Abstract
Introduction
Invasive listeriosis most often presents as bacteremia or neurolisteriosis. Cerebral infection mostly manifests as meningitis or meningoencephalitis, but cerebral abscesses are a rare manifestation.
Case presentation
We present the rare case of a 51-year old patient with progressive right sided hemiparesis caused by a cerebral abscess due to Listeria monocytogenes infection. The initially suspected cerebral ischemia or bleeding was ruled out. Magnetic resonance imaging led to the suspected diagnosis of an angiocentric lymphoma. An open cerebral biopsy revealed an intracranial abscess formation. After abscess evacuation and identification of Listeria monocytogenes, anti-infective treatment with ampicillin and gentamicin was started. After repeated cerebral imaging with signs of ongoing tissue inflammation after 6 weeks we chose to prolong the therapy with oral amoxicillin until resolution of signs of intracerebral inflammation after 12 weeks, documented by repeated cerebral magnetic resonance imaging. During hospitalization, the patient was diagnosed with diabetes mellitus type II and treatment was initiated. The patient was discharged without any persistent neurologic deficits.
Discussion
For the treatment of bacterial brain abscesses, 4–6 weeks of intravenous antimicrobial treatment after surgical drainage are recommended. However, first line therapy of invasive cerebral listeriosis is not well established. We decided to use a combined treatment using ampicillin and gentamicin, followed by prolonged oral treatment due to ongoing tissue inflammation.
Conclusion
No evidence-based treatment recommendations are available for brain abscess caused by Listeria monocytogenes. We report a case with favorable outcome after anti-infective ampicillin- and gentamicin-based therapy. Systematic assessment of treatment would be desirable.
Keywords: Immunodeficiency, Hemiparesis, Gentamicin, Ampicillin
Introduction
Invasive listeriosis, most often caused as foodborne infection by the saprophytic gram-positive facultative anaerobic bacteria Listeria monocytogenes (LM), presents mostly as bacteremia or neurolisteriosis. Cerebral infection often manifests as meningitis or meningoencephalitis [1], [2]. However, the formation of cerebral abscesses are extremely rare and solely account for approximately 1 % of all listeria infections [3].
Despite listeriosis being a relatively rare disease, invasive listeriosis is diagnosed with increasing incidence. Especially elderly and immunocompromised patients are at greatest risk [4], [5]. In 2020, the notification rate in the European Union was 0.42 cases per 100,000 people [4]. The analysis of ready-to-eat food and human invasive listeriosis confirmed that more than 90 % of infections were caused by ingestion of ready-to-eat food preparations [4].
Listeria monocytogenes can access the central nervous system (CNS) via retrograde axonal transport and crossing of the blood-brain barrier. When utilizing the cranial nerves, LM can cause rhombencephalitis, leading to multiple cranial nerve disorders in up to 15 % of patients [6], [7]. Furthermore, blood-borne LM is able to cross the blood-brain barrier, leading to meningitis, meningoencephalitis, rhombencephalitis or, in rare cases, abscess formation (about 10 % of neurolisteriosis cases) [8]. Clinical presentation of listeria meningitis is characterized by high fever, nuchal rigidity and seizures as well as movement disorders. High case-fatality rates of encephalitis caused by listeriosis are reported [10].
Patient presentation
In May 2021, a 51-year-old patient was admitted with new onset of right sided hemiparesis and facial palsy. Three days prior to admission, the patient reported rigidity of the right ankle without any traumatic events. During the following day, he experienced progressive hemiparesis, starting in the right leg. Other symptoms like fever, nuchal rigidity, shivering, headache and photosensitivity were denied. There were no recent dental or ear infections. A sudden worsening of symptoms was experienced at the day of admission.
At the time of hospitalization, preexisting conditions were negated, and no immunodeficiency was known in the medical history. The patient denied continuous prescribed or regular non-prescribed medication or illicit drug use, but indicated long-term nicotine abuse. A frequent consumption of dairy products was reported. It is unknown if these were pasteurized.
Upon initial presentation, the neurological examination confirmed central facial nerve palsy, which mainly affected the movement of the right corner of the mouth. Other cranial nerve examinations as well as motor function of the left sided facial muscles were unremarkable. There were no clinical signs of meningism. The right sided hemiparesis was graded as followed: right arm (average strength) 4/5, hip flexion 3/5, knee extension 2/5, knee flexion 2/5, foot lifting/lowering as well as big toe lifting/lowering paralytic. There was no sensory loss. Tympanic body temperature at the time of hospitalization was not elevated (36.8 °C).
