Abstract
Background
X-linked adrenoleukodystrophy (ALD) is a rare metabolic and neurodegenerative disorder belonging to the group of leukodystrophies, with an estimated incidence around 1:25 000 newborns worldwide, mostly among men. Childhood Cerebral ALD (CCALD) is the most severe form with a poor prognosis if not properly treated during the first years of life. Currently, only allogeneic hematopoietic stem cell transplantation (allo-HSCT) is widely available for CCALD treatment. To date, there is a lack of data regarding CCALD epidemiology, natural history, and current management in France. This knowledge is crucial for the development of new therapies such as gene therapies. In this context, the French National Health Data System (SNDS) is a particularly indicated database to collect information meeting these needs. A non-interventional, national, real-life, retrospective study was performed using secondary data from the national ALD registry (LEUKOFRANCE) and SNDS. CCALD patients detected between 2009 and 2018 and successfully matched between LEUKOFRANCE and SNDS were included in this study. Index date was defined as the first CCALD event detected during study period. Subgroups of patients with sufficient follow-up (6 months) and history (1 year) available around index date were analyzed to assess CCALD burden and natural history.
Results
52 patients were included into the matched cohort. Median annual incidence of CCALD was estimated at 4 patients. Median age at CCALD diagnosis was 7.0 years. Among patients without allo-HSCT, five-year overall survival was 66.6%, with 93.3% of them presenting at least one CCALD symptom and 62.1% presenting a least one major functional disability (MFD). Among patients with allo-HSCT, five-year overall survival was 94.4%, with only 11.1% of patients presenting CCALD symptoms, and 16.7% of presenting a MFD. Mean annualized costs were almost twice as important among patients without allo-HSCT, with 49,211€, 23,117€, respectively. Costs were almost exclusively represented by hospitalizations.
Conclusions
To the best of our knowledge, this is the most up to date study analyzing CCALD epidemiology, clinical and economic burden in France. The necessity of a precocious management with HSCT highlight the potential benefits of including an expanded screening program among newborns, coupled with family screenings when a mutation is detected.
Keywords: Adrenoleukodystrophy, CCALD, Epidemiology, Claims, Registry, HSCT, SNDS, Care management, Neurodegenerative diseases, Health cost
Background and rationale
X-linked adrenoleukodystrophy (ALD) is a rare metabolic and neurodegenerative disorder belonging to the group of leukodystrophies. It results in the production of a dysfunctional adrenoleukodystrophy protein (ALDP), leading to an accumulation of very long chain fatty acids in plasma and tissues, causing axonal damage, cerebral demyelination, and adrenal insufficiency. ALD overall incidence rate, including hemizygotes and heterozygotes who are frequently symptomatic, is estimated between 1:15 000 and 1:33 000 newborns worldwide [1–4]. Most frequently observed clinical manifestations are in line with three main ALD phenotypes. The most frequent and mildest form is an isolated adrenal insufficiency, or Addison disease, observed among around 50% of X-ALD patients, leading to hypotension, hypoglycemia, fatigue, or joint pain. The second main form of X-ALD is Adrenomyeloneuropathy (AMN), an adult-onset slowly progressive myelopathy and peripheral neuropathy, that begins between the ages of 20 and 40 with full penetrance beyond age 60 in men and may also affect heterozygous females. Finally, the most rapidly progressive and devastating form of ALD form of X-ALD is Childhood Cerebral ALD (CCALD), occurring within the first 10 years of life. CCALD has an insidious onset and leads to important neurologic disorders in addition to Addison disease. The main neurologic manifestations occurring during disease progression include hyperactive behavior, auditory and visual impairment, hemiparesis, cerebellar ataxia or spastic tetraparesia which can become highly debilitating within weeks or months. This suddenly progressive disease form is observed for around 40% of boys and adolescents affected with CCALD, with a very low life expectancy without treatment. Less invasive phenotypes will have the same evolution pattern with an onset around 10 to 15 years of age [5–7].
Apart from family screening, the majority of patients are diagnosed when they already have symptoms, a time when the disease is too advanced to offer a curative treatment. Aside from the treatment of the Addison disease, which relies on adrenal hormone supplementation, patients will benefit from symptomatic and palliative treatment (pain, spasticity, nutritional disorders, swallowing disorders, orthopedic complications, physiotherapy) to allow improving their quality of life. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative treatment approved in France, which has been shown to have a beneficial effect on clinical indices of disease and long-term survival [8]. However, it must be performed at the earliest stage of cerebral demyelination process to be effective [5, 6]. An international consensus confers optimal eligibility for HSCT by modern standards: neurological functional score (NFS) ≤ 1, demyelinating score (Loes score) of 0.5 to ≤ 9, and gadolinium enhancement in cerebral imaging [9–11]. For more severe patients, allo-HSCT is not recommended, as allo-HSCT will need weeks to months to be effective and will not tackle the rapid disease evolution, leading to irreversible brain damage. Only a limited number of patients will actually present all required conditions to benefit from an allo-HSCT, which remains associated to a significant mortality risk, reaching up to 15–25% in children during the first year post HSCT, due to graft failure, graft versus host disease (GVHD) and opportunistic infections [12]. Recently, ex vivo gene therapy has been developed for CCALD and evaluated as an interesting alternative to allograft. Clinical trials performed in CCALD children demonstrate that this treatment, if performed in the same indications than allo-HSCT, provides similar efficacy [13].
