Abstract
This study analyses the spontaneous electroencephalogram (EEG) brain activity of 14 children diagnosed with Autism Spectrum Disorder (ASD) compared to 18 children with normal development, aged 5–11 years. (i) Power Spectral Density (PSD), (ii) variability across trials (coefficient of variation: CV), and (iii) complexity (multiscale entropy: MSE) of the brain signal analysis were computed on the resting state EEG. PSD (0.5–45 Hz) and CV were averaged over different frequency bands (low-delta, delta, theta, alpha, low-beta, high-beta and gamma). MSE were calculated with a coarse-grained procedure on 67 time scales and divided into fine, medium and coarse scales. In addition, significant neurophysiological variables were correlated with behavioral performance data (Kaufman Brief Intelligence Test (KBIT) and Autism Spectrum Quotient (AQ)). Results show increased PSD fast frequency bands (high-beta and gamma), higher variability (CV) and lower complexity (MSE) in children with ASD when compared to typically developed children. These results suggest a more variable, less complex and, probably, less adaptive neural networks with less capacity to generate optimal responses in ASD children.
Keywords: Autism spectrum disorder, Multiscale entropy, Power spectral density, Variability, Resting-state
Introduction
Autism spectrum disorder (ASD) is one of the most prevalent neurodevelopmental disorders in childhood. ASD presents a high degree of heritability (64–91%) (Muhle et al. 2004; Tick et al. 2016). Its etiology is still unknown and although some studies point to genetic alterations, most diagnosed children are non-syndromic. The main symptomatology is characterized by socio-communicative deficits, restricted interests, and stereotyped behaviors (American Psychiatric Association 2013). These clinical signs can be present as early as 12 months, however, diagnosis is currently delayed until the age of 4 years or older (Wang et al. 2013). In the time up to diagnosis, subtle changes in brain function may precede behavioral symptoms that indicate developmental problems. This leads to the need to find potential biomarkers for early detection that would help and support diagnosis (Simon et al. 2017). In this sense, non-invasive techniques for human brain research, such as electroencephalography (EEG), are proposed as an advantageous alternative. The resting state condition require little environmental demands and subject involvement (Wang et al. 2013), which accommodates the clinical characteristics of children diagnosed with ASD, such as their high sensitivity (Simon et al. 2017).
Neurophysiological studies with EEG which have analyzed neural correlates of ASD use linear measures, such as Power Spectral Density (PSD) (Chan et al. 2007; DiStefano et al. 2019; Pierce et al. 2021). The PSD measured as relative power (relationship between bands) or absolute power (degree of electrophysiological activity present in a specific band) has supported the U-shaped curve hypothesis in ASD patients proposed by Wang et al. (2013). The U-shaped curve hypothesis refers to higher power in both low-frequency bands (e.g. delta and theta; Daoust et al. 2004; Chan et al. 2007; Pop-Jordanova et al 2010), and high-frequency bands, (e.g. beta and gamma; Orekhova et al. 2007; Rojas and Wilson 2014), and reduced power in medium-frequency bands (e.g. alpha; Dawson et al. 1995; Chan et al. 2007) for ASD with respect to controls. Nevertheless, to show the complex and variable intrinsic activity underlying neural networks, it becomes necessary to complement PSD analyses with other more specific measures of neural activity. In recent decades, research has increasingly focused on nonlinear measures of EEG, such as brain signal variability and complexity (Takahashi 2013; Garrett et al. 2013; Van Noordt and Willoughby 2021).
Variability is one of the most basic measures of the EEG signal. It estimates the range of values over which the signal oscillates through the standard deviation (SD) or the coefficient of variation (CV) (Garrett et al. 2013; Grady and Garrett 2018). Studies have shown variability increase in normal development (Garrett et al. 2013; Angulo-Ruiz et al. 2021), as well as oscillatory and pathological dependencies (Reviewed in Angulo-Ruiz et al. 2021, 2022). A previous study showed greater relative variability (CV) across trials in the delta band in children with attention deficit hyperactivity disorder (ADHD) compared to normally developing children (Angulo-Ruiz et al. 2022).
Entropy is the main measure of analysis of temporal complexity of brain signals (Kolmogorov 1958; Pincus 1991, 1995; Richman and Moorman 2000). Among all types of entropy (Takahashi 2013), multiscale Entropy (MSE) is one of the most direct measures of intrinsic physiological complexity, allowing the detection of long-range temporal correlations, and reflecting atypical EEG patterns in brain disease (Costa et al. 2002, 2005, Takahashi 2013, 2009; Mizuno et al. 2010; Garrett et al. 2013; Simon et al. 2017; Shen et al. 2021). High and sustained values of complexity at all scales would indicate optimal system performance, while low values would indicate random or a highly predictable signal structure (Papaioannou et al. 2021).
The MSE studies have shown increases during development (McIntosh et al. 2008; Lippe et al. 2009; Garrett et al. 2013; Van Noordt and Willoughby 2021), increases (in fine scales) and decreases (in coarse scales) as a function of the scale range during normal development (Szostakiwskyj et al. 2017; Angulo-Ruiz et al. 2022), as well as decreases with normal aging (Takahashi et al. 2009). In addition, recent studies report partially shared variance between the MSE coarse-graining process and spectral density bands (PSD) in both normal (Bosl et al. 2022) and pathological populations (Angulo-Ruiz et al. 2022).
Studies of clinical populations have often reported abnormal physiological complexity (Takahashi 2013; Shen et al. 2021). Lower complexity compared to typical development has been described in neurodevelopmental disorders (Chu et al. 2017; Angulo-Ruiz et al. 2022). Namely, patterns of lower complexity in ASD children (Bosl et al. 2011; Liu et al. 2017; Kang et al. 2019) and adults (Catarino et al. 2011; Milne et al. 2019) has been reported. However, some studies show increased complexity (Bosl et al. 2017; Takahashi et al. 2016) and/or possible influences of symptom severity (Takahashi et al. 2016; Hadoush et al. 2019). Regardless of the direction of MSE abnormality, these findings could be consistent with inefficient information processing and atypical neural connectivity (McIntosh et al. 2008; Takahashi 2013; O'Reilly et al. 2017).
In this context, our study aims to provide an integrative approach to the analysis of the EEG brain signal of children diagnosed with ASD in comparison to normally developing children. We employ spectral power density (PSD), relative PSD, EEG power variability across trials (CV) and complexity (MSE) analyses. We hypothesized that children with ASD would show lower complexity values compared to the normative group, while showing an increase in EEG variability. For PSD, we expect higher amplitude values in the low (low-delta and delta) and high (high beta and gamma) frequency bands, and lower values in the middle frequencies (theta and alpha) in the ASD group when compared to controls. We expect a possible relationship between PSD and MSE suggesting a partially shared common source of variance. We aim to characterize differences between the ASD and the control groups using these neurophysiological metrics and to possibly provide a tool for better classification and early diagnosis of ASD.
Methods
Participants
Thirty-four children participated in this study. The initial sample of the group diagnosed with ASD consisted of 15 children. One child was eliminated after signal processing due to EEG artifacts. The age of the 14 children analyzed was between 5 and 10 years (M = 8.36, SD = 1.22, 1 female). They were recruited from two private centers in Seville, one dedicated to the evaluation and treatment of ASD, and the other from a school with a special education program whose parents reported in detail the diagnosis of ASD. In both cases, the diagnosis was made by expert psychiatrists and clinical psychologists (not directly involved in this study) using CIE-10, DSM-IV, and DSM-V. In each referral private center, the diagnosis was confirmed using Autism Diagnostic Observation Schedule-Generic (ADOS-G; Lord et al. 2000).
The typically development or control group was composed of 19 children, who were recruited from different schools in Seville. The parents did not report neurological diseases, signs of epileptic discharge, learning difficulties, or developmental delays in the children. One child was excluded due to technical problems, thus the final sample was composed of 18 children aged between 5 and 11 years (M = 7.89, SD = 1.99, 3 females). There were no significant differences between the groups (control and ASD) neither in age (t(30) = -1.05, p = 0.28, d = 0.39), nor in gender (t(30) = -0.79, p = 0.44, d = 0.30). Given the absence of significant gender ratio, and the low number of females, the gender factor was excluded for further analysis.
