Abstract
Background:
In trials, hospital walking programs have been shown to improve functional ability after discharge, but little evidence exists about their effectiveness under routine practice conditions.
Objective:
To evaluate the effect of implementation of a supervised walking program known as STRIDE (AssiSTed EaRly MobIlity for HospitalizeD VEterans) on discharge to a skilled-nursing facility (SNF), length of stay (LOS), and inpatient falls.
Design:
Stepped-wedge, cluster randomized trial. (ClinicalTrials.gov: NCT03300336)
Setting:
8 Veterans Affairs hospitals from 20 August 2017 to 19 August 2019.
Patients:
Analyses included hospitalizations involving patients aged 60 years or older who were community dwelling and admitted for 2 or more days to a participating medicine ward.
Intervention:
Hospitals were randomly assigned in 2 stratified blocks to a launch date for STRIDE. All hospitals received implementation support according to the Replicating Effective Programs framework.
Measurements:
The prespecified primary outcomes were discharge to a SNF and hospital LOS, and having 1 or more inpatient falls was exploratory. Generalized linear mixed models were fit to account for clustering of patients within hospitals and included patient-level covariates.
Results:
Patients in pre-STRIDE time periods (n = 6722) were similar to post-STRIDE time periods (n = 6141). The proportion of patients with any documented walk during a potentially eligible hospitalization ranged from 0.6% to 22.7% per hospital. The estimated rates of discharge to a SNF were 13% pre-STRIDE and 8% post-STRIDE. In adjusted models, odds of discharge to a SNF were lower among eligible patients hospitalized in post-STRIDE time periods (odds ratio [OR], 0.6 [95% CI, 0.5 to 0.8]) compared with pre-STRIDE. Findings were robust to sensitivity analyses. There were no differences in LOS (rate ratio, 1.0 [CI, 0.9 to 1.1]) or having an inpatient fall (OR, 0.8 [CI, 0.5 to 1.1]).
Limitation:
Direct program reach was low.
Conclusion:
Although the reach was limited and variable, hospitalizations occurring during the STRIDE hospital walking program implementation period had lower odds of discharge to a SNF, with no change in hospital LOS or inpatient falls.
Primary Funding Source:
U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (Optimizing Function and Independence QUERI).
Inactivity during hospitalization has been recognized as a key contributor to hospital-associated disability and other harms for decades (1). Low mobility has been linked to delirium, falls, longer lengths of stay (LOS), greater risk for readmission, and functional decline resulting in discharge to skilled-nursing facilities (SNFs) (2–4). Discharges from hospitals to postacute care facilities increased nearly 50% between 1996 and 2010 (5). Even when intended for short-term rehabilitation, admission to a SNF after hospitalization is a major risk factor for long-term institutionalization (6). Despite clear evidence of a negative effect of low mobility in the hospital on patients and costs to the health system, gaps remain in clinical practices promoting mobility in the hospital (7–9).
Walking programs have emerged as a strategy for reducing functional decline among hospitalized older adults. Building on observational studies suggesting benefits of inpatient mobility programs (10, 11), 3 recent randomized controlled trials (RCTs) demonstrated that daily ambulation can improve function and walking ability at discharge (12, 13) and prevent loss of community mobility 1 month after hospital discharge (14). In a single-center clinical demonstration program, we observed that participants in a supervised walking program called STRIDE (AssiSTed EaRly MobIlity for HospitalizeD VEterans) were less likely to be discharged to a SNF compared with similar older adults who did not participate (15). Currently published RCTs lack information on the effect of walking programs on discharge to postacute care facilities, a critically important outcome for patients, families, and health systems.
Although previous trials provided solid evidence of benefits to patients when mobility interventions were delivered by research teams, it is unclear how robust effects would be under usual practice conditions. There is a lack of information on the effect of walking programs when hospitals are not provided with additional resources, but rather must rely on existing staff for clinical delivery. For hospitals to successfully launch new clinical programs, especially those that require coordination among many providers and changes in workflow, active implementation support is often required, but there is a knowledge gap on how best to provide this. Within Veterans Affairs (VA), many hospitals expressed interest in starting STRIDE walking programs, which presented an opportunity to generate evidence on the program’s effect and to study a strategy for supporting hospitals in implementing their new program.
We conducted a stepped-wedge, cluster randomized controlled trial (SW-CRT) in 8 hospitals. An SW-CRT was selected to facilitate hospital recruitment and enhance the acceptability of a randomized evaluation; it is the most efficient design to address our goal of examining both effectiveness and implementation (16, 17). We hypothesized that patients hospitalized after implementation of a STRIDE walking program would be less likely to be discharged to a SNF and have shorter LOS compared with similar hospitalizations that occurred before STRIDE implementation. We examined inpatient falls as an exploratory outcome. A secondary goal was to evaluate implementation with support guided by the Replicating Effective Programs (REP) framework, as assessed by program reach and fidelity.
Methods
Design Overview
This SW-CRT was conducted at 8 VA hospitals between 20 August 2017 and 19 August 2019. An overview of the study rationale and evaluation plan has been published previously (17). Reporting was guided by the Consolidated Standards of Reporting Trials (specific to SW-CRT).
