Abstract
Background:
Identifying predictors of drug use recurrence (DUR; formerly referred to as ‘relapse’) is critical to combat the addiction epidemic. Wearable devices and phone-based applications for obtaining self-reported assessments in the patient’s natural environment (e.g., ecological momentary assessment; EMA) have been used in various healthcare settings. However, the utility of combining these technologies to predict DUR in substance use disorder (SUD) has not yet been explored. This study investigates the combined use of wearable technologies and EMA as a potential mechanism for identifying physiological/behavioral biomarkers of DUR.
Methods:
Participants, recruited from an SUD treatment program, were provided with a commercial wearable device that continuously monitors biometric signals (e.g., heart rate/variability [HR/HRV], sleep characteristics). They were also prompted daily to complete an EMA via phone-based application (EMA-APP) that included questionnaires regarding mood, pain, and craving.
Results:
Seventy-seven participants are included in this pilot study (34 participants experienced a DUR during enrollment). Wearable technologies revealed that physiological markers (HRV) were significantly elevated in the week prior to DUR relative to periods of sustained abstinence (p<0.001). Results from the EMA-APP revealed that those who experienced a DUR reported greater difficulty concentrating, exposure to triggers associated with substance use, and increased isolation the day prior to DUR (p<0.001). Compliance with study procedures during the DUR week was lower than any other period of measurement (p<0.001).
Conclusions:
These results suggest that data acquired via wearable technologies and the EMA-APP may serve as a method of predicting near-term DUR, thereby potentially prompting intervention before drug use occurs.
Keywords: ecological momentary assessment, craving, wearable device, physiological, relapse
1. Introduction
In 2021, there were >107,000 overdose deaths in the United States (U.S.), the most in recorded history, and >75% of these overdose deaths involved opioids, many of which were synthetic opioids such as fentanyl (Ahmad et al., 2022). The increase in overdose deaths is not merely a coincidence given the added stress, limited and restricted access to in-person treatment, and social distancing mandates (perpetuating further isolation and withdrawal) that accompanied the COVID-19 pandemic. Unsurprisingly, with restricted access to in-person treatment, there has been a significant and “drastic decline” in individuals receiving care from substance use disorder (SUD) treatment facilities (Cantor et al., 2021). With the increasing number of overdose deaths, increasing prevalence in substance use as a form of coping, and restrictions preventing in-person services for SUD, the need for remote treatment and telehealth has become more important than ever. As a result, the U.S. saw a 154% increase in telehealth visits at the onset of the pandemic (March 2020) relative to the previous year (Koonin et al., 2020). Telehealth has been shown to have high patient satisfaction and similar effectiveness to in-person treatment for patients with SUD (Lin et al., 2019). Despite teletherapy being a viable treatment option for patients with SUD, the U.S. has continued to see increases in substance use, overdose, and substance-related deaths (Ahmad et al., 2022). Therefore, there is an urgent need to expand treatment strategies and integrate remote mechanisms for more intensive patient monitoring, intervention, and assessment. Examples of existing remote tools include the use of ecological momentary assessment (EMA) and wearable physiological-monitoring technologies (D’Souza et al., 2019; Fletcher et al., 2011; Preston et al., 2018). The data derived from these assessment/monitoring methods allows clinicians and researchers to understand better the patterns of drug use reoccurrence (DUR; formerly referred to as relapse) with the goal of developing technologies to predict DUR in order to proactively intervene before drug use occurs.
EMA is a remote data collection tool that offers patients the opportunity to answer subjective questions about their mood, behavior, craving, and experiences via electronic devices (e.g., cell phones, tablets, computers) at different times of the day in their natural environments (Preston et al., 2018). Wearable technology is a broad term that refers to devices worn by the user that have capabilities of monitoring/measuring physiological responses and processes. While EMA provides researchers with valuable subjective responses from patients, wearable physiological monitoring technology provides objective physiological and sleep data. Wearable technology devices, such as smart watches (Apple, Fitbit, Garmin), smart rings (Oura), and chest straps (Garmin, Polar), measure physiological indices such as heart rate (HR), heart rate variability (HRV), blood pressure, body temperature, sleep, and activity. Through advanced computational modeling, the possibility exists that these data can be utilized to predict changes in mood, craving, or substance use so that preventative measures can be implemented prior to DUR. For example, a phone application (APP) was created and synced with patient wearable devices; when “unusual arousal events” were detected cognitive behavioral therapy-based messages were automatically sent to the patient’s phone (Fletcher et al., 2011). Another study using electrocardiogram found that low resting high-frequency HRV may serve as an objective proxy of SUD severity, thus measurements of HRV via wearable devices may have utility in predicting DUR (D’Souza et al., 2019).
While there is an abundance of literature related to EMA and SUD, reports of wearable technology and SUD, or EMA combined with wearable technology, are sparse. For those studies that exist, most lack the longitudinal data necessary for development of algorithms to predict risk of DUR. A recent proof-of-concept study, which combined wearable biosensors to detect respiratory depression with self-reported overdose events in an individual’s natural environment, found that combined data acquisition was feasible (Roth et al., 2021). The current pilot study aims to further investigate the feasibility and utility of combined EMA and wearable technology for continuous daily remote monitoring of patients receiving opioid use disorder (OUD) treatment. Our objectives included acquisition of preliminary evidence characterizing differences in behavioral and physiological responses/measures among those who experience a DUR compared to those who successfully sustain abstinence. For those who experience a DUR, we aimed to identify potential predictors of DUR by evaluating changes in behavioral or physiological metrics along with study compliance during the time preceding DUR.
