Abstract
Background
Excessive body fat may be a major cause of insulin resistance and diabetes. But body weight reduction by energy restriction may simultaneously reduce both fat and muscle. Skeletal muscle is an important organ for glucose metabolism regulation, and loss of muscle may deteriorate glucose metabolism. Therefore, it is preferable to predominantly reduce fat without significant loss of muscle with weight loss in patients with type 2 diabetes. Previously, the anti-diabetic agent glucagon-like peptide-1 receptor agonists (GLP-1RAs) liraglutide and semaglutide given by injection were reported to decrease fat with less effect on muscle in diabetic patients. Recently oral semaglutide was developed and was reported to decrease body weight, but the effect on muscle has not been fully evaluated.
Methods
This was a non-interventional retrospective longitudinal study. We evaluated the effect of 24-week treatment with oral semaglutide on body fat and muscle mass in 25 Japanese patients with type 2 diabetes. Laboratory examination and body composition test by bioelectrical impedance analysis (BIA) were performed at baseline, 12 weeks, and 24 weeks, and the effects on glycemic control and body composition were assessed.
Results
Hemoglobin A1c significantly decreased at 12 weeks and further ameliorated at 24 weeks (8.7±0.87% at baseline; 7.6±1.00% at 12 weeks; 7.0±0.80% at 24 weeks; mean ± standard error (SE)). While body fat significantly decreased (28.3 ± 1.52 kg at baseline; 26.8 ± 1.59 kg at 12 weeks; 25.5 ± 1.57 kg at 24 weeks; mean ± SE), whole-body lean mass was not significantly changed (48.1 ± 1.92 kg at baseline; 47.7 ± 1.93 kg at 12 weeks; 47.6 ± 1.89 kg at 24 weeks; mean ± SE). Furthermore, the appendicular skeletal muscle index (SMI) defined as appendicular skeletal muscle mass (ASM)/height squared (units; kg/m2) was also unchanged.
Conclusion
The 24-week treatment with oral semaglutide ameliorated glycemic control with reduction of body fat but not muscle mass in Japanese patients with type 2 diabetes.
Keywords: Glucagon-like peptide-1, Oral semaglutide, Body composition, Fat mass, Muscle mass, Type 2 diabetes
Introduction
Excess body fat may be a major cause of insulin resistance and deterioration of glucose metabolism [1, 2]. Thus, body weight (BW) reduction by life-style modification, especially dietary energy restriction, is an important therapy in patients with type 2 diabetes and obesity/overweight to restore insulin sensitivity and ameliorate glycemic control. However, BW reduction by energy restriction may simultaneously reduce muscle mass as well as body fat [3]. Skeletal muscles handle about 40-45% of oral glucose intake, and account for up to 80-85% of insulin-mediated glucose disposal. Consequently, loss of muscle mass may lead to elevation of plasma glucose [4, 5]. In addition, a decrease in muscle mass may be associated with an increased risk of sarcopenia and frailty, especially in older patients. Therefore, it is preferable to predominantly reduce body fat without significant loss of muscle mass when losing weight.
Sodium glucose cotransporter 2 (SGLT2) inhibitor is an oral anti-diabetic agent and has been reported to have a lowering effect on BW as well as a preventive effect for cardiovascular and renal complications [6, 7]. However, a previous report showed that treatment of the SGLT2 inhibitor dapagliflozin 10 mg/day for 24 weeks in patients with type 2 diabetes led to a mean reduction of body fat and muscle mass by 2.2 kg and 1.1 kg, respectively, as assessed by dual energy X-ray absorptiometry (DXA) [8]. We also used DXA and investigated the effect of SGLT2 inhibitor ipragliflozin 50 mg/day for 24 weeks in obese Japanese patients with type 2 diabetes. We found a mean reduction in body fat and muscle mass of 1.8 kg and 1.7 kg, respectively [9]. These findings suggest that SGLT2 inhibitors may have catabolic effects on both fat and muscles.
