Abstract
Objective:
To compare the effectiveness of mRNA vaccines (BNT162b2 vs mRNA-1273) against COVID-19 infection among patients with systemic autoimmune rheumatic diseases (SARDs) on immunomodulatory medications.
Methods:
We identified patients with SARDs being treated with DMARDs and/or glucocorticoids in the Mass General Brigham healthcare system who received either BNT162b2 or mRNA-1273 as their initial vaccine series. Patients were followed until positive SARS-CoV-2 test, death, or 2/22/2022. We compared the risk of breakthrough infection between BNT162b2 and mRNA-1273 vaccine recipients using time stratified, overlap propensity score–weighted Cox proportional hazard models.
Results:
We identified 9838 patients with SARDs who received BNT162b2 or mRNA-1273. Demographic and clinical characteristics were similar in both groups after overlap weighting: mean age 61 years, 75% female, 54% with rheumatoid arthritis, and 74% receiving conventional and 43% receiving biologic DMARDs. Of 5516 BNT162b2 and 4322 mRNA-1273 recipients, 446 and 329 had a breakthrough infection, respectively. The corresponding time-stratified PS weighted rate difference of breakthrough infection was 0.71 (95%CI: −0.70, 2.12) per 1000 person months with a weighted HR of 1.12 (95%CI: 0.90, 1.39). When follow-up was censored prior to the Omicron wave, there was a trend towards higher breakthrough risk with BNT162b2 vs mRNA-1273 (weighted HR 1.34, 95%CI: 0.91, 1.98).
Conclusion:
Among SARD patients, the risk of breakthrough COVID-19 infection is similar after receiving either BNT162b2 or mRNA-1273. Patients with SARDs initiating the vaccine series should be encouraged to receive whichever mRNA vaccine is available.
INTRODUCTION
Patients with systemic autoimmune rheumatic diseases (SARDs) who use immunomodulatory treatments are at increased risk for severe COVID-19 (1, 2). SARS-CoV-2 vaccines reduce the risk of COVID-19 and severe outcomes in the immunocompetent and immunosuppressed populations (3, 4). However, many immunomodulator users have a blunted immune response to vaccination and are at increased risk for breakthrough infection compared to immunocompetent individuals (5–7). In this context, studies have demonstrated that the response to vaccination in immunomodulator users can be improved by temporarily holding immunomodulators and by administering additional vaccine doses (6). Little is known, however, regarding the comparative effectiveness of vaccines on the risk of breakthrough infection among immunomodulator users and whether one vaccine may be preferred.
A previous study found that the mRNA-1273 (Moderna) vaccine may have greater effectiveness against breakthrough infection when compared with BNT162b2 (Pfizer-BioNTech) in the population of Veterans Affairs beneficiaries (8). More recently, a large study of immunomodulator users found that those who received mRNA-1273 had a greater humoral immunologic response than those who received BNT162b2 (9). Little is known, however, regarding other potential differences in the immunologic response to mRNA-1273 or BNT162b2 in SARDs that may impact efficacy against clinical outcomes. Additionally, studies have suggested that recipients of mRNA-1273 vs BNT162b2 with a history of rheumatic disease had a lower risk of breakthrough infection but were susceptible to important sources of confounding, including the timing of vaccination (3, 10).
The objective of the current study is to assess the comparative effectiveness of BNT162b2 versus mRNA-1273 on the risk of breakthrough infection among patients with SARDs using immunomodulators.
PATIENTS AND METHODS
Study design, data source, and study population
In this observational cohort study, we compared the risk of COVID-19 breakthrough infection in individuals with SARDs on immunomodulatory medications who received BNT162b2 to those who received the mRNA-1273 vaccine. Patients with a diagnosis of a SARD who received either a BNT162b2 or mRNA-1273 vaccine were identified from the Mass General Brigham (MGB) Enterprise Data Warehouse (EDW). MGB is a multi-center healthcare system that includes a total of 14 hospitals, including two tertiary care hospitals (Massachusetts General Hospital and Brigham and Women’s Hospital), as well as other primary care and specialty outpatient centers in the greater Boston, Massachusetts area. This study was approved by the MGB Institutional Review Board (2020P000833). Patient consent was not required for this project as it did not meet the definition of Human Interaction/Intervention research and was deemed Health/Medical Records research.
