Abstract
The development and bottom-up assembly of synthetic cells with a functional cytoskeleton sets a major milestone to understand cell mechanics and to develop man-made machines on the nano- and microscale. However, natural cytoskeletal components can be difficult to purify, deliberately engineer and reconstitute within synthetic cells which therefore limits the realization of multifaceted functions of modern cytoskeletons in synthetic cells. Here, we review recent progress in the development of synthetic cytoskeletons made from deoxyribonucleic acid (DNA) as a complementary strategy. In particular, we explore the capabilities and limitations of DNA cytoskeletons to mimic functions of natural cystoskeletons like reversible assembly, cargo transport, force generation, mechanical support and guided polymerization. With recent examples, we showcase the power of rationally designed DNA cytoskeletons for bottom-up assembled synthetic cells as fully engineerable entities. Nevertheless, the realization of dynamic instability, self-replication and genetic encoding as well as contractile force generating motors remains a fruitful challenge for the complete integration of multifunctional DNA-based cytoskeletons into synthetic cells.
Keywords: synthetic cell, bottom-up synthetic biology, cytoskeleton, DNA nanotechnology, giant unilamellar lipid vesicle, DNA origami
1. Introduction
Bottom-up synthetic biology cultivates an engineering approach to assemble the first synthetic cell from molecular building blocks. An essential component of every modern eukaryotic cell is the cytoskeleton because it drives cell motility, stability and intracellular transport, among other functions [1]. In this regard, major efforts have been made concerning the reconstitution of natural cytoskeletons and their auxiliary proteins into cell-sized confinement [2]. However, protein purification and functional reconstitution can be challenging and the deliberate engineering of proteins to implement specific functions is only at the verge of being considered for practical applications. DNA nanotechnology offers a chemically functionalizable, programmable and versatile tool [3]. In addition, it is especially exciting to build a synthetic cell from completely synthetic building blocks and to test our knowledge on natural cytoskeletons by comparing it to rationally engineered mimics thereof. Here, we will review current progress in the design and assembly of DNA-based cytoskeletons and discuss their use for the engineering of synthetic cells with a synthetic cytoskeleton.
DNA nanotechnology allows one to programmably assemble DNA strands into higher-order structures using the complementary base pairing rules. These structures can be classified into several basic building blocks, including DNA linkers, Y-motifs, DNA tiles and DNA origami, which again can be assembled into larger architectures like DNA lattices and DNA nanotubes in a hierarchical manner [4]. The potential to engineer functional parts using these programmable and biocompatible building blocks has been especially interesting for use in bottom-up synthetic biology [3]. Examples include, among others, DNA-based scramblases [5], ion channels [6] and porins [7,8], cell–cell linkers [9,10], assembly platforms [11,12] or adhesion sites [13]. In recent years, DNA-based mimics of cytoskeletal elements and cortices have become a particularly prominent example.
The challenge towards the assembly of a DNA-based cytoskeleton is that it should not just perform one function but execute several different tasks on demand. DNA nanotubes are an important early type of DNA nanostructures [14] and visually resemble cytoskeletal elements. At the same time, the advances in DNA nanotechnology go hand-in-hand with the establishment of reliable and easy-to-use encapsulation techniques into cell-sized compartments by means of microfluidics or other techniques [15,16]. Giant unilamellar vesicles (GUVs) are a popular compartment choice for harbouring synthetic cellular components, as they mimic the lipid bilayer membrane of natural cells. Various strategies exist for GUV formation, which have been developed further to optimize encapsulation properties, e.g. octanol-assisted liposome assembly (OLA) [17], continuous droplet interface crossing encapsulation (cDICE) [18,19] or the droplet-stabilized GUV formation method (dsGUVs) [20,21]. Here, we want to review the recent progress and development of DNA nanostructures for their use as a DNA cytoskeleton within cell-sized confinement and in particular GUVs. We will focus on five different key functions of natural cytoskeletons and how these can be mimicked or potentially even surpassed by DNA cytoskeletons. The main functionalities include (i) mechanical control and morphology control, (ii) reversible assembly, (iii) symmetry breaking, (iv) intracellular transport and (v) force generation as illustrated in figure 1.
Figure 1.
Engineering synthetic cytoskeletons with DNA nanotechnology. Engineerable functions include (i) mechanical control and morphology control, (ii) reversible assembly, (iii) symmetry breaking, (iv) intracellular transport and (v) force generation.
2. Functional DNA cytoskeletons
The key challenge for building DNA cytoskeletons is to reach the same sophistication as natural cytoskeletal elements using DNA as an entirely different building material with distinct chemical and physical properties. As a starting point, we first explore already existing DNA nanostructures that mimic one specific key property of natural cytoskeletons. Then, we discuss how they could be merged into one multifunctional synthetic cytoskeleton for a synthetic cell and which elements are still missing, providing exciting directions for future research.
2.1. Shape control and stability
Natural cells are mechanically stable in order to resist stress from the environment. However, their mechanical properties strongly depend on the cell type, i.e. a red blood cell is more deformable than an epithelial cell [22]. This goes hand in hand with the degree to which the cellular morphology is determining the cell’s function. The viscoelastic properties of cells are governed by their cytoskeletal cortex, which forms a crosslinked network underneath the cell’s plasma membrane. On the contrary, the rigidity of a GUV as synthetic cell container is only tuneable to a very limited extent by the choice of lipids, e.g. by changing their fatty acid tail length, saturation, charge or headgroup modifications [23]. In order to change the mechanical properties and the local morphology of GUVs, versatile DNA nanostructures have been developed.
