Abstract
Lymph nodes (LNs) are frequently the first sites of metastasis. Currently, the only prognostic LN assessment is determining metastatic status. However, there is evidence suggesting that LN metastasis is facilitated by the formation of a pre‐metastatic niche induced by tumour derived extracellular vehicles (EVs). Therefore, it is important to detect and modify the LN environmental changes. Earlier work has demonstrated that neutrophil extracellular traps (NETs) can sequester and promote distant metastasis. Here, we first confirmed that LN NETs are associated with reduced patient survival. Next, we demonstrated that NETs deposition precedes LN metastasis and NETs inhibition diminishes LN metastases in animal models. Furthermore, we discovered that EVs are essential to the formation of LN NETs. Finally, we showed that lymphatic endothelial cells secrete CXCL8/2 in response to EVs inducing NETs formation and the promotion of LN metastasis. Our findings reveal the role of EV‐induced NETs in LN metastasis and provide potential immunotherapeutic vulnerabilities that may occur early in the metastatic cascade.
Keywords: cancer, lymph node, metastasis, neutrophils, NETs
Illustrative demonstration of the LNs premetastatic niche formation induced by EVs and NETs. Primary tumour constantly secretes EVs, which were actively uptaken by LECs. LECs subsequently secretes CXCL8 or CXCL2 upon EV reception. CXCL8 and CXCL2 are both neutrophil chemoattractants and potent NETs inducers. The following neutrophil recruitment and NETs formation lead to increased LN metastasis burden.
1. INTRODUCTION
Solid organ tumours are often treated with two goals in mind. The first is local tumour control, which is often carried out by surgical removal. The second is to prevent the development of metastasis, the major limitation to survival (Anand et al., 2021; Kashkooli & Soltani, 2021). Accordingly, whenever possible, primary tumours are excised along with regional lymph nodes (LN) (Hoshino & Lyden, 2017; Steeg, 2016). Regional LN resection improves survival and allows for accurate disease staging (Wang et al., 2021). This is of critical importance, as systemic therapies (e.g., cytotoxic chemotherapy, immunotherapy), are prescribed on the basis of risk factors for the development of fatal distant disease (Cools‐Lartigue et al., 2015). The presence of LN metastases is one of the strongest predictors of the subsequent development of fatal systemic disease in many cancer types such as gastric cancer, lung cancer and melanoma (Nathanson et al., 2017). Thus, understanding the mechanisms of LN metastasis is of major therapeutic interests (Steeg, 2016). Currently, the only relevant prognostic feature is the presence or absence of overt metastatic tumour cells within the LNs. However, this solely tumour centric system overlooks the role of immune and non‐tumour compartments within the LNs (Arrichiello et al., 2022). Understanding this process before distant metastasis formation is critical, as no treatment strategy to date has reliably prevented the emergence of distant metastasis.
For metastatic colonisation to occur, the conditions within the LNs are optimised for tumour cell deposition and growth, a process known as pre‐metastatic niche formation (Gupta & Massagué, 2006; Kataru et al., 2019), which includes lymphoangiogenesis and the activation of various immune cells (García‐Silva et al., 2021; Kerjaschki, 2005; Soler‐Cardona et al., 2018). Neutrophils are resident cells within LNs (Lok et al., 2019). Under normal physiologic condition, LN neutrophils play a role in host defence and antigen presentation (Barnes et al., 2020; De Meo & Spicer, 2021; Shahzad et al., 2022). However, in the context of malignancy, inflammation, defined as elevated neutrophil activity, and infiltration, are associated with reduced patient survival (Lawati et al., 2020; Ocana et al., 2017; Wu et al., 2020).
Inflammation is recognized as one of the hallmarks of cancer, facilitating functions necessary for tumour progression (Coussens & Werb, 2002; Dvorak, 2015; Greten & Grivennikov, 2019). These include proliferation, resistance to therapy, and sequestration of tumour cells within distant organ sites (Abdel‐Latif et al., 2009; Colotta et al., 2009; Deyell et al., 2021; Vyas et al., 2014; Zhao et al., 2021). Neutrophils are the most abundant leukocyte in humans and play a primary role in host defence to infection, however, in the context of malignancy, neutrophils are a major driver of inflammation (Amulic et al., 2012, Hsu et al., 2020; Sagiv et al., 2015; Wu et al., 2020). They mediate functions necessary for cancer progression, such as tumour cell proliferation, invasion, and metastasis (Engblom et al., 2017; Park & Nam, 2020; Spicer et al., 2012). Specifically, neutrophils can induce resistance to cytotoxic chemotherapy, radiotherapy, and immunotherapy (Chen et al., 2022; Jaillon et al., 2020; Kotecha et al., 2022; Rayes et al., 2019; Zhang et al., 2020). Neutrophils can trap and support malignant cells within distant organs. This has been attributed to the elaboration of neutrophil extracellular traps (NETs)—extracellular strands of DNA decorated with biologically active peptides (Cools‐Lartigue et al., 2013; Shahzad et al., 2022). These NETs play a role in defence to infection and represent a primary effector function of neutrophils under normal physiologic conditions (Brinkmann et al., 2004; Malech et al., 2014). Our group and others have demonstrated that NETs act in cancer progression and have shown profound reduction of metastasis that occurs following NETs inhibition (Cools‐Lartigue et al., 2013, Demers & Wagner, 2013, Najmeh et al., 2017, Rayes et al., 2019; Rayes et al., 2020; Tohme et al., 2016). Since then, many of the mechanisms by which neutrophils facilitate tumour progression have been attributed to tumour‐NETs interactions (Park et al., 2016; Shinde‐Jadhav et al., 2021; Wang et al., 2021).
Neutrophils exhibit great plasticity to environmental cues and the process of NETs release is believed to be mediated by tumour secretion of numerous kinds of factors, including extracellular vesicles (EVs) (Giese et al., 2019; Iba & Ogura, 2018; Jaillon et al., 2020). Tumour‐derived EVs have been shown to be actively uptaken and transported by lymphatic vessels and are able to prepare a pre‐metastatic niche in sentinel LNs for impending melanoma metastasis (Broggi et al., 2019; Hood et al., 2011; Srinivasan et al., 2016). Moreover, EVs can polarize neutrophils from an anti‐tumour (N1) phenotype to a pro‐tumour (N2) phenotype (Zhang et al., 2018). EVs can also directly induce the formation of NETs (Leal et al., 2017). However, there is no direct evidence suggesting that EV‐mediated NETs production has a role in LN metastasis development. Thus, a better understanding of the microenvironmental changes initiating LN metastasis is needed to improve clinical understanding and intervention. Currently, no therapeutic modalities exist that selectively mitigate the consequences of neutrophil mediated inflammation.
In the current study, we demonstrate that lymphatic neutrophil accumulation and NETs deposition is associated with reduced survival in human gastroesophageal adenocarcinoma (GEA) patients. Furthermore, we demonstrate that lymphatic neutrophil accumulation and NETs deposition precede and are necessary for efficient LN metastatic outgrowth. Finally, we describe the role tumour derived EVs play in establishing a regional inflammatory microenvironment through the induction of lymphatic endothelial elaboration of CXCL8.
2. METHODS
The detailed antibody and reagent list is in Table S4.
2.1. Patients
This study investigated surgical lymph node of 175 patients with gastroesophageal adenocarcinoma (Table S1) who were treated at the McGill University Health Centre (MUHC), Montréal, Canada. All the studies were conducted according to the relevant regulatory standards, upon approval by the Research Ethics Board Office at McGill University.
2.2. Animal experiments
C57BL/6 (Charles River Laboratories) and PAD4–/– (gift of Alan Tsung, The Ohio State University Comprehensive Cancer Centre) mice of both sexes were used for all experiments at 7–10 weeks old. All experiments were performed with the Veterinary Authority of the Institutional Animal Care and Use Committee at the RI‐MUHC. For all tumour models, 250k cancer cells in 100 μL PBS were injected into the left flank of the mice. The tumour was allowed to grow in the mice for 7 (H59) or 10 (B16F10) days as premetastatic stage or 14 days as post‐metastatic stage. For the neutrophil depletion, experimental group mice received intravenous injection of 100 μg anti‐Ly6G on day 0, 2, 6, 8, 10, 12 post tumour inoculation, and 100 μg of anti‐Rat‐kappa light chain on day 1, 5, 7, 11, 13 post tumour inoculation. Control group only received anti‐Rat‐kappa light chain injection. For the knockout experiments, PAD4–/– mice were compared to age and sex matched C57BL/6 mice. For the NEi treatment, experimental group mice received daily gavage of 2.2 mg/kg of neutrophil elastase inhibitor Sivelestat resuspended in saline. Control mice received saline gavage.
2.3. EV isolation, labelling and mouse injection
A549, BEAS‐2B and B16F10 cells were purchased from ATCC and were cultured in media with EV‐free FBS (ultracentrifuged for 18 h at 100,000 × g). The cells were cultured without any stimulation for 48–72 h until they reached 70%–80% confluency. The supernatant was collected and cells were pelleted by centrifugation at 300 × g for 10 min. The supernatant was centrifuged at 20,000 × g for 20 min. EVs were then harvested by centrifugation at 100,000 × g for 70 min. The EV pellet was resuspended in 20 mL of PBS and collected by ultracentrifugation at 100,000 × g for 70 min. For fluorescence labelled EVs, supernatant was concentrated using Amicon Ultra‐15 Centrifugal Filter Units with 100 KDa filter size, incubated with 1.0 mM CM‐DiI or 50 μM CFSE for 2 h before this first 100,000 × g centrifugation. All EVs were isolated and verified by nanoparticle tracking assay, transmission electron microscopy and western blot according to MISEV guidelines (Théry et al., 2018). These results were included in Figure S5 and we have submitted all relevant data of our experiments to the EV‐TRACK knowledgebase (EV‐TRACK ID: EV230586) (Van Deun et al., 2017).
For EV pre‐treatments (Figure 4c, d), 10 μg EV in 30 μL PBS was injected into the ipsilateral footpad every other day before tumour inoculation. For the EV localization experiments, 10 μg CM‐Dil labelled EV were injected 24 h before sacrificing mice. For the EV injection no‐tumour bearing mice, 10–30 μg EV in 30 μL PBS was injected into the ipsilateral footpad every other day for three times before sacrificing.
FIGURE 4.
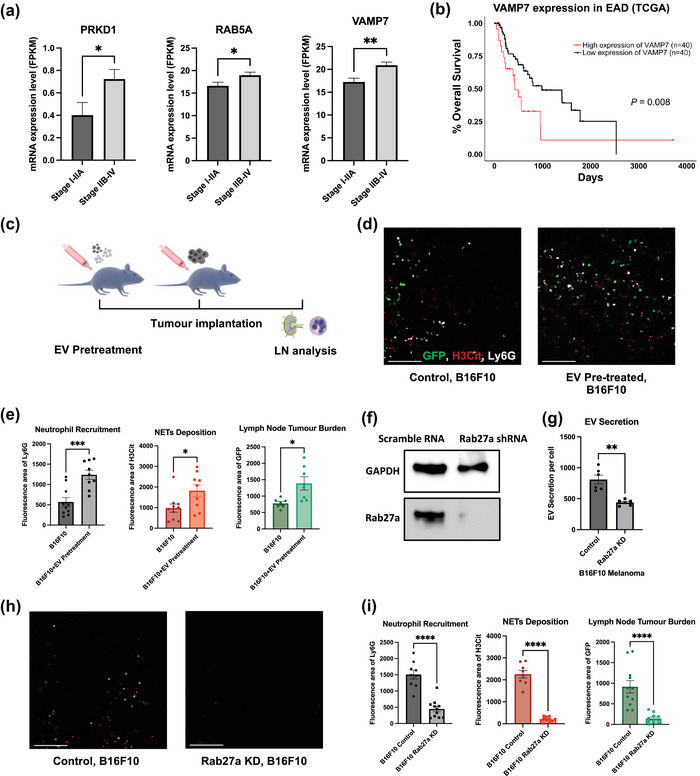
EVs are essential for LNs NETs formation and metastasis. (a) Statistical analysis of TCGA mRNA data of EV synthesis related genes in primary esophageal adenocarcinoma (EAD) tumour tissue. n = 15 (Stage I‐IIA), n = 65 (Stage IIb‐IV). (b) Kaplan‐Meier survival curves comparing the survival of EAD patients with low versus high levels of VAMP7 mRNA in tumour. (c) Schematic illustration of EV pre‐treatment in animal models. (d) Representative images of tumour draining LNs on day 14 post tumour inoculation for B16F10, with or without pre‐treatment of EVs Scale bars represent 100μm. (e) Quantification of the area of neutrophils (Ly6G), NETs (H3Cit) and tumour (GFP) in day 14 LN for B16F10, with or without EV pre‐treatment. (f) Representative Western Blot images indicating the knockdown of Rab27a expression in B16F10 cells. (g) Nanoparticle Tracking Analysis (NTA) indicates Rab27a knockdown in B16F10 cells leads to decreased EV secretion. (h) Representative images of tumour draining LNs on day 14 post tumour inoculation for B16F10, comparing mice injected with B16F10 infected with lentivirus containing scramble RNA or Rab27a shRNA. Scale bars represent 100μm. (i) Quantification of the area of neutrophils (Ly6G), NETs (H3Cit) and metastasis (GFP) in day 14 LNs for B16F10, comparing mice injected with B16F10 infected with lentivirus contain scramble RNA or Rab27a shRNA. Each data point is an image analysed, mouse n =10. Data shown as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.005; **** P < 0.001 by Mann–Whitney t test.
