Summary
About 95% of cervical cancers worldwide are caused by human papillomavirus (HPV). Cervical cancer is preventable and curable if it is detected and treated early. We reviewed the latest national cervical cancer indicators, and barriers to HPV vaccination and cervical cancer screening in 21 Asian National Cancer Centers Alliance (ANCCA) member countries. Half (n = 11, 52%) of the countries have introduced HPV vaccination for girls as part of their national vaccination programme, three countries reported coverage of over 90%. Most ANCCA member countries have cervical cancer screening programmes, only five countries reported screening uptake of over 50%. The barriers to HPV vaccination coverage and cervical cancer screening participation have been identified. Ensuring health service accessibility and affordability for women, addressing sociocultural barriers, and strengthening the healthcare system and continuum of care are essential to increase HPV vaccination and cervical cancer screening coverage.
Keywords: Cervical cancer, Elimination, HPV vaccination, Screening, Barriers, ANCCA, Asia
Background
Cervical cancer is preventable and also curable if it is detected and treated early.1 According to the International Agency for Research on Cancer (IARC), approximately 95% of cervical cancer cases worldwide are caused by persistent infections with oncogenic strains of HPV.2 In 2020, the WHO Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem was adopted by the World Health Assembly, to reduce cervical cancer incidence to below 4 per 100,000 women by the end of the century. The 90-70-90 targets to be met by 2030 include: 90% of girls to be vaccinated against the HPV by age 15 years; 70% of women to be screened with a high-performance test by 35 and again at 45 years of age; and 90% of women identified with cervical cancer or its precursor lesions to be given appropriate treatment.3
In 2020, about 90% of cervical cancer deaths globally occurred in low-and middle-income countries (LMICs).4 The case fatality rate of cervical cancer is high in LMICs where more than 60% of women with cervical cancer die from the disease. This is double the fatality rate seen in many high-income countries (<30%).3 About three-quarters of countries in Asia are considered as LMICs. There is remarkable disparity in terms of cervical cancer incidence and mortality across Asia. GLOBOCAN estimated that about 352,000 new cases of cervical cancer in 2020 occurred in Asia, accounting for 58% of new cervical cancer cases worldwide. About 200,000 women in Asia died from cervical cancer in 2020; this comprised 59% of cervical cancer deaths worldwide.4
The Asian National Cancer Centers Alliance (ANCCA) was established in 2005 by several Asian national cancer centres from the Western Pacific and South Asia regions, to collaborate on cancer prevention, control and research. We conducted a narrative review of the published literature on cervical cancer burden, HPV vaccination, cervical cancer screening, barriers to HPV vaccination and cervical cancer screening among ANCCA member countries.
Methods
We reviewed published scientific articles, national health surveys (e.g. WHO STEPwise approach to noncommunicable disease risk factors surveillance [STEPS]), and international publications such as those from the WHO, IARC, UNICEF, HPV Information Centre. The goals of the literature review were to 1) gather the latest and most representative country-specific indicators on cervical cancer incidence and mortality, HPV vaccination and cervical cancer screening; and 2) identify barriers to HPV vaccination and cervical cancer screening in the Asian settings.
We extracted age standardised cervical cancer burden data from GLOBOCAN 2020 and cross checked with published data from ANCCA countries. Latest statistics on HPV vaccination and cervical cancer screening were extracted from publications of national and international agencies such as STEPS, WHO and UNICEF databases (Supplementary information Appendix A).
Using a standardised data collection form and questionnaire (Supplementary information Appendix B), an online survey was conducted among ANCCA member countries to identify the common barriers to HPV vaccination and cervical cancer screening, and country-specific information. The survey was administered from January to March 2023 among ANCCA members from Bangladesh, Bhutan, Brunei, Cambodia, China, India, Indonesia, Iran, Japan, Laos, Malaysia, Mongolia, Myanmar, Nepal, Pakistan, Philippines, Singapore, South Korea, Sri Lanka, Thailand and Vietnam. Information and literature on barriers which have significant impact on the HPV vaccination and cervical cancer screening uptake in their countries were collated and summarised. These included barriers related to resources, healthcare system, sociocultural attitudes and knowledge.3 Recommendations to address such barriers were also identified to improve HPV vaccination and cervical cancer screening coverage. The experts from the 21 ANCCA member countries consisted of oncologists, gynaecologists, public health physicians and academics affiliated with the national cancer centres or health departments. They provided country-specific data using a standardised data collection form (Supplementary information Appendix B).
Search strategy and selection criteria.
Published material for this narrative review study were identified through searches of PubMed and Google Scholar using combinations of the search terms “human papillomavirus vaccine”, “cervical cancer screening”, “Asia”, “immunisation programme”, “coverage”, “age”, “barriers”, “Bangladesh”, “Bhutan”, “Brunei”, “Cambodia”, “China”, “India”, “Indonesia”, “Iran”, “Japan”, “Korea”, “Laos”, “Malaysia”, “Mongolia”, “Myanmar”, “Nepal”, “Pakistan”, “Philippines”, “Singapore”, “Sri Lanka”, “Thailand” and “Vietnam” from 2010 through 2023. Publications were also identified through searches of the authors’ own files and from established sources such as the national and international health websites. Authors also reviewed relevant national reports published in their national languages where applicable. The final reference list was generated on the basis of originality and relevance to the broad scope of this review study.
