Abstract
Background
Esketamine (ESK) nasal spray, taken with oral antidepressant therapy, is approved for the treatment of depressive symptoms in adults with major depressive disorder (MDD) with acute suicidal ideation or behavior. In pooled analyses of two pivotal phase 3 studies, ASPIRE I and II, remission rates were consistently higher among patients with MDD with active suicidality who were treated with ESK + standard of care (SOC) versus placebo (PBO) + SOC at all time points in the double-blind and most time points in the follow-up phases. The current analysis of the ASPIRE data sets assessed the effect of ESK + SOC versus PBO + SOC on additional remission-related endpoints: time to achieving remission and consistent remission, proportion of patients in remission and consistent remission, and days in remission.
Methods
Post hoc analysis of pooled data from ASPIRE I and II (N = 451). Remission and consistent remission were defined as Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≤ 12 at any given visit or two consecutive visits, respectively. Combined endpoints utilizing Clinical Global Impression-Severity of Suicidality-revised version [CGI-SS-r] ≤ 1 (i.e., not suicidal/questionably suicidal) along with the remission and consistent remission definitions (i.e., MADRS total score ≤ 12) were also examined.
Results
The median times to remission and consistent remission of MDD were significantly shorter in ESK + SOC versus PBO + SOC (15 versus 23 [p = 0.005] and 23 versus 50 days [p = 0.007], respectively) and a greater proportion of patients in ESK + SOC achieved remission and consistent remission by Day 25 (65.2% versus 55.5% and 54.2% versus 39.8%, respectively). Similar results were obtained using the combined endpoint for both remission definitions. The median percent of days in remission during the double-blind treatment phase was significantly greater in ESK + SOC (27.1% or 5 days) versus PBO + SOC (8.3% or 2 days; p = 0.006), and the significant difference was maintained during follow-up.
Conclusion
Treatment with ESK + SOC versus PBO + SOC resulted in significantly shorter time to remission, greater proportion of patients in remission, and greater percent of days in remission using increasingly rigorous definitions of remission. These findings underscore the clinical benefits of ESK for adults with MDD with suicidality.
Trial registration
ClinicalTrials.gov registry NCT03039192 (registered February 1, 2017) and NCT03097133 (registered March 31, 2017).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-023-05017-y.
Keywords: Esketamine nasal spray, Depressive disorder, Suicidal ideation, Remission, Antidepressant agents
Introduction
Depression is a devastating psychiatric illness that is a main cause of disability worldwide [1]. In 2017, there were roughly 264 million individuals worldwide living with depression [2]. Major depressive disorder (MDD) can lead to suicide and is the most prevalent psychiatric diagnosis among those who have taken their own life [3, 4]. While the main objective of MDD treatment is remission of depressive symptoms, reduction of suicidality is also an important treatment goal.
The presence of active suicidal ideation with intent in patients with MDD constitutes a psychiatric emergency requiring urgent treatment of the underlying disease (ie, MDD). Current standard practice for these patients frequently includes hospitalization to ensure close monitoring along with treatment of symptoms of MDD with an antidepressant medication and development of a comprehensive crisis management plan before discharge from inpatient care [5]. Hospitalization, however, if accessible or acceptable by patients, is generally temporary, and patients remain at high risk of rehospitalization [6] and death by suicide [7, 8] after discharge. Indeed, American Psychiatric Association guidelines describe hospitalization not as a treatment for suicidality by itself, but rather a setting from which evaluation and treatment can be facilitated [9]. While standard antidepressants are often effective in treating severe depressive symptomatology, they typically take 4–6 weeks to achieve full clinical efficacy, which limits their utility in crisis situations and creates a treatment gap for patients in need of urgent symptom control. Patients thus remain vulnerable early in the course of treatment [10]. These data underscore the need for fast-acting treatment options that can reduce depressive symptoms rapidly in a psychiatric emergency.
In 2020, the U.S. Food and Drug Administration approved SPRAVATO® (esketamine [ESK] nasal spray), taken with an oral antidepressant, for the treatment of depressive symptoms in adults with MDD with acute suicidal ideation or behavior [11]. A similar indication has recently been approved in Europe [12]. ESK, the S-enantiomer of ketamine and an N-methyl-D-aspartate receptor antagonist, acts through a primary mechanism that differs from that of traditional monoaminergic antidepressants [13, 14]. It has a 4-week treatment regimen and is the first approved medication that significantly reduces depressive symptoms within 24 h. Thus, it provides a novel treatment option to quickly improve symptoms while a longer-term, comprehensive care plan can be established and an oral antidepressant can exert full effect [15]. ESK nasal spray approval in this indication was based on two phase 3, double-blind, multicenter registration trials in patients with MDD and active suicidal ideation with intent, ASPIRE I [16] and ASPIRE II [17]. In both trials, patients who received ESK nasal spray, given in addition to comprehensive standard of care (SOC) treatment (ESK + SOC), exhibited a significantly greater reduction in depressive symptoms than those who received placebo (PBO) nasal spray plus SOC (PBO + SOC) [18]. This treatment effect was observed as early as 4 h after the first dose of ESK and generally remained throughout the 4-week double-blind treatment phase. In the ASPIRE studies, severity of suicidality was improved rapidly in both ESK + SOC and PBO + SOC arms, but the treatment difference on this endpoint was not statistically significant 24 h after the first dose. This may be due in part to the non-specific clinical benefit from hospitalization and high clinical contact with study participants or the method used to evaluate rapid change in suicidality.