In particular, several studies have shown that GUVs can be mechanically stabilized by crosslinking DNA nanostructures on the membrane surface. One way to achieve an increasing mechanical stability of lipid vesicles is to use so-called DNA Y-motifs or triskelia that can hybridize with one another to form micrometre-scale lattices. These lattices have been bound to lipid membranes via charge-mediated interactions with positively charged lipids [24]. The gel-like shell outside or inside GUVs, likely composed of multiple layers of DNA, was shown to endow GUVs with higher mechanical stability leading to higher survival rates after osmotic shock [24] (figure 2a). A different approach is the targeted functionalization of individual DNA strands with hydrophobic moieties that incorporate in the lipid bilayer of GUVs [28] or natural cells [29]. These hydrophobic anchors include tocopherol, palmitoyl, porphyrin or, most commonly, cholesterol [29]. By modifying the DNA triskelia with cholesterol, a similar enhancement of vesicle stability has been used to coat the lipid membrane inspired by the protein clathrin [30]. The clathrin-inspired DNA lattice coating of vesicles can also be used for triggered cargo release based on toehold-mediated strand displacement reactions [31]. Whether the attachment of DNA nanostructures to lipid bilayers requires hydrophobic tags or can be achieved by electrostatic interactions only crucially depends on the buffer condition, the lipid composition as well as the DNA structures themselves. In particular at low concentrations of divalent ions, hydrophobic tags are often needed for accurate attachment [32].
Figure 2.
DNA nanostructures for mechanical support and morphology control of giant unilamellar vesicles. (a) DNA Y-motifs polymerizing into lattices that stabilize GUVs from the inside [24]. Scale bar: 10 μm. (b) Flat and curved DNA origami structures to scaffold lipid membranes [25]. (c) DNA origami rods lead to spike-like membrane protrusions mediated via divalent ions [26]. Scale bar: 5 μm. (d) pH-sensitive deformation of GUVs via polymerized DNA origami [27]. Scale bar: 10 μm.
DNA origami has often been employed as an alternative strategy for the deformation and scaffolding of lipid membranes. DNA origami depends on the folding of a long scaffold strand with hundreds of short staple strands and allows the programmable formation of tens of nanometre- to micrometre-sized structures [33]. In the context of scaffolding lipid membranes, flat DNA origami structures as well as designs with positive or negative curvature have been used (figure 2b) [25]. Based on the DNA origami curvature, the attachment to GUVs resulted in outward tubules (concave), invaginations (convex) or no deformation (no curvature). This concept has also been expanded on DNA origami nanosprings with highly curved geometries that induce vesicle tubulation [34]. Note that membrane curvature is introduced in any case when polymers like DNA are absorbed on the membrane [35]; curved DNA origami is not a prerequisite. Instead of DNA origami curvature, the GUV deformation can also be controlled by the ionic strength of the solution, which regulates the DNA origami diffusion on the lipid membrane [36]. This effect was exploited to create spike-like membrane protrusions depending on the magnesium-ion concentration with DNA origami rods (figure 2c) [26,37]. In addition to curvature and ionic strength, a third approach for GUV deformation relies on DNA origami oligo- and polymerization into bigger clusters, which in turn also have a higher persistence length and lower diffusion [38]. As an additional trigger, the deformation of GUVs via DNA origami polymerization can also be combined with pH-sensitive triplex motifs, which makes the DNA origami attach in a pH-dependent manner (figure 2d) [27].
Finally, the scope of membrane deformation has also been expanded on the use of DNA four-arm motifs [39] and DNA nanotubes to control GUV morphology [40,41].
2.2. Reversible assembly
Many cytoskeletal functions rely on the reversible assembly of actin, intermediate filaments and microtubules. Most prominently, microtubules polymerize in the presence of GTP leading to the property of dynamic instability, where the microtubule ends grow and shrink on their ends depending on GTP hydrolysis [42]. To implement similar features and in particular the stimuli-responsive reversible assembly of DNA nanostructures, several groups used DNA nanotubes as model system.
DNA nanotubes are made up of individual DNA tiles that can hybridize with one another due to complementary overhangs [14]. These serve as modification sites for inducing the assembly and disassembly of DNA nanotubes. One approach towards is this aim is using toehold-mediated strand displacement (figure 3a). Upon addition of an invader strand that binds to the DNA tile overhang, they are inactivated leading to DNA nanotube disassembly [43]. Their reassembly can be induced by addition of an anti-invader strand that binds the invader strand and reactivates the DNA tile. This mechanism can be repeated several times. However, it requires the continuous addition of DNA strands in higher concentrations making it undesirable for many assembly–disassembly cycles. As an alternative trigger for more assembly–disassembly cycles of DNA nanotubes pH can be used (figure 3b). This approach makes use of a so-called triplex motif that forms pH-sensitive Hogsteen interactions and can thus bind a partially complementary strand at low pH values [44]. This, in turn, leads to the disassembly of DNA nanotubes. After changing the pH again, the DNA triplex closes and initiates DNA nanotube reassembly. Similarly, antibodies can be used for the reversible assembly by binding antigen-functionalized DNA strands (figure 3c) [46]. Going one step further, DNA nanotubes have not only been assembled reversibly but also reconstituted within cell-sized water-in-oil droplets [45,47]. The assembly within confinement was coupled to transcription of a DNA template leading to DNA nanotube assembly and simultaneous disassembly of DNA nanotubes due to RNase H-mediated degradation (figure 3d) [45]. RNAse H has also been used to reorganize DNA nanotubes into defined homo- and block copolymers [48]. To closer mimic natural cytoskeletons, however, also physiological triggers like ATP [47,49] or proteins like nucleolin [47] can be employed to induce DNA nanotube assembly–disassembly cycles. This mechanism uses DNA aptamers for DNA filament assembly and can, in principle, also be expanded to other DNA aptamers. Finally, as a non-invasive trigger for the reversible assembly of DNA nanotubes also light has been shown to induce DNA nanotube disassembly–assembly cycles within GUVs (figure 3e) [40].
Figure 3.
Reversible assembly of DNA nanotubes. Reversible DNA nanotube assembly can be controlled via toehold-mediated strand displacement (a) [43], via pH by using a triplex motif (b) [44], via antibodies by using antigen-functionalized DNA (c), via enzymes (d) [45] or via light (e) [40]. Scale bars: 10 μm (a,e), 20 μm (d), 5 μm (c).
Transferring similar concepts apart from DNA nanotubes, only recently, massive strand displacement has been used to induce shape changes of DNA origami that can fold and evolve into different forms upon stimulus presence [50].