Behavioral tests were conducted to all children who participated in this study (Table 1). Participant attrition and nonresponse (i.e. parents’ incapacity of bringing their child to the assessment day or returning the questionnaires) lead to some missing psychometric data. SCQ, and AQ were filled by ASD tutors or parents.
Table 1.
Results of descriptive analysis of behavioral psychometric tests
| Group | SCQ | KBIT (Matrices subtest) | AQ |
|---|---|---|---|
| Control | N = 18 (M = 30.22, SD = 5.28) | N = 17 (M = 42.76, SD = 13.70) | |
| ASD | N = 11 (M = 21.55, SD = 7.16) | N = 13 (M = 28, SD = 8.77) | N = 14 (M = 87.64, SD = 22.50) |
The clinical group was assessed with the Social Communication Questionnaire (SCQ-lifetime; Rutter et al. 2003), a screening questionnaire for parents based on the Autism Diagnostic Interview-Revised (ADI-R; Lord et al. 1994; Berument et al. 1999; Yau et al. 2016) to determine the presence of the disorder. A cut-off point of 15 points was established to confirm the presence of the disorder (Mercader and Miranda 2016). The total score of the questionnaire showed that 10 subjects of the clinical group scored above the cut-off point and only one scored below. Three parents did not return the questionnaire. However, the clinical diagnosis prevailed and all the 14 ASD referred children were included for further analysis.
The parent version of the Autism Spectrum Quotient (AQ; Baron-Cohen and Wheelwright 2004) was used in both groups to measure the expression of autistic characteristics and to corroborate the diagnoses made by the clinical centers. A cut-off point of 76 was established to confirm the presence of ASD symptoms (Auyeung et al. 2008). Seventeen children from the control group (one missing) and four from the ASD group scored below the cut-off point (76). While 10 children from the ASD group scored above 76. However, the clinical diagnosis prevailed and the 14 children with ASD referred were included for further analysis. Total AQ scores showed significant differences between groups (t(29) = -6.84, p < 0.001, d = 2.41), with ASD group presenting a higher mean score (M = 87.64, SD = 22.50) than control group (M = 42.76, SD = 13.70).
In addition, the Matrices subtest of the Kaufman Brief Intelligence Test (KBIT; Kaufman and Kaufman 2004) was employed to measure nonverbal cognitive skills. As this measure does not require participants to give a verbal response, it is considered the most appropriate given the age and diagnosis of the participants. Given the heterogeneity and inequality among cognitive profiles typical of this clinical population, KBIT scores were only used to characterize the sample and not to exclude individuals due to low IQ (Jarrold and Brock 2004; Ruiz-Martínez et al. 2020). Direct KBIT scores showed no differences between groups (t(29) = 0.813, p = 0.427, d = 0.31).
Following the guidelines of the Declaration of Helsinki, written informed consent was obtained from the parents. They received both written information and an explanation from the researcher about the protocol to be followed (prior to the experimental day, see Experimental session). The aim of the protocol was to make parents aware of the experimental requirements of the children, and thus minimize anxiety in the session. The experimental protocol was approved by the biomedical research ethics committee of the autonomous community of Andalucía.
Experimental Session
Before the experimental session each parent was provided with a "storyboard" explaining the order of the experimental session from beginning to end. A photograph of each researcher and recording setting was also included. Parents of children with ASD were requested to practice at home with a swimming cap for 20–30 min while watching a movie without sound.
Spontaneous EEG activity was obtained in an eyes-open condition for 3 min in a soundproof room decorated with child-friendly elements to establish a suitable environment for the children. The subjects were instructed to look at a cross on the screen and to remain in a relaxed position, blinking as little as possible. Nineteen electrodes according to the international 10–20 system were used (ELECTROCAP) (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Cz, Fz, Pz), including a ground electrode. An average reference was used and due to the high somatosensory reactivity of the ASD group, ocular and mastoid electrodes were avoided. To record blink artifacts, the signal amplitude of the frontal electrodes was used. Data were recorded at a gain of 20,000 and at a sampling rate of 1024 Hz in direct current using an analog-to-digital acquisition and analysis system (ANT) without any filtering. Impedance was kept below 10 Kilo-Ohms.
Data Analysis
The EEGLAB toolbox (Delorme and Makeig 2004) and the Matlab R2021b software package were employed for EEG data analysis. A notch filter of 47–53 Hz was applied (EEGLAB function: eegfiltnew) and average reference was used. An Artifact Subspace Reconstruction (ASR) algorithm (EEGLAB Function: clean rawdata) was used to correct portions of data with a standard deviation exceeding by 20 times the one of the calibration data. To correct for eye, blinks, muscle and other movement artefacts, an independent component analysis (ICA) was performed in EEGLAB (Function: pop_runica). Components carrying artefacts were tracked by ICLabel classification (Pion-Tonachini et al. 2019) and removed. All epochs (2000 ms) with ± 120 μV of the 19 channels were rejected (EEGLAB Function eegthresh). Subjects had a range of selected epoch between 14 and 90 for analysis (M = 73.69, SD = 19.62), and a range of 14 to 18 accepted components (M = 15.66, SD = 1.06). There was a significant difference between groups in the number of accepted epochs (t(30) = 2.93, p = 0.016, d = 0.99, mean control = 81.72, SD = 10.55; mean ASD = 63.36, SD = 23.84), but no group differences were found for the number of selected components. There was not a significant correlations of the number of epochs with age.
LPSD and Relative PSD Analysis
Mean (M) and standard deviation (SD) of the logarithm of the absolute PSD (LPSD) across trials were calculated with the EEGLAB spectopo function. This uses the Matlab pwelch function while applying a Hamming window. LPSD was calculated in 2-s windows with 2048 sampling points and a sampling rate of 1024 Hz. The frequency resolution of the LPSD spectrum was 0.5 Hz. LPSD was averaged over different frequency ranges: low-delta (0.5–1 Hz), delta (1.5–3 Hz), theta (4–7.5 Hz), alpha (8.5–13 Hz), low-beta (13.5–20 Hz), high-beta (20.5–30 Hz) and gamma (30.5–45 Hz).
To obtain the absolute PSD (LPSD = 10 * Log (PSD)), needed for computing the relative PSD, the logarithm of the LPSD was removed (PSD = eLPSD/10). The relative PSD spectrum was obtained from absolute PSD. The relative PSD of each electrode and subject was calculated according to the following formula:
where X(fi) is the relative PSD for a given frequency. PSD (fi) is the absolute PSD for a given frequency. ΣPSD(fi) is the sum of the absolute PSD at all frequencies considered (0.5–45 Hz in 0.5 Hz steps).
Variability Across Trials
To obtain a measure of the variability of PSD across trials, the mean of the absolute PSD (M) in a given frequency band and its corresponding trial-by-trial standard deviation (SD) were divided to obtain the CV across trials (CV = SD/M).
Multiscale Entropy Analysis
Using the Matlab function MultiscaleSampleEntropy (Malik 2022), we calculate Multiscale Entropy (MSE) for all channels. Malik (2022) based his function on the MSE method of Costa et al. (2005), which calculates sample entropy (SE; Richman and Moorman 2000) at multiple time scales using a coarse-grained procedure. MSE measures signal complexity (Garrett et al. 2013) by dividing the EEG signal into non-overlapping windows of a different number of samples. Each time scale is defined by averaging the different neighboring points (p) of the original time series (of length τ). Thus, the repetition frequency of m-point versus m + 1-point patterns is evaluated. It is necessary to define a similarity limit (r) to delimit the tolerance range in which individual neighboring points are considered similar (k). This similarity limit is normalized by the standard deviation (SD) of the EEG k < r × SD (Malik 2022). SE is then calculated for each time scale (Malik 2022):
Therefore, MSE is calculated using a coarse-grained process that filters out high frequencies with increasing scales (Kosciessa et al. 2020; Bosl et al. 2022). This process has recently been related to power spectral analysis using Haar wavelet approximations (Bosl et al. 2022). Thus, MSE low scales would contain all frequencies found in the EEG signal, whereas high scales would only contain low frequencies (Bosl et al. 2022).
Following recommendations provided in previous studies on EEG signal complexity (Richman and Moorman 2000; McIntosh et al. 2008; Miskovic et al. 2016; Kloosterman et al. 2019; Kosciessa et al. 2020), the parameters used in this study were m = 2 and r = 0.5. MSE was calculated for 67 time scales. The latter corresponding to 30 time points obtained by collapsing 67 consecutive sampled points (0.97656 ms x 67scales = 65.4 ms), in each 2 s trial. A complete description of the number of points, sample periods and frequencies included in each MSE scale are described in supplementary table 1.
