Abstract
BACKGROUND
Probiotics have shown promise in alleviating symptoms of diarrhea-predominant irritable bowel syndrome (IBS-D); however, the certainty of evidence is low. Well-powered randomized controlled dose-ranging trials are warranted on promising single-strain candidates.
AIM
To investigate the clinical efficacy of Lactiplantibacillus plantarum (L. plantarum) Lpla33 (DSM34428) in adults with IBS-D.
METHODS
This is a randomized, double-blind, placebo-controlled, multi-center, and dose-ranging study. Three hundred and seven adults, 18-70 years of age, with IBS-D, according to Rome IV criteria, were allocated (1:1:1) to receive placebo or L. plantarum Lpla33 at 1 × 109 (1B) or 1 × 1010 (10B) colony-forming units/d over an 8-wk intervention period. The primary outcome was the change in IBS severity scoring system (IBS-SSS) total score after 8 wk, while secondary and exploratory outcomes included abdominal pain severity, IBS related quality of life, stool and microbial profile, and perceived stress.
RESULTS
IBS-SSS was significantly reduced, after 8 wk, in participants receiving L. plantarum 1B (-128.45 ± 83.30; P < 0.001) and L. plantarum 10B (-156.77 ± 99.06; P < 0.001), compared to placebo (-58.82 ± 74.75). Further, a dose-ranging effect was observed, with a greater absolute reduction in the L. plantarum 10B group (P < 0.05). A reduction in sub-scores related to abdominal pain, abdominal distension, bowel habits, and quality of life was observed in both L. plantarum groups compared to placebo (P < 0.001). Further, 62.5% and 88.4% of participants administered L. plantarum 1B and 10B, respectively, were classified as stool consistency responders based on a reduction in diarrheal stool form, as compared to 26.3% in the placebo group (P < 0.001). In contrast, no significant shifts were observed in microbial diversity.
CONCLUSION
L. plantarum Lpla33 (DSM34428) is well tolerated and improves IBS symptom severity with a dose-ranging effect and a corresponding normalization of bowel habits in adults with IBS-D.
Keywords: Randomized clinical trial, Diarrhea-predominant irritable bowel syndrome, Microbiome, Probiotic, Lactiplantibacillus plantarum
Core Tip: The current study is unique in its dose-ranging assessment of a Lactiplantibacillus plantarum (L. plantarum) probiotic strain in a well-powered multi-center randomized controlled trial in adults with diarrhea-predominant irritable bowel syndrome (IBS-D). L. plantarum Lpla33 (DSM34428) was well tolerated and significantly improved global IBS symptom scores as compared to placebo, both at an absolute level and in the number of clinically relevant responders. Further, a dose-ranging effect was observed in global IBS symptom scores, abdominal pain severity, quality of life, and normalization of diarrheal stool type.
INTRODUCTION
Irritable bowel syndrome (IBS) is a chronic disorder of gut-brain interaction, characterized by abdominal pain in association with altered bowel habits[1]. Current estimates report a global prevalence of 4% to 9%, based on Rome IV or Rome III diagnostic criteria, respectively[2]. IBS is further classified according to the predominant stool pattern, with diarrhea predominant subtype (IBS-D) reported to be the most common, affecting approximately 40% of adults with IBS[3]. Pathophysiology is multi-factorial and may include altered gastrointestinal (GI) microbiota, motility or barrier function, immune activation, neurotransmitter imbalance, or visceral hypersensitivity[4,5]. In addition to GI symptoms, IBS significantly impacts quality of life (QoL) and is associated with work absenteeism and avoidance of daily activities[6].
IBS-D treatment options include United States Food and Drug Administration approved pharmacologic therapies such as rifaximin, eluxadoline, and alosetron[4]. However, clinical management is challenging due to chronic daily administration, safety concerns, and the heterogeneity of symptoms to address. Increasing evidence supports a link between psychological processes and IBS symptomology via altered gut-brain interaction, and there is a growing application of psychological interventions for IBS[7]. Within the gut, dysbiosis, including reduced abundance of lactobacilli and Bifidobacterium[8,9], has been reported and is linked to features of IBS-D such as intestinal permeability and low-grade inflammation[6,10]. There are conflicting reports regarding the efficacy of fecal microbiota transplantation in IBS, with enriched lactobacilli, Streptococcus, Dorea, and Ruminococcaceae reported as favorable bacterial profiles for donors[11].
Probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit to the host[12]. Select strains are hypothesized to alleviate symptoms of IBS by promoting intestinal barrier function, modulating markers of inflammation, regulating immune cell balance, preventing pathogen adherence, or producing bioactive molecules such as short chain fatty acids (SCFAs), neurotransmitters, or their precursors[5]. A recent meta-analysis of probiotics for adults with IBS-D assessed ten randomized controlled trials evaluating 943 participants[13]. Probiotics were deemed safe and superior to placebo regarding global IBS-D symptoms, abdominal pain, and abdominal distension, but had no beneficial effect on QoL, stool frequency, or flatulence[13]. Additionally, the certainty of evidence was very low to low, due to heterogeneity between studies, and differences in study population, strain selection, dose, dosage form, and duration of intervention[13]. Another recent systematic review and meta-analysis accounted for probiotic strain-specific efficacy in IBS and included 42 randomized controlled trials evaluating 3856 participants[14]. The meta-analysis highlighted single-strains and combinations showing efficacy in at least one IBS outcome measure; however, most studies did not assess outcomes by IBS subtype[14]. As a result, there is a need for well-powered ran-domized controlled trials on promising single strain candidates with an IBS-D focus.
The present study investigated a Lactiplantibacillus plantarum (L. plantarum) strain with several relevant attributes, including GI persistence, maintenance of intestinal barrier function, and anti-pathogenic, anti-inflammatory, and bile salt hydrolase activity (Chr. Hansen internal data). The objective was to assess the efficacy and tolerability of L. plantarum Lpla33 (DSM34428), with respect to IBS symptomology in a randomized, double-blind, placebo-controlled, multi-center, dose-ranging study in adults with IBS-D.
MATERIALS AND METHODS
Study population and setting
Females and males, ages 18 to 70 years, meeting Rome IV diagnostic criteria for IBS-D were recruited from 12 gastroenterology specialized centers across India. As per Rome IV, included participants had recurrent abdominal pain, at least once a week for the last 3 mo, with onset of symptoms for at least 6 mo, and with at least two of the following criteria: Pain related to defecation or associated with change in stool frequency or stool form. Participants also had a history of abnormal bowel movements wherein diarrhea (type 6 or 7) was the predominant (> 25%) stool pattern on the Bristol stool scale (BSS). Further, an electronic diary was used during a 14-d placebo run-in period, wherein it was re-confirmed that predominant stool type was type 6 or 7 on the BSS. Additionally, participants had an abdominal pain severity-numeric rating score (APS-NRS) of ≥ 4 (on an 11-point scale) and an IBS severity scoring system (IBS-SSS) total score of > 175 (on a 500-point scale) prior to and after a 14-d placebo run-in period. Dietary preferences of all participants included non-vegetarian options.
The exclusion criteria included a history of organic gastrointestinal disease, including inflammatory bowel disease or ischemic colitis, a history of surgical resection of the GI tract, acute gastroenteritis or complications from infectious enteritis, a recent diagnosis of Helicobacter pylori infection, a history of gluten or lactose intolerance, a history of malignant tumors, or a history of neurological or psychiatric conditions. A current or recent history (i.e., past 2 years) of smoking as well as heavy drinking, defined as more than 14 or 7 units per week, for males and females, respectively, was also an exclusion criterion. Additionally, individuals with type I or II diabetes, or uncontrolled hypertension were excluded, as well as individuals with abnormal thyroid-stimulating hormone (< 0.35 mIU/L or > 4.94 mIU/L) or hemoglobin (< 6.21 mmol/L) at screening. The use of probiotic/prebiotic supplements or herbal medicines for gut health, was not permitted within 4 wk prior to screening or during the study. Prohibited medications during the study period included antibiotics, antidiarrheals, or other medications affecting gastric motility and anti-depressants, among others. Lastly, participants who were pregnant or intended to get pregnant or breastfeeding were excluded. All participants provided their voluntary, written, informed consent prior to their inclusion.
