Summary
Cre-mediated recombination is frequently used for cell type-specific loss of function (LOF) studies. A major limitation of this system is recombination in unwanted cell types. CRISPR interference (CRISPRi) has been used effectively for global LOF in mice. However, cell type-specific CRISPRi, independent of recombination-based systems, has not been reported. To test the feasibility of cell type-specific CRISPRi, we produced two novel knock-in mouse models that achieve gene suppression when used together: one expressing dCas9::KRAB under the control of a cell type-specific promoter and the other expressing a single guide RNA from a safe harbor locus. We then compared the phenotypes of mice in which the same gene was targeted by either CRISPRi or the Cre-loxP system, with cell specificity conferred by Dmp1 regulatory elements in both cases. We demonstrate that CRISPRi is effective for cell type-specific LOF and that it provides improved cell type-specificity compared to the Cre-loxP system.
Subject areas: Genetics, Molecular Genetics
Graphical abstract
Highlights
-
•
In vivo global gene knockdown by CRISPRi can be as effective as gene knockout
-
•
CRISPRi can be adapted to perform in vivo cell type-specific loss of function
-
•
Cell type-specific loss of function using CRISPRi is more specific than Cre-loxP
-
•
Knockdown by cell type-specific CRISPRi persists up to 12 months of age
Genetics; Molecular Genetics
Introduction
Cell type-specific loss of function (LOF) studies have been the cornerstone of skeletal research since the development of Cre-driver strains that target bone-forming osteoblasts and bone-resorbing osteoclasts. Since the early 2000s, various Cre-driver strains that target different stages of the osteoblast lineage were produced by placing Cre expression under the control of cell type-specific gene regulatory elements.1,2,3,4,5,6,7,8,9,10,11 However, it has been difficult to identify any genes that are expressed exclusively in a single cell type. In fact, most genes thought to be cell-type specific are also expressed in several other cell types and tissues. For example, Dmp1 and Sost are commonly referred to as osteocyte-specific genes.12,13,14,15,16,17 Osteocytes, which differentiate from osteoblasts, are embedded in the bone matrix, and orchestrate bone resorption and formation. We and others have used transcriptional regulatory elements of the Dmp1 and Sost genes to produce Dmp1-Cre and Sost-Cre mouse strains.6,8,9,10,11 However, it is now clear that these two genes are also expressed in several additional cell types in bone and other tissues, albeit at lower levels.18,19,20,21 Accordingly, analysis of the cell types targeted by the Dmp1-Cre and Sost-Cre transgenes has shown that these Cre models are not as cell type-specific as once thought.10,22,23,24,25,26 Specifically, while Sost-Cre is specific to osteocytes within the osteoblast lineage, it also recombines floxed alleles in hematopoietic cells.10 On the other hand, Dmp1-Cre has been shown to recombine floxed alleles in additional cell types in bone, such as osteoblasts and Cxcl12-abundant reticular (CAR) cells,22,23,24,25,26 and other tissues such as gastrointestinal mesenchymal cells.22 Because gene inactivation in these other cell types may contribute to the observed phenotypes, it is essential to develop LOF models with improved cell type-specificity.
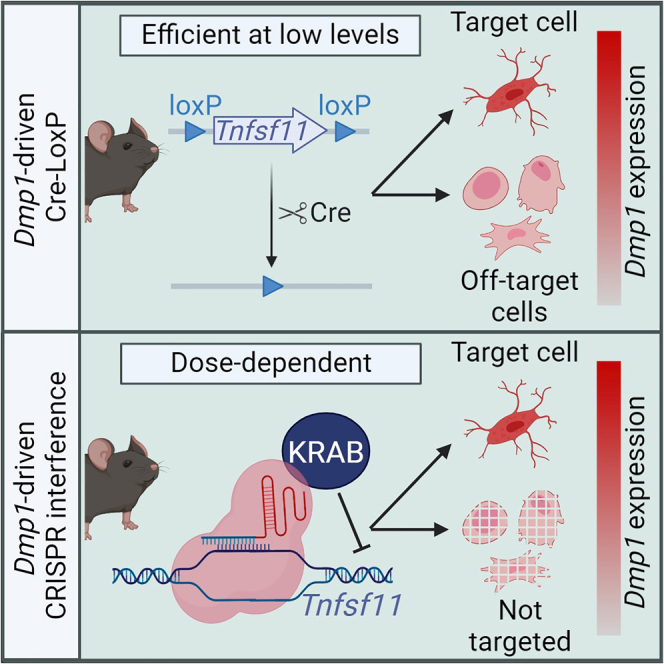
CRISPR interference (CRISPRi) is a recently developed approach to perform LOF.27,28 This technique utilizes a catalytically inactive Cas9 (dCas9) fused to a transcriptional repressor domain to suppress target genes. The dCas9::repressor fusion protein is guided to a region within 100 bp flanking the transcriptional start site (TSS) of the target gene using a single guide RNA (sgRNA) complementary to that region.29 Unlike the Cre-loxP system or the original CRISPR-Cas9 system, CRISPRi-based suppression does not cut or irreversibly modify the target DNA. Instead, CRISPRi-based LOF is achieved by inhibition of target gene transcription. In vitro studies have shown that the level of target gene suppression by CRISPRi is influenced by the location of the sgRNA relative to the TSS,28,29 as well as the expression level of the system components, namely dCas9::KRAB and sgRNA.27,30,31,32 We have recently demonstrated that a single transgene expressing CRISPRi components was able to globally suppress a target gene, Tnfsf11, in vivo.33 We created transgenic mouse lines that expressed dCas9::KRAB and a Tnfsf11 sgRNA ubiquitously and constitutively but at varying levels (low, medium, and high). Comparison of these different lines showed that the level of Tnfsf11 suppression correlated with the expression of the CRISPRi components. Specifically, high levels of dCas9::KRAB and sgRNA were required to suppress a target gene via CRISPRi. This is in contrast to what is observed with the Cre-loxP system, in which even low-level Cre expression can be sufficient to recombine floxed alleles, contributing to recombination in unwanted cell types.34
To allow for cell type-specific CRISPRi, a Cre-dependent dCas9::KRAB model, namely R26-LSL-dCas9-KRAB, has been created.35 In this model, a transgene containing a CAG promoter followed by a loxP-stop-loxP (LSL) cassette and DNA encoding the dCas9::KRAB fusion protein was inserted into the Rosa safe harbor locus. Following Cre recombinase exposure, and in association with expression of a sgRNA at a different locus, this model can suppress expression of target genes. However, the cell type-specificity of this model is determined by the specificity of the Cre-driver strain used to delete the LSL cassette and, therefore, has the same lack of specificity as Cre-loxP models.
Based on the observed dose-dependence of CRISPRi that we observed previously, we hypothesized that expression of dCas9::KRAB under the control of transcriptional regulatory elements of a relatively cell type-specific gene would suppress targets only in cells that express high levels of that gene, thereby improving the specificity of LOF studies. While Dmp1 is expressed by various cell types, its expression in osteocytes is much higher than in other cell types.36,37 Based on this, we sought to determine if Dmp1-driven dCas9::KRAB would provide improved specificity compared to a Dmp1-Cre transgene. To test this, we performed LOF of a well-characterized gene (Tnfsf11) with both Dmp1-driven Cre-loxP and Dmp1-driven CRISPRi systems and compared the resultant phenotypes and specificity of Tnfsf11 suppression.
Results
Expression of a sgRNA from the ROSA26 locus
Although expression of CRISPRi components via a single transgene can suppress target genes in vivo,33 this approach requires production and screening of multiple founder lines for sufficient transgene expression for each new target. To avoid insertion site and copy number effects associated with transgenic approaches, and to facilitate efficient use of CRISPRi in vivo, we envisioned production of two murine models, each expressing one of the two CRISPRi components: one a knock-in model which expresses dCas9::KRAB and the other a knock-in model expressing one or more sgRNAs from a safe harbor locus.
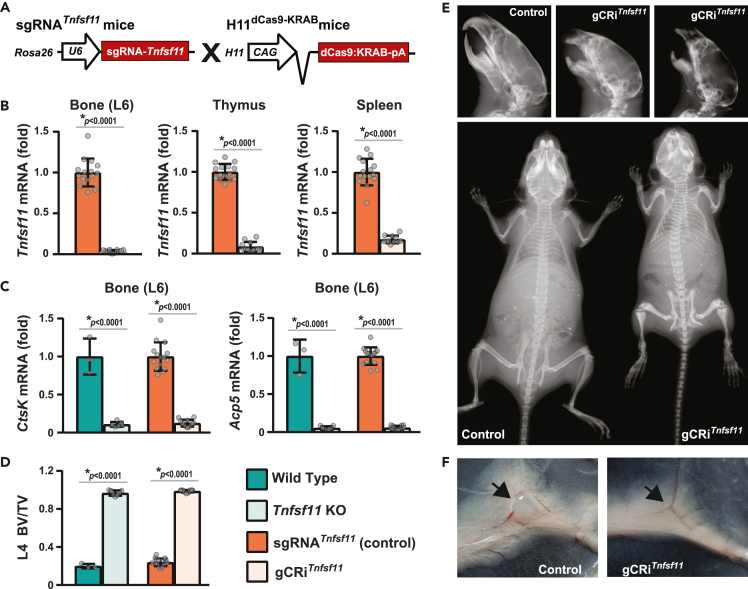
Previous studies have shown that sgRNA expression level may be a limiting factor in target suppression by CRISPRi.27,32 To determine if sgRNA expression from a safe harbor locus is sufficient for CRISPRi, we introduced a cassette expressing a Tnfsf11 sgRNA into the murine Rosa26 locus. We crossed the resulting mice, designated sgRNATnfsf11, with mice that globally and constitutively express dCas9::KRAB, designated H11dCas9::KRAB 38,39 (Figure 1A). In mice hemizygous for both alleles, designated gCRiTnfsf11, ubiquitous expression of both CRISPRi components suppressed Tnfsf11 in the bone, thymus, and spleen by more than 90% (Figure 1B).