High complexity would be defined by high values of SE indicating a low repetition of patterns of length “m” over “m + 1” and an optimally functioning system (Papaioannou et al. 2021). Low complexity would indicate random or high similarity of patterns indicating information poverty (McIntosh et al. 2008; Garrett et al. 2013).
Statistical Analysis
ANOVA
To reduce the dimensionality of the data, neighboring electrode values of each calculated parameter (LPSD, CV, relative PSD and MSE) were collapsed into 9 areas (Fig. 1). Also, PSD (logarithm and relative) and CV were collapsed into different frequency bands (see Table 2 for the EEG frequency ranges used). The MSE, in turn was organized based on three different types of scales, corresponding to the temporal sampling of scales proposed by Szostakiwskyj et al. (2017). Thus, fine scales were considered from scale 1 (0.98 ms and 2048 time points per trial) to scale 25 (24.4 ms and 81 time points), medium scale from scale 26 (25.4 ms and 78 time points) to scale 45 (43.9 ms and 45 time points) and, finally, coarse scale from scale 46 (44.9 ms and 44 time points) to scale 67 (65.4 ms and 30 time points), see supplementary table 1 for parameters of the different scales.
Fig. 1.
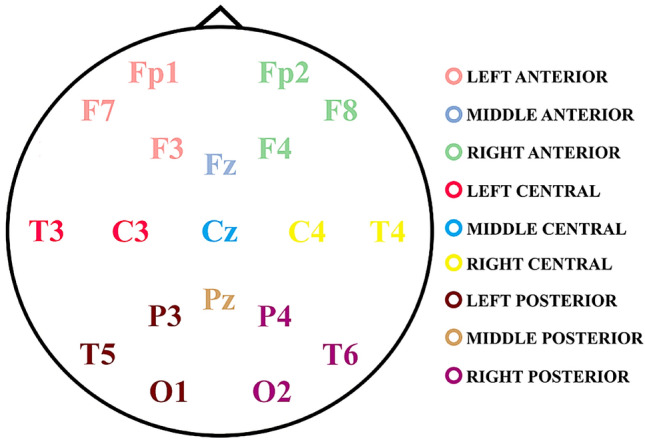
Localization and collapse of electrodes by regions. The colors indicate the nine defined scalp areas for electrodes collapse
Table 2.
Significant results were obtained for the logarithm of the PSD (LPSD)
| BANDS | RM-ANOVA | t-tests |
|---|---|---|
|
Low-delta (0.5–1 Hz) |
Within-subjects: Laterality x group p = 0.006 F(1.81, 52.44) = 6.14, ηp2 = 0.175, power = 0.846 |
Left-medial: t(30) = − 3.02, p = 0.008, d = 1.15 Medial-right: t(30) = 3.02, p = 0.008, d = 1.06 |
|
Delta (1.5–3 Hz) |
Within-subjects: Laterality x group p < 0.001 F(1.93, 55.82) = 9.75, ηp2 = 0.252, power = 0.974 |
Left-medial: t(30) = − 3.89, p < 0.001, d = 1.37 Medial-right: t(30) = 3.89, p < 0.001, d = 1.36 |
|
Theta (4–7.5 Hz) |
Within-subjects: Laterality x group p < 0.001 F(1.76, 50.97) = 13.08, ηp2 = 0.311, power = 0.992 Anterior–posterior x group p = 0.055 F(1.94, 56.32) = 4.54, ηp2 = 0.135, power = 0.742 |
Medial: t(30) = 4.65, p < 0.001, d = 1.66 Central: t(30) = 3.16, p = 0.011, d = 1.14 Posterior: t(30) = 2.60, p = 0.021, d = 0.95 |
|
Alpha (8.5–13 Hz) |
Within-subjects: Laterality x group p = 0.015 F(1.74, 50.53) = 4.85, ηp2 = 0.143, power = 0.737 |
Left-medial: t(30) = -2.53, p = 0.025, d = 0.89 Medial-right: t(30) = 2.75, p = 0.025, d = 0.97 |
|
Low-beta (13.5–20 Hz) |
Within-subjects: Laterality x group p = 0.002 F(1.99, 57.91) = 7.85, ηp2 = 0.213, power = 0.942 Anterior–posterior x group p = 0.055 F(1.80, 52.31) = 5.19, ηp2 = 0.152, power = 0.778 |
Left-medial: t(30) = -2.97, p = 0.014, d = 1.10 Medial-right: t(30) = 3.90, p = 0.001, d = 1.38 Anterior–posterior: t(30) = -3.23, p = 0.009, d = 1.14 |
|
High-beta (20.5–30 Hz) |
Between-subjects Group p = 0.042 F(1, 29) = 7.16, ηp2 = 0.198, power = 0.734 Within-subjects: Laterality x group p = 0.005 F(1.96, 56.89) = 6.22, ηp2 = 0.177, power = 0.873 |
Right: t(30) = -3.39, p = 0.006, d = 1.18 |
|
Gamma (30.5–45 Hz) |
Between-subjects: Group p = 0.041 F(1,29) = 8.81, ηp2 = 0.233, power = 818 Within-subjects: Laterality x group p = 0.005 F(1.99, 57.97) = 6.03, ηp2 = 0.172, power = 0.867 |
Right: t(30) = -3.30, p = 0.007, d = 1.15 |
All p-values of the RM-ANOVA and t-test were corrected by FDR correction. The RM-ANOVA factors used were: group of subjects, anterior–posterior, laterality, and covariate (age in days). The RM-ANOVA was computed independently for each frequency band. The effect size for RM-ANOVA was computed by eta partial squared (ηp2), and for posthoc t-tests by means of Cohen’s d
Data were analyzed using Repeated Measures ANOVAs (RM-ANOVA) with age (in days) as a covariate in the Statistical Package for the Social Sciences 25 (SPSS). RM-ANOVAs size effects were directly computed in SPSS using eta partial squared (ηp2). In addition, false discovery rates (FDR) were used to correct for multiple comparisons (Benjamini and Hochberg 1995). Only significant results for the factors considered in the RM-ANOVA that included the group factor and survived FDR correction were reported and discussed, given the main purpose of the report.
For mean PSD (LPSD and relative PSD) and CV, RM-ANOVA analysis was performed independently for the seven frequency bands (low-delta, delta, theta, theta, alpha, low-beta, high-beta and gamma). Age in days was used as a covariate, group (control and ASD) as a between-subjects factor, and anteroposterior (anterior, central and posterior) and lateral (left, medial and right) areas as within-subjects factors.
For MSE, the between-subjects factor was group (control and ASD), the covariate was age (in days) and the within-subjects factors were scale type (fine, medium and coarse), anteroposterior areas (anterior, central and posterior) and lateral areas (left, medial and right).
For posthoc analysis, Student's t-tests were computed with Cohen's d as the effect size metrics (Cohen 1988). All p-values were corrected for multiple comparisons using the FDR (Benjamini and Hochberg 1995).
Spearman Correlation Between MSE and Relative PSD
In order to verify the relationship between the spectral power (PSD) and the MSE (Bosl et al. 2022), we performed a Spearman correlation between relative PSD vs. MSE, with age (in days) when controlling correlation by age.
EEG Metrics vs. Behavioral Correlational Analysis
For the behavioral vs. EEG correlation analyses, only group significant EEG metrics were used. Psychometric tests used for correlation were those completed by both groups (control and ASD), such as the KBIT (as a measure of non-verbal general intelligence) and the AQ (to characterize subjects with ASD). Spearman partial correlations controlling for age were performed for all subjects without distinguishing between groups, given the small sample size. P-values were corrected by FDR (Benjamini and Hochberg 1995).