Injection of glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) and SGLT2 inhibitors are thought to have a similar effect on BW reduction in patients with type 2 diabetes. In obese Caucasian type 2 diabetic patients, daily injection of GLP-1RA liraglutide showed similar decrease in body fat levels with less effect on muscle loss when compared with SGLT2 inhibitors [10]. We also previously reported that daily injection of liraglutide for 24 weeks (started at 0.3 mg and titrated to 0.9 mg) in obese Japanese patients with type 2 diabetes showed a decrease in abdominal visceral fat (AVF) and intrahepatic lipid (IHL) by a mean of 11.9% and 49.2%, respectively, as assessed by whole abdominal computed tomography (CT) and proton magnetic resonance spectroscopy (1H-MRS) [11]. However, we found no significant reduction of muscle mass as assessed by DXA. Semaglutide has 94% structural homology with human GLP-1 and was initially approved for once weekly injection type of GLP-1RA. Oral semaglutide has been developed as a co-formulation of semaglutide with an absorption enhancer, sodium N-(8-[2-hydroxybenzoyl] amino) caprylate and is the first oral GLP-1RA to be approved as an anti-diabetic agent [12]. The PIONEER trials compared oral semaglutide with placebo or active comparators in a wide range of patients and background therapies and reported the efficacy and safety of oral semaglutide. The PIONEER 1, 4, 5, and 8 trials showed a significant lowering effect on BW compared with placebo, and the PIONEER 2, 3, 4, and 7 trials also revealed a greater effect of oral semaglutide on BW reduction than the active comparators (dipeptidyl peptidase 4 (DPP-4) inhibitor sitagliptin, SGLT2 inhibitor empagliflozin, or injection type of GLP-1RA liraglutide) [13]. However, the effect of oral semaglutide on body fat and muscle mass has not been fully evaluated. Thus, we investigated the effect of 24-week treatment with oral semaglutide on body fat and muscle mass in Japanese patients with type 2 diabetes.
Materials and Methods
Participants
This was a non-interventional retrospective longitudinal study. The study population comprised patients with type 2 diabetes who attended the outpatient clinic at Yokohama General Hospital (Yokohama, Japan).
Between December 2021 and July 2022, oral semaglutide was started at 3 mg daily, titrated up to 14 mg, and continued for 24 weeks. Because the combined use of DPP-4 inhibitors and oral semaglutide was not approved, in patients using DPP-4 inhibitors, this was replaced by oral semaglutide, and other anti-diabetic agents were continued at same dosage. In the patients without DPP-4 inhibitors, oral semaglutide was added, and the other agents were kept at same dosage. The attending doctors started oral semaglutide at 3 mg daily and carefully titrated up to 14 mg, considering laboratory data and symptoms and signs of side effects.
The inclusion criteria were patients aged 20 to 80 years and with a hemoglobin A1c (HbA1c) variation less than 0.5% in the previous 6 months. The exclusion criteria were as follows: 1) malignancy, severe renal disease (estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2), or severe liver disease; 2) abnormal state with extracellular water (ECW) such as systemic edema or dehydration; 3) presence of metals in body, such as cardiac pacemaker, metal plate, or metallic stent and; 4) being considered to be unsuitable for the study by the attending doctors.
This study was approved by the ethics committee of Yokohama General Hospital (No. 202205) and was performed in accordance with the principles of the Declaration of Helsinki. Because this was a non-interventional study, informed consent was obtained in the form of an opt-out on our website.
