Abstract
Background:
Back pain prevalence and burden increase with age; approximately one-third of U.S. adults 65 years of age and older experience lower back pain (LBP). For chronic low back pain (cLBP), typically defined as lasting three months or longer, many treatments for younger adults may be inappropriate for older adults given their greater prevalence of comorbidities with attendant polypharmacy. While acupuncture has been demonstrated to be safe and effective for cLBP in adults overall, few studies of acupuncture have either included or focused on adults ≥ 65 years old.
Methods:
The BackInAction study is a pragmatic, multi-site, three-arm, parallel-groups randomized controlled trial designed to test the effectiveness of acupuncture needling for improving back pain-related disability among 807 older adults ≥ 65 years old with cLBP. Participants are randomized to standard acupuncture (SA; up to 15 treatment sessions across 12 weeks), enhanced acupuncture (EA; SA during first 12 weeks and up to 6 additional sessions across the following 12 weeks), and usual medical care (UMC) alone. Participants are followed for 12 months with study outcomes assessed monthly with the primary outcome timepoint at 6 months.
Discussion:
The BackInAction study offers an opportunity to further understand the effectiveness, dose-dependence, and safety of acupuncture in a Medicare population. Additionally, study results may encourage broader adoption of more effective, safer, and more satisfactory options to the continuing over-reliance on opioid- and invasive medical treatments for cLBP among older adults.
Trial Registration:
ClinicalTrials.gov Identifier: NCT04982315. Clinical trial registration date: July 29, 2021
Keywords: Chronic low back pain, acupuncture, pragmatic clinical trial, older adults
Introduction
Back pain is the leading cause of disability worldwide with both prevalence and burden increasing with age [1, 2]. About $86 billion is spent annually on direct costs of medical care for back/neck pain with markedly escalating costs in Americans 65 and older [3]. Despite these large investments in back-pain care [4], the health and functioning of Americans with back pain has deteriorated [3]. Chronic low back pain (cLBP), typically defined as lasting three months or longer, is the most consequential type of back pain. Approximately one-third of U.S. adults 65 and older experience lower back pain (LBP) [5], with symptoms and disability for many persisting for a year or more [6]. Many cLBP treatments considered appropriate for younger adults may not be appropriate for older adults given their greater prevalence of comorbidities with attendant polypharmacy [7]. In addition, burgeoning imaging rates reveal incidental pathology in many cases, placing older adults at risk for inappropriate invasive treatments [8-10]. Normal age-related physiological changes place older adults at substantially increased risk for adverse effects with commonly used LBP treatments [11-13] and medications such as nonsteroidal anti-inflammatory drugs [14], muscle relaxants, and opioids [6, 8, 14, 15]. Older populations have increased susceptibility to adverse events linked to opioids (e.g., delirium, sedation, dizziness, constipation, and falls) [16]. Opioid-related deaths in people 65 years and older increased 635% in the 15 years from 2001 to 2016 [17]. Opioids are also associated with increased disability, medical costs, subsequent surgery and continued opioid use [18]. Where the American College of Physicians guidelines for LBP recommends acupuncture as one first-line option for acute, subacute and cLBP, there are no specific data on the use of acupuncture for older adults [19, 20].
Thus, research is needed to clarify the cLBP treatments that are safe and effective for older adults. Studies focused on nonpharmacologic treatments may be especially beneficial, given concerns about medication safety with this population and research suggesting that older adults are open to trying nonpharmacological therapies [21, 22]. Acupuncture treatment has an excellent safety profile [23, 24] with few adverse events (AEs) reported across many large acupuncture studies involving thousands of patients and hundreds of acupuncture providers. These studies suggest that minor transient AEs such as bleeding or needle pain are the most common AEs (1-5 out of 100 patients), while serious AEs, such as pneumothorax, persistent nerve pain, or infection are very rare 1-3 in one million treatments) [23-26]. Further, there is evidence that acupuncture may improve outcomes beyond impact on pain and pain-related functioning such as sleep [27] and emotional symptoms [28]. Those with chronic pain report considering a variety of outcomes beyond pain important for their lives including decreases in fatigue and cognitive difficulties, improvements in emotional well-being, and ability to participate in everyday activities [29]. Such outcomes may be particularly important to older adults and may be better supported by acupuncture than by more conventional medication management.
While acupuncture has been found to be safe and effective for cLBP in adults overall, few acupuncture studies have focused on adults 65 years of age and older. Further, the optimal dose of acupuncture is unknown for adults with cLBP as is the impact of “maintenance treatment”, when acupuncture treatments are continued after improvement in pain or functioning has been achieved. Both dosage and the impact of maintenance treatment may differ for older adults who often have more comorbidities and, thus, may take longer to improve.
The goal of this pragmatic clinical trial is to address the critical gap in evidence on the effectiveness of acupuncture treatment for older adults with cLBP. Importantly, the study presented here was funded in response to a National Institutes of Health Request for Applications (https://grants.nih.gov/grants/guide/rfa-files/RFA-At-19-005.html) that anticipated at the time of request that evidence on improvements in health outcomes derived from the funded research would assist the Centers for Medicaid and Medicare Services (CMS) in determining Medicare coverage for acupuncture for cLBP. Yet, CMS began covering acupuncture for Medicare patients with cLBP in January 2020 based on the existing data on the effectiveness and low-risk profile of acupuncture that had been established in studies of the general population. However, the CMS reimbursement criteria has narrow allowances for which providers can be reimbursed for acupuncture that precludes the vast majority of those licensed and providing such services in the US. For the narrower band of allowed providers, Medicare covers 12 acupuncture needling sessions over 3 months for patients with cLBP with an additional 8 sessions if the patient shows improvement, not to exceed a total of 20 sessions in a 12-month period [30]. The current study presents an opportunity to further understand the effectiveness, dose-dependence, and safety of acupuncture in a Medicare population experiencing cLBP and, as such, may inform future revisions to current CMS coverage policy.