Among patients with MDD and suicidal ideation or behavior, the incidence of suicide attempts during a major depressive episode is 21-fold higher than during remission and the length of time spent in a major depressive episode is a risk factor for suicide attempts [19]. This suggests that remission of MDD should be an urgent treatment goal for patients with acute suicidality. This objective is further supported by American Psychiatric Association practice guidelines for patients with suicidal behaviors that identify treatment of the underlying illness as a main goal [9]. In the pooled analyses of ASPIRE trials, remission rates were consistently higher among patients treated with ESK + SOC at all time points in the double-blind and most time points in the follow-up phases, but time to remission and time spent in remission as clinical endpoints have not yet been fully explored to assess the effect of ESK in the ASPIRE trials. Furthermore, the consistency of remission, which is critical to provide relief to patients, has not been evaluated in the ASPIRE data sets.
Aims of the study
This post hoc analysis assessed the effect of ESK + SOC versus PBO + SOC on time to achieving remission and consistent remission (as assessed by the Montgomery-Åsberg Depression Rating Scale [MADRS] total score alone or combined with an assessment of suicidality by the Clinical Global Impression-Severity of Suicidality-revised version [CGI-SS-r] scale) as well as time spent in remission, using pooled data from two pivotal phase 3 trials, ASPIRE I and ASPIRE II.
Methods
Patients
This post hoc analysis used data pooled from two identically designed, double-blind, randomized, placebo-controlled, multicenter, phase 3 studies: ASPIRE I (NCT03039192) and ASPIRE II (NCT03097133) [16, 17]. In both studies, eligible patients (aged 18–64 years of age) met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [20] criteria for MDD without psychotic features as assessed by the Mini International Neuropsychiatric Interview [21]. Inclusion criteria included moderate to severe MDD (MADRS total score > 28), current suicidal ideation with intent in the past 24 h, and clinically warranted psychiatric hospitalization. Full inclusion and exclusion criteria have been previously published [16, 17].
Study design
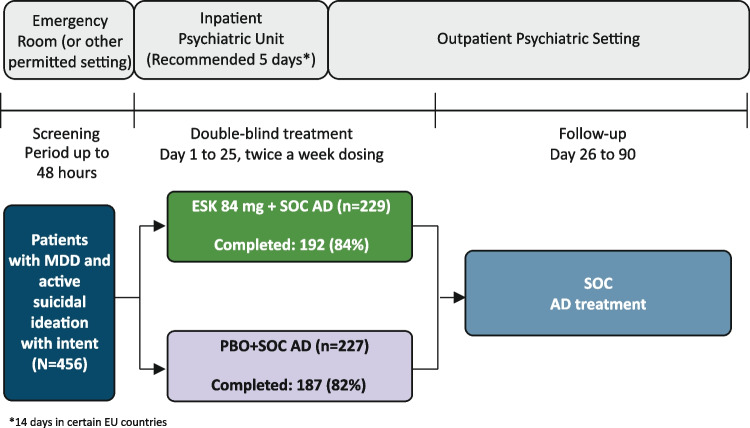
ASPIRE I was conducted from June 2017 to December 2018 in the United States, Europe, Asia, and South Africa. ASPIRE II was conducted from June 2017 to April 2019 in North America, South America, and Europe. Both studies consisted of three phases: (1) a 24- to 48-h screening phase to assess patients’ eligibility for study enrollment, (2) a 4-week double-blind treatment phase (days 1–25), and (3) a 9-week post treatment follow-up phase (days 26–90). During the 4-week double-blind treatment phase, patients were randomly assigned (1:1) to receive either ESK 84 mg or PBO nasal spray twice weekly, in addition to comprehensive SOC treatment (initial psychiatric hospitalization for a recommended ≥ 5 days and newly initiated or optimized oral antidepressant(s), per clinical judgement and practice guidelines) (Fig. 1). After the first dose, a one-time dose reduction to 56 mg ESK or PBO was allowed due to tolerability issues. Dose titrations/adjustment of SOC antidepressants occurred during the first 2 weeks of double-blind treatment. During the 9-week follow-up phase, patients continued SOC antidepressant treatment and discontinued ESK or PBO.
Fig. 1.
Study design of ASPIRE I and ASPIRE II. Two patients in each treatment group were excluded from these analyses because they did not receive a dose of intranasal study drug after randomization. Changes in MADRS total score and CGI-SS-r were assessed at 4 and 24 h post first dose, twice per week pre-dose during the double-blind phase, and at varied time intervals during the follow-up phase (days 28–39: twice weekly; days 46–53: weekly; days 67–90: biweekly). AD, antidepressant; ESK, esketamine; MDD, major depressive disorder; PBO, placebo; SOC, standard of care
Assessments
Depressive symptoms were assessed by the MADRS total score (range: 0–60) [22, 23]. Suicidality was assessed by the CGI-SS-r derived from the Suicide Ideation and Behavior Assessment Tool [24]. The CGI-SS-r (range: 0, normal, not at all suicidal to 6, among the most extremely suicidal patients) is a one-item, clinician-rated assessment of the current severity of a patient’s suicidal ideation and behavior. For both the MADRS and the CGI-SS-r scales, higher scores indicate worse symptomatology. Changes in MADRS total score and CGI-SS-r were assessed at 4 h and 24 h post first dose, twice per week pre-dose during the double-blind phase, and at varied time intervals during the follow-up phase (days 28–39: twice weekly; days 46–53: weekly; days 67–90: biweekly). Post dose assessment visits (ie, 4 h post dose on Day 1 and Day 25) were not included in the analysis performed for this publication because this post hoc analysis aimed to evaluate remission instead of rapid symptom reduction immediately after dosing.
