Abstract
Background
Assisted reproduction techniques (ART), such as in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI), can help subfertile couples to create a family. It is necessary to induce multiple follicles, which is achieved by follicle stimulating hormone (FSH) injections. Current treatment regimens prescribe daily injections of FSH (urinary FSH either with or without luteinizing hormone (LH) injections or recombinant FSH (rFSH)).
Recombinant DNA technologies have produced a new recombinant molecule which is a long‐acting FSH, named corifollitropin alfa (Elonva) or FSH‐CTP. A single dose of long‐acting FSH is able to keep the circulating FSH level above the threshold necessary to support multi‐follicular growth for an entire week. The optimal dose of long‐acting FSH is still being determined. A single injection of long‐acting FSH can replace seven daily FSH injections during the first week of controlled ovarian stimulation (COS) and can make assisted reproduction more patient friendly.
Objectives
To compare the effectiveness of long‐acting FSH versus daily FSH in terms of pregnancy and safety outcomes in women undergoing IVF or ICSI treatment cycles.
Search methods
We searched the following electronic databases, trial registers and websites from inception to June 2015: the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Menstrual Disorders and Subfertility Group (MDSG) Specialized Register, MEDLINE, EMBASE, PsycINFO, CINAHL, electronic trial registers for ongoing and registered trials, citation indexes, conference abstracts in the ISI Web of Knowledge, LILACS, Clinical Study Results (for clinical trial results of marketed pharmaceuticals), PubMed and OpenSIGLE. We also carried out handsearches.
Selection criteria
We included all randomised controlled trials (RCTs) comparing long‐acting FSH versus daily FSH in women who were part of a couple with subfertility and undertaking IVF or ICSI treatment cycles with a GnRH antagonist or agonist protocol.
Data collection and analysis
Two review authors independently performed study selection, data extraction and assessment of risk of bias. We contacted trial authors in cases of missing data. We calculated risk ratios for each outcome, and our primary outcomes were live birth rate and ovarian hyperstimulation syndrome (OHSS) rate. Our secondary outcomes were ongoing pregnancy rate, clinical pregnancy rate, multiple pregnancy rate, miscarriage rate, any other adverse event (including ectopic pregnancy, congenital malformations, drug side effects and infection) and patient satisfaction with the treatment. Trials reported all outcomes, except patient satisfaction with the treatment.
Main results
We included six RCTs with a total of 3753 participants and we graded the quality of the included studies as moderate. All studies included women with an indication for COS as part of an IVF/ICSI cycle with age ranging from 18 to 41 years. A comparison of long‐acting FSH versus daily FSH did not show evidence of difference in effect on overall live birth rate (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.84 to 1.07; 2363 participants, five studies; I² statistic = 44%) or OHSS (RR 1.00, 95% CI 0.74 to 1.37; 3753 participants, six studies; I² statistic = 0%). We compared subgroups by dose of long‐acting FSH. There was evidence of a reduced live birth rate in women who received lower doses (60 to 120 μg) of long‐acting FSH compared to daily FSH (RR 0.70, 95% CI 0.52 to 0.93; 645 participants, three studies; I² statistic = 0%). There was no evidence a difference between the groups in live births in the medium dose (150 to 180 μg) subgroup (RR 1.03, 95% CI 0.90 to 1.18; 1685 participants, four studies; I² statistic = 6%). There was no evidence of a difference between the groups in the clinical pregnancy rate (any dose), ongoing pregnancy rate (any dose), multiple pregnancy rate (any dose), miscarriage rate (low or medium dose), ectopic pregnancy rate (any dose), congenital malformation rate, congenital malformation rate; major or minor (low or medium dose).
Authors' conclusions
The use of a medium dose (150 to 180 μg) of long‐acting FSH is a safe treatment option and equally effective compared to daily FSH in women with unexplained subfertility. There was evidence of reduced live birth rate in women receiving a low dose (60 to 120 μg) of long‐acting FSH compared to daily FSH. Further research is needed to determine whether long‐acting FSH is safe and effective for use in hyper‐ or poor responders and in women with all causes of subfertility.
Plain language summary
Long‐acting FSH versus daily FSH for women undergoing assisted reproduction
Review question
The aim of this Cochrane review was to compare the effectiveness and safety of weekly (long‐acting) follicle stimulating hormone (FSH) compared to daily FSH in women undergoing in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) treatment cycles. Background
When assisted reproduction techniques such as IVF and ICSI are performed, the fertilisation of the egg takes place outside the woman's body. Multiple eggs are needed to increase the availability of fertilised eggs. The growth of multiple eggs is achieved by stimulation of the ovary with FSH. There is a risk of overstimulation (ovarian hyperstimulation syndrome or OHSS). OHSS is a serious adverse effect which can cause illness or death.
Current treatment regimens to stimulate the growth of multiple eggs prescribe daily injections of FSH during the first seven days of treatment. A new treatment is now available which replaces these injections with just a single injection. This new treatment can be more patient friendly, as daily injections may cause discomfort and be a physical burden to the women.
Study characteristics
We included six randomised controlled trials comparing weekly versus daily FSH in 3753 women undertaking controlled ovarian stimulation as part of an IVF/ICSI cycle. Their age ranged from 18 to 41 years. The studies used different dosages of weekly FSH ranging from 60 to 240 μg. Five studies reported live birth rate and all six studies reported OHSS rate (our primary outcomes). The evidence is current to June 2015.
Key results
There was no evidence of a difference in live birth rates between medium dose (150 to 180 μg) weekly FSH and daily FSH. There was evidence of a reduced live birth rate in women who received lower doses (60 to 120 μg) of weekly FSH when compared to daily FSH. Only one study used a high dose of weekly FSH, so we cannot make conclusions about this dosage group.There was no evidence of a difference between the groups in OHSS rate. We concluded that medium dose (150 to 180 μg) weekly FSH is a safe treatment option and is as effective in terms of life birth rate as daily FSH injections.
Quality of the evidence
The quality of the evidence was graded as moderate. Five out of six studies were funded by the same drug manufacturer.
Summary of findings
Summary of findings for the main comparison. Long‐acting FSH (all doses) versus daily FSH for women undergoing assisted reproduction.
| Long‐acting FSH (all doses) versus daily FSH for women undergoing assisted reproduction | ||||||
|
Patient: Women undergoing assisted reproduction Settings: clinic Intervention: Long‐acting FSH (all doses) Control: daily FSH | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Daily FSH | Long‐acting FSH (all doses) | |||||
| Live birth rate ‐ Low dose (60 to 120 μg) | 352 per 1000 | 246 per 1000 (183 to 327) | RR 0.7 (0.52 to 0.93) | 645 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Live birth rate ‐ Medium dose (150 to 180 μg) | 255 per 1000 | 263 per 1000 (229 to 301) | RR 1.03 (0.9 to 1.18) | 1685 (4 studies) | ⊕⊕⊕⊝ moderate2 | |
| Live birth rate ‐ High dose (240 μg) | 375 per 1000 | 161 per 1000 (45 to 570) | RR 0.43 (0.12 to 1.52) | 33 (1 study) | ⊕⊝⊝⊝ very low3 | |
| OHSS ‐ Low dose (60 to 120 μg) | 47 per 1000 | 57 per 1000 (26 to 125) | RR 1.22 (0.56 to 2.66) | 645 (3 studies) | ⊕⊕⊕⊝ moderate4 | |
| OHSS ‐ Medium dose (150 to 180 μg) | 63 per 1000 | 60 per 1000 (43 to 85) | RR 0.96 (0.68 to 1.35) | 3075 (5 studies) | ⊕⊕⊝⊝ low5 | |
| OHSS ‐ High dose (240 μg) | 0 per 1000 | 0 per 1000 (0 to 0) | RR 1.73 (0.09 to 32.75) | 33 (1 study) | ⊕⊝⊝⊝ very low3 |
|
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level for imprecision, due to low event rate 2 Downgraded one level as two studies (including the largest study) at high risk of attrition bias 3 Downgraded two levels for imprecision due to very low event rate and downgraded a further level for high risk of attrition bias
4 Downgraded for imprecision as confidence intervals compatible with clinically meaningful benefit in either arm or with no effect
5 Downgraded for imprecision as confidence intervals compatible with clinically meaningful benefit in either arm or with no effect, and downgraded a further level for high risk of attrition bias in two studies
Background
For definitions of terminology see the Glossary (Appendix 1).
Description of the condition
Infertility affects 10% to 15% of couples trying to conceive (Evers 2002; Gnoth 2005). Assisted reproduction techniques (ART), such as in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI), can help these couples to create a family. In ART it is necessary to induce multiple follicles. This is achieved by controlled ovarian stimulation (COS) with follicle stimulating hormone (FSH) injections.
Current treatment regimens prescribe daily injections of FSH (urinary FSH with or without luteinizing hormone (LH) injections or recombinant FSH (rFSH)). The FSH injections are usually started from cycle day two. Prevention of a premature ovulation due to a LH surge can be accomplished with gonadotropin‐releasing hormone (GnRH) agonists or GnRH antagonists. GnRH agonists (GnRHa) are the most commonly used adjuvants for COS (Tur‐Kaspa) Maheshwari 2011 reported no evidence of a statistically significant difference in live birth rate amongst various protocols (long, short or ultrashort) of GnRHa for pituitary down‐regulation in assisted reproduction treatments. Some clinicians consider antagonists to be the first choice in COS due to their immediate action, lack of side effects (e.g. lower incidence of ovarian hyperstimulation syndrome (OHSS)), the need for fewer injections and the same live birth rate as with agonists (Al‐Inany 2011; Tarlatzis 2007). Antagonist injections start on day five or six (see Figure 1) whereas agonist injections in a long protocol start two to four weeks prior to the stimulation (see Figure 2). Short and ultrashort GnRHa protocols start at day 1 or 2.
1.
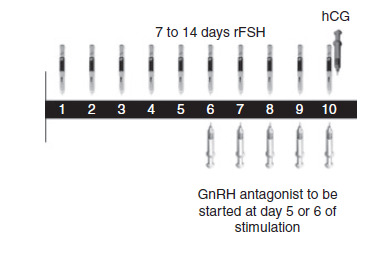
Schematic representation of therapeutic interventions during ovarian stimulation with daily FSH in a GnRH antagonist protocol (Source: de Greef 2010). Copyright © 2010 Wiley: reproduced with permission.
2.
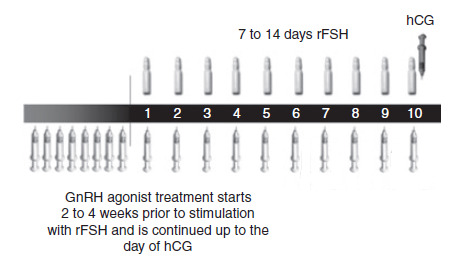
Schematic representation of therapeutic interventions during ovarian stimulation with daily FSH in a GnRH agonist protocol (Source:de Greef 2010). Copyright © 2010 Wiley: reproduced with permission.
FSH and GnRH agonist or antagonist injections are continued up to and including the day the leading follicle reaches 18 to 20 mm (Heineman 2007). Then, ovulation is induced by a human chorionic gonadotropin (hCG) injection (see Figure 1 and Figure 2). Two days (34 to 36 hours) later, several oocytes are ready for ovum pick‐up. After the pick‐up, the oocytes are fertilised by IVF or ICSI. One, two or sometimes three embryos are transferred two to five days later (Kovacs 2011).
Description of the intervention
Daily injections of daily FSH are required to maintain steady‐state levels of FSH in the blood that are above the threshold for follicular development and ongoing maturation, due to its relatively short half‐life and rapid metabolic clearance (Duijkers 2002). The daily subcutaneous administration and side effects of the daily FSH preparations can cause discomfort and be a physical burden to the patient. Many couples withdraw prematurely from IVF or ICSI due to emotional distress, which limits their chances of pregnancy. A study showed withdrawal of 40% of non‐pregnant couples after just one cycle of IVF due to emotional distress (Schröder 2004). For this reason, a patient‐friendly therapy regimen should be developed.
Recombinant DNA technologies have produced a new recombinant molecule which consists of the α‐subunit of human FSH and a hybrid subunit consisting of the carboxyl‐terminal peptide of the β‐subunit of human chorionic gonadotrophin (hCG) coupled with the FSH β‐subunit. This molecule is a long‐acting FSH, named corifollitropin alfa (Elonva) or FSH‐CTP (Fauser 2009; Koper 2008). A single injection of long‐acting FSH on the first day of the stimulation can replace the first seven daily injections of daily FSH and fewer injections can make assisted reproduction more acceptable to these women.
The administration of long‐acting FSH involves one subcutaneous injection on the first day of COS. The dose of long‐acting FSH should be as low as possible to avoid OHSS but high enough to support COS over the seven days. de Greef 2010 investigated 100 µg for women weighing less than 60 kg and 150 µg for women weighing over 60 kg and the doses were proven to be adequate. The optimal dose of long‐acting FSH is still under investigation. From day seven, the same treatment protocol as daily FSH is used (see Figure 3; Figure 4).
3.
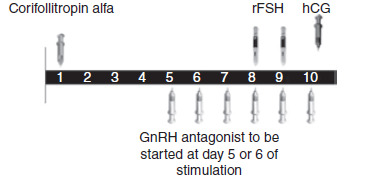
Schematic representation of therapeutic interventions during ovarian stimulation with long‐acting FSH (Corifollitropin alfa) in a GnRH antagonist protocol (Source: de Greef 2010). Copyright © 2010 Wiley: reproduced with permission.
4.
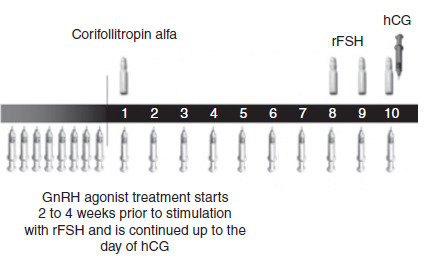
Schematic representation of therapeutic interventions during ovarian stimulation with long‐acting FSH (Corifollitropin alfa) in a GnRH agonist protocol (Source: de Greef 2010). Copyright © 2010 Wiley: reproduced with permission.
How the intervention might work
Long‐acting FSH has, compared with daily FSH, an approximately two‐fold longer elimination half‐life and an almost four‐fold extended time to peak serum levels (Devroey 2009; Duijkers 2002). Due to this pharmacokinetic profile, a single dose of long‐acting FSH is able to keep the circulating FSH level above the threshold necessary to support multi‐follicular growth for an entire week (Devroey 2009; Koper 2008). As such, a single injection of long‐acting FSH can replace seven daily FSH injections during the first week of COS.
Why it is important to do this review
The development of this new treatment regimen may provide similar or better success rates with fewer injections. It may help to reduce the treatment burden and make the therapy more patient friendly. On the other hand, it could also be more costly. This is an update of a Cochrane review first published in 2012. It considered the evidence from RCTs for the use of long‐acting FSH on pregnancy and safety outcomes.
Objectives
To compare the effectiveness of long‐acting FSH versus daily FSH in terms of pregnancy and safety outcomes in women undergoing IVF or ICSI treatment cycles.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). Only trials that were clearly randomised were included. We planned to include cross‐over trials but we did not find any cross‐over trials comparing long‐acting FSH with daily FSH.
Types of participants
Subfertile couples with an indication for COS as part of an IVF/ICSI cycle.
Types of interventions
Trials comparing long‐acting FSH versus daily FSH were eligible for inclusion. Any dose was included. We included both GnRH antagonist or agonist protocol.
Types of outcome measures
Primary outcomes
Effectiveness
Live birth rate per woman randomised, defined as the delivery of one or more living babies after 20 completed weeks of gestation. When there were multiple live births (e.g. twins or triplets), we counted these as one live birth event
Adverse
OHSS rate per woman randomised
Secondary outcomes
Effectiveness
Ongoing pregnancy rate per woman randomised, defined as evidence of a gestational sac with fetal heart motion at 12 weeks, confirmed by ultrasound
Clinical pregnancy rate per woman randomised, defined as the presence of a gestational sac with or without a fetal heart beat, confirmed by ultrasound
Adverse
Multiple pregnancy rate per woman randomised, counted as one live birth event
Miscarriage rate per woman randomised
Any other adverse event per woman randomised (including ectopic pregnancy, congenital malformation, drug side effects and infection)
Process
Patient satisfaction with the treatment
Search methods for identification of studies
We sought all published and unpublished RCTs studying long‐acting FSH versus daily FSH. We used the following search strategy, without language restriction and in consultation with the Cochrane Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator.
Electronic searches
We searched the following electronic databases, trial registers and websites using Ovid software:
Cochrane MDSG Specialized Register of Controlled Trials (from inception to June 2015) (Appendix 2)
Cochrane Central Register of Controlled Trials (CENTRAL) (from inception to June 2015) (Appendix 3)
MEDLINE (1950 to June 2015) (Appendix 4)
EMBASE (from inception to June 2015) (Appendix 5)
PsycINFO (1982 to June 2015) (Appendix 6)
CINAHL (from inception to June 2015) (Appendix 7)
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials that appears in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We combined the EMBASE search with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN).