We identified patients at least 18 years or older who were Massachusetts residents and received a BNT162b2 or mRNA-1273 vaccine dose between the date they were made available and February 2, 2022. We limited our study population to Massachusetts residents (as obtained by primary address in the electronic health record [EHR]) because vaccination data from the Massachusetts state database was populated in the EHR and all immunizations administered in Massachusetts are required by law to be reported to the Massachusetts Immunization Information System (11). The index date was the date of the first mRNA vaccine administration. From this population, we included patients if: (1) they had at least two instances of a rheumatic disease ICD-10 code within two years of the index date (one within the previous 12 months); and (2) a conventional, biologic, or targeted synthetic disease modifying anti-rheumatic drug (DMARD), prescription/administration within 12 months of the index date, and/or (3) a prescription for a minimum of 30 pills of either prednisone or methylprednisolone within 6 months of the index date (Supplemental Table 2). The positive predictive value (PPV) for a similar rules-based algorithm to identify rheumatoid arthritis was 86% (12). We reviewed 50 random patients who met our algorithm and 45 had physician-confirmed SARDs, resulting in a PPV of approximately 90%. Patients with osteoarthritis, fibromyalgia, or crystalline arthritis without another concomitant SARD diagnosis were excluded.
We included patients who received at least two doses of BNT162b2 or at least two doses of mRNA-1273. Those who received a Ad26.CoV2.S (Janssen/Johnson & Johnson) vaccine at any time or who received mixed doses (e.g. an individual who received an initial dose of Ad26.CoV2.S and then a dose of mRNA-1273 or BNT162b2) of any vaccine type were excluded from analysis. We included patients with a COVID-19 infection prior to the index date. Patients were followed from index date until the date of positive SARS-CoV-2 test result (PCR or antigen), death, or end of follow-up (2/22/2022).
Outcomes
The primary outcome was SARS-CoV-2 breakthrough infection, defined as: 1) a positive SARS-CoV-2 polymerase chain reaction (PCR) or antigen test from a nasopharyngeal or respiratory specimen, and/or 2) a positive COVID-19 flag in the EHR on or after the index date. In MGB, a COVID-19 flag indicates a confirmed diagnosis of SARS-CoV-2 infection and captures patients with a confirmed positive test outside of our healthcare system. We also included patients flagged as having COVID-19 based on a positive home rapid antigen assay reported to providers or clinics. In some cases, results from tests performed outside of MGB were automatically pulled into the electronic data warehouse because of a linkage across other healthcare systems that also use Epic as an EHR.
Covariates
Data regarding dates and presence of ICD codes, medication prescriptions, demographics, and comorbidities were extracted from the MGB EDW, as previously described (10, 13). The patient’s primary rheumatic disease diagnosis was based on ICD-10 codes. In some cases, patients had codes associated with multiple rheumatic diseases. In scenarios where one disease is often secondary to or associated with a primary condition (e.g., antiphospholipid syndrome in systemic lupus erythematosus), the patient was considered to have the primary disease (e.g., systemic lupus erythematosus). In cases where there was a discrepancy (e.g., giant cell arteritis and ANCA-associated vasculitis), the disease associated with the ICD code used most frequently was considered the primary diagnosis. Cases in which ICD codes that can coexist (e.g., seronegative spondyloarthopathy and giant cell arteritis) or can exist as overlap disease (e.g., systemic lupus erythematosus and rheumatoid arthritis) were categorized as “multiple” primary rheumatic diseases. Since many patients had ICD codes for both giant cell arteritis and polymyalgia rheumatica, we considered this as a single combined category. Medication data documented or prescribed in the EHR, was extracted as structured data within 12 months of the index date. Medications were categorized as being a conventional synthetic DMARD, biologic DMARD, targeted synthetic DMARD, or oral glucocorticoid. Baseline characteristics including demographics (including race/ethnicity as obtained from the EHR), comorbidities as defined by ICD-10 codes, smoking history, and body mass index (BMI) were extracted from the electronic data warehouse and assessed in the one year prior to the index date. We excluded 107 patients with missing BMI and 1 patient with missing smoking status. The Charlson Comorbidity Index (CCI) was calculated using all available data from comorbidities as ascertained by ICD-10 in the one year prior to the index date (14).
