Abstract
To investigate the mechanism by which ginsenoside Rg3 regulates oxidative stress (OS) and inflammation through NF/KB pathway to delay mouse liver injury.
This work randomized Balbc mice as four groups: Normal, D-gal, Rg3-L, Rg3-H. Paraffin-embedded liver tissue sections were prepared, later, BAX/BCL-2 protein expression was observed by HE, Sirius red, TUNEL and immunofluorescence to detect apoptotic injury and α-SMA/TGF-β protein expression to detect fibrosis, and liver inflammation-related protein NF-KB was detected.
HE and TUNEL staining showed that Rg3 reduced necrotic cells and fibrosis in liver-injured mice, Rg3 increased anti-inflammatory cytokine IL-18 and reduced TNF-α, IL-1β and IL-6 expression. Conclusion: Ginsenoside Rg3 can effectively antagonize D-gal’s role in mouse liver injury, and its mechanism may be associated with regulating inflammatory pathway by Rg.
Keywords: Rg3, red ginseng, D-gal, liver injury
INTRODUCTION
As estimated by statics from the Global Burden of Disease, there were over 2 million individuals dying from hepatopathy in 2010, mostly consisting of acute hepatitis, liver cancer and cirrhosis, occupying approximately 4% of all death cases worldwide [1]. As a body organ with critical role in metabolism, liver injury may result from a variety of factors, such as drug toxicity, hepatitis viral infection, bacterial metabolites, industrial chemicals or drinking [2]. Liver apoptosis and chronic fibrosis are important stages in the development of liver disease [3]. How to reverse liver damage during this stage is an important tool in preventing liver disease. Continuous injection of D-gal induces excessive oxidative free radical generation in the body and is a classical model of near-natural ageing [4], which is why it has been extensively utilized for exploring aging mechanisms and anti-ageing drugs [5]. The global population with the age of more than 65 years may elevate from 524 million to approximately 1.5 billion during 2020-2050. Older people are particularly vulnerable to chronic diseases, especially damage to organs [6].
Ageing-induced liver damage is mainly characterized by excessive inflammation and dysregulation of enzymatic activity in the liver. Regulation of the conversion of hepatic stellate cells to myofibroblasts is essential to prevent senescence-induced liver fibrosis [7]. Liver fibrosis is accompanied by lipid deposition and overproduction of alpha-smooth muscle protein (α-SMA). Due to excessive inflammatory factor expression, inflammatory factors infiltrate the liver tissue and induce excess transforming growth factor-beta (TGF-β) generation [8], which induces the activation of myofibroblasts. In addition to this, dysregulated levels of oxidation in the liver further induce liver damage. Among other things, ROS are transmitted to mitochondria via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system as well as the respiratory chain [9, 10]. Apart from mitochondria, overexpression of cytochrome P450 2E1 (CYP2E1) and 4-Hydroxynonenal (4-HNE) further damage hepatocytes [11].
In addition, a number of natural components or products are analyzed in clinical studies because of the possible medicinal effects. Ginseng is the root of the natural medicinal plant ginseng. Ginseng has long been used as an ethnic medicine in Asia for over 2000 years [12, 13]. Interestingly, processed ginseng, red ginseng, has better antioxidant activity. Studies have reported that red ginseng has been widely used for a variety of conditions such as neurodegeneration, gastroenteritis, liver damage, and aging [14]. Chemical composition studies have shown that the Rg3 content in red ginseng is 8.6 times higher relative to that in regular ginseng. Due to the good antioxidant and anti-inflammatory activities of Rg3, it is hypothesized that it can mitigate the ageing process of the liver by resisting hepatic oxidative stress (OS).
MATERIALS AND METHODS
Materials
This study acquired 20 (R)-G-Rg3 (purity≥98.0%, HPLC) and evaluated the quality according to our prior description. In addition, the present work obtained D-gal from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), whereas BCA protein assay kit (P0010) and RIPA lysis buffer (P0013B) in Beyotime Biotechnology Co., Ltd. (Jiangsu, China). Additionally, we purchased alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), high/low density lipoprotein cholesterol (LDL-H/LDL-C), and hematoxylin-eosin (H&E) kits in Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China). The present work acquired Mouse IL-1β, Mouse IL-6, Mouse IL-18, Mouse TNF-α ELISA kits in Multi Science Biotechnology Co., Ltd. (Hangzhou, China).