Results
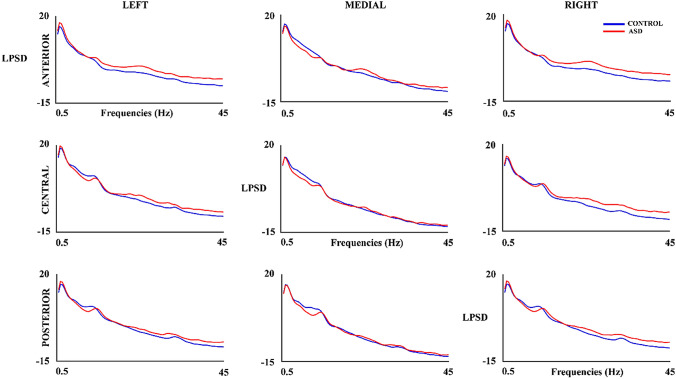
Figure 2 shows the LPSD in all considered areas for the control and ASD groups. The RM-ANOVA (Table 2 and in summary Table 6) shows main and interaction group effects (with FDR correction) of high-beta and gamma bands. Mean of high-beta LPSD of ASD (M = − 3.52, SD = 2.18) was higher than the LPSD of the control group (M = − 4.99, SD = − 1.23). A similar pattern was obtained for the gamma band (ASD: M = − 6.95, SD = 2.31; control: M = − 8.60, SD = − 1.56). All bands showed laterality x group interaction effects. T-test comparison (Table 2) between left and medial areas showed higher mean to ASD group in low-delta (M = 0.93, SD = 1.60), delta (M = 0.80, SD = 1.18), alpha (M = 0.82, SD = 0.92), and low-beta bands (M = 2.05, SD = 1.30) than control group (low-delta: M = − 0.52, SD = 1.11; delta: M = − 0.65, SD = 0.92; alpha: M = 0.074, SD = 0.753; low-beta: M = 0.97, SD = 0.48). The difference between medial and right areas of the mean of LPSD was higher in control group in low-delta (M = 0.168, SD = 1.25), delta (M = 0.48, SD = 0.91), alpha (M = − 0.07, SD = 0.934), and low-beta bands (M = − 1.02, SD = 0.94) than ASD group (low-delta: M = − 1.37, SD = 1.63; delta: M = − 1.01, SD = 1.25; alpha: M = − 1.02, SD = 1.03; low-beta: M = − 2.37, SD = 1.02). The posthoc analyzed in the theta band showed higher mean in medial areas (p < 0.001) in control group (M = 8.23, SD = 1.25) than ASD group (M = 6.20, SD = 1.19). In high-beta and gamma bands the mean of LPSD in right areas (high-beta: p = 0.006; gamma: p = 0.007) was higher in ASD group (high-beta: M = − 2.09, SD = 2.19; gamma: M = − 5.08, SD = 2.52) than control group (high-beta: M = − 4.30, SD = 1.50; gamma: M = − 7.59, SD = 1.79). Only theta and low-beta bands showed an interaction effects between the group with the anterior–posterior factor. The central (p = 0.011) and posterior (p = 0.021) areas showed higher mean of LPSD in control group (central: M = 7.07, SD = 1.35; posterior: M = 8.22, SD = 1.52) than ASD group (central: M = 5.67, SD = 1.09; posterior: M = 6.97, SD = 1.08) in theta band. The low-beta band showed higher differences between anterior and posterior areas in the ASD group (M = 0.37, SD = 1.08) with respect to control group (M = − 0.79, SD = 0.951).
Fig. 2.
Logarithm of the Power Spectral Density (LPSD) in control and ASD subjects
Table 6.
Summary of RM-ANOVA results for all calculated parameters
| Parameters | Between-subjects | Within-subjects (interaction with the group) |
|---|---|---|
| LPSD |
High-beta (ASD > C) Gamma (ASD > C) |
Low-delta: -Diff. Left-Medial (ASD > C) -Diff. Medial-Right (C > ASD) Delta: -Diff. Left-Medial (ASD > C) -Diff. Medial-Right (C > ASD) Theta: -Medial (C > ASD) -Central (C > ASD) -Posterior (C > ASD) Alpha: -Diff. Left-Medial (ASD > C) -Diff. Medial-Right (C > ASD) Low-beta: -Diff. Left-Medial (ASD > C) -Diff. Medial-Right (C > ASD) -Diff. Anterior–Posterior (ASD > C) High-beta: -Right (ASD > C) Gamma: -Right (ASD > C) |
| Relative PSD | Theta (C > ASD) | |
| CV |
Theta (ASD > C) Alpha (ASD > C) |
|
| MSE | C > ASD |
Medium (C > ASD) Coarse (C > ASD) Left (C > ASD) Right (C > ASD) |
In brackets: control > ASD (C > ASD) or ASD > control (ASD > C). Diff differences
RM-ANOVA for relative PSD (Table 3 and in summary Table 6) with FDR correction showed main group effect in theta band where the highest relative PSD values were for the control group (M = 2.33, SD = 0.44) compared to ASD group (M = 1.79, SD = 0.57). No group significant interaction effects were obtained for any frequency band.
Table 3.
Significant results obtained in the RM-ANOVA of the relative PSD with factors: group of subjects, anterior–posterior and laterality, and age in days like covariate
| BANDS | Relative PSD |
|---|---|
|
Theta (4–7.5 Hz) |
Between-subjects: Group p = 0.029 F(1, 29) = 9.65, ηp2 = 0.250, power = 0.851 |
The RM-ANOVA was computed independently for each frequency bands. For the calculation of relative PSD, the logarithm was eliminated. P-value with FDR correction
The RM-ANOVA of the CV across trials (Table 4 and in summary Table 6) with FDR correction showed group main effects in the theta and alpha bands. In the theta band, the CV was higher in the ASD group (M = 1.85, SD = 0.108) compared to the control group (M = 1.79, SD = 0.030). Similarly, the CV in the alpha band was higher in the ASD group (M = 1.94, SD = 0.099) than in the control group (M = 1.83, SD = 0.060).
Table 4.
Significant results obtained in the RM-ANOVA of the Coefficient of variation (CV) across trials with factors: group of subjects, anterior–posterior and laterality, and age in days like covariate
| BANDS | CV |
|---|---|
|
Theta (4–7.5 Hz) |
Between-subjects: Group p = 0.044 F(1, 29) = 7.08, ηp2 = 0.196, power = 0.730 |
|
Alpha (8.5–13 Hz) |
Between-subjects: Group p = 0.008 F(1, 29) = 12.79, ηp2 = 0.306, power = 0.933 |
The RM-ANOVA was computed independently for each frequency bands. For the calculation of CV across trials, the logarithm of the LPSD was eliminated. All the p-values with FDR correction
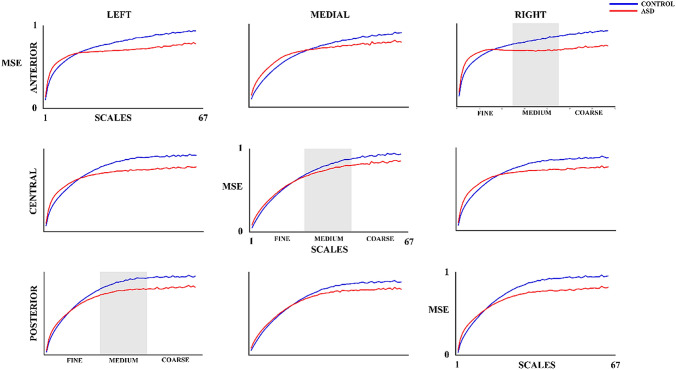
Figure 3 shows the MSE results for the control and ASD groups. The MSE increases as the scale increases for both groups. However, as the MSE values of the control group exhibit a constant rise, the MSE of the ASD group shows a rise in fine scales and a plateau from the medium scales upwards. The RM-ANOVA of the MSE (Table 5 and in summary Table 6) shows a main group effect with the control group presenting a higher mean MSE (M = 0.79, SD = 0.059) than the ASD group (M = 0.74, SD = 0.082). In addition, group differences in the medium scales (t(30) = 2.44, p = 0.030, d = 0.78) and coarse scales (t(30) = 3.49, p = 0.005, d = 1.19) are found based on the interaction effect results (with FDR correction). The MSE in both medium and coarse scales was higher in control group (medium: M = 0.86, SD = 0.077; coarse: M = 0.92, SD = 0.062) than ASD group (medium: M = 0.79, SD = 0.099; coarse: M = 0.82, SD = 0.101). In addition, significant differences were obtained in the interaction of the effects of laterality x group factors. These differences were due to a higher MSE in controls (left: M = 0.79, SD = 0.060; right: M = 0.78, SD = 0.064) than in ASD group (left: M = 0.73, SD = 0.084; right: M = 0.72, SD = 0.077) for both left (t(30) = 2.48, p = 0.030, d = 0.82) and right (t(30) = 2.45, p = 0.030, d = 0.85) lateral areas.
