16(2) https://www.asjp.cerist.dz/en/downArticle/448/16/2/197722 ISSN: 1112–7546, EISSN: 2676–2196. [Google Scholar]
- Ben Hsouna, A., Ghneim-Herrera, T., Ben Romdhane, W., et al. Early effects of salt stress on the physiological and oxidative status of the halophyte Lobularia maritima. Functional Plant Biology, 47(10), 912-924. [DOI] [PubMed]
- Ben Hsouna, M., Hfaiedh, S., Ben Slima, W.B., Romdhane, B.B., Akacha, M., Bouterra, M.T., Dhifi, W., Mnif, W., Brini, F., Ben Saad, R., & Ben Salah, R. Antioxidant and hepatoprotective effects of novel heteropolysaccharide isolated from Lobularia maritima on CCl4-induced liver injury in rats. Food Science & Nutrition, 10(7), [DOI] [PMC free article] [PubMed]
- Benignus V.A., Bushnell P.J., Boyes W.K. Toward cost-benefit analysis of acute behavioral effects of toluene in humans. Risk analysis: an official publication of the Society for Risk Analysis. 2005;25(2):447–456. doi: 10.1111/j.1539-6924.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- Beyer W.F., Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 1987;161(2):559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Bié J., Sepodes, Fernandes P.C.B., Ribeiro M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds. 2023;3:40–72. doi: 10.3390/compounds3010005. [DOI] [Google Scholar]
- Bingham, E., Cohrssen, B. and Patty, F. A. 2012. Patty's Toxicology, Volume Set, Sixth Edition. John Wiley & Sons, Washington, USA.
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bucciantini M., Leri M., Nardiello P., Casamenti F., Stefani M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants (Basel). 2021 Jun 29;10(7):1044. doi: 10.3390/antiox10071044. PMID: 34209636; PMCID: PMC8300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege J.A., Aust S.D. Microsomal lipid peroxidation. Biomembranes - Part C: Biological Oxidations. 1978:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Burja B., Kuret T., Janko T., Topalović D., Živković L., Mrak-Poljšak K., Spremo-Potparević B., Žigon P., Distler O., Čučnik S., Sodin-Semrl S., Lakota K., Frank-Bertoncelj M. Olive Leaf Extract Attenuates Inflammatory Activation and DNA Damage in Human Arterial Endothelial Cells. Frontiers in cardiovascular medicine. 2019;6:56. doi: 10.3389/fcvm.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagas M.D.S.S., Behrens M.D., Moragas-Tellis C.J., Penedo G.X.M., Silva A.R., Gonçalves-de-Albuquerque C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid Med Cell Longev. 2022 Sep;6(2022):9966750. doi: 10.1155/2022/9966750. PMID: 36111166; PMCID: PMC9470311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun O., Oter S., Korkmaz A., Armutcu F., Kanter M. The oxidative and morphological effects of high concentration chronic toluene exposure on rat sciatic nerves. Neurochem. Res. 2005;30(1):33–38. doi: 10.1007/s11064-004-9683-6. [DOI] [PubMed] [Google Scholar]
- Cruz S.L., Montoya Cruz M.T., Rivera-García A.L., Woodward J.J. Review of toluene action: Clinical evidence, animal studies and molecular targets. Journal of Drug and Alcohol Research. 2014;3:235840. doi: 10.4303/jdar/235840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cicco P., Maisto M.G.C., Tenore, Ianaro A. Olive Leaf Extract, from Olea europaea L., Reduces Palmitate-Induced Inflammation via Regulation of Murine Macrophages Polarization. Nutrients. 2020;12(12):3663. doi: 10.3390/nu12123663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roos B., Zhang X., Rodriguez Gutierrez G., Wood S., Rucklidge G.J., Reid M.D., Duncan G.J., Cantlay L.L., Duthie G.G., O'Kennedy N. Anti-platelet effects of olive oil extract: in vitro functional and proteomic studies. Eur. J. Nutr. 2011;50(7):553–562. doi: 10.1007/s00394-010-0162-3. [DOI] [PubMed] [Google Scholar]
- Elgebaly H.A., Mosa N.M., Allach M., El-Massry K.F., El-Ghorab A.H., Al Hroob A.M., Mahmoud A.M. Olive oil and leaf extract prevent fluoxetine-induced hepatotoxicity by attenuating oxidative stress, inflammation and apoptosis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;98:446–453. doi: 10.1016/j.biopha.2017.12.101. [DOI] [PubMed] [Google Scholar]