Animal and experiments
The present work obtained the 8-week-old ICR male mice weighing 24-26 g) from Changchun ESI Laboratory Animal Technology Co., Ltd (SCXK(JI)-2020-0001, China) and raised them within the pathogen-free rearing room under the 60.0% ± 10.0% humidity, 22.0° C±2.0° C and light/dark cycle of 12-h/12-h. The animals were allowed to eat food and drink water freely. At 1-week following domestication, animals were randomized as 4 groups (n=6): Normal, D-gal, D-gal+Rg3 (25 mg/kg) group and D-gal+Rg3 (50 mg/kg). For the remaining mice excluding the Normal group, intraperitoneal injection of D-gal (600 mg/kg) was given for two weeks consecutively for modeling liver senescence. Rg3-treated mice were given gavage from the fourth week of feeding.
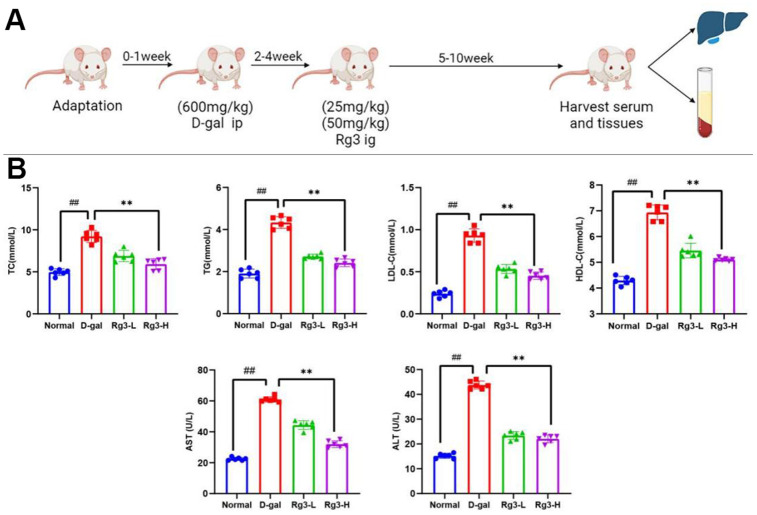
This work calculated doses and determined treatment schedules according to our preliminary results together with additional prior findings. (Figure 1A).
Figure 1.
(A) Sketch map showing D-galactose mediated mouse aging and damage models. (B) Serum lipid metabolism and liver function indexes in mice. Results are represented by mean±S.D. #p< 0.05, ##p<0.01 vs. Normal group. *p<0.05, **p<0.01 vs. D-gal group.
Evaluation of serum biochemical indexes
This study used ALT and AST kits to detect the main indexes of liver function [15]. Briefly, after mice were killed, blood sampling and centrifugation at 1000 × g and 4° C for a 10-min period were completed to collect serum. Later, the kit-derived specific substrate was added into the 96-well plates under 37° C for a 30-min period. Absorbance (OD) value was measured at 510 nm. Additionally, TC, TG, HDL-C and LDL-C levels in serum were also determined for analyzing liver lipid metabolism function [16, 17].
Immunofluorescence (IF)
After deparaffinage, liver sections were subject to rehydration, immersion in 0.3%H2O2 for 5min to eliminate endogenous peroxidase, and 15-min incubation with citrate buffer (pH 6.0) to achieve antigen recovery by boiling. Later, 0.1%Triton X-100 was added to permeate sections, followed by treated with 10% goat serum for non-specific antigen blocking [18].
After washing with PBS, secondary antibodies (1:200) including Alexa Fluor 488-labeled goat anti-rat and 594-labeled goat anti-rabbit were added to further incubate sections. The samples were incubated for a 10-min period within the DAPI working solution under ambient temperature in the humid chamber in dark. The fluorescence microscope was employed to capture images. Signal intensities were analyzed using ImageJ software and determined through calculating mean unit intensity to control [18].