Setting and Participants
Eligible hospitals had a minimum average daily census of 20 general medicine patients per day and agreed to start a STRIDE program using their own clinical personnel. Details about hospital recruitment strategies and characteristics of participating hospitals have been published elsewhere (17, 18).
STRIDE was considered a usual part of clinical care at participating hospitals so patient referrals to the program were at the discretion of the treating teams. No patient-level consent was required to be included in study analyses. We evaluated hospitalizations involving patients who were aged 60 years or older and admitted for 2 or more business days to a participating medical ward without a bedrest order. Because one of our prespecified primary outcomes was discharge destination, we focused on patients who were community dwelling before hospitalization and excluded hospitalizations involving transfers with other acute care facilities or patient deaths. A small number of patients who resided in U.S. territories were excluded due to incomplete data.
Randomization, Intervention, and Implementation Framework
Hospitals were randomly assigned to a 3-month window in which to launch their STRIDE program. Enrollment and randomization occurred in 2 distinct blocks of 4, with 2 hospitals randomly assigned to each sequence (Supplement Figure 1, available at Annals.org). STRIDE is a supervised walking program for older adult inpatients that includes a 1-time gait and balance assessment followed by daily supervised walks for the duration of the hospital stay. Clinicians were not blinded to whether patients received the STRIDE intervention.
Based on a preliminary assessment of barriers to STRIDE implementation and input from clinical and operational VA partners, REP was used as the overarching implementation framework (19). REP provides a structure for specifying core elements of a program to be disseminated and for operationalizing elements that can be adapted to local settings. Core elements of STRIDE were defined as 1) being proactive, with no baseline functional deficits required; 2) enrolling early, ideally within 24 hours of admission; 3) providing supervised walking, up to 20 minutes daily until discharge; and 4) having dedicated staff assigned to conduct walks (20). All sites were offered a series of 6 implementation support calls and 1 in-person site visit, according to a prespecified schedule, in the 3- to 4-month period before program launch. After program launch, sites participated in 5 additional scheduled calls with implementation specialists to troubleshoot barriers to implementation, review data about STRIDE activity, and plan for sustainability. Hospitals could access additional technical assistance over e-mail, and each hospital participated in a final call to “graduate” the program, transition data monitoring, and celebrate accomplishments.
Measures
Data Source
We defined measures using several data sources including the VA’s Corporate Data Warehouse, a repository of VA electronic health record (EHR) and VA-purchased care data (21). We also examined data from health care claims and the Minimum Data Set from the Centers for Medicare & Medicaid Services (CMS), obtained via data use agreement with the VA Information Resource Center.
Outcome Measures
Prespecified primary outcomes were 1) discharge to a SNF as assessed using VA care (delivered and purchased) and CMS data files and 2) LOS measured in days. Inpatient falls (exploratory outcome) were extracted using a combination of structured text fields known as health factors and International Classification of Diseases, 10th Revision (ICD-10) codes. To ensure we only measured falls that occurred during the hospital admission, we excluded falls identified via ICD-10 code with a present on admission indicator and conducted chart reviews.
Measures of Program Reach
Data on STRIDE walks were extracted from health factors that were generated from STRIDE-specific templated notes in the EHR. Walks that occurred with physical therapy (PT) or other hospital personnel were not counted in the definition of STRIDE walks. To evaluate program reach, we examined the percentage of patients with any documented STRIDE walk during an eligible hospitalization. Among hospitalizations with any documented STRIDE walk, we examined fidelity by determining the percentage of eligible hospital days that the patient received a “full dose” of the program, defined as 2 or more documented walks, or 1 walk for more than 5 minutes. Eligible hospital days began on the date of the STRIDE gait assessment or first walk and continued until discharge. We also summarized time and distance documented in the EHR for each STRIDE walk.
Demographic and Clinical Measures
Patient-level demographic and clinical characteristics were extracted from the EHR and selected based on their relevance to outcomes and recovery after hospitalization. The JEN Frailty Index (JFI), a risk score (0 to 13) designed to predict long-term institutionalization (22), was used as a measure of functional status and calculated from VA and CMS diagnosis codes in the year before admission. To measure chronic disease burden, we used the concurrent Nosos score from the fiscal year of admission. Nosos scores, centered around a value of 1, incorporate diagnostic and demographic information and were designed for risk adjustment and to predict costs (23).
Ethics Approval
Activities to support STRIDE implementation were considered nonresearch operations activities as defined in Veterans Health Administration (VHA) Handbook 1058.05. Individual-level outcome measurement was approved as research by the Institutional Review Board of the Durham VA. The study was registered at ClinicalTrials.gov (NCT03300336).
Statistical Analysis
We followed a cross-sectional incomplete stepped-wedge design, including outcomes from a patient’s first eligible hospitalization in pre- or post-STRIDE time periods only (Supplement Figure 1). Sample size was based on patient-level analyses evaluating the effect of STRIDE on the binary outcome discharged to a SNF versus discharged to home. Estimates were derived empirically via simulation using SAS 9.4, assuming a baseline discharge rate to a SNF of 20% and intracluster correlations ranging from 0.02 to 0.06 for patients within the same hospital. After generating 1000 simulated data sets under the stepped-wedge design shown in Supplement Figure 1, we fit generalized linear mixed models with a logit link and assessed the effect of interest using 2-sided tests with a type I error rate of 0.05. A total sample of 2800 patients (50 per hospital per time period; 350 per hospital) resulted in at least 80% power with an α of 0.05 to detect a 10% decrease in discharges to SNFs. Additional details are provided in the Supplement (available at Annals.org).