Assessment of general characteristics and body composition
At the start of oral semaglutide treatment in the outpatient clinic, height and BW were measured and body mass index (BMI) was calculated. Blood and urine were sampled for general laboratory tests at baseline, 12 weeks, and 24 weeks of oral semaglutide treatment, and patient characteristics such as diabetic medication was recorded. Then, bioelectrical impedance analysis (BIA) was performed with the body composition analyzer MC-780MA-N (Tanita Corp., Tokyo, Japan). Because abnormal ECW and metals inside the body may affect body composition measurements by BIA and make it difficult to accurately evaluate the obtained data, we set these points in the exclusion criteria. To assess muscle mass, the appendicular skeletal muscle mass (ASM) was calculated as the sum of the lean mass of the arms and legs. The appendicular skeletal muscle index (SMI) was defined as ASM/height squared (units; kg/m2). We used whole-body lean mass and SMI as indices of muscle mass. The BIA device is equipped with the calculation system of phase angle (PhA) and ECW to total body water (TBW) ratio (ECW/TBW). BIA measures whole-body impedance, i.e., the opposition of the body to an alternating current. Impedance consists of two components: resistance (R) and reactance (Xc); R is derived from the amount of intracellular water (ICW) and ECW, whereas Xc is dependent on the integrity of cell membranes. PhA can be simply calculated as an arctangent by using the raw data of R and Xc at a frequency of 50 kHz, as follows: (Xc/R) × 180°/π. Because both PhA and ECW/TBW were known to be associated with muscle mass and strength [14, 15], PhA and ECW/TBW were also measured.
Statistical analysis
Data were expressed as mean ± standard error (SE). Differences between baseline and 12 and 24 weeks were assessed by Tukey’s type multiple comparison test. All analyses were performed with the SPSS version 21 software package (IBM, Tokyo, Japan). Statistical significance was defined as a P value less than 0.05.
Results
We included 25 patients with type 2 diabetes (male, 14; female, 11) aged 28 to 78 years (54.1 ± 2.7, mean ± SE). Regarding smoking and alcohol history, current smoking was in six patients, previous smoking in three patients, and non-smoking in 16 patients, and drinking in 11 patients and non-drinking in 14 patients, respectively. But, the information about consumption of daily smoking and alcohol was not obtained.
Eighteen patients were taking an DPP-4 inhibitor, which was replaced with oral semaglutide, and the other seven patients had oral semaglutide added to their medications. The dosages of other anti-diabetic agents were not changed during the study period. The percentage treated with the various agents was as follows: insulin injection, 20%; sulfonyl urea, 24%; metformin, 36%; glinides, 16%; pioglitazone, 4%; α-glucosidase inhibitors, 4%; and SGLT2 inhibitors, 20%.
All patients had mild abdominal fulness, abdominal discomfort, and sense of appetite decrease at the beginning state, but they were mild and tolerable level. Thus, the attending doctors carefully titrated and maintained dosage of oral semaglutide that the patients could continue. At 24 weeks, dosage of oral semaglutide was 3 mg in three patients, 7 mg in 12 patients, and 14 mg in 10 patients.
Baseline characteristics are shown in Table 1. Systolic and diastolic blood pressure were unchanged. Serum low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol were decreased at 12 weeks and 24 weeks from baseline levels, whereas serum triglyceride was unchanged. eGFR at 24 weeks was decreased from baseline and 12-week levels.
Table 1. Clinical Characteristics of Participants.
| Baseline | 12 weeks | 24 weeks | |
|---|---|---|---|
| Age, years | 54.1 ± 2.7 | ||
| Height, cm | 165.5 ± 1.6 | ||
| SBP, mm Hg | 140.5 ± 4.1 | 140.8 ± 4.1 | 137.6 ± 4.6 |
| DBP, mm Hg | 81.1 ± 2.7 | 81.5 ± 2.5 | 78.9 ± 3.3 |
| LDL-cholesterol, mg/dL | 132.5 ± 7.1 | 120.6 ± 6.5** | 122.3 ± 7.3** |
| Triglyceride, mg/dL | 178.1 ± 14.8 | 164.6 ± 13.3 | 157.8 ± 15.4 |
| HDL-cholesterol, mg/dL | 48.5 ± 1.8 | 45.8 ± 1.7* | 46.5 ± 1.7** |
| eGFR, mL/min/1.73 m2 | 69.2 ± 4.1 | 68.9 ± 4.1 | 64.2 ± 3.6***, ## |
Data are expressed as mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs. baseline, #P < 0.05 vs. 12 weeks, ##P < 0.01 vs. 12 weeks. SBP: systolic blood pressure; DBP: diastolic blood pressure; LDL: low-density lipoprotein; HDL: high-density lipoprotein; eGFR: estimated glomerular filtration rate; SE: standard error.
