Summary
Biological evidence supports plasma methemoglobin as a biomarker for anemia-induced tissue hypoxia. In this translational planned substudy of the multinational randomized controlled transfusion thresholds in cardiac surgery (TRICS-III) trial, which included adults undergoing cardiac surgery requiring cardiopulmonary bypass with a moderate-to-high risk of death, we investigated the relationship between perioperative hemoglobin concentration (Hb) and methemoglobin; and evaluated its association with postoperative outcomes. The primary endpoint was a composite of death, myocardial infarction, stroke, and severe acute kidney injury at 28 days. We observe weak non-linear associations between decreasing Hb and increasing methemoglobin, which were strongest in magnitude at the post-surgical time point. Increased levels of post-surgical methemoglobin were associated with a trend toward an elevated risk for stroke and exploratory neurological outcomes. Our generalizable study demonstrates post-surgical methemoglobin may be a marker of anemia-induced organ injury/dysfunction, and may have utility for guiding personalized approaches to anemia management. Clinicaltrials.gov registration NCT02042898.
Subject areas: Health sciences, Surgery, Cardiovascular medicine, Public health
Graphical abstract
Highlights
-
•
Sub-analysis of multinational prospective cardiac surgical cohort
-
•
Anemia associated with rises in methemoglobin
-
•
Rises in methemoglobin associated with elevated risk for neurological outcomes
-
•
Methemoglobin may be a marker for anemia-induced brain hypoxia
Health sciences; Surgery; Cardiovascular medicine; Public health;
Introduction
Perioperative anemia is prevalent and is associated with morbidity and mortality; and the risk for these outcomes increases with severity of anemia.1,2 Furthermore, patients undergoing cardiac surgical procedures have an increased risk for anemia due to cardiopulmonary bypass-associated hemodilution and bleeding.3 Currently, hemoglobin concentration (Hb) thresholds are used to define the severity of anemia and for guiding its management4,5; however, the biological implications of these thresholds are unclear, particularly at the level of the individual patient. Humans and studied mammals have a robust adaptive cardiovascular response to acute anemia, with the goal of maintaining optimal vital organ perfusion.1,6 Experimental studies demonstrate that important hypoxic cellular mechanisms are activated during acute,7 subacute,8 and chronic9 anemia, in response to a reduction in blood oxygen content. Anemia-induced tissue hypoxia is biologically sensed via multiple mechanisms, including hypoxia-inducible factor (HIF) and neuronal nitric oxide synthase (nNOS). These cellular mechanisms contribute to optimize oxygen delivery and hypoxic cellular adaptation to promote survival.10 However, these adaptive mechanisms eventually become overwhelmed as indicated by evidence of increased organ injury and mortality observed at progressively lower Hb levels.1,11
In the surgical setting, the most available acute treatment for anemia is the administration of red blood cell (RBC) transfusion. However, blood transfusion is an intervention that includes high osmolar fluid, red cells (variable degree due to age) and also debris that may exert an inflammatory response. The decision to transfuse is complex and should occur only when its net benefit outweighs the risks associated with anemia.12 Recent clinical practice guidelines recommend RBC transfusion in cardiac surgery when Hb falls below 75 g/L (severe anemia)5,13; however, there is evidence that the optimal Hb threshold for transfusion may vary within subgroups of patients undergoing cardiac surgery. Most recently, data from the large randomized Transfusion Requirements in Cardiac Surgery (TRICS-III) trial suggest that a higher Hb threshold for transfusion may be favored among younger patients, and a lower Hb threshold may be favored in older patients.14 Therefore, a biomarker of anemia-induced tissue hypoxia may provide better insight on the degree of anemic stress experienced by specific vital organs, including the brain and heart, and its impact on each individual patient. Utilizing a biomarker of anemia-induced tissue hypoxia may better inform transfusion management, as opposed to the current “imprecise science” of using Hb alone.
Plasma methemoglobin has been proposed as a biomarker for anemia-induced tissue hypoxia.15,16 A number of physiological mechanisms are hypothesized to contribute to the oxidation of hemoglobin to methemoglobin during anemic hypoxia.15 There are three primary mechanisms which have been proposed for the generation of methemoglobin during anemia. First, there is an increased level of deoxyhemoglobin in the microcirculation during acute anemia, which may readily be oxidized to methemoglobin. Second, there is upregulation of nNOS during acute anemia, which may contribute to increased levels of nNOS-derived nitric oxide in the vasculature, further promoting oxidation of hemoglobin to methemoglobin.15,16 Third, deoxyhemoglobin itself may act as a nitrite reductase, and in the process of nitric oxide generation, methemoglobin is produced as a byproduct.15
Additionally, we have observed a small but consistent increase in methemoglobin levels proportional to acute reductions in Hb across preclinical animal models of hemodilutional anemia.16 Similar associations have been observed in a small single-center cohort studies evaluating patients undergoing cardiac surgery on cardiopulmonary bypass; however, these studies have been unable to evaluate the relationship between methemoglobin and postoperative clinical outcomes.17,18 The biological plausibility for these mechanisms and consistent evidence, in the context of the relative ease, low cost, and rapid turn-around-time for results, make methemoglobin a viable potential biomarker for measuring anemia-induced tissue hypoxia.
We therefore conducted this planned substudy nested within TRICS-III trial to: (1) investigate the relationship between Hb and methemoglobin values; and (2) evaluate the association between methemoglobin and postoperative outcomes at 28 days. We hypothesized that methemoglobin may be a biological marker of anemia-induced tissue hypoxia, which may inform the degree of anemia-induced tissue injury experienced by vital organs, including the brain, heart, and kidney.