Study design and procedures
The study was conducted in accordance with the ethical principles that have their origins in the current version of the Declaration of Helsinki, the International Council on Harmonization E6 Good Clinical Practice, and all applicable local regulatory requirements. The study was approved and monitored by an independent ethics committee (Approval No: VED/P-20/22/JUL/2021; Address: ACEAS, Ahmedabad, Gujarat, India). The trial was prospectively registered on clinicaltrials.gov under study number NCT04950296.
This is a prospective, randomized, double-blind, placebo-controlled, multi-center, and parallel-arm study, including two doses of the probiotic product and one placebo arm. The study population was recruited from physician databases of individuals with IBS symptoms within the gastroenterology specialized centers. Participants visited the clinical sites for screening at least 14 d prior to planned randomization. At screening, the study was explained to participants in their local language, following which written informed consent was obtained. Participants were assessed for inclusion-exclusion criteria; a clinical examination was performed, and medical history, concomitant medication, demographic details, and vitals were recorded. Participants meeting the screening criteria underwent a 2-wk placebo run-in period to observe pre-study symptomology and to identify placebo responders, defined as a decrease of more than 25% in APS-NRS. Participants not meeting the placebo responder criteria were eligible for randomization (day 0) and subsequent visits at day 28 ± 2 and day 56 ± 2 over an 8-wk intervention period.
Participants were randomized (1:1:1), without stratification, to receive either placebo, L. plantarum (1B), or L. plantarum (10B). Block randomization (block size of six) was performed using Stats Direct software (Version 3.1.17), generating distinct alphanumeric codes, with every block containing two of each arm in random sequence. The study sites were allocated randomization codes in series, and the sites dispensed blinded product and corresponding randomization code, in sequential order of participant qualification. The randomization codes were concealed from site investigators, as well as site and study teams involved in study conduct and evaluation, with blinding codes secured in tamper-evident sealed envelopes.
Study products
Placebo and L. plantarum capsules were prepared in compliance with standard operating and quality control procedures at Chr. Hansen Inc. (Wausau, WI, United States). Microbiological analyses were confirmed following production. L. plantarum 1B and 10B capsules were formulated with the lyophilized strain and microcrystalline cellulose and contained a potency of not less than (NLT) 1 × 109 colony-forming units (CFU)/capsule or 1 × 1010 CFU/capsule, respectively. Placebo capsules were formulated with microcrystalline cellulose. All capsules were size 1 hypromellose, contained minimal but identical quantities of magnesium stearate and silica as flow aids, were identical in mass, appearance, and taste, and were bottled in identical sealed 112 mL CSP bottles (Aptar CSP Technologies Inc., Auburn, AL, United States) containing 35 capsules per bottle. Participants took the allotted study product orally, one capsule daily before lunch, with a glass of water. Compliance was assessed via participants’ investigational product diary and confirmed by examining unused study product at each visit.
Outcome measures
On days 0, 28, and 56 visits, study outcomes were assessed, a clinical examination was conducted, study product was dispensed or collected, and concomitant medication usage was recorded. Metronidazole (400 mg/d) was provided as a rescue medication in the case of severe pain and/or frequent loose stool, as it has been shown to provide symptom relief in IBS without affecting rectosigmoid motility[15]. Study product compliance was assessed via participants’ investigational product diary and confirmed by examining unused study product at each visit. A dietary diary was completed by participants over the 14 d prior to each study visit, recording dietary intake for two weekdays and one weekend day. Dietary information was processed via HealthifyMe software[16] for average daily intake of calories and macronutrients over the study period.
The pre-specified ranking of primary and secondary outcomes is listed in Supplementary Table 1. The primary outcome was the change in IBS symptom severity, assessed via the IBS-SSS total score[17] from baseline to day 56. Scores on the IBS-SSS range from 0 to 500, with five domains related to abdominal pain severity and frequency, abdominal distention, dissatisfaction with bowel habits, and interference with QoL. A decrease of 95 points has been associated with clinically meaningful improvement[18], and was used for classification of IBS-SSS responders.
Among secondary outcomes, abdominal pain severity was assessed via an 11-point numeric rating scale (APS-NRS)[18], with 10 representing most severe pain and 0 representing no pain. Post-screening APS-NRS scores were submitted using a digital diary on a weekly basis. An average of the prior weeks’ score was considered as the score for the corresponding visit.
Stool consistency was assessed via the BSS, a validated ordinal scale of stool types[19] ranging from 1 through 7, with types 6-7 being indicative of diarrhea. Stool consistency responders were defined as a decrease of 50% or more vs baseline in the number of days per week with at least one types 6-7 bowel movement[20].
IBS related QoL was assessed via the IBS-QoL, a 34-item questionnaire wherein the individual responses are summed and transformed to a 0-100 scale, with increasing scores indicating improved IBS specific QoL[21]. Lastly, mental stress was assessed using the perceived stress scale (PSS), consisting of 10 items and a total score ranging from 0-40, with higher scores indicating higher perceived stress[22].
Microbial profiling
Participants collected fecal samples, as close as possible but prior to the day 0 and day 56 visits, inside a biome collector device and ultimately a barcoded stool collection tube with preservation buffer. Stool collection tubes were stored at the individual sites, with regular shipments to a central laboratory, wherein they were shipped approximately every 8 wk to CosmosID (Germantown, MD, United States) for analysis. Cold chain (2-8 °C) was maintained during the entire process with data loggers used to confirm temperature variations during shipment.
Sample DNA isolation and quantification, library preparation, and shotgun sequencing of fecal samples were performed by CosmosID. DNA from samples was isolated using the QIAGEN DNeasy PowerSoil Pro Kit, according to the manufacturer’s protocol. DNA samples were quantified using the GloMax Plate Reader System (Promega) using the QuantiFluor® dsDNA System (Promega) chemistry. DNA libraries were prepared using the Nextera XT DNA Library Preparation Kit (Illumina) and IDT Unique Dual Indexes with total DNA input of 1 ng. Genomic DNA was fragmented using a proportional amount of Illumina Nextera XT fragmentation enzyme. Unique dual indexes were added to each sample followed by 12 cycles of PCR to construct libraries. DNA libraries were purified using AMpure magnetic beads (Beckman Coulter) and eluted in QIAGEN EB buffer. DNA libraries were quantified using Qubit 4 fluorometer and Qubit dsDNA HS Assay Kit. Libraries were then sequenced on an Illumina NextSeq 2000 platform (2 × 150 bp).
Statistical analysis
The sample size calculation was based on the primary outcome. A reduction in IBS-SSS score of at least 15% in the L. plantarum 1B group as compared to placebo, was considered a relevant treatment effect[23]. The standard deviation of the primary outcome was estimated at 30%. A placebo effect was estimated at 20%-25%. Assuming a 90% study power and a two-sided statistical significance level of 5%, the sample size was estimated to be approximately 86 participants per group. With an estimated 15% drop-out rate, 100 participants per group were planned to be randomized.
The primary and secondary outcomes (Supplementary Table 1) were assessed on the intention-to-treat (ITT) population. Descriptive statistics are presented as the mean (SD) for continuous variables or as a percentage for qua-litative variables. Data normality was assessed using the Shapiro-Wilk test. Differences between groups for demographic and clinical characteristics at baseline were analyzed using one-way ANOVA for continuous variables or Pearson chi-square test for categorical variables. An analysis of covariance (ANCOVA) was assessed for continuous outcomes on the absolute change from baseline to subsequent visit. Dunnett’s test for multiple comparisons was applied to assess statistical significance for each L. plantarum dose group as compared to placebo. A two-sample t-test was used to compare the difference between the two L. plantarum dose groups. Categorical outcomes were assessed via a Pearson chi-square test. The associations between efficacy parameters were assessed via Pearson correlation coefficients. Descriptive and inferential statistics were performed using Statistical Package for the Social Sciences (SPSS, IBM®) Python 3.0 (Armonk, NY, United States).
Microbial profile stacked bar figures were generated using the R package ggpubr. Linear Discriminant Analysis Effect Size (LEfSe) was calculated with a Kruskal-Wallis alpha value of 0.05, a Wilcoxon alpha value of 0.05, and a logarithmic LDA score threshold of 2.0.