Analyses were performed using SAS 9.4. For our outcomes of discharge to a SNF (vs. discharge to home), LOS (count data), and inpatient falls (≥1 falls vs. 0), we fit generalized linear mixed models using PROC GLIMMIX (SAS) to account for the clustering of patients within hospitals. For discharge to a SNF and 1 or more inpatient falls, a binomial distribution with a logit link was used; for LOS, a negative binomial distribution with a log link was used. We examined a range of models from the standard stepped-wedge model, which assumes that correlation between observations in a cluster is the same regardless of treatment and duration between time periods (24), to models that allow variation in the secular trend over time (25) as well as treatment-effect heterogeneity across clusters (26, 27). All models included fixed effects for treatment and time; a time-varying treatment indicator variable of 0 for pre-STRIDE time periods and 1 for post-STRIDE with dummy-coded indicators was used to represent the individual 8 time periods. Akaike information criteria was used to select the best fit model (28). In the final selected models, we included patient-level covariates for sociodemographics, baseline health conditions, and characteristics of the eligible hospitalizations. More detailed analyses are described in the Supplement.
Role of the Funding Source
The funder, VA Quality Enhancement Research Initiative, did not determine the study design, conduct, or reporting.
Results
Hospital and Patient Characteristics
Participating hospitals were in the Northeast, Midwest, and Southern regions of the United States with rurality of the patient population ranging from 8% to 80%. Daily internal medicine census ranged from 32 to 120 patients. Hospitalizations meeting eligibility criteria during the study period in each of 8 participating hospitals are presented in Figure 1. During pre- or post-STRIDE time periods, 13 217 patients had at least 1 eligible hospitalization, including 14.7% (n = 1938) with 2, and 6.1% (n = 809) with 3 or more. Characteristics for all eligible pre- and post-STRIDE hospitalizations (n = 17 237; n = 8167 in pre-STRIDE, n = 9070 in post-STRIDE) are presented in Supplement Table 1 (available at Annals.org).
Figure 1. Study flow diagram.
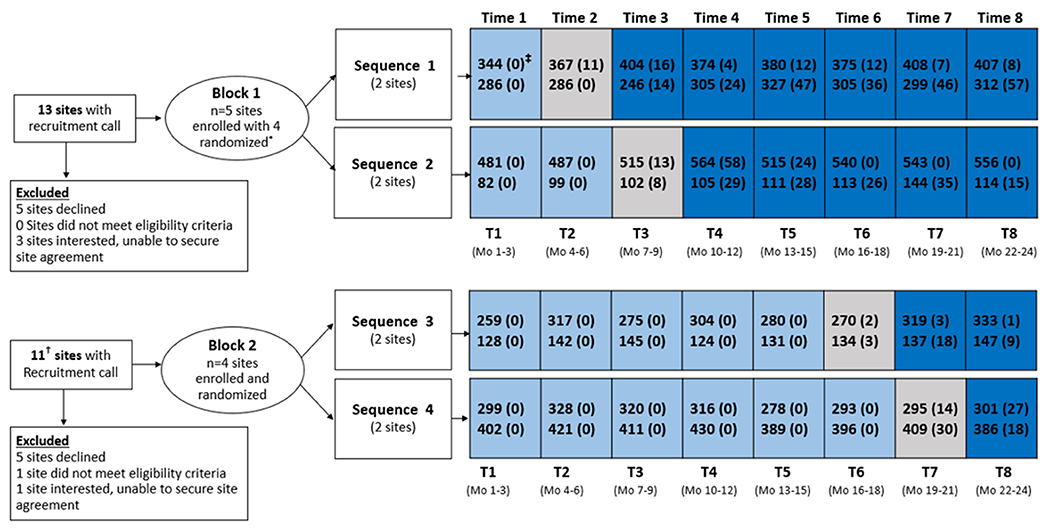
Light blue indicates pre-STRIDE periods, gray indicates implementation period (STRIDE launch), dark blue indicates post-STRIDE periods.
* One of 4 randomized sites decided not to participate 2 months after randomization, citing inadequate staff capacity, and was replaced with an additional site that was able to follow the stepped wedge sequence
† Two of these sites also had a recruitment call in Block 1 (declined both times)
‡ Numbers within time interval represent the number of eligible hospitalizations; number of eligible hospitalizations with at least one STRIDE walk is presented in parentheses
Table 1 presents the patient characteristics for the 6722 and the 6141 patients (n = 12 863 unique patients) in our main analysis who had their first hospitalization occur in the pre- and post-STRIDE phase of the study, respectively. Patients who had their first hospitalization in the implementation period but had subsequent eligible hospitalizations (n = 354) are not represented in Table 1 or primary analyses. Both patient groups (pre- and post-STRIDE) shared similar baseline sociodemographic and health characteristics. There were observed differences in the percentages of patients from a rural area and to a lesser degree those receiving PT during their hospital stay.
