Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is an inherited non-malignant lymphoproliferative disorder characterized by heterozygous mutations within the first apoptosis signal receptor (FAS) signaling pathway. Defects in FAS-mediated apoptosis cause an expansion and accumulation of auto-reactive CD4 negative and CD8 negative (double negative) T-cells, leading to cytopenias, splenomegaly, lymphadenopathy, autoimmune disorders, and a greatly increased lifetime risk of lymphoma. The differential diagnosis of ALPS includes infection, other inherited immunodeficiency disorders, primary and secondary autoimmune syndromes, and lymphoma. The most consistent pathologic feature is a florid paracortical expansion of double negative T-cells in lymph nodes. A presumptive clinical diagnosis can be made based on symptoms and a constellation of laboratory test results. However, a definitive diagnosis requires ancillary testing and enables disease subclassification. Recognition of ALPS is critical as treatment with immunosuppressive therapies can effectively reduce or ameliorate symptoms in a majority of patients.
The first apoptosis signal receptor (FAS) pathway regulates apoptosis and is critical for proper development and functioning of the immune system.1 Autoimmune lymphoproliferative syndrome (ALPS) is an inherited lymphoproliferative disorder caused by heterozygous mutations in genes within the FAS pathway.2,3 ALPS was first recognized as an entity in 1992, when Sneller et. al. identified similar symptoms in children suffering from familial non-malignant lymphadenopathy and splenomegaly, and two recently created mouse strains which had previously been shown to harbor loss of function mutations in FAS or Fas ligand (FASL).4 Although ALPS classically results from mutations in FAS itself, mutations in several other genes have been described and the use of gene-based nomenclature is now recommended when describing all new cases.5 ALPS shows characteristic pathologic findings and its recognition is important to both avoid a false diagnosis of malignancy and to steer patients towards immunosuppressive therapies which often ameliorate symptoms.6 Here we examine the clinical spectrum of ALPS, highlight its pathologic features, review diagnostic pitfalls, and discuss clinical management.
Pathophysiology
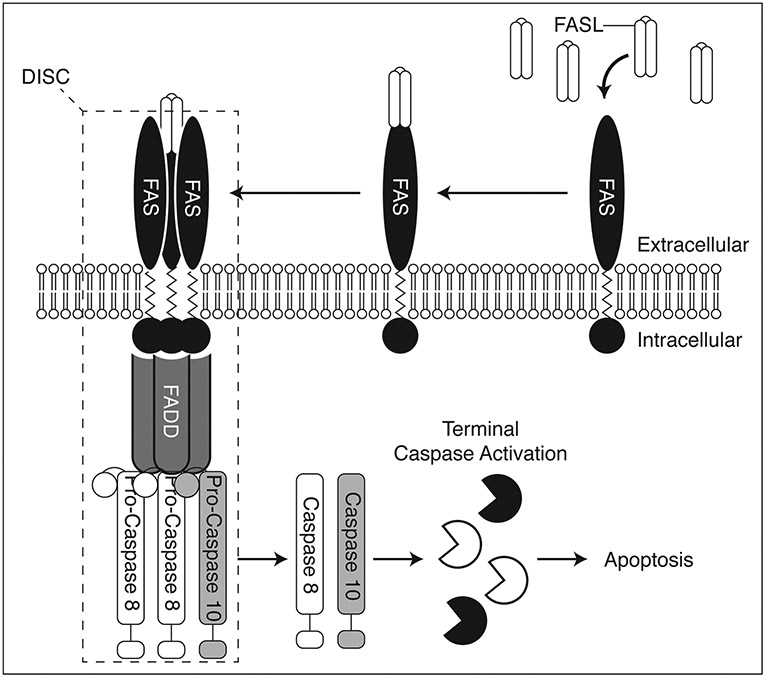
The histopathological findings in ALPS nicely correlate with our understanding of the underlying disease biology (Figure 1). Under normal conditions, T-cell activation induces expression of FASL which can bind to FAS receptors on the same or nearby cells. This event results in clustering of FAS receptors and binding of FAS-associated protein with death domain (FADD). FADD then recruits Pro-Caspase 8 and Pro-Caspase 10 to form the death-inducing signaling complex (DISC), which can propagate a signal leading to terminal caspase activation and apoptosis. This process is known as activation-induced cell death (AICD).7
Figure 1.