2. Methods
2.1. Participants
Ninety-six participants were enrolled, 19 of whom were excluded from the analysis for the following reasons: 14 participants had <14 days of total data and five participants’ DUR status could not be determined. Reasons for undetermined DUR status included: deceased (n=3), dismissed from clinic with no drug screen or recorded reason for dismissal (n=1), and did not show for drug screen (n=1). The final evaluable sample size included 77 participants. All procedures were approved by the Institutional Review Board of West Virginia University.
2.2. Study design
Participants were recruited from the West Virginia University Comprehensive Opioid Addiction Treatment (COAT) program, which utilizes a structured group-based, multidisciplinary, and multimodal approach, and were enrolled between 09/09/2019 – 12/31/2020. Inclusion criteria required that participants be actively enrolled in the COAT program, at least 18 years of age, able to provide informed consent, English speaking, and willing to comply with study procedures. All participants were under the same experimental condition (i.e., daily EMA and physiological data collected in their outpatient natural environment). Once written informed consent was obtained, participants were enrolled and provided with a phone app (EMA-APP) developed at the Rockefeller Neuroscience Institute (RNI) that prompted them to complete daily questionnaires. Participants were also provided with a wearable device (Garmin watch) to wear throughout the duration of the study. During the enrollment process, study staff met one-on-one with each participant and aided with setting up both phone applications (e.g., how to navigate to “RNI NeuroCAT” (EMA-APP) and “Garmin Connect” phone applications). The EMA-APP was built to work on both iOS and Android mobile operating systems, available in Apple Store and Google Play Store and included self-service installation by the participants. User accounts were created on the back-end prior to participant enrollment and participants were assigned unique usernames and instructed how to log into the application with those credentials. When new features were developed for the EMA-APP, an industrial standard software development life cycle methodology was followed when releasing major and minor updates. Examples of these updates included new features, fixes to bugs, and updates to the third-party libraries.
Once participants were logged in, they received instructions on how to navigate the application and complete the questionnaires. They were then provided with a unique username and email address assigned by study staff for use with the Garmin Connect application. Participants were also provided with the “Daily Smartphone RNI App and Garmin Smart Watch User Manual” (included in Supplementary Material) which included detailed instructions on how to navigate the phone applications, how to charge the Garmin Device, and contact information for the study team. Participants were informed that study participation would extend for a duration of one year, but they could discontinue at any time if desired.
2.3. Assessments, measures, and sources of data
2.3.1. Demographic and clinical characteristics
Participant characteristics were obtained from the electronic medical records and included age at study enrollment, sex, multidisciplinary SUD clinic dismissal date/reason (if applicable), and any reported DURs (documented in clinical notes from the participant’s psychiatrist or therapist, urine toxicology results and/or self-report).
2.3.2. Physiological monitoring
Participants were provided and asked to wear commercially available wearable technology (Garmin vívosmart 4) for the duration of the study. The wearable device utilizes multiple sensors to monitor HR (constant and daily resting), blood oxygen saturation, stress activity, and sleep. Activity is measured via step count by accelerometry, and HR and HRV is measured using photoplethysmography. The Garmin Stress score is derived from HRV, which measures the time between heartbeats; the root mean square of successive HRV over three minutes is calculated into a score from 1–100 (1 is very low stress and 100 is very high stress).
2.3.3. Ecological momentary assessment (EMA)
Subjective wellness was assessed via EMA-APP and participants received daily notifications which prompted their completion of morning (12:00–11:59 a.m.) and afternoon/evening (12:00–11:59 p.m.) questionnaires. Assessed subjective metrics included stress, fatigue, mood, pain, and substance craving and answer options were in Likert scale format, with levels of “Strongly disagree,” “Disagree,” “Neither agree nor disagree,” “Agree,” and “Strongly agree.” Specific items included in the questionnaire can be found in Supplementary Material – Appendix A.
2.3.4. Participant compliance
Participant compliance was measured via a point system and based on the number of questionnaires/assessments completed (up to 2 points per day) and wearing/syncing of the wearable device (up to 0.5 points per day). Participants were compensated based on the percentage of points earned in a calendar month and paid via gift card for their participation. Payment schedules were as follows: >70% ($100), 50–69% ($50) and <50% ($25).
2.4. Data Storage
Data acquired from the EMA-APP were automatically uploaded and stored in an approved application programming interface (API). Data acquired from the Garmin wearable device were connected with the EMA-APP API via Garmin’s Connect Cloud. HR data was uploaded in 1-minute intervals along with various daily summary metrics (e.g., average HR and Garmin Stress score) into each user’s profile which also contained the EMA-APP data.
2.5. Statistical analyses
Analyses and data formatting procedures were conducted using R software version 4.1 (R Core Team, 2021) as well as JMP Pro 15 (SAS Institute, Cary, NC). Data was downloaded from Smartabase (EMA-APP Cloud) into R through the neon package (Pross and Day, 2019) where, using the tidyverse package (Wickham, 2019), it was merged and organized into the “wide format” data file used for generating results.