2.3. Symmetry breaking
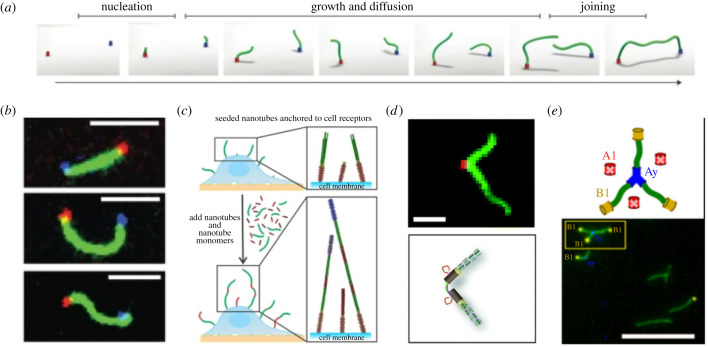
Symmetry breaking within biological systems is essential for cell motility and polarity [51]. Both of these features of natural cells are tightly connected to the cytoskeleton. Therefore, symmetry breaking behaviour is also desirable for synthetic reconstituted DNA cytoskeletons. In order to achieve this asymmetry, DNA origami and DNA nanotubes have been combined within one DNA nanostructure. Here, a hollow cylindrical DNA origami serves as a polymerization seed for DNA nanotubes [52,53]. Using this principle, each end of a DNA nanotube can be addressed separately and in an asymmetric way [54]. As a first proof-of-principle this allowed the end-to-end joining of DNA nanotubes that grew from two different DNA origami seeds to connect molecular landmarks (figure 4a,b) [55]. The individually polymerizing DNA nanotubes connect over time after reaching a certain length and diffusion time. As a first application DNA origami-seeded DNA nanotubes have been attached on the outer membrane of natural cells to measure the shear flow of the surrounding solution (figure 4c) [56]. Inserting the DNA origami seed/DNA nanotube constructs into the membrane of lipid vesicles can even lead to influx of ions and small molecules through the DNA nanotube [59,60]. Recently, DNA origami seed/DNA nanotube conjugates have also been used to transduce mechanical signals into GUVs [61]. In this work, the externally induced clustering of transmembrane DNA origami seeds has been used to induce the rearrangement of DNA nanotubes inside GUVs.
Figure 4.
Asymmetric growth of DNA nanotubes. (a) Schematic illustration of DNA nanotubes growing from a DNA origami seed [55]. (b) Asymmetrically grown DNA nanotubes connect molecular landmarks [55]. (c) DNA nanotubes growing on the cell surface acting as shear force sensors [56]. (d) DNA nanotubes growing in angled directions determined by the DNA origami seed conformation [57]. (e) Hierarchical growth of DNA nanotubes via terminus inactivation [58]. Scale bars: 5 μm (b,d), 10 μm (e).
Showcasing the unique programmability of DNA origami, one can even go one step further and design seeds that allow two (figure 4d)[57] or even three (figure 4e) [58] DNA nanotubes to grow in a predefined direction. In analogy to natural cytoskeletons, these could act as centrioles or cytoskeletal binding proteins that govern and direct the shape of the cytoskeletal filaments.
2.4. Intracellular transport
Cytoskeletal filaments transport cargo and in particular vesicles within all eukaryotic cells. Owing to motion of molecular motors they serve as filament tracks to direct the transport of cargo to organelles. [1]. Despite the fact that the implementation of cargo transport with DNA nanotechnology poses great challenges due to the absence of motor proteins, there exist many different strategies to move cargo along DNA nanostructures.
An easy-to-implement strategy is the use of toehold-mediated strand displacement to engineer DNA walkers [62,63]. A major drawback from mechanisms based on strand displacement reactions is the slow timescale of the invasion, i.e. the displacement of one strand by another with higher binding affinity, which is typically of the order of minutes. Current state-of-the-art strand displacement reactions yield rate constants of the order of 1 min−1, which limits the transport speed. However, by changing the design of the DNA walker to allow a cartwheeling motion transport velocities of the order of 100 nm min−1 can be obtained [51]. Still, the size of the cargo is limited to small molecules [64] or gold nanoparticles [65] which can be attached to the single-stranded DNA. A different mechanism uses the hydrolytic activity of RNase H, which selectively cleaves RNA–DNA duplexes and induces a burnt-bridge motion of silica particles [66,67], gold nanoparticles [68], glass beads [69] or DNA origami (figure 5a) [70]. The achieved velocities of the RNase H-mediated mechanism are between 40 nm min−1 and 2 μm min−1. In order to apply this strategy for synthetic DNA cytoskeletons, SUVs were attached to DNA nanotubes and their motion along the filament track induced via RNase H (figure 5b) [47]. In contrast to the use of an enzyme to generate cargo transport, several studies have also used electric fields as transport-inducing stimulus. Electric fields are a suitable trigger for the manipulation of DNA due to its negative charge. The transport can be directed in either a rotational motion using a robotic DNA origami arm [73] or translational motion by designing a tubular DNA origami transport system (figure 5c) [71]. While this mechanism allows about five times faster transport than DNA walker-based strategies, the transport distance is limited by the length of the DNA origami tube, which currently is about 3 μm. Additionally, the velocity of DNA-based motors is still slow compared with natural motors which move with velocities of the order of 1 μm s−1. An alternative to the presented approaches is the use of natural motors to move on DNA nanotubes [74]. By modifying the natural motor protein dynein with DNA-binding domains and the DNA nanotube with recognition sites, the protein motors can move DNA nanotubes reminiscent to gliding assays of actin filaments or microtubules (figure 5d) [72]. Moreover, by attaching the engineered motors to DNA origami, the DNA origami can be transported with velocities of 20 nm s−1 along the DNA nanotube track. By implementing the previously described branching of DNA nanotubes (figure 2e) the cargo can also be sorted based on the choice of the DNA binding site. However, the use of proteins poses its own challenges because they are difficult to purify, engineer for specific functions and combine with other functional sets of proteins.
Figure 5.
Cargo transport with DNA nanotechnology. (a) DNA origami roll along a surface using an RNase H-mediated burnt-bridge mechanism [70]. (b) The same mechanism can be used for the autonomous transport of SUVs along DNA nanotubes [47]. (c) DNA origami transport system using electric fields [71]. (d) Engineered protein motors on DNA nanotubes move DNA origami cargo [72].
All in all, these versatile strategies to induce cargo transport along DNA nanostructures make up a large toolbox for further studies within the confinement of GUVs.