- El-Nabi Kamel M.A., Shehata M. Effect of toluene exposure on the antioxidant status and apoptotic pathway in organs of the rat. Br. J. Biomed. Sci. 2008;65(2):75–79. doi: 10.1080/09674845.2008.11732801. [DOI] [PubMed] [Google Scholar]
- Eriksson H.P., Andersson E., Schiöler L., Söderberg M., Sjöström M., Rosengren A., Torén K. Longitudinal study of occupational noise exposure and joint effects with job strain and risk for coronary heart disease and stroke in Swedish men. BMJ Open. 2018;8(4):e019160. doi: 10.1136/bmjopen-2017-019160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan W.Q., Moline J., Kim H., Mannino D.M. Exposure to loud noise, bilateral high-frequency hearing loss and coronary heart disease. Occup. Environ. Med. 2016;73(1):34–41. doi: 10.1136/oemed-2014-102778. [DOI] [PubMed] [Google Scholar]
- Gannouni N., Mhamdi A., Tebourbi O., El May M., Sakly M., Rhouma K.B. Qualitative and quantitative assessment of noise at moderate intensities on extra-auditory system in adult rats. Noise Health. 2013;15(67):406–411. doi: 10.4103/1463-1741.121236. [DOI] [PubMed] [Google Scholar]
- Golmohammadi R., Darvishi E. The combined effects of occupational exposure to noise and other risk factors - a systematic review. Noise Health. 2019;21(101):125–141. doi: 10.4103/nah.NAH_4_18. doi: 10.4103/nah.NAH_4_18. PMID: 32719300; PMCID: PMC7650855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas V., Papoti V.T., Exarchou V., Tsimidou M.Z., Gerothanassis I.P. Contribution of flavonoids to the overall radical scavenging activity of olive (Olea europaea L.) leaf polar extracts. J. Agric. Food Chem. 2010;58:3303–3308. doi: 10.1021/jf903823x. [DOI] [PubMed] [Google Scholar]
- Grub S., Persohn E., Trommer W.E., Wolf A. Mechanisms of cyclosporine A-induced apoptosis in rat hepatocyte primary cultures. Toxicol. Appl. Pharmacol. 2000;163(3):209–220. doi: 10.1006/taap.1999.8887. [DOI] [PubMed] [Google Scholar]
- Haft J.I., Fani K. Stress and the induction of intravascular platelet aggregation in the heart. Circulation. 1973 Jul;48(1):164–169. doi: 10.1161/01.cir.48.1.164. PMID: 4781235. [DOI] [PubMed] [Google Scholar]
- Hahad O., Prochaska J.H., Daiber A., Muenzel T. Oxidative Medicine and Cellular Longevity. 2019. Environmental Noise-Induced Effects on Stress Hormones, Oxidative Stress, and Vascular Dysfunction: Key Factors in the Relationship between Cerebrocardiovascular and Psychological Disorders; p. 4623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halifeoglu I., Canatan H., Ustundag B., Ilhan N., Inanc F. Effect of thinner inhalation on lipid peroxidation and some antioxidant enzymes of people working with paint thinner. Cell Biochem. Funct. 2000;18(4):263–267. doi: 10.1002/1099-0844(200012)18:4<263::AID-CBF882>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Hassan A.A., Bel Hadj Salah K., Fahmy E.M., Mansour D.A., Mohamed S.A.M., Abdallah A.A., Ashkan M.F., Majrashi K.A., Melebary S.J., El-Sheikh E.A., El-Shaer N. Olive Leaf Extract Attenuates Chlorpyrifos-Induced Neuro- and Reproductive Toxicity in Male Albino Rats. Life (Basel, Switzerland) 2022;12(10):1500. doi: 10.3390/life12101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinners R.G., Burkart J.K., Punte C.L. Animal inhalation exposure chambers. Arch. Environ. Health. 1968;16(2):194–206. doi: 10.1080/00039896.1968.10665043. [DOI] [PubMed] [Google Scholar]
- Lee O.H., Lee B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010;101:3751–3754. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Lee M.Y., Lin B.F., Chan M.H., Chen H.H. Increased behavioral and neuronal responses to a hallucinogenic drug after adolescent toluene exposure in mice: Effects of antipsychotic treatment. Toxicology. 2020;445 doi: 10.1016/j.tox.2020.152602. [DOI] [PubMed] [Google Scholar]
- Mehri S., Abnous K., Khooei A.R., Mousavi S.H. Protective effect of crocin against toluene-induced oxidative damage in rat liver and kidney. Iran. J. Basic Med. Sci. 2015;18(11):1103–1110. [Google Scholar]
- Mhamdi, F., Gannouni, N., Ben Attia, T., Mhamdi, A. 2021. Biomonitoring of coexposure to mixture of organic solvents and noise on hearing among industry workers. Journal of Prevention and Ergonomics. Vol:9, N°:3/2021. ISSN: 1112-7546. EISSN: 2676-2196. https://www.asjp.cerist.dz/en/downArticle/448/16/1/175176.
