Summary
Immunization of pregnant women with Group B Streptococcus (GBS) capsular polysaccharide (CPS) conjugate vaccine (CV) could protect young infants against invasive GBS disease. We evaluated the immunogenicity of investigational five GBS monovalent (serotypes Ia, Ib, II, III, and V) CPS-tetanus toxoid (TT)-CV with adjuvant and GBS pentavalent CPS-TT-CV with adjuvant (GBS5-CV-adj) and without adjuvant (GBS5-CV-no-adj), in Balb/c mice. Aluminum phosphate was the adjuvant in the formulations, where included. The homotypic immunoglobulin G (IgG) geometric mean concentration (GMC) and opsonophagocytic activity (OPA) geometric mean titer (GMT) did not differ after the third dose of the GBS5-CV-adj vaccine compared with the monovalent counterparts for all five serotypes. The GBS5-CV-adj induced higher post-vaccination serotype-specific IgG GMCs and OPA GMTs compared to GBS5-CV-no_adj. The GBS5-CV with and without adjuvant should be considered for further development as a potential vaccine for pregnant women to protect their infants against invasive GBS disease.
Subject areas: Medical microbiology, Immunology
Graphical abstract
Highlights
-
•
Immunogenicity study of GBS pentavalent CPS-TT conjugate vaccine
-
•
Penta- and mono-valent conjugates elicited comparable functional IgG for all serotypes
-
•
Higher functional IgG evoked with pentavalent with adjuvant than without adjuvant
Medical microbiology; Immunology.
Introduction
Group B Streptococcus (GBS), also known as Streptococcus agalactiae, colonizes the vagina or rectum in approximately 18% of pregnant women globally,1 which is a major risk factor for invasive GBS disease during the first week of life (early onset disease) in their newborns.1,2 Ascending GBS infection in utero in pregnant women could also cause stillbirth and preterm delivery.3,4 The global estimated incidence of invasive GBS disease among infants less than 90 days of age is 0.49 per 1000 live births (95% confidence interval [CI], 0.43–0.56), with the highest incidence in the Africa (1.12 per 1000 live births).2 Globally, case fatality risk from invasive GBS disease in infants is 8.4% (95% CI, 6.6%–10.2%), and highest in Africa (18.9%).2 Maternal intrapartum antibiotic prophylaxis (IAP) is effective in reducing the incidence of early-onset diseases,5 however, its implementation is limited in low resource countries due to logistical constraints and cost effectiveness considerations.5,6 Immunization of pregnant women with a GBS vaccine is an alternate strategy for protection against early onset disease, and could also protect against late onset disease (7–89 days age) through trans-placental acquisition of protective antibodies by the fetus.7 Also, a maternal GBS vaccine could reduce the risk of recto-vaginal GBS colonization in pregnant women,5,8,9,10 and consequently may protect against GBS associated preterm labor and stillbirths.
Group B Streptococcus is covered with a sialic acid-rich capsular polysaccharide (CPS), based on which it is classified into ten serotypes (Ia, Ib, and II–IX).11 Globally, five serotypes (Ia, Ib, II, III, and V) account for 98% of all maternal GBS colonisation serotypes,1,12 and 96% of infant invasive GBS disease causing serotypes.2,13 With a potential role in immune evasion by inhibiting complement deposition and phagocytosis, CPS is crucial for pathogenesis, and is considered as a prime vaccine target epitope.8,14,15 Capsular polysaccharide-protein conjugate vaccines generate strong and functional CPS-specific immunoglobulin G (IgG) response.16,17,18,19 An effective multivalent CPS-protein conjugate vaccine covering serotypes Ia, Ib, II, III, and V targeted at pregnant women could protect their young infant against invasive GBS disease.2,20 Currently, there is no licensed vaccine against GBS.5 To date, a trivalent CPS-cross reactive material 197 (CRM197) conjugate vaccine has been evaluated in pregnant women,21,22 and more recently a hexavalent CPS-CRM197 conjugate vaccine has been evaluated in phase 1 studies in non-pregnant women and is currently under investigation in pregnant women.23,24 The objective of the current study was to determine the immunogenicity of pentavalent GBS CPS-tetanus toxoid (TT) conjugate vaccine (GBS5-CV), including serotypes Ia, Ib, II, III, and V; which was formulated with and without aluminum hydroxide as an adjuvant, in Balb/c mice. The homotypic serotype specific immune responses induced by the GBS5-CV with adjuvant (GBS5-CV-adj) was compared with monovalent GBS CPS-TT conjugate vaccines with serotype Ia (mGBS-Ia-CV-adj), Ib (mGBS-Ib-CV-adj), II (mGBS-II-CV-adj), III (mGBS-III-CV-adj), and V (mGBS-V-CV-adj), each formulated with adjuvant. We further compared immunogenicity of GBS5-CV-adj and GBS5-CV without adjuvant (GBS5-CV-no_adj) formulations. Also, we investigated immunogenicity of unconjugated pentavalent GBS CPS formulation with adjuvant (GBS5-CPS-adj) compared with GBS5-CV-adj formulation.
Results
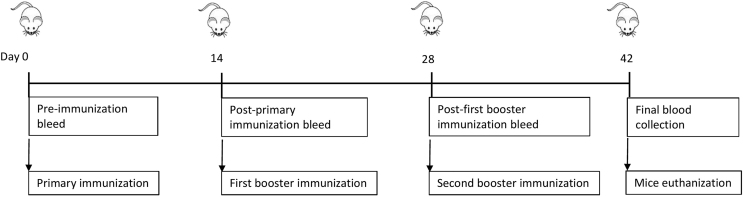
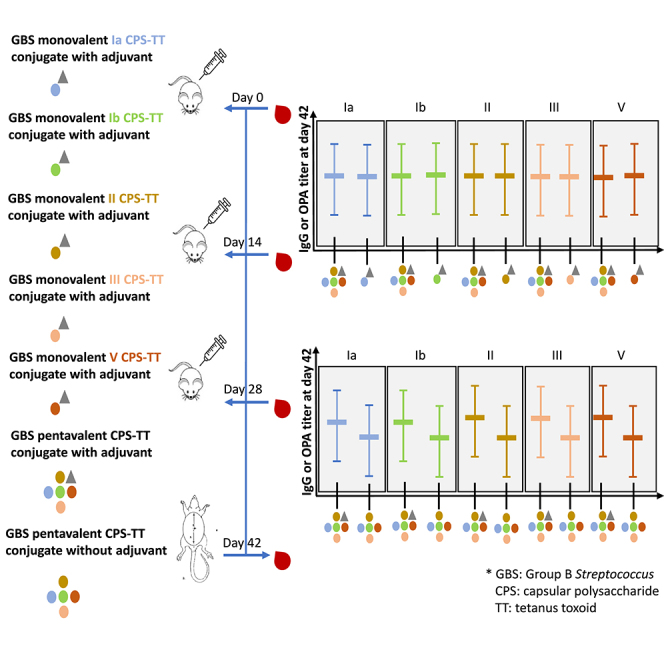
The current study was an intervention study to access the GBS vaccines immunogenicity in 6–8 weeks old female BALB/c mice. The CPS and CPS-TT conjugates of serotypes Ia, Ib, II, III and V were characterised before formulating the vaccines. All five CPS contained no significant burden of residual protein and nucleic acid (all less than 3%); Table S1. All conjugates contained no significant burden of endotoxin (<0.1 EU/μg); Table S2. The nuclear magnetic resonance evaluation of the CPS and conjugates revealed that sialic acid portion of the CPS in the conjugate were fully sialylated and 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) conjugation chemistry had no impact on the CPS sialylation (unpublished data). The study was designed with nine vaccination groups and included monovalent GBS CPS-TT conjugates with adjuvant, pentavalent GBS CPS-TT conjugate formulated with and without adjuvant and an unconjugated pentavalent CPS vaccine with adjuvant. Vaccine diluent with adjuvant was also included as the placebo group. Aluminum phosphate was the adjuvant in the formulations, where included. Each mouse was injected intramuscularly with a specific vaccine formulation three times in a two-week interval at day 0, day 14 (D14), and day 28 (D28) prior to which blood was collected from the tail vein on the same days. Mice euthanization and final blood collection through cardiac puncture was done at two weeks after the third dose at day 42 (D42), Figure 1.