RESULTS
Study parameters
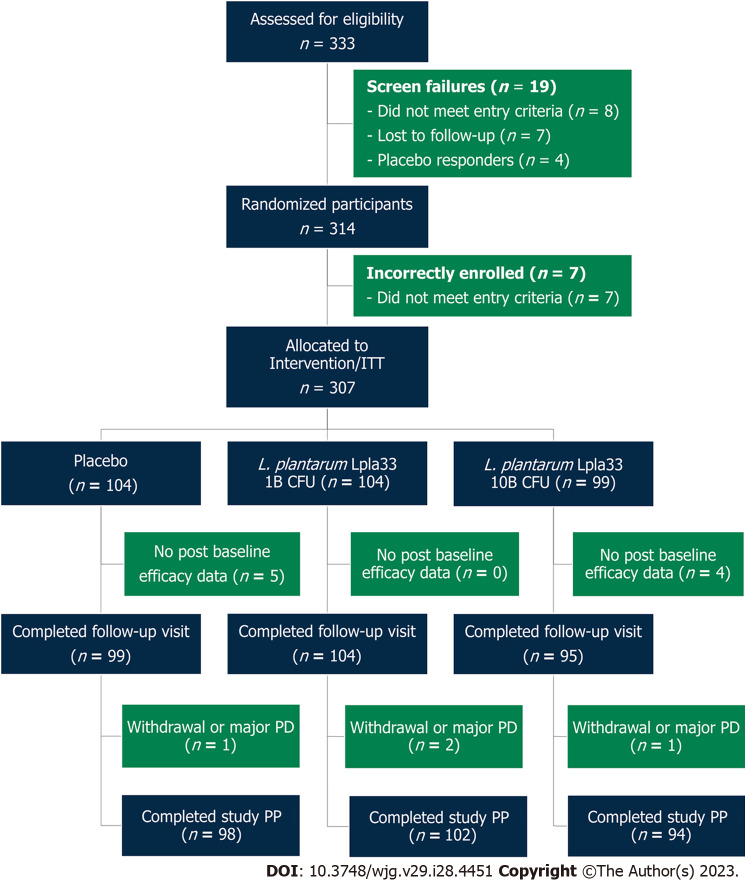
A total of 333 screened participants were assessed for eligibility and 314 participants were randomized in the study (Figure 1). Enrolment and intervention occurred continuously from September 2021 through May 2022. Seven participants were incorrectly enrolled, without meeting entry criteria, and were excluded. Three hundred and seven participants were randomized as part of the ITT population, with 104, 104, and 99 allocated to the placebo, L. plantarum 1B, and L. plantarum 10B groups, respectively. A total of 298 participants completed the first follow-up visit, while nine participants were lost to follow-up with no post-baseline efficacy data. Study attrition rates over the intervention period were 5 (4.8%), 2 (1.9%), and 4 (4.0%) for the placebo, L. plantarum 1B, and L. plantarum 10B groups, respectively. A total of 294 participants completed the study per protocol.
Figure 1.
Participant flow chart. ITT: Intention-to-treat; PD: Protocol deviation; PP: Per protocol; L. plantarum: Lactiplantibacillus plantarum.
Baseline characteristics
The screening and baseline characteristics of the ITT population are presented in Table 1. The three groups had similar demographic and clinical characteristics with no differences between groups (P > 0.05). The mean ages of participants were 39.5, 38.7, and 40.5 years across the three groups with a nearly even distribution of females and males. Participants met Rome IV criteria for IBS-D and demonstrated similar baseline mean IBS-SSS scores of 308.7, 315.2, and 302.9 for the placebo, L. plantarum 1B, and L. plantarum 10B groups, respectively (P > 0.05). At baseline, 43.4%, 41.4%, and 47.4% of participants in the placebo, L. plantarum 1B, and L. plantarum 10B groups, respectively, had moderate (175-300) IBS-SSS profiles, while 56.6%, 58.7%, and 52.6% of participants in the placebo, L. plantarum 1B, and L. plantarum 10B groups, respectively, had severe (> 300) IBS-SSS profiles.
Table 1.
Baseline demographics and clinical characteristics of the intention-to-treat population, n (%)
|
|
Placebo 1B (n = 104)
|
L. plantarum 1B (n = 104)
|
L. plantarum 10B (n = 99)
|
P value |
|
mean (SD) or n (%)
|
mean (SD) or n (%)
|
mean (SD) or n (%)
|
||
| Age (yr) | 39.50 (13.26) | 38.66 (11.25) | 40.46 (11.15) | 0.56191 |
| Female allocation | 46 (44.23) | 50 (48.08) | 44 (44.44) | 0.82322 |
| Height (m) | 1.60 (0.07) | 1.59 (0.08) | 1.61 (0.07) | 0.17751 |
| Weight (kg) | 63.57 (9.10) | 63.09 (9.01) | 63.25 (8.50) | 0.92561 |
| BMI (kg/m2) | 24.83 (3.15) | 25.01 (3.31) | 24.46 (2.92) | 0.45061 |
| Systolic BP (mmHG) | 119.68 (8.27) | 119.76 (8.68) | 118.88 (8.79) | 0.72231 |
| Diastolic BP (mmHG) | 79.29 (5.06) | 79.21 (6.30) | 78.75 (4.62) | 0.74291 |
| Fasting glucose (mmol/L) | 5.16 (0.66) | 5.01 (0.65) | 5.13 (0.62) | 0.19611 |
| IBS-SSS aggregate score | 308.70 (70.91) | 315.20 (62.79) | 302.85 (61.06) | 0.40901 |
| APS-NRS score | 7.14 (0.69) | 7.14 (0.77) | 7.17 (0.86) | 0.96481 |
| IBS-QoL aggregate score | 36.19 (17.97) | 37.83 (17.25) | 37.54 (17.02) | 0.77871 |
| PSS score | 22.06 (4.29) | 22.42 (4.12) | 21.93 (4.31) | 0.69121 |
Between group comparison; one-way ANOVA.
Between group comparison; Pearson chi-square test.
BMI: Body mass index; BP: Blood pressure; IBS-SSS: Irritable bowel syndrome-severity scoring system; APS-NRS: Abdominal pain severity-numeric rating scale; IBS-QoL: Irritable bowel syndrome-quality of life; PSS: Perceived stress scale; L. plantarum: Lactiplantibacillus plantarum.
IBS symptom severity
A significant between-group difference was observed in the primary outcome, change in IBS-SSS total score after 56 d, for both L. plantarum groups when compared to placebo (Table 2). On day 56, a greater reduction in IBS-SSS total score was observed in the L. plantarum 1B (-128.45 ± 83.30) and L. plantarum 10B (-156.77 ± 99.06) groups, as compared to placebo (-58.82 ± 74.75) (P < 0.001). Similarly, a greater reduction in IBS-SSS total score was observed on day 28 in the L. plantarum 1B (-66.37 ± 72.27) and L. plantarum 10B (-85.80 ± 89.53) groups, as compared to placebo (-35.68 ± 60.81) (P < 0.01). Comparing the two dose groups, the L. plantarum 10B group demonstrated a significantly greater reduction in IBS-SSS total score at day 56 (P < 0.05). End-of-study IBS-SSS profiles were considered either in remission or mild (< 175) in 48.1% and 72.6% of participants in the L. plantarum 1B and L. plantarum 10B groups, respectively. as compared to 11.1% in the placebo group (P < 0.001).
Table 2.