Table 1.
Patient Characteristics of Those With First Hospitalization During Pre- and Post-STRIDE Implementation Time Periods
| Characteristic | Pre-STRIDE (n = 6722) |
Post-STRIDE (n = 6141) |
|---|---|---|
| Baseline sociodemographic and health | ||
|
| ||
| Mean age (SD), y | 72.9 (8.9) | 72.9 (8.7) |
|
| ||
| Male, n (%) | 6532 (97.2) | 5908 (96.2) |
|
| ||
| Black race,* n (%) | 1958 (29.6) | 1673 (28.0) |
|
| ||
| Hispanic/Latino ethnicity,* n (%) | 343 (5.2) | 111 (1.8) |
|
| ||
| Social vulnerability,† n (%) | 1304 (19.4) | 1081 (17.6) |
|
| ||
| Rural residence,* n (%) | 860 (12.8) | 1314 (21.4) |
|
| ||
| Mean functional status, JEN Frailty Index*‡ (SD) | 6.6 (1.9) | 6.3 (1.9) |
|
| ||
| Mean chronic disease burden, Nosos score* (SD) | 6.6 (4.6) | 6.3 (4.4) |
|
| ||
| Depression,† n (%) | 2831 (42.1) | 2506 (40.8) |
|
| ||
| Dementia,† n (%) | 1234 (18.4) | 1050 (17.1) |
| Hospitalization characteristics | ||
|
| ||
| Mean nutritional status, albumin (SD)*§ | 3.3 (0.6) | 3.3 (0.6) |
|
| ||
| Hospital diagnoses, n (%) | ||
| Chronic heart failure | 2028 (30.2) | 1954 (31.8) |
|
| ||
| Stroke | 464 (6.9) | 408 (6.6) |
|
| ||
| Diabetes | 3050 (45.4) | 2685 (43.7) |
|
| ||
| Cancer | 1302 (19.4) | 1151 (18.7) |
|
| ||
| Delirium on admission, n (%) | 540 (8.0) | 508 (8.3) |
|
| ||
| Bedrest order, n (%) | 230 (3.4) | 315 (5.1) |
|
| ||
| Order for benzodiazepines, n (%) | 667 (9.9) | 621 (10.1) |
|
| ||
| Physical therapy, n (%) | 3243 (48.2) | 3306 (53.8) |
STRIDE = AssiSTed EaRly MobIlity for HospitalizeD VEterans.
Missing data. Observations removed from denominator in percentage calculations. (Number missing in pre-STRIDE, number missing in post-STRIDE): Black race (118, 170); Hispanic/Latino ethnicity (89, 120); rural residence (1, 2); JEN Frailty Index (1, 4); Nosos (0, 1); nutritional status, albumin (129, 128).
Assessed in the 2 years before hospital discharge.
Score (possible range, 0-13) calculated from diagnosis codes in Veterans Affairs and Centers for Medicare & Medicaid Services data files in the year before hospitalization.
Result from albumin test closest to admission date during hospitalization. If no test result during the hospitalization was available, the closest albumin test result to the admission date in the 365 days prior was used.
Program Implementation, Participant, and Walk Characteristics
Numbers of eligible hospitalizations with at least 1 documented STRIDE walk in each of 8 participating hospitals are presented in Figure 1. In post-STRIDE, 6.3% of all eligible hospitalizations (n = 574 of 9070) had documentation of direct program reach (≥1 STRIDE walk). Program reach across the 8 hospitals ranged from 0.6% to 22.7% with median reach of 6.8%. Characteristics of patients receiving at least 1 walk in post-STRIDE time periods, compared with those who did not, are provided in Supplement Table 2 (available at Annals.org).
Table 2 characterizes STRIDE walk activity during hospitalizations of patients who were eligible, walked at least once, and agreed to further participation (n = 555; n = 19 were ineligible or declined STRIDE). STRIDE activity occurred on 48.3% of 2841 eligible hospital days. On 29.7% of these hospital days, the fidelity benchmark reflecting a “full dose” of STRIDE was achieved, that is, 2 or more walks per day or a single walk of more than 5 minutes in duration. Program fidelity across the 8 hospitals ranged from 0% to 47.7% with median fidelity of 24.8%.
Table 2.
Walk Characteristics for 555* Hospitalizations With STRIDE Activity Used in Fidelity Assessment
| Walk Characteristics | Value, % |
|---|---|
| Individual STRIDE walks (n = 1788) | |
| Distance | |
|
| |
| 1–250 ft | 40.6 |
|
| |
| 251–500 ft | 29.3 |
|
| |
| 501–750 ft | 10.8 |
|
| |
| 751–1000 ft | 8.1 |
|
| |
| ≥1001 ft | 11.2 |
|
| |
| Duration | |
| 1–5 min | 54.2 |
|
| |
| 6–10 min | 31.8 |
|
| |
| 11–15 min | 8.7 |
|
| |
| ≥16 min | 5.3 |
|
| |
| STRIDE activity on eligible hospital days (n = 2841) | |
| 2 walks or 1 walk >5 min in duration (met fidelity benchmark) | 29.7 |
|
| |
| 1 walk ≤5 min in duration (did not meet fidelity benchmark) | 18.6 |
|
| |
| No walk | 51.7 |
STRIDE = AssiSTed EaRly MobIlity for HospitalizeD VEterans.