Also we checked the following electronic sources for trials:
Trial registers for ongoing and registered trials: 'Current Controlled Trials' (http://www.controlled‐trials.com/); 'ClinicalTrials.gov', a service of the US National Institutes of Health (http://clinicaltrials.gov/ct2/home)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://www.who.int/trialsearch/Default.aspx)
Citation indexes (http://scientific.thomson.com/products/sci/)
Conference abstracts and other trials in the Web of Science (http://wokinfo.com/) (Appendix 8)
LILACS database, as a source of trials from the Portuguese and Spanish speaking world (htpp://regional.bvsalud.org/php/index.php?lang=en) (choose 'LILACS' in 'all sources' drop‐down box)
PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), the random control filter for PubMed was taken from the searching chapter of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
OpenGrey database for grey literature from Europe (www.opengrey.eu).
Searching other resources
We handsearched the reference lists of articles retrieved by the search and contacted experts in the field and manufacturers of long‐acting FSH in order to obtain any additional relevant data.
Data collection and analysis
We conducted data collection and analyses in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (AP, CF) independently scanned the titles and abstracts of articles retrieved by the search and removed those that were very clearly irrelevant. Full texts of all potentially eligible studies were retrieved. Two review authors independently examined the full text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We discussed any disagreement or doubt as to whether a study was eligible for inclusion or not with a third review author (JK) and achieved consensus. A list of the excluded studies and the reasons for exclusion are provided in the 'Characteristics of excluded studies' table.
Data extraction and management
We extracted data from eligible studies using a data extraction form we had designed and pilot‐tested. Where studies had multiple publications, we used the main trial report as the reference and additional details were supplemented from secondary papers. As there were studies with four arms, three dosage arms and one control group, it was necessary to divide the control group by three. If the study did not report the outcomes per dosage subgroup, we divided the intervention group by three. Where this number was even, then we made an arbitrary decision to increase or reduce the number of cases by one in one of the three groups. We corresponded with study investigators in order to resolve any data queries, as required. Two review authors independently extracted the data. A third review author resolved any disagreements.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies using the Cochrane 'Risk of bias' assessment tool, which recommends the explicit reporting of the following domains:
-
Random sequence generation (selection bias)
Adequate: use of central computer randomisation, independent central randomisation office, on‐site computer from which assignment could only be determined after entering patient data, random number table or serially numbered and sealed opaque envelopes
Inadequate: no random sequence generation
Unclear: insufficient information about the process of sequence generation
-
Allocation concealment (selection bias)
Adequate: sequentially numbered and identical drug containers were used
Inadequate: use of non‐opaque envelopes or systematic methods (e.g. date of birth, medical record number, day of the week presenting)
Unclear: insufficient information about the process of allocation concealment
-
Blinding of participants, researchers and care providers (performance bias)
Adequate: blinding of the participants, researchers and the care providers, or incomplete or no blinding was used but was not likely to influence outcomes
Inadequate: no blinding or incomplete blinding was used and likely to influence the outcomes
Unclear: insufficient information about the process of blinding the participants, researchers and care providers
-
Blinding of the outcome assessor (detection bias)
Adequate: blinding of the researchers or incomplete blinding had no effect on the outcome measurement
Inadequate: no blinding of the researchers, or incomplete blinding had influence on the outcomes
Unclear: insufficient information about the process of blinding the outcome assessor
-
Incomplete outcome data (attrition bias)
Adequate: there were no missing data, or reasons for missing data may not influence the outcomes
Inadequate: reasons for missing data may influence the outcomes
Unclear: insufficient information about the completeness of outcome data
-
Selective outcome reporting (reporting bias)
Adequate: all pre‐specified outcomes in the protocol have been published, or no protocol available but it was clear all pre‐specified outcomes were reported
Inadequate: not all pre‐specified outcomes in the protocol were reported
Unclear: insufficient information about the process of outcome reporting
-
Other potential sources of bias
Adequate: the study was free of other biases
Inadequate: other biases were present
Unclear: insufficient information about the other sources of bias
Two review authors assessed these seven domains as at 'low' (adequate), 'high' (inadequate), or 'unclear' risk of bias (unclear). The assessments made by the two review authors were compared and we resolved any disagreements by consensus or by discussion with a third review author. We presented the conclusion in the 'Risk of bias' tables and incorporated these results into the interpretation of review findings by means of sensitivity analyses.
Measures of treatment effect
We used the dichotomous data measures and expressed the results in the control and intervention groups of each study as risk ratios (RR) with 95% confidence intervals (CI).
Unit of analysis issues
The primary analysis was per woman randomised. All included studies reported data per woman. We counted multiple live births (e.g. twins or triplets) as one live birth event.
Dealing with missing data
In the case of missing data from the included studies, we contacted the original investigators to request the relevant missing data. We did not received the requested data so we made an imputation of individual values for the primary outcomes only. Live births were assumed not to have occurred in participants without a reported outcome. We analysed all data on an intention‐to‐treat (ITT) basis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. We used the I² statistic to assess the impact of the heterogeneity on the meta‐analysis. We interpreted the result of the I² statistic as follows:
0% to 40%, might not be important
30% to 60%, may represent moderate heterogeneity
50% to 90%, may represent substantial heterogeneity
75% to 100%, considerable heterogeneity (Higgins 2011)
Assessment of reporting biases
We searched for within trial selective reporting, such as trials failing to report obvious outcomes or reporting them in insufficient detail to allow inclusion. We sought published protocols to look for any pre‐planned outcomes that may not have been reported and compared the outcomes between the protocol and the final published study. We planned to undertake informal assessment if included studies failed to report the primary outcome of live birth, but all studies reported live birth.
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise the potential impact by ensuring a comprehensive search for eligible studies. We were alert for duplication of data. To investigate the potential for publication bias, we planned to use a funnel plot if there were 10 or more studies in an analysis. However, due to the small number of studies per subgroup this was not possible.
Data synthesis
We performed statistical analyses using RevMan 2014. We used a fixed‐effect model to combine the data from primary studies. We planned to perform a random‐effects meta‐analysis in the case of substantial heterogeneity, but this was not necessary.
Subgroup analysis and investigation of heterogeneity
We analysed data in the following subgroups:
Dose of long‐acting FSH:
Low (60 to 120 μg)
Medium (150 to 180 μg)
High (240 μg)
We planned to do subgroup analyses on: women's age; weight; body mass index (BMI); day of starting GnRH antagonist; and poor responders to ovarian stimulation. However, we were unable to perform these subgroup analyses due to insufficient information.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcomes to determine whether the conclusions were robust regarding the eligibility and analysis of studies. We explored whether exclusion of trials with high risk of bias had an impact on the results.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, Characteristics of ongoing studies sections.
Results of the search
See: study flow diagram Figure 5.
5.
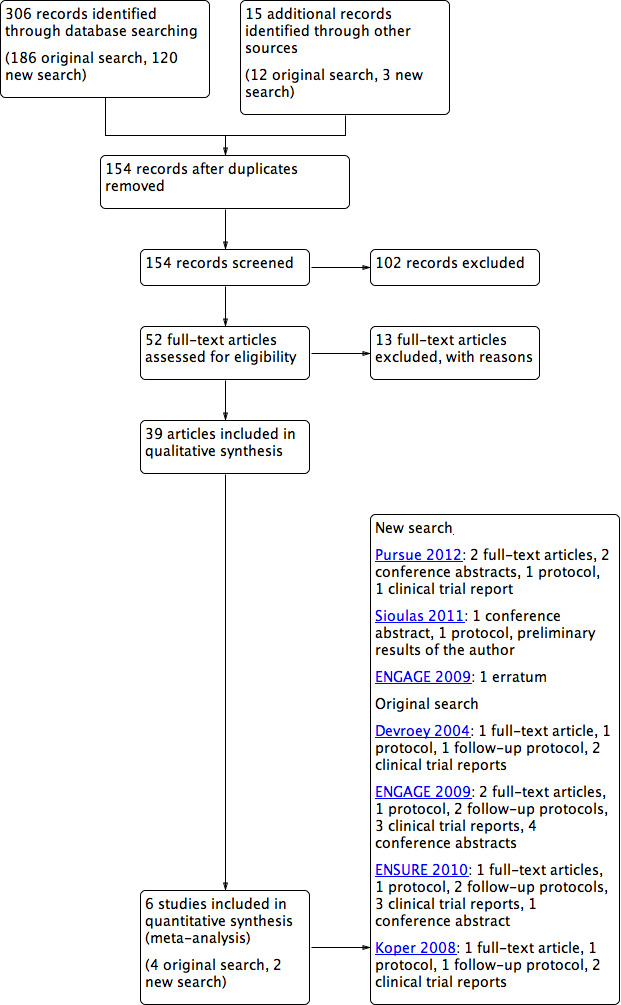
Study flow diagram.
The original search was done on 10 October 2011 (for our search strategy see Methods). Using the search strings stated in Appendix 2 to Appendix 8, we retrieved 107 articles. We also searched LILICS, metaRegister of Controlled Trials‐active registers, WHO ICTRP, clinicaltrials.gov, clinicalstudyresults.org, OpenSIGLE and PubMed (MeSH terms used) using the keywords 'corifollitropin', 'corifollitropin alfa', 'corifollitropin alpha', 'ORG 36286', 'Elonva', 'FSH‐CTP', 'long‐acting FSH', 'long acting FSH'. We retrieved 76 reports, and identified another three reports by using other methods, such as handsearching. After removal of duplicates, we screened titles and exclued clearly irrelevant articles. We retrieved the remaining studies in full‐text or were conference abstracts, protocols or clinical study results. The reports that did not appear to meet our inclusion criteria were excluded. Five trials met our inclusion criteria, and one study was a duplicate (Fauser 2009). We also found two ongoing trials and one conference abstract with preliminary results (Sioulas 2011). For details see the Characteristics of ongoing studies section.
On clinicaltrials.gov we found the protocols of the four included studies and six protocols of follow‐up studies of the original included studies. We found clinical study reports of all protocols on clinicalstudyresults.org. We included four trials and the data of four conference abstracts, six protocols and six reports of clinical study results in our meta‐analyses. Overall, we included four trials.
A new search was performed on 08 June 2015. We used the search strings stated in Appendix 2 to Appendix 8 and retrieved 120 articles. We also searched LILICS, metaRegister of Controlled Trials‐active registers, WHO ICTRP, clinicaltrials.gov, clinicalstudyresults.org, OpenGrey and PubMed (MeSH terms used) using the keywords 'corifollitropin', 'corifollitropin alfa', 'corifollitropin alpha', 'ORG 36286', 'Elonva', 'FSH‐CTP', 'long‐acting FSH', 'long acting FSH'. This search retrieved three additional records. After screening titles and full‐text articles we found two trails meeting our inclusion criteria. Two studies, which were ongoing at the time of the original search, are included in our updated meta‐analysis (Pursue 2012; Sioulas 2011). We also found the study protocol of both studies and one conference abstract with follow‐up data. We also included an erratum of ENGAGE 2009.
Included studies
Study design and setting
We included six RCTs in the review. Five were multi‐centre trials, conducted in Europe (Austria, Belgium, Czech Republic, Denmark, Finland, France, Norway, Poland, Spain, Sweden, The Netherlands, UK), North America (Canada, USA) and Asia (Korea, Taiwan). One one‐centre clinical trial was conducted in Greece (Sioulas 2011). We included two four‐arm trials (Devroey 2004; Koper 2008) and four two‐arm trials (ENGAGE 2009; ENSURE 2010; Pursue 2012; Sioulas 2011). As there were studies with four arms, three dosage arms and one control group, it was necessary to divide the control group by three. If the study did not report live births per dosage subgroup we divided the intervention group by three. Where this number was even, then we made an arbitrary decision to increase or reduce the number of cases by one in one of the three groups.
Participants
A total of 3753 women participated in the included studies, 2054 women in the intervention groups and 1699 women in the control groups. The age of the included participants ranged from 18 to 42 years, and the range of BMI was 17 to 32 kg/m².
The inclusion criteria differed slightly between the studies regarding age, BMI and weight. ENSURE 2010 included women with a body weight ≤ 60 kg and BMI of 18 to 32 kg/m². ENGAGE 2009 included women weighing > 60 kg and ≤ 90 kg with BMI 18 to 32 kg/m². The three other studies included women weighing between 50 to 90 kg and BMI 18.0 to 32.0 kg/m² (Devroey 2004; Koper 2008; Pursue 2012). Sioulas 2011 included women weighing between 60 to 90 kg and with a BMI 18.0 to 32.0 kg/m². There were differences for the inclusion and exclusion criteria between the protocols and the published articles. Koper 2008 reported an inclusion age range of 20 to 39 years in the article while they stated 18 to 39 years in the protocol. Devroey 2004 reported none of the exclusion criteria as stated in the protocol. All studies excluded poor responders, patients with a history of OHSS or polycystic ovary syndrome (PCOS) (hyper‐responders) and women with a cause for subfertility.
We have presented a summary in Table 2; for details see the Characteristics of included studies tables.
1. Summary of characteristics of included studies.
| Study ID | Participant age (years) | Participant BMI (kg/m²) | Participant weight (kg) | Start GnRH antagonist | No. of embryos transferred | Poor responders |
| Devroey 2004 | 18 to 39 | 18 to 29 | 50 to 90 | Leading follicle ≥ 14 mm | ≤ 3 | Excluded |
| ENGAGE 2009 | 18 to 36 | 18 to 32 | > 60 and ≤ 90 | Day 5 | 1 or 2 | Excluded |
| ENSURE 2010 | 18 to 36 | 18 to 32 | < 60 | Day 5 | 1 or 2 | Excluded |
| Koper 2008 | 20 to 39 Protocol: 18 to 39 |
17 to 31 | 50 to 90 | Day 5 | ≤ 3 | Excluded |
| Pursue 2012 | 35 to 42 | 18 to 32 | > 50 | Day 5 | 2 | Excluded |
| Sioulas 2011 | 18 to 36 | 18 to 32 | 60 to 90 | Both agonist and antagonist protocol used, day not stated | 2 | Excluded |
Interventions
Five included studies compared long‐acting FSH with daily FSH and followed by a GnRH antagonist protocol. Sioulas 2011 used both GnRH agonist and antagonist protocol. The studies varied in initial dose of long‐acting FSH administered: 454 women received a low dose (60 to 120 μg) (Devroey 2004; ENSURE 2010; Koper 2008), 1575 women received a medium dose (150 to 180 μg) (Devroey 2004; ENGAGE 2009; Koper 2008; Pursue 2012; Sioulas 2011) and 25 women received a high dose (240 μg) (Devroey 2004). All studies used daily FSH for the control group: three studies used 150 IU daily FSH (Devroey 2004; ENSURE 2010; Koper 2008) and ENGAGE 2009 used 200 IU daily FSH. Pursue 2012 used daily FSH 300 IU. ENSURE 2010 and ENGAGE 2009 used a bodyweight adjusted dose of long‐acting and daily FSH.
GnRH antagonist was administered subcutaneously. Devroey 2004 started on the day the leading follicle reached 14 mm. Four other studies started on day 5. Sioulas 2011 did not state day of administration (ENGAGE 2009; ENSURE 2010; Koper 2008; Pursue 2012). See Table 2.
The number of transferred embryos varied from one, two or three embryos; see Table 2.
Outcomes
Primary outcomes
Effectiveness
Five clinical trial reports reported live birth rate, with additional data obtained by follow‐up studies of the original trial.
Adverse
All six include studies reported OHSS. We reported the total number of OHSS cases, including mild, moderate and severe cases.
Secondary outcomes
Effectiveness
Five studies reported clinical pregnancy rate. These five studies stated both the number of clinical pregnancies (defined as presence of gestational sac confirmed by ultrasound) and the number of ongoing pregnancies (defined as evidence of a gestational sac and heartbeat at 12 weeks confirmed by ultrasound). We reported both outcomes per women randomised. If results after both fresh and frozen embryo transfer were given, we reported the ongoing pregnancy rate after fresh embryo transfer.
Adverse
Five studies reported multiple pregnancy rate. Devroey 2004 reported three sets of twins in the intervention groups; we assumed that one twin pregnancy occurred in each intervention group. Three studies reported miscarriage rate (ENGAGE 2009; ENSURE 2010; Sioulas 2011). Three studies reported ectopic pregnancy rate as an adverse event (Devroey 2004; ENGAGE 2009; ENSURE 2010). Three studies reported congenital malformations (major and minor) per live born infant (ENGAGE 2009; ENSURE 2010; Pursue 2012). Major malformations were defined as any congenital malformation that causes functional impairment or requires surgical correction. Minor malformations were defined as any congenital malformation not classified as major.
We had insufficient data to report any other adverse events.
Process
None of the included studies reported patient satisfaction with the treatment.
Excluded studies
We excluded 13 studies from the review. Eleven studies were not RCTs (Croxtall 2011; de Lartigue 2011; Fatemi 2010; Ledger 2009; Loutradis 2010; Mahmoud Youssef 2012; Norman 2011; Prados 2011; Rombauts 2012; Seyhan 2011; Talmor 2013). We excluded Balen 2004 because the included women did not undergo IVF or ICSI after the stimulation. Leader 2013 did not report outcomes of interest to this review.
See the Characteristics of excluded studies table for details.
Risk of bias in included studies
We assessed the risk of bias for each included trial and recorded these results in the 'Risk of bias' table (see Characteristics of included studies). We summarised our findings in the 'Risk of bias' graph (Figure 6) and 'Risk of bias' summary (Figure 7).
6.
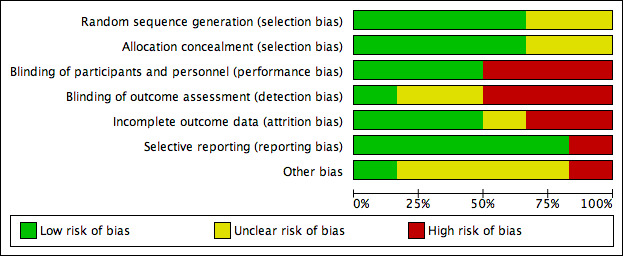
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
7.