Figure 1.
Global suppression of Tnfsf11 using CRISPRi causes osteopetrosis
(A) sgRNATnfsf11 mice were crossed with H11dCas9-KRAB mice to globally suppress Tnfsf11.
(B–F) Gene expression and skeletal analysis of Tnfsf11 knock-out mice (Tnfsf11 KO, n = 7), their wild type littermates (n = 3); H11dCas9-KRAB; sgRNATnfsf11 mice (gCRiTnfsf11, n = 12), and their littermate controls (sgRNATnfsf11 or Control, n = 15) were performed at 5 weeks of age. Both sexes were included. B, Tnfsf11 mRNA levels were measured in the bone (lumbar vertebrae 6, L6), thymus, and spleen of gCRiTnfsf11 mice and their littermate controls by quantitative real-time PCR (qRT-PCR). ∗p < 0.0001 as calculated by unpaired t-test with Welch’s correction (L6 and spleen), or by Rank-Sum test (thymus). Data presented as mean ± standard deviation (SD). C, CtsK and Acp5 mRNA levels were measured in bones of Tnfsf11 KO, gCRiTnfsf11 mice, and their littermate controls by qRT-PCR. For all qRT-PCR analyses, mRNA levels were normalized to mouse Actb and indicated as fold (normalized to the mean of the corresponding littermate control values). D, Vertebral cancellous bone volume over tissue volume (BV/TV) of Tnfsf11 KO mice, gCRiTnfsf11 mice and their littermate controls were measured by μCT analysis of lumbar vertebra 4 (L4). C and D, Data presented as mean ± SD, ∗p < 0.0001 comparing Tnfsf11 KO or gCRiTnfsf11 to their corresponding littermates; #p < 0.05 comparing wild type to sgRNA, or Tnfsf11 KO to gCRiTnfsf11 as assessed by one-way ANOVA with Tukey adjustment. E, Skull and whole body X-ray images were generated at five weeks of age. F, Arrows point to the presence or lack of inguinal lymph nodes in the control and gCRiTnfsf11 mouse images. Data presented as mean + SD.
Tnfsf11 encodes the TNF-family cytokine known as receptor activator of NF-κB ligand (RANKL). RANKL produced by cells of the mesenchymal lineage is necessary for the differentiation and survival of osteoclasts from myeloid progenitors.23,40,41 Global suppression of Tnfsf11 in gCRiTnfsf11 mice decreased osteoclast formation to a level equivalent to deletion of Tnfsf11 from the mouse genome (Tnfsf11 KO mice), as indicated by the profoundly reduced expression of the osteoclast marker genes CtsK and Acp5 (Figure 1C). Similar to what is observed in Tnfsf11 KO mice, lack of osteoclasts in gCRiTnfsf11 mice caused osteopetrosis indicated by high bone mass, lack of tooth eruption, and misshapen bones (Figures 1D and 1E). Independent of its role in osteoclast formation and survival, Tnfsf11 is also required for lymph node formation.41 CRISPRi-mediated Tnfsf11 suppression was also sufficient to prevent lymph node development in gCRiTnfsf11 mice (Figure 1F). Together, these results demonstrate that mice hemizygous for sgRNATnfsf11 express sgRNA at sufficient levels to potently suppress a target gene in the presence of dCas9::KRAB.
Conditional deletion of Tnfsf11 versus suppression of Tnfsf11 via CRISPRi
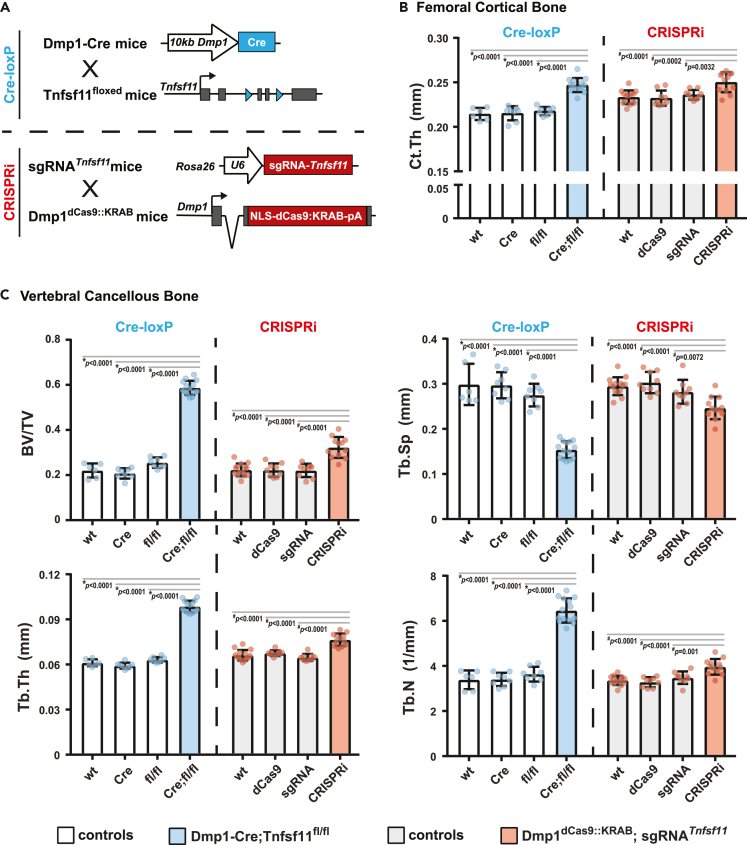
We next determined whether dCas9::KRAB expression from the endogenous Dmp1 locus is sufficient, together with expression of the sgRNA from the Rosa26 locus, for CRISPRi. To do this, we inserted a dCas9::KRAB coding sequence into the endogenous Dmp1 locus-producing Dmp1dCas9::KRAB mice. We selected Dmp1 to drive dCas9::KRAB expression because this gene is expressed at high levels in osteocytes.12,36,37 To suppress Tnfsf11 in osteocytes, we crossed the Dmp1dCas9::KRAB mice with sgRNATnfsf11 mice. Dmp1dCas9::KRAB;sgRNATnfsf11 (Ot-CRiTnfsf11) mice and their littermate controls (wild type, Dmp1dCas9::KRAB, and sgRNATnfsf11) were born at expected mendelian ratios and were grossly indistinguishable from one another (Figure S1A).
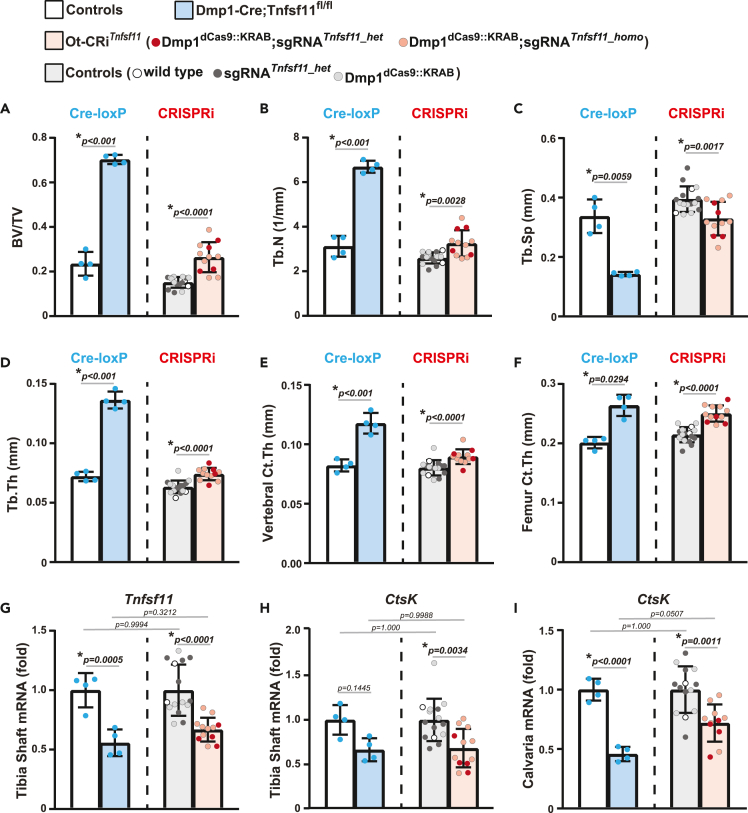
At 6 months of age, microCT analysis was used to compare the skeletal phenotypes of mice whose Tnfsf11 gene was inactivated using Dmp1-driven Cre-loxP deletion (Dmp1-Cre;Tnfsf11 fl/fl) to mice whose Tnfsf11 expression was suppressed using Dmp1-driven CRISPRi (Ot-CRiTnfsf11) (Figure 2; Figures S1B and S1C). The skeletal phenotype of Dmp1dCas9::KRAB or sgRNATnfsf11 hemizygous mice was indistinguishable from wild-type littermates suggesting that expression of dCas9::KRAB or the sgRNA alone does not alter bone mass (Figures 2B and 2C). While the magnitude of the effect was different, both Tnfsf11 deletion and suppression increased cortical thickness (Figure 2B) and cancellous bone volume compared to littermate controls (Figure 2C). LOF of Tnfsf11 by either system also led to similar architectural changes. Specifically, in both models, the increase in vertebral cancellous bone volume was associated with elevated trabecular number and thickness, and a resultant reduction in trabecular separation (Figure 2C). However, in both female and male mice, the high bone mass phenotype was more pronounced in mice whose Tnfsf11 gene was deleted using Dmp1-Cre (Figures 2, S1B, and S1C). The difference in the magnitude of the skeletal changes may be due to greater cell type-specificity of the Dmp1-driven CRISPRi system, the fact that CRISPRi does not completely suppress target genes, or both.