Time to remission and percent of days in remission
Two definitions of remission were evaluated, remission and consistent remission, that were defined as a MADRS total score ≤ 12 at any given visit and for two consecutive visits, respectively. Time to remission was calculated as the time (in days) between the randomization date and the first time the patient achieved remission. Time to consistent remission was calculated as the time (in days) between the randomization date and the date of the first of two consecutive visits where remission was achieved. For each definition of remission of MDD, a single criterion (i.e., MADRS total score ≤ 12) and a combined endpoint (i.e., MADRS total score ≤ 12 and CGI-SS-r ≤ 1 [not suicidal/questionably suicidal]) were examined. For consistent remission, both criteria (i.e., MADRS total score ≤ 12 and CGI-SS-r ≤ 1) had to be met for two consecutive visits. Patients were censored at the date of the last non-missing assessment if no event was identified over the entire study follow-up (days 1–90). Intermittent missingness was treated as a non-response. Monotone missingness was censored at the last non-missing assessment visit.
Number of days in remission (based on MADRS total score ≤ 12) was calculated for the double-blind treatment and follow-up phases. Percent of days in remission was calculated as the number of days in remission divided by the number of days in the study. When calculating the number of days in remission, the last observation carried backward was used within the double-blind treatment and post treatment follow-up phases separately (i.e., the last observed remission or non-remission status was carried backward until the visit with a non-missing value), due to the intermittent assessments at scheduled visits per protocol. For monotone missingness, values were not included in the number of days in the study.
Statistical analysis
The analysis set included all randomized patients from ASPIRE I and ASPIRE II. The Kaplan–Meier product-limit method was used to estimate the median time (95% confidence interval [CI]) to remission of MDD from baseline. Univariate and multivariable Cox proportional hazards regression models were used to estimate hazard ratio (HR). The multivariable Cox hazards regression model included treatment and baseline score as covariates, and analysis center and SOC antidepressant treatment at baseline as stratification factors. For the number of days in remission, a Mann–Whitney U test was used to compare the median percent of days in remission between treatment arms. No adjustment for multiple comparisons was made.
Results
A total of 451 (ESK + SOC: 226; PBO + SOC: 225) patients from the pooled studies were included in this analysis. Patient demographics and baseline characteristics were well balanced between the ESK + SOC and PBO + SOC groups (Table 1). Two patients in each treatment group were excluded from these analyses because they did not receive a dose of intranasal study drug after randomization. Notably, patients had a baseline mean MADRS total score of 40 (severe depression) and about 90% of patients were moderately to extremely suicidal.
Table 1.
Baseline demographic and clinical characteristics
| Characteristics | PBO + SOC (N = 225) | ESK + SOC (N = 226) |
|---|---|---|
| Age, mean (SD), years | 39.6 (13.1) | 40.5 (12.9) |
| Women, n (%) | 140 (62.2) | 134 (59.3) |
| MADRS total scorea, mean (SD) | 40.4 (6.0) | 40.3 (5.6) |
| CGI-SS-ra, n (%) | ||
| Questionably suicidal | 6 (2.7) | 6 (2.7) |
| Mildly suicidal | 17 (7.6) | 16 (7.1) |
| Moderately suicidal | 61 (27.1) | 64 (28.4) |
| Markedly suicidal | 84 (37.3) | 86 (38.2) |
| Severely suicidal | 55 (24.4) | 46 (20.4) |
| Extremely suicidal | 2 (0.9) | 7 (3.1) |
| Prior suicide attempt (lifetime)a, n (%) | 140 (62.2) | 144 (64.0) |
| Suicide attempt within the last month, n (%) | 55 (24.4) | 68 (30.1) |