2.4. Human peripheral blood neutrophil isolation
Human blood was collected into heparin tubes and was diluted in PBS and layered over Lymphocyte Separation Media. After centrifugation at 800 × g for 30 min at RT, the pellet containing neutrophils and red blood cells (RBCs) was collected and resuspended in 3% Dextran and left at RT to sediment RBCs. After 30 min, the supernatant is collected and centrifuged at 450 × g at 4°C for 5 min. Remaining RBCs in the neutrophil rich pellet are then lysed using BD Pharm Lyse Lysing buffer. The obtained pellet is then washed and resuspended in cold RPMI media (Wisent Bioproducts). Purity and viability of the obtained neutrophils were verified through Methylene blue and Trypan blue staining respectively.
2.5. In vitro EV uptake and Boyden chamber neutrophil migration assay
Lymphatic endothelial cells (LECs) were purchased from PromoCell (C‐12216) and were culture in MV2 media (PromoCell, C‐22022). The LECs were treated with 10 μg/mL A549/BEAS‐2B EV, 5 μg/mL LPS, 100 nm Liposome control of same number of particles (determined by NTA) or PBS of the same volume in MV2 media for 24 h. For the in vitro EV uptake experiments, the EVs were pre‐labelled with CFSE and the LECs were fixed with 2% paraformaldehyde before proceeding with immunofluorescence staining and imaging. For the Boyden Chamber assays, fresh MV2 media were added to the LEC cultures. 72 h later, the conditioned media (CM) was collected, centrifuged at 450 × g at 4°C for 5 min to pellet down cells. The supernatant was then transfer to the bottom chamber of the transwell. One million neutrophil was layered in the top chamber and left for migration. Twenty‐four hours later, migrated neutrophil in the bottom chamber was resuspended and count by Methylene blue stain. This assay was repeated three times with triplicates.
2.6. Multiplex ELISA of CXCL8
The A549/BEAS‐2B cell lines were incubated in corresponding media with 0.5% FCS for 72 h to collect CM. The LEC CM were collect in the same way as the Boyden chamber experiments. CXCL8 amount in the CM were incubated with fluorescence multiplex ELISA kit from RayBioTech and quantified using Q‐analyser from the same manufacturer.
2.7. LEC CM induced NETs formation
LEC CM was collected in the same way as in the Boyden chamber experiments and transferred to Nunc Lab‐Tek II Chamber Slide (Thermo Scientific), 200k neutrophils were added to the CM and NETs formation was performed at 37°C. After 24 h, the supernatant was discarded, and the slides were gentle washed. The slides were fixed, immunofluorescence stained and imaged. Percentage of NETosing neutrophils (H3Cit positive neutrophils) were quantified.
2.8. EV induced NETs formation in health control and GEA patients
Health control or GEA patient neutrophils were isolated as mentioned before. Every 200k neutrophil were treated with 10 μg/mL A549 EV and incubated at 37°C for 4 h. After 4 h, SYTOX Green was added to a final dilution of 1:1000 and fluorescence intensity were acquired Tecan Infinity F200. Proneness to NETose was quantified as the relative fluorescence intensity of SYTOX Green of EV treated wells to untreated wells. Representative images were acquired using EVOS cell imaging systems (Thermo Scientific).
3. RESULTS
3.1. Lymphatic NETs are associated with reduced survival in gastroesophageal cancer patients
Our group has previously demonstrated that neutrophil accumulation and NETs deposition promotes the development of metastasis at distant organ sites (Cools‐Lartigue et al., 2013; Najmeh et al., 2017; Rayes et al., 2019). Here, we sought to determine if this process was conserved in LN metastasis, an earlier yet treatable stage during cancer progression. To characterize the pattern of lymphatic neutrophil recruitment and NETs deposition, we constructed tissue microarrays (TMAs) from 175 GEA surgical samples (Figure 1a, Table S1). The TMAs contained both patients with LN metastasis (N+) and without (N0). Within an N+ patient, not all nodes harbour metastatic cancer. Those that do are designated N+met (71 samples), while those that do not are designated N+neg (91 samples). Conversely, within an N0 patient, all regional nodes are free of cancer (13 samples).
FIGURE 1.
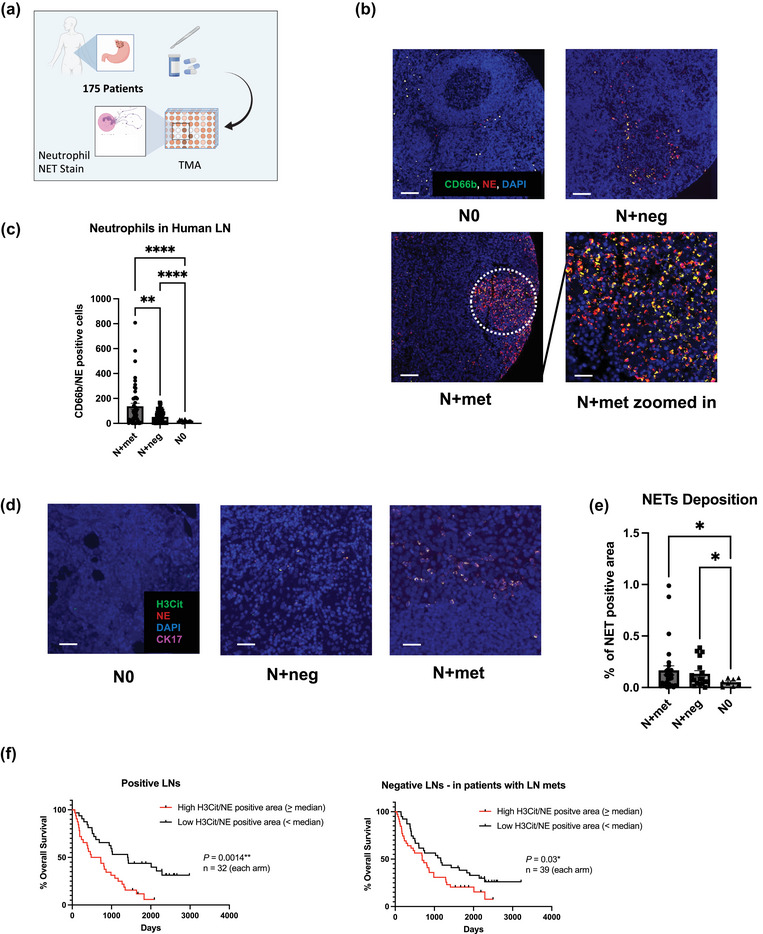
Neutrophils and NETs in regional lymph nodes (LNs) of gastroesophageal adenocarcinoma (GEA) patients. (a) Schematic illustration of the TMA constructions. Sample size: LNs from node negative patients (N0) = 13, tumour negative LNs from node positive patients (N+neg) = 91 and tumour positive LNs (N+met) = 71. Each patient sample corresponds to one tissue core on TMA slides. (b) Representative images of neutrophils in different patient LNs. Scale bars represent 100 μm. White circle indicates the zoomed area shown in bottom right. Scale bars represent 50μm. (c) Quantification of LN neutrophils (CD66b/NE double positive cells) per TMA core for N+met, N+neg and N0 LNs. (d) Representative images of NETs deposition pattern in N+met, N+neg and N0 LNs. Scale bars represent 50μm. (e) Quantification of %NETs positive area per core in N+met, N+neg and N0 LNs. Data shown as mean ± SEM. *, P < 0.05; **, P < 0.01; **** P < 0.001 by Brown‐Forsythe ANOVA test. (f) Kaplan‐Meier survival curves comparing survival of nodal positive GEA patients with low versus high levels of median lymphatic NETs positive area, in both tumour positive and negative LNs. P value by Log‐rank (Mantel‐Cox) test.
The TMAs were stained with neutrophil markers CD66b and neutrophil elastase (NE), NETs marker citrullinated histone H3 (H3Cit) and GEA epithelial marker cytokeratin 7 (CK7). We found that neutrophils (CD66b/NE double positive cells) were present and progressively recruited to regional LNs in N+ patients (Figure 1b). By quantification, we demonstrated that cancer positive nodes (N+met) have significantly higher neutrophil counts compared to cancer negative nodes (N+met vs. N+neg, 137.0 vs. 51.77, p = 0.0033) (N+met vs. N0, 137.0 vs. 21.23, p < 0.0001) (Figure 1c). Notably, the neutrophil count in N+neg is significantly higher than within N0 (51.77 vs. 21.23, p < 0.0001). This suggests the possibility that LN neutrophil recruitment may occur prior to lymphatic neoplastic invasion, which we sought to investigate using an animal model.
In addition, we assessed the relationship between the peripheral blood neutrophil‐lymphocyte ratio (NLR), a standardized method of neutrophil quantification (Lawati et al., 2021, 2020), and LN NLR (Figure S1c). We found that patients with a blood NLR > 4, which has previously been defined as elevated, also have a significantly higher LN NLR (0.02430 vs. 0.01374, p = 0.0451). This result suggests the dynamic correlation between lymphatic neutrophil accumulation and systemic inflammation.
Having shown that neutrophil accumulation within the regional LNs can occur alongside tumour LN infiltration, we therefore sought to determine if NETs deposition also takes place within the LN. Mirroring the same trend shown in nodal neutrophil infiltration, both N+met and N+neg nodes have higher levels of % NETs deposition area per core compared to N0 nodes (Figure 1d, e). (0.05134 vs. 0.1663, p = 0.0372) (0.05134 vs. 0.1330, p = 0.0401). However, LN NETs level is not associated with neoadjuvant therapies or systemic metastasis (Figure S1a, b). Moreover, we found that increased LN NETs quantity was significantly associated with reduced overall survival, both in N+met (hazard ratio [HR], 2.633, 604 vs. 1415.5 days, p = 0.0014) and N+neg (hazard ratio [HR], 1.680, 688 vs. 1161 days, p = 0.03) LNs (Figure 1f).
Collectively, these results suggest a correlation between lymphatic neutrophil accumulation, NETs deposition, and ultimately survival in patients with GEA, which points to a pro‐tumorigenic role of neutrophils and NETs in the progression of regional lymphatic disease. In addition, our data suggests that changes favourable for lymphatic metastasis may occur prior to nodal neoplastic ingress.
3.2. Neutrophil accumulation and NETs deposition precede overt lymphatic metastasis in vivo
The findings from GEA patients’ samples suggests that the changes favourable to lymphatic metastasis occur prior to nodal neoplastic ingress. Therefore, we developed an animal model in order to investigate the kinetics of LN NETs deposition. Briefly, two cancer cell lines, H59 and B16F10 were injected into the flanks of C57bl/6 mice. We specifically used two different tumour cell lines to study this phenomenon under different tumour environments and avoid cell specific phenomenology. In addition, these two cell lines are ideal models as they generate stable LN metastasis. Mice were sacrificed at day 7/10 and day 14 in order to reflect both a pre‐metastatic and post‐metastatic settings. Subsequently, the tumour draining ipsilateral inguinal LNs were resected and analysed (Figure 2a).
FIGURE 2.