Findings
Cervical cancer incidence and mortality among ANCCA member countries
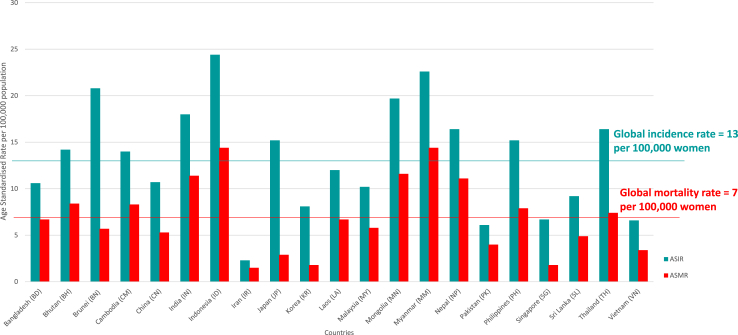
Among ANCCA member countries, the age-standardised incidence rates varied from 2.3 per 100,000 women in Iran to 24.4 per 100,000 women in Indonesia; and the age-standardized mortality rates ranged from 1.5 per 100,000 women in Iran to 14.4 per 100,000 women in Indonesia and Myanmar (Table 1, Fig. 1). In comparison, the global age-standardised incidence rate and mortality rate for cervical cancer in 2020 were 13 per 100,000 women and 7 per 100,000 women, respectively.5
Table 1.
Cervical cancer burden and HPV vaccination indicators in ANCCA members.
| Population (thousand) | ASIR | ASMR | HPV vac proga | Year HPV vaccine introduced | Target age (school year) | HPV vac rateb | Population of girls aged 15 (thousand) | |
|---|---|---|---|---|---|---|---|---|
| Bangladesh | 166,427 | 10.6 | 6.7 | O | 2016 | 10–15 | ND | 1616 |
| Bhutan | 770 | 14.2 | 8.4 | N | 2010 | 12–18 | 97 | 7 |
| Brunei Darussalam | 440 | 20.8 | 5.7 | N | 2012 | 11–16 | 97 | 3 |
| Cambodia | 16,296 | 14 | 8.3 | O | 2017 | 9 | ND | 150 |
| China | 1,423,998 | 10.7 | 5.3 | O | 2016 | ND | ND | 7511 |
| India | 1,389,966 | 18 | 11.4 | O | 2008 | 9–14 | ND | 12 027 |
| Indonesia | 270,826 | 24.4 | 14.4 | N | 2017 | 11–16 | 6 | 2219 |
| Iran | 86,990 | 2.3 | 1.5 | O | 2019 | 9–14 | ND | 588 |
| Japan | 125,543 | 15.2 | 2.9 | N | 2013 | 12–16 | 16 | 530 |
| Korea, Republic of | 51,858 | 11.4 | 1.7 | N | 2016 | 11–12 | 87 | 211 |
| Lao PDR | 7266 | 12 | 6.7 | N | 2020 | 10–14 | 87 | 73 |
| Malaysia | 33,004 | 10.2 | 5.8 | N | 2010 | 13 | 85 | 442 |
| Mongolia | 3322 | 19.7 | 11.6 | O | 2012 | 11–15 | ND | 23 |
| Myanmar | 53,228 | 22.6 | 14.4 | N | 2020 | 9–10 | 89 | 442 |
| Nepal | 28,999 | 16.4 | 11.1 | O | 2016 | 11–13 | ND | 307 |
| Pakistan | 225,113 | 6.1 | 4.0 | O | 2019 | ND | ND | 2464 |
| Philippines | 111,288 | 15.2 | 7.9 | P | 2015 | 9–14 | 28 | 1072 |
| Singapore | 5894 | 6.7 | 1.8 | N | 2010 | 12–13 | 93 | 24 |
| Sri Lanka | 21,683 | 9.2 | 4.9 | N | 2017 | 10–11 | 71 | 178 |
| Thailand | 71,389 | 16.4 | 7.4 | N | 2017 | 11–12 | 80 | 396 |
| Vietnam | 96,204 | 6.6 | 3.4 | P | 2019 | 9–13 | ND | 668 |
Abbreviation: ND = No published data available.
ASIR = Age standardized incidence rate of cervical cancer per 100,000 women; ASMR = Age standardized mortality rate of cervical cancer per 100,000 women.
Types of HPC vaccination programme: N = Nationwide HPV vaccination or cervical cancer screening program implementation; P = Provincial/Regional/State HPV vaccination or cervical cancer screening program implementation; O = Other types of vaccination or screening programme e.g. private or opportunistic.
Latest HPV vaccination coverage of girls of target age groups received at least 1 dose of HPV vaccine.
Fig. 1.
Cervical cancer burden among ANCCA member countries.