| SOC AD monotherapy at baseline, n (%) | 108 (48.0) | 104 (46.0) |
| SOC AD augmentation therapy at baseline, n (%) | 117 (52.0) | 122 (54.0) |
AD Antidepressant, CGI-SS-r Clinical Global Impression–Severity of Suicidality–revised, ESK Esketamine, MADRS Montgomery-Åsberg Depression Rating Scale, PBO Placebo, SD Standard deviation, SOC Standard of care
aN = 225 in ESK + SOC
Time to remission of major depressive disorder
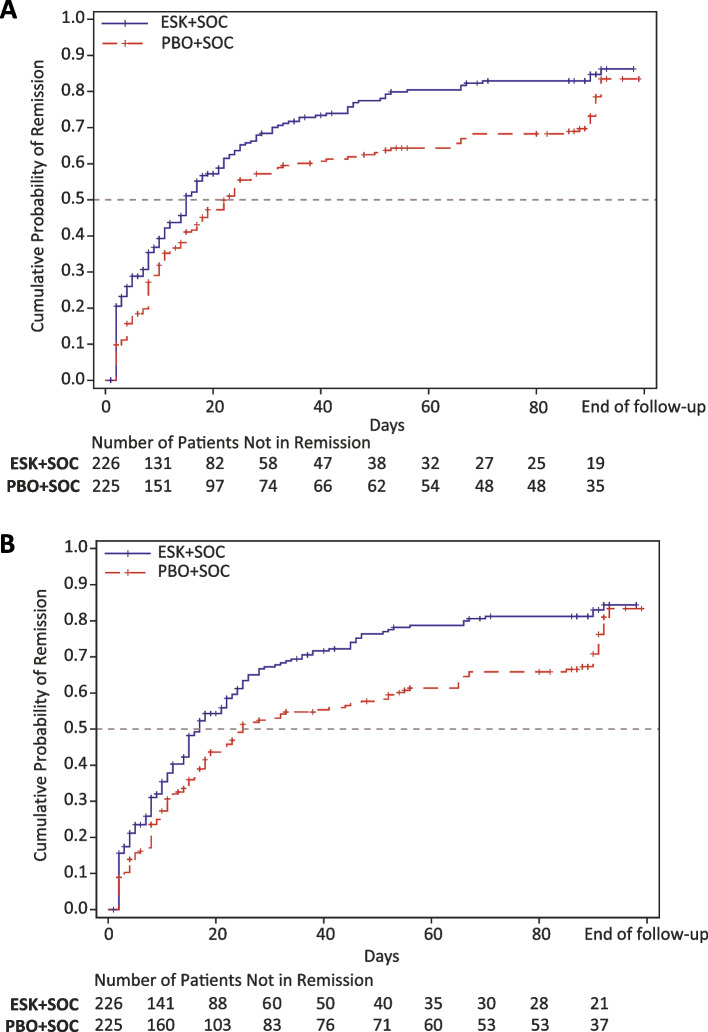
Time to remission of MDD (MADRS total score ≤ 12) was significantly shorter in patients treated with ESK + SOC versus PBO + SOC: median time, 15 versus 23 days; adjusted HR (95% CI), 1.47 (1.13, 1.92); p = 0.005 (Fig. 2A, Table 2). As shown in Fig. 2A, the ESK + SOC group had a higher cumulative probability of achieving remission at any given time compared to the PBO + SOC group. Similarly, time to achieving both MADRS total score ≤ 12 (remission of MDD) and CGI-SS-r ≤ 1 (not suicidal/questionably suicidal) was significantly shorter in the ESK + SOC group versus the PBO + SOC group: median time, 17 versus 25 days; adjusted HR (95% CI), 1.51 (1.15, 1.98); p = 0.003 (Fig. 2B, Table 2).
Fig. 2.
Kaplan–Meier curves of (A) time to remission of MDD based on MADRS and (B) time to remission of MDD based on the combined endpoint of MADRS and CGI-SS-r for the ESK + SOC and PBO + SOC groups. Cumulative probability of remission is the cumulative probability of achieving remission by criterion (A) or (B) by a given time. The numbers of patients at risk (ie, who have not yet remitted) for each of the groups are shown below the survival curves. CGI-SS-r, Clinical Global Impression-Severity of Suicidality-revised version; ESK, esketamine; MADRS, Montgomery-Åsberg Depression Rating Scale; PBO, placebo; SOC, standard of care
Table 2.
Time to remission and consistent remission of MDD
|
ESK + SOC Median (95% CI) N = 226 |
PBO + SOC Median (95% CI) N = 225 |
Unadjusted HR (95% CI) | P-value | Adjusted HRa (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Time to remission of MDD | ||||||
| Using MADRS total score ≤ 12 as a single criterion | ||||||
| Days to remission | 15 (12, 18) | 23 (17, 28) | 1.36 (1.10, 1.70) | 0.006 | 1.47 (1.13, 1.92) | 0.005 |
| Using MADRS total score ≤ 12 and CGI-SS-r ≤ 1 as a combined endpoint | ||||||
| Days to remission | 17 (15, 22) | 25 (19, 45) | 1.39 (1.12, 1.74) | 0.003 | 1.51 (1.15, 1.98) | 0.003 |
| Time to consistent remission of MDD | ||||||
| Using MADRS total score ≤ 12 as a single criterion | ||||||
| Days to remission | 23 (21, 28) | 50 (32, NE) | 1.63 (1.27, 2.08) | 0.0001 | 1.50 (1.12, 2.00) | 0.007 |
| Using MADRS total score ≤ 12 and CGI-SS-r ≤ 1 as a combined endpoint | ||||||
| Days to remission | 25 (22, 36) | 52 (32, NE) | 1.52 (1.18, 1.96) | 0.001 | 1.42 (1.06, 1.91) | 0.020 |
CGI-SS-r Clinical Global Impression–Severity of Suicidality–revised, CI Confidence interval, ESK Esketamine, HR Hazard ratio, MADRS Montgomery-Åsberg Depression Rating Scale, MDD Major depressive disorder, NE Not estimable, PBO Placebo, SOC Standard of care
aAdjusted for baseline score of individual measure, with analysis center and SOC antidepressant treatment as randomized stratification factors
Based on the single criterion of MADRS total score ≤ 12, the cumulative probability of remission was 65.2% in the ESK + SOC group versus 55.5% in the PBO + SOC group by day 25 (ie, end of the double-blind treatment phase); and 86.3% in the ESK + SOC group versus 83.5% in the PBO + SOC group by day 90 (ie, end of the follow-up phase) (p = 0.006). Based on the combined endpoint of MADRS total score ≤ 12 and CGI-SS-r ≤ 1, the cumulative probability of remission was 63.4% in the ESK + SOC group versus 51.3% in the PBO + SOC group by day 25; and 84.4% in the ESK + SOC group versus 83.4% in the PBO + SOC group by day 90 (p = 0.003).