Further diagnostic work-up and course of disease
The laboratory results demonstrated elevated serum glucose levels as well as an elevated HbA1C-value of 9.1 % (76 mmol/L, reference: 20–42 mmol/L), leading to the diagnosis of diabetes mellitus type II. Treatment was initiated with needs-based rapid-acting insulin, which was changed to oral antidiabetics (metformin) according to endocrinological recommendation.
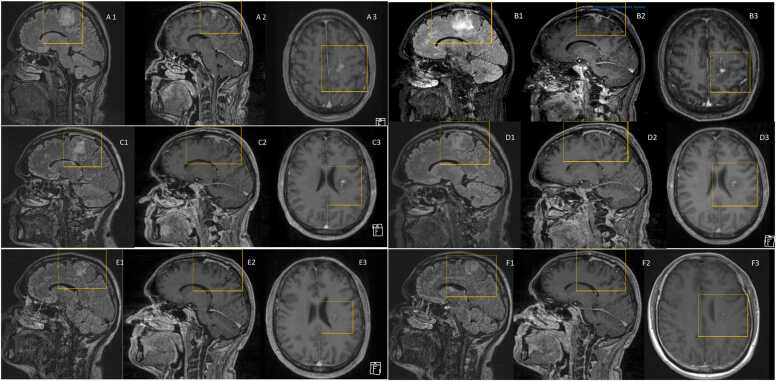
Color-coded duplex sonography excluded hemodynamically relevant stenosis of the brain supplying arteries, which was confirmed in the time-of-flight magnetic resonance angiography. Magnetic resonance imaging (MRI) of the brain showed signal enhancement in the left frontal lobe, extending to the corona radiata (Fig. 1A). This finding lead to the tentative diagnosis of an infarction of the Arteria cerebri anterior. Therefore, the patient was treated with 100 mg acetylsalicylic acid daily and admitted to the stroke unit. Lumbar puncture revealed elevated glucose levels (109 mg/dL, normal range 40–70 mg/dL) in the cerebrospinal fluid (CSF), as well as a leukocyte count of 2/µL with no pathological cells. Lactate (1,7 mmol/L, normal range 1.1–2.4 mmol/L) and total protein (0.39 g/L, normal range 0.15–0.45 g/L) in the CSF were within normal range.
Fig. 1.
T1 sequences of contrast-enhanced MRI-scans of the brain; A: MRI at the time of presentation. A1: sagittal flair imaging. A2: sagittal T1 imaging with contrast agent. A3: coronal T1 imaging with contrast agent. B: MRI 4 weeks after treatment initiation. B1: sagittal flair imaging. B2: sagittal T1 imaging with contrast agent. B3: coronal T1 imaging with contrast agent. C: MRI 8 weeks after treatment initiation. C1: sagittal flair imaging. C2: sagittal T1 imaging with contrast agent. C3: coronal T1 imaging with contrast agent. D: MRI 12 weeks after treatment initiation. D1: sagittal flair imaging. D2: sagittal T1 imaging with contrast agent. D3: coronal T1 imaging with contrast agent. E: MRI 17 weeks after treatment initiation. E1: sagittal flair imaging. E2: sagittal T1 imaging with contrast agent. E3: coronal T1 imaging with contrast agent. F: MRI 30 weeks after treatment initiation. F1: sagittal flair imaging. F2: sagittal T1 imaging with contrast agent. F3: coronal T1 imaging with contrast agent.
Daily transcranial Doppler ultrasonography excluded cerebral vessel flow accelerations at all times.
One day later, repeated analysis of the MRI led to the differential diagnosis of an angiocentric lymphoma. The cranial computed tomography (CT) scan two days after the initial MRI confirmed constant lesions and excluded cerebral infarction. Therefore, therapy with acetylsalicylic acid was discontinued. Contrast-enhanced CT scan of the chest and abdomen excluded extracranial lesions.
For confirmation of the suspected malignant disease, an open cerebral biopsy was performed six days after admission. After opening of the dura mater, an encapsulated, irregular formed abscess reaching from the left medial frontal gyrus to the basal ganglia was resected during surgery. A drainage was not necessary. Pus and tissue samples were collected for further examination. After surgery, calculated intravenous anti-infective treatment with vancomycin (1 g twice daily) and meropenem (2 g thrice daily) was started.
Microbiological and histological examination
Cultural findings of an intraoperative swab revealed LM infection of serogroup IV B. Resistance testing using agar diffusion confirmed trimethoprim/sulfamethoxazole resistance while showing sensitivity for erythromycin. Epsilometer tests confirmed sensitivity for ampicillin (minimal inhibitory concentration 0.5 mg/L) and meropenem (minimal inhibitory concentration 0.125 mg/L), according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) standards. Histological testing confirmed tissue samples from dura mater with meningothelial proliferation and lymphocytic reaction within the vessels.