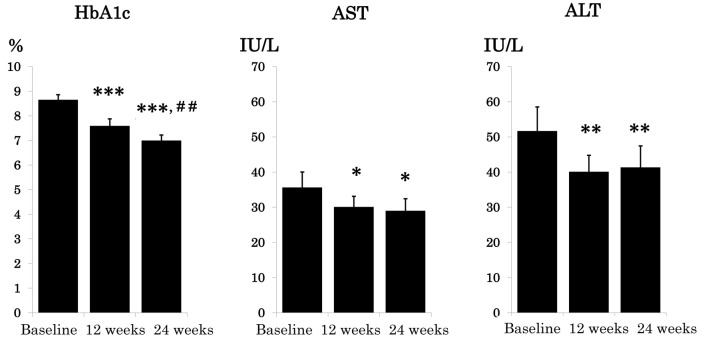
Changes in HbA1c, serum aspartate aminotransferase (AST), and serum alanine aminotransferase (ALT) are shown in Figure 1. HbA1c significantly decreased by 12 weeks and was further ameliorated by 24 weeks (8.7±0.17% at baseline; 7.6±0.20% at 12 weeks; 7.0±0.16% at 24 weeks). Both AST and ALT were significantly decreased at 12 weeks and kept the same level at 24 weeks.
Figure 1.
Effect of 24-week oral semaglutide treatment on glycemic control and serum liver enzymes. Data are expressed as mean ± SE. *P < 0.05. **P < 0.01, ***P < 0.001 vs. baseline and ##P < 0.01 vs. 12 weeks. HbA1c: hemoglobin A1c; AST: aspartate aminotransferase; ALT: alanine aminotransferase; SE: standard error.
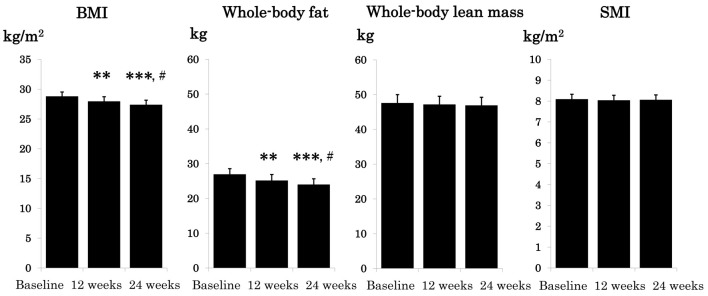
Changes in BMI, whole-body fat, whole-body lean mass, and SMI are shown in Figure 2. BMI and whole-body fat significantly decreased by week 12 and was further reduced by week 24 (BMI and whole-body fat; 29.3 ± 0.68 and 28.3 ± 1.52 at baseline; 28.5 ± 0.72 and 26.8 ± 1.59 at 12 weeks; 28.0 ± 0.71 kg/m2 and 25.5 ± 1.57 kg at 24 weeks). Whereas, whole-body lean mass and SMI remained unchanged during the study period (whole-body lean mass and SMI; 48.1 ± 1.92 and 8.1 ± 0.20 at baseline; 47.7 ± 1.93 and 8.1 ± 0.20 at 12 weeks; 47.6 ± 1.89 kg and 8.1 ± 0.20 kg/m2 at 24 weeks).
Figure 2.
Effect of 24-week oral semaglutide treatment on body mass index and body composition. Data are expressed as mean ± SE.**P < 0.01, ***P < 0.001 vs. baseline and #P < 0.05 vs. 12 weeks. BMI: body mass index; SMI: skeletal muscle index; SE: standard error.
The attending doctors asked the patients about changes in eating and exercise in every visit, and all patients answered to continue diet and exercise therapy with same level according to advise and instructions from the attending doctors and medical staff. However, we did not use a checking sheet for daily diet consumption and a calorie counter to measure daily exercise performance. Thus, it was unclear whether eating and exercise habit was changed by oral semaglutide treatment.
Changes in PhA and ECW/TBW are shown in Table 2. The right side of the body PhA (R-PhA), the left side of the body PhA (L-PhA), and the ECW/TBW were unchanged during the study period.