The first outcome of interest was whether behavioral data differed between participants who experienced a DUR during their time in the study and participants who maintained abstinence. Participants were assigned to two groups based on information from their medical records: those who experienced a DUR during enrollment (DUR+) and those who maintained abstinence throughout enrollment (DUR−). If a DUR occurred during the study, the dates and details of DUR were obtained from medical records.
Analysis #1 – Differences in EMA-APP responses were compared between DUR+ and DUR−. The assumption of independence was violated since participants submitted responses to the survey multiple times (and at varying rates) over the course of the study. Thus, to create a sample with independent observations, a summary statistic for each participant was created by calculating the proportion of times a participant selected either “Agree” or “Strongly agree” for each question. To ensure an accurate estimate, only participants who submitted 50 or more survey responses were included, resulting in a final dataset containing 52 participants: 23 DUR+ and 29 DUR−. To avoid issues of collinearity the analysis commenced with a null model and used a stepwise logistic regression model with forward selection. The outcome variable was a participant’s DUR status.
The second outcome of interest was whether changes in measures occurred during the time preceding and following the DUR. Each DUR event was expanded to a “DUR Week,” which included the DUR date and three days prior to, and post, DUR. Nine additional time periods were also created: one for abstinence, one for each of the four weeks prior to the “DUR Week” and one for each of four weeks after the “DUR Week.” Linear mixed effects models (LMM) were constructed, which allowed for a repeated measures analysis of variance and post hoc multiple comparisons (if necessary) to be calculated across time points. The compliance period for the random effects included all data collected through the preceding month of the participant’s first DUR and for one month following the DUR event. Given the focus on investigating differences across the time periods surrounding a DUR, only participants who experienced a DUR (DUR+) were included in these analyses.
Analysis #2 – Daily Garmin Stress Scores were aggregated for each participant to obtain a Weekly Garmin Stress Score. Only data from before, during, or after a participant’s first DUR are included. An LMM was constructed using the DUR periods as a fixed effect and Weekly Garmin Stress Score as the dependent variable. As participants recorded multiple days of data, observations included in the LMM are not independent, thus necessitating the inclusion of this random effect.
Analysis #3 – Compliance with EMA-APP completion and wearable device participation: A seven-day moving average was constructed per subject for survey and Garmin compliance. This was done for modelling purposes to create a continuous metric rather than a binary comparison. Garmin compliance refers the proportion of available data from the Garmin wearable device during a 7-day period (including the date of data collection along with the previous six days) for each individual participant. Next, the dataset was filtered to include only observations before or during a participant’s first DUR, providing 4,137 observations from 34 participants for the analysis. The data were entered into an LMM, with DUR period defined as the predictor, and Garmin seven-day moving average as the response.
3. Results
3.1. Sample characteristics
Participants (n=77) were 37.3±9.1 years of age, 59.7% were female (n=46), and 40.3% were male (n=31). During the study, 44.2% (n=34) experienced DUR (DUR+) while 55.8% (n=43) maintained abstinence (DUR−), based on self-report, verified via a urine toxicology or through clinical notes. The most common substances involved in the DUR included methamphetamine (n=13), cannabis (n=11), alcohol (n=5), opioids (n=4), benzodiazepines (n=3), and cocaine (n=3); five participants used multiple substances during the DUR event.
3.2. Differences in EMA-APP responses between DUR+ versus DUR−
Following the methods described in Analyses #1, results indicate that the three included questions provide a significant amount of information compared to the null model (p<0.001) (Table 1a). One question (“Have you been outside in last day”) came from the morning survey (p<0.05) and two questions from the afternoon survey trended towards significance (‘difficulty concentrating’; p=.07; ‘Encountered a trigger’, p=.06, Table 1b). The parameter estimates indicate DUR+ participants were more likely to report difficulties in concentration, were more prone to encountering triggers, and were less likely to go outside (and thus perhaps more prone to isolation). Construction of a receiver operating characteristic (ROC) curve (based on the selected model) resulted in an area under the curve (AUC) score of 82.6%, indicating good performance in classifying those who were DUR+ versus DUR− (Figure 1).