Simplified diagram depicting the FAS pathway involved in activation-induced cell death (AID). FAS ligand (FASL) binds FAS, causing clustering of FAS. This recruits FAS-associated protein with death domain (FADD) and Pro-Caspases 8 and 10, which together with FAS constitute the death-inducing signaling complex (DISC). The DISC then propagates a pro-apoptotic signal via the activation of terminal caspases.
In healthy individuals, peripheral T-cells that react with self antigens undergo repeated stimulation which leads to increased FASL production, DISC formation, and AICD. This system prevents the expansion of autoreactive T-cells which can lead to autoimmune disease. In contrast, ALPS patients have mutations in members of the FAS pathway which render their T-cells defective in AICD. These mutations are typically inherited in an autosomal dominant manner, although select mutations may be inherited in an autosomal recessive fashion or occur sporadically over an individual’s lifetime. ALPS patients accumulate self-reactive T-cells which are classically negative for both CD4 and CD8 (double negative), and positive for the alpha-beta (α/ß) T-cell receptor (TCR). These double negative T-cells resemble terminally differentiated T-cells but are highly proliferative due in part to the upregulation of mammalian target of rapamycin (mTOR) and other growth pathways.8 Interestingly, disease penetrance is variable in ALPS and asymptomatic mutation positive persons may have severe lymphocyte apoptosis defects but do not demonstrate an increase in double negative T-cells and show no evidence of disease. This has led to a ‘double hit’ hypothesis in ALPS pathogenesis, where the second hit may represent an additional genetic mutation or exposure to environmental factors. The reason why some mutation positive individuals develop ALPS while others do not remains an area of active research.
Clinical Features
ALPS-associated mutations show an incomplete penetrance that varies by both the affected gene and the mutation type, with the most common FAS mutations showing a reported progression to ALPS of approximately 60%.9 Affected patients develop disease at a median age of 11.5 months, and demonstrate a spectrum of clinical findings as a consequence of non-malignant lymphoid hyperplasia and the progressive accumulation of autoreactive B and T lymphocytes.10 The classic presentation is an adolescent with non-infectious, non-malignant lymphadenopathy and splenomegaly, which are present in 95% and 90% of cases respectively. Splenomegaly may be profound (greater than 10X normal size) and necessitate chest bracing to minimize the risk of splenic rupture. Hepatomegaly is present in up to 50% of cases.
The accumulation of autoreactive T-cells triggers a constellation of autoimmune symptoms that varies by patient. Immune-mediated cytopenias are the most common and may affect any lineage. The most frequent of these is autoimmune hemolytic anemia which can be severe. Also common are idiopathic thrombocytopenic purpura (ITP) resulting in excessive bruising and bleeding, and autoimmune neutropenia which can persist even after splenectomy. Cytopenias are often accompanied by a concurrent increase or decrease in serum IgG levels (70% of patients). New cytopenias in ALPS patients have been reported in patients as old as 54 years and it remains unclear whether the frequency and severity of cytopenias improves with age, as is the case with many pediatric autoimmune cytopenias.11 Autoimmune syndromes involving solid organs are much less common, but reported sites of involvement include: kidneys (glomerulonephritis), liver (autoimmune hepatitis), connective tissue (systemic lupus erythematosus), eyes (uveitis), thyroid (thyroiditis), skin (urticarial rash), and nervous system (Guillain-Barre syndrome).10,12-14
ALPS patients also have a greatly increased risk for the development of secondary malignancies which does not diminish with age, extends across mutation types, and represents a significant cause of morbidity and mortality.15 This includes a 150-fold increased risk for Hodgkin lymphomas and a 14-fold increased risk for non-Hodgkin lymphomas. Lymphomas in ALPS patients typically retain the heterozygous FAS mutations and are defective in FAS-mediated killing.