Results
Cohort description
The open-labeled, pragmatic TRICS-III non-inferiority trial19 included 5243 adults undergoing cardiac surgery requiring cardiopulmonary bypass (CPB) with a moderate-to-high risk of death (European System for Cardiac Operative Risk Evaluation [EuroSCORE] I ≥ 6) between January 20, 2014 to March 20, 2017 from 73 sites in 19 countries.14,20 The TRICS-III cohort included 208 patients randomized in the TRICS-II pilot trial. Of the 5092 patients included in the modified intention-to-treat population, 3056 had available pre-surgical Hb and methemoglobin values (samples collected intraoperatively, before CPB) and were included in the pre-surgical analysis, and of which 2049 had available post-surgical Hb and methemoglobin values (samples collected at ICU admission) and were included in the post-surgical analysis. A patient flow chart is provided in Figure S1, and the distribution of methemoglobin concentrations before and after surgery is provided in Figure S2.
Patient characteristics are presented in Table 1, and are similar to the primary TRICS III cohort14: 64% were male, 51.9% underwent CABG procedures, the average age was 72.6 years, BMI was 27.9 kg/m2, EuroSCORE I was 7.9, preoperative Hb was 131 g/L. The average pre-surgical hemoglobin was 122 g/L and methemoglobin was 0.8%. Additionally, RBC transfusion was administered in 2% of patients preoperatively, and 37% of patients intraoperatively.
Table 1.
Patient characteristics
| Preoperative Characteristics | |
|---|---|
| Allocated to Restrictive Strategy | 1527/3056 (50.0) |
| Age, years | 72.6 ± 9.8 |
| Male Sex | 1958/3056 (64.1) |
| Ethnicity | |
| African descent | 33/2902 (1.1) |
| Asian | 74/2902 (2.5) |
| Caucasian | 2679/2902 (92.3) |
| Other | 116/2902 (4.0) |
| Body Mass Index, kg/m2 | 27.9 ± 5.1 |
| EuroSCORE I | 7.9 ± 1.9 |
| Previous Cardiac Surgery | 364/3056 (11.9) |
| Recent Myocardial Infarction (≤90 days) | 708/3056 (23.2) |
| Emergency Surgery | 38/3056 (1.2) |
| Left Ventricular Function | |
| Good | 1944/3053 (63.7) |
| Moderate | 884/3053 (29.0) |
| Poor | 187/3053 (6.1) |
| Very Poor | 38/3053 (1.2) |
| Diabetes | 823/3056 (26.9) |
| Pulmonary Hypertension | 247/3052 (8.1) |
| Renal Functione | |
| Normal (CC > 85 mL/min) | 1054/2901 (36.3) |
| Moderate (CC > 50 and <85) | 1338/2901 (46.1) |
| Severe (CC < 50) | 482/2901 (16.6) |
| Dialysis (regardless of CC) | 27/2901 (0.9) |
| Treated Hypertension | 2286/3056 (74.8) |
| Preoperative Aspirin Use | 1624/3052 (53.2) |
| Preoperative Anticoagulant Use | 732/3056 (24.0) |
| Preoperative Hemoglobin, g/L | 131.3 ± 17.2 |
| Preoperative RBC Transfusion | 61/3056 (2.0) |
| Operative Characteristics | |
|---|---|
| Planned Surgery | |
| CABG Only | 752/3054 (24.6) |
| CABG and Valve | 594/3054 (19.4) |
| Other Surgery and CABG | 240/3054 (7.9) |
| Valve Only | 905/3054 (29.6) |
| Other Surgery | 563/3054 (18.4) |
| Pre-Surgical Hemoglobin g/L | 122.0 ± 18.0 |
| Pre-Surgical Methemoglobin, (%) | 0.8 ± 0.4 |
| Duration of cardiopulmonary bypass | 122.8 ± 61.0 |
| Intraoperative vasoactive medication for ≥1h duration | 2142/3008 (71.2) |
| Intraoperative RBC transfusionj | 1142/3055 (37.4) |
| Clinical Outcomes | |
|---|---|
| Composite Outcome | 375/3054 (12.3) |
| Death | 96/3053 (3.1) |
| Myocardial Infarction | 200/3054 (6.5) |
| Stroke | 46/3054 (1.5) |
| Severe Acute Kidney Injury | 104/3054 (3.4) |
| Encephalopathy | 38/3054 (1.2) |
| Seizure | 51/3054 (1.7) |
| Delirium | 367/3054 (12.0) |
| Hospital Length of Stay | 10.6 ± 6.1 |
Values presented as n (%) or mean ± standard deviation. There were 154 missing values for ethnicity; 5 missing values for EuroSCORE I; 3 missing values for left ventricular function; 4 missing values for pulmonary hypertension; 155 missing values for renal function; 4 missing values for preoperative aspirin use; 1 missing value for preoperative hemoglobin; 2 missing values for planned surgery; 2 missing values for the composite outcome; 3 missing values for death; 2 missing values for myocardial infarction; 2 missing values for stroke; 2 missing values for severe acute kidney injury; 2 missing values for encephalopathy; 2 missing values for seizure; 2 missing values for delirium; 16 missing values for hospital length of stay. CC, creatinine clearance; RBC, red blood cell; CABG, coronary artery bypass graft surgery.
A comparison of characteristics between included and excluded patients is presented in Table S1. The overall balance of covariate distribution between patients included and excluded in both pre-surgical and post-surgical analyses suggest that these cohorts may be representative of the whole trial population with respect to demographic factors, EuroSCORE components, operative factors, and outcomes.
Association between hemoglobin and methemoglobin is strongest at post-surgical time point
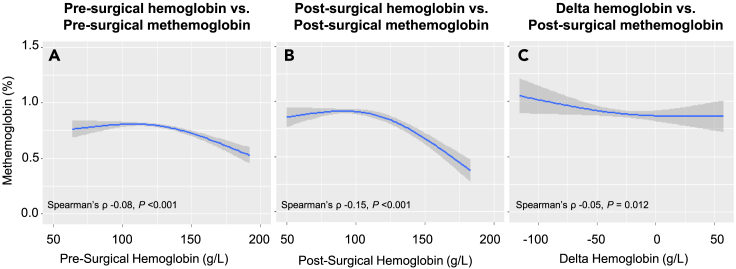
Pre-surgical hemoglobin and methemoglobin levels were 122 ± 18 g/L and 0.81 ± 0.44%, and post-surgical hemoglobin and methemoglobin levels were 100 ± 18 g/L and 0.90 ± 0.47%. The correlations between Hb and methemoglobin are presented in Figure 1, and scatterplots are presented in Figure S3. Negative correlations were observed at the pre-surgical and postsurgical time points, and between delta Hb and post-surgical methemoglobin. Although these correlations may be described as very weak, each achieved statistical significance. The strongest correlation was observed when comparing values at post-surgical time point (post-surgical spearman’s ρ: −0.15 vs. pre-surgical spearman’s ρ: −0.08 and delta spearman’s ρ: −0.05).