Table 2. Changes of PhA and ECW/TBW Ratio.
| Baseline | 12 weeks | 24 weeks | |
|---|---|---|---|
| R-PhA | 5.90 ± 0.16 | 5.84 ± 0.16 | 5.80 ± 0.15 |
| L-PhA | 5.75 ± 0.15 | 5.68 ± 0.14 | 5.69 ± 0.14 |
| ECW/TBW | 0.43 ± 0.01 | 0.44 ± 0.01 | 0.42 ± 0.02 |
Data are expressed as mean ± SE. R-PhA: right phase angle; L-PhA: left phase angle; ECW/TBW: extracellular water/total body water ratio; SE: standard error.
Discussion
The present study demonstrated four findings. First, HbA1c was remarkably improved, as shown in Figure 1 (from 8.7±0.2% at baseline to 7.0±0.2% at 24 weeks) suggesting amelioration of glycemic control. Second, while BMI and whole-body fat were decreased, whole-body lean mass and SMI remained unchanged. Third, serum liver enzyme AST and ALT were reduced. Fourth, R-PhA, L-PhA, and ECW/TBW were unchanged.
The age of the participants widely ranged from 28 to 78 years, but no significant correlations between age and changes in whole-body fat (r = -0.104, P = 0.620) and whole-body lean mass (r = -0.071, P = 0.736) for 24 weeks were observed. Thus, the effect of oral semaglutide on body composition may be independent of age. We did not measure AVF by abdominal CT and IHL by 1H-MRS in the present study, so it remains unclear whether AVF and IHL were reduced by oral semaglutide treatment. However, we previously performed a post-hoc analysis combined with our three prospective studies for 24 weeks to evaluate the effect of anti-diabetic agents (DPP-4 inhibitor sitagliptin, SGLT2-inhibitor ipragliflozin, sulfonylurea glimepiride, and GLP-1RA liraglutide) on IHL content by 1H-MRS [16]. We obtained the results indicating that the changes in BMI, ALT, and HbA1c were independently correlating factors with the change in IHL content from multiple regression analysis with stepwise forward selection method. Moreover, the multiple coefficient of determination (R2) and adjusted R2 with combination of these three factors were very high (0.855 and 0.829). In the present study, BMI, ALT, and HbA1c were significantly reduced during the study period, suggesting that IHL content as well as body fat may be reduced by the treatment of oral semaglutide. We previously reported that the injection type of GLP-1RA, liraglutide significantly reduced both AVF and IHL [11]. Thus, further study measuring AVF and IHL may clarify the effect of oral semaglutide on AVF and IHL.
Gibbons et al previously evaluated the effect of 12-week oral semaglutide treatment on body composition measured by air displacement plethysmography, and they showed that BMI and whole-body fat but not whole-body lean mass were significantly reduced (whole-body fat, -2.6 ± 2.5 kg; whole-body lean mass, -0.1 ± 1.7 kg, mean ± standard deviation (SD)) [17]. Thus, they concluded that reduction in BMI may be principally due to body fat reduction. Although the study period was shorter and the analysis method of body composition was different, the results were consistent with the present study. Meier et al performed a post hoc analysis of the PIONEER Studies 1-8 and showed that body weight loss with oral semaglutide was mediated predominantly by effects other than gastrointestinal adverse events [13]. They discussed that the majority of the weight loss was most likely mediated through the direct effects of GLP-1 agonism on appetite-regulating system. Thus, we speculate that all patients in the present study may decrease daily consumption of diet.