Gross Pathology
In ALPS, the lymph nodes and spleen are enlarged and the cut surfaces show a diffuse parenchymal expansion.
Histology
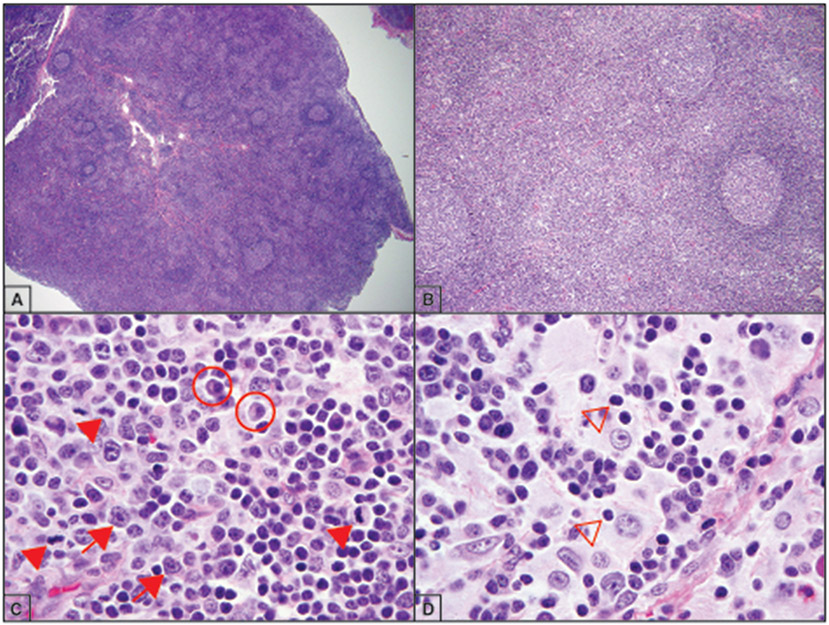
The most consistent histological findings in ALPS are present in the lymph nodes, where there is follicular hyperplasia and a florid paracortical expansion of small proliferative lymphocytes, immunoblasts, and plasma cells (Figure 2A-B).6 Mitotic figures are frequent in the paracortical zones, consistent with the proliferative nature of these cells (Figure 2C). Overall architecture is preserved, which helps to differentiate it from T-cell lymphoma. Lymph nodes may also demonstrate a marked expansion of sinus histiocytes lacking Langerhans-type features and show phagocytosis of lymphocytes (emperipolesis), features more commonly associated with sinus histiocytosis with massive lymphadenopathy (SHML) (Figure 2D).16,17
Figure 2.
Lymph nodes in autoimmune lymphoproliferative syndrome (ALPS) patients often show characteristic changes. A-B) Enlarged lymph node with a florid follicular hyperplasia composed of small mature-appearing lymphocytes (hematoxylin-eosin, original magnification ×2 and ×10). C) The paracortical lymphocytes are highly proliferative (arrowheads), and may be associated with both immunoblasts (arrows) and plasma cells (circled) (hematoxylin-eosin, original magnification ×100). D) Sinus histiocytosis with emperipolesis (arrow heads) may be seen in a minority of cases (hematoxylin-eosin, original magnification ×100).
Immunohistochemistry (IHC) demonstrates increased numbers of paracortical double negative T-cells which are CD3 positive, CD4 negative, and CD8 negative. These double negative T-cells are positive for HLADR, an indicator of prior activation, although interestingly they are also frequently positive for CD45RA which is a marker of naïve T-cells.6 There is no loss of pan T-cell antigens (CD2, CD5, CD7), in contrast to T-cell lymphoma. Staining for Ki67 is elevated in the paracortical zones. Though typically not performed in the clinical setting, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining shows a normal level of apoptosis within germinal centers with greatly decreased or absent apoptosis in the paracortex. When present, the expanded sinus histiocytes may be positive for S100, again mimicking SHML.