Figure 1.
Correlations between hemoglobin versus methemoglobin before and after surgery
(A–C) The correlation between: pre-surgical hemoglobin versus pre-surgical methemoglobin (Panel A); post-surgical hemoglobin versus post-surgical methemoglobin (Panel B); and Delta (difference between pre-surgical and post-surgical) hemoglobin versus post-surgical methemoglobin (Panel C) in the whole cohort. The plotted lines of best fit and 95% confidence intervals were estimated using restricted cubic splines. p value for non-linearity: p < 0.001 at pre-surgical time point, p < 0.001 at post-surgical time point. p = 0.67 for delta.
There was statistical evidence for non-linear associations between pre-surgical Hb and methemoglobin and post-surgical Hb and methemoglobin values (Table 2). The association was present at non-anemic levels at the pre-surgical time point (adjusted change in methemoglobin at non-anemic level [Hb 150 g/L vs. 125 g/L]: −0.11%, 95% confidence interval [CI] −0.15% to −0.08%), and plateaued as anemia increased in severity. At the post-surgical time point, this association increased in magnitude and was present in the non-anemic and mildly anemic levels (adjusted change in methemoglobin at non-anemic level: −0.24%, 95% CI -0.30% to −0.18%; mild anemia [Hb 125 g/L vs. 100 g/L]: −0.19%, 95% CI -0.24% to −0.15%), and plateaued as anemia increased in severity. There was evidence of a linear association between delta Hb and post-surgical methemoglobin (adjusted change in methemoglobin −0.04%, 95% CI -0.07% to −0.01 per 25 g/L increase in Hb).
Table 2.
Association between hemoglobin and methemoglobin before and after surgery
| Unadjusted change in methemoglobin per 25 g/L difference in hemoglobin concentration (95% CI) | Adjusted change in methemoglobin per 25 g/L difference in hemoglobin concentration (95% CI) | |
|---|---|---|
| Pre-Surgical Hb vs. Pre-Surgical MetHb | ||
| No anemia (150 g/L vs. 125 g/L) | −0.11 (−0.15 to −0.08)a | −0.12 (−0.16 to −0.08)a |
| Mild anemia (125 g/L vs. 100 g/L) | −0.02 (−0.05 to 0.01) | −0.01 (−0.04 to 0.02) |
| Moderate anemia (100 g/L vs. 75 g/L) | 0.02 (−0.03 to 0.06) | 0.03 (−0.02 to 0.07) |
| Severe anemia (75 g/L vs. 50 g/L) | 0.02 (−0.03 to 0.06) | 0.03 (−0.02 to 0.07) |
| Post-Surgical Hb vs. Post-Surgical MetHb | ||
| No anemia (150 g/L vs. 125 g/L) | −0.24 (−0.30 to −0.19)a | −0.24 (−0.30 to −0.18)a |
| Mild anemia (125 g/L vs. 100 g/L) | −0.20 (−0.24 to −0.15)a | −0.19 (−0.23 to −0.15)a |
| Moderate anemia (100 g/L vs. 75 g/L) | 0.02 (−0.03 to 0.07) | 0.07 (0.00–0.13) |
| Severe anemia (75 g/L vs. 50 g/L) | 0.06 (0.00–0.12) | 0.02 (−0.03 to 0.07) |
| Delta Hb vs. Post-Surgical MetHb | ||
| per 25 g/L increase in Hb (linear) | −0.02 (−0.05 to 0.00) | −0.03 (−0.06 to −0.01)a |
There was statistical evidence for non-linear associations between pre-surgical hemoglobin versus pre-surgical methemoglobin, and post-surgical hemoglobin versus post-surgical methemoglobin; therefore, hemoglobin was modeled as a non-linear term in these models. Models adjusted for age, sex, diabetes status, preoperative renal function, preoperative left ventricular function, baseline chronic pulmonary disease, planned surgery, and red blood cell transfusion. CI, Confidence interval; Hb, hemoglobin concentration; MetHb, methemoglobin.
p < 0.05.
The relationship between hemoglobin and methemoglobin was strongest in magnitude at the post-surgical time point; thus, only post-surgical values evaluated for subsequent analyses.
Increases in post-surgical methemoglobin associated with elevated risk for postoperative clinical outcomes
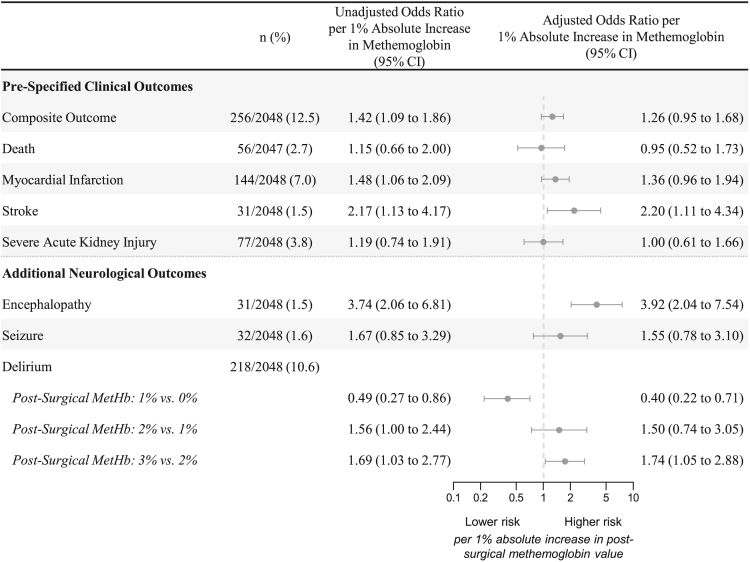
The incidence of clinical outcomes is presented in Figure 2, and the rates are similar to the primary TRICS-III cohort.14 Post-surgical methemoglobin concentration was associated with an increased unadjusted risk of the primary composite outcome (odds ratio [OR]: 1.42, 95% CI 1.09 to 1.86), myocardial infarction (OR: 1.48, 95% CI 1.06 to 2.09) and stroke (OR: 2.17, 95% CI 1.13 to 4.17) per 1% increase in post-surgical methemoglobin. Following adjustment, there was a trend toward an increased risk for the primary composite outcome (ORadj: 1.26, 95% CI 0.95 to 1.68) and myocardial infarction (ORadj: 1.36, 95% CI 0.96 to 1.94), and the association with stroke persisted (ORadj: 2.20, 95% CI 1.11 to 4.34) per 1% increase in post-surgical methemoglobin.