Methods
Study Overview
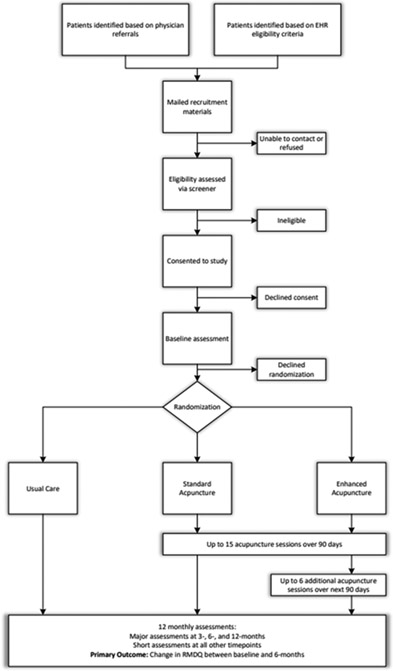
The BackInAction study is designed to test the effectiveness of acupuncture needling for improving back pain-related disability among 807 older adults (65 years of age and older) with cLBP. The study is designed as a three-arm pragmatic trial to evaluate the impact of the following treatments on back-pain-related disability (Figure 1):
Figure 1.
BackInAction Study Design and Participant Flow
Standard course of acupuncture (up to 15 treatment sessions across 12 weeks; standard acupuncture [SA] arm)
Standard course of acupuncture plus a maintenance treatment phase (up to 6 additional sessions across the following 12 weeks; “enhanced” acupuncture [EA] arm)
Usual medical care alone (UMC arm).
Consistent with the pragmatic focus of the design, the study includes patients from a variety of healthcare systems (HCSs) in different regions of the country, primarily utilizes community acupuncturists working in their everyday clinic settings. Somewhat less-pragmatic features of approach and design were specified in the funding announcement and represent required features of the study design as determined in partnership with the National Center for Complementary and Integrative Health (NCCIH) and CMS and include: restriction of acupuncture to needling only, specification of number and timing of acupuncture sessions across the SA and EA arms, and a 6-month primary outcome timepoint.
Study settings and sample
Participants will be enrolled from four HCSs that represent a variety of healthcare delivery types and geographical regions. Two of our participating HCSs, Kaiser Permanente Washington (KPWA) and Kaiser Permanente Northern California (KPNC), are integrated care delivery systems. Sutter Health (SH) is largely a fee-for-service HCS also in Northern California. Our fourth site is the Institute for Family Health (IFH), a network of Federally Qualified Healthcare Centers (FQHCs) in New York City. Collectively these HCSs serve an ethnically, racially, and socioeconomically diverse population. While our overall recruitment yielded 807 consented participants, our power calculations were based on enrolling at least 789 participants with some overage allowed for to accommodate those who might still be in the recruitment pipeline when this threshold was reached. The planned distribution of our target sample of 789 patients across the participating HCSs considers each site’s ability to enroll a racially, ethnically, and socioeconomically diverse sample as well as variations in size of the HCSs and, thus, availability of eligible patients (KPNC target: 288, SH target: 204, KPWA target: 174, IFH target: 123). The study eligibility criteria (Table 1) are broad with few exclusions to increase the generalizability of the findings.
Table 1.
Inclusion and exclusion criteria for the BackInAction study cohort
| Inclusion Criteria | Exclusion Criteria |
|---|---|
Electronic Health Record (EHR) determined:
|
|
EHR = electronic health record; PEG= Pain intensity, pain interference with Enjoyment of life, pain interference with General activity (3-item scale); PCP = primary care provider; SH = Sutter Health; KPNC = Kaiser Permanente Northern California, KPWA = Kaiser Permanente Washington, IFH = Institute for Family Health, cLBP = chronic low back pain.
Recruitment and enrollment
Recruitment (and eligibility) of Study Acupuncturists
The selection of acupuncturists and location of study acupuncture services is intended to mirror real-world service delivery across our participating HCSs. For KPWA, KPNC, and SH community practicing acupuncturists among the existing HCS referral networks will be contacted to ascertain interest and qualifying experience. In these HCSs, recruitment will be prioritized to match targeted areas of patient recruitment and provide broad geographic coverage such that enrolled patients had one or more study acupuncturists close to their primary care provider to reduce transportation barriers. At the New York City-based IFH FQHCs, acupuncturists were hired to provide services directly in the IFH primary care clinics.
All trial acupuncturists are required to be licensed and qualified to practice in the state where care is provided and have the equivalent of 5 years of clinical experience post-licensing in treating older adults with cLBP and multi-morbidities.
All study acupuncturists are required to complete study provider training including an orientation to the trial and trainings on pertinent trial logistics, HIPAA for research, human subjects’ protections, expectations for aligning study treatments with consensus intervention protocol[33] and a review of safety for acupuncture needling in older adults.
Recruitment and Consenting of Study Participants
The BackInAction trial uses two main recruitment methods for potential participants. In the first recruitment method, all four sites query the EHR to identify patients potentially meeting study eligibility criteria as specified above. One of the sites (KPNC) requires that PCPs have the option to opt-out a prospective participant from the study, so they are given the opportunity to do this after patients are identified as potentially eligible from the EHR. Patients are then mailed recruitment materials and are given either/both options to call or mail in a postcard to the study team to opt-in or out of the research. Proactive calls are routinely made by study staff to these patients to complete the study screener necessary for determining eligibility.
The second recruitment method is used by IFH as their primary recruitment method. Patients are identified as potential candidates for the study by their PCP, either through personal knowledge of the patient’s condition or based on study initiated best practice alerts, which let providers know that a patient might be eligible for the study. These patients are referred to the study team who confirm full EHR-eligibility as described above and are proactively outreached to complete the eligibility screener by phone.