Irritable bowel syndrome symptom severity total and domain-specific scores over the intervention period
|
Placebo (n = 104)
|
L. plantarum 1B (n = 104)
|
L. plantarum 10B (n = 99)
|
||||
|
mean (SD)
|
mean (SD)
|
P vs Placebo1
|
mean (SD)
|
P vs Placebo1
|
P vs 1B dose2
|
|
| IBS-SSS total score | ||||||
| Day 0 | 308.70 (70.91) | 315.95 (62.07) | 302.85 (61.06) | |||
| AbsΔ (Day 28) | -35.68 (60.81) | -66.37 (72.27) | 0.0098 | -85.80 (89.53) | < 0.0001 | 0.0956 |
| AbsΔ (Day 56) | -58.82 (74.75) | -128.45 (83.30) | < 0.0001 | -156.77 (99.06) | < 0.0001 | 0.0298 |
| Abdominal pain severity | ||||||
| Day 0 | 67.05 (17.11) | 68.80 (13.40) | 67.07 (13.77) | |||
| AbsΔ (Day 28) | -9.24 (19.67) | -15.45 (18.36) | 0.0830 | -20.76 (22.76) | < 0.0001 | 0.0736 |
| AbsΔ (Day 56) | -14.38 (21.18) | -28.91 (20.36) | < 0.0001 | -36.52 (24.39) | < 0.0001 | 0.0176 |
| Abdominal pain duration | ||||||
| Day 0 | 41.11 (14.13) | 42.60 (14.55) | 39.37 (13.75) | |||
| AbsΔ (Day 28) | -5.35 (13.50) | -8.46 (12.05) | 0.1975 | -7.89 (12.62) | 0.0484 | 0.7463 |
| AbsΔ (Day 56) | -8.38 (13.75) | -15.29 (13.72) | 0.0006 | -19.68 (14.91) | < 0.0001 | 0.0315 |
| Abdominal distension | ||||||
| Day 0 | 65.08 (18.11) | 66.31 (15.11) | 63.07 (14.34) | |||
| AbsΔ (Day 28) | -8.66 (18.19) | -14.69 (17.68) | 0.0600 | -18.55 (22.62) | < 0.0001 | 0.1849 |
| AbsΔ (Day 56) | -14.00 (20.76) | -29.33 (21.64) | < 0.0001 | -35.45 (24.00) | < 0.0001 | 0.0598 |
| Bowel habits | ||||||
| Day 0 | 68.32 (15.64) | 69.35 (13.70) | 66.72 (14.55) | |||
| AbsΔ (Day 28) | -6.45 (12.02) | -14.36 (18.66) | 0.0020 | -19.52 (21.48) | < 0.0001 | 0.0714 |
| AbsΔ (Day 56) | -11.19 (16.63) | -27.73 (19.54) | < 0.0001 | -32.31 (25.12) | < 0.0001 | 0.1561 |
| Effect on quality of life | ||||||
| Day 0 | 67.13 (15.85) | 68.90 (14.47) | 66.62 (12.85) | |||
| AbsΔ (Day 28) | -5.97 (12.03) | -13.40 (17.48) | 0.0060 | -19.08 (21.13) | < 0.0001 | 0.0395 |
| AbsΔ (Day 56) | -10.86 (15.74) | -27.19 (19.19) | < 0.0001 | -32.81 (23.66) | < 0.0001 | 0.0690 |
ANCOVA using Dunnett’s test adjustment with treatment as factor and baseline as covariate vs placebo.
Two-sample t-test comparing dose groups.
IBS-SSS: Irritable bowel syndrome-severity scoring system; AbsΔ: Absolute change; L. plantarum: Lactiplantibacillus plantarum.
Participants receiving L. plantarum 1B capsules reported significant reductions in the scores of IBS-SSS individual domains over the intervention period, as compared to placebo, including abdominal pain severity (-28.91 ± 20.36), abdominal pain duration (-15.29 ± 13.72), abdominal distension (-29.33 ± 21.64), bowel habits (-27.73 ± 19.54) and QoL (-27.19 ± 19.19) (P < 0.001). Similarly, participants receiving L. plantarum 10B capsules reported significant reductions in IBS-SSS domain specific scores, as compared to placebo, including abdominal pain severity (-36.52 ± 24.39), abdominal pain duration (-19.68 ± 14.91), abdominal distension (-35.45 ± 24.00), bowel habits (-32.31 ± 25.12), and QoL (-32.81 ± 23.66) (P < 0.001). Comparing the two dose groups, the L. plantarum 10B group demonstrated a greater reduction in abdominal pain severity and duration scores over the intervention period (P < 0.05).
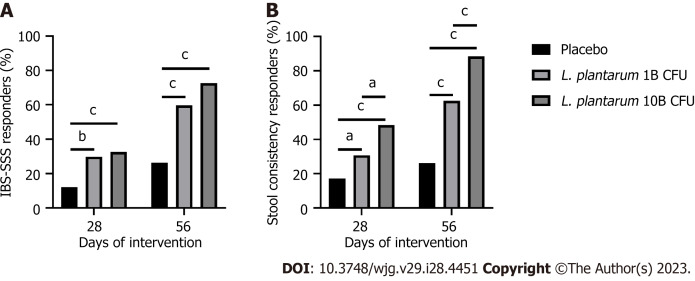
After 56 d, 59.6% and 72.6% of participants met 95-point reduction thresholds[18] in the L. plantarum 1B and L. plantarum 10B groups, respectively, as compared to 26.3% in the placebo group (P < 0.001) (Figure 2A). A post-hoc assessment showed 83.7% and 87.4% of participants met 50-point IBS-SSS reduction thresholds[17] in the L. plantarum 1B and L. plantarum 10B groups, respectively, as compared to 46.5% in the placebo group (P < 0.001). Additionally, 2.9% and 2.1% of participants demonstrated an increase in IBS-SSS score in the L. plantarum 1B and L. plantarum 10B groups, respectively, as compared to 18.2% in the placebo group (P < 0.001).
Figure 2.
Responder profile over the intervention period. A: Percentage of irritable bowel syndrome-severity scoring system (IBS-SSS) responders, as defined by a decrease of 95 points or more in the IBS-SSS total score from baseline, in participants receiving placebo or Lactiplantibacillus plantarum (L. plantarum) (1B or 10B CFU) capsules; B: Percentage of stool consistency responders, as defined by a decrease of at least 50% in the number of days per week with at least one stool that has a consistency of type 6 or 7 compared with baseline, in participants receiving placebo or L. plantarum (1B or 10B CFU) capsules. Between group comparison via Pearson chi-square test. aP < 0.05; bP < 0.01; cP < 0.001. L. plantarum: Lactiplantibacillus plantarum.
Abdominal pain severity, quality of life, and perceived stress
Baseline APS-NRS scores were 7.14 ± 0.69, 7.14 ± 0.77, and 7.17 ± 0.86 for the placebo, L. plantarum 1B, and L. plantarum 10B groups, respectively (P > 0.05). On day 56, a greater reduction in APS-NRS score (Table 3) was observed in the L. plantarum 1B (-1.83 ± 1.38) and L. plantarum 10B (-2.39 ± 1.47) groups, as compared to placebo (-0.94 ± 1.36) (P < 0.001). The mean reduction in the L. plantarum 10B group exceeded a clinically meaningful threshold of 30%[20] over the intervention period.
Table 3.
Abdominal pain severity, quality of life, and perceived stress scores over the intervention period
|
Placebo (n = 104)
|
L. plantarum 1B (n = 104)
|
L. plantarum 10B (n = 99)
|
||||
|
mean (SD)
|
mean (SD)
|
P vs Placebo1
|
mean (SD)
|
P vs Placebo1
|
P vs 1B dose2
|
|
| APS-NRS score | ||||||
| Day 0 | 7.14 (0.69) | 7.14 (0.77) | 7.17 (0.86) | |||
| AbsΔ (Day 28) | -0.61 (1.12) | -0.87 (1.06) | 0.1356 | -0.92 (0.90) | 0.0733 | 0.7089 |
| AbsΔ (Day 56) | -0.94 (1.36) | -1.83 (1.38) | < 0.0001 | -2.39 (1.47) | < 0.0001 | 0.0057 |
| IBS-QoL total score | ||||||
| Day 0 | 36.19 (17.97) | 37.83 (17.25) | 37.54 (17.02) | |||
| AbsΔ (Day 28) | 4.20 (14.19) | 8.82 (17.38) | 0.0208 | 17.65 (19.47) | < 0.0001 | 0.0009 |
| AbsΔ (Day 56) | 4.90 (14.29) | 17.47 (19.54) | < 0.0001 | 28.78 (23.64) | < 0.0001 | 0.0003 |
| PSS score | ||||||
| Day 0 | 22.06 (4.29) | 22.42 (4.12) | 21.93 (4.31) | |||
| AbsΔ (Day 28) | -0.12 (3.92) | -2.38 (4.54) | < 0.0001 | -2.76 (4.69) | < 0.0001 | 0.5690 |
| AbsΔ (Day 56) | -0.46 (4.14) | -4.33 (6.51) | < 0.0001 | -5.59 (6.43) | < 0.0001 | 0.1708 |
ANCOVA using Dunnett’s test adjustment with treatment as factor and baseline as covariate vs placebo.