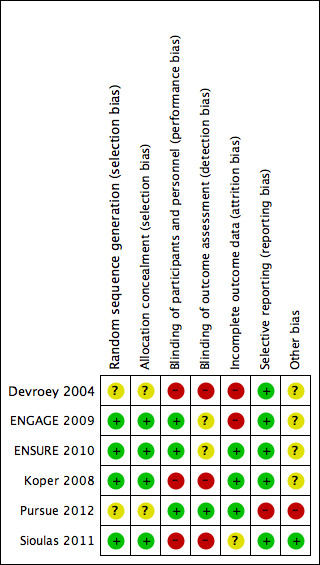
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We contacted the study authors for supplementary information. Five studies had the same contact author; we did not receive any of the requested data (Devroey 2004; ENGAGE 2009; ENSURE 2010; Koper 2008; Pursue 2012). One contact author provided us with the requested data (Sioulas 2011).
Allocation
Random sequence generation
All six included studies were randomised. We judged four studies at low risk of selection bias. Three of four used randomly permutated blocks with an undisclosed fixed block size (ENGAGE 2009; ENSURE 2010; Koper 2008). Sioulas 2011 randomised by telephone to a person totally irrelevant of the unit, by answering to random numbers which had been chosen before by the couple and provider. Thus we judged this trial at low risk of bias. We judged two studies at unclear risk of selection bias related to random sequence generation because the study authors did not report the method of randomisation used (Devroey 2004; Pursue 2012).
Allocation concealment
Three studies concealed allocation by using central remote allocation and we judged these studies to be at low risk of bias (ENGAGE 2009; ENSURE 2010; Koper 2008). Two studies did not describe the method of allocation concealment and we judged these to be unclear risk of bias (Devroey 2004; Pursue 2012; Koper 2008).
Blinding
Blinding of participants and personnel
All six studies reported their method of blinding. Two studies were open‐label trials (Devroey 2004; Koper 2008) and were judged at high risk of bias. One study was not blinded, and we judged this study at high risk of bias (Sioulas 2011). Three studies were double‐blind and described use of a double‐dummy placebo and were thus we deemed them to be at low risk of performance bias (ENGAGE 2009; ENSURE 2010; Pursue 2012).
Blinding of outcome assessment
Pursue 2012 blinded the outcome assessor, and we judged this trial at low risk of bias. Two studies did not report the blinding of outcome assessors and we judged them to be at unclear risk of bias (ENGAGE 2009; ENSURE 2010). One study was not blinded (Sioulas 2011). The other two studies were open‐label trials and for this reason we judged them to be at high risk of detection bias (Devroey 2004; Koper 2008).
Incomplete outcome data
Two studies were at high risk of attrition bias. Devroey 2004 did not report reasons for all withdrawals. They reported in their protocol six participants treated with long‐acting FSH during this trial; these treated participants were not analysed in their publication. We decided to analyse 105 participants in our meta‐analysis (99 participants analysed in the published paper and six participants treated as stated in the protocol). We found ENGAGE 2009 to be at high risk of bias because the trial had a high unexplained drop‐out rate. Three studies were judged to be at low risk of bias because they reported all numbers and reasons for withdrawals (ENSURE 2010; Koper 2008; Pursue 2012). We used preliminary data of Sioulas 2011, and as no dropouts were reported, risk of bias was unclear.
Selective reporting
We were able to obtain the protocols for all included studies and all pre‐specified outcomes were reported in either the published articles or unpublished data on clinical study reports (clinicalstudyresults.gov). We judged five studies to be at low risk of reporting bias. We judged Pursue 2012 at high risk of bias because they didn't report the outcomes as stated in the original study protocol.
Other potential sources of bias
We judged five trials at unclear risk of other bias because they were funded by Schering‐Plough (NV Organon). ENGAGE 2009 also received fees and grants from Ferring, Bessins, Serono, Merck Serono, IBSA, Wyeth, Schering, Ardana, Andromed, Pantrhei Bioscience and Preglem. The protocol of Pursue 2012 stated "The investigator agreed not to publish or publicly present any interim results of the trial without the prior written consent of the sponsor. The investigator further agreed to provide to the sponsor 45 days prior to submission for publication or presentation, review copies of abstracts or manuscripts for publication that report(s) any results of the trial", so we judged it to be at high risk of bias. Sioulas 2011 did not received any pharmaceutical fees or grants, and we judged it at low risk of bias.
Effects of interventions
See: Table 1
We did not conduct all the subgroup analyses as stated in our protocol. We had insufficient data to conduct the analyses (see Table 2). We compared long‐acting FSH (all doses) versus daily FSH with the following subgroups:
Dose of long‐acting FSH:
Low (60 to 120 μg)
Medium (150 to 180 μg)
High (240 μg)
Primary outcomes
1.1 Live birth rate
Five trials reported the numbers of live births. There was no evidence of a difference between long‐acting FSH versus daily FSH. Moderate heterogeneity was detected (RR 0.95, 95% CI 0.84 to 1.07; five studies, 2363 participants; I² statistic = 44%).
1.1.1 Low dose
There was evidence of a reduced live birth rate in women who received lower doses (60 to 120 μg) of long‐acting FSH compared to daily FSH (RR 0.70, 95% CI 0.52 to 0.93; 645 participants, three studies; I² statistic = 0%; moderate quality evidence).
1.1.2 Medium dose
There was no evidence of a difference between the groups (RR 1.03, 95% CI 0.90 to 1.18; 1685 participants, four studies; I² statistic = 6%, moderate quality evidence).
1.1.3 High dose
There was no evidence of a difference between the groups (RR 0.43, 95% CI 0.12 to 1.52; 33 participants, one study; very low quality evidence).
See Analysis 1.1 and Figure 8 for details.
1.1. Analysis.
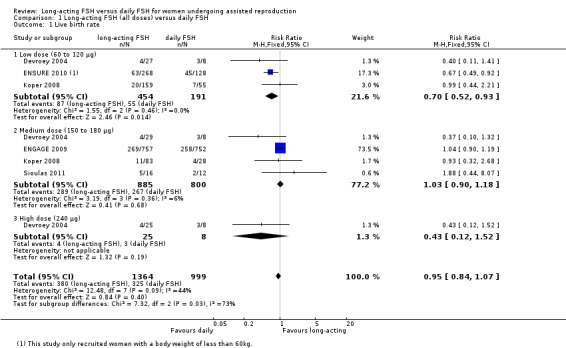
Comparison 1 Long‐acting FSH (all doses) versus daily FSH, Outcome 1 Live birth rate.
8.
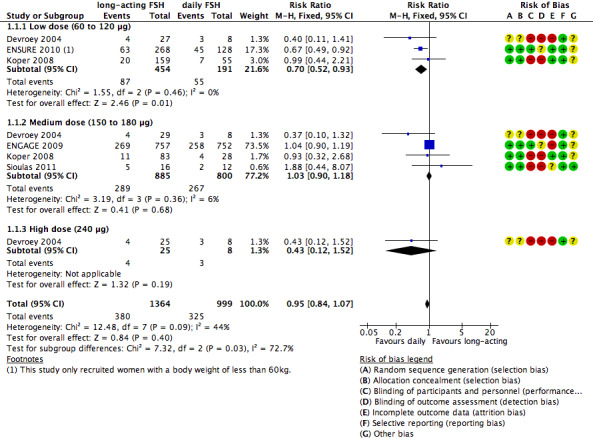
Forest plot of comparison: 1 Long‐acting FSH (all doses) versus daily FSH, outcome: 1.1 Live birth rate.
1.2 Ovarian hyperstimulation syndrome (OHSS)
All six trials reported this primary adverse event. There was no evidence of a difference between the groups for this adverse outcome (RR 1.00, 95% CI 0.74 to 1.37; 3753 participants, six studies; I² statistic = 0%).
1.2.1 Low dose
There was no evidence of a difference between the groups (RR 1.22, 95% CI 0.56 to 2.66; 645 participants, three studies; I² statistic = 0%; moderate quality evidence).
1.2.2 Medium dose
There was no evidence of a difference between the groups (RR 0.96, 95% CI 0.68 to 1.35; 3075 participants, five studies; I² statistic = 9%; low quality evidence).
1.2.3 High dose
There was no evidence of a difference between the groups (RR 1.73, 95% CI 0.09 to 32.75; 33 participants, one study; low quality evidence).
See Analysis 1.2 and Figure 9 for details.
1.2. Analysis.
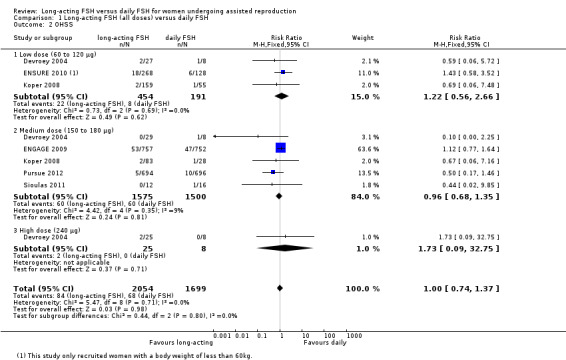
Comparison 1 Long‐acting FSH (all doses) versus daily FSH, Outcome 2 OHSS.
9.
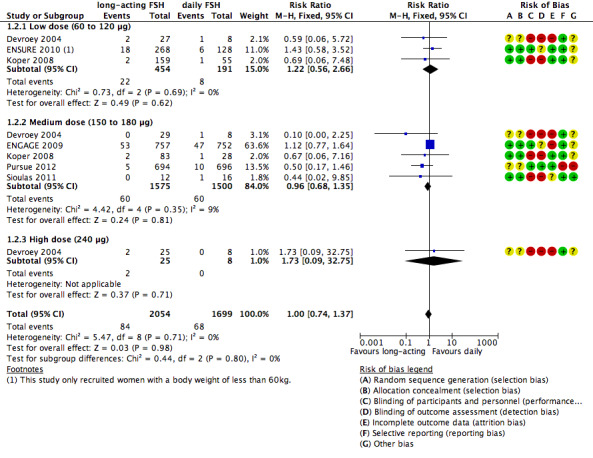
Forest plot of comparison: 1 Long‐acting FSH (all doses) versus daily FSH, outcome: 1.2 OHSS.
Secondary outcomes
1.3 Ongoing pregnancy rate
All six included studies reported ongoing pregnancy rate. There was no evidence of a difference between the groups (RR 0.95, 95% CI 0.86 to 1.04; 3753 participants, six studies; I² statistic = 19%).
1.3.1 Low dose
There was no evidence of a difference between the groups (RR 0.78, 95% CI 0.59 to 1.04; 645 participants, three studies; I² statistic = 13%).
1.3.2 Medium dose
There was no evidence of a difference between the groups (RR 0.98, 95% CI 0.88 to 1.09; 3075 participants, five studies; I² statistic = 0%).
1.3.3 High dose
There was no evidence of a difference between the groups (RR 0.48, 95% CI 0.18 to 1.28; 33 participants, one study).
See Analysis 1.3 for details.
1.3. Analysis.
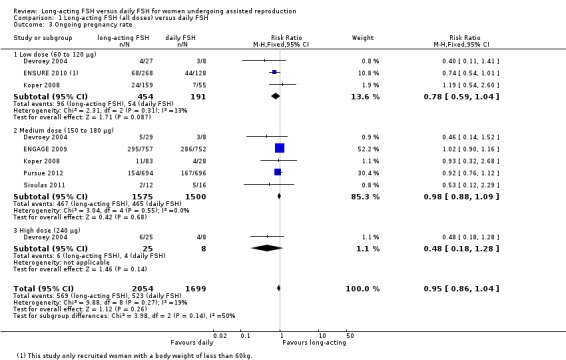
Comparison 1 Long‐acting FSH (all doses) versus daily FSH, Outcome 3 Ongoing pregnancy rate.
1.4 Clinical pregnancy rate
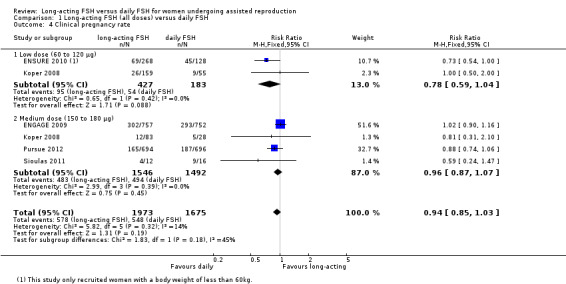
Five trials reported clinical pregnancy rate. There was no evidence of a difference between the groups (RR 0.94, 95% CI 0.85 to 1.03; 3648 participants, five studies; I² statistic = 14%).
1.4.1 Low dose
There was no evidence of a difference between the groups (RR 0.78, 95% CI 0.59 to 1.04; 610 participants, two studies; I² statistic = 0%).
1.4.2 Medium dose
There was no evidence of a difference between the groups (RR 0.96, 95% CI 0.87 to 1.07; 3038 participants, four studies; I² statistic = 0%).
1.4.3 High dose
We were unable to conduct high dose subgroup analysis as no data were available.
See Analysis 1.4 for details.
1.4. Analysis.
Comparison 1 Long‐acting FSH (all doses) versus daily FSH, Outcome 4 Clinical pregnancy rate.
1.5 Multiple pregnancy rate
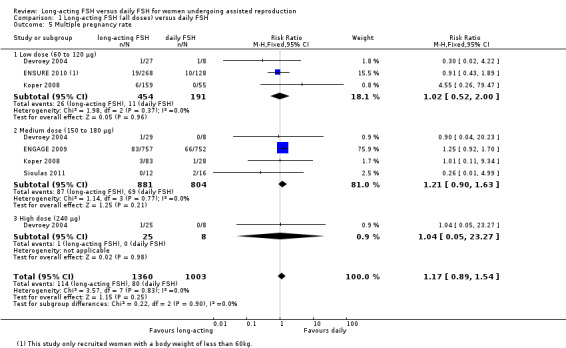
Five studies reported the adverse event multiple pregnancy rate. There was no evidence of a difference between the groups (RR 1.17, 95% CI 0.89 to 1.54; 2363 participants = 2363, five studies; I² statistic = 0%).
1.5.1 Low dose
There was no evidence of a difference between the groups (RR 1.02, 95% CI 0.52 to 2.00; 645 participants, three studies; I² statistic = 0%).
1.5.2 Medium dose
There was no evidence of a difference between the groups (RR 1.21, 95% CI 0.90 to 1.63; 1685 participants, four studies; I² statistic = 0%).
1.5.3 High dose
There was no evidence of a difference between the groups (RR 1.04, 95% CI 0.05 to 23.27; 33 participants, one study).
See Analysis 1.5 for details.
1.5. Analysis.
Comparison 1 Long‐acting FSH (all doses) versus daily FSH, Outcome 5 Multiple pregnancy rate.
1.6 Miscarriage rate
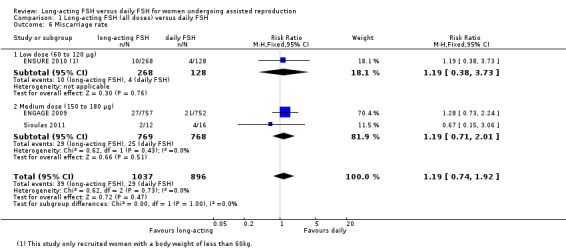
Three studies reported the adverse event of miscarriage rate. There was no evidence of a difference between the groups (RR 1.19, 95% CI 0.74 to 1.92; 1933 participants, three studies; I² statistic = 0%).
1.6.1 Low dose
There was no evidence of a difference between the groups (RR 1.19, 95% CI 0.38 to 3.73; 396 participants, one study).
1.6.2 Medium dose
There was no evidence of a difference between the groups (RR 1.19, 95% CI 0.71 to 2.01; 1537 participants, two studies; I² statistic = 0%).
1.6.3 High dose
No data were available to conduct high dose subgroup analysis.
See Analysis 1.6 for details.
1.6. Analysis.
Comparison 1 Long‐acting FSH (all doses) versus daily FSH, Outcome 6 Miscarriage rate.
1.7 Ectopic pregnancy rate
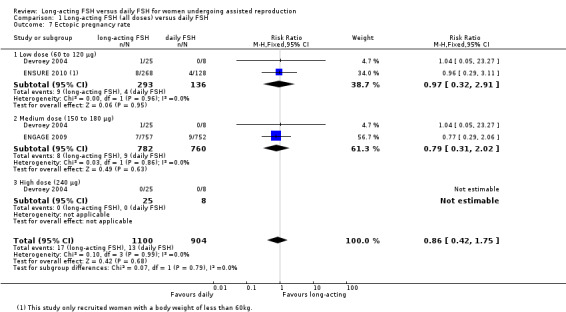
Three studies reported the adverse event ectopic pregnancy rate. There was no evidence of a difference between the groups(RR 0.86, 95% CI 0.42 to 1.75; 2004 participants, three studies; I² statistic = 0%).
1.7.1 Low dose
There was no evidence of a difference between the groups (RR 0.97, 95% CI 0.32 to 2.91; 429 participants, two studies; I² statistic = 0%).
1.7.2 Medium dose
There was no evidence of a difference between the groups (RR 0.79, 95% CI 0.31 to 2.02; 1542 participants, two studies; I² statistic = 0%).