TUNEL staining
Mouse livers were taken and embedded in paraffin, followed by cutting in 4-μm sections with the rotary microtome, dewaxing in xylene as well as dehydrated with ethanol. Liver apoptotic damage was assessed by the TUNEL Apoptosis detection kit in line with specific protocols, and DAPI staining was calculated to determine total nuclear number [19].
Statistical analysis
SPSS18.0 software (IBM) was employed for statistical analysis. T test was utilized to analyze significant difference for two groups, while one-way ANOVA combined with Tukey’s test for three groups. Results were represented by mean±SD of 3 separate assays. P<0.05 stood for statistical significance [20, 21].
RESULTS
Rg3 alleviated abnormal liver function and lipid metabolism markers in mice
As shown in Figure 1B, TC, TG, HDL-C and LDL-C levels in serum of D-gal group remarkably increased (P<0.01). Their levels were decreased after Rg3 addition, which indicated that Rg3 could alleviate the abnormal liver lipid metabolism induced by aging.
ALT and AST represent major indicators of liver function. In this study, their contents of liver injury group remarkably elevated by D-gal injection (P<0.01), indicating the successful construction of mouse liver injury model. At 5-week post-Rg3 treatment (25 and 50 mg·kg−1), ALT and AST contents in serum of Rg3 treatment group decreased (P<0.01).
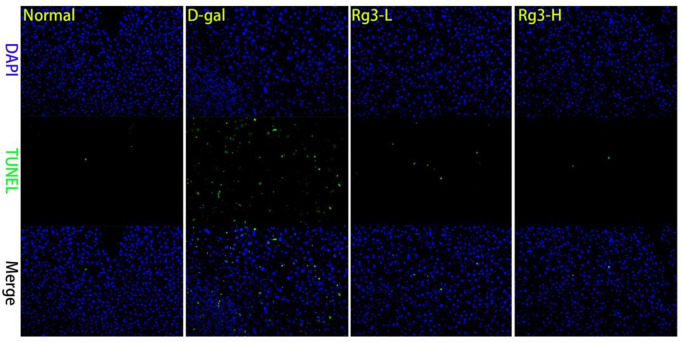
Rg3 alleviates D-Gal-induced mouse liver apoptosis and fibrosis
For investigating how Rg3 affected liver necrosis and fibrosis caused by aging, mice were intraperitoneally injected with D-gal to construct the liver aging mouse model, followed by Rg3 treatment. According to TUNEL staining, lobular destruction, apoptosis, collagen deposition and septal generation of D-gal group significantly elevated relative to Normal group, which were significantly reduced after Rg3 administration (Figure 2).
Figure 2.
Morphopathological section of mouse liver. Mouse liver was stained by TUNEL section.
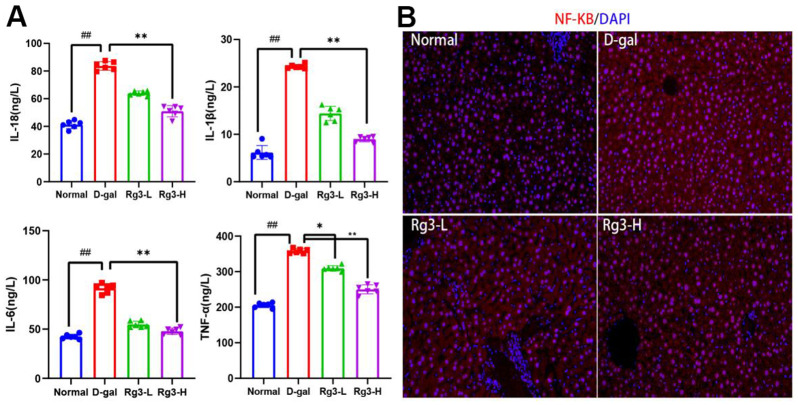
Rg3 ameliorates the inflammatory response to D-GAL mediated liver injury through reducing serum cytokine accumulation in mice
Chronic inflammation in the body represents one of typical aging manifestations. Serum IL-18, IL-6, IL-1β and TNF-α contents were determined (Figure 3A). Compared with Normal group, D-gal exposure dramatically elevated IL-18, IL-6, IL-1β and TNF-α contents, and these changes were reversed after Rg3 treatment (p<0.01). For direct demonstration of the role of D-gal in inducing mouse liver inflammation, NF-κB protein expression was analyzed through IF staining (Figure 3B). NF-κB is a key protein regulating inflammation switch, and its expression level is closely related to cytokine secretion. The results showed that Rg3 antagonized the NF-κB overexpression, which displayed cytoplasmic and nuclear localization within D-Gal-mediated liver tissues.
