Abstract
This randomized clinical trial presents the final 5-year follow-up results of neoadjuvant talimogene laherparepvec (T-VEC) plus surgery in patients with advanced melanoma.
Risk of recurrence after surgical resection remains high for patients with advanced melanoma.1,2 Novel approaches, such as neoadjuvant immunotherapies, may help establish a wide repertoire of antitumoral T-cell responses and enhance patient outcomes. Talimogene laherparepvec (T-VEC), an intralesional oncolytic viral immunotherapy, comprises genetically engineered herpes simplex virus type 1 that replicates in tumor cells and supports recruitment of T cells and natural killer cells.3,4 The phase 3 OPTiM trial of T-VEC monotherapy for advanced melanoma showed a significantly improved durable response over granulocyte-macrophage colony-stimulating factor (16.3% vs 2.1%; P < .001).3 Primary analysis of the safety and efficacy of neoadjuvant T-VEC (NCT02211131) demonstrated a 2-year recurrence-free survival (RFS) of 29.5% for T-VEC plus surgery and 16.5% for surgery alone (overall hazard ratio [HR], 0.75; 80% CI, 0.58-0.96) in patients with advanced, resectable melanoma, persisting at 3 years (overall HR, 0.74; 80% CI, 0.57-0.95).5 Here, we report the study’s final 5-year analysis.
Methods
This phase 2, multicenter, open-label randomized clinical trial enrolled 150 patients from 48 sites in 9 countries starting February 3, 2015. The trial protocol is available in Supplement 1. Patients with resectable stage IIIB to IVM1a melanoma and 1 or more injectable cutaneous, subcutaneous, or nodal lesions were randomized 1:1 to receive 6 doses of neoadjuvant T-VEC then surgery (group 1) or immediate surgery alone (group 2) (eFigure in Supplement 2). Participating sites5 received local institutional review board approval. Participants provided written informed consent. The study followed the CONSORT reporting guideline.