The spleens of ALPS patients show a marked red and white pulp expansion with increased numbers of double negative T-cells.6 The white pulp shows a marked follicular hyperplasia while the red pulp contains increased numbers of lymphocytes, immunoblasts, and plasma cells.
The bone marrow is typically normocellular, although hypercellular and hypocellular cases have been reported.18 Lymphocytosis is the most commonly reported feature, and this may be accompanied by a mild eosinophilic hyperplasia in up to half of cases. Dysplastic changes and hemophagocytosis are not present.
Biopsies from other organs typically show a non-destructive lymphocytic infiltrate composed predominantly of double negative T-cells. Hepatomegaly may be present, and ALPS cases with concurrent periportal fibrosis, extramedullary hematopoiesis, and chronic active hepatitis have been reported.6
Flow Cytometry
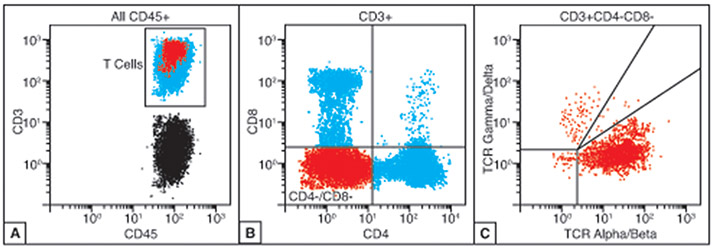
When material is available, flow cytometry can be very helpful in cases of suspected ALPS and is often uniquely well-suited for TCR subtyping. Analysis of the CD45 positive, CD3 positive T-cell population should show a distinct CD4 negative and CD8 negative subset representing the defective double negative T-cells (Figure 3A-B). TCR subtyping of the double negative population showing restricted α/ß TCR expression strongly supports the diagnosis of ALPS (Figure 3C).
Figure 3.
Characteristic flow cytometric findings in autoimmune lymphoproliferative syndrome (ALPS). A-B) The peripheral blood of ALPS patients will show an expanded population of CD3+ T-cells that are negative for both CD4 and CD8 (double negative, highlighted in red). C) The population of double negative T-cells should be positive for alpha-beta (α/ß) T-cell receptor (TCR).
Laboratory Findings
There is no single laboratory abnormality that is diagnostic of ALPS. However, select findings are either required for the diagnosis or strongly support it (Table 1).5 Per the 2010 revised criteria, patients should demonstrate an elevated number of circulating double negative α/ß T-cells comprising greater than 1.5% of total lymphocytes or at least 2.5% of total T-cells. In combination with chronic lymphadenopathy or splenomegaly for greater than 6 months, these constitute the two required findings for a clinical diagnosis of ALPS and should trigger ancillary testing.
Table 1.
Revised Diagnostic Criteria for the Diagnosis of ALPSa
| Required |
|---|
|
| Accessory |
Primary
|
Secondary
|
Abbreviations: ALPS, autoimmune lymphoproliferative syndrome; FAS, first apoptosis signal receptor; FASL, first apoptosis signal receptor ligand; CASP10, caspase 10.
A definitive diagnosis must meet both required criterion and at least one primary accessory criteria. A probable diagnosis can be made based on the presence of both required criterion and at least one secondary accessory criteria.