Figure 3.
Expression levels of inflammatory related factors induced by Rg3 on D-gal in mice. (A) Serum inflammatory cytokines in mice. (B) Immunofluorescence section of NF-KB in mouse liver. Results are represented by mean±S.D. #p<0.05, ##p<0.01 vs. Normal group. *p<0.05, **p<0.01 vs. D-gal group.
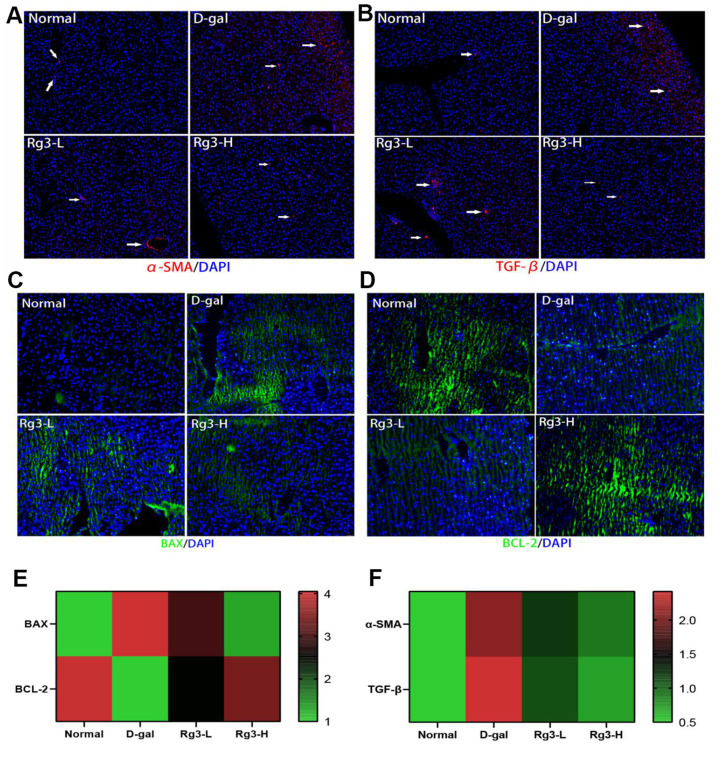
Rg3 plays an antifibrotic role by inhibiting D-Gal-mediated apoptosis
According to our results, the liver fibrosis degree was higher in untreated D-gal group. Therefore, aging induced liver fibrosis. The improvement of liver metabolism by Rg3 may be associated with inhibiting apoptosis while reducing TGF-β [22] and α-SMA secretion [23]. Based on immunofluorescence results, relative to D-gal group, TGF-β and α-SMA secretion in the liver of Rg3 treatment group was alleviated (Figure 4A, 4B). Through determining BAX and BCL-2 protein levels within the liver, we found that BCL-2 (anti-apoptotic protein) expression elevated, while BAX (pro-apoptotic protein) expression markedly declined in Rg3 treatment group (Figure 4C, 4D).
Figure 4.
Immunofluorescence section of mouse liver fibrosis and apoptosis-related factors. (A) Immunofluorescence section of mouse liver with α-SMA. (B) Immunofluorescence section of mouse liver TGF-β. (C) Immunofluorescence section of BAX in mouse liver. (D) Immunofluorescence section of BCL-2 within mouse liver. (E) Immunofluorescence quantification of BAX/BCL-2 within mouse liver. (F) Immunofluorescence quantification of α-SMA/TGF-β in mouse liver.