Two-sample t-test comparing dose groups.
APS-NRS: Abdominal pain severity-numeric rating scale; IBS-QoL: Irritable bowel syndrome-quality of life; PSS: Perceived stress scale; AbsΔ: Absolute change; L. plantarum: Lactiplantibacillus plantarum.
On day 56, a significant increase in IBS-Qol score (Table 3) was observed in the L. plantarum 1B (17.47 ± 19.54) and L. plantarum 10B (28.78 ± 23.64) groups, as compared to placebo (4.90 ± 14.29) (P < 0.001). Similarly, a greater improvement in total IBS-QoL score was demonstrated on day 28 in the L. plantarum 1B (8.82 ± 17.38) and L. plantarum 10B (17.65 ± 19.47) groups, as compared to placebo (4.20 ± 14.19) (P < 0.01). Comparing the two dose groups, the L. plantarum 10B group demonstrated a greater improvement in IBS-QoL at both timepoints (P < 0.001).
Perceived stress, assessed via the PSS, was similarly improved in the L. plantarum 1B and L. plantarum 10B groups, as compared to placebo on both day 28 and day 56 (Table 3). On day 56, a significant reduction in PSS score was observed in the L. plantarum 1B (-4.33 ± 6.51) and L. plantarum 10B (-5.59 ± 6.43) groups, as compared to placebo (-0.46 ± 4.14) (P < 0.001). No significant differences were observed between the dose groups in PSS. Further, a post-hoc analysis showed PSS response to be moderately but significantly correlated to IBS-SSS (r = 0.326, P < 0.001) and APS-NRS scores (r = 0.355, P < 0.001) over the intervention period.
Stool consistency
Figure 2B shows the percent of participants considered IBS-D stool consistency responders, based on a decrease of 50% or more in the number of days per week with at least one types 6-7 bowel movement, as compared to baseline[20]. On day 56, 62.5% and 88.4% of study participants met the criteria of IBS-D stool consistency responders in the L. plantarum 1B and L. plantarum 10B groups, respectively, as compared to 26.3% in the placebo group. Additionally, a greater number of responders were observed in the L. plantarum 10B group as compared to the L. plantarum 1B group (P < 0.001). A significant number of responders were also observed in the L. plantarum 1B (30.8%; P < 0.05 vs placebo) and L. plantarum 10B (48.4%; P < 0.001 vs placebo) groups after day 28.
Microbial profiling
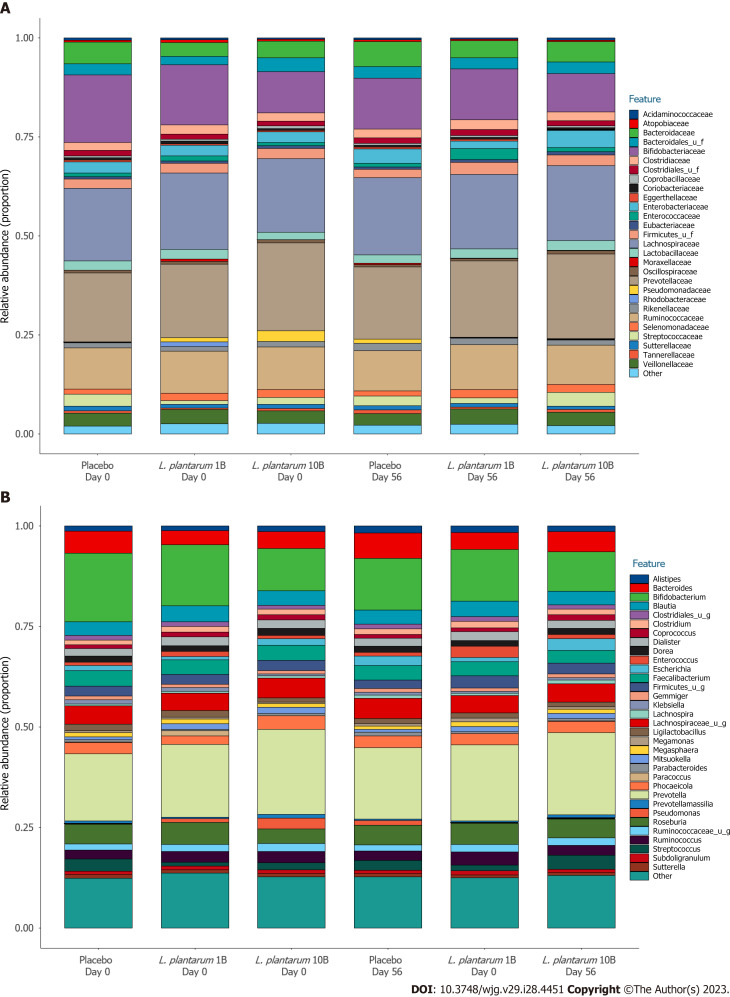
There were no significant differences in alpha (Shannon) diversity or beta diversity between groups over the study period, suggesting no major shifts in microbiome composition. Figure 3 shows the proportional family (A) and genus (B) level abundance in participants receiving placebo, L. plantarum 1B, and L. plantarum 10B, respectively. The most abundant taxa at the phylum level, Firmicutes, Bacteroidetes, and Actinobacteria, did not differ significantly over time in the three groups. Similarly, the most abundant taxa at the family level, Lachnospiraceae, Prevotellaceae, Ruminococcaceae, and Bifidobacteriaceae were stable within group over the intervention period, with the exception of a significant reduction in Ruminococcaceae and Bifidobacteriaceae within the placebo group (LEfSe logarithmic LDA score < -3.0) that was not observed in the L. plantarum groups. Similarly, the placebo group demonstrated a significant reduction in Bifidobacterium, Faecalibacterium, and F. prausnitzii (LEfSe logarithmic LDA score < -3.0) over the study period that was not observed in the L. plantarum groups. LEfSe identified more taxa enriched in the higher dose L. plantarum group at the end of study visit as compared to placebo, including both Lactiplantibacillus and L. plantarum abundance (LEfSe logarithmic LDA score > 2.0).
Figure 3.
Fecal microbial profile over the intervention period. A: Proportional family level abundance in participants receiving placebo or Lactiplantibacillus plantarum (L. plantarum) (1B or 10B CFU) capsules; B: Proportional genus level abundance in participants receiving placebo or L. plantarum (1B or 10B CFU) capsules.
Safety, compliance, and dietary profile
A total of 37 adverse events (AEs) were reported during the study, with seven reported during the run-in phase and 30 reported during the intervention phase. Of the 30 AEs reported post-randomization, 12, 13, and 5 were in the placebo, L. plantarum 1B, and L. plantarum 10B groups, respectively. A total of four AEs were suspected to be related to the study product with one AE (nausea and vomiting) in the L. plantarum 1B group and three AEs (heartburn and hyperacidity) in the placebo group. All AEs reported in the study were considered mild and resolved without complications. Vital signs, including systolic and diastolic blood pressure and pulse rate, were within clinically acceptable ranges over the intervention period with no significant differences between groups (Supplementary Table 2). Overall mean compliance over the study period was 98.7%, 98.4%, and 98.5% for the placebo, L. plantarum 1B, and L. plantarum 10B groups, respectively, with no differences between groups (P > 0.05). Additionally, total caloric and macronutrient intake, including protein, carbohydrates, fat, and fiber, were stable over the intervention period with no significant differences between groups (P > 0.05) (Supplementary Table 3).
DISCUSSION
The present study was a randomized, double-blind, placebo-controlled trial to assess the efficacy of L. plantarum Lpla33 (DSM34428) in adults with IBS-D. The study is unique in its dose-ranging design in a well-powered study across 12 clinical sites. Enrolled participants were primarily 30 to 50 years of age, with a body mass index ranging from normal to borderline overweight and an IBS symptom score of at least moderate severity.