Figure 2.
Comparison of Tnfsf11 loss-of-function using Dmp1-driven Cre-loxP or Dmp1-driven CRISPRi
(A) Dmp1-Cre mice were crossed with Tnfsf11floxed mice to create Dmp1-Cre;Tnfsf11fl/fl mice (Cre;fl/fl, blue bars) and their littermate controls (wild type [wt], Dmp1-Cre [Cre] and Tnfsf11fl/fl [fl/fl] mice, white bars). sgRNATnfsf11 mice were crossed with Dmp1dCas9::KRAB mice to produce Dmp1dCas9::KRAB;sgRNATnfsf11 mice (CRISPRi, red bars) and their littermate controls (wild type [wt], Dmp1dCas9::KRAB [dCas9] and sgRNATnfsf11 [sgRNA] mice, light gray bars).
(B and C) The skeletal phenotype of 6-month-old female mice was compared by μCT analysis. B, Cortical thickness (Ct. Th) was measured at the femoral midshaft. C, Cancellous bone mass, and architecture were analyzed as bone volume over tissue volume (BV/TV), trabecular separation (Tb. Sp), trabecular thickness (Tb. Th), and trabecular number (Tb. N) in lumbar vertebrae 4. Data presented as mean ± SD. For deletion of Tnfsf11 using Cre-loxP n = 6–14 mice/group; ∗p < 0.05 compared to each littermate control using one-way ANOVA with Tukey adjustment. For suppression of Tnfsf11 using CRISPRi n = 9–14 mice/group; #, p < 0.05 compared to each littermate control using one-way ANOVA with Tukey adjustment. Individual Tukey p values for each comparison are provided in the graphs.
Cell type specificity of Dmp1-driven CRISPRi
An optimal approach to compare the specificity of CRISPRi and Cre-loxP systems would be to target Tnfsf11 with Dmp1-driven Cre-loxP or CRISPRi, and then use scRNA-seq to quantify Tnfsf11 deletion or suppression in different cell types. However, in scRNA-seq, the barcoding and sequencing are performed on the 3′ or 5′ end of the mRNA transcripts. In Tnfsf11 fl/fl mice, the exons flanked by loxP sites (exons 3 and 4) are near the middle of the transcript. Thus, the 3′ and 5′ ends of the Tnfsf11 mRNA are still detectable in Dmp1-Cre;Tnfsf11fl/fl mice, even after Cre-mediated deletion of the floxed exons (data not shown). Due to this technical limitation, we could not use scRNA-seq to directly determine from which cell types Dmp1-Cre deletes Tnfsf11. As an alternative, we used a fluorescent Cre-reporter gene to determine which cell types were targeted by Dmp1-Cre and inferred that the floxed Tnfsf11 allele was likely deleted from the same cell types when using Dmp1-Cre mice.
To do this, we marked all Dmp1-Cre targeted cells by activation of a Cre-reporter transgene and then examined whether Dmp1-driven CRISPRi suppressed Tnfsf11 in any of the Cre-targeted cells. If CRISPRi targeted all of the cells targeted by Dmp1-Cre, then all of the reporter-positive cells that express Tnfsf11 should show evidence of reduced Tnfsf11 expression. If any Cre-targeted (reporter-positive) cells do not exhibit reduced Tnfsf11 expression in the CRISPRi mice, then this would constitute evidence that the CRISPRi system targets a narrower range of cells. In other words, it is more cell-type-specific.
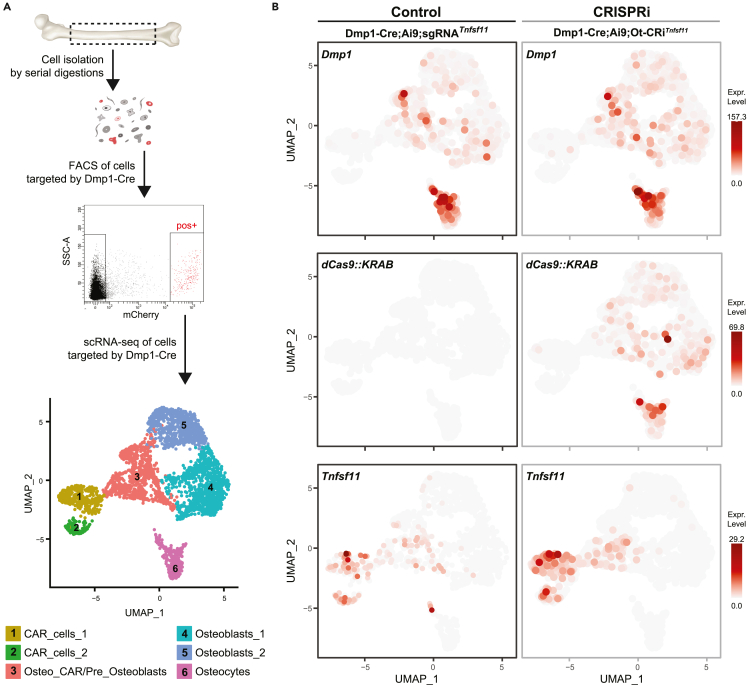
Dmp1-Cre, Ai9, Dmp1dCas9::KRAB mice were crossed with sgRNATnfsf11 mice to obtain Dmp1-Cre;Ai9;Dmp1dCas9::KRAB;sgRNATnfsf11 mice (CRISPRi) and control littermates, Dmp1-Cre;Ai9;sgRNATnfsf11 (Control). Next, we isolated cells from femurs and tibias of CRISPRi and Control mice and sorted the Dmp1-Cre targeted cells by flow cytometry (Figure 3A). In this approach, Cre expression in both Control and CRISPRi mice activated the fluorescent Ai9 reporter. Therefore, in both Control and CRISPRi mice, cells targeted by Dmp1-Cre were TdTomato positive (Figure S2B). We then performed scRNA-seq of the tdTomato-positive cells isolated from each genotype to compare whether Dmp1-driven CRISPRi suppressed Tnfsf11 in the same cell types targeted by Dmp1-Cre (Figures 3 and S2).
Figure 3.
Specificity comparison of Dmp1-driven Cre-loxP or Dmp1-driven CRISPRi using scRNA-seq
(A) Femur and tibia shafts were subjected to serial digestions (see STAR Methods). Cells from fractions 2 to 8 were collected for flow cytometry. tdTomato-positive cells were sorted and used for scRNA-seq. Cell-clustering was performed using cluster-specific markers indicated in Figure S2.
(B) UMAP plot of cells showing Dmp1 (top panel), dCas9::KRAB (middle panel), and Tnfsf11 (bottom panel) expression in cells of control (Dmp1-Cre;Ai9;sgRNATnfsf11) and CRISPRi (Dmp1-Cre;Ai9;Dmp1dCas9::KRAB;sgRNATnfsf11= Dmp1-Cre;Ai9;Ot-CRiTnfsfs11) mice.
Previous studies have shown that the Dmp1-Cre transgene targets osteocytes, osteoblasts, and about 30% of CAR cells.23,26,37 Consistent with these reports, our scRNA-seq analysis of sorted tdTomato-positive cells produced clusters corresponding to CAR cells (clusters 1 and 2), osteo-CAR cells (cluster 3), osteoblasts (clusters 4 and 5), and osteocytes (cluster 6) (Figures 3A and S2). OsteoCAR cells are a subset of CAR cells that express genes associated with osteoblast differentiation, although the relationship to either CAR cells or osteoblasts is uncertain.42
The endogenous Dmp1 gene was highly expressed in osteocytes, with lower levels in osteoblasts and osteo-CAR cells (Figure 3B). Even though the Dmp1-Cre transgene led to activation of the reporter gene in CAR cells (Figure S2B), expression of the endogenous Dmp1 gene was below the level of detection in most CAR cells. The reason for reporter expression in these cells is unclear but may result from low levels of Dmp1-Cre expression that are nonetheless sufficient for recombination or it may reflect the differentiation of osteo-CAR cells into CAR cells. We also cannot rule out the possibility that expression pattern of the Dmp1-Cre transgene is slightly different from that of the endogenous Dmp1 gene. Be that as it may, the expression pattern of dCas9::KRAB mirrored that of the endogenous Dmp1 gene (Figure 3B).