DISCUSSION
Senility related liver fibrosis is a common clinical disease in the elderly. Several studies have shown that the enzyme activity in the liver changes first under the induction conditions of oxidants and inflammatory factors. Rg3 can down-regulate abnormally elevated AST and ALT, thereby improving liver function. As an important place for lipid metabolism, TC, LDL-C, TG and HDLC contents during aging process are abnormal. However, after taking Rg3, the serum lipid marker content of mice gradually recovered to normal [24, 25]. As a key protein in the regulation of inflammatory response, the expression of NF-KB is positively regulated with TNF-α, IL-6, IL-1β and IL-18 [17]. Rg3 can inhibit ROS generation, inhibit mitochondrial swelling and antagonize cellular inflammation, senescence and apoptosis by extracting rat brain mitochondria in vitro and induced by H2O2. Rg3 can also inhibit IL-6 and IL-18 production, and improve cell senescence [12].
In this study, D-gal induced liver tissue apoptosis, hepatocyte damage and severe fibrosis in mice [26]. Serum levels of TNF-α, IL-6, IL-1β and IL-18 were determined through ELISA. Expectedly, serum secretion of inflammatory cytokines was reduced after Rg3 treatment, and NF-KB protein expression in liver was decreased after Rg3 treatment, as measured by immunofluorescence. In addition, extensive research has shown that inflammatory responses promote apoptosis, which was also confirmed in the current study. After treatment with Rg3, Bcl-2/Bax levels were, with Bcl-2 and Bax showing significant up- and down-regulation, separately.
CONCLUSIONS
The present experiments showed that ginsenoside Rg3 attenuated D-gal-mediated mouse liver injury. Based on our results, ginsenoside Rg3 is the potential novel drug used to treat senile liver injury.
Footnotes
AUTHOR CONTRIBUTIONS: Ke Xu and Biwen Hu are responsible for the operation of the experiment and the acquisition of data, xuhui Ding is responsible for data statistical analysis and chart drawing; Zhengyu Zhan is responsible for writing the paper and providing financial support.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
Ethical Statement: The mouse experiments were approved by the Ethics Committee of Nanchang University, and carried out in line with the Guide for the Care and Use of Laboratory Animals.
FUNDING: No funding was provided for this study
This corresponding author has a verified history of publications using a personal email address for correspondence.
REFERENCES
- 1.Sha JY, Li JH, Zhou YD, Yang JY, Liu W, Jiang S, Wang YP, Zhang R, Di P, Li W. The p53/p21/p16 and PI3K/Akt signaling pathways are involved in the ameliorative effects of maltol on D-galactose-induced liver and kidney aging and injury. Phytother Res. 2021; 35:4411–24. 10.1002/ptr.7142 [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Zhang T, Zhao Y, Cai E, Zhu H, Liu S. Panaxynol from Saposhnikovia diviaricata exhibits a hepatoprotective effect against lipopolysaccharide + D-Gal N induced acute liver injury by inhibiting Nf-κB/IκB-α and activating Nrf2/HO-1 signaling pathways. Biotech Histochem. 2020; 95:575–83. 10.1080/10520295.2020.1742932 [DOI] [PubMed] [Google Scholar]
- 3.Xie LY, Yang Z, Wang Y, Hu JN, Lu YW, Zhang H, Jiang S, Li W. 1-O-Actylbritannilactone Ameliorates Alcohol-Induced Hepatotoxicity through Regulation of ROS/Akt/NF-κB-Mediated Apoptosis and Inflammation. ACS Omega. 2022; 7:18122–30. 10.1021/acsomega.2c01681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian S. Metabolomics reveals that alcohol extract of propolis alleviates D-gal-induced skeletal muscle senescence in mice. Food Bioscience. 2022; 49. 10.1016/j.fbio.2022.101885 [DOI] [Google Scholar]