Fig. 3.
Multiscale Entropy (MSE) for 67 scales in control and ASD subjects. The medium scale is highlighted to differentiate the three types of scales
Table 5.
Significant results obtained in the RM-ANOVA of MSE with factors: group of subjects (controls and ASD), scales (fine, medium, and coarse), anterior–posterior, and laterality, and age in days as a covariate. All results with FDR correction
|
Between-subjects Group p = 0.022 F(1, 29) = 5.83, ηp2 = 0.167, power = 0.646 Within-subjects: Scales x group p = 0.001 F(1.26, 36.47) = 10.99, ηp2 = 0.275, power = 0.938 Laterality x group p = 0.019 F(1.88, 54.48) = 4.38, ηp2 = 0.131, power = 0.715 |
Table 6 shows a summary of the RM-ANOVA results.
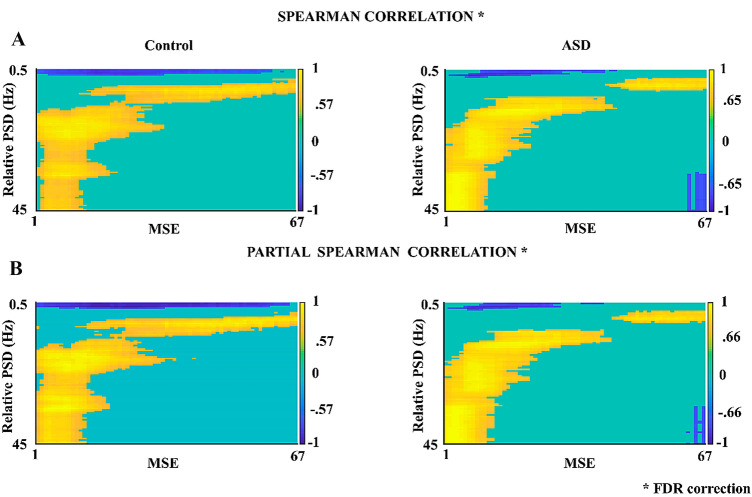
Figure 4 shows the results of the Spearman and partial correlation (controlling for age) between relative PSD (0.5–45 Hz) and MSE (scales 1–67), both with FDR correction. Significant positive and negative correlations are observed both for the control group (positive with cutoff for Rho > 0.57, p < 0.015; negative with cutoff for Rho > -0.57, p < 0.016) and for the ASD group (positive with cutoff for Rho > 0.65, p < 0.014 and negative with cutoff for Rho > -0.66, p < 0.013).
Fig. 4.
A Spearman Correlation between MSE (67 scales) and relative PSD (0.5–45 Hz) in control and ASD subjects. B Partial Spearman Correlation controlled for age (in days) for both groups of subjects. Significant cutoff values for each correlation (with FDR correction) are indicated in the graphs. In this correlation, we calculated up to a total of 67 scales to observe the effect of correlation with increasing scales
The obtained significant positive correlations were observed between frequencies higher than 5 Hz and all MSE scales. Significant negative correlations were also found between low frequencies (< 2.5 Hz) and MSE of all the scales considered, in both groups analyzed. The calculated partial correlations do not show a maturation effect in either group.
Regarding the correlation between EEG neurophysiological metrics (summary in Table 6) and behavioral tests (AQ and KBIT), the results lost significance after correction for multiple comparisons.
Discussion
We analyzed different neurophysiological measures of power, variability and complexity of the EEG in resting state to observe possible alterations between a group of children diagnosed with ASD compared to a normodevelopment group. Previous studies using correlational analyses of MSE vs. age have shown an increase in complexity with age as a product of better adaptability to cognitive demands and possible reconfigurations of the functional network produced by a more variable state (McIntosh et al. 2008, 2010; Van Noordt and Willoughby 2021). A strong dependence of age for the PSD has also been previously described (Reviewed in Rodriguez-Martinez et al. 2021). As such, the present study includes age as a covariate in all the statistical analyses to compensate for any change in the EEG parameters due to age. Our results show an increase in PSD (LPSD and relative) in the theta band in the control group and an increase in high frequency bands (high-beta and gamma in LPSD) in the ASD group, as well as higher variability (CV) across trials, and lower complexity (MSE) compared to typically developed children.
PSD studies have been shown to be a reliable biomarker of EEG maturation, with decreases in the absolute values of brain rhythms throughout development, as well as decreases in low frequency bands and increases in high frequency bands in the relative PSD (Gasser et al. 1988; Segalowitz et al. 2010; Miskovic et al. 2015; Rodríguez-Martínez et al. 2020, 2021). This would be related with the selection of stable neuronal connections during the synaptic pruning process, producing an increase in the efficiency of neuronal transmission throughout development (Whitford et al. 2007).
Our results showed differences between groups in the theta band with higher LPSD and relative PSD values in the control group and, in the high-beta and gamma bands of LPSD with greater power among the ASD group with respect to control. Regarding the alpha band, several studies reported increased or decreased alpha power in children with ASD, high-functioning ASD, or ASD risk populations (Lazarev et al. 2009; Tierney et al. 2012; Carter et al. 2018; DiStefano et al. 2019; Pierce et al. 2021). Our results in this band focus mainly on laterality x group interactions, where the ASD group presents higher levels of LPSD in left vs. medial lateral areas and lower levels in medial vs. right areas. However, our results support the possible biomarker role of gamma band activity as suggested by Rojas and Wilson (2014). These authors suggest gamma activity as a reliable endophenotype of ASD given the weakening of perceptual and cognitive functions in the autism population. However, the direction of amplitude change of gamma activity remains controversial, given that certain studies found an increase of gamma power in ASD (Orekhova et al. 2007, 2008; Machado et al. 2015), while others found a decrease in power (Sheikhani et al. 2009, 2012; Maxwell et al. 2015; Van Hecke et al. 2015). Present results would support the increase in gamma amplitude in ASD. These results would partially support the U-shaped hypothesis of PSD suggested by Wang et al. (2013) in which children with ASD present higher PSD values in low and high-frequency bands compared to a control group, as well as lower values in mid-frequency bands such as the theta band.
EEG signal variability was analyzed based on the absolute PSD CV across trials metric. In this study, the CV, which corresponds to a measure of PSD variability across trials (Angulo-Ruiz et al. 2021), showed an increase in ASD for theta and alpha bands compared to the control group, suggesting greater variability of spontaneous EEG in the ASD group across trials. Similarly, increased neural signal variability has been shown in other clinical populations (Castellanos et al. 2009; Angulo-Ruiz et al. 2022). As CV solely corresponds to a first-order measure of variability, complexity measurements related to multiplicity of EEG patterns at different temporal scales, such as MSE, are more suitable for assessing abnormal physiological EEG signals (Takahashi 2013; Chu et al. 2017; Papaioannou et al. 2021; Shen et al. 2021).
The ASD group shows a slowdown in the increase of MSE with scales from the middle scales onwards yielding lower entropy values from these scales with respect to controls, implying a defective functional system (McIntosh et al. 2008; Papaioannou et al. 2021; Van Noordt and Willoughby 2021). Both increase and decrease of complexity in ASD subjects have been shown in the literature. Bosl et al. (2017) in EEG, and Takahashi et al. (2016) in Magnetoencephalography (MEG) have reported increases in MSE in the ASD group. Lower EEG complexity was also found in this clinical group (Bosl et al. 2011; Chu et al. 2017; Liu et al. 2017; Kang et al. 2019). This reduction of complexity in MSE in ASD would imply a reduced adaptability, given the reduction of possible patterns in the EEG, possibly related to a less variable repertoire of neural network dynamics that would impair behavioral adaptation (McIntosh et al. 2008; Takahashi 2013; O'Reilly et al. 2017).