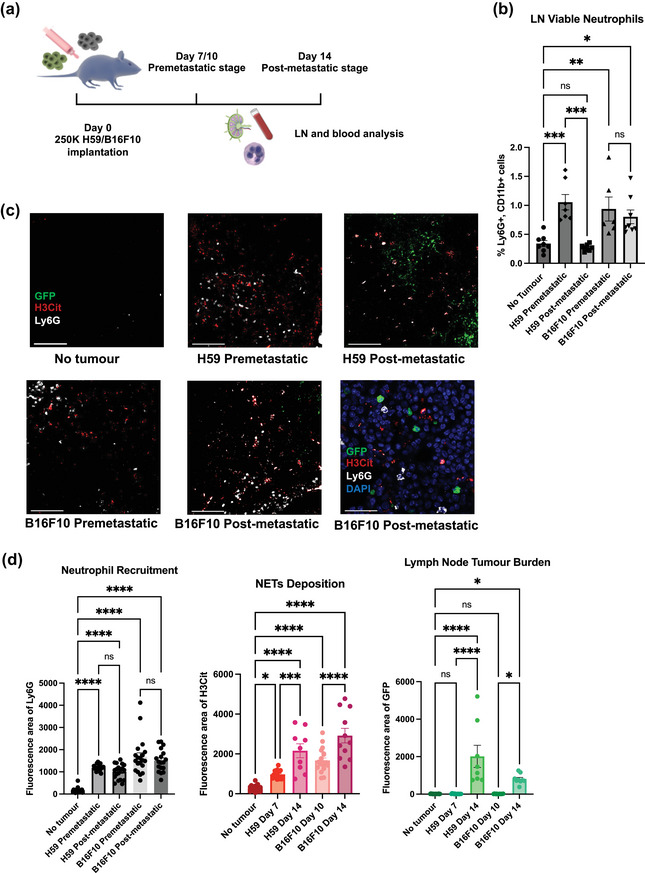
The dynamic of LN neutrophil recruitment and NET deposition in mouse model. (a) Schematic illustration of the animal model construction. 250k H59 or B16F10 cells were injected into the flank of C57bl/6 mice (b) Percentage of LN viable neutrophils within all viable LN cells on a time course by flow cytometry. (c) Representative images of LNs on a time course for both H59 lung cancer and B16F10 melanoma. Scale bars represent 100μm. Zoomed NETs details are shown in bottom right. Scale bars represent 20 μm. (d) Quantification of the area of LN neutrophils (Ly6G), NETs (H3Cit) and tumour (GFP). Each data point is an image analysed, mouse n =10. Data shown as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.005; **** P < 0.001 by one‐way ANOVA.
Using flow cytometry, we analysed LN immune cells on a time course (Figure 2b, S2a, b) and we observed a significant increase in the percentage of viable neutrophils (Viability Dye eFlour780−CD11b+Ly6G+) within all LN cells at premetastatic stage. At this stage, all LNs were devoid of neoplastic cells (Figure 2d, quantified by GFP area). At the post‐metastatic stage, we observed a relative drop or stability of viable neutrophil numbers, for H59 and B16F10 cells, respectively.
The drop or stability of viable neutrophil numbers in post‐metastatic LNs could possibly indicate NETosis, the process of NETs formation, occurring during the post metastatic stage. In fact, this was confirmed by immunofluorescence microscopy (Figure 2c). Compared to non‐tumour bearing LNs, we found significantly higher neutrophil infiltration (Ly6G area) and NETs deposition (H3Cit or neutrophil elastase/NE area) in both pre‐metastatic and post‐metastatic LNs (Figure 2d, S3d, e). The discordance between flow cytometry quantification of viable neutrophil numbers and immunofluorescence (IF) quantification of Ly6G area in pre‐ and post‐ metastatic LNs (Figure 2b, d) is because IF quantification of Ly6g signals includes both viable and NETosing neutrophils.
To further confirm the occurrence of NETosis, not neutrophil apoptosis, we performed IF staining of cleaved caspase‐3 and annexin V assay by flow cytometry. We did not observe apoptotic neutrophils in LNs or any difference among all stages (Figure S2c, d).
Moreover, we also visualised CD169+ macrophages (Figure S2e) and did not observe their colocalization with GFP signals, indicating that the GFP signals are not macrophage‐uptaken debris. To remove immunogenic effects of GFP, we also repeated the experiments with non‐GFP tagged cell lines (S2f).
In addition, we observed that increase of neutrophil percentage in blood (general neutrophilia) only occurred post‐LN‐metastasis (Figure S2g), suggesting that local LN inflammation precedes systemic inflammation. Most importantly, the observation that neutrophil accumulation and NETs deposition precede overt lymphatic metastasis suggests that cues from the primary tumour drive the formation of a favourable neutrophil/NET rich environment, which might be required for tumour cells to metastasize. Correspondingly, we did not observe an increase in LN neutrophil infiltration or NETs deposition using a low‐grade cell line, B16F1, that does not induce LN metastasis in our animal model system (Figure S3a–c), suggesting this LN NET formation is also associated with the malignancy of tumour cells.
3.3. Neutrophil depletion and NETs inhibition abolish LN metastasis
We hypothesized that NETs play a central role in facilitating LN metastasis. Therefore, we sought to demonstrate this through a number of experiments demonstrating reduced LN metastasis in the absence of NETs (Figure 3a).
FIGURE 3.
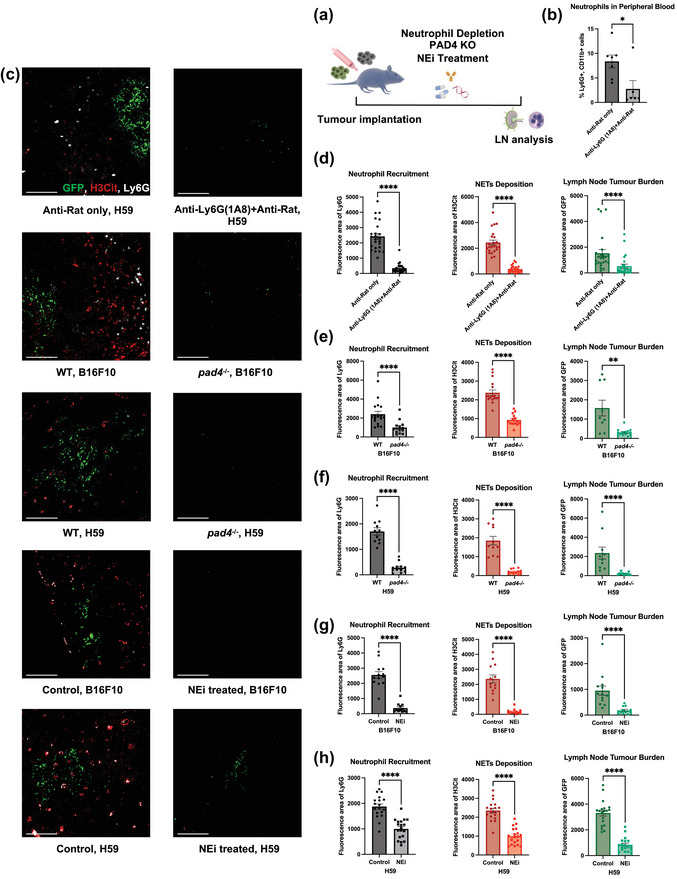
Neutrophil depletion and NETs inhibition abolishes LN metastasis. (a) Schematic illustration of the treatments in animal models. (b) Flow cytometry on blood leukocyte indicates sufficient depletion of neutrophils. (c) Representative images of tumour draining LNs on day 14 post tumour inoculation for both H59 and B16F10, comparing wildtype/no treatment mice to neutrophil‐depleted, pad4 knockout or NEi treated mice. Scale bars represent 100 μm. (d)–(h) Quantification of the area of neutrophils (Ly6G), NETs (H3Cit) and metastasis (GFP) in fay 14 LNs for both H59 and B16F10, comparing wildtype/no treatment mice to neutrophil‐depleted, pad4 knockout or NEi treated mice. Each data point is an image analysed, mouse n =10. Data shown as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.005; **** P < 0.001 by Mann‐Whitney t test.
To determine the role of neutrophils in LN metastasis, first, we depleted circulating neutrophils using anti‐Ly6G antibody as previously described (Faget et al., 2018). We observed a significant reduction in circulating neutrophils on day 14 (Figure 3b) (8.359% vs. 2.755, p = 0.023), suggesting significant depletion. When we assessed the LNs, a significant decrease in neutrophil infiltration, NETs deposition, and metastatic burden was noted (Figure 3c, d) (neutrophil area 2276 vs. 236, NETs area 2109 vs. 294, tumour area 1155 vs. 229.3, all p < 0.0001). The observation that systemic neutrophil depletion could abolish LN neutrophil recruitment corroborates the association between elevated circulating and lymphatic neutrophils observed earlier in TMAs (Figure S1c).
Next, to demonstrate that NET deposition is required for the effective establishment of LN metastases, we used Peptidyl arginine deiminase 4 (PAD4) knockout mice (Pad4 −/−), which are unable to form NETs but have normal circulating neutrophil level (Figure S4a). PAD4 catalyses histone hypercitrullination during NET formation, which is required for histone decondensation (Claushuis et al., 2018). As expected, we observed diminished neutrophil recruitment, NETs formation and metastatic burden in the tumour draining LNs of Pad4−/− compared to wild‐type (WT) mice (Figure 3c, e and f; S3d, e) (B16F10, neutrophil area 2123 vs. 854.6 p < 0.0001, NETs area 2195 vs. 831.6 p < 0.0001, tumour area 1061 vs. 302.8 p = 0.0026) (H59, neutrophil area1701 vs. 278.1, NETs area 1849 vs. 208.9, tumour area 1837 vs. 174.7, all p < 0.0001). These findings support the essential roles of neutrophils and NETs in the establishment of LN metastases.
The requirement of NETs for the development of LN metastasis highlights a potential therapeutic vulnerability. NETs can be pharmacologically targeted with the neutrophil elastase inhibitor Sivelestat (NEi), which reduced NETs formation. Sivelestat and other NEis are orally bio‐available compounds currently under several clinical trials for various autoimmune and respiratory diseases (Kawasaki & Aikawa, 2014; Sahebnasagh et al., 2020). NEi can inhibits the function of neutrophil elastase, a key regulator and component of NETs (Huang et al., 2020). We treated mice daily with NEi and observed a significant decrease in LN NETs deposition and metastatic disease burden (Figures 3c,g,h and S3d, e) (B16F10, neutrophil area 2444 vs. 243.6, NETs area 2014 vs. 130.1, tumour area 812.9 vs. 130.2, all p <0.0001) (H59, neutrophil area 1701 vs. 278.1, NETs area 1849 vs. 208.9, tumour area 3305 vs. 841.7, all p <0.0001), providing further support for the role of NETs in establishing LN metastasis.
Surprisingly, LN neutrophil recruitment decreased both in pad4−/− and NEi treated mice. We hypothesized one possible explanation is that a positive feedback loop between neutrophil recruitment/NETs deposition and LN tumour growth exists, as tumour cells can directly induce NETosis (Cools‐Lartigue et al., 2013). Pad4−/− and NEi treated mice had much lower LN tumour burden, which could result in decreased neutrophil recruitment. To address this, we performed neutrophil migration assays towards tumour cell conditioned media, and we found the number of migrated neutrophils is dose‐dependent to the number of tumour cells (Figure S4e).
Collectively, these results suggest that the recruitment of neutrophils and the deposition of NETs within LNs are necessary for the establishment of nodal metastasis. Disruption of NETs deposition alone through neutrophil depletion or pharmacologic blockade was sufficient to reduce the formation of detectable LN disease.