Interested patients who are determined to be eligible through EHR and telephone screening are consented to the study using either oral, written, or electronic written consent procedures according to the local site’s requirements. Consented patients are asked to complete a telephone baseline assessment and are then randomized to one of the three study arms as described below. An example of the consent and HIPPA authorization forms used are available in supplemental materials. All study procedures and databases are in compliance with federal and state laws and guidance documents regarding protection of human subjects' privacy. All data transfers will be conducted via a secure, HIPPA-compliant website.
Randomization and Blinding
The study employs stratified blocked randomization, in which participants are individually randomized to the UMC, SA, or EA arms in a 1:1:1 ratio, stratified by site (IFH, KPNC, KPWA, and SH), age group (65-74, 75-84, 85+), and sex (male, female/other). Block sizes of 3 and 6 are randomly varied throughout the randomization list. The study Biostatistician computer-generates the sequence of potential block randomizations within each stratum and securely provides randomization files to the study programmer who implements them into a database randomization module. Participants are randomized upon completion of the baseline interview, where stratification variables are collected, using the REDCap database randomization module, but the database only shows if the participant is randomized to usual care or acupuncture but not whether acupuncture-assigned participants are randomized to SA versus EA. This maintains participants’ blinding to receipt of maintenance acupuncture for the first 3 months. To maintain interviewer blinding, baseline interviewers who randomize a given participant will not conduct any of their follow-up assessments. Approximately 10 weeks into the study, all participants randomized to an acupuncture arm and their acupuncturists will be informed whether or not they will receive additional maintenance treatment sessions via unblinded study personnel who do not conduct follow-up assessments.
This is an unmasked trial for participants, although the participants assigned to SA and EA will only know they are randomized to acupuncture, and not SA or EA, at the time of randomization.
Study Interventions
The study includes two active intervention arms: (1) Standard course of acupuncture (up to 15 sessions of treatment across 12 weeks; SA arm) and (2) Standard course of acupuncture plus a maintenance phase of treatment (up to 6 additional sessions across the following 12 weeks; EA arm). Both of these active interventions are compared to (3) a UMC-only arm. Participants in the UMC arm of the study are asked to avoid acupuncture over the course of the study (although all participants are asked to report any out-of-study acupuncture they have received at the 12-month follow-up assessment). All participants in the study will have access to UMC and those randomized to receive acupuncture are not charged for study-related acupuncture visits. Participants randomized to receive acupuncture can discontinue treatment at any time.
As a pragmatic trial, our intention is to evaluate the benefits of acupuncture as delivered in everyday healthcare settings where acupuncture is routinely provided. Study acupuncturists are expected to follow treatment guidelines reflected in the consensus intervention manual developed during the initial year of project funding and pilot work [33] that sought to strike a balance between standardization and flexibility for the study acupuncture intervention [34, 35]. Further details about key parameters of the development of the study acupuncture intervention guidelines are reported in Nielsen et al 2021 [33] and Table 2 summarizes key features of the treatment approach based on consensus of the Acupuncture Advisory Panel and organized according to the STRICTA-checklist, an extension of the CONSORT statement for reporting acupuncture trials [36]. Lastly, due to the pragmatic nature of the trial, no specific intervention retention efforts were undertaken – we expect these to be variable across acupuncturists reflecting “real-world” acupuncture office procedures (e.g., an office may have appointment reminder phone calls).
Table 2.
Key Features of Acupuncture Treatments (Standard and Maintenance Phases)
| STRICTA* Domain | BIA Treatment Parameters |
|---|---|
| Treatment Rationale | |
| Treatment Rationale: Style of Acupuncture | Traditional East Asian Medicine, typically including an interview and palpation before providing acupuncture treatment. Exclusively microsystem focused treatments NOT permitted. |
| Treatment Rationale: Basis for Treatment | Modified Delphi Process made recommendations based on treatment information from large clinical trials of chronic low back pain and other published literature on treatments provided in advance to panel members [REFs] |
| Treatment Rationale: Variability of Treatment | Variability in treatment to respond to patient’s presentation is expected |
| Needling Details: Needles/Study Participant | Recommend 6 – 20 needles |
| Needling Details | |
| Needling Details: Names of Points | Recommend both local and distal points, selecting from a series of 113 named acupoints (214 acupoints total if counting bilaterally): low back acupoints (29; 58 if bilateral; 4 central for total of 62), acupoints on the mid and upper back (33; 66 if bilateral; 2 central for total of 68), front of the body including distal leg acupoints (33; 66 if bilateral; 6 central points for total of 72) and ear acupoints (6; 12 if bilateral). In addition, ashi points – selected by tenderness to palpation - are permitted. Other acupoints should include the rationale. |
| Needling Details: Depths of Insertion | Recommend needling to 75% of safe needling depth for a particular point; shallow needling prohibited |
| Needling Details: De qi sought? | Recommend obtaining de qi |
| Needling Details: Type of Needle Stimulation | Require manual stimulation only |
| Needling Details: Needle Retention Time | Recommend 20-30 minutes for needling only one side of the body (though needles could be in place for up to 40 minutes) and 10-25 minutes/side for both sides |
| Needling Details: Needle Type | Uncoated needles are recommended. Other needle details such as gauge and length are entirely up to the discretion of the acupuncturist. |
| Treatment Regimen and Components | |
| Treatment Regimen: Number of Treatment Sessions | Standard period: allow no more than 15 treatment sessions, and recommend at least 8. Maintenance period: allow no more than 6 treatment sessions, and recommend at least 4 treatment sessions. |
| Treatment Regimen: Frequency and Duration of Sessions | Standard period: recommend at least 6-8 treatments in the first 60 days and at least 1-2 treatments in the last 30 days. Maintenance period: a minimum of 4 treatments is recommended, no further guidance on temporality. |
| Other Key Descriptors of Treatment | |
| Other Components of Treatment: Adjunctive treatments | No adjunctive treatments are permitted. Life style recommendations are permitted. |
| Other Components of Treatment: Setting and Context of Treatment | In the offices of community acupuncturists (3-sites) or in the primary care clinic (1-site). Acupuncturists required to complete training on the protection of human subjects in the context of research, an orientation to the trial and intervention protocol, a review of best practices for safe administration of acupuncture, and study logistics including getting patients from the study team and recording the treatment. |
| Qualifying Acupuncturist Experience | At least 3 years of experience (5 yrs preferred); experience treating older adults; experience treating chronic low back pain and multimorbidities; willingness to adhere to the study constraints |
STRICTA=Standards of Reporting for Clinical Trials of Acupuncture; STRICTA is an extension of the CONSORT statement for reporting acupuncture interventions in clinical trials.