Figure 1.
Study design
An intervention study in 6–8 weeks old female BALB/c mice with nine vaccination groups and 6 mice per group was designed. Each mouse was injected intramuscularly with the vaccine formulation three times in a two-week interval at day 0, day 14, and day 28 prior to which blood was collected from the tail vein on the same days. Mice euthanization and final blood collection through cardiac puncture was done at two weeks after the third dose at day 42.
Serotype specific seroconversion rates and fold change in immune response
The serotype-specific IgG geometric mean concentrations (GMCs) and seroconversion rates were higher at 14 days post-dose 3 i.e., at day 42 (D42) compared to first (D14) or second dose (D28) of all conjugate vaccine formulations (Tables S3–S7). Seroconversion rates at D42 for serotype Ia, Ib and II were 83.3%, 100%, 100% and 16.6% for the mGBS-Ia-CV-adj, GBS5-CV-adj, GBS5-CV-no_adj and GBS5-CPS-adj vaccines, respectively (Figure S1). For serotype V, seroconversion was 100% in mGBS-V-CV-adj, GBS5-CV-adj, GBS5-CV-no_adj, but 0% in the GBS5-CPS-adj group. For serotype III, 100% seroconversion was evident for each of the vaccine formulation groups.
The fold increase in serotype-specific IgG GMCs between day 0 and D42 ranged from 613 (serotype V) to 1480 (serotype Ia) in the monovalent conjugate vaccines with adjuvant (mGBS-CV-adj) groups; 251 (serotype III) to 1576 (serotype II) in the GBS5-CV-adj group; 81 (serotype V) to 481 (serotype 1a) in GBS5-CV-no_adj group; no fold increase (Serotype V) to 14.47 (serotype III) in the GBS5-CPS-adj group (Tables S3–S7). No serotype specific IgG or opsonophagocytosis activity (OPA) titers were detected in the placebo group.
mGBS-CV-adj compared with GBS5-CV-adj vaccine
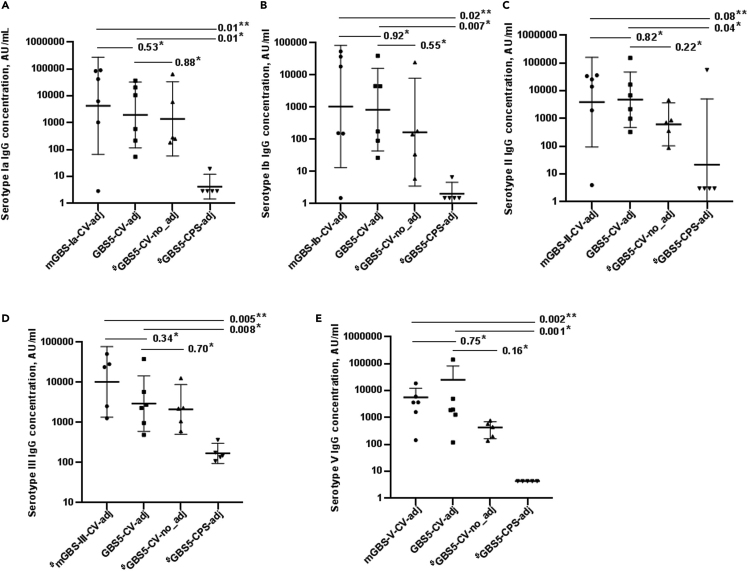
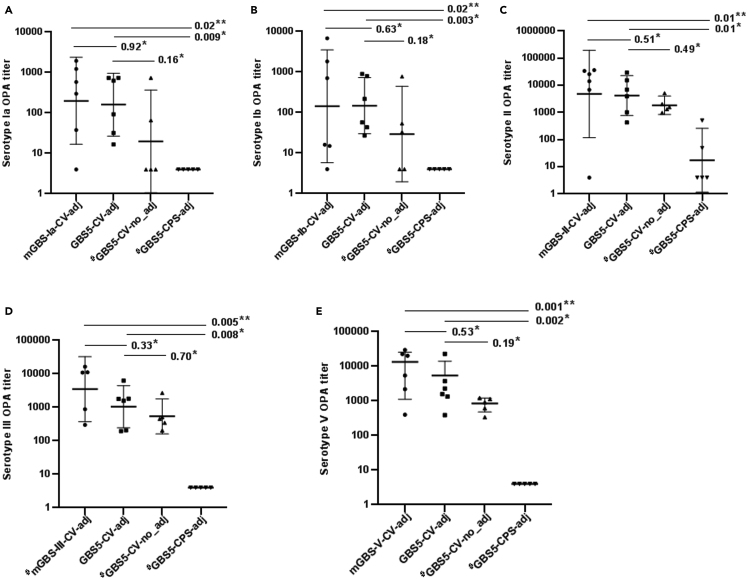
The serotype-specific IgG GMCs increased after the second and third dose of all the conjugate vaccine formulations. At D42, the homotypic GMCs were similar between the mGBS-CV-adj and GBS5-CV-adj vaccine for all serotypes (Figure 2 and Tables S3–S7). Also, the serotype-specific opsonophagocytosis activity (OPA) geometric mean titers (GMTs) at D42 were similar for all serotypes between the mGBS-CV-adj and GBS5-CV-adj vaccines (Figure 3 and Tables S3–S7).
Figure 2.