Table 1a –
Chi-Square Whole Model Test
| Model | Log Likelihood |
DF | Chi-Square | p value |
|---|---|---|---|---|
| Difference | 10.19 | 3 | 20.38 | < 0.001* |
| Full (3 predictors) | 25.51 | |||
| Reduced (0 predictors) | 35.70 |
p<0.05; DF = degrees of freedom
Table 1b –
Parameter Estimates
| Effect | Parameter Estimate (Log Odds Ratio) |
SE | Chi-Square | p value |
|---|---|---|---|---|
| Intercept | 1.90 | 1.806 | 1.11 | 0.293 |
| Difficulty Concentrating | 2.28 | 1.266 | 3.26 | 0.071^ |
| Encountered Trigger | 3.36 | 1.810 | 3.45 | 0.063^ |
| Been Outside in Last Day | −4.31 | 2.151 | 4.02 | 0.045* |
p<0.10
p<0.05; SE = standard error
Figure 1 –
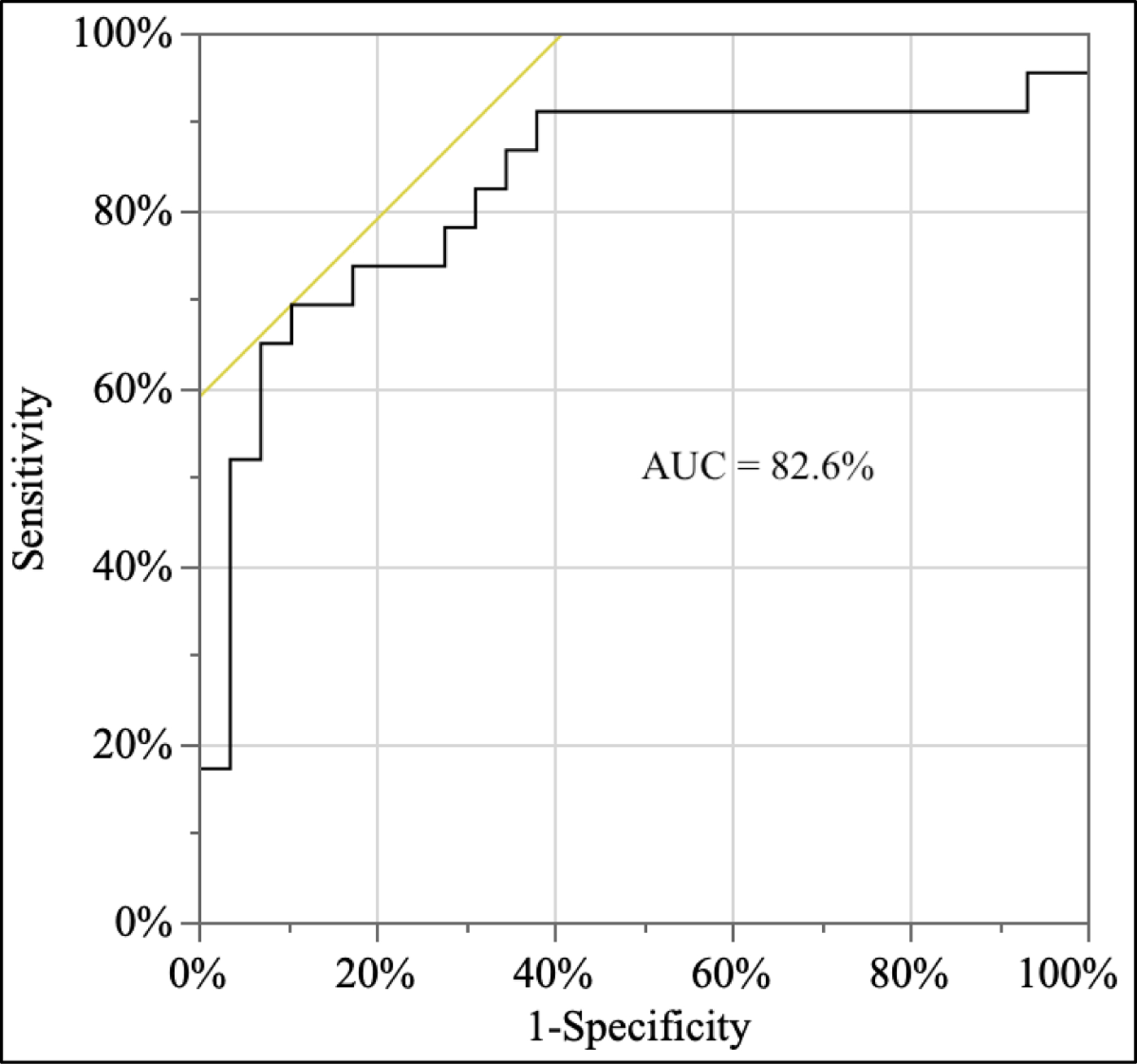
ROC Curve from Final Logistic Regression Model
3.3. Monitoring HRV prior to and following first DUR
Following the methods described in Analyses #2, Weekly Garmin Stress Scores were found to have a right-skewed distribution, which due to non-normality and a fixed 1–100 range could cause bias in the LMM parameter estimates. An Anderson-Darling Normality test returned strong evidence that the scores were not normally distributed, therefore, a Box-Cox transformation was used. This provided a transformation parameter (lambda) of 0.181. Normality was then reassessed, and the Anderson-Darling Normality test statistic was much lower (A2 = 1.09; p = 0.009). Although the assumption of normality was rejected, the right-skew in scores was not nearly as evident. As a result, the LMM provided a better fit and more unbiased estimate of parameters.
As the collected data included repeated measures, we assessed the sphericity assumption. In this case, the sphericity assumption translates to approximately equal variation in Weekly Garmin Stress Score among the specified DUR periods. Using the Brown-Forsythe test for unequal variances resulted in p < 0.0001, giving strong evidence that the variation is not equal across time points. To account for this, a repeated measures covariance matrix was included in the model by specifying the “unequal variances” option in JMP (Supplementary Table 1).