Statistical analyses
Baseline characteristics were compared between patients who received either BNT162b2 or mRNA-1273 and are reported as frequencies and percentages (n, %) or mean and standard deviation (mean, SD). We determined incidence rates (IRs) and 95% confidence intervals (CIs) of COVID-19 infections per 1,000 person-months.
To account for potential confounding introduced by variation in vaccine administration and availability and fluctuation of COVID-19 infection rates over time, we used time-stratified propensity score (PS) overlap weighting. Eligible individuals were allocated into bi-weekly time blocks according to their index dates. We restricted the study period from 12/27/2020 to 05/15/2021 because there were too few patients in time blocks before and after this period to perform the procedure. In each time block, we calculated a PS using logistic regression models and adopted an overlap weighting approach to balance baseline characteristics (15, 16). Potential confounders were selected based on prior literature and clinical expertise and included COVID-19 infection prior to index date, age, sex, race, ethnicity, BMI, smoking status, CCI, rheumatic disease, immunomodulatory medication categories (e.g., biologic DMARD, targeted synthetic DMARD, conventional synthetic DMARD, rituximab), and glucocorticoids (Table 1). Given the known significant effects of B cell depletion on humoral immune responses to mRNA vaccine, we specifically included rituximab exposure as a covariate (17, 18). Because of the size of the sample included, we were unable to incorporate other specific medications into the PS. For each person, we included all medication categories documented or prescribed in the 12 months preceding the index date in the PS to reflect the characteristic and severity of their disease. Patients receiving the BNT162b2 vaccine were weighted by the probability of not receiving the BNT162b2 vaccine (i.e., 1-PS) and those receiving the mRNA-1273 vaccine were weighted by the probability of receiving the BNT162b2 vaccine (i.e., PS). Overlap weights were bounded and smoothed to reduce the influence of individuals at the tails of the PS distribution without making any exclusions. To compare the distribution of covariates before and after weighting, we report standardized mean differences (SMDs).
Table 1.
Demographic and clinical characteristics of SARD patients who received either BNT162b2 or mRNA-1273 vaccine (N=9838)
| Before propensity-score overlap weighting | After propensity-score overlap weightinga | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristic | BNT162b2 (N=5516) | mRNA-1273 (N=4322) | Standardized difference | BNT162b2 (N=5516) | mRNA-1273 (N=4322) | Standardized difference |
|
| ||||||
| Covid prior to index date (%) | 4.5 | 3.8 | 0.0322 | 4.0 | 4.0 | <0.001 |