The study had a roughly equal allocation of females and males, which is in line with prevalence rates for the IBS-D subtype globally[24,25], as well as prior IBS studies in Asia[26-28], and in contrast with a significantly greater female prevalence among constipation-predominant (IBS-C) cohorts[2]. Within South Asia, chronic gut infections, gut microbial dysbiosis, altered intestinal permeability, and inflammation are suggested as contributing factors to IBS and IBS-D[26]. Dietary practices may also play a role, with a generally high consumption of short chain carbohydrates[29].
In the present study, L. plantarum Lpla33, at daily doses of 1 × 109 or 1 × 1010 CFU, reduced IBS-SSS by more than twice the magnitude of the placebo group after 56 d, thus achieving the primary outcome while surpassing a clinically meaningful response[18]. Further, the higher dose L. plantarum group demonstrated a greater effect size than the lower dose L. plantarum group. Previously, an L. acidophilus strain, administered at daily doses of 1 × 109 and 1 × 1010 CFU for 12 wk, exhibited a treatment effect in a post-hoc analysis of IBS participants with elevated abdominal pain levels[30]. However, no dose-response was observed. Similarly, a meta-analysis reported a similar improvement in IBS symptoms when comparing probiotic regimens greater than or less than 1 × 1010 CFU daily[30]. In contrast, a L. gasseri strain resulted in a reduction in abdominal pain severity vs placebo at a daily dose of 1 × 1010 CFU, but had no effect in groups receiving 2 × 108 or 2 × 109 CFU daily[31]. Another meta-analysis looked at 14 different probiotic products over a total of 45 intervention arms, of which 36%, 51%, and 11% used a daily dose of 106-109, 1010, and 1011 CFU, respectively[14]. Of these, a dose response was noted for a 4-strain probiotic combination at 1010 CFU daily[32,33], as well as a B. infantis strain, which demonstrated efficacy at a dose of 108 CFU daily but not at doses of 106 or 1010 CFU daily[34].
Both L. plantarum dose groups displayed a significant number of IBS-SSS responders using either 95-point[18] or 50-point[17] reduction thresholds, which have independently been correlated with clinically relevant responses. Further, the high responder rates across participants with heterogeneous baseline symptoms[35] may also be applicable to the general healthy population experiencing GI discomfort with less frequency or severity[36].
Abdominal pain severity, assessed via the APS-NRS, was also significantly reduced with L. plantarum supplementation, with the higher dose group achieving a clinically relevant reduction of over 30%[20]. It should be noted that the placebo response of approximately 20% in the current study was less than those in prior IBS-D studies, which ranged from 32% to 44%[37-39]. This may have been due, in part, to the placebo run-in period as well as geographic, cultural, or dietary differences. Additionally, the higher-dose L. plantarum group exhibited a mean increase in IBS related QoL of 28.8 points, above the clinically meaningful threshold of 10 points[40]. This is of note given the lower reported QoL in IBS-D as compared to IBS-C[41], in part due to unpredictable defecation habits that limit daily activity. In addition, perceived stress in the L. plantarum groups was reduced compared to placebo. Of note, moderate but significant correlations were observed between reduced IBS symptomology, abdominal pain, and stress levels, with effects possibly linked to a more normalized gut-brain signaling or barrier function.
Rome Foundation reports have supported the concept that the intestinal microbiota is perturbed in IBS[42,43], and both incidence and symptom severity have been inversely associated with microbiome diversity[44,45]. Further, reports have linked IBS pathogenesis to dysbiosis of the microbiota[9,46], which may in turn impact pathogen binding and mucosal barrier integrity. However, there remain no uniform characteristics of an IBS-related gut microbiota[47]. The current study demonstrated a significant reduction in type 6 or 7 stool form via stool consistency responder analysis[20], including a dose effect among the L. plantarum groups. In contrast, no significant shifts in fecal microbial diversity were observed, in line with systematic reviews of probiotic studies showing no effects on alpha diversity, richness, or evenness, as well as composition[48]. Lactiplantibacillus and L. plantarum abundances were enriched at the end of study visit in the higher dose group as compared to placebo. Further, Ruminococcaceae, Bifidobacterium, and F. prausnitzii significantly decreased over the study period in the placebo group. Multi-omics studies have implicated F. prausnitzii, Ruminococcus spp., and Bifidobacterium in IBS treatment response[47], but it is unclear if this played a role here. Overall, the most abundant taxa did not significantly change over time. However, the site of action of L. plantarum Lpla33 is postulated to be proximal to the colon, and fecal samples may not accurately reflect the small intestinal profile or corresponding changes in mucosal inflammation or epithelial function[6].
L. plantarum is a well-documented species, with several prior studies demonstrating efficacy in managing IBS symptomology, albeit with strain specific differences[49-53]. Recent studies with L. plantarum strains have also reported improved QoL and defecation frequency in IBS-D[50,52]. In rodent models, L. plantarum strains have been shown to relieve diarrhea through inflammation modulation and increased SCFAs[54], as well as upregulate the expression of brain-derived neurotrophic factor, serotonin transporter, and intestinal serotonin levels[55]. SCFAs play a critical role in gut homeostasis via mucus production, protection from inflammation, and immunomodulation[56]. Further, SCFA-producing microorganisms can also affect neurotransmitter levels and therefore gut-brain signaling, as well as activate and directly act on intestinal vagal terminals[57]. Additionally, SCFAs, particularly butyrate, may help promote intestinal barrier function[58], a feature of several L. plantarum strains[59]. Bile acid (BA) metabolism may also be implicated, as L. plantarum Lpla33 possesses significant bile salt hydrolase activity. Of note, diarrhea and visceral hypersensitivity have been associated with decreased 7α-dehydroxylation of primary BAs to secondary BAs[60]. Additionally, the ability of L. plantarum Lpla33 to modulate intestinal barrier function, inhibit key pathogens, and moderate inflammatory markers may have played a role in the observed effects.
Limitations of the study include the absence of biomarkers assessing immunity, inflammation, and intestinal barrier function, in part due to the large enrollment across several sites. Additionally, the study assessed fecal samples but not proximal sites of interest or corresponding changes in mucosal profile, inflammation, or epithelial function. Further, the study did not incorporate metabolite profiles in serum, feces, or urine to assess changes in immune or inflammation-related pathways[47]. Additionally, while macronutrient intake was shown to be stable over the study period, the association of diet with the microbial community or metabolite profiles would have been of interest to explore in more detail. Going forward, multi-omics studies should play an important role in further understanding therapeutic mechanisms of probiotic and diet-based interventions in IBS-D. Nevertheless, the study was well-powered to evaluate its primary outcome, incorporated 12 clinical sites and multiple doses, and integrated IBS trial design considerations[61].
CONCLUSION
The present randomized controlled trial demonstrates that L. plantarum Lpla33 (DSM34428) at a dose of 1 × 109 and 1 × 1010 CFU/day was well tolerated and met the primary outcome (reduction in IBS-SSS total score at day 56) in both dose groups compared to placebo. The study also met the first secondary outcome (reduction in IBS-SSS score at day 28) as well as outcomes related to abdominal pain severity, stool normalization, QoL, and perceived stress, when compared to placebo over the intervention period. Lastly, a L. plantarum Lpla33 dose response was observed in several key outcomes in females and males with IBS-D.
ARTICLE HIGHLIGHTS
Research background
Irritable bowel syndrome (IBS) is a disorder of gut-brain interaction characterized by abdominal pain in association with altered bowel habits and further classified by the predominant stool pattern. Global prevalence is high, with diarrhea predominant subtype (IBS-D) considered the most common. IBS-D has a significant impact on quality of life, and clinical management remains challenging due to the variety of symptoms to address.
Research motivation
A recent meta-analysis showed probiotics to be safe and superior to placebo for alleviating global IBS-D symptoms. However, the certainty of evidence is low, due in part to significant heterogeneity between studies. There is therefore a need for well-powered randomized controlled trials on promising probiotic candidates for IBS-D.
Research objectives
To assess the efficacy of a probiotic candidate strain, Lactiplantibacillus plantarum (L. plantarum) Lpla33 (DSM34428), in adults with IBS-D. The primary outcome was the change in the IBS severity scoring system (IBS-SSS) total score after 8 wk. Additional outcomes included the change in abdominal pain severity, IBS-related quality of life, stool and microbial profile, and perceived stress.