When the above required criteria are met, secondary laboratory findings can favor a probable diagnosis of ALPS but are not strictly required. These include elevated plasma soluble FASL (sFASL) >200 pg/mL, elevated plasma interleukin 10 (IL-10), elevated plasma interleukin 18 (IL-18), or elevated plasma vitamin B12.19 Pathologic findings, concurrent cytopenias, and a positive family history are also supportive. However, clinical correlation is critical as a similar signature can be seen in common variable immunodeficiency (CVID) and Evans syndrome (autoimmune hemolytic anemia and thrombocytopenia), both of which are sometimes considered overlap syndromes with ALPS.20,21
Ancillary Testing
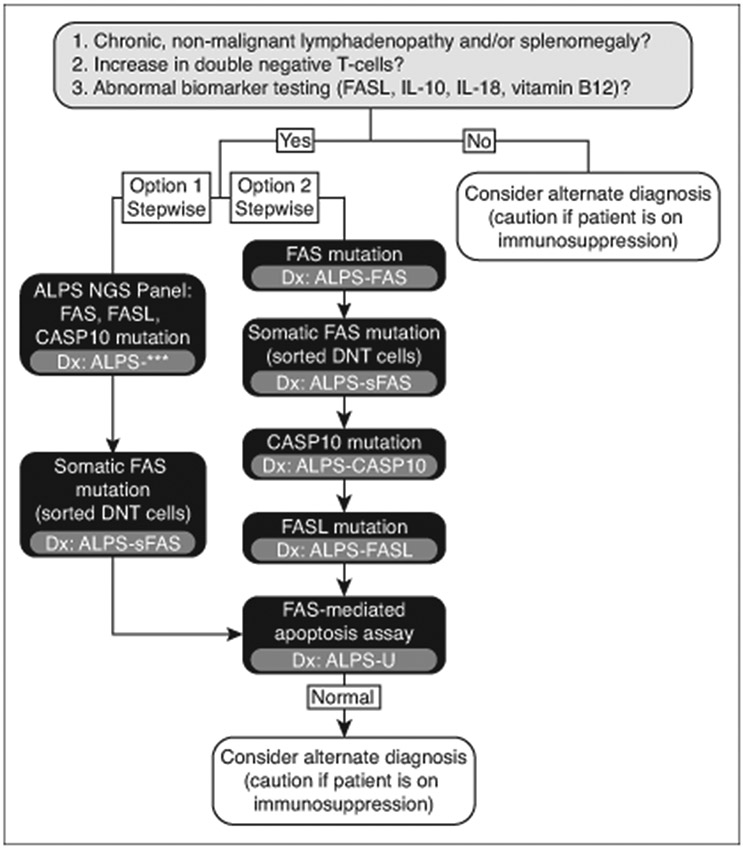
Confirmatory testing should never delay treatment in cases of suspected ALPS. However, testing for ALPS-related mutations or functional testing of patient T-cells is required for a definitive diagnosis per the 2010 revised guidelines (Table 1).5 Testing for ALPS-related mutations also allows for subclassification of the disease (Table 2) which can guide the testing of relatives and potentially provide prognostic information and inform treatment planning. Patients who meet clinical criteria for suspected ALPS should undergo step-wise testing for ALPS-related mutations according to algorithms derived from the current diagnostic criteria (Figure 4). Alternatively, a single multigene panel can be performed. Approximately 30% of ALPS patients do not have an identifiable genetic mutation. In these cases, functional assays remain the gold standard and can detect defective FAS-mediated apoptosis in the T-cells of a vast majority of ALPS patients regardless of the causative mutation. If functional assays are pursued, defective lymphocyte apoptosis should be demonstrated in two separate assays.
Table 2.
Diagnostic Subtypes of ALPSa
| Diagnosis | Definition |
|---|---|
| ALPS-FAS | Meets clinical criteria for ALPS plus germline FAS mutation |
| ALPS-sFAS | Meets clinical criteria for ALPS plus somatic FAS mutation |
| ALPS-FASL | Meets clinical criteria for ALPS plus germline FASL mutation |
| ALPS-CASP10 | Meets clinical criteria for ALPS plus germline CASP10 mutation |
| ALPS-U | Meets clinical criteria for ALPS but no detected genetic lesion |
Abbreviations: ALPS, autoimmune lymphoproliferative syndrome; FAS, first apoptosis signal receptor; FASL, first apoptosis signal receptor ligand; CASP10, caspase 10.
According to the revised 2010 criteria.
Figure 4.
Diagnostic algorithm for autoimmune lymphoproliferative syndrome (ALPS). A presumptive diagnosis of ALPS may be rendered based on clinical history and the results of laboratory testing. A definitive diagnosis requires genetic testing and potentially functional assays. Genetic testing can be performed in a stepwise fashion and may incorporate multigene panels. *** = diagnosis depends on results of multigene testing; IL = interleukin; NGS = next generation sequencing; DNT = double negative T; sFAS = somatic FAS; FASL = FAS ligand; CASP10 = Caspase 10.