1.7.3 High dose
There were no events in either the intervention group or the control group.
See Analysis 1.7 for details.
1.7. Analysis.
Comparison 1 Long‐acting FSH (all doses) versus daily FSH, Outcome 7 Ectopic pregnancy rate.
1.8 Congenital malformation rate
Three studies reported the adverse event congenital malformation rate. There was no evidence of a difference between the groups. We detected moderate heterogeneity (RR 0.96, 95% CI 0.74 to 1.23; 1173 foetuses, three studies; I² statistic = 44%).
1.8.1 Low dose
There was no evidence of a difference between the groups (RR 0.41, 95% CI 0.16 to 1.07; 135 foetuses, one study; I² statistic = 0%).
1.8.2 Medium dose
There was no evidence of a difference between the groups (RR 1.03, 95% CI 0.79 to 1.33; 1038 foetuses, two studies; I² statistic = 0%).
1.8.3 High dose
We were unable to conduct high dose subgroup analysis as no data were available.
See Analysis 1.8 for details.
1.8. Analysis.
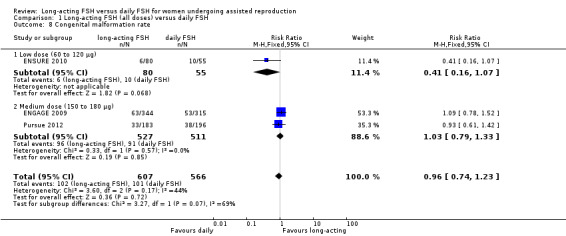
Comparison 1 Long‐acting FSH (all doses) versus daily FSH, Outcome 8 Congenital malformation rate.
1.9 Major congenital malformation rate
Three studies reported the adverse event major congenital malformation rate. There was no evidence of a difference between the groups (RR 0.93, 95% CI 0.55 to 1.57; 1173 foetuses, three studies; I² statistic = 0%).
1.9.1 Low dose
There was no evidence of a difference between the groups (RR 0.69, 95% CI 0.14 to 3.28; 135 foetuses, one study; I² statistic = 0%).
1.9.2 Medium dose
There was no evidence of a difference between the groups and moderate heterogeneity was detected (RR 0.96, 95% CI 0.55 to 1.69; 1038 foetuses, two studies; I² statistic = 32%).
1.9.3 High dose
No data were available to conduct high dose subgroup analysis.
See Analysis 1.9 for details.
1.9. Analysis.
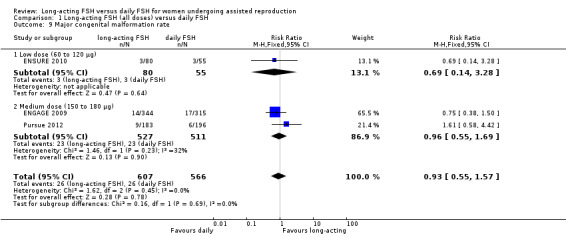
Comparison 1 Long‐acting FSH (all doses) versus daily FSH, Outcome 9 Major congenital malformation rate.
1.10 Minor congenital malformation rate
Three studies reported the adverse event of minor congenital malformation rate. There was no evidence of a difference between the groups and we detected substantial heterogeneity (RR 0.97, 95% CI 0.72 to 1.30; 1173 foetuses, three studies; I² statistic = 62%). We did not perform a sensitivity analysis as the three included studies did not have a high risk of bias.
1.10.1 Low dose
There was no evidence of a difference between the groups (RR 0.29, 95% CI 0.08 to 1.09; 135 foetuses, one study; I² statistic = 0%).
1.10.2 Medium dose
There was no evidence of a difference between the groups and we detected moderate heterogeneity (RR 1.05, 95% CI 0.77 to 1.43; 1038 foetuses, two studies; I² statistic = 46%).
1.10.3 High dose
No data were available to conduct high dose subgroup analysis.
See Analysis 1.10 for details.
1.10. Analysis.
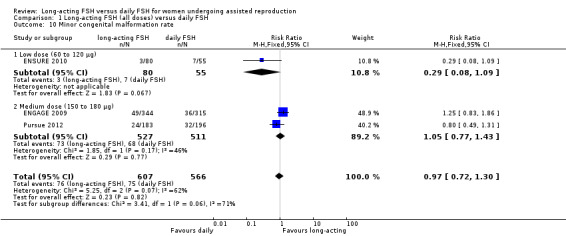
Comparison 1 Long‐acting FSH (all doses) versus daily FSH, Outcome 10 Minor congenital malformation rate.
Discussion
Summary of main results
This Cochrane Review evaluated the effectiveness of long‐acting FSH versus daily FSH on pregnancy and safety outcomes in women undergoing IVF or ICSI treatment cycles. There was evidence of a reduced live birth rate in women who received lower doses (60 to 120 μg) of long‐acting FSH compared to daily FSH but no evidence of difference in effect on live birth rate in women receiving a medium dose (150 to 180 μg) of long‐acting FSH compared to daily FSH. Only one small study used a high dose (240 μg) of long‐acting FSH and at present this is of no clinical value. The meta‐analyses of effectiveness for the outcomes of clinical pregnancy and ongoing pregnancy did not show evidence of a difference of effect between long‐acting and daily FSH. Similarly, there was no evidence of a difference in adverse events for OHSS rate, multiple pregnancy rate, miscarriage rate, ectopic pregnancy rate and congenital malformation (major or minor) between long‐acting FSH and daily FSH. We can conclude that medium dose (150 to 180 μg) long‐acting FSH is a safe treatment option, with no difference in benefits or harm.
See Summary of findings table 1 for a complete overview.
Overall completeness and applicability of evidence
We included all RCTs comparing long‐acting FSH with daily FSH in this Cochrane Review. All six included trials reported on the primary outcomes of live birth rate and OHSS rate. Women at high risk of OHSS (hyper‐responders) were excluded; this provides an explanation for the poor effect of long‐acting FSH on the OHSS rate and the low number of OHSS cases in both treatment groups. Also, all included trials excluded poor responders. For this reason, trials did not provide outcome data on the use of long‐acting FSH in poor responders.
All studies excluded women with an identified cause of subfertility. Therefore, our meta‐analyses were based on women with unexplained subfertility. Our outcomes do not apply to long‐acting FSH in women with a cause of subfertility.
Quality of the evidence
We included six studies with a total of 3753 participants in our meta‐analyses. The number of participants in each study varied between 28 and 1509. We included both two‐arm (one intervention and one control group) and four‐arm (three intervention groups and one control group) studies. All studies reported their outcomes per woman. Only one study performed an ITT analysis but we had sufficient data to perform ITT analyses. We found differences between the inclusion and exclusion criteria as stated in the protocol and those published in the article (see Characteristics of included studies for details). These differences were minor and did not tend to be relevant. The quality of the evidence for each comparison ranged from very low to moderate. The main limitations in the evidence were risk of attrition bias and imprecision.
The included studies differed in the dose of long‐acting FSH. ENGAGE 2009 and ENSURE 2010 used a dose adjusted for participant body weight. ENGAGE 2009 only included women with a body weight above 60 kg and so they used a medium dose of long‐acting FSH. ENSURE 2010 recruited women weighing less than 60 kg and used a low dose of long‐acting FSH for these women. Both Devroey 2004 and Koper 2008 also used a low dose but they included women weighing 50 to 90 kg. This may influence the overall effect in the low dose, long‐acting FSH subgroup in favour of long‐acting FSH.
Only one trial, Devroey 2004, studied a high dose of long‐acting FSH and this treatment subgroup contained only 25 participants. This information is insufficient to make accurate conclusions about the treatment with high dose long‐acting FSH.
No studies reported patient satisfaction for long‐acting FSH versus daily FSH treatment so we are unable to determine if long‐acting FSH treatment is more patient friendly than daily FSH.
Five included trials were sponsored by the same pharmaceutical company and have the same contact author. This may have introduced a bias in favour of long‐acting FSH treatment.
Two studies, Devroey 2004 and Koper 2008, did not blind the participants, personnel and outcome assessors and this caused a high risk of bias. Sioulas 2011 blinded only participants. Two studies did not report all the reasons for withdrawals (Devroey 2004; ENGAGE 2009) and Devroey 2004 did not report six treated participants in their published article, constituting a high risk of bias. We detected some moderate heterogeneity between the studies and subgroups. This can be explained by differences between the inclusion and exclusion criteria for participants, participant characteristics and the small differences between the treatment after the first seven days of COS. The one analysis with substantial heterogeneity did not include studies with a high risk of bias and as a result no sensitivity analysis could be performed.
Potential biases in the review process
We stated in our protocol (Pouwer 2012a) that we would perform different subgroup analyses. Due to insufficient data, we performed only the subgroup analysis for dose of long‐acting FSH. We included a small number of studies and for this reason we did not construct a funnel plot. Therefore, we were unable to estimate the existence of publication or other reporting biases.
Sioulas 2011 published a conference abstract with preliminary results. We wrote to the study authors and received additional data. We included this data in our meta‐analyses, and this may have introduced bias.
The method we adopted to deal with data from four‐arm studies (discussed in detail in Included studies) may have introduced bias.
Two review authors (AWP and CF) extracted all data. AWP compared the extracted data and discussed disagreements and doubts with CF. AWP entered the data into RevMan 2014 and updated the review. These methods may have introduced bias.
Agreements and disagreements with other studies or reviews
Our results are in agreement with all previous reviews (Croxtall 2011; Seyhan 2011; Mahmoud Youssef 2012).
Seyhan 2011 included four RCTs (Devroey 2004; ENGAGE 2009; ENSURE 2010; Koper 2008) and one uncontrolled clinical trial. There was no evidence of a difference between the groups in ongoing pregnancy rate (two RCTs; OR 0.95; 95% CI 0.79 to 1.15) for long‐acting versus daily FSH. Adverse events and OHSS rate seemed to be similar (OR not published).
Mahmoud Youssef 2012 included four RCTs (Devroey 2004; ENGAGE 2009; ENSURE 2010; Koper 2008). There was no evidence of a difference between the groups in ongoing pregnancy rate (two RCTs; OR 0.95, 95% CI 0.79 to 1.15) for long‐acting versus daily FSH. There is evidence of increased number of follicles using long‐acting FSH (four RCTs, weighted mean difference (WMD) 1.99, 95% CI 1.02 to 2.97; P = 0.11). There was no evidence of a difference between the groups in risk of OHSS (four RCTs; OR 1.27, 95% CI 0.72 to 2.22).
Croxtall 2011 included two RCTs (ENGAGE 2009; ENSURE 2010). There was no evidence of a difference between the groups in ongoing pregnancy rate (OR not published). The risk of OHSS in the long‐acting FSH group was 7% versus 6.1% for daily FSH (OR not published).
In large pooled analyses of clinical trials, the incidence of OHSS in both the corifollitropin alfa and rFSH treatment arms was consistent with that expected in the relatively young patient population. Furthermore, there were no clinically relevant differences in pregnancy complications and the incidence of infant adverse events between treatment arms.
Authors' conclusions
Implications for practice.
The use of a medium dose (150 to 180 μg) of long‐acting FSH is a safe treatment option and equally effective compared to daily FSH.
Implications for research.
All current trials excluded poor‐ and hyper‐responders to ovarian stimulation and women with explained subfertility. Therefore, further research is needed to determine if long‐acting FSH can be used in all women with subfertility. There is one ongoing trial about long‐acting FSH in combination with a GnRH agonist treatment (Sioulas 2011). More research is needed to determine the pregnancy and safety outcomes in this treatment combination. There are no studies about patient satisfaction with the treatment and further research should examine this to determine whether the new treatment is more patient friendly than the daily injections regimen.
Feedback
Conversion units for FSH
Summary
Feedback received from Mikael Haggstrom, Physician, Gävle Hospital, Sweden: This is actually the first time I've encountered an article using μg as the unit for FSH rather than IU. When trying to find a conversion factor, the best I found was the following paper: World Health Organization Technical Report Series N0. 565. WHO Expert Committee on Biological Standardization. Twenty‐sixth Report. World Health Organization. Geneva. 1975 URL: http://whqlibdoc.who.int/trs/WHO_TRS_565.pdf According to this paper, 1 μg of FSH would correspond to 0.0088 IU. However, it does not make sense, since the dosages presented in the review article would be extremely small. Does the review article have a different conversion factor between μg and FSH? In such case, is there any explanation why it is different from that given from the WHO paper?
Reply
We thank Dr Haggstrom for his enquiry. We advise that we did not convert FSH doses in the review, as the primary studies expressed the doses in μg.
Contributors
Mikael Haggstrom, Physician, Gävle Hospital, Sweden.
Review authors, Jane Marjoribanks (Cochrane MDSG Feedback Editor).
What's new
| Date | Event | Description |
|---|---|---|
| 18 February 2016 | Amended | Study numbers in abstract corrected, word count reduced in Plain Language Summary |
History
Protocol first published: Issue 1, 2012 Review first published: Issue 6, 2012
| Date | Event | Description |
|---|---|---|
| 16 June 2015 | New search has been performed | We included two new trials (Pursue 2012; Sioulas 2011). We added the outcomes congenital malformation rate, major congenital malformation rate and minor congenital malformation rate. |
| 16 June 2015 | New citation required but conclusions have not changed | The review conclusions have not changed. |
| 20 February 2014 | Feedback has been incorporated | Feedback added re conversion units for FSH |
Acknowledgements
We thank the Cochrane MDSG. In particular we thank Marian Showell (Trials Search Co‐ordinator) for writing and running the search, Vanessa Jordan (NZ Cochrane Fellow), Helen Nagels (Managing Editor of Cochrane MDSG) and Julie Brown (systematic reviewer) for answering our questions.
Appendices
Appendix 1. Glossary
Assisted reproductive technology (ART)
All treatments or procedures that include the in vitro handling of human oocytes and sperm or embryos for the purpose of establishing a pregnancy. This includes, but is not limited to, in vitro fertilization and transcervical embryo transfer, gamete intrafallopian transfer, zygote intrafallopian transfer, tubal embryo transfer, gamete and embryo cryopreservation, oocyte and embryo donation, and gestational surrogacy. ART does not include assisted insemination (artificial insemination) using sperm from either a woman's partner or a sperm donor.
Cancelled cycle
An ART cycle in which ovarian stimulation or monitoring has been carried out with the intent of undergoing ART but which did not proceed to follicular aspiration or, in the case of a thawed embryo, to transfer.
Clinical pregnancy
Evidence of pregnancy by clinical or ultrasound parameters (ultrasound visualization of a gestational sac). It includes ectopic pregnancy. Multiple gestational sacs in one patient are counted as one clinical pregnancy.
Controlled ovarian stimulation (COS)
Medical treatment to induce the development of multiple ovarian follicles to obtain multiple oocytes at follicular aspiration.
Cryopreservation or cryostorage
Freezing and storage of gametes, zygotes or embryos.
Ectopic pregnancy
A pregnancy that occurs outside of the uterus.
Embryo
Product of conception from the time of fertilization to the end of the embryonic stage eight weeks after fertilization (the term pre‐embryo or dividing conceptus, has been replaced by embryo).
Embryo transfer (ET)
Procedure in which embryos are placed in the uterus or fallopian tube.
Fertilisation
The penetration of the ovum by the spermatozoon and fusion of genetic materials resulting in the development of a zygote.
Fetus
The product of conception starting from completion of embryonic development (at eight completed weeks after fertilisation) until birth or abortion.
Follicle
The sac in which an egg develops in the ovary.
Follicle‐stimulating hormone (FSH)
A hormone produced and released from the pituitary gland. In women it stimulates the production of oestrogen and follicles in the ovary ready for ovulation. In men it stimulates the production of sperm.
Gestational age
Age of an embryo or foetus calculated by adding 14 days (two weeks) to the number of completed weeks since fertilisation.
Gestational sac
A fluid‐filled structure containing an embryo that develops early in pregnancy usually within the uterus.
Gonadotrophin releasing hormone (GnRH)
A substance produced by the hypothalamus (part of the brain) to enable the pituitary gland to secrete LH and FSH.
Gonadotropins
Pituitary hormones FSH and LH which stimulate the testes and ovaries.
Human chorionic gonadotrophin (hCG)
A hormone produced by placental tissue that can be measured in the blood and urine of pregnant women.
Hyper‐responder
A women who produces a large number of oocytes (women with PCOS (see polycystic ovary syndrome) or a history of OHSS (see ovarian hyperstimulation syndrome)).
Implantation
The attachment and subsequent penetration by the zona‐free blastocyst (usually in the endometrium) which starts five to seven days following fertilization.
Intracytoplasmic sperm injection (ICSI)
When an egg is surgically removed from a woman and injected with a single spermatozoon is injected through the zona pellucida into the oocyte. If fertilisation is successful the embryo is placed into the woman's uterus. This technique is used when a male partner has a low sperm count or other sperm‐related problem.
Intrauterine
Inside the uterus.
In vitro fertilization (IVF)
An ART procedure which involves extracorporeal fertilization.
Live birth
A birth in which a fetus is delivered with signs of life after complete expulsion or extraction from its mother, beyond 20 completed weeks of gestational age. Live births are counted as birth events (e.g. a twin or triplet live birth is counted as one birth event).
Luteinising hormone (LH)
A hormone produced and released by the pituitary gland. In women it is responsible for ovulation and progesterone production. In men it stimulates the production of testosterone and is involved with the production of sperm cells.
Miscarriage
Spontaneous end of a pregnancy at prior to 20 weeks of gestation.