Serotype specific IgG responses post-third immunization dose (day 42) among study groups
(A–E) Represented on X axis are monovalent conjugate vaccines with adjuvant (mGBS-Ia-CV-adj, mGBS-Ib-CV-adj, mGBS-II-CV-adj, mGBS-III-CV-adj, mGBS-V-CV-adj), pentavalent conjugate vaccine with adjuvant (GBS5-CV-adj) and without adjuvant (GBS5-CV-no_adj), and unconjugated pentavalent CPS vaccine with adjuvant (GBS5-CPS-adj) study groups, IgG concentrations (black dots) in arbitrary units (AU/ml) on Y axis for serotype Ia (A), serotype Ib (B), serotype II (C), serotype III (D), serotype V (E), geometric mean concentration (thick solid line) with 95%CI (thin solid line), and p value by Kruskal-Wallis test comparing all groups (∗∗) and Dunn’s multiple comparisons test (∗), # indicates analysis based on 5 mice.
Figure 3.
Serotype specific opsonophagocytic titers post-third immunization dose (day 42) among study groups
(A–E) Represented on X axis are monovalent conjugate vaccines with adjuvant (mGBS-Ia-CV-adj, mGBS-Ib-CV-adj, mGBS-II-CV-adj, mGBS-III-CV-adj, mGBS-V-CV-adj), pentavalent conjugate vaccine with adjuvant (GBS5-CV-adj) and without adjuvant (GBS5-CV-no_adj), and unconjugated pentavalent CPS vaccine with adjuvant (GBS5-CPS-adj) study groups, opsonophagocytic titers (black dots) on Y axis for serotype Ia (A), serotype Ib (B), serotype II (C), serotype III (D), serotype V (E), geometric mean titer (thick solid line) with 95%CI (thin solid line), and p value by Kruskal-Wallis test comparing all groups (∗∗) and Dunn’s multiple comparisons test (∗), # indicates analysis based on 5 mice.
GBS5-CV-adj vs. GBS5-CV-no_adj vaccine
The mean fold difference in serotype-specific IgG GMCs at D42 was higher in the GBS5-CV-adj compared with GBS5-CV-no_adj group for all serotypes; however, the differences in GMCs were not statistically significant (Ia, 1963 vs. 1395, p = 0.88; Ib, 839 vs. 167.8, p = 0.55; II 4808 vs. 617.4, p = 0.22; III, 2958 vs. 2117, p = 0.70 and V, 2728 vs. 356.2, p = 0.16); Figure 2, and Tables S3–S7. Also, the OPA GMTs were higher in the GBS5-CV-adj compared with GBS5-CV-no_adj groups for all serotypes (Ia, 160 vs. 20, p = 0.16; Ib, 147 vs. 29, p = 0.18; II, 4199 vs. 1848, p = 0.49; III, 1045 vs. 537, p = 0.70; V, 2335 vs. 766, p = 0.19), albeit not significant; Figure 3, and Tables S3–S7. Similarly, after second immunization at D28 mean fold difference in serotype-specific IgG GMCs was higher in the GBS5-CV-adj compared to GBS5-CV-no_adj group, albeit, differences in GMCs were not statistically significant for all five serotypes (Ia, 99.3 vs. 50.1, p = 0.63; Ib, 76.7 vs. 7.7, p = 0.15; II 197.7 vs. 62.1, p = 0.47; III, 1063 vs. 541.4, p = 0.34 and V, 220.3 vs. 27.2, p = 0.16); Tables S3–S7.
GBS5-CV-adj compared with GBS5-CPS-adj vaccine
The GBS5-CV-adj vaccine induced higher serotype specific IgG GMCs compared with the GBS5-CPS-adj vaccine against serotypes Ia (1963 vs. 4.24 AU/ml, p = 0.01), Ib (839 vs. 2.0 AU/ml, p = 0.007), II (4808 vs. 21.7 AU/ml, p = 0.04), III (2958 vs. 170.1 AU/ml, p = 0.008) and V (2728 vs. 4.35, p = 0.001); Figure 2, and Tables S3–S7. Similarly, GBS5-CV-adj induced higher serotype specific OPA GMT’s for all serotypes compared with GBS5-CPS-adj (serotype Ia, 160 vs. 4, p = 0.009; serotype Ib, 147 vs. 4, p = 0.003; serotype II, 4199 vs. 17, p = 0.01; serotype III, 1045 vs. 48, p = 0.008; serotype V, 2335 vs. 4.24, p = 0.002); Figure 3, and Tables S3–S7.
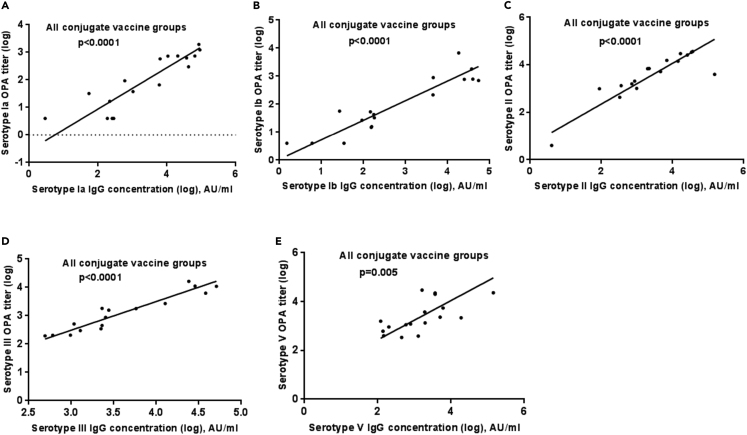
Correlation between homotypic serotype-specific IgG antibody and opsonophagocytic activity
There was a significant association between serotype specific IgG concentration and homotypic OPA titer for all serotypes among the combined conjugate vaccine groups (p < 0.0001 for serotypes Ia, Ib, II, and III; p = 0.005 for serotype V); Figure 4. Positive correlations were also observed for each conjugate vaccine group, ranging between: serotype Ia, r = 0.77–0.89; serotype Ib, r = 0.77–0.87; serotype II, r = 0.65–1; serotype III, r = 0.60–0.82; serotype V, r = 0.6–0.7; except for mGBS-V-CV-adj vaccine group in which no correlation was noted (r = −0.08), Figure S2.
Figure 4.
Deming linear regression analysis between serotype-specific IgG concentrations and opsonophagocytic titers among combined conjugate vaccine groups
(A–E) Represented are IgG concentrations in arbitrary units (AU/ml) on X axis for serotype Ia (A), serotype Ib (B), serotype II (C), serotype III (D), serotype V (E), and serotype specific opsonophagocytic (OPA) titers on Y axis among combined conjugate vaccine study groups including monovalent conjugate vaccines with adjuvant, pentavalent conjugate vaccine with adjuvant and without adjuvant, p values using Deming linear regression analysis.