HPV vaccination coverage among ANCCA members
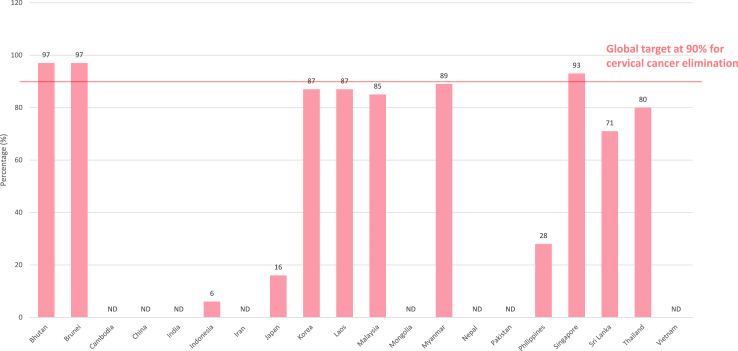
Vaccination against HPV given to adolescent girls prior to sexual initiation has been found to be highly cost-effective in reducing the risk of invasive cervical cancer.2 Worldwide, it was estimated that only 12% of adolescent girls completed the full course of HPV vaccination in 2021, down from 14% in 2019 before COVID-19 pandemic.6 The coverage of the first dose of HPV vaccination was even lower in the Asia region at about 6% in the Western Pacific Region and about 3% in the South East Asia Region.7 About half (n = 11, 52%) of ANCCA member countries have introduced HPV vaccination as part of their national vaccination programme or school health programme. Only three out of 21 (14%) ANCCA member countries (Bhutan, Brunei and Singapore) have reported first dose national vaccination coverage of above 90% for girls by 15 years of age (Table 1, Fig. 2). About half of the ANCCA member countries (n = 11, 52%) reported a lack of national surveillance data on HPV vaccination coverage.
Fig. 2.
HPV vaccination coverage of girls by age 15 years with at least 1 dose ofthevaccine.
A comparative modelling analysis in 78 LMICs found that by meeting the 90% HPV vaccination target, cervical cancer incidence can be reduced by 89% within a century in the LMICs.8 The majority (>80%) of ANCCA member countries are LMICs. Among ANCCA member countries, in the absence of vaccination, 4.2 million cervical cancer cases are predicted to occur among women born between 2005 and 2014. Out of these, with HPV vaccination, an estimated 3.8 million cases can be prevented.9
Cervical cancer screening coverage among ANCCA member countries
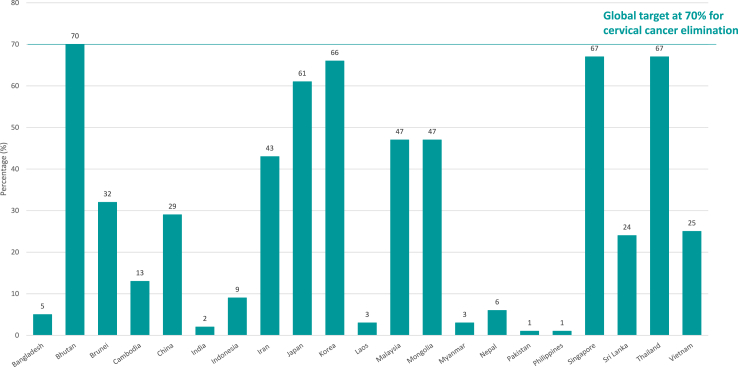
The WHO recommendations for cervical cancer screening prioritise screening women aged 30–49 years in the general population, to detect and treat pre-cancer and malignant cervical lesions early.10 Although most ANCCA member countries (n = 16, 76%) have implemented national cervical cancer screening programme, the coverage has been low, only 5 out of the 21 (24%) ANCCA member countries (Bhutan, Japan, South Korea, Singapore and Thailand) have a screening coverage of over 50% among women aged 30 to 49 in previous 5 years (Fig. 3).
Fig. 3.
Cervical cancer screening coverage in previous 5 years among women aged 30–49.
In the last century, cytology-based cervical cancer screening has been crucial in reducing the incidence and mortality of cervical cancer. HPV testing was subsequently developed with the discovery of the role of HPV in the oncogenesis of cervical cancer. Many countries have moved from VIA and cytology to using HPV testing as the primary screening mode, based on evidence that primary HPV screening provides higher sensitivity to detect pre-neoplastic lesions, better re-assurance with a negative test, and safe prolongation of screening intervals.11 Among ANCCA members, about a third (n = 8, 38%) of countries utilise VIA as the primary screening test, while the rest of the countries use cytology alone or in combination with VIA or HPV test, only 4 countries have rolled out HPV test in the national screening programme (Table 2).
Table 2.
Cervical cancer screening indicators in ANCCA members.