2.5. Force generation
Cytoskeletal filaments can exert forces within natural cells or even cell communities based on their contractility in the presence of motor proteins like myosin II. The quest for DNA nanomachines that can exert similar forces on DNA nanostructures has resulted in quite a few DNA-based applications towards this aim [75,76]. By functionalizing gold nanoparticles with single-stranded DNA and attaching them to two DNA origami filaments these can slide against each other using toehold-mediated strand displacement (figure 6a) [77,80]. Similarly, gold nanoparticles can also rotate DNA origami structures against each other [81–84]. Interestingly, this transition can even be triggered with an external stimulus like light based on the photoswitching properties of azobenzene incorporated into single-stranded DNA [82]. Towards the engineering of rotary DNA motors that mimic the working principle of the F1F0-ATP synthase DNA origami levers have been attached to a mulitlayer DNA origami base [85–87]. Within these, the lever that has a length of up to 290 nm can freely rotate (figure 6b) [78]. Moreover, the lever rotation can also be coupled to mechanical transitions of the DNA origami base. Similarly, also other DNA origami nanostructures can be reconfigured based on DNA origami shape complementarity [88]. A mechanism that does not rely on DNA origami to generate forces with DNA is the condensation of DNA and proteins into liquid-like condensates. There, DNA condensation exerts forces that drive the growth of the protein–DNA condensate by pulling on non-condensed DNA (figure 6c) [79]. Another mechanism that does not rely on DNA origami is the formation and contraction of DNA rings via synthetic peptides [89]. In this work, DNA-binding peptides induce the crosslinking and sliding of DNA nanotubes along one another to induce ring contraction. Mechanisms involving the formation of DNA rings are particularly interesting because they are the most reminiscent of contractile forces leading to the division of natural cells.
Figure 6.
Force generation via DNA nanostructures. (a) Gold nanoparticles induce sliding of DNA origami filaments against each other using toehold-mediated strand displacement [77]. Scale bar: 20 nm. (b) Brownian motion induces the rotary motion of a 10 helix bundle arm [78]. (c) Protein–DNA condensation pulls non-condensed DNA [79].
3. Conclusion and outlook
The development of more and more complex synthetic cells from the bottom up has deepened our understanding of natural cells. A major focus has been the engineering and reconstitution of natural cytoskeletons into cell-sized compartments. Recent advances of DNA nanotechnology in designing programmable micrometre-sized structures that can be interfaced with GUVs open up a new path towards entirely synthetic cytoskeletons for synthetic cells, engineered from nucleic acids rather than proteins. Our continuously increasing understanding of DNA as a building block adds to the comparably easy tuneability of biophysically relevant parameters with DNA. These include size, shape, diameter, charge, functionalization, persistence and rigidity, which can be easily engineered and altered with DNA nanotechnology. Additionally, the use of DNA nanotechnology is straightforward by making use of the large toolbox of versatile structures and corresponding libraries [90].
These advancements have led to different solutions to establish the versatile functions of cytoskeletons with DNA like morphology control, reversible assembly, symmetry breaking, intracellular transport and force generation. As next challenges we identify (i) the incorporation of all these functionalities into a single DNA nanostructure, (ii) the incorporation of these multifunctional cytoskeletons into GUVs, (iii) mechanisms that allow the micrometre-sized contraction of DNA nanostructures and (iv) catalytic activity and self-regeneration.
Even though a key advantage of DNA nanotechnology is the amount of different nanostructures that perform different functions [4], cytoskeletons are multifunctional. This implies that synthetic DNA cytoskeletons should in principle also be made from a single DNA unit with engineered multifunctionality. Polymerizing Y-motifs are some of the easiest DNA nanostructures but albeit their easy handling and incorporation into GUVs also the least complex ones which poses obstacles for, for example, symmetry breaking or intracellular transport. On the other hand, DNA origami are the most complex DNA structures facing opposite problems since they can be engineered for a multitude of different purposes but often suffer from specialized annealing protocols or low concentrations after purification. Therefore, we identify DNA nanotubes, or variants thereof that are not only based on canonical basepairing, as the most promising candidate for synthetic DNA cytoskeletons. Next to their visual resemblance of microtubules they can be encapsulated at high concentrations, form micrometre-sized filaments and have been widely used for different purposes that relate to cytoskeletal function. Additionally, they can also be combined with DNA origami structures to form DNA nanotube/origami hybrids.
The next crucial step towards synthetic cytoskeletons for synthetic cells is the implementation of these into GUVs. Whereas all of the discussed cytoskeletal functions have been achieved in bulk, only some have been realized inside the confinement of lipid vesicles (table 1). Therefore, this poses another challenge for future work in this direction. However, with the many recently emerged GUV formation techniques, it seems reasonable that the amount of reconstituted DNA nanostructures inside GUVs will increase greatly over the next few years.
Table 1.
Cytoskeletal DNA structures for synthetic cells.
| function | out of GUVs | in GUVs | DNA nanostructure | natural counterpart | references |
|---|---|---|---|---|---|
| mechanical stability | yes | yes | Y-motif | actin | [24,30,31] |
| morphology control | yes | yes | DNA origami, DNA nanotubes | BAR proteins | [25–27,38,40,91] |
| reversible assembly | yes | yes | DNA nanotubes | actin, microtubules, IF | [40,43–45] |
| intracellular transport | yes | no | DNA nanotubes | microtubules | [47,72,74] |
| force generation | yes | no | DNA origami, ssDNA | myosin, kinesin, dynein | [77,78] |
After having established the reconstitution of multifunctional synthetic DNA cytoskeletons into GUVs, it will be essential to improve the current strength of forces and transport velocities that can be generated by DNA cytoskeletons. As cytoskeletal forces drive cell motility by balancing filament polymerization and contraction it will be an important step for the future success of DNA cytoskeletons to achieve the active stimuli-responsive remodelling of the GUV membrane from the inside. Moreover, this mechanism can be directly related to the division of GUVs as synthetic cell compartments, e.g. by engineering DNA rings that can contract and induce vesicle fission.