Discussion
This study demonstrated GBS5-CV with or without adjuvant were immunogenic in Balb/c mice. The immune response elicited from the GBS5-CV-adj vaccine was not inferior compared to the GBS monovalent conjugate vaccine formulations. Conjugation with TT was beneficial in evoking a strong CPS specific immune response to the GBS5-CV-adj vaccine. The study further showed that GBS5-CV-adj vaccine immune responses were higher compared with GBS5-CV-no_adj vaccine for all serotypes, albeit, not significant. This study investigated the immunogenicity of GBS5-CV with tetanus toxoid as the carrier protein. To date, a trivalent and a hexavalent conjugate vaccine have been evaluated with CRM197 as the carrier protein.21,22,23,24 A GBS pentavalent conjugate vaccine (serotypes Ia/Ib/II/III/V) would cover 96% of invasive disease causing GBS serotypes in young infants compared to 98% by GBS hexavalent vaccine which includes an additional serotype IV.2,23
Our study compared the homotypic serotype-specific IgG and OPA responses induced by GBS5-CV-adj vaccine to that evoked from the monovalent conjugate vaccine counterparts, necessary for assessing any potential immune interference between serotypes. The homotypic serotype-specific IgG and OPA responses induced by GBS5-CV-adj vaccine were compared with the mGBS-CV-adj formulations, as OPA is considered to measure functional antibody activity against GBS.25 The mean fold change of IgG antibodies was slightly higher in mGBS-CV-adj compared to GBS5-CV-adj vaccine, however, the serotype-specific GMCs and OPA GMTs were similar; indicating the potential of GBS5-CV-adj vaccine to induce a strong and functional IgG response. Our study evidently showed that GBS5-CV-adj and mGBS-CV-adj vaccines were comparable in functional IgG response for all serotypes. Previously, no study compared GBS monovalent- and multivalent-conjugate vaccine (with adjuvant) responses. Only one study reported a comparable IgG response between a bivalent (II and III)-TT conjugate vaccine (without adjuvant) and monovalent-TT conjugate vaccines in humans.26 Furthermore, conjugating TT protein to CPS was beneficial in immunogenicity of GBS5-CV, as a stronger and higher functional serotype-specific IgG response to GBS5-CV-adj vaccine was elicited, compared to GBS5-CPS vaccine. This supports earlier findings with monovalent conjugate vaccines versus uncoupled monovalent vaccines in animal models27 and in human trials.16,17,18,19,28 Prior to this study, we evaluated CDAP and reductive amination (RA) conjugation chemistry on different serotype III conjugate vaccine formulations in Balb/c mice (unpublished data). Similar serotype IgG titer were induced with both CDAP and RA coupled conjugates, however, significantly higher OPA titers were observed for serotype III CPS-TT conjugate using CDAP chemistry compared to RA chemistry (p = 0.01). The higher functional immune responses induced by CDAP than the reductive amination conjugate noted in our study was in agreement with findings for pneumococcal conjugate vaccines.29 Furthermore, synthesis of CDAP coupled bacterial polysaccharide protein conjugates is a much faster and simplified process,30,31 and could be adequately scalable to large quantities at a lower cost.32 Hence, CDAP chemistry was selected as the preferred conjugation chemistry.
Strong correlations between serotype-specific IgG and OPA titers among GBS5-CV-adj and GBS5-CV-no_adj groups confirms the functionality of the anti-capsular IgG evoked by both the vaccines. This is in agreement with positive correlations previously observed for serotype-specific IgG and OPA titers induced with monovalent serotype Ia, Ib, II, and III conjugate vaccines in non-human primates or non-pregnant women,17,28,33 and trivalent conjugate vaccine in pregnant women.34 An exception was mGBS-V-CV-adj in this study, in which a poor correlation between IgG and OPA titers was observed, possibly due to a lower proportion of IgG-relative to IgM-among antibodies elicited by serotype V CPS than those evoked by other CPS serotypes as reported previously.16,35 However, this phenomenon seems to occur with monovalent- and not the pentavalent-serotype V immune response. Notably, in this study serotype V specific IgG and OPA responses to both GBS5-CV-adj and GBS5-CV-no_adj vaccines were concordant, which suggests that most of the induced serotype V specific IgG antibodies were opsonophagocytic. As IgG is the only antibody which significantly crosses the human placenta from mother to baby,36 elevated functional IgG levels induced with the vaccine in mothers could ensure protective IgG antibody levels transmitted to their fetuses or newborns.
This study further demonstrated that GBS5-CV-adj induced slightly higher IgG GMCs and OPA GMTs than GBS5-CV-no_adj vaccine, albeit not significant, for all five serotypes. Nevertheless, a higher mean fold change of IgG antibodies in GBS5-CV-adj compared with GBS5-CV-no_adj vaccine, indicates that inclusion of aluminum phosphate adjuvant could elicit stronger immune response to GBS5-CV. The beneficial effect of aluminum phosphate or MF59 adjuvant on GBS vaccine immune response has varied in previous studies, wherein, trivalent-CRM197 conjugate or monovalent serotype III- TT conjugate vaccine immune responses in humans were not superior in adjuvanted compared with non-adjuvanted vaccine.19,37 The effect of aluminum phosphate adjuvant differed between serotypes in GBS hexavalent-CRM197 conjugate vaccine immune responses in rhesus macaques,23 wherein, no significant differences in functional IgG concentrations were observed between the adjuvanted and non-adjuvanted vaccine groups for serotypes II, III and V, however, higher responses were noted in adjuvanted formulation for serotype Ia, Ib and IV.23
Previous pre-clinical studies have shown that two booster doses with adjuvant are required to induce peak IgG responses in non-human primates,38 and in mice or rabbits for serotype 1b, II and III-conjugate vaccines.39,40 In line with those findings, the majority of the mice in our study had higher immune responses for all serotypes after the second booster doses of GBS5-CV with or without adjuvant and for mGBS-CV-adj vaccine. However, serotype III-conjugate vaccines trials in humans have shown booster doses post primary vaccination are effective in inducing peak IgG response, specifically in individuals with undetectable antibodies to GBS serotype III before the 1st dose of vaccine, while, only a single dose is sufficient in those with pre-existing antibodies.37 Given natural exposure of most women to GBS colonization,1 which induces GBS serotype specific immune responses,41 it is likely that fewer doses of GBS5-CV will be required to reach a peak IgG response. In the study on the trivalent GBS conjugate vaccine, immunized pregnant women remained persistently high in IgG GMCs from the time of delivery through to one-year post-partum, whilst a slight increase was observed during the same period in women who received placebo.22 A similar finding was observed in a recent study of the GBS hexavalent conjugate vaccine, where IgG geometric mean fold rises (approximately 10-fold–56-fold rise) remained substantially high for all doses and formulations at 6 months after vaccination compared to placebo.24 These studies suggest that there could be continuous boosting of antibodies in vaccinated women possibly with triggering of vaccine induced memory immune responses on natural exposure to recto-vaginal GBS colonization. There are still ongoing deliberations on whether a single rather than two dose maternal GBS vaccine regimen would be sufficient, and whether additional doses would be required in subsequent pregnancies.