Figure 2.
Association between post-surgical methemoglobin and clinical outcomes
CI; Confidence interval; MetHb, methemoglobin. There was statistical evidence of a non-linear association only between post-surgical methemoglobin and the additional neurological outcome of delirium; therefore, post-surgical methemoglobin was modeled as a non-linear term for this outcome. Models adjusted for age, sex, diabetes status, preoperative renal function, preoperative left ventricular function, baseline chronic pulmonary disease, planned surgery, red blood cell transfusion, and hemoglobin concentration. The x axis is presented on a log-transformed scale.
Subgroup analyses
RBC transfusion modified the relationship between hemoglobin and methemoglobin at the pre-surgical and post-surgical time points, and between delta Hb and post-surgical methemoglobin (Figure S4). In patients who did not receive RBC transfusion, the relationship between hemoglobin and methemoglobin increased in strength and magnitude; conversely, in patients who received RBC transfusion, there was no observed relationship. The results of the respective subgroup analyses stratified by sex and ethnicity are provided in Figure S4. No evidence of effect modification by transfusion status, sex, nor ethnicity on the association between post-surgical methemoglobin and the primary composite outcome was observed (Figure S5).
Sensitivity analyses
All associations were consistent and persisted in our sensitivity analyses with additional adjustment for ethnicity (excludes patients enrolled in the TRICS-II pilot study; Tables S2 and S3). Additionally, all associations with clinical outcomes were consistent and persisted in our sensitivity analysis evaluating absolute methemoglobin concentration (product of methemoglobin fraction and hemoglobin concentration) as the independent variable (Figure S6).
Post-hoc analyses
Post-surgical methemoglobin was associated with an increased risk of encephalopathy (ORadj: 3.92, 95% CI 2.04 to 7.54 per 1% increase in post-surgical methemoglobin; Figure 2). There was statistical evidence for a non-linear association between post-surgical methemoglobin and delirium where below the threshold of 1%, increases in methemoglobin was associated with a reduction in risk; and above the threshold of 1%, increases in methemoglobin was associated with an elevation in risk. The additive prognostic value of methemoglobin, as compared to hemoglobin concentration alone, for predicting clinical outcomes is presented in Table S4.
Discussion
In this planned substudy of the multinational TRICS-III randomized controlled trial enrolling patients undergoing cardiac surgery on cardiopulmonary bypass with a moderate-to-high risk of death, we observed that perioperative reductions in Hb were associated with subtle increases in methemoglobin, and post-surgical methemoglobin was associated with an elevated risk for stroke and additional exploratory neurological outcomes. These findings were consistent and persisted across all sensitivity analyses.
The association between Hb and methemoglobin was consistent, albeit statistically weak, across all time points. This association was strongest in magnitude in the post-surgical period, similar to previous studies evaluating this relationship in preclinical models of hemodilutional anemia16 and cohort studies in patients undergoing cardiac surgery on cardiopulmonary bypass.17,18 Although, the association between Hb and methemoglobin was most pronounced in the non-anemic and mildly anemic Hb ranges, the linear association between delta Hb and methemoglobin emphasizes that this relationship is consistent across all levels of acute blood loss. Additionally, increases in methemoglobin values were maintained within a normal range (below 1%).21 Our data suggest that methemoglobin may be a marker for acute anemia, and the mechanisms generating anemia-induced methemoglobin production may be tightly regulated to prevent production of supranormal levels of methemoglobin which may further impair tissue oxygen delivery. Of note, the association between Hb and methemoglobin was observed only among non-transfused patients. Stored RBCs may experience higher levels of oxidative stress potentially leading to an accumulation of methemoglobin and reactive oxygen species that may oxidize endogenous hemoglobin to methemoglobin following transfusion.22 More investigation is needed to better understand whether stored RBC is a source of exogenous methemoglobin and/or if it induces production of endogenous methemoglobin during anemia. In addition, these data encourage the future assessment of additional hypoxia-induced biomarkers, including systemic erythropoietin, as clinical indicators of anemia-induced organ injury.18
It is of particular interest that associations were detected between post-surgical methemoglobin and myocardial infarction and stroke, and not for severe acute kidney injury. Under physiological conditions, the heart and brain have the highest metabolic requirements for oxygen and are associated with relatively high proportions of oxygen extraction.23 Therefore, these organs would have relatively high levels of deoxyhemoglobin within the microcirculation following oxygen extraction. Elevated deoxyhemoglobin favors all proposed mechanisms for methemoglobin formation, and therefore may be indicative of hypoxia induced injury in these organs. By contrast, oxygen extraction in the kidney is much lower than that of the brain and heart; thus, may have a lower level of deoxyhemoglobin and may have less of an effect on methemoglobin levels. Although our post-hoc analysis suggests post-surgical methemoglobin may additive prognostic value for predicting myocardial infarction and neurological outcomes; further investigation of methemoglobin as a predictive biomarker is required.