| Age, mean (SD), y | 61 (15) | 61 (15) | 0.0039 | 61 (9) | 61 (11) | <0.001 |
| Female (%) | 74.5 | 75.8 | 0.0283 | 75.2 | 75.2 | <0.001 |
| White (%) | 81.9 | 85.2 | 0.0877 | 84.5 | 84.5 | <0.001 |
| Hispanic or Latinx ethnicity (%) | 7.1 | 5.6 | 0.0406 | 5.7 | 5.7 | <0.001 |
| Body mass index, mean (SD), kg/m2 | 28 (7) | 29 (7) | 0.0541 | 28.6 (4.2) | 28.6 (4.7) | <0.001 |
| Ever smoker (%) | 43.4 | 42.7 | 0.0131 | 43.6 | 43.6 | <0.001 |
| CCI (mean, SD) | 2.6 (3) | 2.3 (2.7) | 0.0783 | 2.5 (1.8) | 2.5 (2.1) | <0.001 |
| Rheumatic disease diagnosis (%) | ||||||
| Rheumatoid arthritis | 52.4 | 54.8 | 0.0483 | 54.1 | 54.1 | <0.001 |
| Other inflammatory arthritis | 16.9 | 17.8 | 0.0225 | 17.0 | 17.0 | <0.001 |
| Systemic lupus erythematosus | 13.5 | 11.6 | 0.0573 | 12.2 | 12.2 | <0.001 |
| Vasculitis | 6.0 | 5.7 | 0.0159 | 5.9 | 5.9 | <0.001 |
| Other rheumatic disease | 5.0 | 4.6 | 0.0187 | 4.7 | 4.7 | <0.001 |
| Multiple rheumatic diseases | 6.2 | 5.7 | 0.0250 | 6.0 | 6.0 | <0.001 |
| Immunomodulatory medications (%) | ||||||
| Conventional synthetic DMARDs | 74.0 | 73.6 | 0.0098 | 74.3 | 74.3 | <0.001 |
| Biologic DMARDs | 42.7 | 43.3 | 0.0128 | 42.5 | 42.5 | <0.001 |
| Rituximab | 2.3 | 2.3 | 0.0035 | 2.1 | 2.1 | <0.001 |
| Targeted synthetic DMARD | 6.7 | 5.7 | 0.0419 | 5.8 | 5.8 | <0.001 |
| Oral glucocorticoid | 11.4 | 9.5 | 0.0621 | 10.0 | 10.0 | <0.001 |
All variables listed were included in the propensity score models
CCI: Charlson Comorbidity Index; DMARD: Disease-Modifying Anti-Rheumatic Drug
Conventional synthetic DMARD: azathioprine, methotrexate, leflunomide, mycophenolic acid, mycophenolate mofetil, sulfasalazine, hydroxychloroquine, chloroquine; Biologic DMARDS: ocrelizumab, abatacept, infliximab, etanercept, adalimumab, certolizumab, golimumab, anakinra, canakinumab, mepolizumab, benralizumab, tocilizumab, sarilumab, secukinumab, ixekizumab, ustekinumab, guselkumab, belimumab, eculizumab; Targeted synthetic DMARD:tofacitinib, baricitinib, upadicinib
We calculated the rate difference (RD) and 95% confidence intervals (CIs) per 1000 person-months and estimated hazard ratios (HRs) and 95% CIs using overlap PS–weighted Cox proportional hazard models of the time from index date to breakthrough infection or date of censoring. We accounted for the competing risk of death using the methods described by Fine and Gray and confirmed that the proportional hazards assumption was met using a Kaplan-Meier method with an inverse probability weighting method.