3.4. EVs are essential for LN NETs formation and metastasis
In the previous experiment, we demonstrated that lymphatic NET deposition occurs prior to, and was required for the ingress of neoplastic cells within LNs, it is possible that signalling pathways are originating from the primary tumour and conducted by tumour derived factors are needed for LN NET deposition. EVs are actively secreted by nearly all eukaryotic cells and are key players in intercellular communication (Becker et al., 2016; Raposo & Stoorvogel, 2013). Studies have shown that EVs are involved in the formation of a pre‐metastatic niche, a fertile microenvironment within target organs favouring neoplastic implantation (Costa‐Silva et al., 2015; Peinado et al., 2017; Urabe et al., 2021). In addition, EVs preferentially accumulate within lymphatic endothelium (Broggi et al., 2019; Srinivasan et al., 2016). Therefore, EVs could provide a link between the primary tumour and the lymphatic inflammatory microenvironment. Interestingly, the expression of genes involved in EV synthesis and secretion are significantly upregulated in oesophageal adenocarcinoma (EAD) patients from The Cancer Genome Atlas Program (TCGA) (Table S2) (Ding et al., 2021; Fader et al., 2009; Hurwitz et al., 2016) with nodal diseases (Figure 4a) (Rab5a 16.61 vs. 18.96 p = 0.0361, PRKD1 0.2598 vs. 0.4705 p = 0.0240, VAMP7 17.23 vs. 20.91 p = 0.0019). Moreover, high expression of VAMP7 is correlated with poor overall survival in EAD patients (hazard ratio [HR] 2.31, 495 vs. 1599 days, p = 0.008), but not PRKD1 or Rab5a (Figure 4b, S6a).
To validate that EVs from the primary tumour are necessary for lymphatic neutrophil recruitment and NETs deposition, we pre‐treated mice with B16F10‐derived EVs before B16F10 tumour inoculation. We validated our EV isolation by nanoparticle tracking assay (NTA), transmission electron microscopy (TEM) and western blot of EV markers (Figure S5a–c). Notably, we used the B16F10 model since B16F10 cells secrete more EVs and are much more susceptible to lentivirus. We found that preexposure to cancer EVs significantly increase nodal neutrophil recruitment and NET deposition as well as LN metastasis burden (Figure 4d and e). (Neutrophil area 568.3 vs. 1238 p = 0.0006, NETs area 974.1 vs. 1821 p = 0.0261, tumour area 779.0 vs. 1387 p = 0.0132).
To further confirm the role of EVs in LN metastasis, we knocked down (KD) the expression of Rab27a in B16F10 cells. Rab27a is a key gene involved in EV secretion (Ostrowski et al., 2009) (Figure 6f). The knockdown approximately halved EV secretion levels (Figure 4g) (787.0 vs. 450.0 p = 0.0022) and resulted in significantly decreased LN neutrophil recruitment, NETs deposition and LN metastasis (Figure 4h and i) (neutrophil area 1508 vs. 447.1, NETs area 2259 vs. 199.8, tumour area 914.4 vs.144.0, all p < 0.0001). Taken together, these data suggest that EVs are essential for LN NETs formation and metastasis.
FIGURE 6.
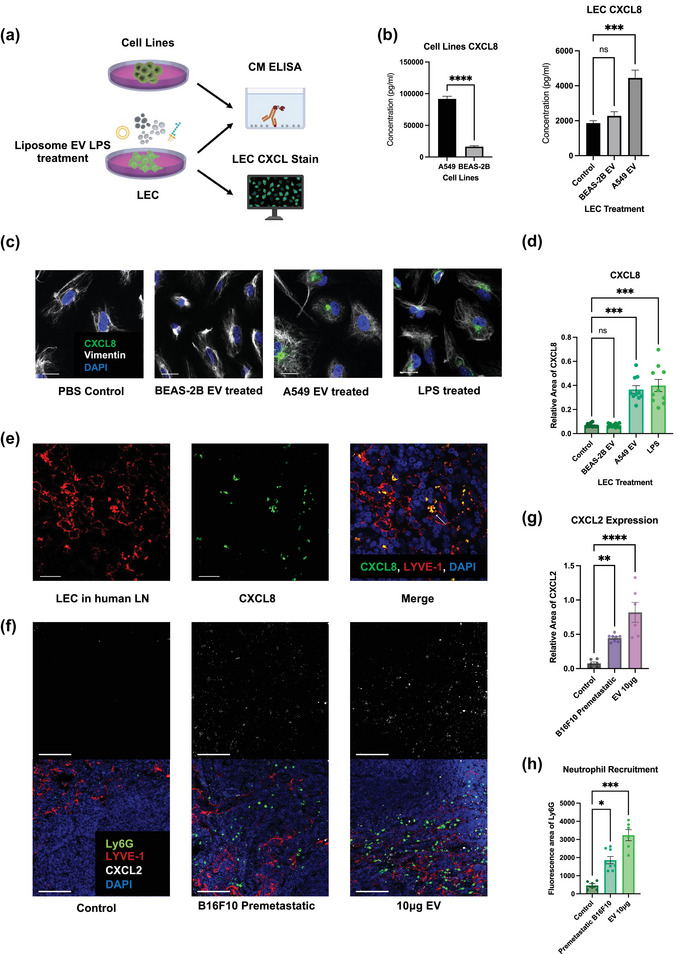
LECs secrete neutrophil chemoattractants and NETs inducers upon EV uptake. (a) Schematic illustration of the ELISA and LEC CXCL8 stain. (b) ELISA of CXCL8 level in conditioned media (CM) of A549, BEAS‐2B and EV‐treated LECs. (c) Representative images of LECs expressing CXCL8 after different kinds of treatments. Scale bars represent 20μm. (d) Quantification of CXCL8 expression in LECs (relative fluorescence area of CXCL8 to DAPI). (e) Representative images of LECs expressing CXCL8 in tumour positive nodes of lung cancer patients. Scale bars represent 20μm. (f) Representative images of LECs expressing CXCL2 and recruiting neutrophils mouse LNs. Scale bars represent 100μm. (g) and (h) Quantification of CXCL2 expression (relative fluorescence area of CXCL2 to LYVE‐1) in LECs and neutrophil recruitment (Ly6G fluorescence area) in mouse LNs. Each data point is an image analysed, mouse n =10. Data shown as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.005; **** P < 0.001 by Mann–Whitney t test, One‐Way ANOVA or Kruskal–Wallis test.
3.5. EV‐Lymphatic endothelium interaction results in LN NETs deposition
Next, we sought to confirm that EVs can be uptaken by the lymphatic system and investigate the downstream mechanism. Similar to the work of Broggi et al (Giese et al., 2019), we found that lymphatic endothelial cells (LECs) exhibit active uptake of EVs both in vivo and in vitro (Figure 5a, b). Surprisingly, we found LECs dramatically enriched for EVs derived from the human lung cancer cell lines A549 compared to EVs from the benign bronchial epithelial cells BEAS‐2B, as quantified by the relative area of CFSE‐EV to DAPI (Figure 5b, c) (0.7436 vs. 0.02310, p < 0.0001). Both A549 and BEAS‐2B cells were used in order to demonstrate that this is specifically a cancer‐related behaviour. Thus, malignant EVs appear to specifically accumulate within lymphatic endothelium.
FIGURE 5.
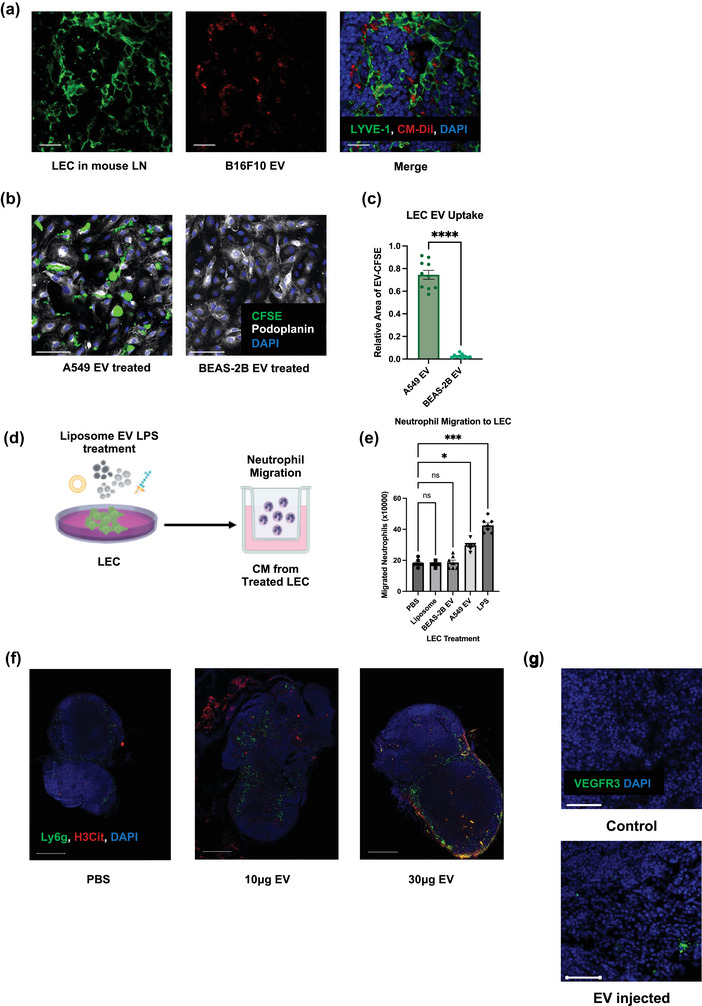
Lymphatic endothelial cells (LECs) are the LN recipient cells of EVs LECs regulate neutrophil recruitment and NETs formation. (a) Representative images of draining LNs after footpad B16F10 EV injection, showing LECs uptaking EVs Scale bars represent 20μm. (b) Representative images of LECs after A549 and BEAS‐2B EV treatment. Scale bars represent 100μm. (c) Quantification of the EV uptake (relative fluorescence area of CFSE to DAPI) in LECs. (d) Schematic illustration of the Boyden chamber transwell assay. (e) Quantification of Boyden chamber transwell assay, showing neutrophil migration towards CM from treated LECs. Data shown as mean ± SEM. *, P < 0.05; ***, P < 0.005; **** P < 0.001 by Mann–Whitney t test or One‐Way ANOVA. (f) Representative images of draining LNs after footpad PBS or different doses of B16F10 EV injection, indicating the subsequent neutrophil recruitment and NETs deposition. Scale bars represent 500 μm. (g) Representative images of draining LNs after footpad PBS or 10 μg B16F10 EV injection, indicating the increased expression of premetastatic marker VEGFR3. Scale bars represent 50 μm.
The consequences of LEC EV uptake were subsequently assessed. We performed Boyden chamber experiments to assess the migration of neutrophils towards A549 EV only or the conditioned media (CM) from LEC pre‐exposed to PBS (vehicle control), liposome (particle control), BEAS‐2B EVs (benign EV control), A549 EVs and LPS (positive control) (Figure 5d). We found that LECs treated with cancer derived EVs could significantly induce neutrophil transwell migration, while EV only or CM from LECs treated with benign cells derived EVs from or liposomes could not (Figure 5e, S4e) (24.93 vs. 18.64/17.5 × 106 neutrophils, p = 0.0489). To further this migration was caused by EV induced LEC secretomes and not EVs themselves, we performed another EV western blot and demonstrated that the purity of our EV isolation; no albumin or cytokine contamination was present (Figure S6b).
Cancer EV‐mediated neutrophil accumulation and NET deposition was subsequently confirmed in vivo. B16F10‐derived EVs were injected into the footpads of non‐tumour bearing mice. Interestingly, cancer EV administration alone was sufficient to drastically increase LN neutrophil infiltration as well as the deposition of NETs in a dose‐dependently manner (Figure 5f and Figure S4b) (PBS vs. 10 μg EV: neutrophil area 12.95 vs. 28.14 P = 0.0026, NETs area 15.57 vs. 18.00 not significant; PBS vs. 30 μg EV: neutrophil area 12.95 vs. 26.00 p = 0.0050, NETs area 15.57 vs. 27.78 p = 0.0093). EV administration also significantly increased LN expression of premetastatic marker VEGFR3 (Figure 5g and S4c) (PBS vs. 10 μg EV: VEGFR area 57.91 vs. 240.9 p = 0.0006).
3.6. Lymphatic secretion of CXCL8/2 induces LN Neutrophil infiltration and NETs formation
Having demonstrated that tumour EVs are sufficient to induce lymphatic neutrophil migration and NETs deposition, we next sought to elucidate the mechanism by which this occurs. First, we collected conditioned media (CM) from LECs that were co‐cultured with EVs from A549 lung cancer cells or BEAS‐2B bronchial epithelial cells (Figure 6a). We then performed a multiplex ELISA targeting common neutrophil chemoattractants (Figures 6b and S4d). Interestingly, LEC production of CXCL8 was radically increased after the treatment of A549 EVs (1865 vs. 4447 pg, p = 0.0004) but of BEAS‐2B EVs Moreover, A549 cells themselves also secreted more CXCL8 compared to BEAS‐2B cells (91652 vs. 16316 pg, p < 0.0001). We also performed immunofluorescence to detect LEC derived CXCL8 and observed a significant increase in CXCL8 synthesis in LECs following A549‐derived EV treatment (Figure 6c, d), quantified by relative area of CXCL8 to DAPI (5.500 vs. 20.40 p = 0.0003). In addition, we also demonstrated the expression of CXCL8 in LECs from surgical LN samples of lung adenocarcinoma patients (Figure 6e).