Time to consistent remission of major depressive disorder
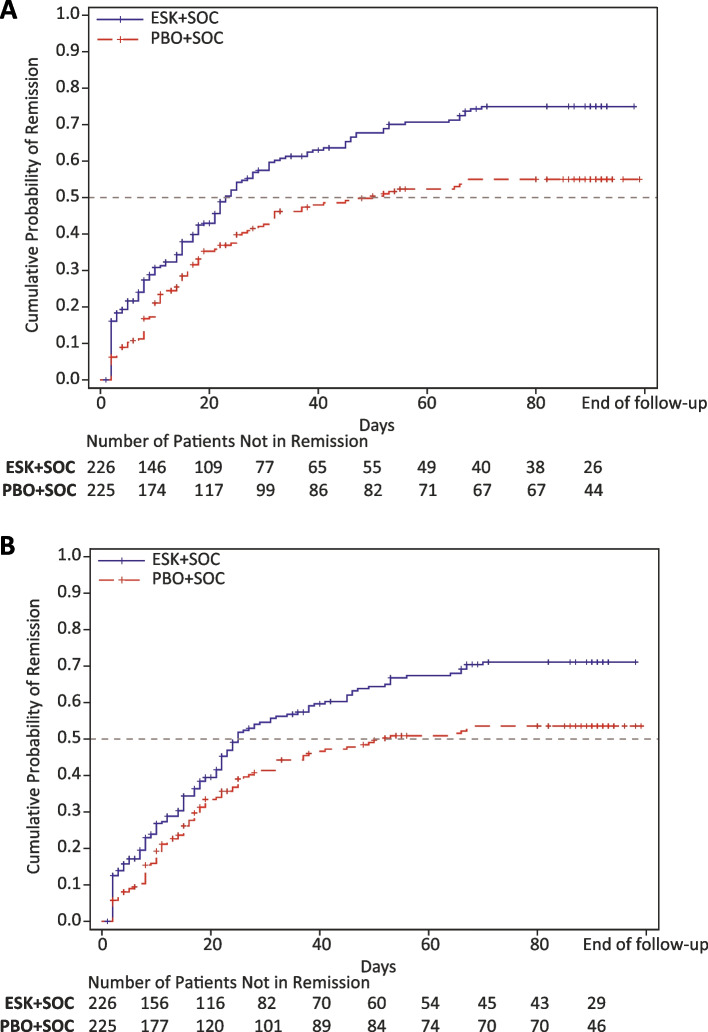
Time to consistent remission of MDD (MADRS total score ≤ 12 for two consecutive visits) was significantly shorter in patients treated with ESK + SOC versus PBO + SOC: median time, 23 versus 50 days; adjusted HR (95% CI), 1.50 (1.12, 2.00); p = 0.007 (Fig. 3A, Table 2). Time to achieving both MADRS total score ≤ 12 and CGI-SS-r ≤ 1 for two consecutive visits was significantly shorter in the ESK + SOC group versus the PBO + SOC group: median time, 25 versus 52 days; adjusted HR (95% CI), 1.42 (1.06, 1.91); p = 0.020 (Fig. 3B, Table 2).
Fig. 3.
Kaplan–Meier curves of (A) time to consistent remission of MDD based on MADRS and (B) time to consistent remission of MDD based on the combined endpoint of MADRS and CGI-SS-r for the ESK + SOC and PBO + SOC groups. Cumulative probability of remission is the cumulative probability of achieving remission by criterion (A) or (B) by a given time. The numbers of patients at risk (ie, who have not yet remitted) for each of the groups are shown below the survival curves. CGI-SS-r, Clinical Global Impression-Severity of Suicidality-revised version; ESK, esketamine; MADRS, Montgomery-Åsberg Depression Rating Scale; PBO, placebo; SOC, standard of care
Based on the single criterion of MADRS total score ≤ 12 for two consecutive visits, the cumulative probability of consistent remission was 54.2% in the ESK + SOC group versus 39.8% in the PBO + SOC group by day 25; and 75.0% in the ESK + SOC group versus 55.0% in the PBO + SOC group by day 90 (p = 0.0001). Based on the combined endpoint of MADRS total score ≤ 12 and CGI-SS-r ≤ 1 for two consecutive visits, the cumulative probability of consistent remission was 51.8% in the ESK + SOC group versus 39.0% in the PBO + SOC group by day 25; and 71.1% in the ESK + SOC group versus 53.5% in the PBO + SOC group by day 90 ( p = 0.001).
Percent of days in remission of major depressive disorder
The percent of days in remission of MDD during the double-blind treatment phase in all randomized patients was significantly greater for the ESK + SOC group versus the PBO + SOC group: median percent of days in remission, 27.1% versus 8.3% (5 days versus 2 days during the 25 days of the double-blind treatment phase), p = 0.006 (Table 3). Similarly, among the patients who achieved remission (MADRS total score ≤ 12) during the double-blind treatment phase, the percent of days in remission across both phases of the trial was significantly greater for the ESK + SOC group versus the PBO + SOC group: median percent days in remission, 58.3% versus 41.7%, p = 0.037 (Table 3).
Table 3.