- 5.Lv H, An B, Yu Q, Cao Y, Liu Y, Li S. The hepatoprotective effect of myricetin against lipopolysaccharide and D-galactosamine-induced fulminant hepatitis. Int J Biol Macromol. 2020; 155:1092–104. 10.1016/j.ijbiomac.2019.11.075 [DOI] [PubMed] [Google Scholar]
- 6.Sarkar C, Mondal M, Al-Khafaji K, El-Kersh DM, Jamaddar S, Ray P, Roy UK, Afroze M, Moniruzzaman M, Khan M, Asha UH, Khalipha AB, Mori E, et al. GC-MS analysis, and evaluation of protective effect of Piper chaba stem bark against paracetamol-induced liver damage in Sprague-Dawley rats: Possible defensive mechanism by targeting CYP2E1 enzyme through in silico study. Life Sci. 2022; 309:121044. 10.1016/j.lfs.2022.121044 [DOI] [PubMed] [Google Scholar]
- 7.Hao M, Ding C, Peng X, Chen H, Dong L, Zhang Y, Chen X, Liu W, Luo Y. Ginseng under forest exerts stronger anti-aging effects compared to garden ginseng probably via regulating PI3K/AKT/mTOR pathway, SIRT1/NF-κB pathway and intestinal flora. Phytomedicine. 2022; 105:154365. 10.1016/j.phymed.2022.154365 [DOI] [PubMed] [Google Scholar]
- 8.Khullar M, Sharma A, Wani A, Sharma N, Sharma N, Chandan BK, Kumar A, Ahmed Z. Acteoside ameliorates inflammatory responses through NFkB pathway in alcohol induced hepatic damage. Int Immunopharmacol. 2019; 69:109–17. 10.1016/j.intimp.2019.01.020 [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Wang Y, Zhang M, Gao Y, Yan Z. Protective Effects of Ginsenosides (20R)-Rg3 on H2 O2 -Induced Myocardial Cell Injury by Activating Keap-1/Nrf2/HO-1 Signaling Pathway. Chem Biodivers. 2021; 18:e2001007. 10.1002/cbdv.202001007 [DOI] [PubMed] [Google Scholar]
- 10.Yang C, He X, Zhao J, Huang W. Hepatoprotection by Ginsenoside Rg1 in alcoholic liver disease. Int Immunopharmacol. 2021; 92:107327. 10.1016/j.intimp.2020.107327 [DOI] [PubMed] [Google Scholar]
- 11.Maksymchuk O, Shysh A, Stroy D. Treatment with omega-3 PUFAs does not increase the risk of CYP2E1-dependent oxidative stress and diabetic liver pathology. Front Endocrinol (Lausanne). 2022; 13:1004564. 10.3389/fendo.2022.1004564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Zeng L, Zhang Y, Qi W, Wang Z, Tian L, Zhao D, Wu Q, Li X, Wang T. Pharmacological properties, molecular mechanisms and therapeutic potential of ginsenoside Rg3 as an antioxidant and anti-inflammatory agent. Front Pharmacol. 2022; 13:975784. 10.3389/fphar.2022.975784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi RJ, Mohamad Zobir SZ, Alexander-Dann B, Sharma N, Ma MK, Lam BY, Yeo GS, Zhang W, Fan TP, Bender A. Combination of Ginsenosides Rb2 and Rg3 Promotes Angiogenic Phenotype of Human Endothelial Cells via PI3K/Akt and MAPK/ERK Pathways. Front Pharmacol. 2021; 12:618773. 10.3389/fphar.2021.618773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JJ, Zhou YD, Liu YB, Wang JQ, Li KK, Gong XJ, Lin XH, Wang YP, Wang Z, Li W. Protective Effect of 20(R)-Ginsenoside Rg3 Against Cisplatin-Induced Renal Toxicity via PI3K/AKT and NF-[Formula: see text]B Signaling Pathways Based on the Premise of Ensuring Anticancer Effect. Am J Chin Med. 2021; 49:1739–56. 10.1142/S0192415X21500828 [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Ren L, Chen S, Zheng H, Yang Y, Gu T, Li Y, Wang C. Longdan Xiegan Tang attenuates liver injury and hepatic insulin resistance by regulating the angiotensin-converting enzyme 2/Ang (1-7)/Mas axis-mediated anti-inflammatory pathway in rats. J Ethnopharmacol. 2021; 274:114072. 10.1016/j.jep.2021.114072 [DOI] [PubMed] [Google Scholar]