In Control mice, Tnfsf11 transcripts were easily detected in CAR cells and some osteo-CAR cells as well as a small portion of osteoblasts and osteocytes (Figure 3B). Notably, Dmp1-driven CRISPRi had no impact on Tnfsf11 expression in CAR cells or osteo-CAR cells (Figure 3B). In contrast, no Tnfsf11 transcripts were detected in osteocytes in the Dmp1-driven CRISPRi mice (Figure 3B). Because the number of osteocytes expressing Tnfsf11 in the control sample was so low, the significance of the lack of Tnfsf11 transcripts in osteocytes of the CRISPRi sample is unclear, although it may reflect suppression by CRISPRi. Nonetheless, these results show that Dmp1-driven CRISPRi did not suppress Tnfsf11 in CAR cells and osteo-CAR cells, whereas Dmp1-Cre recombination clearly occurs in these cell types. Thus, at least in the case of Dmp1-driven transgenes, the CRISPRi-based system targets a more restricted population of cells than the Cre-loxP system.
Dmp1-driven CRISPRi is functional up to 12 months of age
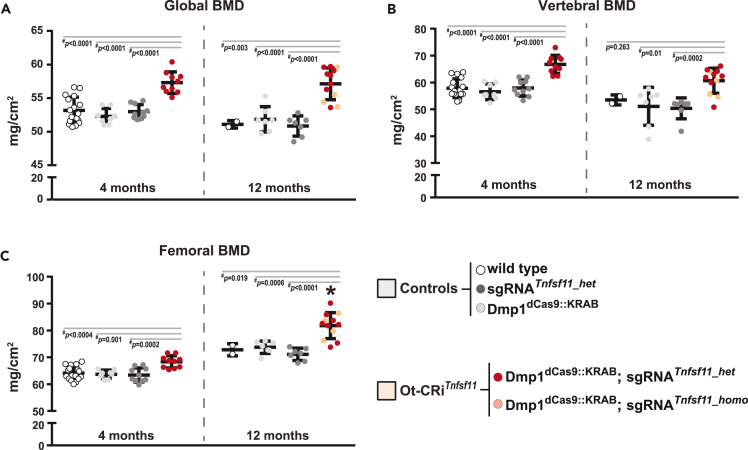
In CRISPRi, dCas9::KRAB has been proposed to facilitate transcriptional suppression of targets by both sterically blocking RNA polymerase binding and elongation, and by recruiting chromatin-modifying complexes for epigenetic suppression.27,28,38,43 Due to the temporal nature of these changes, target gene suppression by CRISPRi is reversible and is dependent on continuous expression of CRISPRi components. In our model, dCas9::KRAB transcription is controlled by Dmp1 regulatory elements. An age-dependent decline in Dmp1 expression has previously been reported.44 Because a decline in Dmp1 expression may translate to a reduction of dCas9::KRAB levels and a resultant decrease in target suppression, we determined if Dmp1-dependent Tnfsf11 suppression persists in older mice. We examined the skeletal phenotype and target suppression of Dmp1dCas9::KRAB;sgRNATnfsf11 (Ot-CRiTnfsf11) mice and their controls at 12 months of age. Bone mineral density (BMD) measurements at 4 and 12 months of age showed that Ot-CRiTnfsf11 mice have higher BMD than controls at all sites examined (Figures 4A–4C). The skeletal phenotype caused by Dmp1-driven deletion of Tnfsf11 becomes more pronounced as mice age.45 Similarly, the increase in femoral BMD in Ot-CRiTnfsf11 mice was more pronounced with age (Figure 4C). MicroCT measurements of 12-month-old Ot-CRiTnfsf11 mice showed increased cancellous bone volume (Figures 5A–5D and S3A–S3D) and increased cortical thickness compared to controls (Figures 5E, 5F, S3E, and S3F). However, in both female and male mice, the high bone mass phenotype was more pronounced in mice whose Tnfsf11 gene was deleted using Dmp1-Cre (Figures 5A–5F and S3).
Figure 4.
The phenotype of Dmp1-driven CRISPRi progresses with age
(A–C) BMD of female Dmp1dCas9::KRAB;sgRNATnfsf11 mice (Ot-CRiTnfsf11) and their littermate controls (wild type, Dmp1dCas9::KRAB, and sgRNATnfsf11 mice) were measured at 4 and 12 months of age. For the 4-month cohort n = 8–17 mice/group, and for the 12-month cohort n = 2–12 mice/group were used. Data presented as mean ± SD. For both ages #,p < 0.05 comparing Ot-CRiTnfsf11 with each control group using one-way ANOVA with Tukey adjustment. Individual Tukey p values for each comparison are provided in the graphs.∗, p < 0.05 for comparison of the change with age (between 12 and 4 months) for the Ot-CRiTnfsf11 group and the change with age within the control groups using t-test (details explained in the Methods Statistics section).
Figure 5.
Dmp1-driven CRISPRi of Tnfsf11 persists up to 12 months of age
The skeletal phenotype of 12-month-old female mice was compared by μCT analysis. For Cre-loxP, blue bars represent Dmp1-Cre;Tnfsf11fl/fl mice (blue bars, n = 4) and white bars represent their littermate controls (n = 4). For CRISPRi, pink bars represent Ot-CRiTnfsf11 mice (n = 12), while the light gray bars represent their littermate controls (n = 15).
(A–D) Cancellous bone mass and architecture were analyzed as bone volume over tissue volume (BV/TV), trabecular separation (Tb. Sp), trabecular thickness (Tb. Th), and trabecular number (Tb. N) in lumbar vertebrae 4 (L4).
(E and F) Cortical thickness (Ct. Th) was measured in L4 (E) and femur (F). A–F, For the samples that have a normal distribution, ∗, p < 0.05 comparing Dmp1-Cre;Tnfsf11fl/fl or Ot-CRiTnfsf11 to their own littermate controls using t-test. For the samples that did not have a normal distribution, namely BV/TV of CRi and femur Ct.Th, Rank-sum was used for comparison. Individual Tukey p values for each comparison are provided in the graphs.
(G–I) Tnfsf11(G) and CtsK (H and I) mRNA levels were measured in tibia shafts (G and H) and calvaria (I) of 12-month-old Dmp1-Cre;Tnfsf11fl/fl mice, Ot-CRiTnfsf11 mice and their corresponding littermate controls by qRT-PCR. For all qRT-PCR analyses, mRNA levels were normalized to mouse Mrsp2. G-I, n = 4–15 mice/group; ∗, p < 0.05 comparing Dmp1-Cre;Tnfsf11fl/fl mice, Ot-CRiTnfsf11 mice and their corresponding littermate controls; #, p < 0.05 comparing Dmp1-Cre;Tnfsf11fl/fl to Ot-CRiTnfsf11, or comparing controls using one-way Anova with Tukey adjustment. Individual Tukey p values for each comparison are provided in the graphs. All data presented as mean ± SD.
Importantly, the high bone mass phenotype of both Dmp1-Cre;Tnsfs11 fl/fl and Ot-CRiTnfsf11 mice were associated with Tnfsf11 suppression (Figure 5G). As mentioned earlier, Tnfsf11 is essential for the formation and survival of osteoclast.41 We and others have shown that deletion of Tnfsf11 from Dmp1-Cre targeted cells reduces osteoclast number, demonstrating that Dmp1-targeted cells support osteoclastogenesis by producing RANKL.23,40 As expected, the lower Tnfsf11 levels observed in both Dmp1-driven CRISPRi and Cre-loxP models reduced osteoclasts as evidenced by a decline in osteoclast-specific gene expression (Figure 5H and 5I). Together, these results demonstrate that target gene suppression by Dmp1-driven CRISPRi persists up to at least 12 months of age in both female and male mice.
Discussion
Here we demonstrate that expression of a sgRNA and dCas9::KRAB from hemizygous safe harbor loci provides global LOF of target genes at a level comparable to that obtained by traditional knockout methodologies. We also show that CRISPRi can be used for cell type-specific LOF studies and that it may provide improved cell type-specificity over the Cre-loxP system. Our studies also set the stage for determining whether CRISPRi can be utilized for temporal, spatial, and progenitor-specific control of gene expression, as well as simultaneous cell type-specific suppression of multiple genes.
We previously showed effective global suppression of Tnfsf11 using CRISPRi via a single transgene. However, significant animal-to-animal variation in the skeletal phenotype was observed within a given transgenic line in that study. Specifically, while most mice exhibited osteopetrosis, some exhibited partial formation of the femoral marrow cavity and partial tooth eruption.33 These variations were likely due to variable transgene expression stemming from the integration site. To avoid this possibility, in the present study, we globally suppressed Tnfsf11 using knock-in mice that express the sgRNA and dCas9::KRAB from safe harbor loci. All mice with global Tnfsf11 suppression exhibited osteopetrosis and lack of tooth eruption in the current study (Figure S4). Moreover, unlike our previous transgene-based CRISPRi, the magnitude of osteoclast inhibition in gCRiTnfsf11 mice was comparable to that observed in Tnfsf11 null mice. These results suggest that the expression of CRISPRi components from safe harbor loci provides improved consistency over transgenic approaches for LOF studies.