19 hospitalizations excluded from fidelity assessment due to STRIDE gait assessment designation as ineligible or declined further STRIDE participation.
Hospitalization Outcomes
For the prespecified coprimary outcome discharge to a SNF, the standard Hussey and Hughes (24) model, including a random intercept for hospital, was the best fit model (correlation within hospital was constant over time). The estimated intracluster correlation was 0.08 and estimated rates of discharge to SNFs were 13% pre-STRIDE and 8% post-STRIDE (Table 3). In the final model, odds of discharge to a SNF were lower among eligible patients hospitalized in post-STRIDE time periods (odds ratio, 0.6 [95% CI, 0.5 to 0.8]) compared with pre-STRIDE (Table 3 and Figure 2). Results were similar in adjusted and unadjusted models (Supplement Tables 3 and 4 and Supplement Figure 2, available at Annals.org). Results from sensitivity analysis including all eligible hospitalizations were similar (Supplement Tables 5, 6a, and 6b, available at Annals.org). There was no difference in the second coprimary outcome, LOS, among eligible patients hospitalized post-STRIDE (rate ratio, 1.0 [CI, 0.9 to 1.1]) compared with pre-STRIDE. Odds of 1 or more inpatient falls (exploratory outcome) were similar among eligible patients hospitalized post-STRIDE (odds ratio, 0.8 [CI, 0.5 to 1.1]) compared with pre-STRIDE.
Table 3.
Effect of Implementation of the STRIDE Program on Discharge to a SNF, LOS, and Inpatient Falls After Adjustment*†
| Outcome | Pre-STRIDE Estimated Mean (95% CI) |
Post-STRIDE Estimated Mean (95% CI) |
Post- vs. Pre-STRIDE | |
|---|---|---|---|---|
| Estimated Difference (95% CI) | P Value | |||
| Discharge to a SNF | 0.13 (0.09–0.19) | 0.08 (0.06–0.13) | OR = 0.6 (0.5–0.8) | <0.001 |
| Length of stay, d | 6.6 (6.2–7.1) | 6.7 (6.3–7.1) | RR = 1.0 (0.9–1.1) | 0.78 |
| Inpatient fall, yes/no | 0.015 (0.008–0.029) | 0.013 (0.006–0.024) | OR = 0.8 (0.5–1.1) | 0.52 |
LOS = length of stay; OR = odds ratio; RR = rate ratio; SNF = skilled-nursing facility; STRIDE = AssiSTed EaRly MobIlity for HospitalizeD VEterans.
Models fit using PROC GLIMMIX include the first hospitalization for a patient that occurred in pre- and post-STRIDE time periods with n = 674 cases deleted due to missing covariates (n = 12 189). Covariates include: age at admission, sex, race, Hispanic/Latino ethnicity, social vulnerability, rural residence, functional status (JEN Frailty Index), chronic disease burden concurrent score (Nosos), depression diagnosis, dementia diagnosis, nutritional status (albumin), chronic heart failure, stroke, diabetes, cancer, delirium on admission, bedrest order during hospitalization, order for benzodiazepines during hospitalization, and physical therapy during stay.
Excluded from models: n = 257 missing albumin (129 pre-STRIDE; 128 post-STRIDE); n = 288 missing race (118; 170); n = 209 missing Hispanic/Latino ethnicity (89; 120); rural residence (1; 2); JEN Frailty Index (1; 4); Nosos (0; 1).
Figure 2.
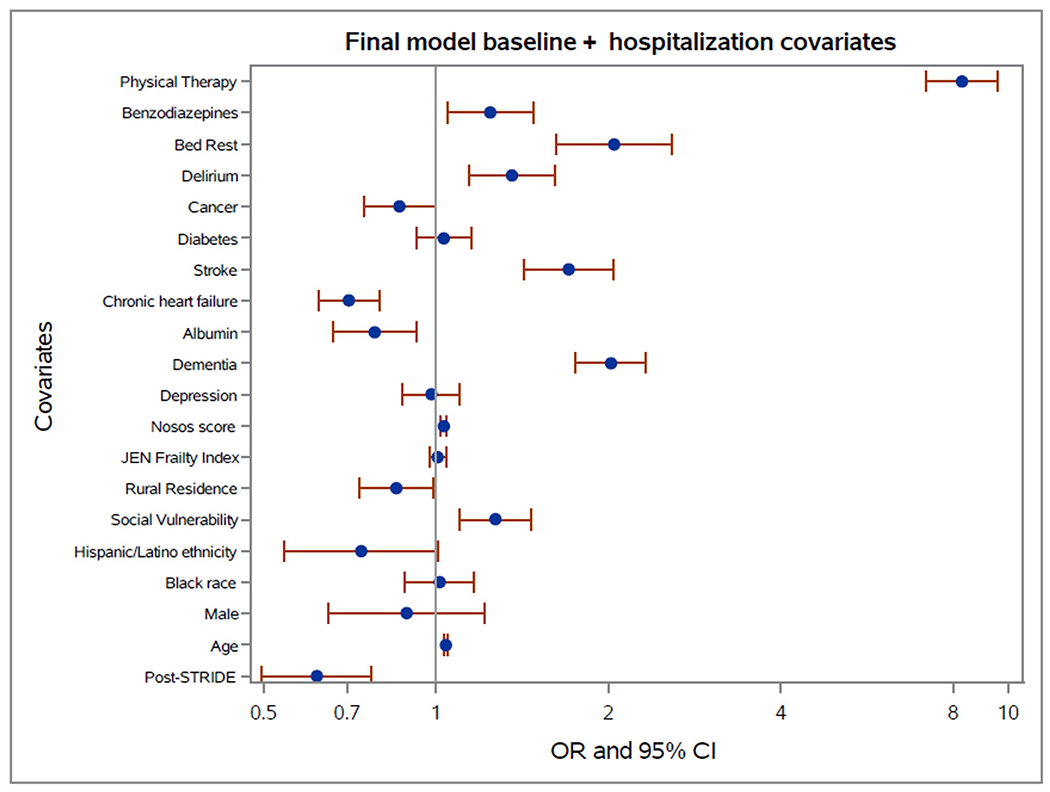
Plots of Odds Ratios and associated 95% Confidence Intervals for covariates from generalized linear mixed model fit using PROC GLIMMIX to discharge to SNF outcome for first hospitalization including all covariates (primary model)
Discussion
Implementation of the STRIDE hospital walking program under real-world settings in 8 VA hospitals was low and variable with participation of potentially eligible patients ranging from 0.6% to 22.7% and 2 hospitals pausing or discontinuing the program after it was launched. Despite this low reach, hospitalizations during the randomized period when STRIDE was implemented had a lower likelihood of discharge to a SNF among hospitalized, community-dwelling older adults. There was no observed effect on LOS or our exploratory outcome of inpatient falls. Participating hospitals received structured guidance to help plan and launch their programs but were responsible for identifying and training their clinical personnel to assess patients and conduct walks.