- 16.Su WY, Fan ML, Li Y, Hu JN, Cai EB, Zhu HY, Song MJ, Li W. 20(S)-ginsenoside Rh1 alleviates T2DM induced liver injury via the Akt/FOXO1 pathway. Chin J Nat Med. 2022; 20:669–78. 10.1016/S1875-5364(22)60201-2 [DOI] [PubMed] [Google Scholar]
- 17.AlAsmari AF, Alharbi M, Alqahtani F, Alasmari F, AlSwayyed M, Alzarea SI, Al-Alallah IA, Alghamdi A, Hakami HM, Alyousef MK, Sari Y, Ali N. Diosmin Alleviates Doxorubicin-Induced Liver Injury via Modulation of Oxidative Stress-Mediated Hepatic Inflammation and Apoptosis via NfkB and MAPK Pathway: A Preclinical Study. Antioxidants (Basel). 2021; 10:1998. 10.3390/antiox10121998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Zhang H, Hou YY, Hu RY, Zhang JJ, Chen X, Wang S, Hu JN, Wang Z, Li W. Arginyl-fructosyl-glucose, a major Maillard reaction product of red ginseng mitigates cisplatin-evoked intestinal toxicity in vivo and in vitro. Food Funct. 2022; 13:11283–97. 10.1039/d2fo00776b [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Zhang D, Li L, Han Y, Dong X, Yang L, Li X, Li W, Li W. Ginsenoside Rg1 ameliorates aging-induced liver fibrosis by inhibiting the NOX4/NLRP3 inflammasome in SAMP8 mice. Mol Med Rep. 2021; 24:801. 10.3892/mmr.2021.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu JN, Wang YM, Zhang H, Li HP, Wang Z, Han M, Ren S, Tang S, Jiang S, Li W. Schisandra B, a representative lignan from Schisandra chinensis, improves cisplatin-induced toxicity: An in vitro study. Phytother Res. 2023; 37:658–71. 10.1002/ptr.7644 [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Chen C, Liu R, Wang X, Zhao Y, Yan Z, Cai E, Zhu H. Potential Myocardial Protection of 3,4-seco-Lupane Triterpenoids from Acanthopanax sessiliflorus Leaves. Chem Biodivers. 2021; 18:e2000830. 10.1002/cbdv.202000830 [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Che J, Cui K, Zhuang W, Li H, Sun J, Chen J, Wang C. Schisantherin A ameliorates liver fibrosis through TGF-β1mediated activation of TAK1/MAPK and NF-κB pathways in vitro and in vivo. Phytomedicine. 2021; 88:153609. 10.1016/j.phymed.2021.153609 [DOI] [PubMed] [Google Scholar]
- 23.Li B, Ma Y, Tan L, Ren H, Wu L, Su Q, Song J, Chen W, Gong P, Jin Y. 20-Hydroxytetraenoic acid induces hepatic fibrosis via the TGF-β1/Smad3 signaling pathway. Toxicol Lett. 2023; 373:1–12. 10.1016/j.toxlet.2022.11.001 [DOI] [PubMed] [Google Scholar]
- 24.Li H, Gao YH, Song L, Chen TF, Zhang GP, Ye ZG, Gao Y, Huo W. Ginsenoside Rg1 protects mice against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced liver injury by inhibiting CYP1A1 through the aryl hydrocarbon receptor. J Ethnopharmacol. 2022; 294:115394. 10.1016/j.jep.2022.115394 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Hou JG, Liu Z, Gong XJ, Hu JN, Wang YP, Liu WC, Lin XH, Wang Z, Li W. Alleviative effects of 20(R)-Rg3 on HFD/STZ-induced diabetic nephropathy via MAPK/NF-κB signaling pathways in C57BL/6 mice. J Ethnopharmacol. 2021; 267:113500. 10.1016/j.jep.2020.113500 [DOI] [PubMed] [Google Scholar]
- 26.Hong W, Hwang-Bo J, Jeon H, Ko M, Choi J, Jeong YJ, Park JH, Kim I, Kim TW, Kim H, Kang SC. A Comparative Study of the Hepatoprotective Effect of Centella asiatica Extract (CA-HE50) on Lipopolysaccharide/d-galactosamine-Induced Acute Liver Injury in C57BL/6 Mice. Nutrients. 2021; 13:4090. 10.3390/nu13114090 [DOI] [PMC free article] [PubMed] [Google Scholar]