Evidence presented here suggests that CRISPRi-based cell type-specific LOF studies provide improved cell type specificity compared with the Cre-loxP system. Specifically, using scRNA-seq analysis we show that two cell types targeted by Dmp1-Cre, namely CAR and Osteo_CAR cells, are not targets of Dmp1-driven CRISPRi. The different methodologies that were used to produce Dmp1-driven Cre-loxP and CRISPRi systems may have contributed to this difference in cell type specificity. Specifically, all currently available Dmp1-Cre driver strains are transgenic mice6,8,9 in which Cre expression is driven by a 10kb or 8kb Dmp1 promoter. In contrast, in our Dmp1-driven CRISPRi model, dCas9::KRAB is inserted into the endogenous Dmp1 locus, and its expression is mediated by endogenous Dmp1 regulatory elements. Therefore, the expression pattern of dCas9::KRAB may mirror that of the endogenous Dmp1 gene better than Dmp1-Cre transgenes. However, we expect Dmp1-driven CRISPRi would also be more specific compared to a knock-in Dmp1-Cre model. Specifically, we show that Dmp1-driven CRISPRi does not alter Tnfsf11 expression in Osteo_CAR cells. As endogenous Dmp1 expression is detected in Osteo_CAR cells, it is reasonable to expect Cre expression and activity in Osteo_CAR cells even if the Cre coding sequence was knocked into the endogenous Dmp1 locus. Because Osteo_CAR cells are targets of Dmp1-Cre, but they are not targeted by Dmp1-driven CRISPRi, Dmp1-driven CRISPRi appears to be more specific compared to the Dmp1-driven Cre-mediated recombination.
Previous in vitro studies show that upon cessation of dCas9::KRAB production, target gene suppression is reversed.31,46 While the length of time required for target expression to return to baseline varies based on the target gene or reporter, for most targets tested 5 to 8 days is sufficient for full reversal.31,46 Herein, we showed that suppression of Tnfsf11 by the Dmp1-CRISPRi system persists at levels comparable to Cre-loxP even at 12 months of age (Figure 5G). However, it is possible that if Dmp1 expression, and hence dCas9::KRAB expression, declines significantly as mice age further, the level of target knockdown may decrease. Therefore, future models may benefit from the use of newer systems such as CRISPRoff,46 which utilizes a combination of ZNF10 KRAB, Dnmt3A, and Dnmt3L protein domains fused to dCas9, to epigenetically suppress targets in a manner stronger and more persistent than dCas9::KRAB alone.
Even with its current limitations, cell type-specific CRISPRi provides significant advantages over current recombination-mediated approaches. Perhaps one of the most useful is that LOF mice can be produced in a single cross. Deletion of loxP-flanked alleles requires two crosses: the first to introduce the Cre driver allele into the loxP-containing mice and the second to produce mice homozygous for the loxP-flanked allele. Targeting multiple loci simultaneously can require several additional crosses. Therefore, the time and cost savings gained by producing mice with a single cross can be substantial. It is also important to consider that introduction of new sgRNA expression cassettes into safe-harbor loci requires significantly less time and money than creating new loxP-flanked alleles using traditional gene targeting in embryonic stem cells. Thus, even though the number of mouse lines expressing sgRNAs is currently much lower than the number of loxP-flanked alleles, production of new sgRNA mice should be relatively efficient.
Further development and improvement of cell type-specific CRISPRi systems has the potential to provide several benefits in addition to improvement of cell type specificity. For example, while tetracycline- or tamoxifen-regulated Cre strains are available to perform temporally controlled cell-type-specific LOF studies, one limitation of these systems is their leakiness (inducer-independent expression of Cre expression at low levels). However, because CRISPRi is dose-dependent and requires high-level expression of system components for target gene suppression, if dCas9::KRAB is expressed under the control of a tetracycline-regulated cell type-specific promoter, low-level ligand-independent expression of dCas9::KRAB would likely not be sufficient for the suppression of target genes. Moreover, the reversible nature of CRISPRi can also be utilized to target LOF specifically to progenitor cells within a given lineage. To accomplish this, dCas9::KRAB expression can be placed under the control of progenitor-specific promoters that are not expressed in cells at later stages of the lineage.
We do not anticipate that the use of CRISPRi will completely replace recombination-based LOF approaches. Instead, CRISPRi can be used as an adjunct approach to answer questions that cannot be addressed using recombination-based gene inactivation. Perhaps the most significant limitation of recombination-based systems is the lack of truly cell-type-specific Cre driver strains. Here we present evidence that CRISPRi can produce gene suppression that is more cell type-specific than Cre-mediated recombination. Furthermore, the potential to suppress genes specifically in progenitor cells is something that is not possible with recombination-based LOF. In our view, these advantages warrant continued development of CRISPRi for LOF studies.
Limitations of the study
One limitation of our study is that it was not possible to directly measure the efficacy of Tnfsf11 suppression in osteocytes of the Dmp1-driven CRISPRi mice. Nonetheless, the reduction in osteoclasts and increase in bone mass in the Dmp1-driven CRISPRi mice were lower in magnitude compared to that achieved by Dmp1-Cre mediated Tnfsf11 deletion (Figures 2 and 5). There are multiple potential explanations for this difference. First, the stronger phenotype in Dmp1-Cre mice may be due to loss of Tnfsf11 function in a broader range of cell types than in the Dmp1-driven CRISPRi mice. Our scRNA-seq results are consistent with this possibility. Second, it is possible that Dmp1-driven Tnfsf11 suppression in osteocytes did not completely eliminate Tnfsf11 production in these cells, whereas Dmp1-Cre mediated deletion did. Global dCas9::KRAB expression using a strong promoter was capable of suppressing Tnfsf11 to a level that mimicked gene deletion. However, it is possible that Dmp1-driven dCas9::KRAB expression was not as effective. Future studies will examine whether sgRNA or dCas9::repressor domain can be modified to increase the potency of suppression. Such modifications may include the use of (i) multiple sgRNAs for the same target gene, (ii) alternative sgRNA design that improves sgRNA stability and sgRNA-dCas9 assembly,47 (iii) bipartite repressor domains such as KRAB-MeCP2,48 or (iv) more potent KRAB domains such as ZIM3 KRAB domain.49
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Trizol Reagent | Life Technologies | Cat No: 15596018 |
| RNAeasy Plus Mini Kit | Qiagen | Cat No: 74136 |
| High-Capacity cDNA Reverse Transcription Kit | Applied Biosystems | Cat No: 4368814 |
| TaqMan Fast Advanced Master Mix | Applied Biosystems | Cat No: 4444964 |
| HBSS | Gibco | Cat No: 14025-076 |
| PBS | Gibco | Cat No: 21600010 |
| EDTA | Invitrogen | Cat No: 15575-020 |
| LiberaseTM | Roche, Sigma | Cat No: LIBTM-RO |
| Deposited data | ||
| scRNA-seq data files | This paper | BioProject PRJNA896097 |
| Experimental models: Organisms/strains | ||
| Mouse: B6.129-Tnfsf11tm1.1Caob/J | The Jackson Laboratory | Strain #:018978, RRID:IMSR_JAX:018978 |
| Mouse: B6N.FVB-Tg(Dmp1-cre)1Jqfe/BwdJ | The Jackson Laboratory | Strain #:023047 RRID:IMSR_JAX:023047 |
| Mouse: B6.Cg-Igs2tm1(CAG-mCherry,-cas9/ZNF10∗)Mtm/J | The Jackson Laboratory | Strain #:030000 RRID:IMSR_JAX:030000 |
| Mouse: B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J | The Jackson Laboratory | Strain #:007909 RRID:IMSR_JAX:007909 |
| Mouse: Tnfsf11-null | Xiong et al.50 | N/A |
| Mouse: sgRNATnfsf11 | This paper | N/A |
| Mouse: Dmp1-dCas9::KRAB | This paper | N/A |
| Oligonucleotides | ||
| Genotyping sgRNATnfsf11 mice: F2: 5′-AAGCACTTGCTCTCCCAAAG-3′ R2: 5′-GGCGGATCACAAGCAATAAT-3′ F3: 5′-GAGGGCCTATTTCCCATGAT-3′ R3: 5′-GGTGTTTCGTCCTTTCCACA-3′ |
This paper | N/A |
| Genotyping Dmp1-dCas9::KRAB mice: F1: 5′-CTTCGCTCTTTCACCCACAT-3′ R1: 5′- TACTGGGAGAGCACAGGACA-3′ R2″ 5′-GGCTCCGATCAGGTTCTTCT-3′ |
This paper | N/A |
| Genotyping Tnfsf11-null mice: RANKL-null-geno-for-1: 5′-CAGCTATGATGGAAGGCTCCTG-3′ RANKL-null-geno-rev-1: 5′-GATTGGCAAGGTAGGGTTCA-3′ RANKL-null-geno-for-2: 5′-TCTCAGGAGCTCCAGGTAAC-3′ RANKL-null-geno-rev-2: 5′-CGCTGGGCCACATCCAACTAA-3′ |
This paper | N/A |
| Taqman Gene Expression Assay Mrsp2 | Life Technologies | Mm00475529_m1 |
| Taqman Gene Expression Assay Actb | Life Technologies | Cat. #4352341E |
| Taqman Gene Expression Assay Tnfsf11 | Life Technologies | Mm00441906_m1 |
| Taqman Gene Expression Assay Acp5 | Life Technologies | Mm00475698_m1 |
| Taqman Gene Expression Assay CtsK | Life Technologies | Mm00484039_m1 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Melda Onal (monal@uams.edu).