Importantly, the study intervention design was also influenced by the requirements established by the funder to align with CMS-expected parameters for Medicare-reimbursable acupuncture treatment under public comment at the time the study was designed and proposed – namely, that the intervention be restricted to needling. Consequently, we asked study acupuncturists to avoid using other specific modalities of treatment that are often used as part of acupuncture therapy in Chinese medicine as adjuncts to needling (e.g., electroacupuncture, moxibustion, application of heat, Gua Sha, cupping, or herbal medicine). Finally, inclusion of 2 active acupuncture intervention arms, SA and EA, was in accordance with the funder and CMS’ interest in better understanding the frequency and duration of needling sessions necessary for therapeutic effect in older adults with cLBP and the relative benefits of including a maintenance phase of treatment. We selected the maximum number of 15 sessions allowed for the SA arm (as well as for the first phase of treatment for those receiving maintenance sessions in the EA arm) based on prior trials’ practices and findings and the belief of acupuncturists that older adults with multi-morbidities may take longer to improve. We will encourage acupuncturists to treat patients for a minimum of 8 treatments in the SA arm/phase of treatment, which is based on data from meta-analyses [37] and from the AADDOPT-2 trial [38]. We allow variability in the number of treatment sessions so that the acupuncturist and patient can, within the study guidelines, engage the number of treatment sessions optimal for any particular participant. The EA arm allows for up to 6 additional acupuncture needling treatment over the next 3-month period anticipating that the frequency of sessions will be tapered during the maintenance phase. An intent-to-treat analysis will be used to analyze all participants, regardless of the number of treatments administered.
Primary and Secondary Outcomes
Our approach to measurement includes adoption of many recommendations by the NIH Research Task Force for Low Back Pain [39], the IMMPACT domains for clinical trials of chronic pain [40] and includes the required NIH PRISM HEAL Common Data Elements (CDE) [41]. Outcome measures and their planned administration is summarized in Table 3. Our primary outcome is back-related disability as measured by the change in score from baseline to 6 months in the 24 item Roland-Morris Disability Questionnaire (RMDQ) [44]. As a secondary outcome, we will consider the proportion of individuals who experience a minimally clinically significant difference of a change in RMDQ of 2 or more points. Additional detail about study-related measures is included in the Supplement.
Table 3.
BackInAction study timeline and outcomes
| STUDY PERIOD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enrollment | Randomization | Post-randomization | Closeout | |||||||
| Timepoint | 0 months | 1 month |
2 months |
3 months |
4 months |
5 months |
6 months |
7, 8, 9, 10, & 11 months |
12 months |
|
| ENROLLMENT | ||||||||||
| Eligibility screen | X | |||||||||
| Informed consent | X | |||||||||
| Randomization | X | |||||||||
| INTERVENTIONS: | ||||||||||
| Standard acupuncture |
|
|||||||||
| Enhanced acupuncture |
|
|||||||||
| Usual Care | ||||||||||
| MEASURES: | ||||||||||
| Additional Baseline and Related Descriptive Measures: | ||||||||||
| Patient Characteristics ¥ * | X | |||||||||
| Medical and Back Pain History ¥ * | X | |||||||||
| Expectations of Acupuncture * | X | |||||||||
| Abbreviated COVID questions * | X | X | X | X | ||||||
| Telephone Interview for Cognitive Status (TICS) * | X | |||||||||
| High Impact chronic pain [CDE] * | X | X | ||||||||
| Euro-Quol-5D * | X | X | X | X | ||||||
| Pain Catastrophizing Questionnaire (6-item) [CDE] * | X | X | ||||||||
| Substance Use (TAPS) [CDE] * | X | X | ||||||||
| Primary, Secondary, and Tertiary Measures: | ||||||||||
| Back-related disability (Roland Morris Disability Questionnaire) * 1,2 | X | X | X | X | ||||||
| PEG [CDE] * 2 | X | X | X | X | X | X | X | X | X | |
| Physical Function (PROMIS-6 item) [CDE] * 2 | X | X | X | X | X | X | X | X | X | |
| Anxiety (PHQ-4) [CDE] * 3 | X | X | X | X | ||||||
| Depression (PHQ-4) [CDE] * 3 | X | X | X | X | ||||||
| Sleep Disturbance (PROMIS 6-item) [CDE] * 3 | X | X | ||||||||
| Sleep Duration (PROMIS) * 3 | X | X | X | |||||||
| Patient Global Impression of Change [pain][CDE] * 2 | X | X | X | |||||||
| Fatigue (PROMIS 4-item) * 3 | X | X | X | X | ||||||
| Ability to Participate in Social Roles and Activities (PROMIS 4-item) * 3 | X | X | X | X | ||||||
| Patient Global Impression of Change [overall status] * 3 | X | X | X | X | ||||||
| Treatment-Related Information: | ||||||||||
| Adverse Events ¥ * ‡ | X | X | ||||||||
| Serious Adverse Events ¥ ‡ | X | |||||||||
| Use of acupuncture during study period * | X | |||||||||
| Adherence to Assigned Treatment ‡ | X | X | X | |||||||
| Health Care Utilization: | ||||||||||
| Health Care Utilization and Costs ¥ # * | X | X | X | X | ||||||
| Pain-related Health Services, Products & Self-Management Practices * | X | X | X | X | ||||||
| Daily Exercise and Job-related Activity * | X | X | X | X | ||||||
Data source: ¥EHR, *PRO, ‡treatment records, and #Medicare fee schedule; CDE: HEAL Common Data Elements (required for all HEAL trials)
Primary
secondary, and
tertiary measures
Baseline measures closely precede randomization
Statistical Analysis
The main study aim is to evaluate the effectiveness of standard acupuncture (SA) and acupuncture plus maintenance (EA) in improving back-related disability (RMDQ) relative to Usual Medical Care (UMC) alone at 6 months post randomization. Secondary analyses include evaluating back-related disability at 3- and 12-months post randomization. We will conduct a longitudinal analysis including the continuous outcome change in RMDQ from baseline (primary outcome) measured at all follow-up times in one model estimated using generalized estimating equations (GEE) [42]. We will assume an independent working correlation matrix and calculate standard errors using the robust sandwich estimator to account for within-person and within-provider correlation (as some participants may see the same acupuncturist). All models will adjust for baseline RMDQ score, age, sex, race and HCS. All analyses, unless otherwise specified, will follow an intent-to-treat approach, including all individuals as randomized regardless of their engagement with, or exposure to, the intervention.