Post-surgical methemoglobin was associated with an increased risk for stroke and the additional neurological outcomes of encephalopathy and delirium at 28 days. Immediately following the onset of anemia, physiological adaptions are activated to preserve brain oxygen balance, including: (1) an increase in cardiac output, systematic vascular resistance, and a redirection of blood flow to the brain; (2) enhanced oxygen offloading (extraction) in the brain; and (3) changes in metabolism reducing oxygen demand in the brain.24 Despite these adaptations, there is biological evidence of anemia-induced brain tissue hypoxia with progressively severe levels of anemia.11 Additionally, blunting of the cardiovascular responses to anemia (beta blockade) has been demonstrated to exacerbate anemia-induced tissue hypoxia, specifically in the brain.25 This suggests that the brain may be particularly susceptible to anemia-induced tissue hypoxia, which may increase the risk for postoperative brain dysfunction and injury.26 Additionally, the mechanism(s) for anemia-induced generation of methemoglobin may be adaptive and enhance brain oxygen delivery when methemoglobin is maintained within a normal range.15,16 However, the brain may be extremely sensitive to the reduction in oxygen carrying capacity from anemia-induced generation of methemoglobin, and these mechanisms may become maladaptive when this normal range is exceeded. This may explain the non-linear relationship between post-surgical methemoglobin and the delirium, where a reduced risk was observed below the threshold of 1%, and an increased risk was observed above the threshold of 1%. Although post-surgical increases in methemoglobin were associated with a non-significant elevated risk for seizure, it is unclear whether this association was mediated by anemia-induced tissue hypoxia. Larger, adequately powered studies are needed to confirm the association between post-surgical methemoglobin and neurological outcomes and to elucidate the mechanism(s) contributing to this effect.
Current clinical practice guidelines support RBC transfusion at a restrictive Hb threshold (< 75 g/L) in patients free of symptoms of anemia.5,13 However, Hb and symptoms of anemia may not accurately reflect the imbalance of oxygen supply and demand in the brain.27 Post-surgical methemoglobin may have utility in providing unique insight on a patient’s degree of anemia-induced brain tissue hypoxia. Interestingly, a recent small randomized trial evaluated central venous oxygen saturation (SvO2) as a biological marker for measuring the severity of anemia and for guiding RBC transfusion.28 SvO2-guided transfusion was successful at reducing blood transfusion exposure as compared to Hb-guided strategy; however, it was associated with a non-significant but numerically higher risk for stroke. The incidence of stroke may potentially be reduced by incorporating post-surgical methemoglobin interpretation in the SvO2-guided transfusion strategy. Further investigation is needed to evaluate whether methemoglobin may be warranted for guiding perioperative RBC transfusion when interpreted in conjunction with SvO2 and/or other exploratory measures of anemia-induced tissue hypoxia (such as urinary oxygen tension,29 erythropoietin; 18 and HIF30) for optimizing postoperative outcomes and blood utilization.
Limitations of the study
Although we provide results from a large, contemporary, prospective multinational cohort with clearly defined clinical outcomes, and statistical adjustment for clinically important factors, our study has limitations which should be noted. The weakness of the correlation between methemoglobin and hemoglobin may limit the clinical importance of this relationship, and may provide support for the exploration for other biological markers, such as erythropoietin, as a marker for anemia-induced tissue hypoxia. Additionally, it is likely that the mechanisms affecting methemoglobin levels during cardiac surgery are multifactorial, and include factors which may not be related to anemia-induced tissue hypoxia (such as use of vasopressors, or HIF-pathway activation via inflammation). The relationship between anemia, HIF-pathway activation, and methemoglobin was a working hypothesis based on data from animal studies, and may not directly translate into clinical practice.
There may be potential bias and limiting of generalizability of our findings due to missing data in our study. Pre-surgical and post-surgical methemoglobin and hemoglobin data were not available for all patients. To address this, we performed a comparison of characteristics between those who were and were not included in the analysis which revealed that the groups were very similar to each other and to the primary TRICS-III cohort; suggesting that missing data had a very limited impact on the generalizability of our findings.
Our study may not be adequately powered for detecting the clinical outcomes. However, the hypothesis-generating association between post-surgical methemoglobin and stroke persisted across all analyses, including: (1) models adjusted for preoperative and intraoperative characteristics; (2) models including additional adjustment for ethnicity; (3) models evaluating post-surgical absolute methemoglobin concentration as the independent variable. Additionally, the consistent elevated risk between post-surgical methemoglobin and additional neurological outcomes, in the context of the biological plausibility for the mechanism contributing to this effect, provide rationale that this association may not be due to chance. However, due to the observational design of this study, we are unable to exclude all potential bias due to residual confounding in this study.
In patients undergoing cardiac surgery on cardiopulmonary bypass with a moderate-to-high risk of death, post-surgical methemoglobin may be a marker of anemia-induced organ stress, and values may be associated with an increased risk for clinical outcomes. Further investigation is needed for assessing the utility of methemoglobin as a biomarker for guiding perioperative transfusion when interpreted in conjunction with other measures of anemia.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| R: A language and environment for statistical computing. | R Foundation for Statistical Computing, Vienna, Austria | version 3.6.3 |
Resource availability
Lead contact
Further information and reasonable requests for resources should be directed to and will be fulfilled by the lead contact, C. David Mazer (David.Mazer@unityhealth.to).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Transfusion Requirements in Cardiac Surgery III (TRICS-III) was a pragmatic, open-labelled, non-inferiority trial randomizing adults ≥18 years of age undergoing cardiac surgery requiring CBP with a moderate-to-high risk of death (EuroSCORE I ≥ 6) to either a restrictive transfusion strategy (transfuse if Hb was <75 g/L) or liberal strategy (transfuse if Hb was <95 g/L in the operating room or intensive care unit, or <85 g/L on the ward). The trial protocol19 and primary results14,20 have been previously published. This trial recruited patients from 73 sites in 19 countries spanning 6 continents. The TRICS-III cohort included patients randomized in the TRICS-II pilot trial, whose data were kept blinded until the completion of the TRICS-III trial. Appropriate research ethics board approval was obtained for each site, and informed written consent was obtained from each participant. A list of participating investigators is provided in the supplementary appendix (Data S1). Data on ethnicity were not collected in the pilot TRICS-II trial, and gender data were not collected.