Research methods
Adults meeting Rome IV diagnostic criteria for IBS-D were recruited from 12 gastroenterology specialized centers across India. In this randomized, double-blind, placebo-controlled, multi-center, parallel-arm, and dose-ranging study, a total of 307 adults meeting the inclusion criteria were allocated (1:1:1) to receive placebo or L. plantarum Lpla33 at one of two doses [1 × 109 colony-forming units (CFU)/d (1B) or 1 × 1010 CFU/d (10B)] over 8 wk.
Research results
The primary outcome, IBS-SSS total score, was significantly reduced after 8 wk in participants receiving L. plantarum compared to placebo (P < 0.001), with a dose-ranging effect when comparing the two L. plantarum groups (P < 0.05). In total, 59.6% and 72.6% of participants in the L. plantarum 1B and L. plantarum 10B groups, respectively, were considered significant responders based on a 95-point reduction threshold, as compared to 26.3% in the placebo group (P < 0.001). Additionally, 62.5% and 88.4% of participants administered L. plantarum 1B and 10B, respectively, demonstrated a significant reduction in diarrheal stool form as compared to 26.3% in the placebo group (P < 0.001).
Research conclusions
L. plantarum Lpla33 is well tolerated and demonstrates dose-ranging efficacy in alleviating IBS symptom severity with a corresponding normalization of bowel habits in adults with IBS-D.
Research perspectives
Future research should incorporate multi-omics analyses and associated biomarkers to better understand the mechanisms of action involved.
ACKNOWLEDGEMENTS
The authors thank the site investigators (Dr. R. Dargad, Dr. S. Deshpande, Dr. J. Patel, Dr. S. Narang, Dr. P. Rahate, Dr. S. Kulkarni, Dr. A. Khanna, Dr. S. Khanna, Dr. A. Jangid, Dr. A. Pandit, Dr. R. Kushwaha, and Dr. N. Jillawar) for enabling the study, and monitors for overseeing the conduct of the study. We thank the Vedic Lifesciences team, including project, quality assurance, and data managers, as well as the consulting statistician, for professional management of the study. We thank the CosmosID team for their microbial profiling analysis and expertise. Lastly, we are thankful to J. Jensen and team for overseeing the production and quality control of the study products.
Footnotes
Institutional review board statement: This study was approved and monitored by an independent ethics committee (Approval No: VED/P-20/22/JUL/2021).
Clinical trial registration statement: This study was prospectively registered on clinicaltrials.gov under study number NCT04950296. The registry URL is as follows: https://clinicaltrials.gov/ct2/show/NCT04950296.
Informed consent statement: All study participants provided their voluntary written informed consent prior to their inclusion.
Conflict-of-interest statement: The study was funded by Chr. Hansen A/S. Christopher J Martoni, Anders Damholt and Gregory J Leyer are employees of Chr. Hansen A/S but were not involved in the study conduct, data management, or statistical analysis.
CONSORT 2010 statement: The authors have read the CONSORT 2010 Statement, and the manuscript was prepared and revised according to the CONSORT 2010 Statement.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: June 9, 2023
First decision: June 14, 2023
Article in press: July 11, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: McFarland LV, United States; Wen XL, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YX
Contributor Information
Christopher J Martoni, Clinical Development, Human Health, Chr. Hansen A/S, Hoersholm 2970, Denmark. cachma@chr-hansen.com.
Shalini Srivastava, Clinical Development, Vedic Lifesciences, Mumbai 400053, India.
Anders Damholt, Clinical Development, Human Health, Chr. Hansen A/S, Hoersholm 2970, Denmark.
Gregory J Leyer, Scientific Affairs,Human Health, Chr. Hansen A/S, Hoersholm 2970, Denmark.
Data sharing statement
The anonymized data presented in this study are available on request from the corresponding author.
References
- 1.Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133–146. doi: 10.1016/S2468-1253(16)30023-1. [DOI] [PubMed] [Google Scholar]
- 2.Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:908–917. doi: 10.1016/S2468-1253(20)30217-X. [DOI] [PubMed] [Google Scholar]
- 3.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Moshiree B, Heidelbaugh JJ, Sayuk GS. A Narrative Review of Irritable Bowel Syndrome with Diarrhea: A Primer for Primary Care Providers. Adv Ther. 2022;39:4003–4020. doi: 10.1007/s12325-022-02224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dale HF, Rasmussen SH, Asiller ÖÖ, Lied GA. Probiotics in Irritable Bowel Syndrome: An Up-to-Date Systematic Review. Nutrients. 2019;11 doi: 10.3390/nu11092048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altomare A, Di Rosa C, Imperia E, Emerenziani S, Cicala M, Guarino MPL. Diarrhea Predominant-Irritable Bowel Syndrome (IBS-D): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients. 2021;13 doi: 10.3390/nu13051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakur ER, Shapiro J, Chan J, Lumley MA, Cully JA, Bradford A, El-Serag HB. A Systematic Review of the Effectiveness of Psychological Treatments for IBS in Gastroenterology Settings: Promising but in Need of Further Study. Dig Dis Sci. 2018;63:2189–2201. doi: 10.1007/s10620-018-5095-3. [DOI] [PubMed] [Google Scholar]
- 8.Bellini M, Gambaccini D, Stasi C, Urbano MT, Marchi S, Usai-Satta P. Irritable bowel syndrome: a disease still searching for pathogenesis, diagnosis and therapy. World J Gastroenterol. 2014;20:8807–8820. doi: 10.3748/wjg.v20.i27.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The Microbiome and Irritable Bowel Syndrome-A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol. 2019;10:1136. doi: 10.3389/fmicb.2019.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns GL, Talley NJ, Keely S. Immune responses in the irritable bowel syndromes: time to consider the small intestine. BMC Med. 2022;20:115. doi: 10.1186/s12916-022-02301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69:859–867. doi: 10.1136/gutjnl-2019-319630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Chen N, Niu F, Li Y, Guo K, Shang X, E F, Yang C, Yang K, Li X. Probiotics therapy for adults with diarrhea-predominant irritable bowel syndrome: a systematic review and meta-analysis of 10 RCTs. Int J Colorectal Dis. 2022;37:2263–2276. doi: 10.1007/s00384-022-04261-0. [DOI] [PubMed] [Google Scholar]
- 14.McFarland LV, Karakan T, Karatas A. Strain-specific and outcome-specific efficacy of probiotics for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. EClinicalMedicine. 2021;41:101154. doi: 10.1016/j.eclinm.2021.101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nayak AK, Karnad DR, Abraham P, Mistry FP. Metronidazole relieves symptoms in irritable bowel syndrome: the confusion with so-called 'chronic amebiasis'. Indian J Gastroenterol. 1997;16:137–139. [PubMed] [Google Scholar]
- 16.Vashisht T, Shenoy S, Cherian M. HealthifyMe App. [cited 12 June 2023]. Available from: https://www.healthifyme.com/in/
- 17.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel B, Bolus R, Harris LA, Lucak S, Naliboff B, Esrailian E, Chey WD, Lembo A, Karsan H, Tillisch K, Talley J, Mayer E, Chang L. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Aliment Pharmacol Ther. 2009;30:1159–1170. doi: 10.1111/j.1365-2036.2009.04144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693–703. doi: 10.1111/apt.13746. [DOI] [PubMed] [Google Scholar]
- 20.Food and Drug Administration. Guidance for Industry: Irritable Bowel Syndrome-Clinical Evaluation of Drugs for Treatment. [cited 12 June 2023]. Available from: https://www.fda.gov/media/78622/download .
- 21.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 22.Cohen S. Perceived stress in a probability sample of the United States. The social psychology of health. Sage Publications, Inc. 1988. [cited 12 June 2023]. Available from: https://psycnet.apa.org/record/1988-98838-002 .