To date, there is a lack of data regarding CCALD epidemiology, natural history, and current management in France. This knowledge is crucial for the development of new therapies. In addition, from health policies perspective, there is a need of comprehensive and recent data on CCALD impact on public health, based on healthcare resource use and related costs from Health Insurance and Collective perspectives. In this context, the French National Health Data System (Système National des données de Santé, SNDS) is a particularly indicated database to collect information meeting these needs.
Objectives
In order to answer these questions, four objectives were set up.
The main objective was (objective A) to determine the number of patients with CCALD included in a matched cohort based on SNDS data and French registry of Leukodystrophies (LEUKOFRANCE), between 2009 and 2018, overall and per year, and to describe their characteristics.
Secondary objectives are listed as follows:
to describe CCALD natural history, and notably (i) the delay between ALD diagnosis and the occurrence of the CCALD phenotype, (ii) the nature and frequency of morbidity CCALD-related events and (iii) the overall survival and most common causes of death (objective B);
to assess the CCALD care management, including medical procedures and therapies, and to describe the disease evolution depending on the therapeutic management, notably according to HSCT status (objective C);
to assess health care resource use of CCALD patients and related costs, form the Health Insurance and the collective perspective (objective D).
Methods
Study design
The study was a non-interventional, national, real-life, retrospective study with secondary use of two sources of existing data. The primary source was the national leukodystrophies registry, called LEUKOFRANCE registry, which includes patients referred to the French reference centers for leukodystrophies (LEUKOFRANCE). The CCALD patients extracted from this registry were probabilistically linked to the medico-administrative database of the French Health Insurance (French System of Health Data - Système National des Données de Santé, SNDS) as a secondary source. Patients with a CCALD-related event (diagnosis and/or treatment) recorded between January 1st 2009 and June 31st 2018 and successfully matched between LEUKOFRANCE registry and SNDS data were included in this study. Index date was defined as the date of first CCALD diagnosis, allowing to distinguish prevalent patients with an index date captured using LEUKOFRANCE registry data prior to 2009, and incident patients (i.e., with an index date during study period).
A first subgroup of patients was followed-up for a minimum of 6 months until December 31st, 2018 (corresponding to the end of study period) or death, whichever occurs first, to assess clinical and economic burden of the disease (objectives C and D). Among them, those having at least 1 year of historical data before index date were extracted to assess CCALD natural history (objective B).
Data sources
LEUKOFRANCE registry
LEUKOFRANCE registry belongs to the Reference Centre for Leukodystrophies and Rare Leukoencephalopathies (LEUKOFRANCE). In 2013 (corresponding to the middle of the study period), almost 2,000 families were part of LEUKOFRANCE registry, gathering phenotypic, imaging, molecular, clinical resources, and data, as well as almost 30,000 biological samples. When considering historical data retrospectively included, LEUKOFRANCE registry allows access to more than 30 years of data, with first record available dating back to 1988. Among data of interest, sociodemographic characteristics, date of diagnosis, symptom onset, imagery, and biology results, as well as ALD related events and treatments were analyzed.
SNDS
The national health data system, SNDS (Système National des Données de Santé) is managed by the national health insurance since its implementation in 2017 [14]. It gathers and allows the linkage of all reimbursed healthcare consumptions from inpatient and outpatient databases. It covers every subjects affiliated to one of the compulsory health insurance plans, representing nearly 99% of French residents [15, 16]. Each data is returned in an individualized way using a unique pseudonymized identification number called NIR. Data collected include administrative information on the beneficiaries, all reimbursed ambulatory health care services (e.g. health professionals, date, nature and number of care performed, biology acts, reimbursable medical devices, dispensed drugs – recorded with their respective classification system…) as well as those performed in healthcare facilities (type of facility, dates and modes and entry and discharge, diagnoses, procedures…) [17, 18].
Study population
Study population included all CCALD patients successfully linked from LEUKOFRANCE registry and SNDS and will be referred as LEUKOFRANCE-CCALD cohort.
SNDS cohort included male beneficiaries, with at least one diagnosis code potentially related to ALD, using the international classification of diseases – 10 revision (ICD-10) codes, and aged less than 25 years old at the first ICD10 coded recorded. ICD-10 codes used for ALD detection included E71.3, G13, G31.8, G31.9, G37.8, G37.9, G53.8, G64, G94, G95, and G99 [19]. Exhaustive list and code detail are available in supplementary appendix.