Method details
Protocolized pre-surgical and post-surgical laboratory measurements of Hb and methemoglobin were collected for this planned substudy. The pre-surgical sample was obtained in the operating room, after induction of anesthesia and prior to initiation of cardiopulmonary bypass. The post-surgical sample was obtained upon admission to the intensive care unit. Patients missing or having negative Hb and/or methemoglobin values were excluded from the study.
The first objective of this study was to investigate the relationship between Hb and methemoglobin. For this, we performed three separate analyses: 1) pre-surgical: comparing pre-surgical Hb versus pre-surgical methemoglobin; 2) post-surgical: comparing post-surgical Hb versus post-surgical methemoglobin; and 3) delta: comparing the difference between pre-surgical and post-surgical Hb versus post-surgical methemoglobin. The second objective of this study was to evaluate the association between methemoglobin and postoperative clinical outcomes. The primary analysis evaluated the relationship between hemoglobin in grams per litre, and methemoglobin as a percentage of total hemoglobin, their respective most common units of measure. The primary clinical outcome was a composite of death, myocardial infarction, stroke, and severe acute kidney injury until the first of hospital discharge or 28 days after index surgery as defined in the TRICS-III trial. Secondary outcomes were the individual components of the primary composite outcome. Clinical outcomes were evaluated by a central adjudication committee using definitions provided in the supplementary appendix (Data S2). Additional neurological outcomes included postoperative encephalopathy, seizure and delirium.
The association between methemoglobin and clinical outcomes were evaluated only at the timepoint for which the relationship between Hb and methemoglobin was strongest in order to reduce multiple comparisons, potentially leading to spurious associations between methemoglobin and clinical outcomes.
Quantification and statistical analysis
This was an analysis of the modified intention-to-treat population of the TRICS-III trial, which included all randomized patients except patients who withdrew consent or did not undergo their planned surgery. The sample size was determined by the TRICS-III cohort. Patient characteristics were described using frequency and proportion for categorical variables, and mean and standard deviation for continuous variables.
Spearman rank correlation coefficients were used to describe the correlation of each relationship between Hb and methemoglobin. Adjusted linear regression models were used to evaluate the association between Hb and methemoglobin (first study objective) and adjusted logistic regression models were used to evaluate the association between methemoglobin and clinical outcomes (second study objective). The covariates used for adjustment were selected a priori based on clinical sensibility and literature search, and included: age, sex, diabetes status, preoperative renal function, preoperative left ventricular function, baseline chronic pulmonary disease, planned surgery, red blood cell transfusion, and hemoglobin concentration. Ethnicity data were not collected during the pilot TRICS-II trial, and thus was not included as a covariate for adjustment in our primary analysis.
All models were initially fit with the independent variable (Hb for study objective 1) or methemoglobin for study objective 2) expressed with a cubic spline with 3-knots placed at the 10th, 50th, and 90th quantiles to account for potential non-linear associations. If there was evidence of a non-linear association between the independent variable and outcome, it was expressed with spline terms. Conversely, if there was no evidence of a non-linear association between the independent variable and outcome, it was expressed as a linear term.
Effect estimates with 95% confidence intervals are presented from regression models. A 2-tailed p value of <0.05 was taken to be statistically significant. Analyses were performed with R software (v3.6.3)
Subgroup, sensitivity, and post-hoc analyses
Subgroup analyses were performed to evaluate if there was any evidence of effect modification on our study objectives due to RBC transfusion status, sex, and ethnicity. For these analyses, we fitted separate adjusted regression models that included an interaction term between the independent and subgroup variables. p values were obtained from a chi-squared tests adjusted for all interactions in the model.
We performed a sensitivity analysis with additional adjustment for ethnicity. Additionally, we evaluated the association between absolute concentration of methemoglobin (calculated by multiplying the methemoglobin fraction by Hb) and clinical outcomes.
To further investigate the observed association between post-surgical methemoglobin and stroke, we evaluated the association between post-surgical methemoglobin and additional neurological outcomes. Additionally, to evaluate the additive prognostic value of methemoglobin, as compared to hemoglobin concentration alone, for predicting clinical outcomes, we compared the continuous net reclassification improvement statistic and area under the receiver operating curve for these respective models.
Additional resources
The TRICS-III trial is registered at Clinicaltrials.gov, registration: NCT02042898.
Acknowledgments
The authors thank the TRICS investigators and participants for their contributions to the study. The complete author list of co-authors, including TRICS-III investigators, is available in the appendix (Data S1). This study was supported by the: Canadian Institutes of Health Research (232416 and 301852); Canadian Blood Services Canada (Kenneth J. Fyke Award); National Health and Medical Research Council of Australia (1085942); Health Research Council of New Zealand (16/353); and Merit Awards from the Department of Anesthesiology and Pain Medicine, University of Toronto (to G.M.T.H and C.D.M). The funders had no role in the collection, analysis, interpretation, and presentation of data.
Author contributions
Conceptualization: N.M., G.M.T.H., C.D.M.; Methodology, N.M., G.M.T.H., N.S., D.T.K., D.N.W., S.V., and C.D.M.; Formal Analysis, N.M.; Investigation, G.M.T.H., N.S., R.S.K., H.F.F, R.A.B., P.C., R.S., D.F., C.S.A., A.R., A.J.G., B.K., J.D.L., E.M., D.K., J.C.T., T.S., S.V., and C.D.M.; Writing – Original Draft, N.M., G.M.T.H., and C.D.M.; Writing – Review & Editing, N.M., G.M.T.H., N.S., R.S.K., H.F.F, R.A.B., P.C., R.S., D.F., C.S.A., A.R., A.J.G., B.K., J.D.L., E.M., D.K., J.C.T., T.S., D.T.K., D.N.K., S.V., and C.D.M.; Visualization, N.M.; Supervision, G.M.T.H., N.S., D.T.K., D.N.W., S.V., and C.D.M.; Funding Acquisition, N.S. and C.D.M.