Oocyte
The egg from a woman's ovary.
Ova
A woman’s reproductive cell, also known as egg or oocyte.
Ovarian hyperstimulation syndrome (OHSS)
A condition that occurs from fertility drugs when a large number of follicles in the ovary are stimulated to develop and ovulate. This stimulation causes an enlargement of the ovaries.
Ovulation
The release of an egg/ova from an ovarian follicle.
Ovulation induction
Medical treatment to produce ovulation.
Ovulatory hCG (human chorionic gonadotrophin)
Hormone given to trigger ovulation in assisted reproduction.
Polycystic ovary syndrome (PCOS)
When a woman has enlarged ovaries with multiple cysts and the surface of the ovary is thickened. The woman may ovulate infrequently or not at all.
Poor responder
A women who require large doses of medication to stimulate the ovary but produce less than an optimal number of oocytes.
Premature LH‐surge
In a normal menstrual cycle an increase in LH‐levels (LH‐surge) is needed to start ovulation. In IVF/ICSI cycles it is important that the ovulation does not start before the oocytes are mature enough to be retrieved. A LH‐surge that occurs too early is called premature and is an unwanted event in IVF/ICSI cycles.
Recombinant (as in recombinant FSH)
Is a naturally occurring hormone which has been made in the laboratory with the use of DNA technology. Recombinant technology examines the DNA sequence of a hormone. The sequence is then placed inside certain bacteria (bacterial factories), which produce a protein from the DNA sequence. This protein is then taken from the bacteria and packaged as a hormone.
Semen
A thick white fluid produced in the reproductive organs of men that usually contains the sperm cells produced in the testicles.
Spermatozoa/sperm
Male reproductive cells found in semen.
Subfertility
Failure to achieve pregnancy after at least one year of unprotected coitus.
Subcutaneous
Under the skin.
Ultrasound
Radiology sounds waves of a high frequency used to examine the inside of the body. Ultrasound is also used to visualise the developing foetus in the uterus to check size, growth and the presence of abnormalities.
We obtained most definitions from the MDSG module 2008 glossary.
Appendix 2. Cochrane MDSG search strategy
Date of search: 10 October 2011 Search update: 08 June 2015
Keywords CONTAINS "IVF"or"ICSI" or "subfertility" or "in vitro fertilisation" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "assisted conception" or "assisted reproduction" or "ART" or "infertility" or "IUI" or "Intrauterine Insemination" or "artificial insemination" or "ovarian hyperstimulation" or "ovarian stimulation" or "ovulation induction"or"COH" or "controlled ovarian " or "insemination" or "insemination‐intrauterine" or Title CONTAINS "IVF"or"ICSI" or "subfertility" or "in vitro fertilisation" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "assisted conception" or "assisted reproduction" or "ART" or "infertility" or "IUI" or "Intrauterine Insemination" or "artificial insemination" or "ovarian hyperstimulation" or "ovarian stimulation" or "ovulation induction"or"COH" or "controlled ovarian " or "insemination" or "insemination‐intrauterine"
AND
Keywords CONTAINS "corifollitropin alfa" or Title CONTAINS "corifollitropin alfa"
Appendix 3. CENTRAL search strategy
EBM Reviews ‐ Cochrane Central Register of Controlled Trials (CENTRAL) Date of search: 10 October 2011 Search update: 08 June 2015
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (1533) 2 embryo transfer$.tw. (853) 3 in vitro fertili?ation.tw. (1282) 4 ivf‐et.tw. (244) 5 (ivf or et).tw. (5804) 6 icsi.tw. (626) 7 intracytoplasmic sperm injection$.tw. (389) 8 (blastocyst adj2 transfer$).tw. (63) 9 exp reproductive techniques, assisted/ or exp insemination, artificial/ or exp ovulation induction/ (2133) 10 assisted reproduct$.tw. (373) 11 artificial insemination.tw. (53) 12 iui.tw. (270) 13 intrauterine insemination$.tw. (373) 14 ovulation induc$.tw. (414) 15 ovarian hyperstimulation.tw. (524) 16 COH.tw. (117) 17 (ovari$ adj2 stimulat$).tw. (702) 18 superovulat$.tw. (127) 19 infertil$.tw. (1688) 20 subfertil$.tw. (127) 21 (ovari$ adj2 induction).tw. (25) 22 or/1‐21 (8905) 23 corifollitropin alfa.tw. (12) 24 corifollitropin alpha.tw. (1) 25 org 36286.tw. (2) 26 org36286.tw. (0) 27 FSH carboxy terminal peptide.tw. (1) 28 FSH‐CTP.tw. (5) 29 FSH CTP.tw. (5) 30 long acting follitropin.tw. (0) 31 Elonva$.tw. (0) 32 sustained follicle stimulat$.tw. (0) 33 long acting fsh.tw. (3) 34 long acting follicle stimulating hormone.tw. (0) 35 or/23‐34 (14) 36 22 and 35 (7)
Appendix 4. MEDLINE search strategy
Ovid MEDLINE®In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE®Daily and Ovid MEDLINE (1948 to present) Date of search: 10 October 2011 Search update: 08 June 2015
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (29168) 2 embryo transfer$.tw. (7138) 3 in vitro fertili?ation.tw. (14924) 4 ivf‐et.tw. (1669) 5 (ivf or et).tw. (154947) 6 icsi.tw. (4487) 7 intracytoplasmic sperm injection$.tw. (4173) 8 (blastocyst adj2 transfer$).tw. (424) 9 exp reproductive techniques, assisted/ or exp insemination, artificial/ or exp ovulation induction/ (47003) 10 assisted reproduct$.tw. (7212) 11 artificial insemination.tw. (4355) 12 iui.tw. (1012) 13 intrauterine insemination$.tw. (1571) 14 ovulation induc$.tw. (3109) 15 ovarian hyperstimulation.tw. (3292) 16 COH.tw. (894) 17 (ovari$ adj2 stimulat$).tw. (4178) 18 superovulat$.tw. (2718) 19 infertil$.tw. (36915) 20 subfertil$.tw. (2981) 21 (ovari$ adj2 induction).tw. (200) 22 or/1‐21 (227905) 23 corifollitropin alfa.tw. (22) 24 corifollitropin alpha.tw. (1) 25 org 36286.tw. (4) 26 org36286.tw. (0) 27 FSH carboxy terminal peptide.tw. (2) 28 FSH‐CTP.tw. (7) 29 FSH CTP.tw. (7) 30 long acting follitropin.tw. (1) 31 Elonva$.tw. (3) 32 sustained follicle stimulat$.tw. (5) 33 long acting fsh.tw. (13) 34 long acting follicle stimulating hormone.tw. (5) 35 or/23‐34 (40) 36 22 and 35 (28) 37 randomized controlled trial.pt. (319965) 38 controlled clinical trial.pt. (83743) 39 randomized.ab. (234955) 40 placebo.tw. (137303) 41 clinical trials as topic.sh. (158838) 42 randomly.ab. (172143) 43 trial.ti. (100880) 44 (crossover or cross‐over or cross over).tw. (52403) 45 or/37‐44 (783234) 46 exp animals/ not humans.sh. (3701210) 47 45 not 46 (723312) 48 36 and 47 (11)
Appendix 5. EMBASE search strategy
Embase.com (1980 to current) Date of search: 10 October 2011 Search update: 08 June 2015
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (42896) 2 embryo$ transfer$.tw. (9711) 3 in vitro fertili?ation.tw. (17136) 4 ivf‐et.tw. (2023) 5 icsi.tw. (6900) 6 intracytoplasmic sperm injection$.tw. (5013) 7 (blastocyst adj2 transfer$).tw. (667) 8 (ivf or et).tw. (299448) 9 exp infertility therapy/ or exp artificial insemination/ or exp intrauterine insemination/ or exp ovulation induction/ (63983) 10 artificial insemination.tw. (4135) 11 intrauterine insemination.tw. (1904) 12 assisted reproduct$.tw. (9674) 13 iui.tw. (1423) 14 ovulation induc$.tw. (3738) 15 (ovari$ adj2 stimulat$).tw. (5358) 16 ovarian hyperstimulation.tw. (4173) 17 COH.tw. (1095) 18 superovulat$.tw. (2637) 19 infertil$.tw. (43284) 20 subfertil$.tw. (3494) 21 (ovari$ adj2 induction).tw. (236) 22 or/1‐21 (385118) 23 exp corifollitropin alfa/ (35) 24 corifollitropin alfa.tw. (46) 25 corifollitropin alpha.tw. (5) 26 FSH carboxy terminal peptide.tw. (1) 27 FSH‐CTP.tw. (12) 28 FSH CTP.tw. (12) 29 long acting follitropin.tw. (1) 30 Elonva$.tw. (23) 31 sustained follicle stimulat$.tw. (9) 32 long acting fsh.tw. (16) 33 long acting follicle stimulating hormone.tw. (5) 34 org 36286.tw. (16) 35 org36286.tw. (0) 36 or/23‐35 (82) 37 Clinical Trial/ (819367) 38 Randomized Controlled Trial/ (290224) 39 exp randomization/ (54690) 40 Single Blind Procedure/ (14260) 41 Double Blind Procedure/ (100996) 42 Crossover Procedure/ (30907) 43 Placebo/ (185441) 44 Randomi?ed controlled trial$.tw. (64940) 45 Rct.tw. (7766) 46 random allocation.tw. (1056) 47 randomly allocated.tw. (15613) 48 allocated randomly.tw. (1708) 49 (allocated adj2 random).tw. (688) 50 Single blind$.tw. (11114) 51 Double blind$.tw. (118240) 52 ((treble or triple) adj blind$).tw. (247) 53 placebo$.tw. (159940) 54 prospective study/ (173744) 55 or/37‐54 (1147610) 56 case study/ (13429) 57 case report.tw. (208273) 58 abstract report/ or letter/ (795123) 59 or/56‐58 (1012785) 60 55 not 59 (1114252) 61 22 and 36 and 60 (35) 62 (2010$ or 2011$).em. (2228857) 63 61 and 62 (24)
Appendix 6. PsycINFO search strategy
PsycINFO (1980 to current) Date of search: 10 October 2011 Search update: 08 June 2015
1 exp reproductive technology/ (1093) 2 in vitro fertili?ation.tw. (433) 3 ivf‐et.tw. (16) 4 (ivf or et).tw. (78691) 5 icsi.tw. (37) 6 intracytoplasmic sperm injection$.tw. (30) 7 (blastocyst adj2 transfer$).tw. (2) 8 assisted reproduct$.tw. (379) 9 artificial insemination.tw. (202) 10 iui.tw. (17) 11 intrauterine insemination$.tw. (12) 12 ovulation induc$.tw. (13) 13 (ovari$ adj2 stimulat$).tw. (42) 14 superovulat$.tw. (5) 15 ovarian hyperstimulation.tw. (8) 16 COH.tw. (44) 17 infertil$.tw. (2154) 18 subfertil$.tw. (50) 19 (ovari$ adj2 induction).tw. (4) 20 or/1‐19 (81563) 21 corifollitropin alfa.tw. (0) 22 corifollitropin alpha.tw. (0) 23 org 36286.tw. (0) 24 org36286.tw. (0) 25 FSH carboxy terminal peptide.tw. (0) 26 FSH‐CTP.tw. (0) 27 FSH CTP.tw. (0) 28 long acting follitropin.tw. (0) 29 Elonva$.tw. (0) 30 sustained follicle stimulat$.tw. (0) 31 long acting fsh.tw. (0) 32 long acting follicle stimulating hormone.tw. (0) 33 exp Follicle Stimulating Hormone/ (71) 34 Follicle Stimulating Hormone$.tw. (406) 35 FSH.tw. (329) 36 rFSH.tw. (0) 37 or/21‐36 (520) 38 20 and 37 (27) 39 random.tw. (33464) 40 control.tw. (261118) 41 double‐blind.tw. (15184) 42 clinical trials/ (5432) 43 placebo/ (2981) 44 exp Treatment/ (495944) 45 or/39‐44 (748851) 46 38 and 45 (12)
Appendix 7. CINAHL search strategy
| Date of search: 10 October 2011 Search update: 08 June 2015 | ||
| # | Results | Query |
| S30 | 24 | S17 and S29 |
| S29 | 1036 | S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 |
| S28 | 0 | "ovarial hyperstimulation" |
| S27 | 216 | "ovarian hyperstimulation" |
| S26 | 17 | "controlled ovarian stimulation" |
| S25 | 103 | "COS" |
| S24 | 31 | "COH" |
| S23 | 298 | "assisted reproduction" |
| S22 | 9 | "assisted reproductive technique" |
| S21 | 157 | "ICSI" |
| S20 | 8 | "Intracytoplasmatic sperm injection" |
| S19 | 20 | (MH "Fertilization in Vitro") |
| S18 | 328 | (MH "Embryo Transfer") |
| S17 | 68 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 |
| S16 | 12 | "recombinant FSH" |
| S15 | 7 | "rFSH" |
| S14 | 53 | (MH "Follicle‐Stimulating Hormone") |
| S13 | 0 | "long‐acting follicle stimulating hormone" |
| S12 | 0 | "long acting follicle stimulating hormone" |
| S11 | 0 | "FSH carboxy terminal peptide" |
| S10 | 0 | "long acting FSH" |
| S9 | 0 | "long‐acting FSH" |
| S8 | 0 | "FSH‐CTP" |
| S7 | 0 | "FSH CTP" |
| S6 | 0 | "ORG36386" |
| S5 | 0 | "ORG 36286" |
| S4 | 2 | "Corifollitropin" |
| S3 | 0 | "Elonva" |
| S2 | 0 | "Corifollitropin alpha" |
| S1 | 2 | "Corifollitropin alfa" |
Appendix 8. Web of Science search strategy
| Date of search: 10 October 2011 Search update: 08 June 2015 | ||
| Set | Results | Search |
| #6 | 15 | #4 AND #3 Refined by: Document Type=( MEETING ) Timespan=All Years |
| #5 | 96 | #4 AND #3 Timespan=All Years |
| #4 | 798 | #2 AND #1 Timespan=All Years |
| #3 | 232,390 | Topic=(embryo transfer) OR Topic=(in vitro fertilisation) OR Topic=(in vitro fertilization) OR Topic=(ivf) OR Topic=(icsi) OR Topic=(intracytoplasmatic sperm injections) OR Topic=(artificial insemination) OR Topic=(intrauterine insemination) OR Topic=(ovulation induction) OR Topic=(COS) OR Topic=(COH) OR Topic=(hyperstimulation) OR Topic=(assisted reproduction) OR Topic=(assited reproduction technique) Timespan=All Years |
| #2 | 60,600 | Topic=(rFSH) OR Topic=(recombinant FSH) OR Topic=(recombinant follicle stimulating hormone) OR Topic=(FSH) Timespan=All Years |
| #1 | 674 | Topic=(Corifollitropin) OR Topic=(Corifollitropin alfa) OR Topic=(Corifollitropin alpha) OR Topic=(Elonva) OR Topic=(ORG36286) OR Topic=(ORG 36286) OR Topic=(FSH‐CTP) OR Topic=(FSH CTP) OR Topic=(long‐acting FSH) OR Topic=(long acting FSH) OR Topic=(FSH carboxy terminal peptide) OR Topic=(long acting follicle stimulating hormone) OR Topic=(long‐acting follicle stimulating hormone) Timespan=All Years |
Data and analyses
Comparison 1. Long‐acting FSH (all doses) versus daily FSH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 5 | 2363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| 1.1 Low dose (60 to 120 μg) | 3 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.93] |
| 1.2 Medium dose (150 to 180 μg) | 4 | 1685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.18] |
| 1.3 High dose (240 μg) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.52] |
| 2 OHSS | 6 | 3753 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.74, 1.37] |
| 2.1 Low dose (60 to 120 μg) | 3 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.56, 2.66] |
| 2.2 Medium dose (150 to 180 μg) | 5 | 3075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.68, 1.35] |
| 2.3 High dose (240 μg) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.09, 32.75] |
| 3 Ongoing pregnancy rate | 6 | 3753 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.04] |
| 3.1 Low dose (60 to 120 μg) | 3 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.04] |
| 3.2 Medium dose (150 to 180 μg) | 5 | 3075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.88, 1.09] |
| 3.3 High dose (240 μg) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.18, 1.28] |
| 4 Clinical pregnancy rate | 5 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.03] |
| 4.1 Low dose (60 to 120 μg) | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.04] |
| 4.2 Medium dose (150 to 180 μg) | 4 | 3038 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.07] |
| 5 Multiple pregnancy rate | 5 | 2363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.89, 1.54] |
| 5.1 Low dose (60 to 120 μg) | 3 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.52, 2.00] |
| 5.2 Medium dose (150 to 180 μg) | 4 | 1685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.90, 1.63] |
| 5.3 High dose (240 μg) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.05, 23.27] |
| 6 Miscarriage rate | 3 | 1933 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.74, 1.92] |
| 6.1 Low dose (60 to 120 μg) | 1 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.38, 3.73] |
| 6.2 Medium dose (150 to 180 μg) | 2 | 1537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.71, 2.01] |
| 7 Ectopic pregnancy rate | 3 | 2004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.42, 1.75] |
| 7.1 Low dose (60 to 120 μg) | 2 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.32, 2.91] |
| 7.2 Medium dose (150 to 180 μg) | 2 | 1542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.31, 2.02] |
| 7.3 High dose (240 μg) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Congenital malformation rate | 3 | 1173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.23] |
| 8.1 Low dose (60 to 120 μg) | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.16, 1.07] |
| 8.2 Medium dose (150 to 180 μg) | 2 | 1038 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.33] |
| 9 Major congenital malformation rate | 3 | 1173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.55, 1.57] |
| 9.1 Low dose (60 to 120 μg) | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.14, 3.28] |
| 9.2 Medium dose (150 to 180 μg) | 2 | 1038 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.55, 1.69] |
| 10 Minor congenital malformation rate | 3 | 1173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.30] |
| 10.1 Low dose (60 to 120 μg) | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.09] |
| 10.2 Medium dose (150 to 180 μg) | 2 | 1038 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.77, 1.43] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Devroey 2004.