| Screening programa | Year started | Target age group | Primary screening testb | Screen 30–49 years: previous 5 years (95% CI)c | Screen 30–49 years: ever (95% CI)d | Population of women aged 30–49 (thousand) | |
|---|---|---|---|---|---|---|---|
| Bangladesh | N | 2006 | 30–60 | VIA | 5 (5–6) | 7 (7–7) | 24,338 |
| Bhutan | N | 2006 | 25-65/30-76 | HPV/Cyto | 70 (66–73) | 82 (80–84) | 109 |
| Brunei Darussalam | N | 2011 | 20–65 | Cyto/HPV | 32 (30–33) | 68 (64–71) | 69 |
| Cambodia | N | 2018 | 30–49 | VIA | 13 (9–19) | 17 (12–24) | 2193 |
| China | N | 2019 | 35–64 | Cyto/HPV | 29 (28–31) | 31 (29–33) | 210,550 |
| India | N | 2016 | 30–65 | VIA/Cyto | 2 (2–2) | 2 (2–2) | 189,736 |
| Indonesia | N | 2008 | 30–50 | VIA | 9 (9–9) | 12 (10–14) | 39,089 |
| Iran | N | 2018 | 30–49 | Cyto | 43 (38–48) | 49 (45–54) | 14,859 |
| Japan | N | 1983 | ≥20 | Cyto | 61 (55–68) | 70 (59–80) | 15,320 |
| Korea, Republic of | N | 1999 | ≥20 | Cyto | 66 (62–71) | 69 (64–74) | 7341 |
| Lao PDR | O | ND | ND | Cyto/VIA | 3 (2–5) | 4 (3–6) | 955 |
| Malaysia | N | 2019 | 30–65 | HPV/Cyto | 47 (42–52) | 54 (46–64) | 4930 |
| Mongolia | N | 2012 | 30–60 | Cyto | 47 (42–52) | 62 (61–64) | 491 |
| Myanmar | O | 2018 | 30–49 | VIA/HPV | 3 (3–3) | 4 (3–4) | 7795 |
| Nepal | O | 2010 | 30–60 | VIA | 6 (5–7) | 10 (9–10) | 3991 |
| Pakistan | O | ND | ND | VIA | 1 (1–1) | 1 (1–1) | 24,933 |
| Philippines | N | 2009 | 25–55 | VIA | 1 (1–1) | 1 (1–1) | 14,221 |
| Singapore | N | 2019 | 25–69 | HPV/Cyto | 67 (64–69) | 69 (66–71) | 894 |
| Sri Lanka | N | 2007 | 35 & 45 | Cyto/HPV | 24 (18–29) | 27 (21–33) | 3024 |
| Thailand | N | 2020 | 30–60 | HPV/Cyto | 67 (64–70) | 77 (71–83) | 10,417 |
| Vietnam | O | 2011 | 21–70 | Cyto/VIA | 25 (22–28) | 31 (31–32) | 15,058 |
Abbreviation: ND = No published data available.
Types of screening programme: N = Nationwide cervical cancer screening program implementation; P = Provincial/Regional/State cervical cancer screening program implementation; O = Other types of screening programme eg private, opportunistic.
Types of screening tests: Cyto = cytology, HPV = Human papillomavirus DNA test, VIA = Visual Inspection with Acetic Acid.
Latest reported national cervical cancer screening coverage among women aged 30–49 in the previous 5 years.
Latest reported national cervical cancer screening coverage among women aged 30–49 in the lifetime ever.
Barriers to HPV vaccination uptake
HPV vaccination was first licensed in 2006 for the prevention of HPV-related diseases and to be given to girls aged 9 years or older before exposure to HPV or onset of sexual activity.2 Since then, all ANCCA member countries have made HPV vaccination available, either in their national or school vaccination programme, or through private out-of-pocket health services. HPV vaccines were first licensed as a three-dose schedule, and then changed to a two-dose schedule in 2014. In 2022, based on immunogenicity and effectiveness data, the WHO through its independent expert advisory group (SAGE) review concluded that single-dose HPV vaccination had an efficacy and durability of protection comparable to a two-dose regimen.2 Despite nearly two decades since the licensing of the HPV vaccine, the improvements in dose schedule, and inclusion of boys in the HPV vaccination programme in several developed countries; the majority of women in the LMICs are still deprived of the opportunity to be vaccinated against HPV.
About half of the ANCCA member countries do not have a national HPV vaccination programme or surveillance data (Table 1). The countries which have HPV vaccination as part of their national vaccination programme with consistently high vaccine uptake, have achieved a reduction in the prevalence of HPV 16/18 and high grade cervical precancers in the population.12 Of the ANCCA countries with national programme for HPV vaccination, Brunei, Malaysia, Mongolia, Myanmar and Singapore have included HPV vaccination as part of their national school health programme.
A shortage of HPV vaccine supplies and a lack of affordability or government subsidies have been highlighted by about half of the ANCCA member countries (12 out of 21). Many have identified that the out-of-pocket payment for the vaccination was not affordable in their country. More than half of ANCCA member countries (n = 11, 52%) reported a lack of national surveillance data on HPV vaccination. This poses a challenge to planning and monitoring of the vaccination programme, as there are no available or accurate data on the uptake of the vaccination in these countries. Other significant barriers which have been reported include inadequate and ineffective distribution of the vaccines, perceived fear of vaccine safety among women, lack of knowledge or awareness on HPV vaccination and cervical cancer prevention, vaccine hesitancy, time related constraints and poor health seeking behaviour.13 Among ANCCA members, about 11 (52%) countries have reported vaccine hesitancy and utilization only when recommended by health care providers. In Japan, although the vaccine has been provided free-of-charge to girls in the target age group since 2013, the coverage remained low due to the suspension of active recommendation by the government.14
Barriers to cervical cancer screening
All ANCCA member countries identified lack of awareness and knowledge of cervical cancer prevention and screening, fear of gynaecological examination, fear of cancer diagnosis and cultural reasons as major barriers. ANCCA member countries (n = 17, 81%) also highlighted existing disparities in health service utilisation across various social groups. More than half of the ANCCA member countries (12 out of 21) identified inadequate accessibility to health facilities in both urban and rural region as a main barrier to screening along with lack of national surveillance data (11 out of 21), and shortage of trained health care professionals (n = 11, 52%) to conduct the necessary screening test. Nearly all (n = 19, 90%) ANCCA member countries have identified that the involvement of multidisciplinary stakeholders such as gynaecologists and community healthcare workers is important in their cervical cancer screening programme.