All in all, the development of synthetic DNA-based cytoskeletons for synthetic cells with unique multifunctionality and straightforward tuneability will be an exciting step in the bottom-up assembly of truly synthetic cells from molecular building blocks. Thereby, DNA cytoskeletons will not only advance our knowledge in DNA nanotechnology but also serve as well-characterized model systems to understand natural cytoskeletons. Along this way, they allow the assembly of rationally engineered complex synthetic cells with applications in biomedicine, cellular biophysics and synthetic biology.
Contributor Information
Kevin Jahnke, Email: kjahnke@g.harvard.edu.
Kerstin Göpfrich, Email: k.goepfrich@zmbh.uni-heidelberg.de.
Data accessibility
This article has no additional data.
Authors' contributions
K.J.: conceptualization, visualization, writing—original draft, writing—review and editing; K.G.: conceptualization, writing—original draft, writing—review and editing.
Both authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Open access funding provided by the Max Planck Society.
K.G. received funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy via the Excellence Cluster 3D Matter Made to Order (EXC-2082/1-390761711), the Max Planck Society and the Hector Fellow Academy. K.J. thanks the Carl Zeiss Foundation, the Joachim Herz Foundation and the Alexander von Humboldt Foundation for financial support.
References
- 1.Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P. 2017. Molecular biology of the cell, ch. 16. New York, NY: W. W. Norton & Company. [Google Scholar]
- 2.Bashirzadeh Y, Liu AP. 2019. Encapsulation of the cytoskeleton: towards mimicking the mechanics of a cell. Soft Matter 15, 8425-8436. ( 10.1039/C9SM01669D) [DOI] [PubMed] [Google Scholar]
- 3.Göpfrich K, Platzman I, Spatz JP. 2018. Mastering complexity: towards bottom-up construction of multifunctional eukaryotic synthetic cells. Trends Biotechnol. 36, 938-951. ( 10.1016/j.tibtech.2018.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeman NC, Sleiman HF. 2017. DNA nanotechnology. Nat. Rev. Mater. 3, 17068. ( 10.1038/natrevmats.2017.68) [DOI] [Google Scholar]
- 5.Ohmann A, Li C-Y, Maffeo C, Nahas KA, Baumann KN, Göpfrich K, Yoo J, Keyser UF, Aksimentiev A. 2018. A synthetic enzyme built from DNA flips 107 lipids per second in biological membranes. Nat. Commun. 9, 2426. ( 10.1038/s41467-018-04821-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langecker M, Arnaut V, Martin TG, List J, Renner S, Mayer M, Dietz H, Simmel FC. 2012. Synthetic lipid membrane channels formed by designed DNA nanostructures. Science 338, 932-936. ( 10.1126/science.1225624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Göpfrich K, et al. 2016. Large-conductance transmembrane porin made from DNA origami. ACS Nano 10, 8207-8214. ( 10.1021/acsnano.6b03759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fragasso A, De Franceschi N, Stömmer P, Van Der Sluis EO, Dietz H, Dekker C. 2021. Reconstitution of ultrawide DNA origami pores in liposomes for transmembrane transport of macromolecules. ACS Nano 15, 12 768-12 779. ( 10.1021/acsnano.1c01669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoenit A, Giudice CL, Hahnen N, Ollech D, Jahnke K, Göpfrich K, Cavalcanti-Adam EA. 2021. Tuning epithelial cell–cell adhesion and collective dynamics with functional DNA-E-cadherin hybrid linkers. Nano Lett. 22, 302-310. ( 10.1021/acs.nanolett.1c03780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffecker IT, Arima Y, Iwata H. 2019. Tuning intercellular adhesion with membrane-anchored oligonucleotides. J. R. Soc. Interface 16, 20190299. ( 10.1098/rsif.2019.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng W, et al. 2016. An autonomous molecular assembler for programmable chemical synthesis. Nat. Chem. 8, 542-548. ( 10.1038/nchem.2495) [DOI] [PubMed] [Google Scholar]
- 12.Gu H, Chao J, Xiao S-J, Seeman NC. 2010. A proximity-based programmable DNA nanoscale assembly line. Nature 465, 202-205. ( 10.1038/nature09026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parolini L, Kotar J, Michele LD, Mognetti BM. 2016. Controlling self-assembly kinetics of DNA-functionalized liposomes using toehold exchange mechanism. ACS Nano 10, 2392-2398. ( 10.1021/acsnano.5b07201) [DOI] [PubMed] [Google Scholar]
- 14.Rothemund PWK, Ekani-Nkodo A, Papadakis N, Kumar A, Fygenson DK, Winfree E. 2004. Design and characterization of programmable DNA nanotubes. J. Am. Chem. Soc. 126, 16 344-16 352. ( 10.1021/ja044319l) [DOI] [PubMed] [Google Scholar]
- 15.Shang L, Cheng Y, Zhao Y. 2017. Emerging droplet microfluidics. Chem. Rev. 117, 7964-8040. ( 10.1021/acs.chemrev.6b00848) [DOI] [PubMed] [Google Scholar]
- 16.Dimova R, Marques CM (eds). 2019. The giant vesicle book. Boca Raton, FL: CRC Press. [Google Scholar]
- 17.Deshpande S, Caspi Y, Meijering AEC, Dekker C. 2016. Octanol-assisted liposome assembly on chip. Nat. Commun. 7, 10447. ( 10.1038/ncomms10447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abkarian M, Loiseau E, Massiera G. 2011. Continuous droplet interface crossing encapsulation (cDICE) for high throughput monodisperse vesicle design. Soft Matter 7, 4610. ( 10.1039/c1sm05239j) [DOI] [Google Scholar]