Mice do not express CXCL8 but do express its analogue CXCL2 (Hol et al., 2009). We found CXCL2 expression colocalised well with LECs and was in proximity to LN neutrophils (Figure 6f). The level of CXCL2 was also extensively increased in the pre‐metastatic condition or following EV injection (Figure 6g), quantified by relative area of CXCL2 to LYVE‐1 (control vs. B16F10 premetastatic 0.07592 vs. 0.4462, p = 0.0058; control vs. EV 10 μg 0.07592 vs. 0.8199, p < 0.0001). This was accompanied by an increase in the number of neutrophils infiltrated into the LNs, quantified by area of Ly6G (Figure. 6h) (control vs. premetastatic B16F10 3.500 vs. 10.88, p = 0.0420; control vs. EV 10 μg 3.500 vs. 17.00, p = 0.0002). Moreover, we found that CXCL2 expression was decreased in post‐metastatic LNs of pad4 −/− and NEi treated mice, which could also contribute the decreased neutrophil recruitment in these conditions (Figure S6c, d).
In addition to being a common chemoattractant of neutrophils, CXCL8 is also a potent NETs inducer (An et al., 2019). To consolidate the conclusion that cancer derived EVs can induce LECs to secrete CXCL8, thus resulting in NETs formation, we exposed healthy control‐derived neutrophils to CM from LECs that were treated with A549‐derived EVs with or without a CXCL8 blocking antibody (Figure 7a). We found that CM from A549 EV treated LECs significantly increased the percentage of neutrophils that were undergoing NETs formation and that CXCL8 blockade significantly attenuated this phenotype (Figure 7b and c) (control vs. A549 EV 7.415% vs. 79.63%, p < 0.0001; control vs. A549 EV+CXCL8 Ab 7.415% vs. 24.64% ns; A549 EV vs. A549 EV+CXCL8 Ab 79.63% vs. 24.64%, p < 0.0001). Moreover, we repeated this assay with ultracentrifuged A549 EV treated LEC CM and did not observed change regarding NETosis. This confirmed that it is LEC derived CXCL8, not remaining EVs, that led to NETosis (Figure S6e, f). Furthermore, since EVs can directly lead to the release of NETs (Leal et al., 2017), we simultaneously exposed GEA patient or healthy control derived neutrophils to A549‐derived EVs and we found that GEA patient derived neutrophils are more likely to form NETs (Figure 7d and e) (39.71 vs. 23.36 p = 0.0040). The increased propensity of NETosis in response to EVs in cancer patients indicates the profundity of neutrophil preexposure to other tumour derived factors in cancer patients and the potential synergy between EVs and cytokines (Demers et al., 2012). Moreover, we performed mass spectrometry on the plasma EV proteins of a small number of GEA patients, with or without nodal metastasis (n = 4 for N0 patients and n = 6 for N+ patients; Table S3). We found that total protein spectra count of HSPD1 and HSP90AB1 are increased in EVs from patient with LN metastasis (Figure S5d).
FIGURE 7.
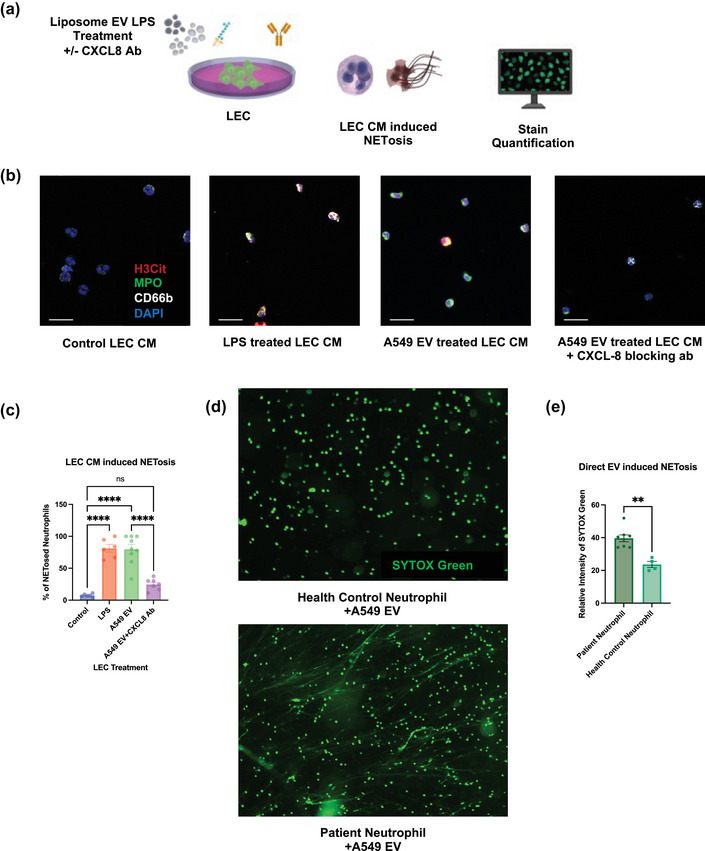
Lymphatic CXCL8 secretion upon EV uptake leads to NETs formation. (a)Schematic illustration of the in vitro NETosis assay induced by CM of treated LECs. (b) Representative images of neutrophils treated with CM from treated LECs. Scale bars represent 20 μm. (c) Quantification of LEC CM induced NETosis (% of H3Cit positive neutrophils). (d) Representative images of indicating the different proneness to form NETs for neutrophils from health control and GEA patients after A549 EV treatment. Scale bars represent 100μm. (d) Quantification of propensity of neutrophil to form NETs induced by EVs (Relative fluorescence intensity of SYTOX Green treated to untreated neutrophil) from health control and GEA patients). Data shown as mean ± SEM.**, P < 0.01; ****, P < 0.001 by Kruskal–Wallis test or Mann–Whitney t test.
4. DISCUSSION
LN metastasis is nearly ubiquitous among solid tumours and portend poor survival (Steeg, 2016). Treating regional LNs via resection forms the basis for modern surgical oncologic treatment approaches. Regional LNs are removed and often trigger the implementation of systemic therapies when they harbour cancer (Hagens et al., 2019). The commonality of LN metastasis among cancers presents a broadly applicable opportunity for treatment. Despite this, little is understood about how LN metastasis actually forms. In particular, the link between the primary tumour site and regional LNs remains poorly described.
In the current study, we set out to demonstrate that similar to conditions of systemic inflammation, a regional inflammatory state can promote the development of LN metastasis. We demonstrated that lymphatic neutrophil accumulation and NET deposition occurs within the tumour LNs of GEA patients. Furthermore, we revealed that the level of lymphatic NETs is an indicator of poor prognosis among these patients. Interestingly, while NETs levels were highest in LNs that had been infiltrated with cancer (N+met), even negative regional nodes (N+neg) demonstrated elevated NETs compared to LNs harvested from patients without nodal metastasis (N0). This finding suggests that regional lymphatic neutrophil accumulation and NETs deposition can occur without the presence of neoplastic cells. Since NET levels were highest in N+met, we surmised that lymphatic neutrophils and NETs present a favourable environment for tumour implantation. As such, perhaps they should represent a treatment target in and of themselves. However, our study has aroused several new research questions, such as the discordance between human and mouse LN neutrophil recruitment patterns, and the mechanism ofdecreased LN neutrophil recruitment in pad4 −/− and NEi treated conditions. As the interaction between neutrophil recruitment, simultaneous NETs deposition, and subsequent LN tumour growth is complicated, an interesting future direction could be long term intravital imaging to capture the entire process of LN premetastatic niche formation or spatial sequencing of LNs.
We show that regional lymphatic inflammation caused by neutrophils results from inflammatory mediators, in the form of EVs, originating from within the primary tumour. This distinct premetastatic phase, within LNs, is characterized by lymphatic neutrophil infiltration and deposition of NETs. Our data confirms that LN metastasis together with the regional LN inflammation is a common behaviour of multiple cancer models, and it is induced by environmental cues from the primary tumour. Correspondingly, in the study by Wang et al, the authors highlight tumour associated neutrophils (TANs) within early gastric cancer resection specimens as independent risk factors for LN metastasis (Wang et al., 2020). Similarly, Hiramatsu et al demonstrate a similar association between the number of neutrophil number in the tumour draining LNs and patient outcome (Tokumoto et al., 2014). In the data presented here, regional lymphatic inflammation by neutrophils appears to have increased as a result of inflammatory mediators in the form of EVs possibly originating from within the primary tumour. The observation of a distinct pre‐metastatic phase has important implications when considering the pathologic staging of neoplasia. LN staging in contemporary clinical practice is binary; nodes are either infiltrated with cancer or not. This study, along with previous work on the prognostic value of looking at immune compartments in pathological samples, suggests that LN infiltration represents a spectrum of disease (Boquet et al., 2022; Galon et al., 2012; Pagès et al., 2018).
EVs derived from the primary tumour play a critical role in establishing a pre‐metastatic niche within LNs. Tumour derived EVs have been shown to rapidly disseminate through the lymphatic system and into regional LNs, particularly during infection (Srinivasan et al., 2016). The observation that EVs can be rapidly taken up by LECs, transported to the lumen of the vessel, and concentrated rapidly within LNs has led to the suggestion that they represent a means of information exchange that can precede the arrival of migrating cells. We posit that an analogous mechanism is taking place between the primary tumour site and regional lymphatics. In support of this, lymphatic fluid from patients with cutaneous melanoma are enriched with EVs derived from their tumours (Broggi et al., 2019). Therefore, an analysis of lymphatic composition may permit the stratification of patients with early as opposed to advanced disease.
Neutrophil depletion abolished both the deposition of presumed NETs and abolished the formation of nodal metastasis all together (Cools‐Lartigue et al., 2013; Najmeh et al., 2017; Rayes et al., 2020). We and others have revealed a pivotal role for neutrophils in the development of metastasis (Shahzad et al., 2022; Spicer et al., 2012). In the current study, we observed widespread NETs in LNs, which led to the formation of an environment permissive to tumour outgrowth. Indeed, NETs have been shown to play a pro‐tumorigenic role through direct effects on tumour cell proliferation, invasion, and escape from dormancy (Albrengues et al., 2018; Najmeh et al., 2017; Park et al., 2016; Rayes et al., 2020), Furthermore, NETs themselves have been shown to induce local immune suppression through the extracellular elaboration of NET associated PD‐L1 (Kaltenmeier et al., 2021). Thus, it is feasible to surmise that NETs support tumour outgrowth through a variety of mechanisms, including but not limited to, tumour cell sequestration, enhanced adhesion, migration, proliferation, and local immune suppression. Here, we confirmed that NETs support the process of LN metastasis by observing the effects of both PAD4 inhibition and NE inhibition on tumour outgrowth.
EVs were sufficient to induce nodal neutrophil ingress in vivo and were necessary for the formation of metastasis. Similar findings have been reported for macrophage recruitment (Sun et al., 2019), lymphangiogenesis (Commerford et al., 2018), and immune suppression (García‐Silva et al., 2021) within LN microenvironment. Indeed, EVs serve as mediators of communication between primary tumour cells and LN cells, resulting in premetastatic changes that create a permissive condition for subsequent tumour cell colonisation. Our work using B16F10 model highlights the essential role of EVs in mediating this NET‐induced LN metastasis. A future direction could be validating this role of EVs in other cancer models, as we have demonstrated premetastatic LN NET deposition is preserved among multiple cancer types.
Moreover, while the process of NET formation has been reported to be regulated by both cytokines, such as CXCL8 and G‐CSF (An et al., 2019; Demers et al., 2012), in addition to EVs (Leal et al., 2017), we are the first to establish a link between the regulation of lymphatic CXCL8 accumulation by tumour derived EVs and subsequent NETs formation. EVs induced the production of CXCL8 leading to neutrophil migration and NET deposition both in vitro and in vivo. This was confirmed through CXCL8 inhibition which diminished much of this phenotype. CXCL8 is a pro‐inflammatory cytokine that predominantly activates and recruits neutrophils (Xiong et al., 2022). Earlier work has characterised the role of CXCL8 as a NET inducer and aggravator in chronic inflammatory diseases. Future work is needed to assess the synergy between tumour‐derived soluble factors and EVs and to investigate the plasticity of neutrophil function in the milieu of cancer from a cancer secretome mindset.