- Miller M.R., Shaw C.A., Langrish J.P. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012;8(4):577–602. doi: 10.2217/fca.12.43. [DOI] [PubMed] [Google Scholar]
- Mohammadi S., Labbafinejad S., Attarchi M. Combined effects of ototoxic solvents and noise on hearing in automobile plant workers in Iran. Arh Hig Rada Toksikol. 2010 Sep;61(3):267–274. doi: 10.2478/10004-1254-61-2010-2013. PMID: 20860967. [DOI] [PubMed] [Google Scholar]
- Montes S., Yee-Rios Y., Páez-Martínez N. Environmental enrichment restores oxidative balance in animals chronically exposed to toluene: Comparison with melatonin. Brain Res. Bull. 2019;144:58–67. doi: 10.1016/j.brainresbull.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Morata T.C., Dunn D.E., Kretschmer L.W., Lemasters G.K., Keith R.W. Effects of occupational exposure to organic solvents and noise on hearing. Scand J Work Environ Health. 1993;19(4):245–254. doi: 10.5271/sjweh.1477. PMID: 8235513. [DOI] [PubMed] [Google Scholar]
- Münzel T., Schmidt F.P., Steven S., Herzog J., Daiber A., Sørensen M. Environmental Noise and the Cardiovascular System. J. Am. Coll. Cardiol. 2018;71(6):688–697. doi: 10.1016/j.jacc.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Murata M., Tsujikawa M., Kawanishi S. Oxidative DNA damage by minor metabolites of toluene may lead to carcinogenesis and reproductive dysfunction. Biochem. Biophys. Res. Commun. 1999;261(2):478–483. doi: 10.1006/bbrc.1999.1041. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health. Manual of Analytical Methods, 4th ed.; National Institute for Occupational Safety and Health: Atlanta, GA. 2003.
- Olmez E., Vural K., Gok S., Ozturk Z., Kayalar H., Ayhan S., Var A. Olive Leaf Extract Improves the Atherogenic Lipid Profile in Rats Fed a High Cholesterol Diet. Phytother. Res. 2015;PTR, 29(10):1652–1657. doi: 10.1002/ptr.5445. [DOI] [PubMed] [Google Scholar]
- Rahmania N., Jafari S.M., Wani T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015;42:150–172. [Google Scholar]
- Romani A., Ieri F., Urciuoli S., Noce A., Marrone G., Nediani C., Bernini R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrient. 2019;11(8):1776. doi: 10.3390/nu11081776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero M., Toral M., Gómez-Guzmán M., Jiménez R., Galindo P., Sánchez M., Olivares M., Gálvez J., Duarte J. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016;7(1):584–593. doi: 10.1039/c5fo01101a. [DOI] [PubMed] [Google Scholar]
- Rubio-Senent F., de Roos B., Duthie G., Fernández-Bolaños J., Rodríguez-Gutiérrez G. Inhibitory and synergistic effects of natural olive phenols on human platelet aggregation and lipid peroxidation of microsomes from vitamin E-deficient rats. Eur. J. Nutr. 2015;54(8):1287–1295. doi: 10.1007/s00394-014-0807-8. [DOI] [PubMed] [Google Scholar]
- Rudrapal M., Khairnar S.J., Khan J., Dukhyil A.B., Ansari M.A., Alomary M.N., Alshabrmi F.M., Palai S., Deb P.K., Devi R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.806470. PMID: 35237163; PMCID: PMC8882865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şahin S., Bilgin M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: a review. J. Sci. Food Agric. 2018;98(4):1271–1279. doi: 10.1002/jsfa.8619. [DOI] [PubMed] [Google Scholar]