- 19.de Cauter LV, et al. 2021. Optimized cDICE for efficient reconstitution of biological systems in giant unilamellar vesicles. ACS Synth. Biol. 10, 1690-1702. ( 10.1021/acssynbio.1c00068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss M, et al. 2017. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat. Mater. 17, 89-96. ( 10.1038/nmat5005) [DOI] [PubMed] [Google Scholar]
- 21.Göpfrich K, Haller B, Staufer O, Dreher Y, Mersdorf U, Platzman I, Spatz JP. 2019. One-pot assembly of complex giant unilamellar vesicle-based synthetic cells. ACS Synth. Biol. 8, 937-947. ( 10.1021/acssynbio.9b00034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otto O, et al. 2015. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat. Methods 12, 199-202. ( 10.1038/nmeth.3281) [DOI] [PubMed] [Google Scholar]
- 23.Dimova R. 2014. Recent developments in the field of bending rigidity measurements on membranes. Adv. Colloid Interface Sci. 208, 225-234. ( 10.1016/j.cis.2014.03.003) [DOI] [PubMed] [Google Scholar]
- 24.Kurokawa C, et al. 2017. DNA cytoskeleton for stabilizing artificial cells. Proc. Natl Acad. Sci. USA 114, 7228-7233. ( 10.1073/pnas.1702208114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franquelim HG, Khmelinskaia A, Sobczak J-P, Dietz H, Schwille P. 2018. Membrane sculpting by curved DNA origami scaffolds. Nat. Commun. 9, 811. ( 10.1038/s41467-018-03198-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franquelim HG, Dietz H, Schwille P. 2021. Reversible membrane deformations by straight DNA origami filaments. Soft Matter 17, 276-287. ( 10.1039/D0SM00150C) [DOI] [PubMed] [Google Scholar]
- 27.Jahnke K, et al. 2021. Proton gradients from light-harvesting E. coli control DNA assemblies for synthetic cells. Nat. Commun. 12, 3967. ( 10.1038/s41467-021-24103-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Q, Grome MW, Yang Y, Lin C. 2019. Engineering lipid membranes with programmable DNA nanostructures. Adv. Biosyst. 4, 1900215. ( 10.1002/adbi.201900215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenit A, Cavalcanti-Adam EA, Göpfrich K. 2021. Functionalization of cellular membranes with DNA nanotechnology. Trends Biotechnol. 39, 1208-1220. ( 10.1016/j.tibtech.2021.02.002) [DOI] [PubMed] [Google Scholar]
- 30.Baumann KN, Piantanida L, García-Nafría J, Sobota D, Voïtchovsky K, Knowles TPJ, Hernández-Ainsa S. 2020. Coating and stabilization of liposomes by clathrin-inspired DNA self-assembly. ACS Nano 14, 2316-2323. ( 10.1021/acsnano.9b09453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumann KN, Schröder T, Ciryam PS, Morzy D, Tinnefeld P, Knowles TPJ, Hernández-Ainsa S. 2022. DNA-liposome hybrid carriers for triggered cargo release. ACS Appl. Bio Mater. 5, 3713-3721. ( 10.1021/acsabm.2c00225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morzy D, Rubio-Sánchez R, Joshi H, Aksimentiev A, Michele LD, Keyser UF. 2021. Cations regulate membrane attachment and functionality of DNA nanostructures. J. Am. Chem. Soc. 143, 7358-7367. ( 10.1021/jacs.1c00166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dey S, et al. 2021. DNA origami. Nat. Rev. Methods Primers 1, 13. ( 10.1038/s43586-020-00009-8) [DOI] [Google Scholar]
- 34.Grome MW, Zhang Z, Pincet F, Lin C. 2018. Vesicle tubulation with self-assembling DNA nanosprings. Angew. Chem. Int. Ed. 57, 5330-5334. ( 10.1002/anie.201800141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikolov V, Lipowsky R, Dimova R. 2007. Behavior of giant vesicles with anchored DNA molecules. Biophys. J. 92, 4356-4368. ( 10.1529/biophysj.106.100032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kocabey S, Kempter S, List J, Xing Y, Bae W, Schiffels D, Shih WM, Simmel FC, Liedl T. 2015. Membrane-assisted growth of DNA origami nanostructure arrays. ACS Nano 9, 3530-3539. ( 10.1021/acsnano.5b00161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuraw-Weston SE, Siavashpouri M, Moustaka ME, Gerling T, Dietz H, Fraden S, Ribbe AE, Dinsmore AD. 2021. Membrane remodeling by DNA origami nanorods: experiments exploring the parameter space for vesicle remodeling. Langmuir 37, 6219-6231. ( 10.1021/acs.langmuir.1c00416) [DOI] [PubMed] [Google Scholar]
- 38.Czogalla A, Kauert DJ, Franquelim HG, Uzunova V, Zhang Y, Seidel R, Schwille P. 2015. Amphipathic DNA origami nanoparticles to scaffold and deform lipid membrane vesicles. Angew. Chem. Int. Ed. 54, 6501-6505. ( 10.1002/anie.201501173) [DOI] [PubMed] [Google Scholar]
- 39.Franceschi ND, Pezeshkian W, Fragasso A, Bruininks BMH, Tsai S, Marrink SJ, Dekker C. 2022. Synthetic membrane shaper for controlled liposome deformation. ACS Nano 17, 966-978. ( 10.1021/acsnano.2c06125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jahnke K, Huth V, Mersdorf U, Liu N, Goepfrich K. 2022. Bottom-up assembly of DNA cytoskeletons for synthetic cells. ACS Nano 16, 7233-7241. ( 10.1021/acsnano.1c10703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arulkumaran N, Singer M, Howorka S, Burns JR. 2023. Creating complex protocells and prototissues using simple DNA building blocks. Nat. Commun. 14, 1314. ( 10.1038/s41467-023-36875-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchison T, Kirschner M. 1984. Dynamic instability of microtubule growth. Nature 14, 1314. ( 10.1038/312237a0) [DOI] [PubMed] [Google Scholar]
- 43.Green LN, Subramanian HKK, Mardanlou V, Kim J, Hariadi RF, Franco E. 2019. Autonomous dynamic control of DNA nanostructure self-assembly. Nat. Chem. 11, 510-520. ( 10.1038/s41557-019-0251-8) [DOI] [PubMed] [Google Scholar]