Days in remission of MDD
| ESK + SOC | PBO + SOC | P-value | |
|---|---|---|---|
| Days in remissiona achieved during DB phase | N = 224 | N = 225 | |
| Median days in remission, DB phase | 5 | 2 | |
| Median % of days in remission, DB phase | 27.1 | 8.3 | 0.006 |
| Days in remissiona for patients who achieved remission during DB phase | N = 138 | N = 117 | |
| Median days in remission, DB + FU phases | 13 | 10 | |
| Median % days in remission, DB + FU phases | 58.3 | 41.7 | 0.037 |
| Days in remissiona achieved during FU phase | N = 189 | N = 185 | |
| Median days in remission, FU phase | 37 | 23 | |
| Median % days in remission, FU phase | 63.5 | 38.1 | 0.049 |
| Days in remissiona for patients who achieved remission during FU phase | N = 145 | N = 127 | |
| Median days in remission, FU phase | 49 | 42 | |
| Median % days in remission, FU phase | 81.0 | 77.8 | 0.333 |
DB Double-blind, ESK Esketamine, FU Follow-up, MDD Major depressive disorder, SOC Standard of care
aRemission defined as MADRS total score ≤ 12
Furthermore, during the follow-up phase (when patients received SOC antidepressant treatment only), the percent of days in remission continued to be greater for the ESK + SOC group versus the PBO + SOC group: median percent of days in remission, 63.5% versus 38.1%, p = 0.049 (Table 3). Among the patients who achieved remission (MADRS total score ≤ 12) during the follow-up phase, the percent of days in remission was also greater for the ESK + SOC group versus the PBO + SOC group: median percent days in remission, 81.0% versus 77.8%, p = 0.333 (Table 3).
Discussion
In ASPIRE I and II, ESK demonstrated a rapid reduction of depressive symptoms in patients with MDD with active suicidal ideation and intent who were experiencing a psychiatric emergency [16, 17]. Here, we evaluated clinically relevant and important endpoints in these same patients. The results of this post hoc analysis demonstrated that, compared to PBO + SOC, ESK + SOC treatment resulted in a higher proportion of patients who achieved remission and consistent remission of MDD, and significantly and consistently shortened the time to both remission and consistent remission. Similar results were obtained when a single criterion of remission (MADRS total score ≤ 12) was used as well as when a more stringent combined endpoint of remission incorporating depressive symptoms (MADRS total score ≤ 12) and severity of suicidality (CGI-SS-r ≤ 1) was used. Patients in the ESK + SOC group also spent a significantly greater percent of days in remission during the double-blind treatment and follow-up phases than patients in the PBO + SOC group.
In practice, temporary hospitalization leaves patients with MDD and suicidality vulnerable in the near term while antidepressant treatment takes weeks to be effective [10]. In addition, compared to patients with depression without suicidality, patients with depression and suicidality are less likely to improve or achieve remission with oral antidepressants [25]. Our results suggest that ESK may address the unmet needs of this population, providing rapid and sustained reduction of symptomatology and attainment of remission.
A large body of literature points to the importance of rapidly achieving remission in patients with MDD and suicidality [26]. Patients with MDD who achieve remission exhibit better functioning and an improved prognosis [27], improved health-related [28, 29] and “back-to-normal” [30] quality of life, and improved functional status [31]. The incidence of suicide is 21-fold lower during remission than during a major depressive episode [19]. American Psychiatric Association guidelines call for addressing the underlying disease (eg, depression) as the main treatment for suicidality [9]. In addition, improvement of depressive symptoms and remission are the most important attributes considered by physicians when assessing the readiness to discharge a patient with MDD and acute suicidal ideation with intent from the hospital [32].
In addition to the humanistic benefits of achieving remission, there are economic benefits as well. In patients with treatment-resistant depression, healthcare resource utilization (HRU) is lower during remission than during a major depressive episode [33]. Another study showed that patients with moderate or severe treatment-resistant depression have greater HRU and higher medical costs than those with a mild form of the illness [34]. Findings are similar in MDD — healthcare costs, HRU, and productivity losses are lower among patients who remit compared to those who do not achieve remission [31, 35, 36].
A strength of our analysis is that multiple, increasingly stringent assessments of remission were used and that the results obtained were robust and consistent. The benefits of ESK + SOC over PBO + SOC were maintained during the follow-up phase, when ESK treatment had been discontinued.
Limitations of this analysis include the post-hoc nature of the evaluation and the lack of adjustment for multiple comparisons. In addition, the suicidality endpoint (CGI-SS-r), while an important component of the remission assessment, did not show a statistically significant difference favoring ESK in the ASPIRE studies. Therefore, the results should be interpreted in line with the exploratory nature of the analysis. Finally, MADRS and CGI-SS-r assessments were performed intermittently, thus the remission analyses used values at each visit or two consecutive visits and days of remission in between two assessments were based on the calculation of last observation carried backward as described in the Methods.
Conclusions
In summary, in this post hoc analysis of patients with MDD with suicidal ideation or behavior, treatment with ESK + SOC, compared to PBO + SOC, resulted in greater proportions of patients achieving remission and significantly shorter time to remission (using increasingly stringent definitions of remission incorporating depressive symptoms and severity of suicidality), as well as a significantly greater percent of time spent in remission. These findings highlight the clinical benefits of ESK treatment to address high unmet needs in adults with MDD with acute suicidality.
Supplementary Information
Additional file 1: List of Institutional Review Boards and Independent Ethics Committees.
Acknowledgements
Yutian Mu, of Evidera-PPD, provided support for the statistical analysis. Writing assistance was provided by Allison Marin, PhD, of System One. Additional editorial support was provided by Harry Ma, PhD, of Janssen Global Services, LLC.
Abbreviations
- AD
Antidepressant
- CGI-SS-r
Clinical Global Impression-Severity of Suicidality-revised
- CI
Confidence interval
- DB
Double-blind
- ESK
Esketamine
- FU
Follow-up
- HR
Hazard ratio
- MADRS
Montgomery-Åsberg Depression Rating Scale
- MDD
Major depressive disorder
- NE
Not estimable
- PBO
Placebo
- SD
Standard deviation
- SOC
Standard of care
Authors’ contributions
DJF and CMC contributed to the study concept and design. LS and SG performed the statistical analysis and prepared the figures. All authors contributed to the interpretation of results. DJF wrote the main manuscript text. All authors participated in the critical revision of the manuscript for important intellectual content and approved the final manuscript.