The GBS5-CV (with or without adjuvant) investigated in this study is the second GBS multivalent conjugate vaccine formulation globally with a broad coverage and the first GBS multivalent vaccine being developed in Africa, a region with the highest GBS invasive disease burden in infants. The GBS5-CV-no_adj formulation conforms to the World Health Organisation preferred product characteristics of a GBS vaccine for immunization of pregnant women.20 The GBS5-CV-adj formulation might possibly induce mild to moderate local reactogenicity as previously reported among the adjuvanted vaccine groups versus non-adjuvanted or placebo groups in a GBS trivalent-CRM197 conjugate vaccine trial among non-pregnant women.19 However, transient, mild to moderate local symptoms are acceptable, as per World Health Organization (WHO) recommendations, for preferred product characteristics of a GBS vaccine.20 Tetanus toxoid as the carrier protein in GBS5-CV has a well-established safety and efficacy in GBS vaccine trials in pregnant women,22,42 and is also a routine WHO recommended vaccine during pregnancy. Furthermore, an added advantage of TT in GBS5-CV is that it is likely to generate a robust anti-TT response post-vaccination, as has been evident with other TT-conjugate vaccines, that could reduce incidences of neonatal tetanus infections.43,44,45 The GBS pentavalent vaccine with TT as carrier protein could be an effective vaccination strategy for prevention of both neonatal GBS and tetanus infections, particularly in high burden regions like Africa.5
Conclusion
This study demonstrated the potential of GBS5-CV with or without adjuvant to induce a robust functional IgG antibody response against five GBS CPS serotypes (Ia, Ib, II, III, and V) and would cover 96% of invasive disease causing GBS serotypes in young infants. Further investigation of GBS5-CV-adj and GBS5-CV-no_adj vaccines in human clinical trials is warranted that could provide further insights into vaccine safety and immunogenicity, with respect to serotype-specific IgG or OPA titer threshold associated with reduced risk of GBS serotype specific disease in infants for vaccine evaluation and licensure.46
Limitations of our study include that we did not evaluate vaccine efficacy in mice challenge experiments and the small number of mice in each group, which could have compromised the power to detect differences between some vaccine groups. Nevertheless, previous studies on the monovalent, trivalent, tetravalent and hexavalent GBS conjugate vaccines have shown good correlation between ability of vaccine to induce opsonophagocytic IgG antibodies in vitro and vaccine protective efficacy against GBS in animal challenge experiments.17,23,34,39,40 The IgM responses in mice could not be evaluated due to very limited sample volume left after performing both IgG and OPA assays. Variations in immune response was noted among mice vaccinated with the same vaccine formulation. However, individuals within inbred strains are not isogenic, and there are genetic variations,47 which are likely to affect the immune responses of mice receiving the same vaccine.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| SECONDARY ANTIBODY IgG for Luminex [R-Phycoerythrin-conjugated AffiniPure Goat Anti-Mouse IgG (subclasses1+2a+2b + 3), Fcγ Fragment Specific] | JACKSON IMMUNORESEARCH | 115-115-164 |
| Bacterial strains for OPA assay | ||
| Serotype Ia | American Type Culture Collection | BAA-1138 |
| Serotype Ib | American Type Culture Collection | BAA-1174 |
| Serotype II | Center for Diseases Control and Prevention (CDC, USA) | SS-619 |
| Serotype III | American Type Culture Collection | BAA-1176 |
| Serotype V | American Type Culture Collection | BAA-611 |
| Biological samples | ||
| Mice Serum | WITS-VIDA | G001-054 |
| Chemicals, peptides, and recombinant proteins | ||
| DMTMM ((4-(4,6-dimethoxy[1,3,5]triazin-2-yl)-4-methyl-morpholinium chloride) | SIGMA | 74104-1G-F |
| Baby rabbit complement | Pel-Freez | 31061–3 |
| Capsular polysaccharides (Ia, Ib, II, III, V) | Biovac | Biovac CPS Ia-V |
| Bacteriological AGAR | SIGMA | A5306 |
| Todd Hewitt Broth | SIGMA | T1438 |
| Experimental models: Cell lines | ||
| HL-60 Cell Line (Human promyelocytic leukemia cell line) | American Type Culture Collection | ATCC CL 240 |
| Experimental models: Organisms/strains | ||
| Female Balb/c Mice (6–8 weeks old) | South African Vaccine Producers (Pty) Ltd | M7 |
| Software and algorithms | ||
| GraphPad Prism version 8.0.02 software | GraphPad Software, San Diego, California | https://www.graphpad.com/scientific-software/prism/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Gaurav Kwatra (gaurav.kwatra@wits-vida.org).
Materials availability
There are restrictions to the availability of GBS vaccines due to intellectual property rights.
Experimental model and study participant details
Animal experiments were conducted in compliance with protocols (reference number: 2020/05/04/B) approved by the Animal Research Ethics Committee of the University of the Witwatersrand. Female Balb/c mice were purchased from South African Vaccine Producers (Pty) Ltd, Sandringham, Johannesburg at 6–8 weeks of age and housed with six mice per cage under standard Wits Research Animal Facility (WRAF) conditions. Mice were housed in a temperature-controlled (20°C–22°C) room on a 12 h light/dark cycle and were fed on a standard rodent chow diet.
Method details
An intervention study in 6–8 week old female Balb/c mice with nine vaccination groups and 6 mice per group was designed. Each mouse was injected intramuscularly with 0.1 mL of a specific vaccine formulation three times in a two-week interval at day 0, day 14, and day 28 prior to which blood (0.1–0.2 mL) was collected from the tail vein on the same days. At day 42, mice were euthanised with an intra-peritoneal injection of pentobarbitone. As soon as the mice were unresponsive, blood was collected via cardiac puncture; Figure 1. Investigated vaccine formulations included monovalent GBS CPS-TT conjugate vaccines with serotype Ia (mGBS-Ia-CV-adj), Ib (mGBS-Ib-CV-adj), II (mGBS-II-CV-adj), III (mGBS-III-CV-adj), and V (mGBS-V-CV-adj), each formulated with adjuvant; pentavalent GBS CPS-TT conjugate vaccine with all serotypes formulated with adjuvant (GBS5-CV-adj) and without adjuvant (GBS5-CV-no_adj). In addition, an unconjugated pentavalent GBS CPS vaccine formulated with TT and adjuvant (GBS5-CPS-adj) was investigated for evaluation of the GBS5-CV-adj immune responses, with the objective to study the effect of tetanus toxoid carrier protein conjugation on pentavalent polysaccharide immunogenicity. Included in the experiments was a control group of six mice who received placebo composed of vaccine diluent and adjuvant. Capsular polysaccharides were conjugated individually to TT using 1-cyano-4-dimethylaminopyridine tetrafluoroborate chemistry, and formulated with 5 mM histidine in sodium chloride buffer as the vaccine diluent, and aluminum phosphate as the adjuvant where included. Analytical data for CPS and CPS conjugates is provided in Tables S1 and S2. Total serotype specific CPS dose was 1ug for each serotype in the monovalent and pentavalent (total 5ug of CPS) formulations and 0.1 mg of aluminum phosphate in formulations which included adjuvant.