5. CONCLUSION
In summary, the data presented sets a scene wherein tumour derived EVs accumulate within lymphatic endothelium. This results in a local chemotactic gradient, involving CXCL8, that promotes neutrophil influx and NET deposition. Furthermore, locally infiltrated neoplastic cells may themselves attract neutrophil ingress and induce NET release by releasing more CXCL8 and EVs. The NET deposition results in a local microenvironment that is permissive and favourable to tumour outgrowth. This was the case in the murine models employed. In addition, there was a clear association between lymphatic NET deposition and reduced survival in gastroesophageal cancer patients. The finding that this may occur across malignant types may highlight NETs as potential therapeutic targets based not on histology but host inflammatory status instead. Given that no treatments to date target host inflammatory status, the results of this study are particularly germane. We highlighted that NETs represent an attractive therapeutic target as they are amenable to pharmacologic inhibition and appear to be involved throughout the metastatic cascade, as well as the therapeutic potentials of targeting EVs and CXCL8. Future works further investigating clinical efficiency of NET targeting agents in treating LN metastasis could lead to major advances in the management of cancer patients.
AUTHOR CONTRIBUTIONS
Xin Su: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; validation; visualization; writing‐original draft; writing‐review & editing. Ariane Brassard: Data curation; formal analysis; investigation; software. Alexandra Bartolomucci: Investigation; writing‐original draft; writing‐review & editing. Iqraa Dhoparee‐Doomah: Data curation; formal analysis; investigation; software. Qian Qiu: Data curation; formal analysis; software; visualization. Thupten Tsering: Investigation; methodology. Ramin Rohanizadeh: Formal analysis; investigation; software; visualization. Olivia Koufos: Data curation. Betty Giannias: Methodology. France Bourdeau: Methodology. Lixuan Feng: Investigation. Julia Messina‐Pacheco: Methodology; resources. Sabrina Leo: Investigation. Veena Sangwan: Writing‐original draft. Daniela Quail: Writing‐review and editing. James Tankel: Writing‐original draft. Jonathan Spicer: Writing‐original draft. Julia Valdemarin Burnier: Conceptualization; methodology; supervision; writing‐original draft; writing‐review and editing. Swneke Donovan Bailey: Supervision; visualization; writing‐original draft; writing‐review and editing. Lorenzo Ferri: Conceptualization; funding acquisition. Jonathan Cools‐Lartigue: Conceptualization; funding acquisition; supervision; writing‐original; writing‐review & editing.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
Supporting information
Table S1 Demographic and clinical characteristics of patients with gastroesophageal adenocarcinoma in the TMA study
Table S2 Demographic and clinical characteristics of patients with oesophageal adenocarcinoma from TCGA
Table S3 Demographic and clinical characteristics of patients with oesophageal adenocarcinoma for plasma EV proteomics study
Table S4 Resources table of antibodies, reagents, cells and viruses.
Figure S1: (a) Quantification of %NETs positive area per LN core, divided by stage and comparing patients who received neoadjuvant therapies or not.
Figure S2: (a) Flow cytometry gating strategies of mouse LN and blood neutrophils.
Figure S3: (a) Representative images of tumour draining LNs on a time course for both B16F1 and B16F10 melanoma cells.
Figure S4: (a) Baseline percentage of neutrophils within blood leukocytes for wildtype and PAD4 knockout mice.
Figure S5: (a) Nanoparticle tracking assay (NTA) showing the size distribution of EVs.
Fig. S6: (a) Kaplan‐Meier survival curves comparing the survival of EAD patients with low versus high levels of PRKD1 or Rab5a mRNA in tumour.
Supporting Information
ACKNOWLEDGEMENTS
The authors would like to acknowledge Drs. Janusz Rak, Ian Watson, John Stagg and all technique platforms at RI‐MUHC, as well as MGH foundation and the Thoracic Surgery Foundation.
Su, X. , Brassard, A. , Bartolomucci, A. , Dhoparee‐Doomah, I. , Qiu, Q. , Tsering, T. , Rohanizadeh, R. , Koufos, O. , Giannias, B. , Bourdeau, F. , Feng, L. , Messina‐Pacheco, J. , Leo, S. , Sangwan, V. , Quail, D. , Tankel, J. , Spicer, J. , Burnier, J. V. , Bailey, S. D. , … Cools‐Lartigue, J. (2023). Tumour extracellular vesicles induce neutrophil extracellular traps to promote lymph node metastasis. Journal of Extracellular Vesicles, 12, e12341. 10.1002/jev2.12341
Contributor Information
Xin Su, Email: xin.su3@mail.mcgill.ca.
Jonathan Cools‐Lartigue, Email: jonathan.cools-lartigue@mcgill.ca.
REFERENCES
- Abdel‐Latif, M. M. , Duggan, S. , Reynolds, J. V. , & Kelleher, D. (2009). Inflammation and esophageal carcinogenesis. Current Opinion in Pharmacology, 9(4), 396–404. 10.1016/j.coph.2009.06.010 [DOI] [PubMed] [Google Scholar]
- Albrengues, J. , Shields, M. A. , Ng, D. , Park, C. G. , Ambrico, A. , Poindexter, M. E. , Upadhyay, P. , Uyeminami, D. L. , Pommier, A. , Küttner, V. , Bružas, E. , Maiorino, L. , Bautista, C. , Carmona, E. M. , Gimotty, P. A. , Fearon, D. T. , Chang, K. , Lyons, S. K. , Pinkerton, K. E. , … Egeblad, M. (2018). Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science, 361(6409), eaao4227. 10.1126/science.aao4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Lawati, Y. , Alkaaki, A. , Luis Ramírez García Luna, J. , Skothos, E. , Mueller, C. , Spicer, J. , Mulder, D. , Ferri, L. , & Cools‐Lartigue, J. (2021). The predictive value of inflammatory biomarkers in esophageal anastomotic leaks. The Annals of Thoracic Surgery, 112(6), 1790–1796. 10.1016/j.athoracsur.2020.12.033 [DOI] [PubMed] [Google Scholar]
- Amulic, B. , Cazalet, C. , Hayes, G. L. , Metzler, K. D. , & Zychlinsky, A. (2012). Neutrophil function: From mechanisms to disease. Annual Review of Immunology, 30(1), 459–489. 10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- An, Z. , Li, J. , Yu, J. , Wang, X. , Gao, H. , Zhang, W. , Wei, Z. , Zhang, J. , Zhang, Y. , Zhao, J. , & Liang, X. (2019). Neutrophil extracellular traps induced by IL‐8 aggravate atherosclerosis via activation NF‐κB signaling in macrophages. Cell Cycle, 18(21), 2928–2938. 10.1080/15384101.2019.1662678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, S. , Chan, T. , Hasan, T. , & Maytin, E. (2021). Current prospects for treatment of solid tumors via photodynamic, photothermal, or ionizing radiation therapies combined with immune checkpoint inhibition (A review). Pharmaceuticals, 14(5), 447. 10.3390/ph14050447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrichiello, G. , Pirozzi, M. , Facchini, B. A. , Facchini, S. , Paragliola, F. , Nacca, V. , Nicastro, A. , Canciello, M. A. , Orlando, A. , Caterino, M. , Ciardiello, D. , Della Corte, C. M. , Fasano, M. , Napolitano, S. , Troiani, T. , Ciardiello, F. , Martini, G. , & Martinelli, E. (2022). Beyond N staging in colorectal cancer: Current approaches and future perspectives. Frontiers in Oncology, 12, 937114. 10.3389/fonc.2022.937114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, B. J. , Adrover, J. M. , Baxter‐Stoltzfus, A. , Borczuk, A. , Cools‐Lartigue, J. , Crawford, J. M. , Daßler‐Plenker, J. , Guerci, P. , Huynh, C. , Knight, J. S. , Loda, M. , Looney, M. R. , McAllister, F. , Rayes, R. , Renaud, S. , Rousseau, S. , Salvatore, S. , Schwartz, R. E. , Spicer, J. D. , … Egeblad, M. (2020). Targeting potential drivers of COVID‐19: Neutrophil extracellular traps. Journal of Experimental Medicine, 217(6), e20200652. 10.1084/jem.20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, A. , Thakur, B. K. , Weiss, J. M. , Kim, H. S. , Peinado, H. , & Lyden, D. (2016). Extracellular vesicles in cancer: Cell‐to‐cell mediators of metastasis. Cancer Cell, 30(6), 836–848. 10.1016/j.ccell.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet, I. , Kassambara, A. , Lui, A. , Tanner, A. , Latil, M. , Lovera, Y. , Arnoux, F. , Hermitte, F. , Galon, J. , & Catteau, A. (2022). Comparison of immune response assessment in colon cancer by immunoscore (Automated digital pathology) and pathologist visual scoring. Cancers, 14(5), 1170. 10.3390/cancers14051170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann, V. , Reichard, U. , Goosmann, C. , Fauler, B. , Uhlemann, Y. , Weiss, D. S. , Weinrauch, Y. , & Zychlinsky, A. (2004). Neutrophil extracellular traps kill bacteria. Science, 303(5663), 1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- Broggi, M. A. S. , Maillat, L. , Clement, C. C. , Bordry, N. , Corthésy, P. , Auger, A. , Matter, M. , Hamelin, R. , Potin, L. , Demurtas, D. , Romano, E. , Harari, A. , Speiser, D. E. , Santambrogio, L. , & Swartz, M. A. (2019). Tumor‐associated factors are enriched in lymphatic exudate compared to plasma in metastatic melanoma patients. Journal of Experimental Medicine, 216(5), 1091–1107. 10.1084/jem.20181618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , He, D. , & Cui, J. (2022). A neutrophil extracellular traps signature predicts the clinical outcomes and immunotherapy response in head and neck squamous cell carcinoma. Frontiers in Molecular Biosciences, 9, 833771. 10.3389/fmolb.2022.833771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claushuis, T. A. M. , van der Donk, L. E. H. , Luitse, A. L. , van Veen, H. A. , van der Wel, N. N. , van Vught, L. A. , Roelofs, J. J. T. H. , de Boer, O. J. , Lankelma, J. M. , Boon, L. , de Vos, A. F. , van ’t Veer, C. , & van der Poll, T. (2018). Role of peptidylarginine deiminase 4 in neutrophil extracellular trap formation and host defense during Klebsiella pneumoniae‐induced pneumonia‐derived sepsis. Journal of Immunology, 201(4), 1241–1252. 10.4049/jimmunol.1800314 [DOI] [PubMed] [Google Scholar]
- Colotta, F. , Allavena, P. , Sica, A. , Garlanda, C. , & Mantovani, A. (2009). Cancer‐related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis, 30(7), 1073–1081. 10.1093/carcin/bgp127 [DOI] [PubMed] [Google Scholar]
- Commerford, C. D. , Dieterich, L. C. , He, Y. , Hell, T. , Montoya‐Zegarra, J. A. , Noerrelykke, S. F. , Russo, E. , Röcken, M. , & Detmar, M. (2018). Mechanisms of tumor‐induced lymphovascular niche formation in draining lymph nodes. Cell Reports, 25(13), 3554–3563. 10.1016/j.celrep.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools‐Lartigue, J. , Spicer, J. , & Ferri, L. E. (2015). Current status of management of malignant disease: Current management of esophageal cancer. Journal of Gastrointestinal Surgery, 19(5), 964–972. 10.1007/s11605-014-2701-3 [DOI] [PubMed] [Google Scholar]