Funding
The study was supported by funding from Janssen Research & Development, LLC. Employees of Janssen, as noted under Authors’ Contributions, were involved in the design, collection, and analysis of the data; interpretation of the results; preparation of the manuscript; and the decision to submit the manuscript for publication. The sponsor also provided funding for the development of the manuscript.
Availability of data and materials
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at: https://www.janssen.com/clinical-trials/transparency. Requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at:
NCT03039192: https://yoda.yale.edu/nct03039192-double-blind-randomized-placebo-controlled-study-evaluate-efficacy-and-safety-intranasal
NCT03097133: https://yoda.yale.edu/nct03097133-double-blind-randomized-placebo-controlled-study-evaluate-efficacy-and-safety-intranasal
Declarations
Ethics approval and consent to participate
Over 60 Institutional Review Boards and Independent Ethics Committees approved the study protocol and amendments. The full list can be found in Additional File 1 as well as in the supplementary materials of the primary publications [16, 17]. The study was conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki, consistent with Good Clinical Practices and applicable regulatory requirements. All patients provided written informed consent before participation in the study.
Consent for publication
Not applicable.
Competing interests
Dong-Jing Fu, Qiaoyi Zhang, Joana Anjo, Marguerite O’Hara, and Carla Canuso are employees of Janssen Research & Development, LLC and may hold Johnson & Johnson company stocks or stock options. Stephane Borentain, Abigail Nash, and Maju Mathews were employees of Janssen Research & Development, LLC at the time of study and manuscript preparation and may hold Johnson & Johnson company stocks or stock options; Abigail Nash is currently employed by Neurocrine Biosciences Inc.; Maju Mathews is currently employed by Perception Neuroscience. Ling Shi and Shien Guo are employees of Evidera-PPD and received research funding support from Janssen Research & Development, LLC.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Depression. 2019. Available from: https://www.who.int/health-topics/depression. Accessed 26 May 2022.
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018;92(10159):1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann S. Epidemiology of Suicide and the Psychiatric Perspective. Int J Environ Res Public Health. 2018;15(7):1425. doi: 10.3390/ijerph15071425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavanagh JT, Carson AJ, Sharpe M, Lawrie SM. Psychological autopsy studies of suicide: a systematic review. Psychol Med. 2003;33(3):395–405. doi: 10.1017/S0033291702006943. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Depression in adults: treatment and management. 2018. [PubMed]
- 6.Cepeda MS, Schuemie M, Kern DM, Reps J, Canuso C. Frequency of rehospitalization after hospitalization for suicidal ideation or suicidal behavior in patients with depression. Psychiatry Res. 2020;285:112810. doi: 10.1016/j.psychres.2020.112810. [DOI] [PubMed] [Google Scholar]
- 7.Chung D, Hadzi-Pavlovic D, Wang M, Swaraj S, Olfson M, Large M. Meta-analysis of suicide rates in the first week and the first month after psychiatric hospitalisation. BMJ Open. 2019;9(3):e023883. doi: 10.1136/bmjopen-2018-023883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenstein M, Kim HM, Ganoczy D, McCarthy JF, Zivin K, Austin KL, et al. Higher-risk periods for suicide among VA patients receiving depression treatment: prioritizing suicide prevention efforts. J Affect Disord. 2009;112(1–3):50–58. doi: 10.1016/j.jad.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Practice Guideline for the Assessment and Treatment of Patients with Suicidal Behaviors. 2003. [PubMed]
- 10.Wasserman D, Rihmer Z, Rujescu D, Sarchiapone M, Sokolowski M, Titelman D, et al. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129–141. doi: 10.1016/j.eurpsy.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Janssen Pharmaceuticals, Inc. SPRAVATO® [Prescribing Information]. Titusville: Janssen Pharmaceuticals, Inc.; 2020.
- 12.SPRAVATO® (Esketamine Nasal Spray) Authorised in Europe for the Rapid Reduction of Depressive Symptoms in a Psychiatric Emergency for Patients with Major Depressive Disorder [press release]. 2021.
- 13.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur U, Pathak BK, Singh A, Chakrabarti SS. Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):417–429. doi: 10.1007/s00406-019-01084-z. [DOI] [PubMed] [Google Scholar]
- 15.Johnson & Johnson. Janssen Announces U.S. FDA Approval of SPRAVATO® (esketamine) CIII Nasal Spray to Treat Depressive Symptoms in Adults with Major Depressive Disorder with Acute Suicidal Ideation or Behavior. 2020. Available from: https://www.jnj.com/janssen-announces-u-s-fda-approval-of-spravato-esketamine-ciii-nasal-spray-to-treat-depressive-symptoms-in-adults-with-major-depressive-disorder-with-acute-suicidal-ideation-or-behavior. Accessed 26 May 2022.