In Asia, several barriers to cervical cancer screening uptake have been reported at the individual level–stigma, lack of awareness about cervical cancer and screening, lack of time and family support for the women, and at the health services level–unaffordability, lack of screening services and self-sampling facilities.15 HPV testing has been recommended as the primary screening test for cervical cancer. It has a high sensitivity exceeding 90% for the detection of high-grade cervical intra-epithelial neoplasia (CIN 2+) lesions. The test offers an objective diagnosis and has demonstrated significant mortality reduction from cervical cancer, compared with cytology and VIA which are subjective tests amenable to interpretation error.10 Triaging protocols recently recommended by the WHO include cytology, practical HPV genotyping, colposcopy and VIA, either alone or in combination (e.g. HPV 16/18 positive women are directly referred for colposcopy while women positive for other HPV types undergo cytology or VIA).9 Reflex cytology triage of HPV-positive cases was less expensive than HPV/cytology co-test as the tests are performed on a smaller number of women with higher prevalence of disease, which improves the test performance.10,12
Impact of COVID-19 pandemic on HPV vaccination and cervical cancer screening
Cancer preventive services such as awareness, vaccination and screening programme were significantly disrupted during the COVID-19 pandemic. The WHO estimated that HPV vaccine coverage dropped by nearly a quarter during the pandemic compared with 2019.16 The COVID-19 pandemic resulted in most ANCCA members (except Bhutan) suspending their national HPV vaccination programme, due to closure of schools during the pandemic and prioritisation of resources to COVID-19 vaccination. Many countries have started to catch-up and even leverage on the COVID-19 vaccination drive to improve HPV vaccination uptake. Similarly, for screening, many ANCCA member countries have experienced lockdowns, suspension of cervical cancer screening, diagnostic services and even cancer treatment services during the pandemic.17
Recommendations to improve vaccination and screening coverage
Several key factors that could improve the uptake of vaccination and screening were identified by our review study. These include political and financial commitment, health system strengthening, integration of screening and treatment services into the primary care package, ensuring care continuum, evidence-based communication, and support for vulnerable populations.
Political and financial commitment
Almost all ANCCA member countries (19 out of 21) identified strong political commitment to be essential in ensuring availability of resources for cervical cancer prevention. This ensures a comprehensive and coordinated approach that involves multi-stakeholder partnerships, long-term planning, adequate funding, to improve infrastructure and facilities, improve accessibility and affordability of vaccination, screening and treatment. A limited health budget and competing priorities have been highlighted as key barriers to achieving elimination of cervical cancer. This is especially relevant in the post COVID-19 era where there are many other competing priorities including overall economic recovery.
HPV vaccination for girls, and cervical screening for women need to be included in primary care services, without imposing financial hardship on the women, as part of the Universal Health Coverage (UHC). Increased development assistance directed at HPV immunisation through multilateral organisations with cooperation from vaccine suppliers, has been shown to provide short term financial and technical support in the pilots and roll out of the programmes in selected geographical areas.18 It is, however, necessary to have a long-term plan to ensure sustainability of the programme even if the assistant funding is discontinued.
ANCCA member countries have also highlighted the important roles of health insurance in increasing vaccination and screening coverage for all women from different income groups. Health economic modelling can be utilised to get stronger buy-ins from stakeholders, to determine cost-effective way of programme implementation based on local settings, infrastructure and capacities, community support, and collaboration with the private sectors and civil society organisations.
Health system strengthening
More than two-third (15 out of 21) of ANCCA member countries highlighted health system strengthening as crucial in improving the uptake of HPV vaccination and cervical cancer screening in their countries. This includes building capacity to improve vaccine supply chains, training manpower, developing national surveillance system, cultivating efficient public-private partnerships, having accountable health financing, and using high-performance laboratory tests, self-sampling facilities, triaging and optimal treatment.12 Governments play an important role in facilitating and supporting the enhancement of the national health information and surveillance systems, the implementation and evaluation of interventions across the cervical cancer care continuum, so as to improve cost effectiveness, accessibility and affordability, and ensure accountability.