The relationship between connectivity and complexity has been shown previously and explained in terms of neural connections (Friston 1996; Sporns et al. 2000; Takahashi 2013). Therefore, the large-scale integration efficiency resulting from synchronization between small and large neuronal populations evolving in different frequency ranges could be reflected in the physiological complexity of the signal (Takahashi 2013). Lower entropy values in ASD suggests abnormal information processing and connectivity, mainly in long-range neural interactions, given the relationship between coarse scales and low frequency bands (Szostakiwskyj et al. 2017; Bosl et al. 2022). The latter argument is supported by the relationship of long range connections with low EEG frequencies, and of short range connections with high EEG frequencies (Lea-Carnall et al. 2016). In this sense, abnormal complexity could support the theory of local overconnectivity and long-range underconnectivity, which is central to the underlying pathology of ASD (Belmonte et al. 2004; Courchesne and Pierce 2005; Geschwind and Levitt 2007; Wass 2011) and other neurodevelopmental disorders such as attention deficit hyperactivity disorder (Clarke et al. 2008). Moreover, impaired connectivity has been found in ASD, such as decrease of electrophysiological connectivity and long-range connections (Rippon et al. 2007; Chan et al. 2011; Duffy and Als 2012; Takahashi 2013; Yuk et al. 2020). Our results support local overconnectivity and long-range underconnectivity hypothesis for ASD due to the decrease of MSE coarse scales (decrease in long range connectivity), and by the increase of highbeta and gamma PSD (increase of local connectivity). Although, it must be indicated that this hypothesis remains as controversial, despite a higher support for the long-range underconnectivity hypothesis (O’Reilly et al. 2017).
The MSE decrease in coarse scales suggests lower complexity and greater signal predictability in ASD with respect to controls. The relationship between PSD and MSE shown in the present report and others (McIntosh et al. 2008, 2010; Szostakiwskyj et al. 2017; Bosl et al. 2022; Angulo-Ruiz et al. 2022) support the statement about the relationship between high frequencies and fine scales, as well as between low frequencies and coarse scales, both in the normo-developmental and clinical group, even when this relationship is controlled by age. In this regard, the relation of scales and frequency bands in our study with a sampling rate of 1024 Hz and 67 scales would include in the coarse scales (coarsest scale ≤ 7.64 Hz bands): the low-delta, delta, theta bands; medium scales: alpha and low-beta bands (in addition to low frequencies); and in the fine scales, high-beta and gamma bands (in addition to low and medium frequencies) (Bosl et al. 2022). The strong relationship between the fine scales of the MSE and high-frequency bands would be related to local connectivity and/or local processing. The same is implied to the relationship between the coarse scales and low-frequency bands with global connectivity or long-range interactions (Szostakiwskyj et al. 2017; Van Noordt and Willoughby 2021; Bosl et al. 2022). Present results of correlations between MSE and PSD suggest that there is some shared variance for these two metrics, in both ASD and controls.
The correlation of the EEG metrics with the AQ and KBIT test were not significant after correction for multiple comparisons. Other studies have shown a relationship between EEG complexity (Bosl et al. 2011), spectral power (Carter et al. 2018) and autistic traits and cognitive brain capacity (McIntosh et al. 2008). However, given the heterogeneity of the disorder, it is possible that there are multiple individual neural profiles in ASD and thus multiple pathways to its features and symptoms (Milne et al. 2019). The small sample size does not permit analysis of differential correlational patterns in both groups of subjects.
This work has some limitations that could influence the reported results. The low sample size and the limited age range could explain the disparity found with other developmental studies, in which specific developmental patterns of power (Segalowitz et al. 2010; Rodríguez-Martínez et al. 2020; Angulo-Ruiz et al. 2021) and complexity (Szostakiwskyj et al. 2017; Angulo-Ruiz et al. 2022) are shown in healthy subjects. Likewise, it is important to include more women in the studies and to analyze the possible existence of gender dependencies, as Huberty et al. (2021) and Cragg et al. (2011) found in their spectral power studies. Statistical power is another considerable limitation given our sample size and low range age, however our results show a minimum power of 0.64 with a large effect size (ηp2 = 0.167), and a maximum power of 0.99 with a large effect size (ηp2 = 0.311) (Cárdenas-Castro and Arancibia-Martini 2014). Although the statistical power and effect sizes were generally high in this report, the results described should be taken with caution due to the sample size. In this sense the loss of significance in some metrics could be due to the effect of a low sample size. Therefore, it is important to consider incorporating a higher number of participants for future experiments. Finally, we suggest a longer period than 3-min resting state recording period for a longer epoch segmentation and a more reliable estimation of MSE.
Conclusions
The higher LPSD values of the ASD group in high-frequency bands, higher variability (CV) across trials, and lower EEG complexity (MSE) would indicate abnormal functioning at the neural and functional level in ASD, which is in line with previous literature on ASD and other neurodevelopmental disorders (Chu et al. 2017; Angulo-Ruiz et al. 2022).
Acknowledgements
This study was supported by grants from the Agencia Estatal de Investigación [PID2019–105618RB-I00]; and the Agencia de Innovación y Desarrollo de la Junta de Andalucía [P20_00537]. We are grateful to the children, adolescents, and young adults who participated in the present study.
Author contributions
Brenda Y. Angulo-Ruiz: Conceptualization, Methodology, Software, Formal analysis, Investigation, Data Curation, Writing – Original Draft. Francisco J. Ruiz-Martínez: Conceptualization, Methodology, Investigation, Resources. Elena I. Rodríguez-Martínez: Methodology, Investigation, Resources. Anca Ionescu: Formal analysis, Data curation, Writing – Review & Editing. David Saldaña. Conceptualization, Resources, Validation. Carlos M. Gómez: Conceptualization, Methodology, Validation, Formal analysis, Writing – Original Draft, Writing – Review & Editing, Supervision, Project administration, Funding acquisition. All authors reviewed the final version of the manuscript.
Funding
Funding for open access publishing: Universidad de Sevilla/CBUA.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Brenda Y. Angulo-Ruiz, Email: bangulo@us.es
Francisco J. Ruiz-Martínez, Email: frmartinez@us.es
Elena I. Rodríguez-Martínez, Email: elisroma@us.es
Anca Ionescu, Email: anca-stefania.ionescu@umontreal.ca.
David Saldaña, Email: dsaldana@us.es.