Differential Diagnosis
ALPS is a rare entity whose true incidence is unknown. As such, careful consideration should be given to alternative disease processes before rendering a presumptive ALPS diagnosis. The differential includes infection, autoimmune diseases, other inherited disorders involving the immune system, and lymphoma.2 Among these, self-limited viral infections are an especially common cause of pediatric lymphadenopathy. These include Epstein-Barr virus (EBV) infection which can also cause splenomegaly, fatigue, and a transient increase in circulating double negative T-cells. While these symptoms may mimic ALPS, EBV infection should show a subset of EBER positive lymphocytes and reactive double negative T-cells that express the γ/δ TCR. In contrast, ALPS is not associated with EBV infection and double negative T-cells express the α/ß TCR.
One of the manifestations of ALPS is the production of self-reactive lymphocytes leading to autoimmune symptoms, commonly cytopenias. As such, the differential for ALPS includes other primary and secondary immune-mediated cytopenias affecting platelets (immune thrombocytopenia), red blood cells (autoimmune hemolytic anemia), or neutrophils (autoimmune neutropenia). Multi-lineage antibody-mediated cytopenias, especially those involving red blood cells and platelets, are often collectively referred to as Evans syndrome.22 Evans syndrome can also be associated with elevated numbers of double negative T-cells and many consider it to be an overlap syndrome with ALPS, although lymphadenopathy is typically absent.20 Follow up studies, if warranted, will fail to reveal an ALPS-associated mutation and FAS-mediated lymphocyte apoptosis will be intact.
Any differential diagnosis for ALPS should include alternative and generally more common inherited conditions which can produce similar symptoms in pediatric patients. The most notable of these is CVID.23 CVID is a poorly understood disorder resulting in immune impairment which has an estimated frequency of 1 in 25,000-50,000 people worldwide. The cause is unknown in up to 90% of cases. CVID patients often present in childhood with recurrent infections due to low levels of circulating antibodies. However, up to 25% also have an autoimmune disorder which typically involves the hematopoietic system. CVID patients tend to have mature B-cells but absent or diminished memory B-cells, and frequently present with lymphadenopathy and splenomegaly. They often have a history of recurrent infections, low or absent numbers of multiple antibody classes, intact ALPS-associated genes, and intact FAS-mediated lymphocyte apoptosis. Additional inherited conditions in the differential diagnosis of ALPS include: X-linked lymphoproliferative syndrome, hyperimmunoglobulin M syndrome, Wiskott-Aldrich syndrome, gray platelet syndrome, and RAS-associated autoimmune leukoproliferative disorder (RALD).24
Finally, the differential of ALPS includes lymphomas, especially T-cell lymphoma.25 The paracortical expansion of double negative T-cells seen in ALPS may be misinterpreted as architectural effacement. This, coupled with splenic and bone marrow involvement by double negative T-cells, can suggest the diagnosis of peripheral T-cell lymphoma. In such cases, demonstration of polyclonality by TCR subtyping or T-cell clonality testing can be helpful in ruling out a clonal neoplasm.
Management
Up to 50% of ALPS patients require immunosuppression for treatment of their autoimmune manifestations. The most important goals of therapy are to resolve life threatening cytopenias and avoid splenectomy.26 Typically, short duration treatment with high dose corticosteroids is successful in the acute setting and patients are transitioned to steroid-sparing immunosuppressive regimens to avoid the side effects of prolonged corticosteroid therapy. Mycophenolate mofetil has been used for this purpose and leads to improved outcome in ALPS patients.27 Rituximab is also useful in ALPS, but should be avoided if possible due to the risk of prolonged hypogammaglobulinemia which appears to be somewhat unique to ALPS patients.28 Both steroid and steroid-sparing treatment may be supplemented with intravenous immunoglobulin (IVIG), if necessary. Recombinant granulocyte-colony stimulating factor (GCSF) may be useful in the management of isolated neutropenia. Work up to rule out lymphoma is warranted in those who develop constitutional signs and symptoms or unusual change to disease course. Fluorodeoxyglucose-positron emission tomography (FDG-PET) is not particularly helpful in distinguishing lymphoma from ALPS since both are PET-avid.