Materials availability
Murine models generated in this study, namely sgRNATnfsf11 and Dmp1-dCas9::KRAB mice, will be made available upon request.
Experimental model and subject details
Generation of mice
In previous studies, we have tested 3 sgRNAs targeting 3 different regions within 150 bp of the Tnfsf11 transcriptional start site and determined the sgRNA that would facilitate efficient suppression of Tnfsf11 via CRISPRi.33 This sgRNA sequence was used to produce to sgRNATnfsf11 mice. These mice were produced via CRISPR/Cas9-mediated knock-in of a U6-sgRNATnfsf11 cassette into the Rosa26 locus. Briefly, Cas9 protein, a sgRNA targeting the Rosa26 locus (sgRNARosa26), and single-strand oligonucleotide donor (ssODN) harboring the U6-sgRNATnfsf11 expression cassette, were used to perform the CRISPR/Cas9-mediated knock-in. sgRNARosa26 (ACTCCAGTCTTTCTAGAAGA, PAM: TGG) was used to drive the production of a double-strand break at the 1st intron of the Rosa loci.51 A 756 bp ssODN was used to knock in the U6-sgRNATnfsf11 sequence into the sgRNARosa26 cleavage site. This ssODN contained the U6 small RNA promoter, sgRNATnfsf11 (GAGCCAATCAGCCTCCAGGA, PAM: GGG),33 and homology arms corresponding to 200 bp upstream and downstream around the sgRNARosa cleavage site. As the upstream homology arm of the ssODN contained the sequence targeted by sgRNARosa26, the ssODN can be a target for CRISPR/Cas9 and sgRNARosa. To avoid this, three nucleotides were changed in the upstream homology arm of the ssODN corresponding to the positions C9A, C13T, and G16A of the sgRNARosa target sequence. Knock-in mice were produced by microinjection of 50 ng/ul Cas9 protein, 30 ng/ul sgRNARosa, and 10 ng/ul ssODN into the pronuclei of C57BL/6J mice. Founders were screened for the presence of the knock-in sequence using the following primers: F1 5′-AAGCACTTGCTCTCCCAAAG-3′; R1 5′-GGCGGATCACAAGCAATAAT -3′. This PCR produced an 803bp band for the knock-in allele and a 447bp band for the wild-type allele. The structure of the knock-in allele was confirmed by DNA sequencing of the PCR product. The progeny of the founders were genotyped using the PCR primers indicated in key resources table. This PCR produced a 447 bp band for wild type allele and a 210bp band for the knock-in allele.
Dmp1-dCas9::KRAB mice were produced by Cyagen. For this purpose, a cassette containing nuclear localization signal-dCas9-KRAB-rabbit beta globin poly-A terminator (NLS-dCas9-KRAB-rBG pA) sequences was inserted upstream of ATG start codon in exon 2 of the endogenous murine Dmp1 gene. The Dmp1 targeting vector used for the knock-in contained homology arms, NLS-dCas9-KRAB-rBG pA cassette, and a Neo cassette flanked by self-deletion anchor sites. This targeting construct was linearized by restriction digestion and electroporated into C57BL/6N ES cells. The electroporated ES cells were subjected to G418 selection followed by expansion of G418-resistant clones. Insertion of the targeting cassette was confirmed by PCR and Southern blot analysis. After the removal of the Neo cassette, ES cells with the correct insertion were introduced into host embryos and transferred into surrogate mothers. Chimeras were bred to wild-type mice to confirm germline transmission. Pups were genotyped by PCR to identify F1 heterozygous mutants. Genotyping was performed with the PCR primers indicated in key resources table. This PCR produced a 202 bp band for wild type allele and a 343bp band for the knock-in allele.
A Tnfsf11-null allele was created as a by-product of generating the Tnfsf11-SR allele using gene editing.50 The null allele contains a single T insertion after the 18th nucleotide of exon 4, which results in truncation of the RANKL protein prior to production of the TNF domain. Genotyping was performed with the PCR primers indicated in key resources table. All 4 primers were used in a single PCR to detect the single T insertion via the following product sizes: WT product = 231 bp, null product = 135 bp.
Murine models
Generation and genotyping of the Tnfsf11floxed 23, Dmp1-Cre6 and H11dCas9KRAB38,39 mice used in this study have been described previously. Briefly, Tnfsf11floxed mice were created by flanking exon 3 and exon 4 of Tnfsf11 with loxP sites. In Dmp1-Cre transgenic mice, a 14 kb Dmp1 promoter fragment, which contains the genomic sequence of the promoter through 17 bp of the initial non-coding region in exon 2, drives the Cre expression. H11dCas9KRAB knock-in mice constitutively active and broadly expressed CMV early enhancer/chicken β actin (CAG) promoter drives the expression of mCherry and dCas9::KRAB from the safe harbor Igs2 locus (Hipp11 or H11). All mice were provided water and food ad libitum and were maintained on a 12-hour light/dark cycle. All animal studies were carried out in accordance with the policies of, and with approval from, the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences. The studies of this manuscript were performed and reported in accordance with ARRIVE guidelines. Sex, number and age of the experimental mice are indicated in each figure legend.
Method details
RNA isolation and gene expression analysis
Murine bones and soft tissues were snap-frozen in liquid nitrogen and stored at −80°C. To prepare RNA, frozen thymus, spleen, lumbar vertebrae, tibia shafts, and calvaria were homogenized in Trizol Reagent (Life Technologies # 15596018). RNA was isolated from soft tissues following the Trizol Reagent’s manufacturer protocol. RNA was isolated from bones using RNAeasy Plus Mini Kit (Qiagen Cat # 74136) according to the manufacturer’s instructions. For all tissues, RNA concentrations were determined using a Nanodrop instrument (Thermo Fisher Scientific). One μg of RNA was used to synthesize cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems Cat. #4368814) according to the manufacturer’s instructions. Relative mRNA levels were determined using multiplex quantitative real-time PCR (qRT-PCR) analysis using TaqMan Fast Advanced Master Mix (Applied Biosystems # 4444964), FAM-labeled TaqMan gene expression assays (Life Technologies) and VIC-labeled mouse Mrsp2 (Mm00475529_m1) or VIC-labeled mouse Actb (beta-actin)(Applied Biosystems Cat. #4352341E). The relative mRNA levels were determined using the comparative cycle threshold (ΔCt) method.52
Skeletal analysis
Bone mineral density (BMD) was measured in live mice by dual-energy x-ray absorptiometry with a PIXImus Mouse Densitometer (GE Lunar Corp., Madison, WI) using the manufacturer’s software as described previously (PMID: 25431114). X-ray images of euthanized mice were taken using a Faxitron imager. Fourth lumbar vertebrae (L4) and femurs were used for the microCT analysis. The femurs and vertebrae were dissected, cleaned of soft tissue, wrapped in saline-soaked gauze, and stored at –20°C. For microCT analysis, bones were thawed and loaded into a 12.3 mm diameter scanning tube filled with saline. The microCT scans were performed on a model uCT40 (Scanco Biomedical) as previously described.53,54 Briefly, medium-resolution scans were obtained (12 μm isotropic voxel size). A Gaussian filter (sigma = 0.8, support = 1) was used to reduce noise, and a threshold of 220 was used for all scans. Nomenclature conforms to recommendations of the American Society for Bone and Mineral Research.55 The femurs were scanned from the distal growth plate to the mid-shaft. The midshaft cortical measurements were performed by drawing contours to measure the cortical thickness on the first 20 midshaft slices. For microCT analysis of the fourth lumbar vertebrae (L4), the whole vertebral body was scanned. Trabecular analysis was performed by drawing contours every 10 slices on the whole space between the 2 growth plates of the vertebrae. Similar to femoral measurements, the vertebral cortical bone thickness was determined on the ventral cortical wall using contours of cross-sectional images, drawn to exclude trabecular bone. Calibration and quality control of the scanner were performed weekly or monthly as previously described.33
scRNA-seq sample preparation
Muscles and soft tissue were removed from freshly isolated femurs and tibias. The periosteum was removed by scraping with a scalpel. Epiphyses were removed above the growth plate. Bones (containing cancellous and cortical compartments) were cut longitudinally and flushed with PBS containing 1% BSA to remove marrow. Bone was then chopped into small fragments and subjected to 5 serial digestions with LiberaseTM (Roche, Sigma Cat. #LIBTM-RO) interspaced with incubation in EDTA. Briefly, for each enzyme digestion, bone fragments were incubated in HBSS containing 2 Wunsch units of LiberaseTM for 20 minutes (min) at 37°C with shaking. The supernatant of each fraction was moved to a new tube on ice. After each enzyme digestion bone fragments were washed with PBS. Bone fragments were then incubated with PBS containing 5 mM EDTA and 0.1% BSA for 20 min at 37°C with shaking. The supernatant of each fraction was transferred to a fresh tube on ice. After each EDTA treatment, bone fragments were washed with HBSS. Cells isolated from each fraction were pelleted at 300 g for 10 min and supernatants were aspirated with a glass pipette. Pelleted cells were resuspended in the FACS sorting buffer (PBS with 1% BSA with 2 mM EDTA) and stored on ice. Fractions 2 to 8 were combined, concentrated, and used for FACS. Approximately 10, 000 TdTomato-positive cells were sorted into a fresh tube. Single cells were captured as droplets using a 10x Genomics Chromium Controller. scRNA-seq libraries were constructed using Chromium Single-Cell 3′ v3 Reagent Kit according to the manufacturer’s instructions.