Administration of T-VEC was by intralesional injection initially at 4.0 mL of 106 plaque-forming units (PFU)/mL at day 1 of week 1 followed by 4.0 mL of 108 PFU/mL at day 1 (±3 days) of weeks 4, 6, 8, 10, and 12 until surgery, no remaining injectable tumors, or intolerance. The investigator’s choice of adjuvant therapy was allowed. There was no formal hypothesis testing. The primary end point was the difference in RFS between groups 1 and 2 at 2 years in the intention-to-treat set (all randomized patients). Secondary end points included overall survival (OS), local RFS, regional RFS, and distant metastasis–free survival (DMFS). Event-free survival (EFS) was added beginning with the 3-year analysis. The snapshot for this analysis occurred on May 30, 2022. Statistical analysis was performed using SAS, version 8.2 software (SAS Institute Inc).
Results
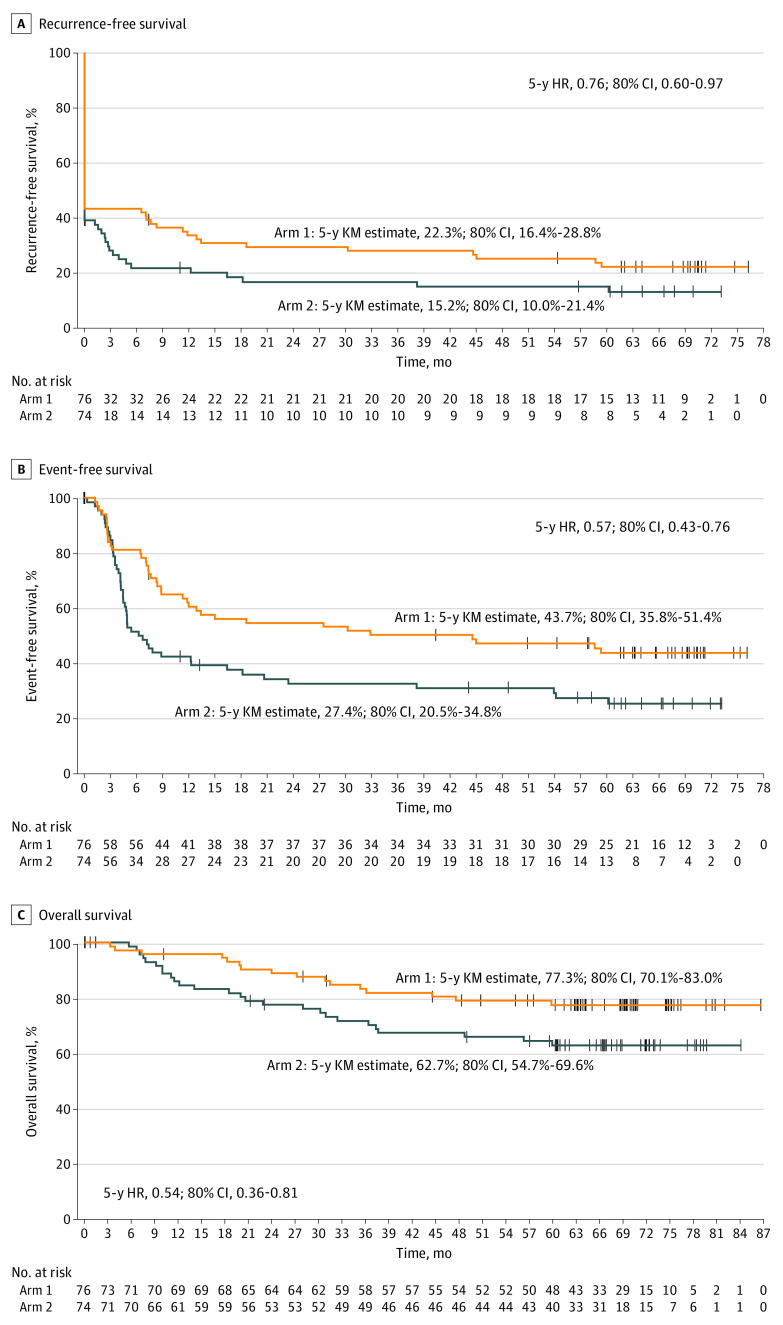
As of May 30, 2022, the median follow-up was 63.3 months (range, 0.1-86.8 months). The treatment groups were similar with respect to disease stage at baseline (group 1: 28 participants [36.8%] with stage IIIB, 30 [39.5%] with stage IIIC, 17 [22.4%] with stage IVM1a; group 2: 26 participants [35.1%] with stage IIIB, 35 [47.3%] with stage IIIC, 13 [17.6%] with stage IVM1a). Also, 10 (13.7%) and 22 (31.9%) received adjuvant therapy in groups 1 and 2, respectively. For groups 1 and 2, respectively, the 5-year Kaplan-Meier estimates were 22.3% and 15.2% (HR, 0.76; 80% CI, 0.60-0.97) for RFS; 43.7% and 27.4% (HR, 0.57; 80% CI, 0.43-0.76) for EFS; and 77.3% and 62.7% (HR, 0.54; 80% CI, 0.36-0.81) for OS (Figure). The HRs for local RFS, regional RFS, and DMFS were 0.82 (80% CI, 0.64-1.06), 0.81 (80% CI, 0.62-1.05), and 0.73 (80% CI, 0.57-0.94), respectively (Table).
Figure. Kaplan-Meier (KM) Estimates of Recurrence-Free Survival, Event-Free Survival, and Overall Survival at 5 Years.