The dataset used for this analysis contained observations from 2,025 days, obtained from the participants who experienced a DUR. Overall, significant differences were found in Weekly Garmin Stress Score during the DUR periods (F9, 347.3 = 3.62; p < 0.001). The least squares (LS) means plot is displayed in Figure 2 and shows the estimates of Weekly Garmin Stress Score given by the LMM (along with 95% confidence interval).
Figure 2 –
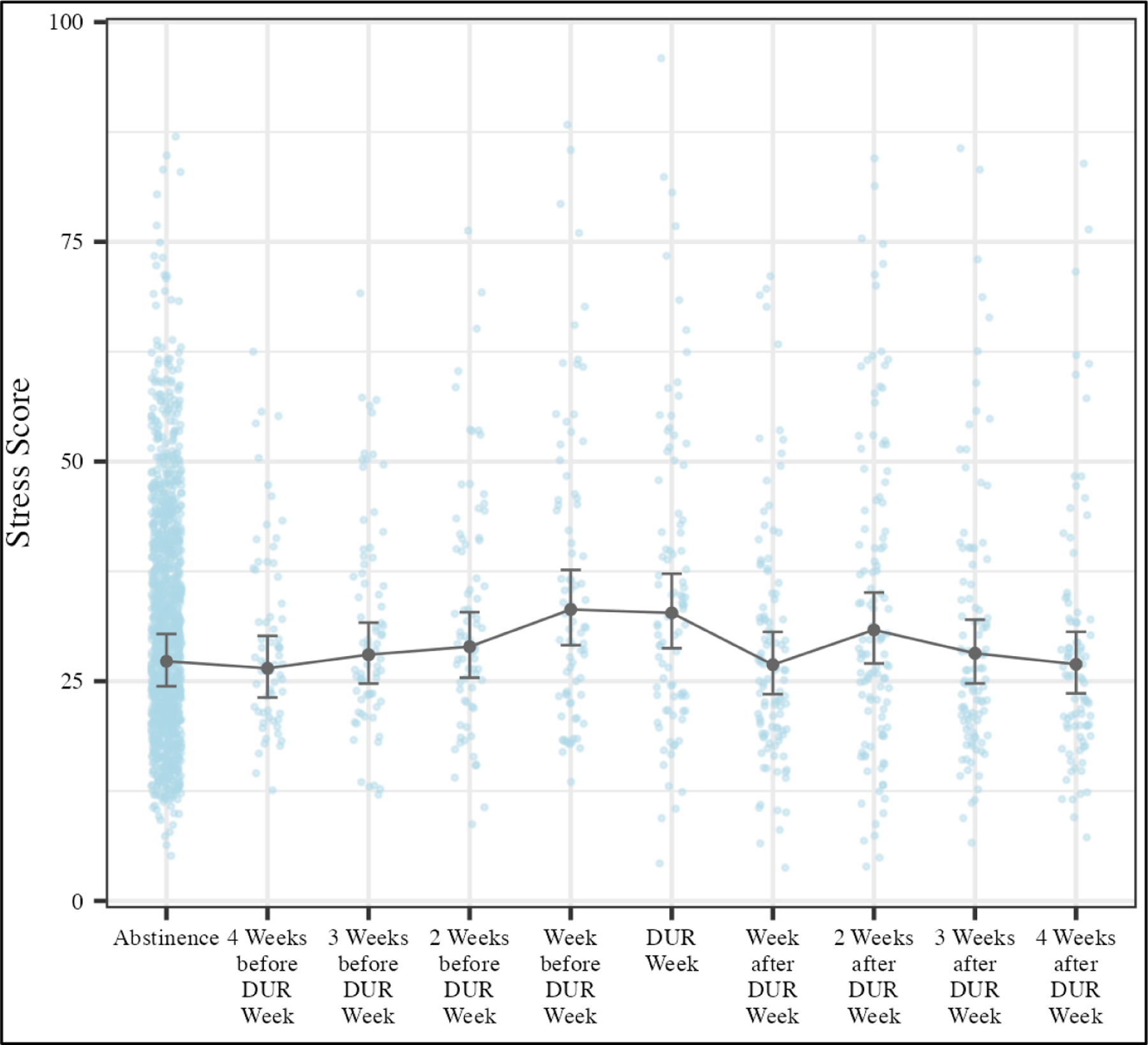
Weekly Garmin Stress Score Estimates as Calculated by a Linear Mixed-Effects Model.
Time periods correspond to the participants first drug use recurrence (DUR) during the study
Due to the significant F statistic, the Dunnett test for comparisons with a control was chosen to further investigate which specific DUR periods were significantly different for Weekly Garmin Stress Score compared with the abstinence period. Dunnett test results showed that participants’ Weekly Garmin Stress Score were significantly elevated in the week prior to DUR and the DUR week compared with the abstinence period (Table 2).