scRNA-seq data analysis
The raw sequencing data (fastq files) were preprocessed by CellRanger software (10x Genomics) version 6.0.1. Reads were aligned on Mus musculus reference genome (mm10) and demultiplexed to generate count tables of transcripts across individual cells. The count tables were further analyzed in R suite software through Seurat version 4.1.0 package.56 Cells with a gene number less than 500, greater 3,500, and having more than 10% of unique molecular identifiers stemming from mitochondrial genes, were discarded from the analysis. Principal component analysis (PCA) was performed on the top 6,000 variable genes of the remaining high-quality cells. To make the data across individual samples comparable, we performed integration across samples using the reciprocal PCA method to minimize the technical batch effect. To identify cell types, clustering analysis of Louvain algorithm with multilevel refinement57 was employed. The clustering result was visualized by Uniform Manifold Approximation and Projection for Dimension Reduction (UMAP) method of two dimensions. The gene-specific markers of individual clusters were identified by the MAST method.58
Quantification and statistical analysis
All values are reported as mean ± standard deviation (STDEV). The statistical tests performed are indicated in figure legends. Briefly, using SAS version 9.4, one-way ANOVA models were fit to the various outcomes with animal group as the predictor variable. Model residuals were examined for normality and constant variance. Transformations such as square root, natural logarithm, negative reciprocal or rank were used when the original scale of data did not meet those assumptions. In case of normality, statistical comparisons of two groups were done by t-test (equal variance) or by t-test with Welch correction (unequal variance). In case where data does not have a normal distribution, statistical comparisons of two groups were done by rank sum test. All pairwise comparisons from each ANOVA were examined and the Tukey method was used to adjust the p values for multiple comparisons.
SAS v9.4 software was used for the comparison of the phenotype effect between the two ages in Figure 4. First, vertebral, femoral, and global BMD were analyzed from the 4 genotypes and 2 ages. Two-way ANOVAs with the factors of genotype and age were performed to check for differences between the 3 control groups at each age. No significant differences were found while the assumptions of normally distributed residuals with equal variance were verified for all 3 BMD sites. Next, having verified no statistically significant differences between the control groups, the control groups were combined with new Two-way ANOVAs with the factors of age (12 months vs. 4 months) and experimental group (experimental vs. control). Again the assumptions of normally distributed residuals with equal variance were verified for all three BMD sites. A contrast was estimated for each model testing for an interaction effect of the experimental group and age, to address if the change between 12 months and 4 months for the experimental group was equal to or different from that change within the control group. t-tests on this contrast were performed for all 3 BMD sites.
Acknowledgments
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) R21AR076575, National Institute of General Medical Sciences (NIGMS) grants P20GM125503, and the UAMS Bone and Joint Initiative. We thank the Genetic Models Core Facility for the production of the sgRNA murine model, the Bone Histology and Imaging Core for their help with tissue collection and analysis, the UAMS Genomics Core for their help with scRNA-seq, and the staff of the UAMS Department of Laboratory Animal Medicine for their help with husbandry and care of mice.
Author contributions
M.O. and C.A.O. designed murine models and experiments. Q.F. helped with the design, production, and sequence identification of the sgRNA murine model. M.O., D.J.L., and N.S.A. performed experiments. M.O., D.J.L., N.S.A, J.A.C., and A.J. contributed to tissue collection. D.J.L., S.B.H., J.A.H., and A.J. performed μCT analysis. M.O., D.J.L., and N.S.A. performed gene expression analysis. T.J.D. performed statistical analysis of data. I.N. performed bioinformatics analysis of the scRNA-seq. M.O. wrote the manuscript. All authors revised the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: July 20, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107428.
Supplemental information
Data and code availability
-
•
scRNA-seq data reported in this work are available at SRA database under accession number BioProject PRJNA896097. This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper can be obtained from the lead contact upon request.
References
- 1.Zhang M., Xuan S., Bouxsein M.L., von Stechow D., Akeno N., Faugere M.C., Malluche H., Zhao G., Rosen C.J., Efstratiadis A., Clemens T.L. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J. Biol. Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 2.Logan M., Martin J.F., Nagy A., Lobe C., Olson E.N., Tabin C.J. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 3.Dacquin R., Starbuck M., Schinke T., Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev. Dyn. 2002;224:245–251. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- 4.Kim J.E., Nakashima K., de Crombrugghe B. Transgenic mice expressing a ligand-inducible cre recombinase in osteoblasts and odontoblasts: a new tool to examine physiology and disease of postnatal bone and tooth. Am. J. Pathol. 2004;165:1875–1882. doi: 10.1016/S0002-9440(10)63240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodda S.J., McMahon A.P. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y., Xie Y., Zhang S., Dusevich V., Bonewald L.F., Feng J.Q. DMP1-targeted Cre expression in odontoblasts and osteocytes. J. Dent. Res. 2007;86:320–325. doi: 10.1177/154405910708600404. [DOI] [PubMed] [Google Scholar]
- 7.Zha L., Hou N., Wang J., Yang G., Gao Y., Chen L., Yang X. Collagen1alpha1 promoter drives the expression of Cre recombinase in osteoblasts of transgenic mice. J Genet Genomics. 2008;35:525–530. doi: 10.1016/S1673-8527(08)60072-7. [DOI] [PubMed] [Google Scholar]
- 8.Powell W.F., Jr., Barry K.J., Tulum I., Kobayashi T., Harris S.E., Bringhurst F.R., Pajevic P.D. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J. Endocrinol. 2011;209:21–32. doi: 10.1530/JOE-10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bivi N., Condon K.W., Allen M.R., Farlow N., Passeri G., Brun L.R., Rhee Y., Bellido T., Plotkin L.I. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J. Bone Miner. Res. 2012;27:374–389. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong J., Piemontese M., Onal M., Campbell J., Goellner J.J., Dusevich V., Bonewald L., Manolagas S.C., O'Brien C.A. Osteocytes, not Osteoblasts or Lining Cells, are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurel D.B., Matsumoto T., Vallejo J.A., Johnson M.L., Dallas S.L., Kitase Y., Brotto M., Wacker M.J., Harris M.A., Harris S.E., Bonewald L.F. Characterization of a novel murine Sost ER(T2) Cre model targeting osteocytes. Bone Res. 2019;7:6. doi: 10.1038/s41413-018-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toyosawa S., Shintani S., Fujiwara T., Ooshima T., Sato A., Ijuhin N., Komori T. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J. Bone Miner. Res. 2001;16:2017–2026. doi: 10.1359/jbmr.2001.16.11.2017. [DOI] [PubMed] [Google Scholar]
- 13.Fen J.Q., Zhang J., Dallas S.L., Lu Y., Chen S., Tan X., Owen M., Harris S.E., MacDougall M. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J. Bone Miner. Res. 2002;17:1822–1831. doi: 10.1359/jbmr.2002.17.10.1822. [DOI] [PubMed] [Google Scholar]
- 14.Kalajzic I., Braut A., Guo D., Jiang X., Kronenberg M.S., Mina M., Harris M.A., Harris S.E., Rowe D.W. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004;35:74–82. doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Brunkow M.E., Gardner J.C., Van Ness J., Paeper B.W., Kovacevich B.R., Proll S., Skonier J.E., Zhao L., Sabo P.J., Fu Y., et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balemans W., Ebeling M., Patel N., Van Hul E., Olson P., Dioszegi M., Lacza C., Wuyts W., Van Den Ende J., Willems P., et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum. Mol. Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 17.van Bezooijen R.L., Roelen B.A.J., Visser A., van der Wee-Pals L., de Wilt E., Karperien M., Hamersma H., Papapoulos S.E., ten Dijke P., Löwik C.W.G.M. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J. Exp. Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magarò M.S., Bertacchini J., Florio F., Zavatti M., Potì F., Cavani F., Amore E., De Santis I., Bevilacqua A., Reggiani Bonetti L., et al. Identification of Sclerostin as a Putative New Myokine Involved in the Muscle-to-Bone Crosstalk. Biomedicines. 2021;9 doi: 10.3390/biomedicines9010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weivoda M.M., Youssef S.J., Oursler M.J. Sclerostin expression and functions beyond the osteocyte. Bone. 2017;96:45–50. doi: 10.1016/j.bone.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshioka H., Okita S., Nakano M., Minamizaki T., Nubukiyo A., Sotomaru Y., Bonnelye E., Kozai K., Tanimoto K., Aubin J.E., Yoshiko Y. Single-Cell RNA-Sequencing Reveals the Breadth of Osteoblast Heterogeneity. JBMR Plus. 2021;5 doi: 10.1002/jbm4.10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terasawa M., Shimokawa R., Terashima T., Ohya K., Takagi Y., Shimokawa H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J. Bone Miner. Metab. 2004;22:430–438. doi: 10.1007/s00774-004-0504-4. [DOI] [PubMed] [Google Scholar]