Group 1 is talimogene laherparepvec (T-VEC) plus surgery, and group 2 is surgery alone. The vertical marks indicate patients who were censored. Time 0 is the time of randomization. HR indicates hazard ratio. A, Recurrence-free survival was defined as the time from randomization to the first of local, regional, or distant recurrence or death, where patients who withdrew before surgery were imputed as events at baseline. In this analysis, R1 and R2 resections were imputed as events at baseline. Patients without an event were censored at their last evaluable tumor assessment. B, Event-free survival was defined as the time from randomization to the first of local, regional, or distant recurrence after surgery; disease progression that precludes surgery; or death from any cause, whichever occurred first. Patients without an event were censored at their last evaluable tumor assessment. Patients with no evaluable tumor assessment were censored at surgery date if they had an R0 surgical outcome or on the randomization date if they had a non-R0 surgical outcome. Patients with a nonmelanoma tumor or a second malignant tumor identified before or at surgery were censored at the day after randomization. C, Overall survival was defined as the time from randomization to the date of death from any cause.
Table. Summary of Survival in the 5-Year Final Analysis.
| No. of events (%) | KM estimate, mo | Treatment difference KM estimate, % (80% CI)c | Overall unstratified HR (80% CI)d | |||
|---|---|---|---|---|---|---|
| Group 1 (n = 76)a | Group 2 (n = 74)b | Group 1a | Group 2b | |||
| LRFS | 53 (69.7) | 56 (75.7) | 28.02 | 21.12 | 6.90 (−2.60 to 16.40) | 0.82 (0.64 to 1.06) |
| RRFS | 50 (65.8) | 54 (73.0) | 32.91 | 24.69 | 8.22 (−1.58 to 18.01) | 0.81 (0.62 to 1.05) |
| DMFS | 54 (71.1) | 61 (82.4) | 27.62 | 14.99 | 12.63 (3.79 to 21.48) | 0.73 (0.57 to 0.94) |
Abbreviations: DMFS, distant metastasis–free survival; HR, hazard ratio; KM, Kaplan-Meier; LRFS, local recurrence-free survival; RRFS, regional recurrence-free survival.
Talimogene laherparepvec plus surgery.
Surgery alone.
Group 1 – group 2.
Group 1 / group 2.
Conclusions
Neoadjuvant therapy is a rapidly evolving field that has shown early signs of dramatic benefits for patients with advanced, resectable melanoma.6 In this analysis, we report durable improvements in 5-year RFS, EFS, DMFS, and OS with neoadjuvant T-VEC plus surgery over standard surgery. These results demonstrate that neoadjuvant T-VEC plus surgery improves cancer-related outcomes vs surgery alone, with acceptable safety. The improvement in survival outcomes is likely the result of an induced systemic immunologic antitumor effect, as shown by elevated CD8+ density after T-VEC treatment.5 Limitations in the trial design, particularly in the definition and assessment of RFS, are detailed in the primary analysis.5 These results support further investigation of neoadjuvant T-VEC combined with checkpoint inhibitors for the treatment of resectable high-risk melanoma.
Trial Protocol and Statistical Analysis Plan
eFigure. Patient Flow Diagram
Data Sharing Statement
References
- 1.Keung EZ, Gershenwald JE. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther. 2018;18(8):775-784. doi: 10.1080/14737140.2018.1489246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng G, Xu W, Atkinson V. Treatment approaches for melanomas that relapse after adjuvant or neoadjuvant therapy. Curr Oncol Rep. 2022;24(10):1273-1280. doi: 10.1007/s11912-022-01288-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780-2788. doi: 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 4.Ramelyte E, Tastanova A, Balázs Z, et al. Oncolytic virotherapy-mediated anti-tumor response: a single-cell perspective. Cancer Cell. 2021;39(3):394-406.e4. doi: 10.1016/j.ccell.2020.12.022 [DOI] [PubMed] [Google Scholar]
- 5.Dummer R, Gyorki DE, Hyngstrom J, et al. Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage IIIB-IVM1a melanoma: a randomized, open-label, phase 2 trial. Nat Med. 2021;27(10):1789-1796. doi: 10.1038/s41591-021-01510-7 [DOI] [PubMed] [Google Scholar]
- 6.Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med. 2021;27(2):301-309. doi: 10.1038/s41591-020-01188-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eFigure. Patient Flow Diagram
Data Sharing Statement