We performed three sensitivity analyses to assess the robustness of our findings. First, we censored follow-up on 12/15/21, the date that Omicron became the dominant variant in Massachusetts. Second, we censored follow-up at the time of a 3rd vaccine dose. Third, we censored follow-up at the time of the earliest of 3rd vaccine dose or 12/15/21. We also performed two negative control analyses to assess for the impact of residual confounding. First, we assessed the risk of COVID-19 within 10 days of the first vaccination when there should be no difference in the risk of breakthrough infection if confounding has been adequately addressed. Second, we compared death not attributed to COVID-19 in each vaccine group since vaccine type should not influence non-COVID related mortality. All P values were two-sided and P<0.05 was considered significant for all tests. All statistical analyses were performed with SAS software, version 9.4
RESULTS
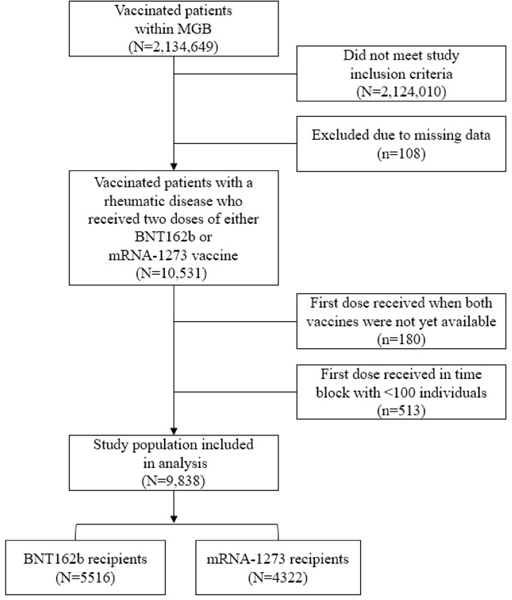
Among the 2.1 million vaccinated patients within MGB, we identified 5,516 who received BNT162b2 and 4,322 who received mRNA-1273 during the study period among patients with SARDs on immunomodulatory medications (Figure 1). Prior to PS weighting (Table 1), the two groups were overall similar. The mean [SD] age was 61 [15] years in each group (SMD 0.03). There were slight differences in the distribution of sex (75% vs. 76%, SMD 0.004), proportion White (82% v.s 85%, SMD 0.09) and Hispanic ethnicity (7% vs. 6%, SMD 0.04). There were also differences in disease specific features, including the CCI (mean 2.6 vs. 2.3, SMD 0.08), proportion with rheumatoid arthritis (52% vs. 55%, SMD 0.05), and proportion using certain immunomodulators, such as targeted synthetic DMARDs (5.7% vs. 6.7%, SMD 0.04) and glucocorticoids (11% vs. 10%, SMD 0.06). A slightly higher proportion of BNT162b2 recipients had a COVID-19 infection prior to vaccination than mRNA-1273 recipients (4.4% vs. 3.8%, SMD 0.03). After propensity score weighting, there were no longer significant differences in the distribution of demographic and disease-specific characteristics, including for DMARD categories, among those who received BNT162b2 vs. mRNA-1273 with all SMD < 0.001 (Table 1).
FIGURE 1.
IDENTIFICATION OF PATIENTS WITH A SYSTEMIC AUTOIMMUNE RHEUMATIC DISEASE WHO RECEIVED EITHER BNT162B2 OR MRNA-1273 VACCINES WITHIN THE MGB SYSTEM
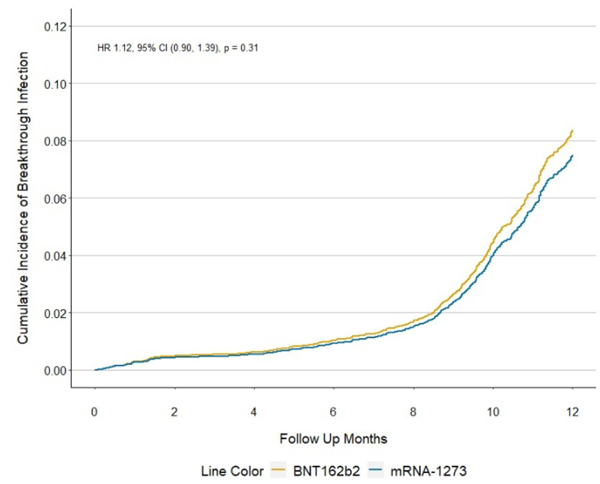
In our primary analysis, inclusive of a time period characterized by dominance of the Omicron variant in Massachusetts, there were 446 breakthrough infections among BNT162b2 recipients and 329 among mRNA-1273 recipients over a mean of 11 and 12 months of follow-up, respectively (Table 2). The weighted incidence of breakthrough infection was 6.83 vs 6.12 per 1,000 person-months in BNT162b2 vs. mRNA 1273 recipients, respectively. There was no statistically significant difference in the rate of breakthrough infection with BNT162b2 vs. mRNA1273 vaccination (weighted rate difference 0.71, 95% CI −0.70, 2.12 per 1,000 person-months) or the hazard ratio when comparing vaccine recipients (weighted HR 1.12, 95% CI 0.90–1.39, Figure 2).
Table 2.