Use of institutional postacute care has grown in the United States, despite uncertain benefits for patients (25). Initiatives to reduce facility-based care after hospitalization align with patient-reported preferences to receive care in the least-restrictive setting. Our findings provide support for the emphasis placed on mobility as 1 of 4 “M”s that require attention to improve the quality of health care delivered to older adults under Age-Friendly Health Systems (26). Importantly, we found that the intervention resulted in fewer discharges to SNFs and did not come at a cost of increased inpatient falls or prolonged LOS. Although we did not observe a reduction in patients experiencing a fall or shorter LOS, our results are consistent with others in providing important reassurance for both patients and health systems about the safety of mobility programs (9, 14, 15, 27). An assessment of the overall importance of an estimated 5% absolute difference in rate of discharge to a SNF is dependent on patient, family, and health system values, preferences, and cost considerations.
In light of low direct program reach, potential mechanisms and alternative explanations for our findings must be considered. Given limited resources, staff may have prioritized patients for whom they perceived STRIDE might have led to the greatest benefit. Patients who interacted with STRIDE walked with the program at least once on about half of eligible hospital days, which is similar to the amount of walking that resulted in functional benefits in a previous trial (14). Another study has shown that implementation of a structured mobility program led to an increase in the amount patients walked on their own (28). However, STRIDE only affected a very small fraction of eligible hospitalized patients suggesting that the positive physiologic effects of walking, such as enhanced lower extremity muscle strength and aerobic fitness, are unlikely to be the primary mechanism. Beyond physical effects, it is possible that STRIDE influenced patient and/or clinician’s decision making and confidence in discharging patients to home with home health support rather than being admitted to an institution for rehabilitation. For patients not directly receiving STRIDE walks, it is possible that program implementation indirectly affected them by influencing hospital culture around mobility. Previously published findings from our interviews with site staff and leadership suggest that implementing a STRIDE program was viewed as a platform to address staff biases and procedures that were hindering patient mobility and to “jumpstart” positive changes in mobility practices more broadly (20).
Given our study design, our findings could be affected by confounders. Overall, we found that our inference for the effectiveness of STRIDE was similar in adjusted and unadjusted models. Receipt of PT in the hospital warrants special consideration because it is strongly associated with discharge to a SNF, and there is a possibility that STRIDE may have increased exposure to PT. However, our study implementation specialists maintained close relationships with participating hospitals and reported that STRIDE was more commonly considered for patients without an indication for PT. We found a somewhat stronger odds ratio when including a covariate for PT during hospitalization suggesting that PT was unlikely to have accounted for the differences in discharge to a SNF. Our models accounted for the clustering nature of the stepped-wedge design and the confounding effect of time; however, as with all stepped-wedge trials, we cannot rule out the possibility of an unrecognized underlying temporal trend that contributed to our findings.