- Samson J., Sheeladevi R., Ravindran R. Oxidative stress in brain and antioxidant activity of Ocimum sanctum in noise exposure. Neurotoxicology. 2007;28(3):679–685. doi: 10.1016/j.neuro.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Skogstad M., Johannessen H.A., Tynes T., Mehlum I.S., Nordby K.C., Lie A. Systematic review of the cardiovascular effects of occupational noise. Occupational medicine (Oxford, England) 2016;66(6):500. doi: 10.1093/occmed/kqw113. [DOI] [PubMed] [Google Scholar]
- Sørensen M. Aircraft noise exposure and hypertension. Occup. Environ. Med. 2017;74(2):85–86. doi: 10.1136/oemed-2016-103988. [DOI] [PubMed] [Google Scholar]
- Tas U., Ogeturk M., Kuloglu T., Sapmaz H.I., Kocaman N., Zararsiz I., Sarsilmaz M. HSP70 immune reactivity and TUNEL positivity in the liver of toluene-inhaled and melatonin-treated rats. Toxicol. Ind. Health. 2013;29(6):514–522. doi: 10.1177/0748233712440138. [DOI] [PubMed] [Google Scholar]
- Toukh M., Gordon S.P., Othman M. Construction noise induces hypercoagulability and elevated plasma corticosteroids in rats. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2014;20(7):710–715. doi: 10.1177/1076029613483168. [DOI] [PubMed] [Google Scholar]
- Trigui A., Msallem M. IRESA (Ministère de l’Agriculture), Institut de l’Olivier, Tunisia Volume I. 2002. Oliviers de Tunisie : Catalogue des Variétés Autochtones & Types Locaux : Identification variétale & Caractérisation morpho-pomologique des Ressources Génétiques Oléicoles de Tunisie; p. 159. [Google Scholar]
- Tsantila N., Karantonis H.C., Perrea D.N., Theocharis S.E., Iliopoulos D.G., Antonopoulou S., Demopoulos C.A. Antithrombotic and antiatherosclerotic properties of olive oil and olive pomace polar extracts in rabbits. Mediators Inflamm. 2007;2007:36204. doi: 10.1155/2007/36204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H., Togar B., Polat E. Olive leaf extract modulates permethrin induced genetic and oxidative damage in rats. Cytotechnology. 2012;64(4):459–464. doi: 10.1007/s10616-011-9424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R., Etienne N., Alonso M.G., de Pascual-Teresa S., Minihane A.M., Weinberg P.D., Rimbach G. Antioxidant and anti-atherogenic activities of olive oil phenolics. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition. 2005;75(1):61–70. doi: 10.1024/0300-9831.75.1.61. [DOI] [PubMed] [Google Scholar]
- Vezza T., Rodríguez-Nogales A., A lgieri F., Garrido-Mesa J., Romero M., Sánchez M., Toral M., Martín-García B., Gómez-Caravaca A.M., Arráez-Román D., Segura-Carretero A., Micol V., García F., Utrilla M.P., Duarte J., Rodríguez-Cabezas M.E., Gálvez J. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019;150:104487. doi: 10.1016/j.phrs.2019.104487. [DOI] [PubMed] [Google Scholar]
- Wayman W.N., Woodward J.J. Exposure to the Abused Inhalant Toluene Alters Medial Prefrontal Cortex Physiology. Neuropsychopharmacology : official publication of the American College of. Neuropsychopharmacology. 2018;43(4):912–924. doi: 10.1038/npp.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P., Huang L., Zhang C., Zhang Y. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods. 2015;16:460–471. [Google Scholar]
- Yateem H., Afaneh I., Al-Rimawi F. Optimum conditions for oleuropein extraction from olive leaves. Int. J. Appl. Sci. Technol. 2014;4:153–157. [Google Scholar] [Google Scholar]