Risk of COVID-19 breakthrough infection among SARD patients who received either BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccine
| Population | Number of COVID-19 breakthrough infection events | Mean follow up, months | Incidence ratea, per 1000 person-months | Rate differencea (95%CI), per 1000 person-months | Hazard ratioa (95%CI) BNT162b2 vs mRNA-1273 | |||
|---|---|---|---|---|---|---|---|---|
| BNT162b2 | mRNA-1273 | BNT162b2 | mRNA-1273 | BNT162b2 | mRNA-1273 | |||
|
| ||||||||
| N | 5516 | 4322 | 5516 | 4322 | 5516 | 4322 | ||
| Primary analysis | ||||||||
| Any infection | 446 | 329 | 11 | 12 | 6.83 | 6.12 | 0.71 (−0.70, 2.12) | 1.12 (0.90, 1.39) |
| Sensitivity analyses | ||||||||
| Pre-Omicron (12/15/2021) | 158 | 94 | 9 | 10 | 2.89 | 2.16 | 0.73 (−0.24, 1.71) | 1.34 (0.91, 1.98) |
| Censor at 3rd vaccine | 212 | 132 | 8 | 9 | 4.22 | 3.36 | 0.82 (−0.43, 2.07) | 1.31 (0.94, 1.83) |
| Pre-Omicron and censor at 3rd vaccine | 118 | 71 | 8 | 8 | 2.49 | 1.95 | 0.55 (−0.45, 1.55) | 1.33 (0.84, 2.09) |
| Negative control analyses | ||||||||
| Infection within 10 days of first vaccine | 6 | 5 | 12 | 12 | 0.06 | 0.09 | −0.03 (−0.18, 0.12) | 0.67 (0.09, 5.28) |
| Death due to non-COVID-19 causes | 97 | 60 | 11 | 12 | 1.28 | 1.27 | 0.01 (−0.61, 0.63) | 1.01 (0.62, 1.64) |
Time-stratified PS overlap weighted
Propensity score model: covid prior to index date, age, sex, race, ethnicity, BMI, smoking status, Charlson
Comorbidity Index, rheumatic disease diagnosis, immunomodulatory medication
FIGURE 2.
CUMULATIVE INCIDENCE OF COVID-19 BREAKTHROUGH INFECTIONS AMONG SARD PATIENTS ACCORDING TO VACCINE TYPE (MRNA-1273 VS BNT162B2) THROUGH FEBRUARY 22, 2022
We performed several sensitivity analyses and two negative control analyses to assess the robustness of our observations (Table 2). When we limited follow-up to the pre-Omicron era, there was a non-statistically significant trend toward higher risk of breakthrough infection associated with BNT162b2 vs. mRNA1273 (weighted HR 1.34, 95% CI 0.91, 1.98). Similar estimates were observed when we censored at the time of a 3rd mRNA vaccine dose and when we censored at either the time of a 3rd mRNA vaccine or the date when Omicron became the dominant strain in Massachusetts. There was no difference in the risk of breakthrough infection within 10 days of the index date when comparing BNT162b2 vs. mRNA-1273 vaccine recipients (weighted HR 0.67, 95%CI 0.09, 5.28). There was also no difference in recipients of BNT162b2 vs. mRNA-1273 in the risk of death from causes other than COVID-19 (weighted HR 1.01, 95%CI 0.62, 1.64).
DISCUSSION
Among patients with SARDs on immunomodulatory medications, there was no statistical difference in the risk of breakthrough infection after two doses of either BNT162b2 or mRNA1273. There was a trend in the pre-Omicron era towards mRNA1273 providing a greater protection against breakthrough infection, but this did not reach statistical significance. While ongoing efforts are made to further reduce the risk of breakthrough infection and severity among patients with SARDs, choice of mRNA vaccine type does not appear to be a strong factor driving this risk. Patients with SARDs initiating the vaccine series should be encouraged to receive whichever mRNA vaccine is available.