Few countries in the region are producing HPV vaccines and cervical cancer screening test kits. In India, the manufacturing of quadrivalent vaccine (Cervavax®) was approved by the Drug Controller General in 2022.19 A bivalent HPV vaccine (Cecolin®) manufactured in China has been WHO pre-qualified,20 and another recombinant HPV bivalent vaccine (Walrinvax™) was licensed in China in 2022.21 These new vaccines are expected to mitigate the supply shortages and improve affordability. Managing the logistics of vaccine implementation needs to be strengthened in LMICs to ensure effective distribution, delivery and management of HPV vaccination.22
With the discovery of the role of HPV in the aetiology of cervical cancer, HPV testing has gradually replaced cytology test as the primary screening tool, due to its higher sensitivity in detecting pre-neoplastic lesions, better re-assurance with a negative test, and safe prolongation of screening intervals.10 Clinically validated HPV assays and triage protocols of screen positive cases should be available prior to implementation of primary HPV screening.10,12
Integration of services into a primary care package and ensuring continuum of care
More than half of the ANCCA member countries (n = 13, 62%) identified improving accessibility of community healthcare facilities as crucial to increase screening uptake. Services integrated into existing sexual and reproductive health services, HIV care and treatment clinics, antenatal care, well women clinics and school-based health outreach are points of entry for reaching women and girls. Several countries are also exploring HPV self-sampling with 13 out of 21 ANCCA member countries identifying it as a key element for increasing screening coverage. HPV self-sampling has a significant logistic advantage and is as accurate as provider sampling, especially when a PCR based test is used.23 Self-sampling was found to be accepted by women in many Asia countries.24
People-centred referral and tracking mechanisms are needed to minimise inconvenience to patients and reduce the defaulting rate to follow-up or referrals. A robust referral linkage should be established to ensure timely, affordable and accessible management of cervical diseases; a single-visit screen-and-treat approach is recommended to reduce the lost to follow-up of the screened posititves.9 A thorough understanding of the bottlenecks in services pertaining to vaccination, screening, referral and treatment pathway, in relation to both service delivery and community perspectives, is needed to establish synergy in all the three pillars of disease prevention, and to ensure continuum of care. Clinical management protocols should be developed in accordance with the availability of manpower and infrastructure to allow optimum resource utilisation in a standardised way. Linkage of the protocols with clear definition of criteria will ensure that screening and treatment services can be conducted in a standardised and well organised manner.
Tele-mentoring communities or online knowledge-sharing tool like ECHO (Extension of Community Health Outcomes)25 are important avenues for knowledge exchange among healthcare professionals to ensure quality of care. Such platforms were increasingly utilised during the COVID-19 pandemic by connecting gynaecologists, nurses and primary healthcare workers in the community. These virtual meetings allowed ground-level healthcare professionals to stay up-to-date when formal or in-person meetings were not feasible.
Evidence-based communication to raise awareness and remove misinformation
The majority of the ANCCA member countries (17 out of 21) highlighted the important role schools play in providing the necessary information to girls and parents to encourage vaccination uptake. Health education campaigns targeted at parents, healthcare providers, and schools can help increase awareness and knowledge about the vaccine. The provision of relevant and easy to understand information on HPV vaccination by healthcare providers was also identified as an important element in raising awareness on the benefits of HPV vaccination and screening. Community awareness programmes on cervical cancer screening and involvement of community stakeholders in providing awareness have been found to increase vaccine uptake especially in remote areas in Asia.26 Local communities, especially women, must be engaged and empowered to lead the development of these critical programmes, serve as allies, counter misinformation or stigmatization, and support those needing more complex treatment. Improving health literacy, knowledge and awareness of cervical cancer prevention and control will help to empower women in taking the necessary actions to prevent cervical cancer.27
Advocacy efforts need to be sustained with accountability and transparency. Connections between the health and non-health stakeholders need to be established within the community. If there are funding constraints, these established connections will ensure continuity of the programme in the community, for example, by identifying synergy with other health or community programme. Addressing barriers to cervical cancer prevention and control requires a multifaceted approach to increase awareness of the effectiveness and safety of the HPV vaccine, benefits of screening and early detection and management of diseases, and to address social stigma and misinformation. A broad approach will be required to break down cultural barriers by involving community and religious leaders, as well as social influencers.
Support for the women, families and vulnerable populations
Almost all ANCCA members (19 out of 21) identified the importance of community stakeholders' engagement in providing support for women to take up vaccination and screening. In some parts of Asia, educating women is considered a low priority leading to poor health literacy among women.28 Despite the success of school-based vaccination programmes, it is important to ensure that girls who are not attending school are also vaccinated. A targeted approach is needed to reach the hard-to-reach segment of the population, including retention of girls in secondary education, and supporting migrant or marginalised women, and provision of subsidies to women who cannot afford HPV vaccination, screening and treatment. The marginalised populations are at higher risk of HPV infection and cervical cancer, particularly if they are HIV positive. Populations with high HIV prevalence have some of the highest cervical cancer rates; greater effort is needed to achieve elimination in such settings as part of HIV/STI/HPV reduction strategies.3 Studies have shown that migrant populations have lower health care accessibility and enrolment in the health insurance system; engaging stakeholders from the migrant's population is important to disseminate correct information on HPV vaccination and cervical cancer screening.29
Strengths and limitations
This review provides a critical insight into the current status of cervical cancer prevention, the key barriers and recommendations among the ANCCA member countries in order to achieve the targets for the elimination of cervical cancer. The review provides an update of the progress made by each country in working towards their HPV vaccination and cervical cancer screening coverage targets. We have summarised the latest national data, and provided an overview of the key factors affecting HPV vaccination and cervical cancer screening in the 21 Asian countries.