- Cools‐Lartigue, J. , Spicer, J. , McDonald, B. , Gowing, S. , Chow, S. , Giannias, B. , Bourdeau, F. , Kubes, P. , & Ferri, L. (2013). Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. Journal of Clinical Investigation, 123(8), 3446–3458. 10.1172/jci67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa‐Silva, B. , Aiello, N. M. , Ocean, A. J. , Singh, S. , Zhang, H. , Thakur, B. K. , Becker, A. , Hoshino, A. , Mark, M. T. , Molina, H. , Xiang, J. , Zhang, T. , Theilen, T.‐M. , García‐Santos, G. , Williams, C. , Ararso, Y. , Huang, Y. , Rodrigues, G. , Shen, T.‐L. , … Lyden, D. (2015). Pancreatic cancer exosomes initiate pre‐metastatic niche formation in the liver. Nature Cell Biology, 17(6), 816–826. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens, L. M. , & Werb, Z. (2002). Inflammation and cancer. Nature, 420(6917), 860–867. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meo, M. L. , & Spicer, J. D. (2021). The role of neutrophil extracellular traps in cancer progression and metastasis. Seminars in Immunology, 57, 101595. 10.1016/j.smim.2022.101595 [DOI] [PubMed] [Google Scholar]
- Demers, M. , Krause, D. S. , Schatzberg, D. , Martinod, K. , Voorhees, J. R. , Fuchs, T. A. , Scadden, D. T. , & Wagner, D. D. (2012). Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer‐associated thrombosis. Proceedings of the National Academy of Sciences, 109(32), 13076–13081. 10.1073/pnas.1200419109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers, M. , & Wagner, D. D. (2013). Neutrophil extracellular traps: A new link to cancer‐associated thrombosis and potential implications for tumor progression. Oncoimmunology, 2(2), e22946. 10.4161/onci.22946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyell, M. , Garris, C. S. , & Laughney, A. M. (2021). Cancer metastasis as a non‐healing wound. British Journal of Cancer, 124(9), 1491–1502. 10.1038/s41416-021-01309-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, H. , Li, L. X. , Harris, P. C. , Yang, J. , & Li, X. (2021). Extracellular vesicles and exosomes generated from cystic renal epithelial cells promote cyst growth in autosomal dominant polycystic kidney disease. Nature Communications, 12(1), 4548. 10.1038/s41467-021-24799-xim [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak, H. F. (2015). Tumors: Wounds that do not heal—Redux. Cancer Immunology Research, 3(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom, C. , Pfirschke, C. , Zilionis, R. , Da Silva Martins, J. , Bos, S. A. , Courties, G. , Rickelt, S. , Severe, N. , Baryawno, N. , Faget, J. , Savova, V. , Zemmour, D. , Kline, J. , Siwicki, M. , Garris, C. , Pucci, F. , Liao, H. W. , Lin, Y. J. , Newton, A. , & Pittet, M. J. (2017). Osteoblasts remotely supply lung tumors with cancer‐promoting SiglecFhigh neutrophils. Science, 358(6367), eaal5081. 10.1126/science.aal5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader, C. M. , Sánchez, D. G. , Mestre, M. B. , & Colombo, M. I. (2009). TI‐VAMP/VAMP7 and VAMP3/cellubrevin: two v‐SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research, 1793(12), 1901–1916. 10.1016/j.bbamcr.2009.09.011 [DOI] [PubMed] [Google Scholar]
- Faget, J. , Boivin, G. , Ancey, P.‐B. , Gkasti, A. , Mussard, J. , Engblom, C. , Pfirschke, C. , Vazquez, J. , Bendriss‐Vermare, N. , Caux, C. , Vozenin, M.‐C. , Pittet, M. J. , Gunzer, M. , & Meylan, E. (2018). Efficient and specific Ly6G+cell depletion: A change in the current practices toward more relevant functional analyses of neutrophils. 10.1101/498881 [DOI]
- Galon, J. , Pagès, F. , Marincola, F. M. , Thurin, M. , Trinchieri, G. , Fox, B. A. , Gajewski, T. F. , & Ascierto, P. A. (2012). The immune score as a new possible approach for the classification of cancer. Journal of Translational Medicine, 10(1). 10.1186/1479-5876-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Silva, S. , Benito‐Martín, A. , Nogués, L. , Hernández‐Barranco, A. , Mazariegos, M. S. , Santos, V. , Hergueta‐Redondo, M. , Ximénez‐Embún, P. , Kataru, R. P. , Lopez, A. A. , Merino, C. , Sánchez‐Redondo, S. , Graña‐Castro, O. , Matei, I. , Nicolás‐Avila, J. Á. , Torres‐Ruiz, R. , Rodríguez‐Perales, S. , Martínez, L. , Pérez‐Martínez, M. , … Peinado, H. (2021). Melanoma‐derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR‐dependent mechanism. Nature Cancer, 2(12), 1387–1405. 10.1038/s43018-021-00272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese, M. A. , Hind, L. E. , & Huttenlocher, A. (2019). Neutrophil plasticity in the tumor microenvironment. Blood, 133(20), 2159–2167. 10.1182/blood-2018-11-844548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten, F. R. , & Grivennikov, S. I. (2019). Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity, 51(1), 27–41. 10.1016/j.immuni.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, G. P. , & Massagué, J. (2006). Cancer metastasis: Building a framework. Cell, 127(4), 679–695. 10.1016/j.cell.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Hagens, E. R. C. , van Berge Henegouwen, M. I. , van Sandick, J. W. , Cuesta, M. A. , van der Peet, D. L. , Heisterkamp, J. , Nieuwenhuijzen, G. A. P. , Rosman, C. , Scheepers, J. J. G. , Sosef, M. N. , van Hillegersberg, R. , Lagarde, S. M. , Nilsson, M. , Räsänen, J. , Nafteux, P. , Pattyn, P. , Hölscher, A. H. , Schröder, W. , Schneider, P. M. , … Gisbertz, S. S. (2019). Distribution of lymph node metastases in esophageal carcinoma [TIGER study]: study protocol of a multinational observational study. BMC Cancer, 19(1). 10.1186/s12885-019-5761-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol, J. , Wilhelmsen, L. , & Haraldsen, G. (2009). The murine IL‐8 homologues KC, MIP‐2, and LIX are found in endothelial cytoplasmic granules but not in Weibel‐Palade bodies. Journal of Leukocyte Biology, 87(3), 501–508. 10.1189/jlb.0809532 [DOI] [PubMed] [Google Scholar]
- Hood, J. L. , San, R. S. , & Wickline, S. A. (2011). Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Research, 71(11), 3792–3801. 10.1158/0008-5472.can-10-4455 [DOI] [PubMed] [Google Scholar]
- Hoshino, A. , & Lyden, D. (2017). Lymphatic detours for cancer. Nature, 546(7660), 609–610. 10.1038/546609a [DOI] [PubMed] [Google Scholar]
- Hsu, B. E. , Shen, Y. , & Siegel, P. M. (2020). Neutrophils: Orchestrators of the malignant phenotype. Frontiers in Immunology, 11, 00. 10.3389/fimmu.2020.01778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Zhang, H. , Onuma, A. E. , & Tsung, A. (2020). Neutrophil elastase and neutrophil extracellular traps in the tumor microenvironment. Tumor Microenvironment, 13–23. 10.1007/978-3-030-44518-8_2 [DOI] [PubMed] [Google Scholar]
- Hurwitz, S. N. , Conlon, M. M. , Rider, M. A. , Brownstein, N. C. , & Meckes, D. G. (2016). Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. Journal of Extracellular Vesicles, 5(1), 31295. 10.3402/jev.v5.31295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba, T. , & Ogura, H. (2018). Role of extracellular vesicles in the development of sepsis‐induced coagulopathy. Journal of Intensive Care, 6(1), 68. 10.1186/s40560-018-0340-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillon, S. , Ponzetta, A. , Di Mitri, D. , Santoni, A. , Bonecchi, R. , & Mantovani, A. (2020). Neutrophil diversity and plasticity in tumour progression and therapy. Nature Reviews Cancer, 20(9), 485–503. 10.1038/s41568-020-0281-y [DOI] [PubMed] [Google Scholar]
- Kaltenmeier, C. , Yazdani, H. O. , Morder, K. , Geller, D. A. , Simmons, R. L. , & Tohme, S. (2021). Neutrophil extracellular traps promote T cell exhaustion in the tumor microenvironment. Frontiers in Immunology, 12, 785222. 10.3389/fimmu.2021.785222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataru, R. P. , Ly, C. L. , Shin, J. , Park, H. J. , Baik, J. E. , Rehal, S. , Ortega, S. , Lyden, D. , & Mehrara, B. J. (2019). Tumor lymphatic function regulates tumor inflammatory and immunosuppressive microenvironments. Cancer Immunology Research, 7(8), 1345–1358. 10.1158/2326-6066.cir-18-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, Y. , & Aikawa, N. (2014). Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Therapeutics and Clinical Risk Management, 621, 00. 10.2147/tcrm.s65066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki, D. (2005). The crucial role of macrophages in lymphangiogenesis. Journal of Clinical Investigation, 115(9), 2316–2319. 10.1172/jci26354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha, K. , Singla, A. , Townend, P. , & Merrett, N. (2022). Association between neutrophil‐lymphocyte ratio and lymph node metastasis in gastric cancer: A meta‐analysis. Medicine (Baltimore), 101(25):e29300. 10.1097/MD.0000000000029300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawati, A. L. , Cools‐Lartigue, Y. , Ramirez‐GarciaLuna, J. , L, J. , Molina‐Franjola, J. C. , Pham, D. , Skothos, E. , Mueller, C. , Spicer, J. , & Ferri, L. (2020). Dynamic alteration of neutrophil‐to‐lymphocyte ratio over treatment trajectory is associated with survival in esophageal adenocarcinoma. Annals of Surgical Oncology, 27(11), 4413–4419. 10.1245/s10434-020-08521-7 [DOI] [PubMed] [Google Scholar]
- Leal, A. C. , Mizurini, D. M. , Gomes, T. , Rochael, N. C. , Saraiva, E. M. , Dias, M. S. , Werneck, C. C. , Sielski, M. S. , Vicente, C. P. , & Monteiro, R. Q. (2017). Tumor‐derived exosomes induce the formation of neutrophil extracellular traps: Implications for the establishment of cancer‐associated thrombosis. Scientific Reports, 7(1), 6438. 10.1038/s41598-017-06893-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok, L. S. C. , Dennison, T. W. , Mahbubani, K. M. , Saeb‐Parsy, K. , Chilvers, E. R. , & Clatworthy, M. R. (2019). Phenotypically distinct neutrophils patrol uninfected human and mouse lymph nodes. Proceedings of the National Academy of Sciences, 116(38), 19083–19089. 10.1073/pnas.1905054116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malech, H. L. , DeLeo, F. R. , & Quinn, M. T. (2014). The role of neutrophils in the immune system: An overview. Methods in Molecular Biology, 3–10. 10.1007/978-1-62703-845-4_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi Kashkooli, F. , & Soltani, M. (2021). Evaluation of solid tumor response to sequential treatment cycles via a new computational hybrid approach. Scientific Reports, 11(1). 10.1038/s41598-021-00989-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najmeh, S. , Cools‐Lartigue, J. , Rayes, R. F. , Gowing, S. , Vourtzoumis, P. , Bourdeau, F. , Giannias, B. , Berube, J. , Rousseau, S. , Ferri, L. E. , & Spicer, J. D. (2017). Neutrophil extracellular traps sequester circulating tumor cells via β1‐integrin mediated interactions. International Journal of Cancer, 140(10), 2321–2330. Portico. 10.1002/ijc.30635 [DOI] [PubMed] [Google Scholar]
- Nathanson, S. D. , Rosso, K. , Chitale, D. , & Burke, M. (2017). Chapter 13 – Lymph node metastasis∗∗funded in part by the nathanson/rands chair in breast cancer research. Artwork by Kelly Rosso, MD, and Dhananjay Chitale, MD. In: Ahmad A. (Ed.), Introduction to cancer metastasis. Academic Press, 235–261. [Google Scholar]
- Ocana, A. , Nieto‐Jiménez, C. , Pandiella, A. , & Templeton, A. J. (2017). Neutrophils in cancer: Prognostic role and therapeutic strategies. Molecular Cancer, 16(1), 137. 10.1186/s12943-017-0707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski, M. , Carmo, N. B. , Krumeich, S. , Fanget, I. , Raposo, G. , Savina, A. , Moita, C. F. , Schauer, K. , Hume, A. N. , Freitas, R. P. , Goud, B. , Benaroch, P. , Hacohen, N. , Fukuda, M. , Desnos, C. , Seabra, M. C. , Darchen, F. , Amigorena, S. , Moita, L. F. , & Thery, C. (2009). Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature Cell Biology, 12(1), 19–30. 10.1038/ncb2000 [DOI] [PubMed] [Google Scholar]