Carlos M. Gómez, Email: cgomez@us.es
References
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. 10.1176/appi.books.9780890425596
- Angulo-Ruiz BY, Muñoz V, Rodríguez-Martínez EI, Gómez C. Absolute and relative variability changes of the resting state brain rhythms from childhood and adolescence to young adulthood. Neurosci Lett. 2021;749:135747. doi: 10.1016/j.neulet.2021.135747. [DOI] [PubMed] [Google Scholar]
- Angulo-Ruiz BY, Muñoz V, Rodríguez-Martínez EI, Cabello-Navarro C, Gómez C. Multiscale entropy of ADHD children during resting state condition. Cogn Neurodyn. 2022 doi: 10.1007/s11571-022-09869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Wheelwright S, Allison C. The autism spectrum quotient: children’s version (AQ-child) J Autism Dev Disord. 2008;38:1230–1240. doi: 10.1007/s10803-007-0504-z. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The emphaty quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34(2):163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bosl W, Tierney A, Tager-Flusberg H, Nelson C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med. 2011 doi: 10.1186/1741-7015-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl WJ, Loddenkemper T, Nelson CA. Nonlinear EEG biomarker profiles for autism and absence epilepsy. Neuropsychiatr Electrophysiol. 2017;3:1. doi: 10.1186/s40810-017-0023-x. [DOI] [Google Scholar]
- Bosl WJ, Loddenkemper T, Vieluf S. Coarse-graining and the Haar wavelet transform for multiscale analysis. Biolectron Med. 2022 doi: 10.1186/s42234-022-00085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas-Castro M, Arancibia-Martini H (2014) Statistical power and effect size calculating in G*power: complementary analysis of statistical significance testing and its application in psychology. Salud & Sociedad: Latin American Journal on Health & Social Psycology, ISSN-e 0718-7475, 5,2, pp 210–224. https://dialnet.unirioja.es/servlet/articulo?codigo=4945415
- Carter LV, Tomlinson SB, Chang SA, Naples AJ, McPartland JC. Resting-state alpha power is selectively associated with autistic traits reflecting behavioral rigidity. Sci Rep. 2018;8(1):11982. doi: 10.1038/s41598-018-30445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Kelly C, Milham MP. The restless brain: attention-deficit/hyperactivity disorder, resting state functional connectivity and intrasubject variability. Can J Psychiatry. 2009;54(10):665–672. doi: 10.1177/070674370905401003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino A, Churches O, Baron-Cohen S, Andrade A, Ring H. Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin Neurophysiol. 2011;122(12):2375–2383. doi: 10.1016/j.clinph.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Chan AS, Sze SL, Cheung MC. Quantitative electroencephalographic profiles for children with autistic spectrum disorder. Neuropsychology. 2007;21(1):74–81. doi: 10.1037/0894-4105.21.1.74. [DOI] [PubMed] [Google Scholar]
- Chan AS, Han YMY, Sze SL, Cheung M, Leung WW, Chan RCK, To C. Disordered connectivity associated with memory deficits in children with autism spectrum disorders. Res Autism Spectr Disord. 2011;5:237–245. doi: 10.1016/J.RASD.2010.04.005. [DOI] [Google Scholar]
- Chu YJ, Chang CF, Shieh JS, Lee WT. The potential application of multiscale entropy analysis of electroencephalography in children with neurological and neuropsychiatric disorders. Entropy. 2017;19:428. doi: 10.3390/e19080428. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Heaven PC, McCarthy R, Selikowitz M, Byrne MK. EEG coherence in adults with attention-deficit/hyperactivity disorder. Int J Psychophysiol. 2008;67(1):35–40. doi: 10.1016/j.ijpsycho.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale: Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]
- Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89:068102. doi: 10.1103/physrevlett.89.068102. [DOI] [PubMed] [Google Scholar]
- Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev Lett. 2005;71(021906):753. doi: 10.1103/physrevlett.89.068102. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Cragg L, Kovacevic N, McIntosh AR, Poulsen C, Martinu K, Leonard G, Paus T. Maturation of EEG power spectra in early adolescence: a longitudinal study. Dev Sci. 2011;14(5):935–943. doi: 10.1111/j.1467-7687.2010.01031.x. [DOI] [PubMed] [Google Scholar]
- Daoust AM, Limoges E, Bolduc C, Mottron L, Godbout R. EEG spectral analysis of wakefulness and REM sleep in high functioning autistic spectrum disorders. Clin Neurophysiol. 2004;115(6):1368–1373. doi: 10.1016/j.clinph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Dawson G, Klinger LG, Panagiotides H, Lewy A, Castelloe P. Subgroups of autistic children based on social behavior display distinct patterns of brain activity. J Abnorm Child Psychol. 1995;23(5):569–583. doi: 10.1007/BF01447662. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open-source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- DiStefano C, Dickinson A, Baker E, Jeste SS. EEG data collection in children with ASD: the role of state in data quality and spectral power. Res Autism Spectr Disord. 2019;57:132–144. doi: 10.1016/j.rasd.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy FH, Als H. A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls - a large case control study. BMC Med. 2012;10:64. doi: 10.1186/1741-7015-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Theoretical neurobiology and schizophrenia. Br Med Bull. 1996;52(3):644–655. doi: 10.1093/oxfordjournals.bmb.a011573. [DOI] [PubMed] [Google Scholar]
- Garrett DD, Samanez-Larkin GR, MacDonald SWS, Lindenberger U, McIntosh AR, Grady CL. Moment-to-moment brain signal variability: a next frontier in human brain mapping? Neurosci Biobehav Rev. 2013;37:610–624. doi: 10.1016/j.neubiorev.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T, Verleger R, Bacher P, Sroka L. Development of the EEG of school-age children and adolescents. I. analysis of band power. Electroencephalogr Clin Neurophysiol. 1988;69:91–99. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Grady CL, Garrett DD. Brain signal variability is modulated as a function of internal and external demand in younger and older adults. Neuroimage. 2018;169:510–523. doi: 10.1016/j.neuroimage.2017.12.031. [DOI] [PubMed] [Google Scholar]
- Hadoush H, Alafeef M, Abdulhay E. Brain Complexity in Children with Mild and Severe Autism Spectrum Disorders: Analysis of Multiscale Entropy in EEG. Brain Topogr. 2019;32(5):914–921. doi: 10.1007/s10548-019-00711-1. [DOI] [PubMed] [Google Scholar]
- Huberty S, Carter LV, Van Noordt SJR, Bedford R, Pickles A, Desjardins JA, Webb SJ, BASIS ASDm. Elsabbagh M. Association between spectral electroencephalography power and autism risk and diagnosis in early development. Autism Res. 2021;14(7):1390–1403. doi: 10.1002/aur.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrold C, Brock J. To match or not to match? Methodological issues in autism-related research. J Autism Dev Disord. 2004;34(1):81–86. doi: 10.1023/b:jadd.0000018078.82542.ab. [DOI] [PubMed] [Google Scholar]
- Kang J, Chen H, Li X, Li X. EEG entropy analysis in autistic children. J Clin Neurosci. 2019;62:199–206. doi: 10.1016/j.jocn.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. KBIT: Kaufman Brief Intelligence Test (KBIT Spanish Version) Madrid: ASD Editions; 2004. [Google Scholar]
- Kloosterman NA, Kosciessa JQ, Lindenberger U, Fahrenfort JJ, Garrett DD. Boosting brain signal variability underlies liberal shifts in decision bias. Biorxiv. 2019 doi: 10.1101/834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmogorov AN. New metric invariant of transitive dynamical systems and endomorphisms of Lebesgue spaces. Dokl Russ Acad Sci. 1958;119:851–864. [Google Scholar]
- Kosciessa JQ, Kloosterman NA, Garrett DD. Standard multiscale entropy reflects neural dynamics at mismatched temporal scales: What’s signal irregularity got to do with it? Plos Comput Biol. 2020;16:e1007885. doi: 10.1371/journal.pcbi.1007885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarev VV, Pontes A, de Azevedo LC. EEG photic driving: right-hemisphere reactivity deficit in childhood autism. A pilot study. Int J Psychophysiol. 2009;71(2):177–183. doi: 10.1016/j.ijpsycho.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Lea-Carnall CA, Montemurro MA, Trujillo-Barreto NJ, Parkes LM, El-Deredy W. Cortical resonance frequencies emerge from network size and connectivity. PLoS Comput Biol. 2016;12(2):e1004740. doi: 10.1371/journal.pcbi.1004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe S, Kovacevic N, McIntosh AR. Differential maturation of brain signal complexity in the human auditory and visual system. Front Hum Neurosci. 2009;3:48. doi: 10.3389/neuro.09.048.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Chen Y, Chen D, Li C, Qiu Y, Wang J. Altered electroencephalogram complexity in autistic children shown by the multiscale entropy approach. Neuroreport. 2017;28(3):169–173. doi: 10.1097/WNR.0000000000000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Conteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule. Los Angeles: Western Psychological Services; 2000. [Google Scholar]
- Machado C, Estévez M, Leisman G, Melillo R, Rodríguez R, DeFina P, Hernández A, Pérez-Nellar J, Naranjo R, Chinchilla M, Garófalo N, Vargas J, Beltrán C. QEEG spectral and coherence assessment of autistic children in three different experimental conditions. J Autism Dev Disord. 2015;45(2):406–424. doi: 10.1007/s10803-013-1909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik J (2022) Multiscale sample entropy. https://www.mathworks.com/matlabcentral/fileexchange/62706-multiscale-sample-entropy, MATLAB Central File Exchange. Accessed 12 Jan 2022