Previous studies have established that certain immunomodulator users have a blunted humoral immune response to SARS-CoV-2 vaccines, including the BNT162b2 and mRNA1273 vaccines and that this can increase the risk of breakthrough infection (6). A recent study found that among immunomodulator users, mRNA-1273 may yield a greater level of humoral immunity than BNT162b2. In the population managed in the Veterans Affairs system in the US, investigators found a small but statistically significant association between BNT162b2 vs. mRNA1273 with the risk of breakthrough infection (RR: 1.27 95%CI: 1.15, 1.42) over 24 weeks (8). This population was different than ours since it included patients who were, on average, 10 years older and the majority of whom were male. Given these differences and observations regarding the blunted immunogenicity of mRNA vaccines in immunomodulator users compared to the general population (3, 4), it has been unclear whether BNT162b2 or mRNA1273 provides greater protection against breakthrough infection. Our study expands upon prior findings by estimating similar effectiveness of both currently available mRNA vaccines on the risk of breakthrough infection. However, our study may have been too small to detect modest statistically significant differences between the vaccine types.
A study by Widdifield et al examined COVID-19 vaccine effectiveness in patients with rheumatoid arthritis, ankylosing spondylitis, psoriasis, and inflammatory bowel disease and found that mRNA-1273 tended to have greater effectiveness than BNT162b2 (3). Several important aspects distinguish our study. First, all patients in our analysis were immunomodulator users whereas these data were unavailable in the study by Widdifield et al, so the balance of this important confounder between groups is unknown. Second, our study used robust methods to account for the differences in the temporal availability and uptake of these vaccines which could impact associations. Third, our study extended follow up to include a time period characterized by Omicron variant dominance which is now known to be associated with greater immune evasiveness (19). Finally, theirs was focused on vaccine effectiveness (i.e., vaccination vs not vaccinated for risk of infection) while ours assessed the risk of breakthrough infection between vaccine types.
In mid-December 2021, the Omicron variant became the dominant strain in Massachusetts and mRNA vaccines are now known to provide less protection against this variant because of spike protein mutations. Because of the known immune evasiveness associated with the Omicron variant (19), we also examined outcomes limited to the pre-Omicron era and found that there was a trend towards mRNA1273 having potentially greater efficacy during time periods characterized by dominance of Alpha and Delta variants. Collectively, these results do raise the question of whether the enhanced immunogenicity observed in patients with mRNA-1273 vs. BNT162b2 provides greater protection for infection against variants more closely related to the original SARS-CoV-2 strain used to develop vaccines (9). Our findings did not achieve statistical significance but should be evaluated again in the future in the context of the anticipated use of variant-specific vaccines.
Our study has a number of notable strengths. First, we systematically identified patients with SARDs who used immunomodulators prior to vaccination as well as test-confirmed COVID-19 breakthrough infection using data from tests conducted in the healthcare setting and at home. Second, we used time-stratified PS overlap weighting to account for potential confounding introduced by variation in vaccine availability and fluctuation of COVID-19 infection rates over time as well as potential differences in demographic, lifestyle, and clinical characteristics. Third, we assessed the robustness of our findings in two negative control analyses to investigate any potential strong impact of unmeasured confounding.
Despite these strengths, our study has certain limitations. First, we identified patients who had been prescribed an immunomodulator prior to receiving an mRNA vaccine, however we did not have data on whether an individual was actually taking their prescription at the time of vaccination, the dose of steroid at the time of vaccination, or whether patients had temporarily held their treatments around the time of vaccination, as has been recommended (20). Regardless, we would not expect the patterns of holding medications to differ among those who received either mRNA vaccine. Second, the number of patients in potential subgroups of interest (e.g., specific immunomodulator use) was small, limiting our ability to compare mRNA vaccine effectiveness among users of immunomodulators associated with different degrees of blunted immune responses and to incorporate some of these specific variables into our propensity scores. Third, we did not have data regarding antibody or other immunologic responses to the vaccine. Fourth, we may not have detected all breakthrough infections, particularly those who tested positive on home rapid antigen tests, but never reported to their MGB physician. While this may underestimate the absolute rate of breakthrough infections, it is unlikely that this varies by vaccine type, so should not affect our results. Fifth, we did not have data regarding disease activity, however, would not expect this to vary by vaccine type. Further studies with registry data may be necessary to explore disease activity, vaccination, and breakthrough infections.