On the other hand, Volpe et al recently reported that once-weekly semaglutide injection treatment for 6 months significantly decreased both fat mass index (FMI), defined as whole-body fat/height squared (units; kg/m2), and SMI [18]. But the degree of reduction was much different between FMI and SMI (baseline FMI, 17.10 ± 0.99, variation of FMI at 6 months, -3.04 ± 0.43 kg/m2; baseline SMI, 10.36 ± 0.27, variation of SMI at 6 months, -0.51 ± 0.14 kg/m2; mean ± SD) measured by BIA. Furthermore, they showed that handgrip strength measured by a manual hydraulic dynamometer and PhA and ECW/TBW measured by BIA were not significantly changed during the study period. Taken together, the authors discussed that semaglutide once-weekly injection remarkably decreased body fat with non-clinically relevant change in muscle mass and strength. Recently we also reported that R-PhA, L-PhA, and the ECW/TBW were closely associated with muscle mass and handgrip strength in the patients with type 2 diabetes [19]. In the present study, we found no significant changes in R-PhA, L-PhA, and ECW/TBW as shown in Table 2. Thus, although we did not directly measure muscle strength, it is likely that oral semaglutide does not induce loss of muscle strength. The majority of the body fat loss seen with oral semaglutide was thought to be mediated through the direct effects of GLP-1 agonism on appetite-regulating system in brain, rather than gastrointestinal adverse effects [13, 20]. However, the exact mechanism of why whole-body lean mass and SMI were not decreased by oral semaglutide remains unclear.
Human vascular endothelial cells express abundant GLP-1 receptor as well as insulin receptor [21]. Recently Wang et al reported that GLP-1RA infusion increased microvascular blood volume by about 30% and 40% in skeletal and cardiac muscle, respectively, with no change in flow velocity, leading to significant increase in microvascular blood flow in both skeletal and cardiac muscle in obese human subjects [22]. These actions of GLP-1 may increase delivery of oxygen, nutrients, and hormones, such as insulin, to the myocytes, which is important for maintaining muscle mass and function. In fact, a recent study showed that GLP-1 enhances insulin-mediated whole-body glucose uptake in the glucose clamp study of human subjects [23]. In addition, a previous study showed that GLP-1 directly induced myogenesis via a cAMP-dependent complex network in skeletal muscles of rodents [24]. Furthermore, previous reports showed that GLP-1RA directly activates glucose transport in rat and mouse skeletal muscle cells [25, 26]. Taken together, GLP-1 may have an anabolic action on skeletal muscle tissue. Exercise is an important factor regulating muscle volume and strength and we previously reported that resistant exercise increased muscle strength in older patients with type 2 diabetes in 48 weeks prospective interventional study [27]. However, in the present study, we did not check daily exercise in the patients. Thus, further basic and clinical investigations including assessment of exercise habit may clarify the clinical effect of semaglutide treatment on skeletal muscle mass and function.
Alhindi and Avery recently reported the efficacy and safety of oral semaglutide compared to injection of semaglutide and the other injection of GLP-1RA comparators by network meta-analysis [28]. They showed that oral semaglutide was non-inferior to injection of semaglutide and superior to other comparators in regarding HbA1c and body weight. However, there has been no report to directly compare injection type of semaglutide and oral semaglutide regarding body composition. To our knowledge, this is the first report that 24-week treatment of oral semaglutide decreased body fat without affecting muscle mass in Japanese patients with type 2 diabetes. Because oral semaglutide is more acceptable for patients with type 2 diabetes than injection type of GLP-1RA, further study should be required for clarification of pharmacological significance and clinical usefulness of oral semaglutide.
The present study has several limitations. First, it was a non-interventional retrospective longitudinal analysis of a small number of patients with type 2 diabetes for short terms without control group. Thus, it is unclear whether the obtained results are applicable to a large population for a longer term. A future prospective interventional study with large number of patients comparing with control group may confirm the present results. Second, we did not check the changes in diet and exercise and did not evaluate appetite change. Thus, the exact mechanism of body fat reduction remains unclear. Third, we did not directly assess both muscle strength such as handgrip or leg extension, AVF, and IHL. Based on our previous studies, we discussed that muscle strength might not be decreased, and IHL may be reduced considering the lack of change in PhA and ECW/TBW and significant decrease in BMI, HbA1c, and ALT, respectively. Nevertheless, exact changes in muscle strength, AVF, and IHL were not evaluated, and thus a further study needs direct measurement to confirm these points. Fourth, we did not evaluate whether there was an additive or synergistic action of oral semaglutide with other anti-diabetic agents on body composition. As we showed in the Result section, many patients were already treated with other anti-diabetic agents and kept the same dosage during the study period. Thus, a future study in a large sample should investigate drug interaction on body composition.