- 23.Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044–1060. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]
- 24.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 25.Adeyemo MA, Spiegel BM, Chang L. Meta-analysis: do irritable bowel syndrome symptoms vary between men and women? Aliment Pharmacol Ther. 2010;32:738–755. doi: 10.1111/j.1365-2036.2010.04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman MM, Mahadeva S, Ghoshal UC. Epidemiological and clinical perspectives on irritable bowel syndrome in India, Bangladesh and Malaysia: A review. World J Gastroenterol. 2017;23:6788–6801. doi: 10.3748/wjg.v23.i37.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbara G, Feinle-Bisset C, Ghoshal UC, Quigley EM, Santos J, Vanner S, Vergnolle N, Zoetendal EG. The Intestinal Microenvironment and Functional Gastrointestinal Disorders. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Martoni CJ, Srivastava S, Leyer GJ. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 Improve Abdominal Pain Severity and Symptomology in Irritable Bowel Syndrome: Randomized Controlled Trial. Nutrients. 2020;12 doi: 10.3390/nu12020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewawasam SP, Iacovou M, Muir JG, Gibson PR. Dietary practices and FODMAPs in South Asia: Applicability of the low FODMAP diet to patients with irritable bowel syndrome. J Gastroenterol Hepatol. 2018;33:365–374. doi: 10.1111/jgh.13885. [DOI] [PubMed] [Google Scholar]
- 30.Lyra A, Hillilä M, Huttunen T, Männikkö S, Taalikka M, Tennilä J, Tarpila A, Lahtinen S, Ouwehand AC, Veijola L. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22:10631–10642. doi: 10.3748/wjg.v22.i48.10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JY, Park YJ, Lee HJ, Park MY, Kwon O. Effect of Lactobacillus gasseri BNR17 on irritable bowel syndrome: a randomized, double-blind, placebo-controlled, dose-finding trial. Food Sci Biotechnol. 2018;27:853–857. doi: 10.1007/s10068-017-0296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kajander K, Hatakka K, Poussa T, Färkkilä M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387–394. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 33.Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 34.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang P, Peskoe S, Byrd R, Smith P, Breslin R, Chow SC. Statistical Evaluation of Absolute Change versus Responder Analysis in Clinical Trials. Acta Mater Med. 2022;1:320–332. doi: 10.15212/amm-2022-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.EFSA Panel on Nutrition; Novel Foods and Food Allergens (EFSA NDA Panel) Turck D, Castenmiller J, De Henauw S, Hirsch-Ernst KI, Kearney J, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Thies F, Tsabouri S, Vinceti M, Bresson JL, Burlingame B, Dean T, Fairweather-Tait S, Heinonen M, Neuhäuser-Berthold M, Nowicka G, Sanz Y, Sjödin A, Stern M, Tomé D, Van Loveren H, Willatts P, Martin A, Strain JJ, Heng L, Valtueña Martínez S, Siani A. Guidance on the scientific requirements for health claims related to muscle function and physical performance: (Revision 1) EFSA J. 2018;16:e05434. doi: 10.2903/j.efsa.2018.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishaque SM, Khosruzzaman SM, Ahmed DS, Sah MP. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2018;18:71. doi: 10.1186/s12876-018-0788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skrzydło-Radomańska B, Prozorow-Król B, Cichoż-Lach H, Majsiak E, Bierła JB, Kosikowski W, Szczerbiński M, Gantzel J, Cukrowska B. The Effectiveness of Synbiotic Preparation Containing Lactobacillus and Bifidobacterium Probiotic Strains and Short Chain Fructooligosaccharides in Patients with Diarrhea Predominant Irritable Bowel Syndrome-A Randomized Double-Blind, Placebo-Controlled Study. Nutrients. 2020;12 doi: 10.3390/nu12071999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skrzydło-Radomańska B, Prozorow-Król B, Cichoż-Lach H, Majsiak E, Bierła JB, Kanarek E, Sowińska A, Cukrowska B. The Effectiveness and Safety of Multi-Strain Probiotic Preparation in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Study. Nutrients. 2021;13 doi: 10.3390/nu13030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passos MC, Lembo AJ, Conboy LA, Kaptchuk TJ, Kelly JM, Quilty MT, Kerr CE, Jacobson EE, Hu R, Friedlander E, Drossman DA. Adequate relief in a treatment trial with IBS patients: a prospective assessment. Am J Gastroenterol. 2009;104:912–919. doi: 10.1038/ajg.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh P, Staller K, Barshop K, Dai E, Newman J, Yoon S, Castel S, Kuo B. Patients with irritable bowel syndrome-diarrhea have lower disease-specific quality of life than irritable bowel syndrome-constipation. World J Gastroenterol. 2015;21:8103–8109. doi: 10.3748/wjg.v21.i26.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbara G, Grover M, Bercik P, Corsetti M, Ghoshal UC, Ohman L, Rajilić-Stojanović M. Rome Foundation Working Team Report on Post-Infection Irritable Bowel Syndrome. Gastroenterology. 2019;156:46–58.e7. doi: 10.1053/j.gastro.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattarai Y, Muniz Pedrogo DA, Kashyap PC. Irritable bowel syndrome: a gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol. 2017;312:G52–G62. doi: 10.1152/ajpgi.00338.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shrestha B, Patel D, Shah H, Hanna KS, Kaur H, Alazzeh MS, Thandavaram A, Channar A, Purohit A, Venugopal S. The Role of Gut-Microbiota in the Pathophysiology and Therapy of Irritable Bowel Syndrome: A Systematic Review. Cureus. 2022;14:e28064. doi: 10.7759/cureus.28064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J Gastroenterol. 2016;22:2219–2241. doi: 10.3748/wjg.v22.i7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng QX, Yau CE, Yaow CYL, Chong RIH, Chong NZ, Teoh SE, Lim YL, Soh AYS, Ng WK, Thumboo J. What Has Longitudinal 'Omics' Studies Taught Us about Irritable Bowel Syndrome? A Systematic Review. Metabolites. 2023;13 doi: 10.3390/metabo13040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8:52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143–1147. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Jung K, Kim A, Lee JH, Cho D, Seo J, Jung ES, Kang HJ, Roh J, Kim W. Effect of Oral Intake of Lactiplantibacillus plantarum APsulloc 331261 (GTB1(TM)) on Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2022;14 doi: 10.3390/nu14102015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 2010;10:16. doi: 10.1186/1471-230X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Yu X, Yu L, Tian F, Zhao J, Zhang H, Qian L, Wang Q, Xue Z, Zhai Q, Chen W. Lactobacillus plantarum CCFM8610 Alleviates Irritable Bowel Syndrome and Prevents Gut Microbiota Dysbiosis: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Trial. Engineering . 2021;7:376–385. [Google Scholar]
- 53.Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012–4018. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yue Y, He Z, Zhou Y, Ross RP, Stanton C, Zhao J, Zhang H, Yang B, Chen W. Lactobacillus plantarum relieves diarrhea caused by enterotoxin-producing Escherichia coli through inflammation modulation and gut microbiota regulation. Food Funct. 2020;11:10362–10374. doi: 10.1039/d0fo02670k. [DOI] [PubMed] [Google Scholar]
- 55.Ranuh R, Athiyyah AF, Darma A, Risky VP, Riawan W, Surono IS, Sudarmo SM. Effect of the probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: role of intestinal microbiota on the gut-brain axis. Iran J Microbiol. 2019;11:145–150. [PMC free article] [PubMed] [Google Scholar]
- 56.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamamah S, Aghazarian A, Nazaryan A, Hajnal A, Covasa M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines. 2022;10 doi: 10.3390/biomedicines10020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang RX, Lee JS, Campbell EL, Colgan SP. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc Natl Acad Sci U S A. 2020;117:11648–11657. doi: 10.1073/pnas.1917597117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mujagic Z, de Vos P, Boekschoten MV, Govers C, Pieters HH, de Wit NJ, Bron PA, Masclee AA, Troost FJ. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci Rep. 2017;7:40128. doi: 10.1038/srep40128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei W, Wang HF, Zhang Y, Zhang YL, Niu BY, Yao SK. Altered metabolism of bile acids correlates with clinical parameters and the gut microbiota in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2020;26:7153–7172. doi: 10.3748/wjg.v26.i45.7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller LE. Study design considerations for irritable bowel syndrome clinical trials. Ann Gastroenterol. 2014;27:338–345. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized data presented in this study are available on request from the corresponding author.