A key element for successfully executing the SW-CRT design was for all hospitals to adhere to their assigned time period for STRIDE launch according to the randomization schedule (17). This was accomplished through implementation specialists working directly with points of contact at each hospital to provide technical assistance and interactive problem solving, guided by the REP framework. All hospitals launched within their assigned implementation period (17) and 6 of 8 hospitals offered STRIDE continuously through the postimplementation period. Sites reported that receiving guidance in how to engage leadership and other stakeholders in making collaborative decisions about STRIDE staffing models and receiving logistic support, such as training and documentation templates, were particularly valuable (20). In our study, hospitals were provided with evidence-informed implementation support and yet uptake of STRIDE was low overall, and variable across hospitals. These findings point to the urgency of additional research to advance our understanding of how implementation of hospital mobility programs like STRIDE affect delivery of other types of care and how to deliver the right “dose” of tailored support to optimize implementation and sustainment of evidence-based clinical programs (29).
Our study has limitations. We had a small number of clusters in the SW-CRT. Almost all hospitalizations involved male patients, consistent with the older adult population served by VA hospitals. Clinicians treating hospitalized patients at enrolled sites were not blinded; however, they were not informed of study eligibility criteria nor of study outcomes being assessed. We were only able to measure STRIDE walks that were documented using STRIDE-specific templated EHR notes, which likely underestimated program reach. Patient walks that were not documented or that occurred on their own or with other health care providers were not captured. For identifying potentially eligible hospitalizations, we lacked clinical information such as patients’ baseline functional status. Therefore, we did not anticipate 100% reach; patients with too low or high functional status may not benefit from a supervised walking program. We also observed STRIDE being used in hospitalizations that were not part of our evaluation (involving younger patients and those admitted to surgical services, for example). We used data sources from VA and CMS to construct our primary outcome of discharge destination, but it is possible that we could have missed some facility discharges.
Despite limited direct program reach, hospitalizations occurring during the implementation period of the STRIDE program had lower odds of discharge to a SNF but did not affect LOS. Health systems should consider hospital walking programs as a reasonable means to improve quality of care for older adults. Further development of strategies to support hospitals in implementation of new clinical programs are needed to enhance their effect.
Supplementary Material
Acknowledgment:
The Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT) [CIN 13-410] at the Durham VA Health Care System provided expert advice and consultation as well as support for some technical resources for this study. Support for VA/CMS data was provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). This work is dedicated to the memory of Elizabeth P. Mahanna, MPH.
Financial Support:
By the U.S. Department of Veterans Affairs Quality Enhancement Research Initiative (Optimizing Function and Independence QUERI, QUE-16-170).
Footnotes
Presented in part at the Gerontological Society of America Annual Scientific Meeting, Indianapolis, Indiana, 6 November 2022.
Note: Dr. Hastings and Dr. Coffman had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study was approved as exempt research by the Institutional Review Board of the Durham VA Health Care System (#2040).
Disclaimer: The views expressed in this article of those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. government.
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-3679.
Data Sharing Statement:
The following data will be made available with publication: Deidentified participant data. Access to data will be granted consistent with VA policy; data may be made available via inquiry to Susan N. Hastings, MD, MHSc (susan.hastings@va.gov). The following supporting documents will be made available: the protocol, with publication (see the Study Protocol). The protocol is also available for requests consistent with VA policy, and may be made available via inquiry to Susan Hastings, MD (susan.hastings@va.gov). The protocol may be redacted to remove information from ongoing studies that are not yet published. The statistical code is available in the Supplement. These data will be made available to: Anyone requesting the data in a manner consistent with VA policy. Types of analysis: For any purpose consistent with VA policy. Mechanisms: Via agreement consistent with VA policy. Restrictions: Any sharing of data is subject to VA policy.
References
- 1.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306:1782–1793. doi: 10.1001/jama.2011.1556 [DOI] [PubMed] [Google Scholar]
- 2.Fisher SR, Graham JE, Ottenbacher KJ, et al. Inpatient walking activity to predict readmission in older adults. Arch Phys Med Rehabil. 2016;97:S226–S231. doi: 10.1016/j.apmr.2015.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong IC, Healy R, Bao B, et al. Assessment of patient ambulation profiles to predict hospital readmission, discharge location, and length of stay in a cardiac surgery progressive care unit. JAMA Netw Open. 2020;3:e201074. doi: 10.1001/jamanetworkopen.2020.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavon JM, Sloane RJ, Pieper CF, et al. Accelerometer-measured hospital physical activity and hospital-acquired disability in older adults. J Am Geriatr Soc. 2020;68:261–265. doi: 10.1111/jgs.16231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke RE, Juarez-Colunga E, Levy C, et al. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175:295–296. doi: 10.1001/jamainternmed.2014.6383 [DOI] [PubMed] [Google Scholar]