- Pagès, F. , Mlecnik, B. , Marliot, F. , Bindea, G. , Ou, F.‐S. , Bifulco, C. , Lugli, A. , Zlobec, I. , Rau, T. T. , Berger, M. D. , Nagtegaal, I. D. , Vink‐Börger, E. , Hartmann, A. , Geppert, C. , Kolwelter, J. , Merkel, S. , Grützmann, R. , Van den Eynde, M. , Jouret‐Mourin, A. , … Galon, J. (2018). International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. The Lancet, 391(10135), 2128–2139. 10.1016/s0140-6736(18)30789-x [DOI] [PubMed] [Google Scholar]
- Park, J. , Wysocki, R. W. , Amoozgar, Z. , Maiorino, L. , Fein, M. R. , Jorns, J. , Schott, A. F. , Kinugasa‐Katayama, Y. , Lee, Y. , Won, N. H. , Nakasone, E. S. , Hearn, S. A. , Küttner, V. , Qiu, J. , Almeida, A. S. , Perurena, N. , Kessenbrock, K. , Goldberg, M. S. , & Egeblad, M. (2016). Cancer cells induce metastasis‐supporting neutrophil extracellular DNA traps. Science Translational Medicine, 8(361). 10.1126/scitranslmed.aag1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.‐Y. , & Nam, J.‐S. (2020). The force awakens: metastatic dormant cancer cells. Experimental & Molecular Medicine, 52(4), 569–581. 10.1038/s12276-020-0423-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado, H. , Zhang, H. , Matei, I. R. , Costa‐Silva, B. , Hoshino, A. , Rodrigues, G. , Psaila, B. , Kaplan, R. N. , Bromberg, J. F. , Kang, Y. , Bissell, M. J. , Cox, T. R. , Giaccia, A. J. , Erler, J. T. , Hiratsuka, S. , Ghajar, C. M. , & Lyden, D. (2017). Pre‐metastatic niches: Organ‐specific homes for metastases. Nature Reviews Cancer, 17(5), 302–317. 10.1038/nrc.2017.6 [DOI] [PubMed] [Google Scholar]
- Raposo, G. , & Stoorvogel, W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. Journal of Cell Biology, 200(4), 373–383. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayes, R. F. , Mouhanna, J. G. , Nicolau, I. , Bourdeau, F. , Giannias, B. , Rousseau, S. , Quail, D. , Walsh, L. , Sangwan, V. , Bertos, N. , Cools‐Lartigue, J. , Ferri, L. E. , & Spicer, J. D. (2019). Primary tumors induce neutrophil extracellular traps with targetable metastasis‐promoting effects. JCI Insight, 4(16). 10.1172/jci.insight.128008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayes, R. F. , Vourtzoumis, P. , Bou Rjeily, M. , Seth, R. , Bourdeau, F. , Giannias, B. , Berube, J. , Huang, Y.‐H. , Rousseau, S. , Camilleri‐Broet, S. , Blumberg, R. S. , Beauchemin, N. , Najmeh, S. , Cools‐Lartigue, J. , Spicer, J. D. , & Ferri, L. E. (2020). Neutrophil extracellular trap–Associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. The Journal of Immunology, 204(8), 2285–2294. 10.4049/jimmunol.1900240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv, J. Y. , Michaeli, J. , Assi, S. , Mishalian, I. , Kisos, H. , Levy, L. , Damti, P. , Lumbroso, D. , Polyansky, L. , Sionov, R. V. , Ariel, A. , Hovav, A.‐H. , Henke, E. , Fridlender, Z. G. , & Granot, Z. (2015). Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Reports, 10(4), 562–573. 10.1016/j.celrep.2014.12.039 [DOI] [PubMed] [Google Scholar]
- Sahebnasagh, A. , Saghafi, F. , Safdari, M. , Khataminia, M. , Sadremomtaz, A. , Talaei, Z. , Rezai Ghaleno, H. , Bagheri, M. , Habtemariam, S. , & Avan, R. (2020). Neutrophil elastase inhibitor (sivelestat) may be a promising therapeutic option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID‐19. Journal of Clinical Pharmacy and Therapeutics, 45(6), 1515–1519. Portico. 10.1111/jcpt.13251 [DOI] [PubMed] [Google Scholar]
- Shahzad, M. H. , Feng, L. , Su, X. , Brassard, A. , Dhoparee‐Doomah, I. , Ferri, L. E. , Spicer, J. D. , & Cools‐Lartigue, J. J. (2022). Neutrophil extracellular traps in cancer therapy resistance. Cancers, 14(5), 1359. 10.3390/cancers14051359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde‐Jadhav, S. , Mansure, J. J. , Rayes, R. F. , Marcq, G. , Ayoub, M. , Skowronski, R. , Kool, R. , Bourdeau, F. , Brimo, F. , Spicer, J. , & Kassouf, W. (2021). Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nature Communications, 12(1), 2776. 10.1038/s41467-021-23086-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler‐Cardona, A. , Forsthuber, A. , Lipp, K. , Ebersberger, S. , Heinz, M. , Schossleitner, K. , Buchberger, E. , Gröger, M. , Petzelbauer, P. , Hoeller, C. , Wagner, E. , & Loewe, R. (2018). CXCL5 facilitates melanoma cell–neutrophil interaction and lymph node metastasis. Journal of Investigative Dermatology, 138(7), 1627–1635. 10.1016/j.jid.2018.01.035 [DOI] [PubMed] [Google Scholar]
- Spicer, J. D. , McDonald, B. , Cools‐Lartigue, J. J. , Chow, S. C. , Giannias, B. , Kubes, P. , & Ferri, L. E. (2012). Neutrophils promote liver metastasis via Mac‐1–mediated interactions with circulating tumor cells. Cancer Research, 72(16), 3919–3927. 10.1158/0008-5472.can-11-2393 [DOI] [PubMed] [Google Scholar]
- Srinivasan, S. , Vannberg, F. O. , & Dixon, J. B. (2016). Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Scientific Reports, 6(1), 2446. 10.1038/srep24436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg, P. S. (2016). Targeting metastasis. Review article. Nature Reviews Cancer, 16, 201. 10.1038/nrc.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, B. , Zhou, Y. , Fang, Y. , Li, Z. , Gu, X. , & Xiang, J. (2019). Colorectal cancer exosomes induce lymphatic network remodeling in lymph nodes. International Journal of Cancer, 145(6), 1648–1659. Portico. 10.1002/ijc.32196 [DOI] [PubMed] [Google Scholar]
- Théry, C. , Witwer, K. W. , Aikawa, E. , Alcaraz, M. J. , Anderson, J. D. , Andriantsitohaina, R. , Antoniou, A. , Arab, T. , Archer, F. , Atkin‐Smith, G. K. , Ayre, D. C. , Bach, J.‐M. , Bachurski, D. , Baharvand, H. , Balaj, L. , Baldacchino, S. , Bauer, N. N. , Baxter, A. A. , Bebawy, M. , … Zuba‐Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7(1), 1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohme, S. , Yazdani, H. O. , Al‐Khafaji, A. B. , Chidi, A. P. , Loughran, P. , Mowen, K. , Wang, Y. , Simmons, R. L. , Huang, H. , & Tsung, A. (2016). Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Research, 76(6), 1367–1380. 10.1158/0008-5472.can-15-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumoto, M. , Tanaka, H. , Ohira, M. , Go, Y. , Okita, Y. , Sakurai, K. , Toyokawa, T. , Kubo, N. , Muguruma, K. , Maeda, K. , Sawada, T. , & Hirakawa, K. (2014). A positive correlation between neutrophils in regional lymph nodes and progression of gastric cancer. Anticancer Research, 34(12), 7129–7136. [PubMed] [Google Scholar]
- Urabe, F. , Patil, K. , Ramm, G. A. , Ochiya, T. , & Soekmadji, C. (2021). Extracellular vesicles in the development of organ‐specific metastasis. Journal of Extracellular Vesicles, 10(9), e12125. Portico. 10.1002/jev2.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deun, J. , Mestdagh, P. , Agostinis, P. , Akay, Ö. , Anand, S. , Anckaert, J. , Martinez, Z. A. , Baetens, T. , Beghein, E. , Bertier, L. , Berx, G. , Boere, J. , Boukouris, S. , Bremer, M. , Buschmann, D. , Byrd, J. B. , Casert, C. , Cheng, L. , … Hendrix, A. (2017). EV‐TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nature Methods, 14(3), 228–232. 10.1038/nmeth.4185 [DOI] [PubMed] [Google Scholar]
- Vyas, D. , Laput, G. , & Vyas, A. (2014). Chemotherapy‐enhanced inflammation may lead to the failure of therapy and metastasis. OncoTargets and Therapy, 1015. 10.2147/ott.s60114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Zhang, H. , Wang, Y. , Brown, Z. J. , Xia, Y. , Huang, Z. , Shen, C. , Hu, Z. , Beane, J. , Ansa‐Addo, E. A. , Huang, H. , Tian, D. , & Tsung, A. (2021). Regulatory T‐cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non‐alcoholic steatohepatitis. Journal of Hepatology, 75(6), 1271–1283. 10.1016/j.jhep.2021.07.032 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Chen, Y. , Gao, Y. , Zhang, H. , Guan, Z. , Dong, Z. , Zheng, Y. , Jiang, J. , Yang, H. , Wang, L. , Huang, X. , Ai, L. , Yu, W. , Li, H. , Dong, C. , Zhou, Z. , Liu, X. , & Yu, G. (2021). Predicting gastric cancer outcome from resected lymph node histopathology images using deep learning. Nature Communications, 12(1), 1637. 10.1038/s41467-021-21674-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhai, J. , Zhang, T. , Han, S. , Zhang, Y. , Yao, X. , & Shen, L. (2020). Tumor‐associated neutrophils can predict lymph node metastasis in early gastric cancer. Frontiers in Oncology, 10, 570113. 10.3389/fonc.2020.570113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M. , Ma, M. , Tan, Z. , Zheng, H. , & Liu, X. (2020). Neutrophil: A new player in metastatic cancers. Frontiers in Immunology, 11, 565165. 10.3389/fimmu.2020.565165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, X. , Liao, X. , Qiu, S. , Xu, H. , Zhang, S. , Wang, S. , Ai, J. , & Yang, L. (2022). CXCL8 in tumor biology and its implications for clinical translation. Frontiers Molecular Bioscience, 9, 723846. 10.3389/fmolb.2022.723846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Shi, H. , Yuan, X. , Jiang, P. , Qian, H. , & Xu, W. (2018). Tumor‐derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Molecular Cancer, 17(1), 146. 10.1186/s12943-018-0898-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Chandra, V. , Riquelme Sanchez, E. , Dutta, P. , Quesada, P. R. , Rakoski, A. , Zoltan, M. , Arora, N. , Baydogan, S. , Horne, W. , Burks, J. , Xu, H. , Hussain, P. , Wang, H. , Gupta, S. , Maitra, A. , Bailey, J. M. , Moghaddam, S. J. , Banerjee, S. , …. McAllister, F. (2020). Interleukin‐17–induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. Journal of Experimental Medicine, 217(12), e20190354. 10.1084/jem.20190354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Wu, L. , Yan, G. , Chen, Y. , Zhou, M. , Wu, Y. , & Li, Y. (2021). Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduction and Targeted Therapy, 6(1), 263. 10.1038/s41392-021-00658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Demographic and clinical characteristics of patients with gastroesophageal adenocarcinoma in the TMA study
Table S2 Demographic and clinical characteristics of patients with oesophageal adenocarcinoma from TCGA
Table S3 Demographic and clinical characteristics of patients with oesophageal adenocarcinoma for plasma EV proteomics study
Table S4 Resources table of antibodies, reagents, cells and viruses.
Figure S1: (a) Quantification of %NETs positive area per LN core, divided by stage and comparing patients who received neoadjuvant therapies or not.
Figure S2: (a) Flow cytometry gating strategies of mouse LN and blood neutrophils.
Figure S3: (a) Representative images of tumour draining LNs on a time course for both B16F1 and B16F10 melanoma cells.
Figure S4: (a) Baseline percentage of neutrophils within blood leukocytes for wildtype and PAD4 knockout mice.
Figure S5: (a) Nanoparticle tracking assay (NTA) showing the size distribution of EVs.
Fig. S6: (a) Kaplan‐Meier survival curves comparing the survival of EAD patients with low versus high levels of PRKD1 or Rab5a mRNA in tumour.
Supporting Information