We will include interactions between indicators of the intervention groups (SA and EA) and indicators of time (6-, and 12-months) to estimate time-specific intervention effects. To gain power, since SA and EA at 3-months are the same intervention (maintenance begins after 3 months) we will use a single acupuncture group (A) indicator at 3-months. For the 6-month timepoint, following a significant omnibus test of all three interventions (see Supplement for multiple comparison control details), we will conduct a sequence of analyses, beginning with a GEE model treating SA and EA as separate groups by including indicators for each acupuncture group (SA and EA) for the 6-month timepoint. We will then assess differences in change in RMDQ at 6-months between the two acupuncture groups: with (EA) and without (SA) maintenance. If a statistically significant (α=0.05) and meaningful difference (>1 point difference) is found between the maintenance (EA) versus no maintenance (SA) groups (Scenario 1), we will further compare each of the acupuncture groups separately to UMC. Scenario 1 assessments will determine if EA is better than SA at 6-months and if either or both acupuncture groups are better than UMC. If acupuncture groups do not differ meaningfully at 6-months (Scenario 2), we will combine acupuncture groups for this time point and run a second GEE model including only an indicator of the combined acupuncture group. If this GEE model shows that acupuncture is better than UMC, we will conclude that acupuncture improved RMDQ at 6-months, but maintenance was not shown to be efficacious. We will follow the same general framework for the 12-month time-point as specified for 6-months. More detail about these quantitative study analyses as well as summaries of collected data and analyses associated with the study formative, process, and economic evaluations is included in the Supplement.
Sample size
The study sample size of at least 789 (263 per arm) was selected to provide at least 90% power to detect a clinically meaningful difference of 2 points between each acupuncture group compared to UMC (pair-wise comparison power) on the primary outcome, the RMDQ at 6 months. Power was calculated via simulation using R software (version 3.6). Estimates were made using GEE and assumed a 20% missing-data rate, a standard deviation (SD) of 6 points in the RMDQ outcome in each arm [43-45], and control of multiple comparisons across the three intervention groups. Additional details of power analyses are included in the Supplement.
Oversight and Safety Monitoring
Institutional Review Board (IRB) Oversight
IRB oversight is provided for the study by a single IRB at KPNC (#1474280; IRB FWA# 00002344) with individual IRBs for each of the HCSs ceding local authority yet reviewing all materials and procedures for alignment with HCS and regional standards and to ensure HIPAA compliance.
Adverse Events
Adverse events will be routinely monitored through acupuncture report, participant surveys, and monthly EHR monitoring. All reportable events, including adverse events, will follow guidelines set forth by the national Office for Human Research Protections and the single IRB at KPNC. Adverse events will be adjudicated without knowledge of randomization assignment by study-designated physicians at each site to determine severity and potential relatedness to acupuncture.
Data Safety and Monitoring Board (DSMB)
The DSMB was established by NCCIH and convened to monitor and advise on the study-related participant safety and data quality. The DSMB meets two times per year to review reports from the study team, and no interim analyses are planned. While there are no strict stopping rules, the DSMB and single IRB will have authority to stop or alter study procedures as needed to ensure participant safety.
Discussion
The BackInAction pragmatic clinical trial addresses one of the most pervasive and costly public health issues in the United States: identifying safe and cost-effective treatments for cLBP among older adults for whom prevalence of the problem is high and pharmacotherapy and its attendant risks make potentially effective nonpharmacologic treatments approaches like acupuncture especially attractive. The trial tests the effectiveness of two different courses of acupuncture needling compared to usual care – a standard course of up to 15 sessions across 12 weeks, and a condition which adds to this standard acupuncture a maintenance phase allowing up to 6 additional sessions across the following 12 weeks. Our study builds upon considerable previous research on the effectiveness of acupuncture needling for cLBP by focusing exclusively on the needs and outcomes of older adults. We employ a design allowing us to explore the optimal dose of acupuncture and effects of including a maintenance phase of treatment by conducting the study within a pragmatic framework that is focused on applicability, broad inclusion, flexibility in intervention implementation, and attention to the outcomes most meaningful to key stakeholders.