- Maxwell CR, Villalobos ME, Schultz RT, Herpertz-Dahlmann B, Konrad K, Kohls G. Atypical laterality of resting gamma oscillations in autism spectrum disorders. J Autism Dev Disord. 2015;45(2):292–297. doi: 10.1007/s10803-013-1842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Kovacevic N, Itier RJ. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput Biol. 2008;4(7):e1000106. doi: 10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Kovacevic N, Lippe S, Garrett D, Grady C, Jirsa V. The development of a noisy brain. Arch Ital Biol. 2010;148:323–337. [PubMed] [Google Scholar]
- Mercader J, Miranda A (2016) Comunicación social en niños con ASD y TDAH. Castejón Costa, Juan Luis (coord.). Psicología y Educación: Presente y Futuro. Alicante: ACIPE, 2016. ISBN 978-84-608-8714-0, pp. 656–664. http://hdl.handle.net/10045/63720
- Milne E, Gomez R, Giannadou A, Jones M. Atypical EEG in autism spectrum disorder: comparing a dimensional and a categorical approach. J Abnorm Psychol. 2019;128(5):442–452. doi: 10.1037/abn0000436. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Chou C-A, Ma X, Fan M, Owens M, Samaya H, Gibb BE. Developmental changes in spontaneous electrocortical activity and network organization from early to late childhood. Neuroimage. 2015;118:237–247. doi: 10.1016/j.neuroimage.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Owens M, Kuntzelman K, Gibb BE. Charting moment-to-moment brain signal variability from early to late childhood. Cortex. 2016;83:51–61. doi: 10.1016/j.cortex.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Takahashi T, Cho RY, Kikuchi M, Murata T, Takahashi K, Wada Y. Assessment of EEG dynamical complexity in Alzheimer’s disease using multiscale entropy. Clin Neurophysiol. 2010;121(9):1438–1446. doi: 10.1016/j.clinph.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- O'Reilly C, Lewis JD, Elsabbagh M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLoS ONE. 2017;12(5):e0175870. doi: 10.1371/journal.pone.0175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, Elam M. Excess of high frequency electroencephalogram oscillations in boys with autism. Biol Psychiatry. 2007;62(9):1022–1029. doi: 10.1016/j.biopsych.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, Nygren G, Gillberg C, Elam M. Sensory gating in young children with autism: relation to age, IQ, and EEG gamma oscillations. Neurosci Lett. 2008;434(2):218–223. doi: 10.1016/j.neulet.2008.01.066. [DOI] [PubMed] [Google Scholar]
- Papaioannou AG, Kalantzi E, Papageorgiou CC, Korombili K, Βokou A, Pehlivanidis A, Papageorgiou CC, Papaioannou G. Complexity analysis of the brain activity in Autism Spectrum Disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD) due to cognitive loads/demands induced by Aristotle's type of syllogism/reasoning. A Power Spectral Density and multiscale entropy (MSE) analysis. Heliyon. 2021;7(9):7984. doi: 10.1016/j.heliyon.2021.e07984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce S, Kadlaskar G, Edmondson DA, McNally KR, Dydak U, Keehn B. Associations between sensory processing and electrophysiological and neurochemical measures in children with ASD: an EEG-MRS study. J Neurodev Disord. 2021;13(1):5. doi: 10.1186/s11689-020-09351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy (ApEn) as a complexity measure. Chaos. 1995;5:110–117. doi: 10.1063/1.166092. [DOI] [PubMed] [Google Scholar]
- Pion-Tonachini L, Kreutz-Delgado K, Makeig S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. Neuroimage. 2019;198:181–197. doi: 10.1016/j.neuroimage.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop-Jordanova N, Zorcec T, Demerdzieva A, Gucev Z. QEEG characteristics and spectrum weighted frequency for children diagnosed as autistic spectrum disorder. Nonlinear Biomedical Physics. 2010;4:4. doi: 10.1186/1753-4631-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JS, Moorman RJ. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278(6):H2039–H2049. doi: 10.1103/physreva.29.975. [DOI] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autism brain: challenges of the ‘new psychophysiology’. Int J Psychophysiol. 2007;63:164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Martínez EI, Angulo-Ruiz BY, Arjona-Valladares A, Rufo M, Gómez-González J, Gómez CM. Frequency coupling of low and high frequencies in the EEG of ADHD children and adolescents in closed and open eyes conditions. Res Dev Disabil. 2020 doi: 10.1016/j.ridd.2019.103520. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Martínez EI, Angulo-Ruíz BY, Ruíz-Martínez FJ, Gómez CM (2021) EEG development during resting state. Factors Affecting Neurodevelopment: Genetics, Neurology, Behavior, and Diet. Elsevier. ISBN 978-0-12-817986-4
- Rojas DC, Wilson LB. Gamma-band abnormalities as markers of autism spectrum disorders. Biomark Med. 2014;8:353–368. doi: 10.2217/bmm.14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Martínez FJ, Rodríguez-Martínez EI, Wilson CE, Yau S, Saldaña D, Gómez CM. Impaired P1 habituation and mismatch negativity in children with autism spectrum disorder. J Autism Dev Disord. 2020;50(2):603–616. doi: 10.1007/s10803-019-04299-0. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Segalowitz SJ, Santesso DL, Jetha MK. Electrophysiological changes during adolescence: a review. Brain Cogn. 2010;72(1):86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sheikhani A, Behnam H, Mohammadi MR, Noroozian M, Mohammadi M. Detection of abnormalities for diagnosing of children with autism disorders using of quantitative electroencephalography analysis. J Med Syst. 2012;36(2):957–963. doi: 10.1007/s10916-010-9560-6. [DOI] [PubMed] [Google Scholar]
- Sheikhani A, Behnam H, Noroozian M, Mohammadi MR, Mohammadi M. Abnormalities of quantitative electroencephalography in children with Asperger disorder in various conditions. Res Autism Spectr Disord. 2009;3(2):538–546. doi: 10.1016/j.rasd.2008.11.002. [DOI] [Google Scholar]
- Shen K, McFadden A, McIntosh AR. Signal complexity indicators of health status in clinical EEG. Sci Rep. 2021;11:20192. doi: 10.1038/s41598-021-99717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DM, Damiano CR, Woynaroski TG, Ibañez LV, Murias M, Stone WL, Wallace MT, Cascio CJ. Neural correlates of sensory hyporesponsiveness in toddlers at high risk for autism spectrum disorder. J Autism Dev Disord. 2017;47(9):2710–2722. doi: 10.1007/s10803-017-3191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Edelman GM. Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural Netw. 2000;13(8–9):909–922. doi: 10.1016/s0893-6080(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Szostakiwskyj JMH, Willatt SE, Cortese F, Protzner AB. The modulation of EEG variability between internally- and externally- driven cognitive states varies with maturation and task performance. PLoS ONE. 2017;12(7):e0181894. doi: 10.1371/journal.pone.0181894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. Complexity of spontaneous brain activity in mental disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:258–266. doi: 10.1016/j.pnpbp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Cho RY, Murata T, Mizuno T, Kikuchi M, Mizukami K, Wada Y. Age-related variation in EEG complexity to photic stimulation: a multiscale entropy analysis. Clin Neurophysiol. 2009;120(3):476–483. doi: 10.1016/j.clinph.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Yoshimura Y, Hiraishi H, Hasegawa C, Munesue T, Higashida H, Minabe Y, Kikuchi M. Enhanced brain signal variability in children with autism spectrum disorder during early childhood. Hum Brain Mapp. 2016;37:1038–1050. doi: 10.1002/hbm.23089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twins studies. J Child Psychol Psyc. 2016;57(5):585–595. doi: 10.1111/jcpp.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLoS ONE. 2012;7(6):e39127. doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hecke AV, Stevens S, Carson AM, Karst JS, Dolan B, Schohl K, McKindles RJ, Remmel R, Brockman S. Measuring the plasticity of social approach: a randomized controlled trial of the effects of the PEERS intervention on EEG asymmetry in adolescents with autism spectrum disorders. J Autism Dev Disord. 2015;45(2):316–335. doi: 10.1007/s10803-013-1883-y. [DOI] [PubMed] [Google Scholar]
- Van Noordt S, Willoughby T. Cortical maturation from childhood to adolescence is reflected in resting state EEG signal complexity. Dev Cogn Neurosci. 2021;48:100945. doi: 10.1016/j.dcn.2021.100945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barstein J, Ethridge LE, Mosconi MW, Takarae Y, Sweeney JA. Resting state EEG abnormal in autism spectrum disorders. J Neurodev Disord. 2013;5:24. doi: 10.1186/1866-1955-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass S. Distortions and disconnections: disrupted brain connectivity in autism. Brain Cogn. 2011;75(1):18–28. doi: 10.1016/j.bandc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 2007;28:228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau SH, Brock J, McArthur G. The relationship between spoken language and speech and nonspeech processing in children with autism: a magnetic event-related field study. Dev Sci. 2016;19(5):834–852. doi: 10.1111/desc.12328. [DOI] [PubMed] [Google Scholar]
- Yuk V, Dunkley BT, Anagnostou E, Taylor MJ. Alpha connectivity and inhibitory control in adults with autism spectrum disorder. Mol Autism. 2020;11(1):95. doi: 10.1186/s13229-020-00400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]