Two sets of peripheral venous blood cultures showed no evidence of bacteremia.
Targeted treatment
The anti-infective therapy was adapted to 2 g ampicillin 4 times per day in combination with 320 mg (equaling 3,59 mg/kg) gentamicin once daily and the patient was transferred to the infectious diseases ward after postoperative monitoring. Here, ampicillin dosage was increased to 2 g 6 times daily. Drug monitoring for gentamicin showed insufficient base (< 0.4 µg/mL) and peak levels (0.5 µg/mL, goal: 3–5 µg/mL). Thus, the gentamicin dosage was stepwise increased to 440 mg daily (equaling 4,94 mg/kg), after repeated drug monitoring [8]. To achieve high peak levels, gentamicin was administered as a single intravenous dose over the course of one hour [9]. Creatinine, aspartate transaminase and alanine transaminase levels were monitored twice a week without evidence of renal impairment or drug-induced liver injury. An audiometric measurement after 4 weeks of treatment showed no signs of ototoxicity. Solely mild hearing loss attributed to working with loud machinery was identified.
After 5 weeks of treatment, repeated cerebral MRI showed equivocal results with on the one hand reduced contrast-enhancement, whereas the flair hyperintensity was progressive compared to the initial MRI scan (Fig. 1B). This was interpreted as sign of ongoing tissue inflammation.
During the sixth week of antimicrobial treatment, the patient reported vertigo. Central and peripheral causes were excluded by cranial CT and investigation by an otolaryngologist.
The patient received physical therapy during hospitalization, resulting in complete regression of the hemiparesis. After six weeks of parenteral anti-infective therapy, the treatment was converted to oral amoxicillin-based therapy and the asymptomatic patient was discharged. Follow-up cerebral MRI eight weeks after initiation of the anti-infective treatment revealed a regressive, but ongoing contrast-enhancement of the brain lesion (Fig. 1C). Therefore, amoxicillin was continued at a dose of 1 g thrice daily until a total duration of anti-infective treatment of 3 months was reached. Afterwards, repeated cerebral MRI scans confirmed regression of the contrast-enhancing area and excluded further abscess formations (Fig. 1D). Final cerebral imaging showed no sign of recurrent abscess formation.
Discussion
In general, brain abscesses caused by LM are a very unusual complication of cerebral listeriosis, which is a rare disease anyway. To our knowledge, in this case, no risk factors for abscess formation in cerebral LM infection are identified. In general, surgical patients are at greater risk to develop cerebral abscesses. In non-surgical patients, solid cancer, dental and upper respiratory tract infections as well as immunomodulation harbor an increased risk of developing a cerebral abscess [11]. However, none of these conditions were present in our patient. Regular consumption of dairy products might be a contributor for the occurrence of LM infection in the presented case. In our patient, the previously unknown diabetes mellitus seems to be the only relevant present risk factor for the development of cerebral abscesses [11], [12], [13].
Due to the rarity of disease and limited experience with treatment of LM brain abscesses, no evidence-based treatment guideline exists. A consensus study for the treatment of bacterial brain abscesses in general recommends 4–6 weeks of intravenous treatment after surgical drainage, without clear consensus for oral treatment continuation [14]. Further, optimal first line therapy of invasive cerebral listeriosis is not well established. However, ampicillin-based treatment is widely used. Combination regimens with ampicillin and aminoglycosides are also common [15]. Due to the severity of the cerebral infection with abscess formation and hardly calculable penetration of anti-infective substances into the inflamed tissue, we decided to start treatment using a combination regime. We opted for once daily gentamicin administration, because of the possible reduction of nephrotoxicity without limitations in effectiveness [16], [17]. Side effects due to gentamicin treatment could be precluded. The potential use of trimethoprim/sulfamethoxazole as an alternative for the combination was not advisable due to bacterial resistance testing. Furthermore, we decided against a possible treatment with erythromycin because of the limited cerebrospinal fluid penetrability.
After repeated MRI scan with signs of ongoing tissue inflammation, we chose to prolong the therapy with oral ampicillin after a total of 6 weeks of parenteral therapy for a total treatment duration of 12 weeks. Repeated contrast-enhanced MRI after 12 weeks of treatment showed resolution of signs of intracerebral inflammation, therefore the treatment was concluded at this point.
Conclusion
Cerebral listeriosis is a rare disease with many remaining uncertainties regarding optimal therapy. No evidence-based treatment recommendations or even structured case series are available for the treatment of brain abscess caused by LM, an even rarer complication of cerebral listeriosis. Here, we report a case with favorable outcome after prolonged anti-infective ampicillin- and gentamicin-based therapy. However, systematic assessment of treatment would be desirable.