Importantly, the impetus for this study emerged from the CMS’s initial interest in and later allowance for Medicare coverage for acupuncture needling. Elements of our approach were intended to align with parameters of Medicare-reimbursable acupuncture treatment including the restriction to needling and the phasing and treatment sessions allowed. While consequently less flexible than some pragmatic frameworks might allow, this does provide opportunity to inform this important expansion of covered healthcare treatment for older adults – the effectiveness of such an approach and the potential facilitators and barriers to provision of such care. Other strengths of the study arise from the pragmatic framework adopted including recruiting older adult patients from a variety of HCSs in different regions of the country, primarily utilizing community acupuncturists working in their everyday clinic settings and includes numerous secondary outcomes of importance to older adults and for which acupuncture has shown some promise. An additional study strength is the balance between rigor (treatment aligned with protocols among positive treatment trials of acupuncture treatment trials) and flexibility for community acupuncturists to adapt their treatment approach to the potentially unique needs of older adults with cLBP and multi-morbidities as well as acupuncturists' preferred practice approach. A further strength of the study is its anticipated large and diverse sample with few exclusion criteria hence, generalizable to the overall older adult population in the USA.
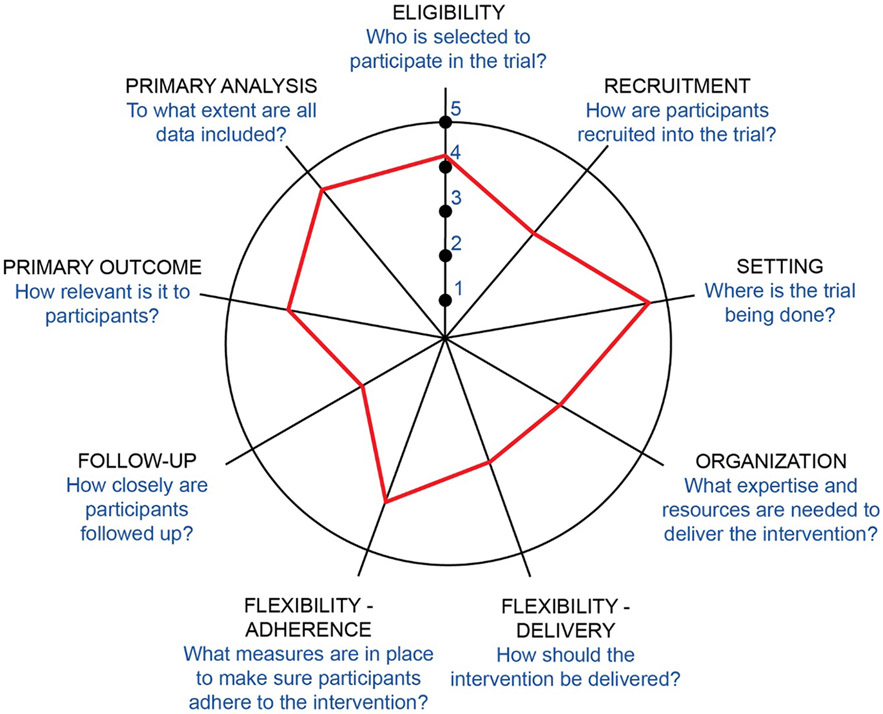
The Pragmatic-Explanatory Continuum Indicator Summary (PRECIS-2) is a tool developed to describe how “pragmatic” a specific trial is with respect to nine key domains [46]. Figure 2 illustrates where the BackInAction pragmatic clinical trial falls on this continuum for each domain (with those items closer to the center [“1”] representing the least pragmatic and items further towards the perimeter [“5”] representing the most pragmatic), as judged collectively by the research team who are authors of this paper. This illustrates that, although the trial is very pragmatic on many of these domains (eligibility, setting, flexibility: adherence, primary outcome, and primary analysis), others are less pragmatic by design as appropriate for efficient recruitment and retention necessary to evaluate key study questions with rigor (recruitment, follow-up, organization, flexibility: adherence).
Figure 2.
BackInAction PRECIS-2 Wheel
In summary, the BackInAction study is a novel pragmatic clinical trial designed to align with key parameters of current Medicare coverage for acupuncture for older adults with cLBP while incorporating pragmatic features to best reflect the diversity of the population of older adults with cLBP and their treatment needs as well as acupuncturists currently delivering such services in everyday practice settings. The current study presents an opportunity to further understand the effectiveness, dose-dependence, and safety of acupuncture in a Medicare population and, as such, may inform future revisions to current CMS coverage policy potentially encouraging adoption of a broader set of more effective, safer, and more satisfactory alternatives to the continuing over-reliance on opioid- and other more invasive medical treatments for cLBP among older adults.
Supplementary Material
Acknowledgements
First and foremost, the authors acknowledge Karen Sherman, who had to step away from the project during the development of this manuscript but was one the original principal investigators on the study. Dr. Sherman’s earlier research on acupuncture interventions was part of the critical foundation for this trial and we would not have been able to initiate the project nor execute the early part of the trial as rigorously without her strong support and involvement. Further, the authors gratefully acknowledge the contribution of the broader study team in carrying out this trial: Matthew Beyrouty, Lisa Dean, Heather Law, Gabriela Sanchez, Juanita Trejo, Julia Anderson, Crystal Aparicio, Crystal Baggott, Mahesh Bulble, Bianca DiJulio, Michelle Goodreau, Gabrielle Gundersen, Laurel Hansell, Alyssa Hernandez, Ariel Jacobs, Douglas Kane, Aidan Nguyen, Luesa Healy, Chi Tran, Sherry Kubitz, Vicki Li, Donna Mah, Talia Maridueña-Urdanigo, Catherine Nasrallah, Ridhima Nerlekar, Phoebe Rosenheim, Estefhany Soto Cossio, Pamela del Valle, Nora Wheat, and Yishi Xian. Finally, we are grateful for the strong support of our NIH colleagues, Lanay Mudd, Basil Eldadah, Qilu Yu, and Robin project scientist, Basel Eldadah, and Robin Boineau
Funding
Research reported in this publication was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under award numbers UG3AT010739 and UH3AT010739. The study sponsor collaborated with study investigators to approve the final study design; however, they had no role in collection, management, analysis, or interpretation of data; writing of the report; or the decision to submit the report for publication. As such, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
National Center for Complementary and Integrative Health, 6707 Democracy Boulevard, Suite 401, Bethesda, Maryland 20892-5475 nccih.nih.gov
Abbreviations:
- AE
adverse event
- CDE
Common Data Elements
- cLBP
chronic low back pain
- CMS
Centers for Medicaid and Medicare Services
- DSMB
Data Safety and Monitoring Board
- EA
enhanced acupuncture
- EHR
electronic health record
- FQHC
federally qualified healthcare center
- GEE
generalized estimating equations
- HCS
healthcare system
- HIPAA
Health Insurance Portability and Accountability Act
- IFH
Institute of Family Health
- IRB
Institutional Review Board
- KPNC
Kaiser Permanente Northern California
- KPWA
Kaiser Permanente Washington
- LBP
lower back pain
- LSD
least significant difference
- MCID
minimal clinically important difference
- NCCIH
National Center for Complementary and Integrative Health
- NIH
National Institutes of Health
- PCP
primary care provider
- PRECIS-2
Pragmatical-Explanatory Continuum Indicator Summary
- RMDQ
Roland-Morris Disability Questionnaire
- SA
standard acupuncture
- SH
Sutter Health
- sIRB
single Institutional Review Board
- UMC
usual medical care
Footnotes
Ethics approval and consent to participate
NCCIH Protocol: Study Protocol: Trial of Acupuncture for Chronic Low Back Pain in Older Adults (BackInAction). Version 1.6. June 2022. Approved by the “Kaiser Permanente Northern California Institutional Review Board” (IRB #00001045, FWA #00002344) the sIRB of record for this study.