In conclusion, 24-week treatment with oral semaglutide ameliorated glycemic control with reduction of body fat but not muscle mass in Japanese patients with type 2 diabetes.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was obtained in the form of an opt-out on our website.
Author Contributions
SU designed the study, participated in data collection, performed the statistical analysis, and wrote the manuscript. YS, SM, and YS participated in data collection and statistical analysis. MS and YT designated the study and edited the manuscript. All authors approved that final version for publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
References
- 1.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 2.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 3.Kim B, Tsujimoto T, So R, Zhao X, Oh S, Tanaka K. Changes in muscle strength after diet-induced weight reduction in adult men with obesity: a prospective study. Diabetes Metab Syndr Obes. 2017;10:187–194. doi: 10.2147/DMSO.S132707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore MC, Cherrington AD, Wasserman DH. Regulation of hepatic and peripheral glucose disposal. Best Pract Res Clin Endocrinol Metab. 2003;17(3):343–364. doi: 10.1016/s1521-690x(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 6.van der Aart-van der Beek AB, de Boer RA, Heerspink HJL. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat Rev Nephrol. 2022;18(5):294–306. doi: 10.1038/s41581-022-00535-6. [DOI] [PubMed] [Google Scholar]
- 7.Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18(8):783–794. doi: 10.1111/dom.12670. [DOI] [PubMed] [Google Scholar]
- 8.Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J. et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 9.Ohta A, Kato H, Ishii S, Sasaki Y, Nakamura Y, Nakagawa T, Nagai Y. et al. Ipragliflozin, a sodium glucose co-transporter 2 inhibitor, reduces intrahepatic lipid content and abdominal visceral fat volume in patients with type 2 diabetes. Expert Opin Pharmacother. 2017;18(14):1433–1438. doi: 10.1080/14656566.2017.1363888. [DOI] [PubMed] [Google Scholar]
- 10.Perna S, Guido D, Bologna C, Solerte SB, Guerriero F, Isu A, Rondanelli M. Liraglutide and obesity in elderly: efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin Exp Res. 2016;28(6):1251–1257. doi: 10.1007/s40520-015-0525-y. [DOI] [PubMed] [Google Scholar]
- 11.Ishii S, Nagai Y, Sada Y, Fukuda H, Nakamura Y, Matsuba R, Nakagawa T. et al. Liraglutide reduces visceral and intrahepatic fat without significant loss of muscle mass in obese patients with type 2 diabetes: a prospective case series. J Clin Med Res. 2019;11(3):219–224. doi: 10.14740/jocmr3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucheit JD, Pamulapati LG, Carter N, Malloy K, Dixon DL, Sisson EM. Oral semaglutide: a review of the first oral glucagon-like peptide 1 receptor agonist. Diabetes Technol Ther. 2020;22(1):10–18. doi: 10.1089/dia.2019.0185. [DOI] [PubMed] [Google Scholar]
- 13.Meier JJ, Bardtrum L, Cheng AYY, Malling B, Montanya E, Wagner L, Pratley RE. Body weight loss with oral semaglutide is mediated predominantly by effects other than gastrointestinal adverse events in patients with type 2 diabetes: A post hoc analysis. Diabetes Obes Metab. 2023;25(4):1130–1135. doi: 10.1111/dom.14957. [DOI] [PubMed] [Google Scholar]
- 14.Custodio Martins P, de Lima TR, Silva AM, Santos Silva DA. Association of phase angle with muscle strength and aerobic fitness in different populations: A systematic review. Nutrition. 2022;93:111489. doi: 10.1016/j.nut.2021.111489. [DOI] [PubMed] [Google Scholar]
- 15.Hioka A, Akazawa N, Okawa N, Nagahiro S. Extracellular water-to-total body water ratio is an essential confounding factor in bioelectrical impedance analysis for sarcopenia diagnosis in women. Eur Geriatr Med. 2022;13(4):789–794. doi: 10.1007/s41999-022-00652-2. [DOI] [PubMed] [Google Scholar]