| Methods | RCT, open‐label four‐arm trial Academic multi‐centre trial; Belgium and The Netherlands Timing: July 2001 to October 2002 (15 months) Ethical approval and informed consent obtained Power calculation carried out (dose of FSH‐CTP) No ITT analysis performed |
|
| Participants | Number of participants as stated in their protocol (unpublished data): 104 (6 subjects treated during stage I part of this trial) Number of participants as stated in published article: 99 (75 intervention, 24 control treated during phase II part of the trial) All treated subjects (104) during the trial (phase I and II) are analysed in the follow‐up study. We decided to analyse 105 subjects (99 subjects analysed in the published paper and 6 subjects treated stated in the protocol) Inclusion criteria as stated in the article Women between 18 and 39 years of age and a regular menstrual cycle (24 to 35 days) and normal body weight (BMI 18 to 29 kg/m²) Inclusion criteria as stated in the protocol Women of couples with an indication for COH before IVF or ICSI, between 18 and 39 years, regular menstrual cycle (24 to 35 days), BMI 18 to 29 kg/m², couples have availability of ejaculatory sperm, willing and able to sign informed consent Exclusion criteria as stated in the article Not reported Exclusion criteria as stated in the protocol History of/or current endocrine abnormality such as PCOS, or polycystic ovaries according to USS, (treated) hyper‐prolactinemia or evidence of ovarian dysfunction, > 3 unsuccessful COH cycles for IVF since last established ongoing pregnancy, history of non‐ or low ovarian response to FSH/hMG treatment, any clinically relevant hormone value outside the reference range during the early follicular phase as measured by the local laboratory (FSH, LH, E2, P, total T, TSH and prolactin), any clinically relevant abnormal laboratory value, any ovarian and/or abdominal abnormality interfering with ultrasound examination, contraindications for the use of gonadotropins, epilepsy, diabetes, cardiovascular, gastro‐intestinal, hepatic, renal, pulmonary, or abdominal disease, history of alcohol or drug abuse within 12 months prior to signing informed consent, hypersensitivity to Orgalutran® or any of its compounds, administration of investigational drugs within three months prior to screening, use of hormonal preparations within one month prior to the start of Org 36286 with the exception of thyroid medication Mean age (years) and SD
Mean weight (kg) and SD: not reported Mean BMI (kg/m²) and SD
Mean duration of subfertility (years) and SD
Withdrawals Intervention Total 16% of participants in intervention groups withdrawn before embryo transfer 120 μg: two subjects who received hCG did not continue with oocyte pick‐up, because absence of sperm and too few pre‐ovulatory follicles 180 μg: one randomised subject dropped‐out before the treatment started, no reason reported. One subject did not received hCG because an excessive response. Before oocyte pick‐up one subject discontinued because too few preovulatory follicles 240 μg: two subjects did not received hCG because an excessive response or a too‐low response Six subjects in the intervention groups who had oocyte retrieval did not proceed with embryo transfer because of fertilisation failure or the recovery of too few or no embryos. Control Total 4.2% of participants in control group withdrawn before embryo transfer One subject in the control group did not received hCG because a too‐low response |
|
| Interventions | Intervention: 120 μg, 180 μg or 240 μg long‐acting FSH Control: 150 IU daily FSH GnRH antagonist was administered sc starting on the day the leading follicle reached 14 mm, until at least 3 follicles ≥ 17 mm No more than 3 embryos were transferred |
|
| Outcomes |
Primary Live birth rate OHSS Secondary Ongoing pregnancy rate Multiple pregnancy rate Adverse events: ectopic pregnancy |
|
| Funding | Funded by NV Organon (now Shering‐Plough) Possible conflicts of interest not stated |
|
| Notes | The study authors reported in their protocol that six subjects were treated with long‐acting FSH during the phase I stage of this trial. Two subjects were treated with 120 μg and four subjects were treated with 180 μg of long‐acting FSH. These six subjects were not reported in their published article. We decided to analyse all participants of this trial instead of the 99 as reported in their article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | "Randomised". No reference to allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reason for one withdrawal not reported, other numbers and reasons reported, for details see Characteristics of included studies. |
| Selective reporting (reporting bias) | Low risk | All planned protocol outcomes reported. |
| Other bias | Unclear risk | Funded by pharmaceutical company |
ENGAGE 2009.
| Methods | RCT, double‐blind two‐arm trial Multicentre trial; 14 centres in North America (USA, Canada), 20 centres in Europe (Spain, UK, Belgium, Czech Republic, Finland, France, Norway, Sweden, Denmark, The Netherlands) Timing June 2006 to January 2008 (20 months) Ethical approval and informed consent obtained Power calculation carried out (total number of participants) No ITT analysis performed |
|
| Participants | Number of participants: 1509 (757 intervention, 752 control) Inclusion criteria as stated in the article Women aged 18 to 36 years with a body weight > 60 kg and ≤ 90 kg, a BMI 18 to 32 kg/m², a menstrual cycle 24 to 35 days, access to ejaculatory sperm and an indication for COS before IVF or ICSI Inclusion criteria as stated in the protocol Women of a couple with an indication for COS before IVF or ICSI, between 18 and 36 years with a regular menstrual cycle (24 to 35 days), body weight > 60 kg an ≤ 90 kg, BMI 18 to 32 kg/m², couples have availability of ejaculatory sperm (donated or cryopreserved sperm, or both, is allowed), willing and able to sign informed consent Exclusion criteria as stated in the article Patients who had a (history of) an endocrine abnormality, an abnormal outcome of blood biochemistry or hematology, an abnormal cervical smear, a chronic disease, relevant ovarian, tubal or uterine pathology that could interfere with the COS treatment (e.g. endometrioma > 0 mm or fibroids ≥ 5 cm), embryo implantation or pregnancy were excluded. Patients who had a history of ovarian hyperresponse (more than 30 follicles ≥ 11 mm) or OHSS, PCOS or a basal antral follicle count of more than 20 on ultrasound (< 11 mm, both ovaries combined). Other exclusion criteria included a previously low ovarian response to FSH or hMG treatment (i.e. cycle cancelled due to insufficient ovarian response or less than four oocytes obtained), an FSH or LH over 12 IU/L in the early follicular phase, more than three consecutive unsuccessful IVF cycles since the last ongoing pregnancy, a history of recurrent miscarriage (three or more), or currently smoking more than five cigarettes per day Exclusion criteria as stated in the protocol History of/or any current (treated) endocrine abnormality, history of ovarian hyper‐response or OHSS, history of/or current PCOS, more than 20 basal antral follicles < 11 mm (both ovaries combined) as measured on USS in the early follicular phase (menstrual cycle day 2 to 5), < 2 ovaries or any other ovarian abnormality (including endometrioma > 10 mm; visible on USS), presence of unilateral or bilateral hydrosalphinx (visible on USS), presence of any clinically relevant pathology affecting the uterine cavity or fibroids ≥ 5 cm, more than three unsuccessful IVF cycles since the last established ongoing pregnancy (if applicable), history of non‐ or low ovarian response to FSH/hMG treatment, history of recurrent miscarriage (3 or more, even when unexplained), FSH > 12 IU/L or LH > 12 IU/L as measured by the local laboratory (sample taken during the early follicular phase: menstrual cycle day 2 to 5), any clinically relevant abnormal laboratory value based on a sample taken during the screening phase, contraindications for the use of gonadotropins (e.g. tumours, pregnancy/lactation, undiagnosed vaginal bleeding, hypersensitivity, ovarian cysts), recent history of/or current epilepsy, HIV infection, diabetes, cardiovascular, gastro‐intestinal, hepatic, renal or pulmonary disease, abnormal karyotyping of the patient or her partner (if karyotyping is performed), smoking more than 5 cigarettes per day, history or presence of alcohol or drug abuse within 12 months prior to signing informed consent, previous use of Org 36286, use of hormonal preparations within 1 month prior to randomisation, hypersensitivity to any of the concomitant medication prescribed as part of the treatment regimen in this protocol, administration of investigational drugs within three months prior to signing informed consent Mean age (years) and SD
Mean weight (kg) and SD
Mean BMI (kg/m²) and SD
Mean duration of subfertility (years) and SD
Withdrawals Total intervention group: 11.2% withdrew before embryo transfer Total control group: 6.4% withdrew before embryo transfer A total of 187 patients failed screening or dropped out due to personal reasons prior to treatment allocation. Three patients (one in the intervention and two in the comparison group) were discontinued prior to the start of treatment (one for personal reasons and two were found to violate entry criteria after randomisation but before commencing treatment). During the treatment, but before the embryo transfer, a total of 130 patients (84 interventional, 46 control) discontinued the treatment, with no reason reported. |
|
| Interventions | Intervention: 150 μg FSH‐CTP Control: 200 IU daily FSH GnRH antagonist was administered sc starting on day 5 up to and including the day of hCG (at least 3 follicles ≥ 17 mm) One or two embryos were transferred Drugs provided by patient itself, partner or medical staff |
|
| Outcomes |
Primary Live birth rate OHSS Secondary Clinical (vital) pregnancy rate Ongoing pregnancy rate Multiple pregnancy rate Miscarriage rate Adverse event: ectopic pregnancy rate and congenital malformation (major and minor) rate |
|
| Funding | Funded by Schering‐Plough Conflicts of interest; fees and grants received from: Organon, Schering‐Plough, Ferring, Bessins, Serono, Merck Serono, IBSA, Wyeth, Schering, Ardana, Andromed, Pantrhei bioscience, Preglem |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation to one of the two arms (1:1 ratio) was done per centre and stratified by age (<32 or ≥ 32 years) by using randomly permutated blocks with a 'undisclosed' fixed block size of four". |
| Allocation concealment (selection bias) | Low risk | "central remote allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded (subject, investigator). "The double‐dummy approach guaranteed the blinding of medication during the trial and prevented any bias in terms of treatment decisions". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High unexplained drop out rate, for details see Characteristics of included studies. |
| Selective reporting (reporting bias) | Low risk | All planned protocol outcomes reported. |
| Other bias | Unclear risk | Pharmaceutical funding |
ENSURE 2010.
| Methods | RCT, double‐blind two‐arm trial Multicentre trial; 14 centres in Europe (Austria, Czech republic, France, Spain, Poland, Sweden, Denmark) and 5 centres in Asia (Korea, Taiwan) Timing: January 2007 to December 2007 (12 months) Ethical approval and informed consent obtained Power calculation carried out (total no. participants) ITT analysis performed |
|
| Participants | Number of participants: 396 (268 intervention, 128 control) Inclusion criteria as stated in the article Women aged 18 to 36 years, weighing ≤ 60 kg and BMI 18 to 32 kg/m², normal menstrual cycle (25 to 34 days), have an indication for ovarian stimulation before IVF or ICSI, access to ejaculatory spermatozoa Inclusion criteria as stated in the protocol Women aged 18 to 36 years, weighing ≤ 60 kg and BMI 18 to 32 kg/m², normal menstrual cycle (25 to 34 days) and have an indication for IVF or ICSI, couples have availability of ejaculatory sperm (donated or cryopreserved sperm, or both, allowed), willing and able to sign informed consent Exlusion criteria as stated in the article Same as those reported in Devroey 2009; History of ovarian hyperresponse to ovarian stimulation (more than 30 follicles > 11 mm) or OHSS, PCOS or more than 20 basal antral follicles on ultrasound (< 11 mm, both ovaries combined), history of no or low ovarian response (i.e. cycle cancelled due to insufficient response of less than four oocytes obtained) or more than three unsuccessful ovarian stimulation cycles since the last established ongoing pregnancy Exclusion criteria as stated in the protocol History of/or any current (treated) endocrine abnormality, history of ovarian hyper‐response or OHSS, history of/or current PCOS, more than 20 basal antral follicles < 11 mm (both ovaries combined) as measured on USS in the early follicular phase (menstrual cycle day 2 to 5), < 2 ovaries or any other ovarian abnormality (including endometrioma > 10 mm; visible on USS), presence of unilateral or bilateral hydrosalphinx (visible on USS), presence of any clinically relevant pathology affecting the uterine cavity or fibroids ≥ 5 cm, more than three unsuccessful IVF cycles since the last established ongoing pregnancy (if applicable), history of non‐ or low ovarian response to FSH/hMG treatment, history of recurrent miscarriage (3 or more, even when unexplained), FSH > 12 IU/L or LH > 12 IU/L as measured by the local laboratory (sample taken during the early follicular phase: menstrual cycle day 2 to 5), any clinically relevant abnormal laboratory value based on a sample taken during the screening phase, contraindications for the use of gonadotropins (e.g. tumours, pregnancy/lactation, undiagnosed vaginal bleeding, hypersensitivity, ovarian cysts), recent history of/or current epilepsy, HIV infection, diabetes, cardiovascular, gastro‐intestinal, hepatic, renal or pulmonary disease, abnormal karyotyping of the patient or her partner (if karyotyping is performed), smoking more than 5 cigarettes per day, history or presence of alcohol or drug abuse within 12 months prior to signing informed consent, previous use of Org 36286, use of hormonal preparations within 1 month prior to randomisation, hypersensitivity to any of the concomitant medication prescribed as part of the treatment regimen in this protocol, administration of investigational drugs within three months prior to signing informed consent. Mean age (years) and SD
Mean weight (kg) and SD
Mean BMI (kg/m²) and SD
Mean duration of subfertility (years) and SD
Withdrawals Intervention Total 8.2% of participants in intervention group All the randomised patients started stimulation, two subjects cancelled the treatment before hCG because of insufficient ovarian response and a patients decision. All hCG treated patients underwent oocyte retrieval, twenty subjects cancelled before embryo transfer, one because the risk of OHSS, one suspicious for tuberculosis, too high ovarian response (5 subjects), no/too few/bad quality oocytes retrieved (2 subjects), no or abnormal fertilisation (4 subjects), no/too few/bad quality embryos for transfer (7 subjects) Control Total 6.3% of participants in control group All the randomised subjects started stimulation, one cancelled before the hCG treatment because too high ovarian response. All hCG treated patients underwent oocyte retrieval, a total of seven subjects discontinued before embryo transfer. The reasons are: no/too few/bad oocytes retrieved (2 subjects), one subject because of too high ovarian response, in one subject no fertilisation was possible, one had no/too few/bad quality embryos, no or abnormal fertilisation (2 subjects) |
|
| Interventions | Intervention: 100 μg long‐acting FSH Control: 150 IU daily FSH GnRH antagonist was administered sc starting on day 5 up to and including the day of hCG (at least 3 follicles ≥ 17 mm) One ore two embryos were transferred |
|
| Outcomes |
Primary Live birth rate Cumulative live birth rate OHSS Secondary Clinical pregnancy rate Ongoing pregnancy rate Multiple pregnancy rate Miscarriage rate Congenital malformation (major and minor) rate |
|
| Funding | Funded by Schering‐Plough Possible conflicts of interest not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation to one of the two treatment groups in a 2:1 ratio (investigational:reference group) was performed at each centre and stratified by age (< 32 or ≥ 32 years) and planned fertilisation procedure (IVF or ICSI) by central remote allocation using randomly permutated blocks with an 'undisclosed' fixed block size of three". |
| Allocation concealment (selection bias) | Low risk | "Randomisation by central remote allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. "To conceal the allocation all patients also started daily sc injection of daily FSH or placebo on the same day, daily active or placebo daily FSH injections were continued through the first seven days of stimulation". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported, for details see Characteristics of included studies. |
| Selective reporting (reporting bias) | Low risk | All planned protocol outcomes reported. |
| Other bias | Unclear risk | Funded by a pharmaceutical company. |
Koper 2008.