Table 2 –
Dunnett Test: Comparison with Abstinence Period for Weekly Garmin Stress Score
| DUR Period | t-ratio | Adjusted p value |
|---|---|---|
| Four weeks before | −0.66 | 0.998 |
| Three weeks before | 0.67 | 0.998 |
| Two weeks before | 1.30 | 0.834 |
| Week before DUR | 3.96 | < 0.001* |
| DUR week | 3.47 | 0.005* |
| Week after DUR | −0.27 | 0.999 |
| Two weeks after | 2.30 | 0.172 |
| Three weeks after | 0.63 | 0.999 |
| Four weeks after | −0.23 | 0.999 |
p<0.05
3.4. Compliance with EMA-APP completion and wearable device participation
Following the methods described in Analyses #3, the response of the LMM, limited to values ranging from 0 to 1, was not normally distributed. Transformations, such as the Box-Cox transformation, were not able to rectify the distribution. The sphericity assumption was checked using a Brown-Forsythe test, which resulted in strong evidence of unequal variance in compliance across DUR periods. Thus, a repeated measures variance-covariance matrix was specified when constructing the LMM (Supplementary Table 2).
A Dunnett test for multiple comparisons with control yielded significant differences from the abstinence period at each time point, with the exception of ‘Three weeks’ and ‘Two weeks’ prior to first DUR. After the low point at the DUR week, there is an upward trend for two weeks, followed by downward trend three weeks after DUR (Table 3).
Table 3 –
Dunnett Test: Comparison with Abstinence Period for Garmin Compliance
| DUR Period | t-ratio | Adjusted p-value |
|---|---|---|
| Four weeks before | −3.101 | 0.018 |
| Three weeks before | −0.878 | 0.982 |
| Two weeks before | −2.002 | 0.325 |
| Week before DUR | −4.452 | < 0.001* |
| DUR week | −7.784 | < 0.001* |
| Week after DUR | −6.638 | < 0.001* |
| Two weeks after | −4.445 | < 0.001* |
| Three weeks after | −5.316 | < 0.001* |
| Four weeks after | −7.235 | < 0.001* |
p<0.05
Results showed a significant overall difference in compliance averages among the specified DUR periods (F9, 487.8 = 12.66; p < 0.001). Specifically, compliance during participants’ DUR week was lower than any other period as displayed in the LS means plot (Figure 3).
Figure 3 –
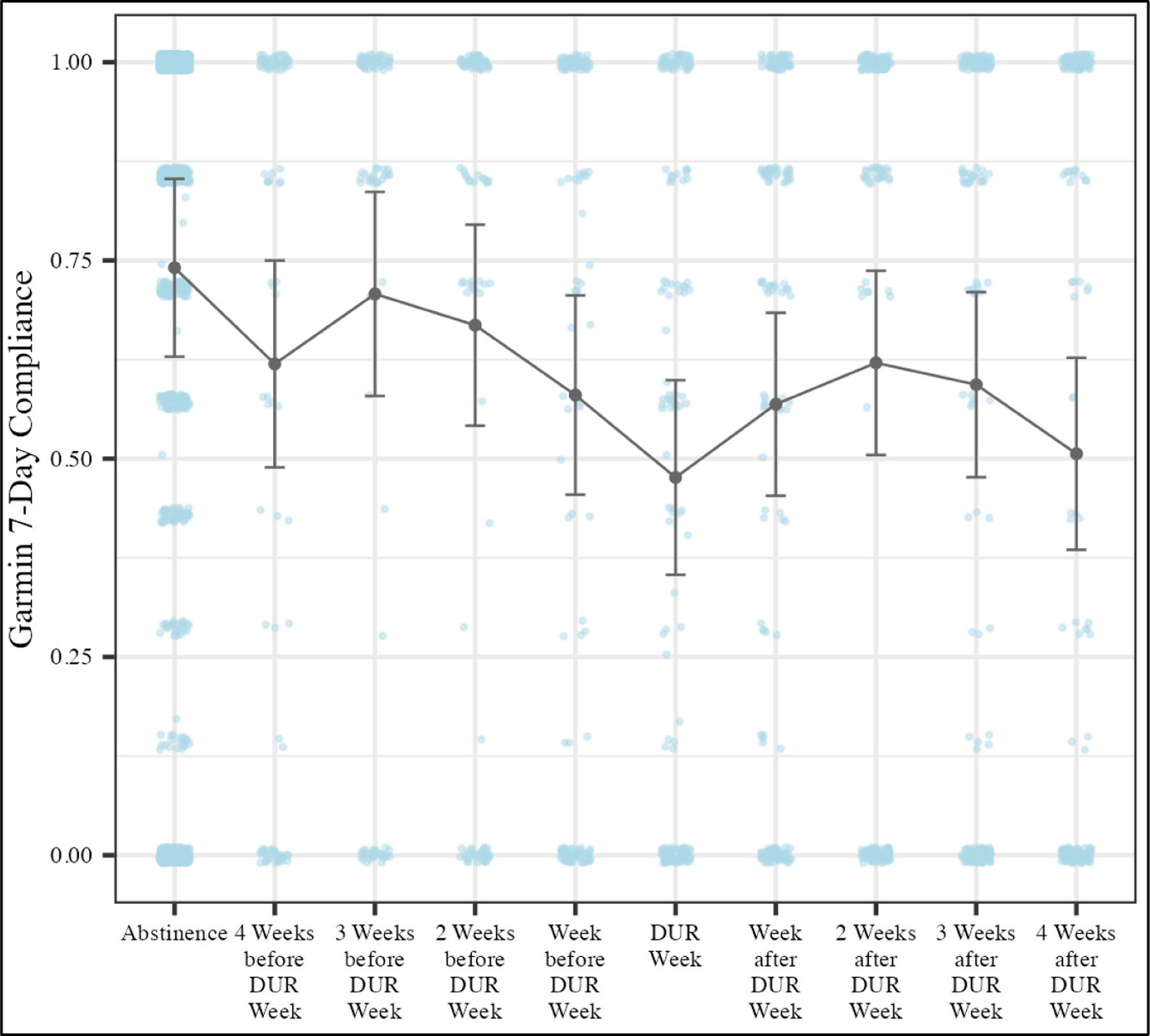
Garmin Compliance LS Means Plot
Time periods correspond to participants first drug use recurrence (DUR) during the study
4. Discussion
The current pilot study investigated the feasibility and utility of the EMA-APP for monitoring daily emotional/behavioral symptoms in combination with wearable technology for monitoring physiological characteristics of patients receiving SUD treatment in their normal environment outside the clinic. Combining these two methods of assessment allows for a more comprehensive understanding of an individual’s behavioral and physiological state during times of abstinence, DUR, and most importantly, during the time preceding DUR when intervention is most needed. Specifically, responses via the EMA-APP provide subjective insights into craving and mood, while wearable technology provides objective physiological information that can be linked with these responses to further inform our understanding of DUR and successful abstinence. Both the EMA-APP and wearable technology allow for day-to-day data collection in patients’ natural environments. Perhaps of greatest importance, the data gathered on craving, mood, and physiological functioning can be combined with machine-learning algorithms to identify patterns and indicators prior to DUR. Once data patterns associated with DUR are established, researchers and clinicians can use them to proactively intervene before DUR occurs. For example, when there is an indication that a patient may be at risk for DUR based on acquired data, patients and providers can be notified accordingly so that immediate precautions can be taken to prevent DUR.
Findings from the current study revealed that enrolled participants were initially compliant with the EMA-APP and wearable device requirements (with reduced compliance over time being a potential indicator of a DUR). While the findings from this study should be considered preliminary, there is evidence that the data acquired via the EMA-APP and wearable devices differs as a function of abstinence/DUR and therefore may serve as a mechanism for detecting the probability of DUR before it occurs. EMA-APP responses revealed that DUR+ patients reported: i) greater difficulty concentrating; ii) exposure to a trigger associated with their substance use; and iii) less outdoor experiences (potentially reflective of isolation and/or social withdrawal). These questions may serve as patient-reported markers for DUR. The wearable device metrics revealed two potential identifiers of increased DUR risk. First, physiological markers of stress were significantly elevated in the week before DUR compared with a time