- 16.Fu DJ, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, et al. Esketamine Nasal Spray for Rapid Reduction of Major Depressive Disorder Symptoms in Patients Who Have Active Suicidal Ideation With Intent: Double-Blind, Randomized Study (ASPIRE I) J Clin Psychiatry. 2020;81(3):19m13191. doi: 10.4088/JCP.19m13191. [DOI] [PubMed] [Google Scholar]
- 17.Ionescu DF, Fu DJ, Qiu X, Lane R, Lim P, Kasper S, et al. Esketamine Nasal Spray for Rapid Reduction of Depressive Symptoms in Patients With Major Depressive Disorder Who Have Active Suicide Ideation With Intent: Results of a Phase 3, Double-Blind, Randomized Study (ASPIRE II) Int J Neuropsychopharmacol. 2021;24(1):22–31. doi: 10.1093/ijnp/pyaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canuso CM, Ionescu DF, Li X, Qiu X, Lane R, Turkoz I, et al. Esketamine Nasal Spray for the Rapid Reduction of Depressive Symptoms in Major Depressive Disorder With Acute Suicidal Ideation or Behavior. J Clin Psychopharmacol. 2021;41(5):516–524. doi: 10.1097/JCP.0000000000001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holma KM, Melartin TK, Haukka J, Holma IAK, Sokero TP, Isometsä ET. Incidence and predictors of suicide attempts in DSM-IV major depressive disorder: a five-year prospective study. Am J Psychiatry. 2010;167(7):801–808. doi: 10.1176/appi.ajp.2010.09050627. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Fifth Edition ed. Washington, D.C.: American Psychiatric Association; 2013.
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33. [PubMed] [Google Scholar]
- 22.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 23.Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA) Br J Psychiatry. 2008;192(1):52–58. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- 24.Alphs L, Fu DJ, Williamson D, Turkoz I, Jamieson C, Revicki D, et al. Suicide Ideation and Behavior Assessment Tool (SIBAT): Evaluation of Intra- and Inter-Rater Reliability, Validity, and Mapping to Columbia Classification Algorithm of Suicide Assessment. Psychiatry Res. 2020;294:113495. doi: 10.1016/j.psychres.2020.113495. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Castroman J, Jaussent I, Gorwood P, Courtet P. Suicidal depressed patients respond less well to antidepressants in the short term. Depress Anxiety. 2016;33(6):483–494. doi: 10.1002/da.22473. [DOI] [PubMed] [Google Scholar]
- 26.Bakish D. New standard of depression treatment: remission and full recovery. J Clin Psychiatry. 2001;62(Suppl 26):5–9. [PubMed] [Google Scholar]
- 27.Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 28.Woo JM, Jeon HJ, Noh E, Kim HJ, Lee SW, Lee KK, et al. Importance of remission and residual somatic symptoms in health-related quality of life among outpatients with major depressive disorder: a cross-sectional study. Health Qual Life Outcomes. 2014;12:188. doi: 10.1186/s12955-014-0188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow W, Doane MJ, Sheehan JJ, Alphs L, Daly K. Patient-centric Outcomes Among Patients With Major Depressive Disorder: Comparisons Across Depression Severity. 31st Annual US Psychiatric & Mental Health Congress; October 25–28; Orlando, FL 2018.
- 30.IsHak WW, Mirocha J, James D, Tobia G, Vilhauer J, Fakhry H, et al. Quality of life in major depressive disorder before/after multiple steps of treatment and one-year follow-up. Acta Psychiatr Scand. 2015;131(1):51–60. doi: 10.1111/acps.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauskopf JA, Simon GE, Kalsekar A, Nimsch C, Dunayevich E, Cameron A. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress Anxiety. 2009;26(1):83–97. doi: 10.1002/da.20505. [DOI] [PubMed] [Google Scholar]
- 32.Voelker J, Wilkinson ST, Katz EG, Nash AI, Daly E, Ali A, et al. A Choice-Based Conjoint Analysis of the Psychiatrist Decision-Making Process Used in Determining When to Discharge Adults With Major Depressive Disorder Hospitalized for Active Suicidal Ideation With Intent. J Nerv Ment Dis. 2022;210(5):373–379. doi: 10.1097/NMD.0000000000001463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denee T, Ming T, Waller J, Bailey T, Rajkovic-Hooley O, Middleton-Dalby C, et al. A retrospective chart review study to quantify the monthly medical resource use and costs of treating patients with treatment resistant depression in the United Kingdom. Curr Med Res Opin. 2021;37(2):311–319. doi: 10.1080/03007995.2020.1857580. [DOI] [PubMed] [Google Scholar]
- 34.Pilon D, Sheehan JJ, Szukis H, Morrison L, Zhdanava M, Lefebvre P, et al. Is clinician impression of depression symptom severity associated with incremental economic burden in privately insured US patients with treatment resistant depression? J Affect Disord. 2019;255:50–59. doi: 10.1016/j.jad.2019.04.100. [DOI] [PubMed] [Google Scholar]
- 35.Dennehy EB, Robinson RL, Stephenson JJ, Faries D, Grabner M, Palli SR, et al. Impact of non-remission of depression on costs and resource utilization: from the COmorbidities and symptoms of DEpression (CODE) study. Curr Med Res Opin. 2015;31(6):1165–1177. doi: 10.1185/03007995.2015.1029893. [DOI] [PubMed] [Google Scholar]
- 36.Chow W, Doane MJ, Sheehan JJ, Alphs L, Daly K. Relationship Between Patient-reported Depression Severity Using the Patient Health Questionnaire-9 (PHQ-9) and Economic Outcomes for Major Depressive Disorder. Academy of Managed Care Pharmacy Nexus Meeting; October 22–25; Orlando, FL 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: List of Institutional Review Boards and Independent Ethics Committees.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at: https://www.janssen.com/clinical-trials/transparency. Requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at:
NCT03039192: https://yoda.yale.edu/nct03039192-double-blind-randomized-placebo-controlled-study-evaluate-efficacy-and-safety-intranasal
NCT03097133: https://yoda.yale.edu/nct03097133-double-blind-randomized-placebo-controlled-study-evaluate-efficacy-and-safety-intranasal