Availability of data and materials
The final datasets analyzed during the current study will be made available according to HEAL DataSharing Policy (https://heal.nih.gov/data/public-access-data). Specific data repository, criteria for data access, and timing of dataset availability will be determined by NIH HEAL leadership.
Competing interests
The authors have no competing interests to disclose.
CReditT author statement
Lynn L. DeBar: Conceptualization, Methodology, Validation, Investigation, Writing – Original Draft, Writing – Review and Editing, Visualization, Supervision, Project administration, Funding acquisition. Morgan Justice: Methodology, Validation, Investigation, Data Curation, Writing – Original Draft, Writing – Review and Editing, Visualization, Supervision, Project administration. Andrew L. Avins: Conceptualization, Methodology, Investigation, Writing – Review and Editing, Supervision, Project administration, Funding acquisition. Raymond Y. Teets: Conceptualization, Methodology, Investigation, Writing – Review and Editing, Supervision, Project administration, Funding acquisition. Katie L. Stone: Investigation, Writing – Review and Editing, Supervision. Alice Pressman: Conceptualization, Methodology, Investigation, Writing – Review and Editing, Supervision, Project administration, Funding acquisition. Andrea Cook: Conceptualization, Validation, Formal Analysis, Methodology, Investigation, Writing – Review and Editing. Robert Wellman: Validation, Formal Analysis, Methodology, Investigation, Writing – Review and Editing. Patricia M. Herman: Conceptualization, Methodology, Writing – Review and Editing. Arya Nielsen: Methodology, Writing – Review and Editing. Clarissa Hsu: Methodology, Investigation, Writing – Review and Editing. Carolyn M. Eng: Methodology, Validation, Investigation, Data Curation, Writing – Original Draft, Writing – Review and Editing, Visualization, Project administration.
References
- 1.Vos T., et al. , Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet, 2017. 390(10100): p. 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blyth FM, et al. , The Global Burden of Musculoskeletal Pain-Where to From Here? Am J Public Health, 2019. 109(1): p. 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin BI, et al. , Expenditures and health status among adults with back and neck problems. Jama, 2008. 299(6): p. 656–64. [DOI] [PubMed] [Google Scholar]
- 4.Deyo RA, et al. , Overtreating chronic back pain: time to back off? J Am Board Fam Med, 2009. 22(1): p. 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackwell DL and Villarroel MA. Tables of Summary Health Statistics for U.S. Adults: 2016 National Health Interview Survey. National Center for Health Statisitics; Table A-5a-c , pages 1–9. 2018. [cited 2022 Sept 17]; Available from: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_A-5.pdf. [Google Scholar]
- 6.Rundell SD, et al. , The clinical course of pain and function in older adults with a new primary care visit for back pain. J Am Geriatr Soc, 2015. 63(3): p. 524–30. [DOI] [PubMed] [Google Scholar]
- 7.Makris UE, et al. , Management of persistent pain in the older patient: a clinical review. Jama, 2014. 312(8): p. 825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner DK, Introduction to Special Series: Deconstructing chronic low back pain in the older adult: shifting the paradigm from the spine to the person. Pain Med, 2015. 16(5): p. 881–5. [DOI] [PubMed] [Google Scholar]
- 9.Jarvik JG, et al. , Association of early imaging for back pain with clinical outcomes in older adults. Jama, 2015. 313(11): p. 1143–53. [DOI] [PubMed] [Google Scholar]
- 10.Powell AC, et al. , Health Care Utilization and Pain Outcomes Following Early Imaging for Low Back Pain in Older Adults. J Am Board Fam Med, 2019. 32(6): p. 773–780. [DOI] [PubMed] [Google Scholar]
- 11.Arnstein P., Balancing analgesic efficacy with safety concerns in the older patient. Pain Manag Nurs, 2010. 11(2 Suppl): p. S11–22. [DOI] [PubMed] [Google Scholar]
- 12.Barkin RL, et al. , Should nonsteroidal anti-inflammatory drugs (NSAIDs) be prescribed to the older adult? Drugs Aging, 2010. 27(10): p. 775–89. [DOI] [PubMed] [Google Scholar]
- 13.Schofield P., et al. , Evidence-based clinical practice guidelines on the management of pain in older people - a summary report. Br J Pain, 2022. 16(1): p. 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper C., et al. , Safety of Oral Non-Selective Non-Steroidal Anti-Inflammatory Drugs in Osteoarthritis: What Does the Literature Say? Drugs Aging, 2019. 36(Suppl 1): p. 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnstein P and Herr K, Risk evaluation and mitigation strategies for older adults with persistent pain. J Gerontol Nurs, 2013. 39(4): p. 56–65; quiz 66-7. [DOI] [PubMed] [Google Scholar]
- 16.Gold LS, et al. , Associations of Early Opioid Use With Patient-reported Outcomes and Health Care Utilization Among Older Adults With Low Back Pain. Clin J Pain, 2018. 34(4): p. 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes T., et al. , The Burden of Opioid-Related Mortality in the United States. JAMA Netw Open, 2018. 1(2): p. e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manchikanti L., et al. , Opioid epidemic in the United States. Pain Physician, 2012. 15(3 Suppl): p. Es9–38. [PubMed] [Google Scholar]
- 19.Chou R., et al. , Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med, 2017. 166(7): p. 493–505. [DOI] [PubMed] [Google Scholar]