However, as highlighted in the results section, many countries lack an established national surveillance system to monitor and report indicators accurately. WHO and UNICEF estimates of cervical cancer burden, HPV vaccination and cervical cancer screening indicators may be incomplete or underestimated in countries where no national cancer registry or surveillance system is available; the use of modelling estimates have their intrinsic assumptions and biases. In addition, disruption to cancer prevention and surveillance programmes during the COVID-19 pandemic could result in delays or inconsistencies in data collection and reporting. An additional limitation related to data collection was that HPV vaccination coverage reported were based on cross-sectional national surveys. These estimates may fluctuate from year to year if the programme is affected by external factors such as funding. There are notable disparities within countries and differences in urban versus rural settings in the interpretation of national data.
This review did not include HPV vaccination for men and other HPV-associated cancers. The current WHO recommendation focuses on vaccinating young girls due to the scale of benefits and limited vaccine supply, however, HPV vaccination for men should be considered when vaccine supply is sufficient to enhance herd immunity and prevent HPV-associated cancers. Biases and limitation in experience of the researchers may also affect the selection of information regarding barriers to vaccination and screening in the various member countries. While this review focuses on HPV vaccination and cervical cancer screening, we have also emphasised the importance of ensuring the continuum of care namely the system for referral, follow-up and treatment, which forms the third pillar of the global strategy to eliminate cervical cancer as a public health problem.
Conclusion
A comprehensive approach is needed to overcome the barriers to HPV vaccination and cervical cancer screening among ANCCA member countries. Addressing health disparities and inequalities requires global and national leadership and commitment to ensure vaccine accessibility and affordability for all women. Multipronged approaches on increasing awareness, addressing cultural and social barriers, improving access to screening services, strengthening healthcare infrastructure, and ensuring high-quality screening services and continuum of care are needed to eliminate cervical cancer as a public health problem in the Asian region.
Contributors
Conceptualization & original draft: Sokking Ong. Data curation: Sokking Ong, Sarah K. Abe, Shyamala Thilagaratnam, Rei Haruyama, Ruchi Pathak, Aliza KC Bhandari, Youn Kyung Chung, Abhishek Shankar, Ashrafun Nessa, Jugder Uranbolor, Julyanti Agustina, Ugyen Tshomo, Clarito Cairo, Mohammed Biglari, Aasim Yusuf, Champadeng Vongdala, Kyaw Kan Kaung, Eshani Fernando, Beauta Rath, Kishore Kumar Pradhananga, Tran Thi Thanh Huong, Suleeporn Sangrajran, Yawei Zhang, Yin Ling Woo. Review, edits & revision: Sokking Ong, Sarah K. Abe, Shyamala Thilagaratnam, Rei Haruyama, Ruchi Pathak, Harindra Jayasekara, Kayo Togawa, Abhishek Shankar, Aliza KC Bhandari, Jeongseon Kim, Abhishek Shankar, Babu Sukumaran, Partha Basu, Yin Ling Woo, William YK Hwang.
Disclaimer
The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of their affiliated institutions. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Declaration of interests
The authors have no conflicts of interest to declare.
Acknowledgements
The authors wish to thank CS Pramesh, Soeko Werdi Nindito, Evlina Suzanna, Tomohiro Matsuda, Laureline Gatellier, Manami Inoue, Maryam Bagherian, Mamak Tahmasebi, Nguyen Huong Giang, Nor Saleha Ibrahim Tamin, Siti Norbayah Yusof, Noraslinah Ramlee, Shareefah Koh Kai Shing, Aida Darwisyah Aiddy Azhan, Erdenekhuu Nansalmaa, Uranchimeg Tsegmid, Tashi Dhendup, Kinley Tshering, Donghyun Kim, Chong Woo Yoo, Hayley Jones, Karen Canfell and Rolando Enrique D. Domingo for their support in the preparation and publication of the paper.
Funding: None to declare.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100860.