Ethical approval
The case report involving a human participant was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments. Written informed consent for publication of clinical details and images was obtained from the patient. A copy of the consent form is available for review upon reasonable request.
Consent
Written informed consent was obtained from the individual participant.
Consent for publication
The participant has consented to the submission of the case report to the journal.
Funding
No funds, grants, or other support were received.
CRediT authorship contribution statement
Report design and implementation: C.W., M.S., B.S., A.F. Data acquisition, analysis and interpretation: C.W., M.S., A.F., C.M. Manuscript drafting as well as approval of the manuscript: All authors.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
Availability of Data
The data supporting the findings of this case report are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
- 1.Tiri B., Priante G., Saraca L.M., Martella L.A., Cappanera S., Francisci D. Listeria monocytogenes brain abscess: controversial issues for the treatment—two cases and literature review. Case Rep Infect Dis. 2018;2018:1–9. doi: 10.1155/2018/6549496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doganay M. Listeriosis: clinical presentation. FEMS Immunol Med Microbiol. 2003;35(3):173–175. doi: 10.1016/S0928-8244(02)00467-4. [DOI] [PubMed] [Google Scholar]
- 3.Henke D, Rupp S, Gaschen V, et al. Listeria monocytogenes Spreads within the Brain by Actin-Based Intra-Axonal Migration. In: Blanke SR (ed.), Infect Immun. Vol. 83(no. 6); 2015, p. 2409–19. 〈 10.1128/IAI.00316-15〉. [DOI] [PMC free article] [PubMed]
- 4.Ricci A., Allende A., Bolton D., et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018;16(1) doi: 10.2903/j.efsa.2018.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Food Safety Authority European centre for disease prevention and control. The European Union One Health 2020 zoonoses report. EFSA J. 2021;19(12):6971. doi: 10.2903/j.efsa.2021.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlech W.F., Acheson D. Foodborne listeriosis. Clin Infect Dis. 2000;31(3):770–775. doi: 10.1086/314008. [DOI] [PubMed] [Google Scholar]
- 7.Uldry P.-A., Kuntzer T., Bogousslavsky J., et al. Early symptoms and outcome of Listeria monocytogenes rhombencephalitis: 14 adult cases. J Neurol. 1993;240(4):235–242. doi: 10.1007/BF00818711. [DOI] [PubMed] [Google Scholar]
- 8.Quereda J.J., Morón-García A., Palacios-Gorba C., et al. Pathogenicity and virulence of Listeria monocytogenes: a trip from environmental to medical microbiology. Virulence. 2021;12(1):2509–2545. doi: 10.1080/21505594.2021.1975526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garraghan F., Fallon R. Gentamicin: dose regimens and monitoring. Antibacterials. 2015 [Google Scholar]
- 10.Mailles A., Stahl J. Infectious encephalitis in France in 2007: a national prospective study. Clin Infect Dis. 2009;49(12):1838–1847. doi: 10.1086/648419. [DOI] [PubMed] [Google Scholar]
- 11.Bodilsen J., Dalager-Pedersen M., van de Beek D., Brouwer M.C., Nielsen H. Risk factors for brain abscess: a nationwide, population-based, nested case-control study. Clin Infect Dis. 2020;71(4):1040–1046. doi: 10.1093/cid/ciz890. [DOI] [PubMed] [Google Scholar]
- 12.Karageorgiou I., Chandler C., Whyte M.B. Silent diabetes mellitus, periodontitis and a new case of thalamic abscess. Case Rep. 2014;2014(jul21 1) doi: 10.1136/bcr-2014-204654. [bcr2014204654-bcr2014204654] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samra Y., Hertz M., Altmann G. Adult listeriosis–a review of 18 cases. Post Med J. 1984;60(702):267–269. doi: 10.1136/pgmj.60.702.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arlotti M., Grossi P., Pea F., et al. Consensus document on controversial issues for the treatment of infections of the central nervous system: bacterial brain abscesses. Int J Infect Dis. 2010;14:S79–S92. doi: 10.1016/j.ijid.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Temple M.E., Nahata M.C. Treatment of listeriosis. Ann Pharmacother. 2000/05 doi: 10.1345/aph.19315. [DOI] [PubMed] [Google Scholar]
- 16.Verpooten G.A., Riuliano R.A., Verbist L., et al. Once-daily dosing decreases renal accumulation of gentamicin and netilmicin. Clin Pharm Ther. 1989 doi: 10.1038/clpt.1989.4. [PMID: 2910634] [DOI] [PubMed] [Google Scholar]
- 17.Prins J.M., Büller H.R., Kuijper E.J., et al. Oncce versus thrice daily gentamicin in patients with serious infections. Lancet. 1993 doi: 10.1016/0140-6736(93)90137-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this case report are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.