Declaration of interests
RSK reports receiving grants from the NIH, speaker honorarium from the Cardiothoracic Research and Education Forum, and reports membership on an advisory board for Bio Products Laboratory. DF reports receiving grants financed by the Erasmus+ program, lecture; honoraria from Werfen Instrumentation Laboratory Comp., the American Society of Anesthesiology, University of Continuing Education Krems, Department for Health Sciences, Medicine and Research, Austria; leadership roles World Federation of Societies of Anesthesiologists, the Romanian Society of Anesthesia and Intensive Care, and European Sepsis Alliance; and has received equipment from Vifor Pharma Romania. AJG reports receiving consulting fees and honoraria from Edwards Lifesciences, and is a non-remunerated member of the executive board for the Society for Enhanced Recovery after Cardiac Surgery. JDL reports receiving grants or contracts from Edwards Lifesciences and the CTSA Seed Grant. SV holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and reports receiving research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, Sun Pharmaceuticals, and the Toronto Knowledge Translation Working Group. He is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization. CDM reports advisory board honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, BioAge and PhaseBio; and DSMB honoraria from Cerus and Takeda. All other authors declare no competing interests.
Published: July 20, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107429.
Contributor Information
Gregory M.T. Hare, Email: greg.hare@unityhealth.to.
C. David Mazer, Email: david.mazer@unityhealth.to.
Supplemental information
Data and code availability
-
•
Data and other materials may be available by request. Please submit requests to the lead contact for consideration. Deidentified participant data will be made available to researchers whose proposed use of the data has been approved, and whose research group includes a qualified statistician or epidemiologist. Data will be provided after completion of a data sharing agreement and protocol registration.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Hare G.M.T., Mazer C.D. Anemia: Perioperative Risk and Treatment Opportunity. Anesthesiology. 2021;135:520–530. doi: 10.1097/aln.0000000000003870. [DOI] [PubMed] [Google Scholar]
- 2.Warner M.A., Shore-Lesserson L., Shander A., Patel S.Y., Perelman S.I., Guinn N.R. Perioperative Anemia: Prevention, Diagnosis, and Management Throughout the Spectrum of Perioperative Care. Anesth. Analg. 2020;130:1364–1380. doi: 10.1213/ane.0000000000004727. [DOI] [PubMed] [Google Scholar]
- 3.Meybohm P., Westphal S., Ravn H.B., Ranucci M., Agarwal S., Choorapoikayil S., Spahn D.R., Ahmed A.B., Froessler B., Zacharowski K. Perioperative Anemia Management as Part of PBM in Cardiac Surgery - A Narrative Updated Review. J. Cardiothorac. Vasc. Anesth. 2020;34:1060–1073. doi: 10.1053/j.jvca.2019.06.047. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . World Health Organization; 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. [Google Scholar]
- 5.Tibi P., McClure R.S., Huang J., Baker R.A., Fitzgerald D., Mazer C.D., Stone M., Chu D., Stammers A.H., Dickinson T., et al. STS/SCA/AmSECT/SABM Update to the Clinical Practice Guidelines on Patient Blood Management. Ann. Thorac. Surg. 2021;112:981–1004. doi: 10.1016/j.athoracsur.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Weiskopf R.B., Viele M.K., Feiner J., Kelley S., Lieberman J., Noorani M., Leung J.M., Fisher D.M., Murray W.R., Toy P., Moore M.A. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 7.Tsui A.K.Y., Marsden P.A., Mazer C.D., Sled J.G., Lee K.M., Henkelman R.M., Cahill L.S., Zhou Y.Q., Chan N., Liu E., Hare G.M.T. Differential HIF and NOS responses to acute anemia: defining organ-specific hemoglobin thresholds for tissue hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R13–R25. doi: 10.1152/ajpregu.00411.2013. [DOI] [PubMed] [Google Scholar]
- 8.Mistry N., Mazer C.D., Sled J.G., Lazarus A.H., Cahill L.S., Solish M., Zhou Y.Q., Romanova N., Hare A.G.M., Doctor A., et al. Red blood cell antibody-induced anemia causes differential degrees of tissue hypoxia in kidney and brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;314 doi: 10.1152/ajpregu.00182.2017. R611–r622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill L.S., Gazdzinski L.M., Tsui A.K., Zhou Y.Q., Portnoy S., Liu E., Mazer C.D., Hare G.M., Kassner A., Sled J.G. Functional and anatomical evidence of cerebral tissue hypoxia in young sickle cell anemia mice. J. Cereb. Blood Flow Metab. 2017;37:994–1005. doi: 10.1177/0271678x16649194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsui A.K.Y., Marsden P.A., Mazer C.D., Adamson S.L., Henkelman R.M., Ho J.J.D., Wilson D.F., Heximer S.P., Connelly K.A., Bolz S.S., et al. Priming of hypoxia-inducible factor by neuronal nitric oxide synthase is essential for adaptive responses to severe anemia. Proc. Natl. Acad. Sci. USA. 2011;108:17544–17549. doi: 10.1073/pnas.1114026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hare G.M.T., Tsui A.K.Y., Ozawa S., Shander A. Anaemia: can we define haemoglobin thresholds for impaired oxygen homeostasis and suggest new strategies for treatment? Best practice & research. Clinical anaesthesiology. 2013;27:85–98. doi: 10.1016/j.bpa.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Shehata N., Mazer C.D. Red cell transfusion in cardiac surgery: what is the right balance? Transfusion. 2019;59:903–904. doi: 10.1111/trf.15200. [DOI] [PubMed] [Google Scholar]