Appendix A. Supplementary data
References
- 1.IARC. IARC Handbooks of Cancer Prevention . International Agency for Research on Cancer; Lyon, France: 2022. Cervical cancer screening. [Google Scholar]
- 2.WHO Human papillomavirus vaccines: WHO position paper December 2022. https://www.who.int/publications/i/item/who-wer9750-645-672
- 3.WHO . World Health Organization; Geneva: 2020. Global strategy to accelerate the elimination of cervical cancer as a public health problem. [Google Scholar]
- 4.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer; Lyon, France: 2020. Global Cancer Observatory: cancer today.https://gco.iarc.fr/today Available from: [Google Scholar]
- 6.WHO Human Papillomavirus (HPV) vaccination coverage. https://immunizationdata.who.int/pages/coverage/hpv.html?CODE=Global&ANTIGEN=PRHPV1_F&YEAR=
- 7.Bruni L., Saura-Lazaro A., Montoliu A., et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med. 2021;144 doi: 10.1016/j.ypmed.2020.106399. [DOI] [PubMed] [Google Scholar]
- 8.Brisson M., Kim J.J., Canfell K., et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet (London, England) 2020;395(10224):575–590. doi: 10.1016/S0140-6736(20)30068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonjour M., Charvat H., Franco E.L., et al. Global estimates of expected and preventable cervical cancers among girls born between 2005 and 2014: a birth cohort analysis. Lancet Public Health. 2021;6(7):e510–e521. doi: 10.1016/S2468-2667(21)00046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd ed. World Health Organization; Geneva: 2021. [PubMed] [Google Scholar]
- 11.Bhatla N., Singhal S. Primary HPV screening for cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2020;65:98–108. doi: 10.1016/j.bpobgyn.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Garland S.M., Kjaer S.K., Muñoz N., et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 Years of real-world experience. Clin Infect Dis. 2016;63(4):519–527. doi: 10.1093/cid/ciw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alfaro K., Maza M., Cremer M., Masch R., Soler M. Removing global barriers to cervical cancer prevention and moving towards elimination. Nat Rev Cancer. 2021;21(10):607–608. doi: 10.1038/s41568-021-00396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haruyama R., Obara H., Fujita N. Japan resumes active recommendations of HPV vaccine after 8.5 years of suspension. Lancet Oncol. 2022;23(2):197–198. doi: 10.1016/S1470-2045(22)00002-X. [DOI] [PubMed] [Google Scholar]
- 15.Devarapalli P., Labani S., Nagarjuna N., Panchal P., Asthana S. Barriers affecting uptake of cervical cancer screening in low-and middle-income countries: a systematic review. Indian J Cancer. 2018;55:318–326. doi: 10.4103/ijc.IJC_253_18. [DOI] [PubMed] [Google Scholar]
- 16.WHO . 2022. COVID-19 pandemic fuels largest continued backslide in vaccinations in three decades.https://www.who.int/news/item/15-07-2022-covid-19-pandemic-fuels-largest-continued-backslide-in-vaccinations-in-three-decades [Google Scholar]
- 17.Villain P., Carvalho A.L., Lucas E., et al. Cross-sectional survey of the impact of the COVID-19 pandemic on cancer screening programs in selected low- and middle-income countries: study from the IARC COVID-19 impact study group. Int J Cancer. 2021;149(1):97–107. doi: 10.1002/ijc.33500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spayne J., Hesketh T. Estimate of global human papillomavirus vaccination coverage: analysis of country-level indicators. BMJ Open. 2021;11(9) doi: 10.1136/bmjopen-2021-052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IARC . 2022. Evaluation of new HPV vaccine by IARC and partners supports recommendation to grant marketing authorization for the vaccine.https://www.iarc.who.int/news-events/evaluation-of-new-hpv-vaccine-by-iarc-and-partners-supports-recommendation-to-grant-marketing-authorization-for-the-vaccine/ [Google Scholar]
- 20.WHO Vaccine Cecolin WHO–prequalification of medical products (IVDs, medicines, vaccines and immunization devices, vector control) https://extranet.who.int/pqweb/content/cecolin%C2%AE
- 21.You T., Zhao X., Hu S., et al. Optimal allocation strategies for HPV vaccination introduction and expansion in China accommodated to different supply and dose schedule scenarios: a modelling study. eClinicalMedicine. 2022;56 doi: 10.1016/j.eclinm.2022.101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toh Z.Q., Licciardi P.V., Russell F.M., Garland S.M., Batmunkh T., Mulholland E.K. Cervical cancer prevention through HPV vaccination in low- and middle-income countries in Asia. Asian Pac J Cancer Prev. 2017;18(9):2339–2343. doi: 10.22034/APJCP.2017.18.9.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbyn M., Smith S.B., Temin S., et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self-samples: updated meta-analyses. BMJ. 2018;363:k4823. doi: 10.1136/bmj.k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poli U.R., Muwonge R., Bhoopal T., Lucas E., Basu P. Feasibility, acceptability, and efficacy of a community health worker-driven approach to screen hard-to-reach periurban women using self-sampled HPV detection test in India. JCO Glob Oncol. 2020;6:658–666. doi: 10.1200/GO.20.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariprasad R., Arora S., Babu R., et al. Retention of knowledge levels of health care providers in cancer screening through telementoring. J Glob Oncol. 2018;4:1–7. doi: 10.1200/JGO.18.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chua B., Ma V., Asjes C., Lim A., Mohseni M., Wee H.L. Barriers to and facilitators of cervical cancer screening among women in Southeast Asia: a systematic review. Int J Environ Res Public Health. 2021;18(9) doi: 10.3390/ijerph18094586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salehiniya H., Momenimovahed Z., Allahqoli L., Momenimovahed S., Alkatout I. Factors related to cervical cancer screening among Asian women. Eur Rev Med Pharmacol Sci. 2021;25(19):6109–6122. doi: 10.26355/eurrev_202110_26889. [DOI] [PubMed] [Google Scholar]
- 28.Lau J., Shrestha P., Shaina Ng J., Jianlin Wong G., Legido-Quigley H., Tan K.K. Qualitative factors influencing breast and cervical cancer screening in women: a scoping review. Prev Med Rep. 2022;27 doi: 10.1016/j.pmedr.2022.101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson de Cuevas R.M., Saini P., Roberts D., et al. A systematic review of barriers and enablers to South Asian women's attendance for asymptomatic screening of breast and cervical cancers in emigrant countries. BMJ Open. 2018;8(7) doi: 10.1136/bmjopen-2017-020892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.