| Methods | RCT, open‐label four‐arm trial Multicentre trial; 14 centres in Europe Timing: May 2003 to May 2004 (12 months) Ethical approval and informed consent obtained Power calculation carried out (no. participants per group) No ITT analyses performed |
|
| Participants | Number of participants: 325 (242 intervention, 83 control) Inclusion criteria as stated in the article Women aged 20 to 39 years with a normal menstrual cycle (24 to 35 days) and a BMI 17 to 31 kg/m² with an indication for COS before IVF or ICSI Inclusion criteria as stated in the protocol Women of couples with an indication for COH and IVF or ICSI, aged 18 to 39 years, normal menstrual cycle (24 to 35 days), BMI 17 to 31 kg/m², with an indication for IVF or ICSI, couples have availability of ejaculatory sperm (donated or frozen sperm is allowed), able and willing to sign informed consent Exclusion criteria as stated in the article Women with a history of OHSS, PCOS, any endocrine abnormality, previous poor response to FSH or hCG, more than three unsuccessful COS cycles since last ongoing pregnancy, fewer than two ovaries, abnormal hormone levels during days 2 to 7 of the menstrual cycle, use of hormonal preparations within 1 month before treatment or previous use of Corifollitropin alfa, were excluded Exclusion criteria as stated in the protocol History of/or any current (treated) endocrine abnormality, history of OHSS, history of/or current PCOS or current polycystic ovaries according to USS (at least 10 follicles of 2‐8 mm in each ovary), more than three unsuccessful COH cycles since the last established ongoing pregnancy (if applicable), history of non‐ or low ovarian response to FSH/hMG treatment, any clinically relevant hormone value outside the reference range during the early follicular phase (menstrual cycle day 2 to 7) as measured by the local laboratory (FSH, LH, E2, P, total T, TSH and prolactin), any clinically relevant abnormal laboratory value, < 2 ovaries, any ovarian and/or abdominal abnormality interfering with ultrasound examination, contraindications for the use of gonadotropins (e.g. tumours, pregnancy/lactation, undiagnosed vaginal bleeding, hypersensitivity, ovarian cysts), epilepsy, diabetes, cardiovascular, gastro‐intestinal, hepatic, renal, pulmonary, or abdominal disease, history of presence of alcohol or drug abuse within 12 months prior to signing informed consent, previous use of Org 36286, use of hormonal preparations within 1 month prior to randomisation, hypersensitivity to Org 32489 (Puregon®) and/or Org 37462 (Orgalutran®) and/or Pregnyl® or any of their components, administration of investigational drugs within three months prior to signing informed consent. Mean age (years) and SD
Mean weight (kg) and SD
Mean BMI (kg/m²) and SD
Mean duration of subfertility (years) and SD
Withdrawals 58 subject were excluded before randomisation, but reasons were not reported. Interventions Total 21.1% of participants in intervention groups withdrew before embryo transfer 60 μg: One subject were excluded before stimulation because PCOS. Twenty‐three subjects did not received hCG because insufficient ovarian response (22 subjects) and one (serious) adverse event. Five subjects were excluded before oocyte retrieval, two because insufficient ovarian response, and two inadequate oocytes retrieved (none, too few or poor quality). Before embryo transfer six subjects withdrew because of no fertilisation (2 subjects, inadequate embryos (3 subjects) and one because ICSI not possible (dead sperm) 120 μg: Three subjects were excluded before the start stimulation because personal reasons, five did not underwent hCG treatment because insufficient ovarian response (2 subjects), risk of hyperstimulation (2 subjects) and one for personal reasons. After oocyte retrieval two were excluded before transfer because no fertilisation or inadequate embryos 180 μg: Before the start of treatment four subjects were excluded because spontaneous pregnancy (2 subjects) and personal reasons (2 subjects). After oocyte retrieval 4 subjects did not undergo embryo transfer because risk of hyperstimulation, no fertilisation and inadequate embryos (2 subjects) Control Total of 20.5% in control group withdrawn before embryo transfer Two subjects did not started stimulation due to a menstrual disorder and personal reasons, six subjects discontinued before the hCG treatment because insufficient ovarian response (5 subjects) and one because personal reasons. Two subjects were excluded before oocyte retrieval because an insufficient ovarian response and inadequate oocytes retrieved. After oocyte retrieval seven subjects dropped‐out because inadequate oocytes retrieved (5 subjects) and in two subjects no fertilisation took place. |
|
| Interventions | Intervention: 60 μg, 120 μg or 180 μg long‐acting FSH Control: 150 IU daily FSH GnRH antagonist was administered sc starting on day 5 up to and including the day of hCG (at least 3 follicles ≥ 17 mm) No more than 3 embryos were transferred |
|
| Outcomes |
Primary Live birth rate OHSS Secondary Clinical (vital) pregnancy rate Ongoing pregnancy rate Multiple pregnancy rate |
|
| Funding | Funded by NV Organon (now Schering‐Plough) Possible conflicts of interest not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation to one of the four arms (1:1:1:1) was stratified by age (<32 or ≥ 32 years) and by centre using a fixed block size of four and a minimization algorithm combined with randomly permutated blocks". |
| Allocation concealment (selection bias) | Low risk | "Randomisation by using a central remote allocation procedure". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and most reasons for withdrawals reported, for details see Characteristics of included studies. |
| Selective reporting (reporting bias) | Low risk | All planned protocol outcomes reported. |
| Other bias | Unclear risk | Funded by a pharmaceutical company. |
Pursue 2012.
| Methods | RCT Double‐blind (subject, caregiver, investigator, outcome assessor) Double‐dummy Two‐arm trial 33 IVF centres in USA |
|
| Participants | Total no of participants: 1390, 694 intervention, 696 control Inclusion criteria as stated in the protocol Willing and able to provide written informed consent for trial P06029 as well as for the Frozen‐Thawed Embryo Transfer (FTET) follow‐up trial P06031, and for the pharmacogenetic analysis (as applicable), female and ≥ 35 to ≤ 42 years of age with indication for COS and IVF/ICSI, body weight ≥ 50.0 kg, BMI ≥18.0 to ≤ 32.0 kg/m², regular spontaneous cycle with variation not outside 24 to 35 days, ejaculatory sperm must be available (donated or cryopreserved sperm, or both, is allowed), results of clinical laboratory tests, cervical smear, physical examination within normal limits or acceptable to the investigator, adhere to trial schedule. Exclusion criteria as stated in the protocol A recent history of/or any current endocrine abnormality, a history of ovarian hyper‐response or OHSS, a history of/or current PCOS, more than 20 basal antral follicles < 11 mm (both ovaries combined) in the early follicular phase, < 2 ovaries or any other ovarian abnormality, unilateral or bilateral hydrosalpinx, intrauterine fibroids ≥ 5 cm or any clinically relevant pathology, which could impair embryo implantation or pregnancy continuation, more than three unsuccessful COS cycles for IVF/ICSI since the last established ongoing pregnancy (if applicable), a history of non‐ or low ovarian response to FSH/hMG treatment, a history of recurrent miscarriage, FSH > 15.0 IU/L or LH > 12.0 IU/L during the early follicular phase, positive for HIV or Hepatitis B, contraindications for the use of gonadotropins or GnRH antagonists, a recent history of/or current epilepsy, thrombophilia, diabetes, cardio‐vascular, gastro‐intestinal, hepatic, renal or pulmonary or auto‐immune disease requiring regular treatment, smoking or recently stopped smoking (i.e. within the last 3 months prior to signing informed consent), a recent history or presence of alcohol or drug abuse, the subject or the sperm donor has known gene defects, genetic abnormalities, or abnormal karyotyping, relevant for the current indication or for the health of the offspring, prior or concomitant medications disallowed by protocol. Withdrawals Corifollitropin alfa Started 694, completed 632, not completed 62. Reasons: 5 adverse event, 11 insufficient ovarian response, 0 risk of OHSS, 4 too high ovarian response, 7 no/too few/bad oocytes, 25 no or abnormal fertilization, 3 no/too few/bad quality embryos, 7 unknown daily FSH Started 696, completed 647, not completed 49. Reasons: 6 adverse event, 17 insufficient ovarian response, 1 risk of OHSS, 2 too high ovarian response, 5 no/too few/bad oocytes, 10 no or abnormal fertilization, 3 no/too few/bad quality embryos, 5 unknown Baseline measures Mean age and SD Intervention 38.0 ± 2.2 Control 38.0 ± 2.2 |
|
| Interventions |
Intervention as stated in protocol: corifollitropin alfa, 100 μg for women weighing ≤ 60 kg, and 150 μg for women weighing > 60 kg Intervention as stated in abstract: corifollitropin alfa 150 μg Control as stated in protocol: daily FSH 150 to 300 IU daily until > 2 follicles are > 18 mm Control as stated in abstract: daily FSH 300 IU daily until 3 follicles reached ≥ 17 mm |
|
| Outcomes |
Outcomes as stated in protocol: Primary: vital pregnancy (assessed by ultrasound at least 35 days after embryo transfer) Secondary: number of oocytes retrieved, live birth rate Outcomes as stated in abstract Primary: vital pregnancy (assessed by ultrasound at least 35 days after embryo transfer) Secondary: number of oocytes retrieved, ongoing pregnancy (≥ 70 days after embryo transfer) and safety evaluations Other publications Congenital malformation (major and minor) rate |
|
| Funding | Funded by Merck, Sharp and Dohme & Co | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded (subject, caregiver, investigator, outcome assessor). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded (subject, caregiver, investigator, outcome assessor). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers and reasons for withdrawal reported, for details see Characteristics of included studies. |
| Selective reporting (reporting bias) | High risk | Live birth rate not reported. |
| Other bias | High risk | Protocol states: "The investigator agreed not to publish or publicity present any interim results of the trial without the prior written consent of the sponsor. The investigator further agreed to provide to the sponsor 45 days prior to submission for publication or presentation, review copies of abstracts or manuscripts for publication that report(s) any results of the trial." |
Sioulas 2011.
| Methods | RCT Single‐blind (participant), no placebo injections Two‐arm trial One‐centre trial January 2011 to December 2015 |
|
| Participants |
Participants Intervention 12 women Control 16 women Inclusion criteria Women aged 18 to 36 years old with a body weight > 60 kg but < 90 kg, BMI of 18 to 32 kg/m², menstrual cycle length of 23 to 35 days, an indication for COS for IVF or ICSI. Exclusion criteria History of an endocrine abnormality, abnormal outcome of blood biochemistry or hematology, abnormal cervical smear, chronic disease, uterine pathology that interfering with the COS treatment (e.g. fibroids ≥ 5 cm). Poor responders. |
|
| Interventions |
Intervention: Corifollitropin alfa 150 μg Control: recombinant FSH 150 to 300 IU according to age, BMI, previous attempts and changed accordingly to ovarian response. Both GnRH agonists (long) and antagonists protocols will be used. Final oocyte maturation will be induced by the administration of hCG on the day that 2 follicles > 18 mm were recognized on the transvaginal ultrasound scan. One or two embryos will be transferred |
|
| Outcomes |
Outcomes as stated in protocol Primary Ongoing pregnancy rate, defined as the presence of fetal heart at ultrasound after 12 gestational weeks Secondary Clinical pregnancy rate, defined as the presence of fetal heart at transvaginal ultrasound at 6+2 gestational weeks Cancellation rate Miscarriage rate Ectopic pregnancy rate Outcomes as stated in preliminary results of the author Live birth rate Clinical pregnancy rate Ongoing pregnancy rate Multiple pregnancy rate Miscarriage rate OHSS rate Cancellation rate |
|
| Funding | Funded by National and Kapodistrian University of Athens | |
| Notes | Outcomes received from the author, study not completed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised by telephone to a person totally irrelevant of the Unit, by answering to random numbers which had been chosen before by the couple and provider". |
| Allocation concealment (selection bias) | Low risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | We received preliminary results from the author. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes as stated in the protocol were reported. |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balen 2004 | No IVF or ICSI performed after stimulation. |
| Croxtall 2011 | Not a RCT (review). |
| de Lartigue 2011 | Not a RCT. |
| Fatemi 2010 | Not a RCT. |
| Leader 2013 | Does not include any outcome of interest. |
| Ledger 2009 | Not a RCT (review). |
| Loutradis 2010 | Not a RCT. |
| Mahmoud Youssef 2012 | Not a RCT (review). |
| Norman 2011 | Not a RCT. |
| Prados 2011 | Not a RCT. |
| Rombauts 2012 | Not a RCT, expert opinion. |
| Seyhan 2011 | Not a RCT (review). |
| Talmor 2013 | Not a RCT. |
Differences between protocol and review
We added the outcomes congenital malformation rate, major congenital malformation rate, and minor congenital malformation rate. In this update we decided to present data using the risk ratio (RR) rather than the Peto odds ratio (OR).
Contributions of authors
AWP and CF extracted data. AWP entered data and wrote the review update. CF helped to draft the review, acted as a clinical expert and commented on the updated review. JK acted as a clinical expert and commented on the updated review.
Sources of support
Internal sources
Cochrane MDSG, New Zealand.
External sources
-
Stichting Nijmeegs Universiteitsfonds, Netherlands.
Scholarship to support students from Radboud University Nijmegen to study, do an internship or research abroad
-
Commissie Voorzieningen Studenten Budget (CVSB), Netherlands.
Grant to subsidise activities of (medical) student organisation and foreign internships of individual students from the medical faculty of Radboud University Nijmegen
Declarations of interest
The authors have no interests to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Devroey 2004 {published and unpublished data}
- Devroey P, Fauser BC, Platteau P, Beckers NG, Dhont M, Mannaerts BM. Induction of multiple follicular development by a single dose of long‐acting recombinant follicle‐stimulating hormone (FSH‐CTP, corifollitropin alfa) for controlled ovarian stimulation before in vitro fertilization. Journal of Clinical Endocrinology and Metabolism May;89(5):2062‐70. [DOI] [PubMed] [Google Scholar]
- NCT00702195. Pregnancy and neonatal follow‐up of ongoing pregnancies established during phase II clinical trials for the development of Org 36286 (corifollitropin alfa). clinicaltrials.gov/show/NCT00702195 (accessed 18 June 2008).
- NCT00702195. Pregnancy and neonatal follow‐up of ongoing pregnancies established during phase II clinical trials for the development of Org 36286 (corifollitropin alfa). www.clinicalstudyresults.org (accessed 27 May 2009).
- NCT00702806. A phase III, open‐label, prospective, randomized, comparative clinical trial to investigate the appropriate dose of a single injection of Org 36286 (corifollitropin alfa) to initiate multiple follicular growth in a controlled ovarian hyperstimulation protocol for IVF or IVF/ICSI. clinicaltrials.gov/show/NCT00702806 (accessed 18 June 2008).
- NCT00702806. A phase III, open‐label, prospective, randomized, comparative clinical trial to investigate the appropriate dose of a single injection of Org 36286 (corifollitropin alfa) to initiate multiple follicular growth in a controlled ovarian hyperstimulation protocol for IVF or IVF/ICSI. www.clinicalstudyresults.org (accessed 11 February 2008).
ENGAGE 2009 {published and unpublished data}
- Behr B, Heijnen E. Corifollitropin alfa versus recombinant FSH treatment for COS in a GnRH antagonist protocol: equal efficacy in terms of oocyte and embryo quality. Human Reproduction, European Society of Human Reproduction and Embryology, ESHRE 25th Annual Meeting; 2009 June 28‐July 1 2009; Amsterdam, The Netherlands. 2009.
- Bonduelle M, Mannaerts B, Leader A, Bergh C, Passier D, Devroey P. Prospective follow‐up of 838 fetuses conceived after ovarian stimulation with corifollitropin alfa: comparative and overall neonatal outcome. Human Reproduction 2012;27(7):2177‐85. [DOI] [PubMed] [Google Scholar]
- Boostanfar R, Devroey P, Oberye J, Mannaerts B. No difference in the cumulative pregnancy rates or live birth rates when comparing corifollitropin alfa with daily rFSH treatment for COS. Human Reproduction. Abstracts of the 26th Annual Meeting of ESHRE; 2010 June 27‐30; Rome. 2010; Vol. 25 Suppl 1(6):i47 Abstract no. O‐119.
- Boostanfar R, Mannarts B, Witjes H, Devroey P. International differences in IVF live birth rates and ongoing pregnancy rates following ovarian stimulation with corifollitropin alfa or recombinant FSH. Fertility and Sterility, Annual Meeting of the American Society for Reproductive Medicine. 2011 Oct 15‐19; Orlando 2011;96(Suppl 3):174. [Google Scholar]
- Devroey P, Boostanfar R, Koper NP, Mannaerts BM, Ijzerman‐Boon PC, Fauser BC, ENGAGE Investigators. Erratum: A double‐blind, non‐inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Human Reproduction 2014;29(5):1116‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devroey P, Boostanfar R, Koper NP, Mannaerts BMJL, Ijzerman‐Boon PC, Fauser BC, ENGAGE Investigators. A double‐blind, non‐inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Human Reproduction 2009;24(12):3063‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi HM, Doody K, Witjes H, Mannaerts B. A high ovarian response does not compromise the change of pregnancy. Human Reproduction, 27th Annual Meeting of the European Society of Human Reproduction and Embryology, ESHRE. Stockholm, Sweden, 2011; Vol. 26, suppl 1:i319 Abstract no. P‐510.
- Fauser BC, Alper MM, Ledger W, Schoolcraft WB, Zandvliet A, Mannaerts BM, Engage Investigators. Pharmacokinetics and follicular dynamics of corifollitropin alfa versus recombinant FSH during ovarian stimulation for IVF. Reproductive BioMedicine Online 2010;21(5):593‐601. [DOI] [PubMed] [Google Scholar]
- Fernandez Sanchez M, Koper N. Equally high ongoing pregnancy rates with corifollitropin alfa and recombinant FSH irrespective of variations in ART procedures. Human Reproduction, Abstracts of the 26th Annual Meeting of ESHRE; 2009 June 28‐July 1; Amsterdam, The Netherlands. 2009; Vol. 24 Suppl 1:i2 O‐005 Oral.
- Hillensjo T, Yeko T, Witjes H, Elbers J, Devroey P. Does treatment flexibility affect clinical outcome of either corifollitropin alfa or daily regimens?. Human Reproduction, 27th Annual Meeting of the European Society of Human Reproduction and Embryology, ESHRE. Stockholm, Sweden, 2011; Vol. 26, issue suppl 1:i303‐4 Abstract no. P468.