- 13.Mueller M.M., Van Remoortel H., Meybohm P., Aranko K., Aubron C., Burger R., Carson J.L., Cichutek K., De Buck E., Devine D., et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA. 2019;321:983–997. doi: 10.1001/jama.2019.0554. [DOI] [PubMed] [Google Scholar]
- 14.Mazer C.D., Whitlock R.P., Fergusson D.A., Hall J., Belley-Cote E., Connolly K., Khanykin B., Gregory A.J., de Médicis É., McGuinness S., et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N. Engl. J. Med. 2017;377:2133–2144. doi: 10.1056/NEJMoa1711818. [DOI] [PubMed] [Google Scholar]
- 15.Hare G.M.T., Tsui A.K.Y., Crawford J.H., Patel R.P. Is methemoglobin an inert bystander, biomarker or a mediator of oxidative stress--The example of anemia? Redox Biol. 2013;1:65–69. doi: 10.1016/j.redox.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsui A.K.Y., Dattani N.D., Marsden P.A., El-Beheiry M.H., Grocott H.P., Liu E., Biro G.P., Mazer C.D., Hare G.M.T. Reassessing the risk of hemodilutional anemia: Some new pieces to an old puzzle. Canadian J. Anaesth. 2010;57:779–791. doi: 10.1007/s12630-010-9329-x. [DOI] [PubMed] [Google Scholar]
- 17.Hare G.M.T., Mu A., Romaschin A., Tsui A.K.Y., Shehata N., Beattie W.S., Mazer C.D. Plasma methemoglobin as a potential biomarker of anemic stress in humans. Canadian J. Anaesth. 2012;59:348–356. doi: 10.1007/s12630-011-9663-7. [DOI] [PubMed] [Google Scholar]
- 18.Hare G.M.T., Han K., Leshchyshyn Y., Mistry N., Kei T., Dai S.Y., Tsui A.K.Y., Pirani R.A., Honavar J., Patel R.P., et al. Potential biomarkers of tissue hypoxia during acute hemodilutional anemia in cardiac surgery: A prospective study to assess tissue hypoxia as a mechanism of organ injury. Canadian J. Anaesth. 2018;65:901–913. doi: 10.1007/s12630-018-1140-0. [DOI] [PubMed] [Google Scholar]
- 19.Shehata N., Whitlock R., Fergusson D.A., Thorpe K.E., MacAdams C., Grocott H.P., Rubens F., Fremes S., Lellouche F., Bagshaw S., et al. Transfusion Requirements in Cardiac Surgery III (TRICS III): Study Design of a Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2018;32:121–129. doi: 10.1053/j.jvca.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Mazer C.D., Whitlock R.P., Fergusson D.A., Belley-Cote E., Connolly K., Khanykin B., Gregory A.J., de Médicis É., Carrier F.M., McGuinness S., et al. Six-Month Outcomes after Restrictive or Liberal Transfusion for Cardiac Surgery. N. Engl. J. Med. 2018;379:1224–1233. doi: 10.1056/NEJMoa1808561. [DOI] [PubMed] [Google Scholar]
- 21.Ludlow J.T., Wilkerson R.G., Nappe T.M. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022. Methemoglobinemia. [Google Scholar]
- 22.Yoshida T., Prudent M., D'Alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood transfusion = Trasfusione del sangue. 2019;17:27–52. doi: 10.2450/2019.0217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolff C.B. Normal cardiac output, oxygen delivery and oxygen extraction. Adv. Exp. Med. Biol. 2007;599:169–182. doi: 10.1007/978-0-387-71764-7_23. [DOI] [PubMed] [Google Scholar]
- 24.Hare G.M.T. Tolerance of anemia: understanding the adaptive physiological mechanisms which promote survival. Transfus. Apher. Sci. 2014;50:10–12. doi: 10.1016/j.transci.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Ragoonanan T.E., Beattie W.S., Mazer C.D., Tsui A.K.Y., Leong-Poi H., Wilson D.F., Tait G., Yu J., Liu E., Noronha M., et al. Metoprolol reduces cerebral tissue oxygen tension after acute hemodilution in rats. Anesthesiology. 2009;111:988–1000. doi: 10.1097/ALN.0b013e3181b87f0e. [DOI] [PubMed] [Google Scholar]
- 26.Hare G.M.T., Tsui A.K.Y., McLaren A.T., Ragoonanan T.E., Yu J., Mazer C.D. Anemia and cerebral outcomes: many questions, fewer answers. Anesth. Analg. 2008;107:1356–1370. doi: 10.1213/ane.0b013e318184cfe9. [DOI] [PubMed] [Google Scholar]
- 27.Balegar V K.K., Low G.K., Nanan R.K. Regional tissue oxygenation and conventional indicators of red blood cell transfusion in anaemic preterm infants. EClinicalMedicine. 2022;46 doi: 10.1016/j.eclinm.2022.101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeroual N., Blin C., Saour M., David H., Aouinti S., Picot M.C., Colson P.H., Gaudard P. Restrictive Transfusion Strategy after Cardiac Surgery. Anesthesiology. 2021;134:370–380. doi: 10.1097/aln.0000000000003682. [DOI] [PubMed] [Google Scholar]
- 29.Silverton N.A., Lofgren L.R., Hall I.E., Stoddard G.J., Melendez N.P., Van Tienderen M., Shumway S., Stringer B.J., Kang W.S., Lybbert C., Kuck K. Noninvasive Urine Oxygen Monitoring and the Risk of Acute Kidney Injury in Cardiac Surgery. Anesthesiology. 2021;135:406–418. doi: 10.1097/aln.0000000000003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viikinkoski E., Jalkanen J., Gunn J., Vasankari T., Lehto J., Valtonen M., Biancari F., Jalkanen S., Airaksinen K.E.J., Hollmén M., Kiviniemi T.O. Red blood cell transfusion induces abnormal HIF-1α response to cytokine storm after adult cardiac surgery. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-01695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data and other materials may be available by request. Please submit requests to the lead contact for consideration. Deidentified participant data will be made available to researchers whose proposed use of the data has been approved, and whose research group includes a qualified statistician or epidemiologist. Data will be provided after completion of a data sharing agreement and protocol registration.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.