Measurement of serotype-specific anti-capsular serum IgG concentration
Quantitative serum GBS serotype-specific CPS IgG antibody concentrations were measured with a bead-based assay on the multiplex Luminex platform. The purified CPS antigens were coupled to the magnetic microsphere beads (Bio-Rad, USA) with the cross-linking agent 4-(4,6-dimethoxy [1,3,5] triazin-2-yl)-4-methyl-morpholinium (DMTMM) as previously described.41 Pooled mice serum from a different vaccine group was used as reference serum for the assay and was assigned a concentration of 1000 arbitrary units (AU) per mL. Assays were performed in true duplicates. Bead fluorescence was read with the Bio-Plex 200 instrument (Bio-Rad) using Bio-Plex manager 5.0 software (Bio-Rad). The optimal serum and secondary antibody dilution was 1:100 and 1:200, respectively. The results for serum CPS IgG were reported in AU per mL with lower detection limits of 5.8, 3.0, 6.1, 23.5 and 8.7 for serotype Ia, Ib, II, III, and V, respectively. For statistical analysis, samples with values below these limits were assigned values of half the lower limit of detection.
Serum opsonophagocytosis activity assay
The functional activity of serum was determined by serotype-specific opsonophagocytosis activity (OPA) assay using the HL-60 cell line as previously described.41 Standard GBS strains were used for the assays (GBS serotype Ia (A909), GBS Serotype Ib (H36B), GBS Serotype II (SS-619), Serotype III (COH1) and serotype V (2603 v/r). Pooled mice serum from different vaccine groups was used as the control in each plate. Briefly, 10000 CFU of serotype-specific GBS wase mixed with an appropriate dilution of heat-inactivated serum sample (10 μL), and incubated for 15 min in an incubator set at 37°C with 5% carbon dioxide (CO2). Baby rabbit complement (10 μL) and Human promyelocytic leukemia (HL-60) cells (40 μL of 1 × 107, cultured for 5 days in the presence of dimethyl formamide) were added to the mixture and incubated for a further hour with agitation in an incubator set at 37°C with 5% CO2,. Aliquots (10 μL) were removed and plated on Todd- Hewitt agar plates for quantitative culture. OPA was expressed as the titer at which the serum dilution yielded 50% killing compared to the bacterial growth in the complementary controls. Continuous titers were determined by interpolating the concentration at which 50% killing of GBS bacteria occurred. The limit of detection was 8. For statistical analysis, samples below the detection were assigned an arbitrary titer of 4.
Quantification and statistical analysis
Sample size
Sample size was based on the Resource equation method.48 Based on the number of groups for each serotype, the sample size needed for the study was 6 mice per group.
Statistical analyses
Seroconversion rates were defined as proportion of vaccinated mice with ≥ 4-fold change in IgG concentration.17,49 Antibody concentrations between groups were compared using Kruskal-Wallis test and Dunn’s multiple comparisons test. For all analyses, p values, geometric means, and 95% CIs were reported. Correlation analysis was performed using Deming linear regression and Spearman correlation test. For all analyses, a p value < 0.05 was considered statistically significant. Data were analyzed using GraphPad Prism version 8.0.02 software (GraphPad Software, San Diego, California).
Acknowledgments
This study was funded by Department of Health and Social Care using UK Aid funding (managed by the Engineering and Physical Sciences Research Council (EPSRC, grant number: EP/R013764/1) through DCVMN International, Switzerland. The views expressed in this publication are those of the author(s) and not necessarily of the those of the funders. We acknowledge staff of Wits research animal facility, Faculty of Health Sciences, University of the Witwatersrand for assisting with animal procedures and care.
Role of the funding source: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
G.K., F.K., N.D., S.A.M., E.M., and S.W. conceptualised and designed the study, P.T., E.M., S.W., F.K., M.W., S.D., P.V.Z, M.J.R., and T.A. developed the vaccine formulations, N.D., G.K performed the pre-clinical experiments, K.J. assisted with animal procedures, L.H. assisted with advice on animal procedures, N.D. performed the lab experiments, N.D., G.K., B.V., and S.A.M. analyzed and interpreted the data, N.D. drafted the manuscript, all authors reviewed and edited the manuscript.
Declaration of interests
N.D., S.A.M, G.K. institution received funding from Biovac. F.K., E.M., S.W., P.T., M.W., S.D., P.V.Z, M.J.R., T.A were employees of Biovac at the time the work was carried out and received funding Department of Health and Social Care using UK Aid for this Study. All other authors declare no conflict of interest.
Published: July 13, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107380.
Contributor Information
Nisha Dhar, Email: nisha.dhar@wits.ac.za.