- 22.Lim J., Burclaff J., He G., Mills J.C., Long F. Unintended targeting of Dmp1-Cre reveals a critical role for Bmpr1a signaling in the gastrointestinal mesenchyme of adult mice. Bone Res. 2017;5 doi: 10.1038/boneres.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong J., Onal M., Jilka R.L., Weinstein R.S., Manolagas S.C., O'Brien C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalajzic I., Matthews B.G., Torreggiani E., Harris M.A., Divieti Pajevic P., Harris S.E. In vitro and in vivo approaches to study osteocyte biology. Bone. 2013;54:296–306. doi: 10.1016/j.bone.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim J., Shi Y., Karner C.M., Lee S.Y., Lee W.C., He G., Long F. Dual function of Bmpr1a signaling in restricting preosteoblast proliferation and stimulating osteoblast activity in mouse. Development. 2016;143:339–347. doi: 10.1242/dev.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J., Link D.C. Targeting of Mesenchymal Stromal Cells by Cre-Recombinase Transgenes Commonly Used to Target Osteoblast Lineage Cells. J. Bone Miner. Res. 2016;31:2001–2007. doi: 10.1002/jbmr.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi L.S., Larson M.H., Gilbert L.A., Doudna J.A., Weissman J.S., Arkin A.P., Lim W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert L.A., Larson M.H., Morsut L., Liu Z., Brar G.A., Torres S.E., Stern-Ginossar N., Brandman O., Whitehead E.H., Doudna J.A., et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert L.A., Horlbeck M.A., Adamson B., Villalta J.E., Chen Y., Whitehead E.H., Guimaraes C., Panning B., Ploegh H.L., Bassik M.C., et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell. 2014;159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontana J., Dong C., Ham J.Y., Zalatan J.G., Car J.M. Regulated Expression of sgRNAs Tunes CRISPRi in E. coli. Biotechnol. J. 2018;13 doi: 10.1002/biot.201800069. [DOI] [PubMed] [Google Scholar]
- 31.Mandegar M.A., Huebsch N., Frolov E.B., Shin E., Truong A., Olvera M.P., Chan A.H., Miyaoka Y., Holmes K., Spencer C.I., et al. CRISPR Interference Efficiently Induces Specific and Reversible Gene Silencing in Human iPSCs. Cell Stem Cell. 2016;18:541–553. doi: 10.1016/j.stem.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Xie Y., Dong Z.C., Jiang X.J., Gong P., Lu J., Wan F. [Levels of sgRNA as a Major Factor Affecting CRISPRi Knockdown Efficiency in K562 Cells] Mol. Biol. 2021;55:86–95. doi: 10.31857/S0026898421010146. [DOI] [PubMed] [Google Scholar]
- 33.MacLeod R.S., Cawley K.M., Gubrij I., Nookaew I., Onal M., O'Brien C.A. Effective CRISPR interference of an endogenous gene via a single transgene in mice. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-53611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt-Supprian M., Rajewsky K. Vagaries of conditional gene targeting. Nat. Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 35.Gemberling M.P., Siklenka K., Rodriguez E., Tonn-Eisinger K.R., Barrera A., Liu F., Kantor A., Li L., Cigliola V., Hazlett M.F., et al. Transgenic mice for in vivo epigenome editing with CRISPR-based systems. Nat. Methods. 2021;18:965–974. doi: 10.1038/s41592-021-01207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong L., Yao L., Tower R.J., Wei Y., Miao Z., Park J., Shrestha R., Wang L., Yu W., Holdreith N., et al. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife. 2020;9 doi: 10.7554/eLife.54695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J.S., Kamath T., Mazur C.M., Mirzamohammadi F., Rotter D., Hojo H., Castro C.D., Tokavanich N., Patel R., Govea N., et al. Control of osteocyte dendrite formation by Sp7 and its target gene osteocrin. Nat. Commun. 2021;12:6271. doi: 10.1038/s41467-021-26571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindtner S., Catta-Preta R., Tian H., Su-Feher L., Price J.D., Dickel D.E., Greiner V., Silberberg S.N., McKinsey G.L., McManus M.T., et al. Genomic Resolution of DLX-Orchestrated Transcriptional Circuits Driving Development of Forebrain GABAergic Neurons. Cell Rep. 2019;28:2048–2063.e8. doi: 10.1016/j.celrep.2019.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoneshiro T., Wang Q., Tajima K., Matsushita M., Maki H., Igarashi K., Dai Z., White P.J., McGarrah R.W., Ilkayeva O.R., et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572:614–619. doi: 10.1038/s41586-019-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J.Q., Bonewald L.F., Kodama T., Wutz A., Wagner E.F., et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 41.Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A.J., Van G., Itie A., et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 42.Baccin C., Al-Sabah J., Velten L., Helbling P.M., Grünschläger F., Hernández-Malmierca P., Nombela-Arrieta C., Steinmetz L.M., Trumpp A., Haas S. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat. Cell Biol. 2020;22:38–48. doi: 10.1038/s41556-019-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilton I.B., D'Ippolito A.M., Vockley C.M., Thakore P.I., Crawford G.E., Reddy T.E., Gersbach C.A. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 2015;33:510–517. doi: 10.1038/nbt.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato S., Hashimoto J., Usami Y., Ohyama K., Isogai Y., Hagiwara Y., Maruyama N., Komori T., Kuroda T., Toyosawa S. Novel sandwich ELISAs for rat DMP1: age-related decrease of circulatory DMP1 levels in male rats. Bone. 2013;57:429–436. doi: 10.1016/j.bone.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Kim H.N., Xiong J., MacLeod R.S., Iyer S., Fujiwara Y., Cawley K.M., Han L., He Y., Thostenson J.D., Ferreira E., et al. Osteocyte RANKL is required for cortical bone loss with age and is induced by senescence. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nunez J.K., Chen J., Pommier G.C., Cogan J.Z., Replogle J.M., Adriaens C., Ramadoss G.N., Shi Q., Hung K.L., Samelson A.J., et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell. 2021;184:2503–2519.e2517. doi: 10.1016/j.cell.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen B., Gilbert L.A., Cimini B.A., Schnitzbauer J., Zhang W., Li G.W., Park J., Blackburn E.H., Weissman J.S., Qi L.S., Huang B. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155:1479–1491. doi: 10.1016/j.cell.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeo N.C., Chavez A., Lance-Byrne A., Chan Y., Menn D., Milanova D., Kuo C.C., Guo X., Sharma S., Tung A., et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat. Methods. 2018;15:611–616. doi: 10.1038/s41592-018-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alerasool N., Segal D., Lee H., Taipale M. An efficient KRAB domain for CRISPRi applications in human cells. Nat. Methods. 2020;17:1093–1096. doi: 10.1038/s41592-020-0966-x. [DOI] [PubMed] [Google Scholar]
- 50.Xiong J., Cawley K., Piemontese M., Fujiwara Y., Zhao H., Goellner J.J., O'Brien C.A. Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy-induced bone loss. Nat. Commun. 2018;9:2909. doi: 10.1038/s41467-018-05244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu V.T., Weber T., Graf R., Sommermann T., Petsch K., Sack U., Volchkov P., Rajewsky K., Kühn R. Efficient generation of Rosa26 knock-in mice using CRISPR/Cas9 in C57BL/6 zygotes. BMC Biotechnol. 2016;16:4. doi: 10.1186/s12896-016-0234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 53.Akel N., MacLeod R.S., Berryhill S.B., Laster D.J., Dimori M., Crawford J.A., Fu Q., Onal M. Loss of chaperone-mediated autophagy is associated with low vertebral cancellous bone mass. Sci. Rep. 2022;12:3134. doi: 10.1038/s41598-022-07157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piemontese M., Almeida M., Robling A.G., Kim H.N., Xiong J., Thostenson J.D., Weinstein R.S., Manolagas S.C., O'Brien C.A., Jilka R.L. Old age causes de novo intracortical bone remodeling and porosity in mice. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 56.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., 3rd, Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blondel V.D., Guillaume J.-L., Lambiotte R., Lefebvre E. Fast unfolding of communities in large networks. J. Stat. Mech. 2008;2008 doi: 10.1088/1742-5468/2008/10/p10008. [DOI] [Google Scholar]
- 58.Finak G., McDavid A., Yajima M., Deng J., Gersuk V., Shalek A.K., Slichter C.K., Miller H.W., McElrath M.J., Prlic M., et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 2015;16:278. doi: 10.1186/s13059-015-0844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
scRNA-seq data reported in this work are available at SRA database under accession number BioProject PRJNA896097. This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper can be obtained from the lead contact upon request.