In conclusion, we found that the BNT162b2 and mRNA1274 vaccines have comparable effectiveness with regard to the risk of breakthrough infection among patients with SARDs using immunomodulators. There may be a benefit to mRNA1274 over BNT162b2, but this was substantially blunted in a time period characterized by Omicron dominance. The choice of mRNA vaccine type is unlikely to strongly influence the risk of breakthrough infection in this population and the decision regarding which mRNA vaccine to receive should be based on availability rather than potential difference in effectiveness.
Supplementary Material
Financial support:
NJP is supported by the Rheumatology Research Foundation. JAS is funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (grant numbers, R01 AR077607, P30 AR070253, and P30 AR072577), the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. ZSW is funded by NIH/NIAMS [K23AR073334 and R03AR078938].
NJP reports consulting fees from FVC Health unrelated to the current work. JAS reports research support from Bristol Myers Squibb and consultancy fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer. ZSW reports research support from Bristol-Myers Squibb and Principia/Sanofi and consulting fees from Zenas Biopharma, Horizon, Sanofi, Shionogi, Viela Bio, and MedPace.
Footnotes
Declaration of interests:
All other authors report no competing interests.
REFERENCES
- 1.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: Results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widdifield J, Kwong JC, Chen S, et al. Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes among individuals with immune-mediated inflammatory diseases tested between March 1 and Nov 22, 2021, in Ontario, Canada: a population-based analysis. Lancet Rheumatol. 2022;4:e430–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tenforde MW, Patel MM, Gaglani M, et al. Effectiveness of a third dose of pfizer-biontech and moderna vaccines in preventing COVID-19 hospitalization among immunocompetent and immunocompromised adults - United States, August-December 2021. MMWR Morb Mortal Wkly Rep. 2022;71:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook C, Patel NJ, D’Silva KM, et al. Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Ann Rheum Dis. 2022;81:289–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paik JJ, Sparks JA, Kim AHJ. Immunogenicity, breakthrough infection, and underlying disease flare after SARS-CoV-2 vaccination among individuals with systemic autoimmune rheumatic diseases. Curr Opin Pharmacol. 2022;65:102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liew J, Gianfrancesco M, Harrison C, et al. SARS-CoV-2 breakthrough infections among vaccinated individuals with rheumatic disease: results from the COVID-19 Global Rheumatology Alliance provider registry. RMD Open. 2022;8: e002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell J, Connolly CM, Chiang TP, et al. Comparison of SARS-CoV-2 antibody response after 2-dose mRNA-1273 vs BNT162b2 vaccines in incrementally immunosuppressed patients. JAMA Netw Open. 2022;5:e2211897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel NJ, Wang X, Fu X, et al. Factors associated with COVID-19 breakthrough infection among vaccinated patients with rheumatic diseases: A cohort study. Semin Arthritis Rheum. 2022;58:152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massachusetts Immunization Information System (MIIS). [Available from: https://www.mass.gov/massachusetts-immunization-information-system-miis].
- 12.Kim SY, Servi A, Polinski JM, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nalichowski R, Keogh D, Chueh HC, et al. Calculating the benefits of a Research Patient Data Repository. AMIA Annu Symp Proc. 2006;2006:1044. [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol. 2019;188:250–7. [DOI] [PubMed] [Google Scholar]
- 16.Thomas LE, Li F, Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. Jama. 2020;323:2417–8. [DOI] [PubMed] [Google Scholar]
- 17.Deepak P, Kim W, Paley MA, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174:1572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jyssum I, Kared H, Tran TT, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 2022;4:e177–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1–8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1731–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 4. Arthritis Rheumatol. 2022;74:e21–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.