- 16.Takemoto A, Sada Y, Oyanagi T, Sasaki Y, Sone M, Tanaka Y. Alanine aminotransferase, body mass index, and hemoglobin A1c may be useful markers for monitoring changes in intrahepatic lipid content in Japanese patients with overweight or obesity and type 2 diabetes. J Endocrinol Metab. 2022;12(2):73–78. [Google Scholar]
- 17.Gibbons C, Blundell J, Tetens Hoff S, Dahl K, Bauer R, Baekdal T. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes Metab. 2021;23(2):581–588. doi: 10.1111/dom.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpe S, Lisco G, Racaniello D, Fanelli M, Colaianni V, Vozza A, Triggiani V. et al. Once-weekly semaglutide induces an early improvement in body composition in patients with type 2 diabetes: a 26-week prospective real-life study. Nutrients. 2022;14(12):2414. doi: 10.3390/nu14122414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyanagi T, Sada Y, Sasaki Y, Sone M, Tanaka Y. Associations of phase angle obtained by bioelectrical impedance analysis with muscle mass and strength in Japanese patients with type 2 diabetes. Endocr J. 2023 doi: 10.1507/endocrj.EJ23-0022. [DOI] [PubMed] [Google Scholar]
- 20.Alorfi NM, Algarni AS. Clinical impact of semaglutide, a glucagon-like peptide-1 receptor agonist, on obesity management: a review. Clin Pharmacol. 2022;14:61–67. doi: 10.2147/CPAA.S374741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117(18):2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 22.Wang N, Tan AWK, Jahn LA, Hartline L, Patrie JT, Lin S, Barrett EJ. et al. Vasodilatory actions of glucagon-like peptide 1 are preserved in skeletal and cardiac muscle microvasculature but not in conduit artery in obese humans with vascular insulin resistance. Diabetes Care. 2020;43(3):634–642. doi: 10.2337/dc19-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdulla H, Phillips B, Wilkinson D, Gates A, Limb M, Jandova T, Bass J. et al. Effects of GLP-1 infusion upon whole-body glucose uptake and skeletal muscle perfusion during fed-state in older men. J Clin Endocrinol Metab. 2023;108(4):971–978. doi: 10.1210/clinem/dgac613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurjar AA, Kushwaha S, Chattopadhyay S, Das N, Pal S, China SP, Kumar H. et al. Long acting GLP-1 analog liraglutide ameliorates skeletal muscle atrophy in rodents. Metabolism. 2020;103:154044. doi: 10.1016/j.metabol.2019.154044. [DOI] [PubMed] [Google Scholar]
- 25.Andreozzi F, Raciti GA, Nigro C, Mannino GC, Procopio T, Davalli AM, Beguinot F. et al. The GLP-1 receptor agonists exenatide and liraglutide activate Glucose transport by an AMPK-dependent mechanism. J Transl Med. 2016;14(1):229. doi: 10.1186/s12967-016-0985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Ni CL, Yao Z, Chen LM, Niu WY. Liraglutide enhances glucose transporter 4 translocation via regulation of AMP-activated protein kinase signaling pathways in mouse skeletal muscle cells. Metabolism. 2014;63(8):1022–1030. doi: 10.1016/j.metabol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto Y, Nagai Y, Kawanabe S, Hishida Y, Hiraki K, Sone M, Tanaka Y. Effects of resistance training using elastic bands on muscle strength with or without a leucine supplement for 48 weeks in elderly patients with type 2 diabetes. Endocr J. 2021;68(3):291–298. doi: 10.1507/endocrj.EJ20-0550. [DOI] [PubMed] [Google Scholar]
- 28.Alhindi Y, Avery A. The efficacy and safety of oral semaglutide for glycaemic management in adults with type 2 diabetes compared to subcutaneous semaglutide, placebo, and other GLP-1 RA comparators: A systematic review and network meta-analysis. Contemp Clin Trials Commun. 2022;28:100944. doi: 10.1016/j.conctc.2022.100944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.