- 44.Green LN, Amodio A, Subramanian HKK, Ricci F, Franco E. 2017. pH-driven reversible self-assembly of micron-scale DNA scaffolds. Nano Lett. 17, 7283-7288. ( 10.1021/acs.nanolett.7b02787) [DOI] [PubMed] [Google Scholar]
- 45.Agarwal S, Klocke MA, Pungchai PE, Franco E. 2021. Dynamic self-assembly of compartmentalized DNA nanotubes. Nat. Commun. 12, 3557. ( 10.1038/s41467-021-23850-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranallo S, Sorrentino D, Ricci F. 2019. Orthogonal regulation of DNA nanostructure self-assembly and disassembly using antibodies. Nat. Commun. 10, 5509. ( 10.1038/s41467-019-13104-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhan P, Jahnke K, Liu N, Göpfrich K. 2022. Functional DNA-based cytoskeletons for synthetic cells. Nat. Chem. 14, 958-963. ( 10.1038/s41557-022-00945-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gentile S, Grosso ED, Pungchai PE, Franco E, Prins LJ, Ricci F. 2021. Spontaneous reorganization of DNA-based polymers in higher ordered structures fueled by RNA. J. Am. Chem. Soc. 143, 20 296-20 301. ( 10.1021/jacs.1c09503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng J, Walther A. 2021. Autonomous DNA nanostructures instructed by hierarchically concatenated chemical reaction networks. Nat. Commun. 12, 5132. ( 10.1038/s41467-021-25450-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi-Gendron C, Fakih FE, Nakazawa K, Chocron L, Endo M, Sugiyama H, Morel M, Rudiuk S, Baigl D. 2022. Isothermal self-assembly of multicomponent and evolutive DNA nanostructures. ChemRxiv. ( 10.26434/chemrxiv-2022-12jqs) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li R, Bowerman B. 2010. Symmetry breaking in biology. Cold Spring Harb. Perspect. Biol. 2, a003475. ( 10.1101/cshperspect.a003475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohammed AM, Schulman R. 2013. Directing self-assembly of DNA nanotubes using programmable seeds. Nano Lett. 13, 4006-4013. ( 10.1021/nl400881w) [DOI] [PubMed] [Google Scholar]
- 53.Barish RD, Schulman R, Rothemund PWK, Winfree E. 2009. An information-bearing seed for nucleating algorithmic self-assembly. Proc. Natl Acad. Sci. USA 106, 6054-6059. ( 10.1073/pnas.0808736106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agrawal DK, Jiang R, Reinhart S, Mohammed AM, Jorgenson TD, Schulman R. 2017. Terminating DNA tile assembly with nanostructured caps. ACS Nano 11, 9770-9779. ( 10.1021/acsnano.7b02256) [DOI] [PubMed] [Google Scholar]
- 55.Mohammed AM, Šulc P, Zenk J, Schulman R. 2016. Self-assembling DNA nanotubes to connect molecular landmarks. Nat. Nanotechnol. 12, 312-316. ( 10.1038/nnano.2016.277) [DOI] [PubMed] [Google Scholar]
- 56.Jia S, et al. 2021. Growth and site-specific organization of micron-scale biomolecular devices on living mammalian cells. Nat. Commun. 12, 5729. ( 10.1038/s41467-021-25890-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohammed AM, Velazquez L, Chisenhall A, Schiffels D, Fygenson DK, Schulman R. 2017. Self-assembly of precisely defined DNA nanotube superstructures using DNA origami seeds. Nanoscale 9, 522-526. ( 10.1039/C6NR06983E) [DOI] [PubMed] [Google Scholar]
- 58.Schaffter SW, Schneider J, Agrawal DK, Pacella MS, Rothchild E, Murphy T, Schulman R. 2020. Reconfiguring DNA nanotube architectures via selective regulation of terminating structures. ACS Nano 14, 13 451-13 462. ( 10.1021/acsnano.0c05340) [DOI] [PubMed] [Google Scholar]
- 59.Dhanasekar NN, Li Y, Schulman R. 2022. The ion permeability of DNA nanotube channels. bioRxiv. ( 10.1101/2022.03.04.482952) [DOI] [Google Scholar]
- 60.Li Y, Maffeo C, Joshi H, Aksimentiev A, Ménard B, Schulman R. 2022. Leakless end-to-end transport of small molecules through micron-length DNA nanochannels. Sci. Adv. 8, eabq4834. ( 10.1126/sciadv.abq4834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jahnke K, Illig M, Scheffold M, Tran MP, Mersdorf U, Göpfrich K. In press. DNA origami signaling units transduce chemical and mechanical signals in synthetic cells. Adv. Funct. Mater. ( 10.1002/adfm.202301176) [DOI] [Google Scholar]
- 62.Yin P, Yan H, Daniell XG, Turberfield AJ, Reif JH. 2004. A unidirectional DNA walker that moves autonomously along a track. Angew. Chem. 116, 5014-5019. ( 10.1002/ange.200460522) [DOI] [PubMed] [Google Scholar]
- 63.Shin J-S, Pierce NA. 2004. A synthetic DNA walker for molecular transport. J. Am. Chem. Soc. 126, 10 834-10 835. ( 10.1021/ja047543j) [DOI] [PubMed] [Google Scholar]
- 64.Wickham SFJ, Bath J, Katsuda Y, Endo M, Hidaka K, Sugiyama H, Turberfield AJ. 2012. A DNA-based molecular motor that can navigate a network of tracks. Nat. Nanotechnol. 7, 169-173. ( 10.1038/nnano.2011.253) [DOI] [PubMed] [Google Scholar]
- 65.Cha T-G, Pan J, Chen H, Salgado J, Li X, Mao C, Choi JH. 2013. A synthetic DNA motor that transports nanoparticles along carbon nanotubes. Nat. Nanotechnol. 9, 39-43. ( 10.1038/nnano.2013.257) [DOI] [PubMed] [Google Scholar]
- 66.Yehl K, Mugler A, Vivek S, Liu Y, Zhang Y, Fan M, Weeks ER, Salaita K. 2015. High-speed DNA-based rolling motors powered by RNase H. Nat. Nanotechnol. 11, 184-190. ( 10.1038/nnano.2015.259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blanchard AT, Bazrafshan AS, Yi J, Eisman JT, Yehl KM, Bian T, Mugler A, Salaita K. 2019. Highly polyvalent DNA motors generate 100 + pN of force via autochemophoresis. Nano Lett. 19, 6977-6986. ( 10.1021/acs.nanolett.9b02311) [DOI] [PubMed] [Google Scholar]