- 6.Goodwin JS, Howrey B, Zhang DD, et al. Risk of continued institutionalization after hospitalization in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1321–1327. doi: 10.1093/gerona/glr171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greysen SR, Patel MS. Web exclusive. Annals for Hospitalists inpatient notes - bedrest is toxic-why mobility matters in the hospital. Ann Intern Med. 2018;169:HO2–HO3. doi: 10.7326/M18-1427 [DOI] [PubMed] [Google Scholar]
- 8.Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med. 2017;177:759–760. doi: 10.1001/jamainternmed.2017.0840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavon JM, Fish LJ, Colón-Emeric CS, et al. Towards “mobility is medicine”: socioecological factors and hospital mobility in older adults. J Am Geriatr Soc. 2021;69:1846–1855. doi: 10.1111/jgs.17109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalisch BJ, Lee S, Dabney BW. Outcomes of inpatient mobilization: a literature review. J Clin Nurs. 2014;23:1486–1501. doi: 10.1111/jocn.12315 [DOI] [PubMed] [Google Scholar]
- 11.Zisberg A, Shadmi E, Sinoff G, et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273. doi: 10.1111/j.1532-5415.2010.03276.x [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: a randomized clinical trial. JAMA Intern Med. 2019;179:28–36. doi: 10.1001/jamainternmed.2018.4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazineo D, Godino L, Decaro R, et al. Assisted walking program on walking ability in in-hospital geriatric patients: a randomized trial. J Am Geriatr Soc. 2021;69:637–643. doi: 10.1111/jgs.16922 [DOI] [PubMed] [Google Scholar]
- 14.Brown CJ, Foley KT, Lowman JD Jr, et al. Comparison of post-hospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial. JAMA Intern Med. 2016;176:921–927. doi: 10.1001/jamainternmed.2016.1870 [DOI] [PubMed] [Google Scholar]
- 15.Hastings SN, Sloane R, Morey MC, et al. Assisted early mobility for hospitalized older veterans: preliminary data from the STRIDE program. J Am Geriatr Soc. 2014;62:2180–2184. doi: 10.1111/jgs.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemming K, Taljaard M. Reflection on modern methods: when is a stepped-wedge cluster randomized trial a good study design choice? Int J Epidemiol. 2020;49:1043–1052. doi: 10.1093/ije/dyaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastings SN, Stechuchak KM, Choate A, et al. Implementation of a stepped wedge cluster randomized trial to evaluate a hospital mobility program. Trials. 2020;21:863. doi: 10.1186/s13063-020-04764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang V, Allen K, Van Houtven CH, et al. Supporting teams to optimize function and independence in veterans: a multi-study program and mixed methods protocol. Implement Sci. 2018;13:58. doi: 10.1186/s13012-018-0748-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilbourne AM, Neumann MS, Pincus HA, et al. Implementing evidence-based interventions in health care: application of the replicating effective programs framework. Implement Sci. 2007;2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastings SN, Choate AL, Mahanna EP, et al. Early mobility in the hospital: lessons learned from the STRIDE program. Geriatrics (Basel). 2018;3. doi: 10.3390/geriatrics3040061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VA Informatics and Computing Infrastructure (VINCI). VA HSR RES 13-457. U.S. Department of Veterans Affairs; 2008. [Google Scholar]
- 22.Kinosian B, Wieland D, Gu X, et al. Validation of the JEN frailty index in the National Long-Term Care Survey community population: identifying functionally impaired older adults from claims data. BMC Health Serv Res. 2018;18:908. doi: 10.1186/s12913-018-3689-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner T, Stefos T, Moran E, et al. Risk Adjustment: Guide to the V21 and Nosos Risk Score Programs. Technical Report 30. Health Economics Resource Center, U.S. Department of Veterans Affairs. February 2016. Accessed at www.herc.research.va.gov/include/page.asp?id=technical-report-risk-adjustment in July 2022. [Google Scholar]
- 24.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28:182–191. [DOI] [PubMed] [Google Scholar]
- 25.Werner RM, Konetzka RT. Trends in post-acute care use among Medicare beneficiaries: 2000 to 2015. JAMA. 2018;319:1616–1617. doi: 10.1001/jama.2018.2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke RE, Ashcraft LE, Manges K, et al. What matters when it comes to measuring Age-Friendly Health System transformation. J Am Geriatr Soc. 2022;70:2775–2785. doi: 10.1111/jgs.18002 [DOI] [PubMed] [Google Scholar]
- 27.Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: a quality-improvement project. J Hosp Med. 2016;11:341–347. doi: 10.1002/jhm.2546 [DOI] [PubMed] [Google Scholar]
- 28.King BJ, Steege LM, Winsor K, et al. Getting patients walking: a pilot study of mobilizing older adult patients via a nurse-driven intervention. J Am Geriatr Soc. 2016;64:2088–2094. doi: 10.1111/jgs.14364 [DOI] [PubMed] [Google Scholar]
- 29.Hughes JM, Zullig LL, Choate AL, et al. Intensification of implementation strategies: developing a model of foundational and enhanced implementation approaches to support national adoption and scale-up. Gerontologist. 2023;63:604–613. doi: 10.1093/geront/gnac130 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following data will be made available with publication: Deidentified participant data. Access to data will be granted consistent with VA policy; data may be made available via inquiry to Susan N. Hastings, MD, MHSc (susan.hastings@va.gov). The following supporting documents will be made available: the protocol, with publication (see the Study Protocol). The protocol is also available for requests consistent with VA policy, and may be made available via inquiry to Susan Hastings, MD (susan.hastings@va.gov). The protocol may be redacted to remove information from ongoing studies that are not yet published. The statistical code is available in the Supplement. These data will be made available to: Anyone requesting the data in a manner consistent with VA policy. Types of analysis: For any purpose consistent with VA policy. Mechanisms: Via agreement consistent with VA policy. Restrictions: Any sharing of data is subject to VA policy.