in which they had demonstrated sustained abstinence (at least one month prior to the DUR week). Therefore, increased psychosocial stressors may manifest in physiological responses outside conscious awareness and may provide important insight to an individual who may be otherwise unaware of how the stress is impacting them. Second, compliance during the participant’s DUR week was lower than any other period of measurement; and there was also a significant decrease in compliance the week prior to the DUR. Thus, reduced compliance and physiological stress response may both serve as risk-markers for DUR and alert the treatment team to reach out to an individual to see if additional support is needed with a potential to create a future close-loop feedback/intervention once further validated.
Overall, the combination of the EMA-APP and wearable technology as complementary tools for SUD treatment has the potential to provide significant longitudinal insights relating to craving and drug use in the patient’s natural environment. The EMA-APP and wearable technologies can provide a more accurate reflection of the individual’s current state and in a more frequent fashion, as opposed to having patients report mood/craving/DUR retrospectively when returning to the hospital or clinic for their scheduled appointments. The importance of improving remote assessment is especially pertinent given the restrictions to in-person treatment secondary to the pandemic and the ever-increasing rates of substance use and substance overdoses. Subjectively, participants reported a positive experience participating in the study and felt that health metrics from the wearable were insightful, helping them to appreciate real-time improvements during abstinence. They also reported working to try and improve their performance on cognitive tasks.
Despite the informative findings, there are some important limitations of this study that must be noted. The study was originally designed to include weekly urine toxicology for confirmation of substance use/non-use and to verify the accuracy of participant self-report of substance use/non-use. However, due to COVID-19 restrictions and clinic visits transitioning to remote teletherapy-based platforms, participants were not regularly presenting onsite; therefore, urine toxicology was not obtained as frequently as initially intended. Notwithstanding, participants were randomly requested to come to clinic so that urine toxicology could be performed as part of their clinical care. When comparing the instances that participants presented to clinic for random urine toxicology acquisition (>900 occasions) and their self-reported substance use/non-use on that day and the proceeding days, there was 100% consistency between their subjective report and the results from objective urine toxicology. This finding, at the very least, demonstrates a proxy for the reliability and consistency of participant self-report, at least with the current sample of participants. Another study limitation is the relatively small sample size along with the exclusion of ~20% of participants (19 out of 96 with <14 days of data) from the overall sample and, of those 77 participants remaining, the exclusion of ~32% of participants from the first analysis (25 out of 77 who did not provide at least 50 responses). Despite this, compliance rates in the current study may have been lower if not for incentivized participation for task completion, which may limit practicality within a purely clinical setting given financial constraints. Additionally, participants enrolled in this study were all patients receiving addiction treatment within the WVU health system. Given the rural nature of West Virginia (and limited addiction treatment services available outside the WVU health system), this may have positively impacted compliance. In larger metropolitan cities where there are more treatment options available there may be a higher likelihood of attrition. Additionally, the finding that compliance was reduced during the DUR week, while informative and potentially a predictor in itself, complicates matters and poses another limitation as we therefore do not have their self-report data in the time immediately approximating the DUR. An additional limitation with defining the DUR date is that many participants only have to report to an appointment on a weekly or biweekly basis. As such, dates associated with DUR events are largely dependent on the subject’s self-report and may lack accuracy based on their recollection. Future studies should not only include a much larger sample but also acquire EMA-APP and wearable device data over a larger time-period. It would be ideal to capture multiple DURs followed by sustained periods of abstinence to determine the consistency of the potential predictors identified in this study.