Splenectomy should be avoided if possible, as it leads to significantly worse outcomes in ALPS patients.29 This is unlike other refractory autoimmune cytopenias of childhood, in which splenectomy is often a mainstay of treatment. Regimens used to treat cytopenias are usually successful in resolving hypersplenism. However, splenectomy may be necessary in cases of uncontrolled hypersplenism that fails medical treatment. It is recommended that such patients remain on long-term antibiotic prophylaxis and maintain their vaccinations.
Recently, the mTOR inhibitor sirolimus was shown to be highly effective in the amelioration of ALPS-related refractory cytopenias in a small multicenter clinical trial.30 Although long-term follow-up data is currently lacking, sirolimus should be strongly considered in cases of treatment-refractory ALPS and may soon be regarded as the recommended first-line steroid-sparing therapy for these patients. Currently, the only known cure for ALPS is hematopoietic stem cell transplant, but this is reserved for severe cases refractory to immunosuppressive regimens.
Prognosis
The major causes of morbidity and mortality in ALPS are splenectomy-related sepsis and the development of lymphomas.29 Many ALPS patients can ultimately control their disease and with long-term treatment, are likely to experience a decrease or complete resolution of their lymphadenopathy, splenomegaly, and autoimmune symptoms. Attempts to correlate select ALPS-related mutations with prognosis have been challenging due to the relatively limited data available, however patients with certain mutations in the intracellular domain of FAS may have a worse prognosis, while those with mutations in the extracellular domain may experience more mild disease.9
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30(2):180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teachey DT, Seif AE, Grupp SA. Advances in the management and understanding of autoimmune lymphoproliferative syndrome (ALPS). Br J Haematol. 2010;148(2):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bride K, Teachey D. Autoimmune lymphoproliferative syndrome: more than a FAScinating disease. F1000Research. 2017;6:1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sneller MC, Straus SE, Jaffe ES, et al. A novel lymphoproliferative/autoimmune syndrome resembling murine lpr/gld disease. J Clin Invest. 1992;90(2):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira JB, Bleesing JJ, Dianzani U, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116(14):e35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim MS, Straus SE, Dale JK, et al. Pathological findings in human autoimmune lymphoproliferative syndrome. Am J Pathol. 1998;153(5):1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. [DOI] [PubMed] [Google Scholar]
- 8.Völkl S, Rensing-Ehl A, Allgäuer A, et al. Hyperactive mTOR pathway promotes lymphoproliferation and abnormal differentiation in autoimmune lymphoproliferative syndrome. Blood. 2016;128(2):227–238. [DOI] [PubMed] [Google Scholar]
- 9.Jackson CE, Fischer RE, Hsu AP, et al. Autoimmune lymphoproliferative syndrome with defective Fas: genotype influences penetrance. Am J Hum Genet. 1999;64(4):1002–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sneller MC, Wang J, Dale JK, et al. Clinical, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89(4):1341–1348. [PubMed] [Google Scholar]
- 11.Infante AJ, Britton HA, DeNapoli T, et al. The clinical spectrum in a large kindred with autoimmune lymphoproliferative syndrome caused by a Fas mutation that impairs lymphocyte apoptosis. J Pediatr. 1998;133(5):629–633. [DOI] [PubMed] [Google Scholar]
- 12.Kanegane H, Vilela MM dos S, Wang Y, Futatani T, Matsukura H, Miyawaki T. Autoimmune lymphoproliferative syndrome presenting with glomerulonephritis. Pediatr Nephrol Berl Ger. 2003;18(5):454–456. [DOI] [PubMed] [Google Scholar]