- Leader A, Pang S, Witjes H, Gordon K, Devroey P. Reduction of daily rFSH dose from stimulation day 8 onward in a corifollitropin alfa or rFSH treatment regimen does not compromise clinical outcome. Human Reproduction, 27th Annual Meeting of the European Society of Human Reproduction and Embryology, ESHRE. Stockholm, Sweden, 2011:i302 Abstract no. P‐465.
- NCT00698800. A phase III, non‐inferiority clinical trial to investigate the efficacy and safety of a single injection of 150 μg Org 36286 (corifollitropin alfa) to induce multifollicular development for ovarian stimulation using daily recombinant FSH as reference. clinicaltrials.gov/show/NCT00698800 (accessed 11 June 2008).
- NCT00698800. A phase III, randomised, double‐blind, active‐controlled, non‐inferiority clinical trial to investigate the efficacy and safety of a single injection of 150 μg Org 36286 (corifollitropin alfa) to induce multifollicular development for ovarian stimulation using daily recombinant FSH as reference. www.clinicalstudyresults.org (accessed 11 July 2008).
- NCT00702273. Follow‐up protocol to collect the outcome of frozen‐thawed embryo transfer cycles after cryopreservation of embryos in clinical trial 38819. clinicaltrials.gov/show/NCT00702273 (accessed 18 June 2008).
- NCT00702273. Follow‐up protocol to collect the outcome of frozen‐thawed embryo transfer cycles after cryopreservation of embryos in clinical trial 38819. www.clinicalstudyresults.org (accessed 16 February 2010).
- NCT00703014. Pregnancy and neonatal follow‐up of ongoing pregnancies established after controlled ovarian stimulation in Clinical Trial 38819 (P05787) for ORG 36286 (Corifollitropin alfa). www.clinicalstudyresults.org (accessed 7 July 2009).
- NCT00703014. Pregnancy and neonatal follow‐up of ongoing pregnancies established after controlled ovarian stimulation in Clinical Trial 38819 for ORG 36286 (Corifollitropin alfa). clinicaltrials.gov/show/NCT00703014 (accessed 18 June 2008).
ENSURE 2010 {published and unpublished data}
- Bonduelle M, Mannaerts B, Leader A, Bergh C, Passier D, Devroey P. Prospective follow‐up of 838 fetuses conceived after ovarian stimulation with corifollitropin alfa: comparative and overall neonatal outcome. Human Reproduction 2012;27(7):2177‐85. [DOI] [PubMed] [Google Scholar]
- Corifollitropin alfa Ensure Study Group: Obruca A, Schenk M, Tews G, Mardesic T, Mrazek M, Meinertz H, et al. Corifollitropin alfa for ovarian stimulation in IVF: a randomized trial in lower‐body‐weight women. Reproductive BioMedicine Online 2010;21(1):66‐76. [DOI] [PubMed] [Google Scholar]
- Hillenjso T, Mauw E. A comparison of corifollitropin vs. recombinant FSH in a GnRH antagonist protocol for controlled ovarian stimulation in women weighing 60 kg or less. Human Reproduction. European Society of Human Reproduction and Embryology, ESHRE 25th Annual Meeting; 2009 June 28‐July 1; Amsterdam. 2009; Vol. 24 Suppl 1:i2.
- NCT00702546. Follow‐up protocol to collect the outcome of frozen‐thawed embryo transfer cycles after cryopreservation of embryos in clinical trial 107012. clinicaltrials.gov/show/NCT00702546 (accessed 18 June 2008).
- NCT00702546. Follow‐up protocol to collect the outcome of frozen‐thawed embryo transfer cycles after cryopreservation of embryos in clinical trial 107012 (P05711). www.clinicalstudyresults.org (accessed November 2009).
- NCT00702624. Pregnancy and neonatal follow‐up of ongoing pregnancies established after controlled ovarian stimulation in clinical trial 107012 for the development of Org 36286 (corifollitropin alfa). clinicaltrials.gov/show/NCT00702624 (accessed 18 June 2008).
- NCT00702624. Pregnancy and neonatal follow‐up of ongoing pregnancies established after controlled ovarian stimulation in clinical trial 107012 for the development of Org 36286 (corifollitropin alfa) (107014/P05710). www.clinicalstudyresults.org (accessed 9 June 2009).
- NCT00702845. A phase III, equivalence clinical trial to investigate the efficacy and safety of a single injection of 100 μg Org 36286 (Corifollitropin alfa) to induce multifollicular development for ovarian stimulation using daily recombinant FSH as reference. clinicaltrials.gov/show/NCT00702845 (accessed 18 June 2008).
- NCT00702845. A phase III, randomized, double‐blind, active‐controlled, equivalence clinical trial to investigate the efficacy and safety of a single injection of 100 μg Org 36286 (Corifollitropin alfa) to induce multifollicular development for controlled ovarian stimulation (COS) using daily recombinant FSH (rFSH) as a reference. www.clinicalstudyresults.org (accessed 11 July 2008).
Koper 2008 {published and unpublished data}
- Corifollitropin Alfa Dose‐finding Study Group: Abyholm T, Anderson AN, Balen AH, Braat DDM, Devroey P, D'Hooge TH, et al. A randomized dose‐response trial of a single injection of corifollitropin alfa to sustain multifollicular growth during controlled ovarian stimulation. Human Reproduction 2008;23(11):2484‐92. [DOI] [PubMed] [Google Scholar]
- NCT005982088. A phase 2, open label, randomized trial to investigate the dose‐response relationship of a single injection or Org 36286 (corifollitropin alfa) to initiate multiple follicular growth in a controlled ovarian hyperstimulation protocol for IVF or ICSI. clinicaltrials.gov/show/NCT005982088 (accessed 10 January 2008).
- NCT005982088. A phase 2, open label, randomized trial to investigate the dose‐response relationship of a single injection or Org 36286 (corifollitropin alfa) to initiate multiple follicular growth in a controlled ovarian hyperstimulation protocol for IVF or ICSI. www.clinicalstudyresults.org (accessed 11 July 2008).
- NCT00702988. Pregnancy and neonatal follow‐up of ongoing pregnancies established during a previous clinical trial for the development of Org 36286 (corifollitropin alfa) (38827/P06056). www.clinicalstudyresults.org (accessed 28 May 2009).
- NCT00702988. Pregnancy and neonatal follow‐up of ongoing pregnancies established during clinical trial 38826 for the development of Org 36286 (corifollitropin alfa). clinicaltrials.gov/show/NCT00702988 (accessed 18 June 2008).
Pursue 2012 {published and unpublished data}
- Boostanfar R, Mannaerts B, Pang S, Fernandez‐Sanchez M, Witjes H, Devroey P, Engage Investigators. A comparison of live birth rates and cumulative ongoing pregnancy rates between Europe and North America after ovarian stimulation with corifollitropin alfa or recombinant follicle‐stimulating hormone. Fertility and Sterility 2012;97(6):1351‐8. [DOI] [PubMed] [Google Scholar]
- Boostanfar R, Mannaerts B, Witjes H, Devroey P. International differences in IVF live birth rates and cumulative ongoing pregnancy rates following ovarian stimulation with corifollitropin alfa or recombinant FSH. Fertility and Sterility 2011;1:S174. [DOI] [PubMed] [Google Scholar]
- Boostanfar R, Yeko T, Shapiro B, Elbers J, Witjes H, Mannaerts B. A large double‐blind efficacy and safety trial of corifollitropin alfa versus daily recombinant FSH in women 35 to 42 years of age undergoing ovarian stimulation prior to IVF or ICSI (pursue trial). Fertility and Sterility 2012;98(Suppl 1):S34. [Google Scholar]
- NCT01144416. A Phase III, Randomized, Double‐blind, Active‐controlled, Non‐inferiority Trial to Investigate the Efficacy and Safety of a single injection of SCH 900962 (Corifollitropin alfa) to Induce Multifollicular Development for Controlled Ovarian Stimulation (COS) Using Daily Recombinant FSH (recFSH) as a Reference in Women aged 35‐42 Years (P06029). clinicaltrials.gov/show/ NCT01144416 (accessed 11 June 2010).
- NCT01144416. Efficacy and safety study of a single injection of SCH 900962 versus daily recFSH injections in women undergoing controlled ovarian stimulation (study P06029 AM1) (PURSUE). clinicaltrials.gov/show/NCT01144416 (accessed 11 November 2013).
- Stegmann B, Bonduelle M, Gordon K, Witjes H, Verweij P. Incidence of congenital malformations after ovarian stimulation with corifollitropin alfa or recombinant FSH: Data from 3 randomized controlled trials. Fertility and Sterility 2013;100(3):S59. [Google Scholar]
Sioulas 2011 {published and unpublished data}
- NCT01319695. Corifollitropin alfa versus recombinant follicle stimulating hormone (FSH) in ovarian stimulation of women undergoing in vitro fertilisation. clinicaltrials.gov/show/NCT01319695 (accessed 8 March 2011).
- Sioulas V, Siristatidis C, Chrelias C, Alexiou E, Vrantza T, Kassanos D. Corifollitropin alfa vs. recombinant follicle stimulating hormone in ovarian stimulation of women undergoing in vitro fertilization: Preliminary results. Fertility and Sterility. 2011; Vol. 96 Suppl:S181 P‐252.
References to studies excluded from this review
Balen 2004 {published and unpublished data}
- Balen AH, Mulders AG, Fauser BC, Schoot BC, Renier MA, Devroey P, et al. Pharmacodynamics of a single low dose of long‐acting recombinant follicle‐stimulating hormone (FSH‐carboxy terminal peptide, corifollitropin alfa) in women with World Health Organization group II anovulatory infertility. Journal of Clinical Endocrinology and Metabolism 2004;89(12):6297‐304. [DOI] [PubMed] [Google Scholar]
Croxtall 2011 {published and unpublished data}
- Croxtall JD, McKeage K. Corifollitropin alfa: a review of its use in controlled ovarian stimulation for assisted reproduction. BioDrugs 2011;25(4):243‐54. [DOI] [PubMed] [Google Scholar]
de Lartigue 2011 {published and unpublished data}
- Lartigue J. Corifollitropin alfa: a new option to treat female infertility. Drugs of Today 2011;47(8):583‐90. [DOI] [PubMed] [Google Scholar]
Fatemi 2010 {published and unpublished data}
- Fatemi HM, Oberyé J, Popovic‐Todorovic B, Witjes H, Mannaerts B, Devroey P. Corifollitropin alfa in a long GnRH agonist protocol: proof of concept trial. Fertility and Sterility 2010;94(5):1922‐4. [DOI] [PubMed] [Google Scholar]
Leader 2013 {published and unpublished data}
- Leader A, Devroey P, Witjes H, Gordon K. Corifollitropin alfa or rFSH treatment flexibility options for controlled ovarian stimulation: a post hoc analysis of the Engage trial. Reproductive Biology and Endocrinology 2013;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ledger 2009 {published and unpublished data}
- Ledger W, Zandvliet A, Mannaerts B. On behalf on the ENGAGE/ENSURE investigators: Two doses of corifollitropin alfa allow for similar exposure and ovarian response in patients weighing ≤60 kg and >60 kg. Molecular Human Reproduction 2009;24(Suppl 1):i114. [Google Scholar]
Loutradis 2010 {published and unpublished data}
- Loutradis D, Vlismas A, Drakakis P. Corifollitropin alfa: a novel long‐acting recombinant follicle‐stimulating hormone agonist for controlled ovarian stimulation. Women's Health 2010;6(5):655‐64. [DOI] [PubMed] [Google Scholar]
Mahmoud Youssef 2012 {published and unpublished data}
- Mahmoud Youssef MA, Wely M, Aboulfoutouh I, El‐Khyat W, Veen F, Al‐Inany H. Is there a place for corifollitropin alfa in IVF/ICSI cycles? A systematic review and meta‐analysis. Fertility and Sterility 2012;97(4):876‐85. [DOI] [PubMed] [Google Scholar]
Norman 2011 {published and unpublished data}
- Norman RJ, Zegers‐Hochschild F, Salle BS, Elbers J, Heijnen E, Marintcheva‐Petrova M, et al. Repeated ovarian stimulation with corifollitropin alfa in patients in a GnRH antagonist protocol: no concern for immunogenicity. Human Reproduction 2011;26(8):2200‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prados 2011 {published and unpublished data}
- Prados N, Pellicer A, Fernandez‐Sanchez M. Corifollitropin alfa: A new recombinant FSH gonadotropin analogue. Expert Review of Obstetrics and Gynecology 2011;6(4):395‐402. [Google Scholar]
Rombauts 2012 {published and unpublished data}
- Rombauts L, Talmor A. Corifollitropin alfa for female infertility. Expert Opinion on Biological Therapy 2012;12(1):107‐12. [DOI] [PubMed] [Google Scholar]
Seyhan 2011 {published and unpublished data}
- Seyhan A, Baris A. The role of corifollitropin alfa in controlled ovarian stimulation for IVF in combination with GnRH antagonist. International Journal of Women's Health 2011;3:243‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talmor 2013 {published and unpublished data}
- Talmor A, McLachlan V, Osianlis T, Sorby KL, Lutjen P. Is longer better? The long and short of it ‐ a matched case control study of long and short acting follicle stimulating hormone. Fertility and Sterility 2013;100(Supplement 3):S17. [Google Scholar]
Additional references
Al‐Inany 2011
- Al‐Inany HG, Youssef MAFM, Aboulghar M, Broekmans FJ, Sterrenburg MD, Smit JG, et al. Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD001750.pub3] [DOI] [PubMed] [Google Scholar]
de Greef 2010
- Greef R, Zandvliet AS, Haan AFJ, Ijzerman‐Boon PCI, Marintcheva‐Petrova M, Mannaerts BMJL. Dose selection of corifollitropin alfa by modeling and simulation in controlled ovarian stimulation. Clinical Pharmacology and Therapeutics 2010;88(1):79‐87. [DOI] [PubMed] [Google Scholar]
Devroey 2009
- Devroey P, Boostanfar R, Koper NP, Mannaerts BMJL, IJzerman‐Boon PC, Fauser BCJM, the ENGAGE Investigators. A double‐blind, non‐inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Human Reproduction 2009;24(12):3063‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duijkers 2002
- Duijkers IJ, Klipping C, Boerrigter PJ, Machielsen CS, Bie JJ, Voortman G. Single dose pharmacokinetics and effects on follicular growth and serum hormones of a long‐acting recombinant FSH preparation (FSH‐CTP) in healthy pituitary‐suppressed females. Human Reproduction 2002;17(8):1987‐93. [DOI] [PubMed] [Google Scholar]
Evers 2002
- Evers JLH. Female subfertility. Lancet 2002;360(9327):151‐9. [DOI] [PubMed] [Google Scholar]
Fauser 2009
- Fauser BC, Mannaerts BM, Devroey P, Leader A, Boime I, Baird DT. Advances in recombinant DNA technology: corifollitropin alfa, a hybrid molecule with sustained follicle‐stimulating activity and reduced injection frequency. Human Reproduction Update 2009;15(3):309‐21. [DOI] [PubMed] [Google Scholar]
Gnoth 2005
- Gnoth C, Godehardt E, Frank‐Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Human Reproduction 2005;20(5):1144‐7. [DOI] [PubMed] [Google Scholar]
Heineman 2007
- Heineman MJ, Evers JLH, Massuger LFAG, Steegers EAP. Obstetrie en Gynaecology. De voortplanting van de mens. Amsterdam: Elsevier Gezondheidszorg, 2007. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kovacs 2011
- Kovacs G. How to Improve your ART Success Rates. An Evidence‐Based Review of Adjuncts to IVF. Cambridge: Cambridge University Press, 2011. [Google Scholar]
Maheshwari 2011
- Maheshwari A, Gibreel A, Siristatidis CS, Bhattacharya S. Gonadotrophin‐releasing hormone agonist protocols for pituitary suppression in assisted reproduction. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD006919.pub3] [DOI] [PubMed] [Google Scholar]
MDSG module 2008
- Farquhar C, Clarke J, Lethaby A, Thomas J, Proctor M, Barlow D, et al. Cochrane Menstrual Disorders and Subfertility Group, About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). Available at www.thecochranelibrary.com 2008, issue 4.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, the Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014.
Schröder 2004
- Schröder AK, Katalinic A, Diedrich K, Ludwig M. Cumulative pregnancy rates and drop‐out rates in a German IVF programme: 4102 cycles in 2130 patients. Reproductive Biomedicine Online 2004;8(5):600‐6. [DOI] [PubMed] [Google Scholar]
Tarlatzis 2007
- Tarlatzis BC, Kolibianakis EM. GnRH agonists vs antagonists. Best Practice & Research. Clinical Obstetrics and Gynaecology 2007;21(1):57‐65. [DOI] [PubMed] [Google Scholar]
Tur‐Kaspa
- Tur‐Kaspa I, Fauser B. The use of GnRH agonist in IVF protocols. IVF Worldwide website accessed 20 May 2015:http://www.ivf‐worldwide.com/survey/the‐use‐of‐gnrh‐agonist‐in‐ivf‐protocols/results‐the‐use‐of‐gnrh‐agonist‐in‐ivf‐protocols.html.
References to other published versions of this review
Pouwer 2012a
- Pouwer AW, Farquhar C, Kremer JA. Long‐acting FSH versus daily FSH for women undergoing assisted reproduction. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009577] [DOI] [PubMed] [Google Scholar]
Pouwer 2012b
- Pouwer AW, Farquhar C, Kremer JA. Long‐acting FSH versus daily FSH for women undergoing assisted reproduction. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009577.pub2] [DOI] [PubMed] [Google Scholar]