Gaurav Kwatra, Email: gaurav.kwatra@wits-vida.org.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to re-analyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Russell N.J., Seale A.C., O'Driscoll M., O'Sullivan C., Bianchi-Jassir F., Gonzalez-Guarin J., Lawn J.E., Baker C.J., Bartlett L., Cutland C., et al. Maternal Colonization With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017;65:S100–S111. doi: 10.1093/cid/cix658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madrid L., Seale A.C., Kohli-Lynch M., Edmond K.M., Lawn J.E., Heath P.T., Madhi S.A., Baker C.J., Bartlett L., Cutland C., et al. Infant Group B Streptococcal Disease Incidence and Serotypes Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017;65:S160–S172. doi: 10.1093/cid/cix656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn J.E., Bianchi-Jassir F., Russell N.J., Kohli-Lynch M., Tann C.J., Hall J., Madrid L., Baker C.J., Bartlett L., Cutland C., et al. Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children: Why, What, and How to Undertake Estimates? Clin. Infect. Dis. 2017;65:S89–S99. doi: 10.1093/cid/cix653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seale A.C., Bianchi-Jassir F., Russell N.J., Kohli-Lynch M., Tann C.J., Hall J., Madrid L., Blencowe H., Cousens S., Baker C.J., et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin. Infect. Dis. 2017;65:S200–S219. doi: 10.1093/cid/cix664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi M., Schrag S.J., Alderson M.R., Madhi S.A., Baker C.J., Sobanjo-Ter Meulen A., Kaslow D.C., Smith P.G., Moorthy V.S., Vekemans J. WHO consultation on group B Streptococcus vaccine development: Report from a meeting held on 27-28 April 2016. Vaccine. 2019;37:7307–7314. doi: 10.1016/j.vaccine.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishihara Y., Dangor Z., French N., Madhi S., Heyderman R. Challenges in reducing group B Streptococcus disease in African settings. Arch. Dis. Child. 2017;102:72–77. doi: 10.1136/archdischild-2016-311419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker C.J. The spectrum of perinatal group B streptococcal disease. Vaccine. 2013;31(Suppl 4):D3–D6. doi: 10.1016/j.vaccine.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Song J.Y., Lim J.H., Lim S., Yong Z., Seo H.S. Progress toward a group B streptococcal vaccine. Hum. Vaccin. Immunother. 2018;14:2669–2681. doi: 10.1080/21645515.2018.1493326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker C.J., Kasper D.L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N. Engl. J. Med. 1976;294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 10.Hillier S.L., Ferrieri P., Edwards M.S., Ewell M., Ferris D., Fine P., Carey V., Meyn L., Hoagland D., Kasper D.L., et al. A Phase 2, Randomized, Control Trial of Group B Streptococcus (GBS) Type III Capsular Polysaccharide-tetanus Toxoid (GBS III-TT) Vaccine to Prevent Vaginal Colonization With GBS III. Clin. Infect. Dis. 2019;68:2079–2086. doi: 10.1093/cid/ciy838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cieslewicz M.J., Chaffin D., Glusman G., Kasper D., Madan A., Rodrigues S., Fahey J., Wessels M.R., Rubens C.E. Structural and genetic diversity of group B streptococcus capsular polysaccharides. Infect. Immun. 2005;73:3096–3103. doi: 10.1128/IAI.73.5.3096-3103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell N.J., Seale A.C., O'Sullivan C., Le Doare K., Heath P.T., Lawn J.E., Bartlett L., Cutland C., Gravett M., Ip M., et al. Risk of Early-Onset Neonatal Group B Streptococcal Disease With Maternal Colonization Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017;65:S152–S159. doi: 10.1093/cid/cix655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianchi-Jassir F., Paul P., To K.N., Carreras-Abad C., Seale A.C., Jauneikaite E., Madhi S.A., Russell N.J., Hall J., Madrid L., et al. Systematic review of Group B Streptococcal capsular types, sequence types and surface proteins as potential vaccine candidates. Vaccine. 2020;38:6682–6694. doi: 10.1016/j.vaccine.2020.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wessels M.R., Rubens C.E., Benedí V.J., Kasper D.L. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc. Natl. Acad. Sci. USA. 1989;86:8983–8987. doi: 10.1073/pnas.86.22.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen V.L., Avci F.Y., Kasper D.L. A maternal vaccine against group B Streptococcus: past, present, and future. Vaccine. 2013;31 Suppl 4:D13–D19. doi: 10.1016/j.vaccine.2012.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker C.J., Paoletti L.C., Rench M.A., Guttormsen H.K., Edwards M.S., Kasper D.L. Immune response of healthy women to 2 different group B streptococcal type V capsular polysaccharide-protein conjugate vaccines. J. Infect. Dis. 2004;189:1103–1112. doi: 10.1086/382193. [DOI] [PubMed] [Google Scholar]
- 17.Baker C.J., Paoletti L.C., Wessels M.R., Guttormsen H.K., Rench M.A., Hickman M.E., Kasper D.L. Safety and immunogenicity of capsular polysaccharide-tetanus toxoid conjugate vaccines for group B streptococcal types Ia and Ib. J. Infect. Dis. 1999;179:142–150. doi: 10.1086/314574. [DOI] [PubMed] [Google Scholar]
- 18.Kasper D.L., Paoletti L.C., Wessels M.R., Guttormsen H.K., Carey V.J., Jennings H.J., Baker C.J. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J. Clin. Invest. 1996;98:2308–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leroux-Roels G., Maes C., Willekens J., De Boever F., de Rooij R., Martell L., Bedell L., Wittke F., Slobod K., Dull P. A randomized, observer-blind Phase Ib study to identify formulations and vaccine schedules of a trivalent Group B Streptococcus vaccine for use in non-pregnant and pregnant women. Vaccine. 2016;34:1786–1791. doi: 10.1016/j.vaccine.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Organization W.H. World Health Organization; 2017. WHO Preferred Product Characteristics for Group B streptococcus Vaccines. [Google Scholar]
- 21.Swamy G.K., Metz T.D., Edwards K.M., Soper D.E., Beigi R.H., Campbell J.D., Grassano L., Buffi G., Dreisbach A., Margarit I., et al. Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in pregnant women and their infants: Results from a randomized placebo-controlled phase II trial. Vaccine. 2020;38:6930–6940. doi: 10.1016/j.vaccine.2020.08.056. [DOI] [PubMed] [Google Scholar]
- 22.Madhi S.A., Cutland C.L., Jose L., Koen A., Govender N., Wittke F., Olugbosi M., Meulen A.S.T., Baker S., Dull P.M., et al. Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in healthy women and their infants: a randomised phase 1b/2 trial. Lancet Infect. Dis. 2016;16:923–934. doi: 10.1016/S1473-3099(16)00152-3. [DOI] [PubMed] [Google Scholar]
- 23.Buurman E.T., Timofeyeva Y., Gu J., Kim J.H., Kodali S., Liu Y., Mininni T., Moghazeh S., Pavliakova D., Singer C., et al. A Novel Hexavalent Capsular Polysaccharide Conjugate Vaccine (GBS6) for the Prevention of Neonatal Group B Streptococcal Infections by Maternal Immunization. J. Infect. Dis. 2019;220:105–115. doi: 10.1093/infdis/jiz062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Absalon J., Segall N., Block S.L., Center K.J., Scully I.L., Giardina P.C., Peterson J., Watson W.J., Gruber W.C., Jansen K.U., et al. Safety and immunogenicity of a novel hexavalent group B streptococcus conjugate vaccine in healthy, non-pregnant adults: a phase 1/2, randomised, placebo-controlled, observer-blinded, dose-escalation trial. Lancet Infect. Dis. 2021;21:263–274. doi: 10.1016/S1473-3099(20)30478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker C.J., Edwards M.S. Group B streptococcal conjugate vaccines. Arch. Dis. Child. 2003;88:375–378. doi: 10.1136/adc.88.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker C.J., Rench M.A., Fernandez M., Paoletti L.C., Kasper D.L., Edwards M.S. Safety and immunogenicity of a bivalent group B streptococcal conjugate vaccine for serotypes II and III. J. Infect. Dis. 2003;188:66–73. doi: 10.1086/375536. [DOI] [PubMed] [Google Scholar]