- 13.Pensati L, Costanzo A, Ianni A, et al. Fas/Apo1 mutations and autoimmune lymphoproliferative syndrome in a patient with type 2 autoimmune hepatitis. Gastroenterology. 1997;113(4):1384–1389. [DOI] [PubMed] [Google Scholar]
- 14.Lim W-K, Ursea R, Rao K, et al. Bilateral uveitis in a patient with autoimmune lymphoproliferative syndrome. Am J Ophthalmol. 2005;139(3):562–563. [DOI] [PubMed] [Google Scholar]
- 15.Straus SE, Jaffe ES, Puck JM, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98(1):194–200. [DOI] [PubMed] [Google Scholar]
- 16.Maric I, Pittaluga S, Dale JK, et al. Histologic features of sinus histiocytosis with massive lymphadenopathy in patients with autoimmune lymphoproliferative syndrome. Am J Surg Pathol. 2005;29(7):903–911. [DOI] [PubMed] [Google Scholar]
- 17.Rudman Spergel A, Walkovich K, Price S, et al. Autoimmune lymphoproliferative syndrome misdiagnosed as hemophagocytic lymphohistiocytosis. Pediatrics. 2013;132(5):e1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Pittaluga S, Price S, et al. Bone marrow findings in autoimmune lymphoproliferative syndrome with germline FAS mutation. Haematologica. 2017;102(2):364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caminha I, Fleisher TA, Hornung RL, et al. Using biomarkers to predict the presence of FAS mutations in patients with features of the autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol. 2010;125(4):946–949.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seif AE, Manno CS, Sheen C, Grupp SA, Teachey DT. Identifying autoimmune lymphoproliferative syndrome in children with Evans syndrome: a multi-institutional study. Blood. 2010;115(11):2142–2145. [DOI] [PubMed] [Google Scholar]
- 21.Savaşan S, Warrier I, Buck S, Kaplan J, Ravindranath Y. Increased lymphocyte Fas expression and high incidence of common variable immunodeficiency disorder in childhood Evans’ syndrome. Clin Immunol Orlando Fla. 2007;125(3):224–229. [DOI] [PubMed] [Google Scholar]
- 22.Norton A, Roberts I. Management of Evans syndrome. Br J Haematol. 2006;132(2):125–137. [DOI] [PubMed] [Google Scholar]
- 23.Abolhassani H, Sagvand BT, Shokuhfar T, Mirminachi B, Rezaei N, Aghamohammadi A. A review on guidelines for management and treatment of common variable immunodeficiency. Expert Rev Clin Immunol. 2013;9(6):561–574; quiz 575. [DOI] [PubMed] [Google Scholar]
- 24.Niemela JE, Lu L, Fleisher TA, et al. Somatic KRAS mutations associated with a human nonmalignant syndrome of autoimmunity and abnormal leukocyte homeostasis. Blood. 2011;117(10):2883–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poppema S, Maggio E, van den Berg A. Development of lymphoma in Autoimmune Lymphoproliferative Syndrome (ALPS) and its relationship to Fas gene mutations. Leuk Lymphoma. 2004;45(3):423–431. [DOI] [PubMed] [Google Scholar]
- 26.Rao VK, Oliveira JB. How I treat autoimmune lymphoproliferative syndrome. Blood. 2011;118(22):5741–5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kossiva L, Theodoridou M, Mostrou G, et al. Mycophenolate mofetil as an alternate immunosuppressor for autoimmune lymphoproliferative syndrome. J Pediatr Hematol Oncol. 2006;28(12):824–826. [DOI] [PubMed] [Google Scholar]
- 28.Cooper N, Davies EG, Thrasher AJ. Repeated courses of rituximab for autoimmune cytopenias may precipitate profound hypogammaglobulinaemia requiring replacement intravenous immunoglobulin. Br J Haematol. 2009;146(1):120–122. [DOI] [PubMed] [Google Scholar]
- 29.Price S, Shaw PA, Seitz A, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123(13):1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klemann C, Esquivel M, Magerus-Chatinet A, et al. Evolution of disease activity and biomarkers on and off rapamycin in 28 patients with autoimmune lymphoproliferative syndrome. Haematologica. 2017;102(2):e52–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]