In conclusion, the findings provide preliminary evidence to support the potential utility of combined EMA-APP and wearable devices for remote monitoring of patients enrolled in SUD treatment and identifying markers to detect and/or predict DUR before the recurrence occurs. Future studies will investigate these potential predictors, along with others, in a larger cohort of individuals over a longer duration of time to address the limitations noted above to enable more conclusive decisions can be made regarding the utility of combined EMA-APP and wearable device technologies in prediction and prevention of DUR.
Supplementary Material
Highlights.
Ecological momentary assessment can provide useful information prior to drug use recurrence (relapse).
Wearable technologies can potentially detect biomarkers of drug use recurrence.
Physiological markers of daily stress were significantly elevated prior to drug use recurrence.
Ecological momentary assessment showed changes in mood and behavior prior to drug use recurrence.
Combining these technologies could aid clinicians to predict and prevent drug use.
Acknowledgements
Supplementary funding from the National Institute of General Medical Sciences Clinical and Translational Research, IDeA (CTR) Award, U54GM104942 was utilized to support this project.
Funding:
National Institute of General Medical Sciences Clinical and Translational Research, Idea (CTR) Award, 5U54GM104942, 2018–2019
Role of funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Role of funding source
The funding source had no other role other than financial support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Declaration of Competing Interest
The authors have no conflicts of interest to disclose/declare.
References
- Cantor J, Kravitz D, Sorbero M, Andraka-Christou B, Whaley C, Bouskill K, Stein BD, 2021. Trends in visits to substance use disorder treatment facilities in 2020. J. Subst. Abuse Treat 127, 108462. 10.1016/j.jsat.2021.108462.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza JM, Wardle M, Green CE, Lane SD, Schmitz JM, Vujanovic AA, 2019. Resting Heart Rate Variability: Exploring Associations With Symptom Severity in Adults With Substance Use Disorders and Posttraumatic Stress. J. Dual Diagn 15(1), 2–7. 10.1080/15504263.2018.1526431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges DF, Powell JW, 1985. Microcomputer analysis of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput 6, 652–655. [Google Scholar]
- Eriksen B, Eriksen CW, 1974. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics 16(1), 143–149. [Google Scholar]
- Fletcher RR, Tam S, Omojola O, Redemske R, Kwan J, 2011. Wearable sensor platform and mobile application for use in cognitive behavioral therapy for drug addiction and PTSD. Annu Int Conf IEEE Eng Med Biol Soc 2011, 1802–1805. 10.1109/IEMBS.2011.6090513. [DOI] [PubMed] [Google Scholar]
- Kirchner WK, 1958. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol 55(4), 352–358. 10.1037/h0043688. [DOI] [PubMed] [Google Scholar]
- Koonin LM, Hoots B, Tsang CA, Leroy Z, Farris K, Jolly T, Antall P, McCabe B, Zelis CBR, Tong I, Harris AM, 2020. Trends in the Use of Telehealth During the Emergence of the COVID-19 Pandemic - United States, January-March 2020. MMWR Morb. Mortal. Wkly. Rep 69(43), 1595–1599. 10.15585/mmwr.mm6943a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LA, Casteel D, Shigekawa E, Weyrich MS, Roby DH, McMenamin SB, 2019. Telemedicine-delivered treatment interventions for substance use disorders: A systematic review. J. Subst. Abuse Treat 101, 38–49. 10.1016/j.jsat.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, 2018. Before and after: craving, mood, and background stress in the hours surrounding drug use and stressful events in patients with opioid-use disorder. Psychopharmacology (Berl.) 235(9), 2713–2723. 10.1007/s00213-018-4966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pross Z, Day J, 2019. Neon: Push/pull data to/from smartabase. R package version. 0.0.0.9000
- R Core Team, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, V., Austria. URL https://www.R-project.org/.
- Roth AM, Tran NK, Cocchiaro B, Mitchell AK, Schwartz DG, Hensel DJ, Ataiants J, Brenner J, Yahav I, Lankenau SE, 2021. Wearable biosensors have the potential to monitor physiological changes associated with opioid overdose among people who use drugs: A proof-of-concept study in a real-world setting. Drug Alcohol Depend. 229(Pt A), 109138. 10.1016/j.drugalcdep.2021.109138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, 2019. Welcome to the tidyverse. Journal of Open Source Software 4:1686, 10.21105/joss.01686 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