- 27.Guttormsen H.K., Wetzler L.M., Finberg R.W., Kasper D.L. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect. Immun. 1998;66:2026–2032. doi: 10.1128/IAI.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker C.J., Paoletti L.C., Rench M.A., Guttormsen H.-K., Carey V.J., Hickman M.E., Kasper D.L. Use of capsular polysaccharide—tetanus toxoid conjugate vaccine for type II group B streptococcus in healthy women. J. Infect. Dis. 2000;182:1129–1138. doi: 10.1086/315839. [DOI] [PubMed] [Google Scholar]
- 29.Poolman J., Frasch C., Nurkka A., Käyhty H., Biemans R., Schuerman L. Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines. Clin. Vaccine Immunol. 2011;18:327–336. doi: 10.1128/CVI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frasch C.E. Preparation of bacterial polysaccharide-protein conjugates: analytical and manufacturing challenges. Vaccine. 2009;27:6468–6470. doi: 10.1016/j.vaccine.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Lees A., Barr J.F., Gebretnsae S. Vol. 8. Vaccines; 2020. Activation of Soluble Polysaccharides with 1-Cyano-4-Dimethylaminopyridine Tetrafluoroborate (CDAP) for Use in Protein-Polysaccharide Conjugate Vaccines and Immunological Reagents. (III Optimization of CDAP Activation). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner A.E.B., Gerson J.E., So H.Y., Krasznai D.J., St Hilaire A.J., Gerson D.F. Novel polysaccharide-protein conjugates provide an immunogenic 13-valent pneumococcal conjugate vaccine for S. pneumoniae. Synth. Syst. Biotechnol. 2017;2:49–58. doi: 10.1016/j.synbio.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paoletti L.C., Pinel J., Kennedy R.C., Kasper D.L. Maternal antibody transfer in baboons and mice vaccinated with a group B streptococcal polysaccharide conjugate. J. Infect. Dis. 2000;181:653–658. doi: 10.1086/315285. [DOI] [PubMed] [Google Scholar]
- 34.Fabbrini M., Rigat F., Tuscano G., Chiarot E., Donders G., Devlieger R., Filippini S., Frigimelica E., Forte P., Wittke F., et al. Functional activity of maternal and cord antibodies elicited by an investigational group B Streptococcus trivalent glycoconjugate vaccine in pregnant women. J. Infect. 2018;76:449–456. doi: 10.1016/j.jinf.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Guttormsen H.K., Paoletti L.C., Mansfield K.G., Jachymek W., Jennings H.J., Kasper D.L. Rational chemical design of the carbohydrate in a glycoconjugate vaccine enhances IgM-to-IgG switching. Proc. Natl. Acad. Sci. USA. 2008;105:5903–5908. doi: 10.1073/pnas.0710799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmeira P., Quinello C., Silveira-Lessa A.L., Zago C.A., Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012;2012 doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paoletti L.C., Rench M.A., Kasper D.L., Molrine D., Ambrosino D., Baker C.J. Effects of alum adjuvant or a booster dose on immunogenicity during clinical trials of group B streptococcal type III conjugate vaccines. Infect. Immun. 2001;69:6696–6701. doi: 10.1128/IAI.69.11.6696-6701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paoletti L.C., Kennedy R.C., Chanh T.C., Kasper D.L. Immunogenicity of group B Streptococcus type III polysaccharide-tetanus toxoid vaccine in baboons. Infect. Immun. 1996;64:677–679. doi: 10.1128/iai.64.2.677-679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paoletti L.C., Wessels M.R., Rodewald A.K., Shroff A.A., Jennings H.J., Kasper D.L. Neonatal mouse protection against infection with multiple group B streptococcal (GBS) serotypes by maternal immunization with a tetravalent GBS polysaccharide-tetanus toxoid conjugate vaccine. Infect. Immun. 1994;62:3236–3243. doi: 10.1128/iai.62.8.3236-3243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wessels M.R., Paoletti L.C., Kasper D.L., DiFabio J.L., Michon F., Holme K., Jennings H.J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J. Clin. Invest. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwatra G., Adrian P.V., Shiri T., Buchmann E.J., Cutland C.L., Madhi S.A. Natural acquired humoral immunity against serotype-specific group B Streptococcus rectovaginal colonization acquisition in pregnant women. Clin. Microbiol. Infect. 2015;21:568.e13–568.e21. doi: 10.1016/j.cmi.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 42.Heyderman R.S., Madhi S.A., French N., Cutland C., Ngwira B., Kayambo D., Mboizi R., Koen A., Jose L., Olugbosi M., et al. Group B streptococcus vaccination in pregnant women with or without HIV in Africa: a non-randomised phase 2, open-label, multicentre trial. Lancet Infect. Dis. 2016;16:546–555. doi: 10.1016/S1473-3099(15)00484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borrow R., Tang Y., Yakubu A., Kulkarni P.S., LaForce F.M. MenAfriVac as an Antitetanus Vaccine. Clin. Infect. Dis. 2015;61:S570–S577. doi: 10.1093/cid/civ512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bröker M. Potential protective immunogenicity of tetanus toxoid, diphtheria toxoid and Cross Reacting Material 197 (CRM197) when used as carrier proteins in glycoconjugates. Hum. Vaccin. Immunother. 2016;12:664–667. doi: 10.1080/21645515.2015.1086048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi M., Vekemans J., Baker C.J., Ratner A.J., Le Doare K., Schrag S.J. Group B Streptococcus vaccine development: present status and future considerations, with emphasis on perspectives for low and middle income countries. F1000Res. 2016;5:2355. doi: 10.12688/f1000research.9363.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madhi S.A., Izu A., Kwatra G., Jones S., Dangor Z., Wadula J., Moultrie A., Adam Y., Pu W., Henry O., et al. Vol. 73. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America; 2021. pp. e1170–e1180. (Association of Group B Streptococcus (GBS) Serum Serotype-specific Anticapsular Immunoglobulin G Concentration and Risk Reduction for Invasive GBS Disease in South African Infants: An Observational Birth-Cohort, Matched Case-Control Study). [DOI] [PubMed] [Google Scholar]
- 47.Chebib J., Jackson B.C., López-Cortegano E., Tautz D., Keightley P.D. Inbred lab mice are not isogenic: genetic variation within inbred strains used to infer the mutation rate per nucleotide site. Heredity. 2021;126:107–116. doi: 10.1038/s41437-020-00361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charan J., Kantharia N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013;4:303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotloff K.L., Fattom A., Basham L., Hawwari A., Harkonen S., Edelman R. Safety and immunogenicity of a tetravalent group B streptococcal polysaccharide vaccine in healthy adults. Vaccine. 1996;14:446–450. doi: 10.1016/0264-410x(95)00147-s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to re-analyze the data reported in this paper is available from the lead contact upon request.