- 68.Bazrafshan A, et al. 2021. DNA gold nanoparticle motors demonstrate processive motion with bursts of speed up to 50 nm per second. ACS Nano 15, 8427-8438. ( 10.1021/acsnano.0c10658) [DOI] [PubMed] [Google Scholar]
- 69.Piranej S, Bazrafshan A, Salaita K. 2022. Chemical-to-mechanical molecular computation using DNA-based motors with onboard logic. Nat. Nanotechnol. 17, 514-523. ( 10.1038/s41565-022-01080-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bazrafshan A, Meyer TA, Su H, Brockman JM, Blanchard AT, Piranej S, Duan Y, Ke Y, Salaita K. 2020. Tunable DNA origami motors translocate ballistically over μm distances at nm s−1 speeds. Angew. Chem. 132, 9601-9608. ( 10.1002/ange.201916281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stömmer P, Kiefer H, Kopperger E, Honemann MN, Kube M, Simmel FC, Netz RR, Dietz H. 2021. A synthetic tubular molecular transport system. Nat. Commun. 12, 4393. ( 10.1038/s41467-021-24675-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ibusuki R, Morishita T, Furuta A, Nakayama S, Yoshio M, Kojima H, Oiwa K, Furuta K. 2022. Programmable molecular transport achieved by engineering protein motors to move on DNA nanotubes. Science 375, 1159-1164. ( 10.1126/science.abj5170) [DOI] [PubMed] [Google Scholar]
- 73.Kopperger E, List J, Madhira S, Rothfischer F, Lamb DC, Simmel FC. 2018. A self-assembled nanoscale robotic arm controlled by electric fields. Science 359, 296-301. ( 10.1126/science.aao4284) [DOI] [PubMed] [Google Scholar]
- 74.Ibusuki R, Shiraga M, Furuta A, Yoshio M, Kojima H, Oiwa K, Furuta K. 2020. Collective motility of dynein linear arrays built on DNA nanotubes. Biochem. Biophys. Res. Commun. 523, 1014-1019. ( 10.1016/j.bbrc.2019.12.125) [DOI] [PubMed] [Google Scholar]
- 75.Endo M, Sugiyama H. 2018. DNA origami nanomachines. Molecules 23, 1766. ( 10.3390/molecules23071766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bath J, Turberfield AJ. 2007. DNA nanomachines. Nat. Nanotechnol. 2, 275-284. ( 10.1038/nnano.2007.104) [DOI] [PubMed] [Google Scholar]
- 77.Urban MJ, Both S, Zhou C, Kuzyk A, Lindfors K, Weiss T, Liu N. 2018. Gold nanocrystal-mediated sliding of doublet DNA origami filaments. Nat. Commun. 9, 1454. ( 10.1038/s41467-018-03882-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bertosin E, Maffeo CM, Drexler T, Honemann MN, Aksimentiev A, Dietz H. 2021. A nanoscale reciprocating rotary mechanism with coordinated mobility control. Nat. Commun. 12, 7138. ( 10.1038/s41467-021-27230-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quail T, Golfier S, Elsner M, Ishihara K, Murugesan V, Renger R, Jülicher F, Brugués J. 2021. Force generation by protein–DNA co-condensation. Nat. Phys. 17, 1007-1012. ( 10.1038/s41567-021-01285-1) [DOI] [Google Scholar]
- 80.Zhan P, Urban MJ, Both S, Duan X, Kuzyk A, Weiss T, Liu N. 2019. DNA-assembled nanoarchitectures with multiple components in regulated and coordinated motion. Sci. Adv. 5, eaax6023. ( 10.1126/sciadv.aax6023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peil A, Xin L, Both S, Shen L, Ke Y, Weiss T, Zhan P, Liu N. 2022. DNA assembly of modular components into a rotary nanodevice. ACS Nano 16, 5284-5291. ( 10.1021/acsnano.1c10160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuzyk A, Schreiber R, Zhang H, Govorov AO, Liedl T, Liu N. 2014. Reconfigurable 3D plasmonic metamolecules. Nat. Mater. 13, 862-866. ( 10.1038/nmat4031) [DOI] [PubMed] [Google Scholar]
- 83.Xin L, Duan X, Liu N. 2021. Dimerization and oligomerization of DNA-assembled building blocks for controlled multi-motion in high-order architectures. Nat. Commun. 12, 3207. ( 10.1038/s41467-021-23532-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Göpfrich K, Urban MJ, Frey C, Platzman I, Spatz JP, Liu N. 2020. Dynamic actuation of DNA-assembled plasmonic nanostructures in microfluidic cell-sized compartments. Nano Lett. 20, 1571-1577. ( 10.1021/acs.nanolett.9b04217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ketterer P, Willner EM, Dietz H. 2016. Nanoscale rotary apparatus formed from tight-fitting 3D DNA components. Sci. Adv. 2, e1501209. ( 10.1126/sciadv.1501209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pumm A-K, et al. 2022. A DNA origami rotary ratchet motor. Nature 607, 492-498. ( 10.1038/s41586-022-04910-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi X, et al. 2022. Sustained unidirectional rotation of a self-organized DNA rotor on a nanopore. Nat. Phys. 18, 1105-1111. ( 10.1038/s41567-022-01683-z) [DOI] [Google Scholar]
- 88.Gerling T, Wagenbauer KF, Neuner AM, Dietz H. 2015. Dynamic DNA devices and assemblies formed by shape-complementary, non-base pairing 3D components. Science 347, 1446-1452. ( 10.1126/science.aaa5372) [DOI] [PubMed] [Google Scholar]
- 89.Illig M, Jahnke K, Scheffold M, Mersdorf U, Drechsler H, Diez S, Göpfrich K. 2023. Self-assembly and contraction of micron-scale DNA rings. bioRxiv. ( 10.1101/2023.03.09.531887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poppleton E, Mallya A, Dey S, Joseph J, Šulc P. 2021. Nanobase.org: a repository for DNA and RNA nanostructures. Nucleic Acids Res. 50, D246-D252. ( 10.1093/nar/gkab1000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franceschi ND, Pezeshkian W, Fragasso A, Bruininks BM, Tsai S, Marrink SJ, Dekker C. 2023. Synthetic membrane shaper for controlled liposome deformation. ACS Nano 17, 966-978. ( 10.1021/acsnano.2c06125) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.