- 20.Qaseem A., et al. , Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Annals of Internal Medicine, 2017. 166(7): p. 514–530. [DOI] [PubMed] [Google Scholar]
- 21.Reid MC, Ong AD, and Henderson CR Jr., Why We Need Nonpharmacologic Approaches to Manage Chronic Low Back Pain in Older Adults. JAMA Intern Med, 2016. 176(3): p. 338–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townley S., et al. , Preparing to implement a self-management program for back pain in new york city senior centers: what do prospective consumers think? Pain Med, 2010. 11(3): p. 405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bäumler P., et al. , Acupuncture-related adverse events: systematic review and meta-analyses of prospective clinical studies. BMJ Open, 2021. 11(9): p. e045961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witt CM, et al. , Safety of acupuncture: results of a prospective observational study with 229,230 patients and introduction of a medical information and consent form. Forsch Komplementmed, 2009. 16(2): p. 91–7. [DOI] [PubMed] [Google Scholar]
- 25.White A., et al. , Survey of adverse events following acupuncture (SAFA): A prospective study of 32,000 consultations. Acupunct Med, 2001. 19: p. 84–92. [DOI] [PubMed] [Google Scholar]
- 26.MacPherson H., et al. , Patient reports of adverse events associated with acupuncture treatment: a prospective national survey. Qual Saf Health Care, 2004. 13(5): p. 349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin X., et al. , Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med, 2017. 37: p. 193–200. [DOI] [PubMed] [Google Scholar]
- 28.Amorim D., et al. , Acupuncture and electroacupuncture for anxiety disorders: A systematic review of the clinical research. Complement Ther Clin Pract, 2018. 31: p. 31–37. [DOI] [PubMed] [Google Scholar]
- 29.Turk DC, et al. , Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain, 2008. 137(2): p. 276–85. [DOI] [PubMed] [Google Scholar]
- 30.cms.gov. Decision Memo for Acupuncture for Chronic Low Back Pain (CAG-00452N). 2020; Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=295; Accessed Sept 17, 2022.
- 31.Krebs EE, et al. , Development and Initial Validation of the PEG, a Three-item Scale Assessing Pain Intensity and Interference. Journal of General Internal Medicine, 2009. 24(6): p. 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callahan CM, et al. , Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care, 2002. 40(9): p. 771–81. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen A., et al. , Acupuncture intervention protocol: Consensus process for a pragmatic randomized controlled trial of acupuncture for management of chronic low back pain in older adults, an NIH HEAL Initiative funded project. Glob Adv Health Med, 2021. 10: p. 2164956121100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnyer RN and Allen JJ, Bridging the gap in complementary and alternative medicine research: manualization as a means of promoting standardization and flexibility of treatment in clinical trials of acupuncture. J Altern Complement Med, 2002. 8(5): p. 623–34. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen A., et al. , Developing and employing a 'responsive manualization' in the 'Acupuncture Approaches to Decrease Disparities in Outcomes of Pain Treatment' comparative effectiveness study. Acupunct Med, 2019. 37(3): p. 184–191. [DOI] [PubMed] [Google Scholar]
- 36.MacPherson H., et al. , Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med, 2010. 7(6): p. e1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacPherson H., et al. , The persistence of the effects of acupuncture after a course of treatment: a meta-analysis of patients with chronic pain. Pain, 2017. 158(5): p. 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKee MD, et al. , Individual vs. Group Delivery of Acupuncture Therapy for Chronic Musculoskeletal Pain in Urban Primary Care—a Randomized Trial. J Gen Intern Med, 2020. 35(4): p. 1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deyo RA, et al. , Report of the NIH Task Force on research standards for chronic low back pain. Phys Ther, 2015. 95(2): p. e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dworkin RH, et al. , Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain, 2010. 149(2): p. 177–93. [DOI] [PubMed] [Google Scholar]
- 41.heal.nih.gov. Common Data Elements (CDEs) Program. 2022. [cited 2022 Sept 17]; Available from: https://heal.nih.gov/data/common-data-elements.
- 42.LIANG K-Y and ZEGER SL, Longitudinal data analysis using generalized linear models. Biometrika, 1986. 73(1): p. 13–22. [Google Scholar]
- 43.Cherkin DC, et al. , A comparison of the effects of 2 types of massage and usual care on chronic low back pain: a randomized, controlled trial. Ann Intern Med, 2011. 155(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cherkin DC, et al. , A Randomized Trial Comparing Acupuncture, Simulated Acupuncture, and Usual Care for Chronic Low Back Pain. Archives of Internal Medicine, 2009. 169(9): p. 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherman KJ, et al. , Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med, 2005. 143(12): p. 849–56. [DOI] [PubMed] [Google Scholar]
- 46.Loudon K., et al. , The PRECIS-2 tool: designing trials that are fit for purpose. Bmj, 2015. 350: p. h2147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.