LEUKOFRANCE cohort included ALD patients alive on January 1st, 2009, with a diagnosis of CCALD before their 18th anniversary.
The creation of the LEUKOFRANCE-CCALD cohort was based on a 2-stepped probabilistic linkage. The first step was to detect patients from SNDS with at least one stay at an ALD reference center (Saint Vincent de Paul hospital and Bicêtre hospitals, Assistance Publique des Hôpitaux de Paris), as every CCALD patients from LEUKOFRANCE registry went to these hospitals. The second step was the probabilistic linkage per se using variables available in both databases. Linkage algorithm used gender, date of birth, region of residence, date of diagnosis, date of specific biology tests (cortisol, VLCFA…) date of brain imagery, date of HSCT among others. Based on literature data and previous experience, a linkage rate of at least 90% was expected [20–22]. A medical review was performed for LEUKOFRANCE patients with multiple matches.
As HSCT remains one of the most effective therapies for CCALD and represents a drastic change in disease management and symptomatology, patients were analyzed according to their HSCT status, between patients who did or did not undergo HSCT. Patients were also analyzed according to the way ALD diagnosis was performed. Screened cases include patients who underwent a precocious targeted test for ALD, mostly due to family history or isolated adrenal insufficiency. Index cases include patients who were fortuitously diagnosed or diagnosed when first symptoms occurred.
Study variables
Variables of interest included patient’s sociodemographic characteristics at index date (age, gender, area of residency…), disease natural history (type and date of CCALD symptoms, Major Functional Disabilities [MFD – blindness, communication loss, enteral nutrition, incontinence, movement loss and wheelchair use], HSCT, death…), disease management (laboratory and imaging, CCALD treatments), healthcare resource use and their related costs (inpatient stays, home hospitalization, reimbursed medical and paramedical cares, outpatient visits, treatments, medical devices, transportations…).
Classifications and codes used for variable detection gather the ICD10 classification for diseases and diagnoses, the Classification Commune des Actes Médicaux (CCAM) for medical procedures [23], the Nomenclature des actes de biologie médicale (NABM) for outpatient laboratory tests [24], the Liste des Produits et Prestations (LPP) for reimbursed medical devices [25]. Drugs were detected using either the international anatomical, therapeutic and chemical classification (ATC), or specific French codes for more detailed information about packaging such as the Code Identifiant de Presentation (CIP) [26, 27].
The exhaustive list of variables and their availability among both databases, as well as coding algorithms used for their detection, are available in Supplementary appendix.
Statistical methods
All analyses have been performed using SAS® version 9.4 (SAS Institute Inc. Cary, NC, USA). Quantitative variables were described in terms of mean, standard deviation, median, quartiles and extreme values. Qualitative variables were described in terms of absolute value frequency and percentage for each category by modality, excluding missing data. In accordance with data protection requirements, clinical categories extracted from SNDS data including less than 11 patients may be either grouped with other categories or not described. Cost categories are not affected by this measure. MFD-free and overall survivals were assessed from CCALD diagnosis. For patients presenting MFD before CCALD diagnosis, MFD was considered to occur at CCALD diagnosis. Overall survival and MFD-free survival were estimated using Kaplan-Meier method. MFD-free survival events of interest were defined for patients with HSCT as the first MFD occurrence or a second HSCT or death, whichever occurred first. For patients without HSCT, first MFD occurrence or death were considered, whichever occurs first. Cost analysis was performed based on health care consumptions of interest identified for the study population. Costs per patient for each health care of interest (i.e., direct medical costs) were extracted from the SNDS database. Costs presented for reimbursement and costs reimbursed by the French Health Insurance were extracted from the above database, to perform the economic analysis from the collective perspective and the French Health Insurance perspective, respectively. Costs are reported in Euros (€), year 2018, with costs prior to 2018 being revalued according to a consumer price index published by the national institute for statistics (INSEE) [28].
Results
CCALD LEUKOFRANCE-SNDS Cohort (Objective A)
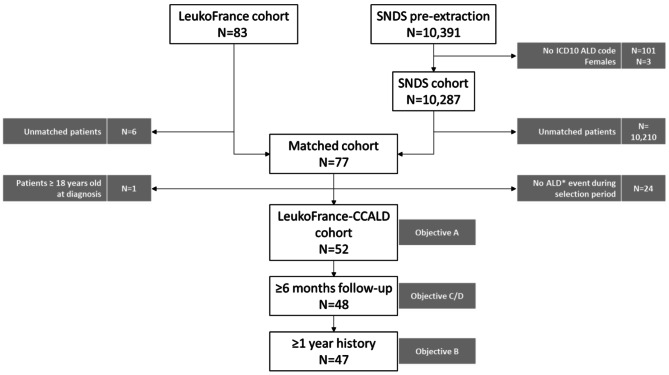
Among the 10,391 patients pre-identified in SNDS database, 10,287 (99.0%) were extracted for matching (SNDS cohort), based on validated ICD-10 codes related to ALD. In the other hand, LEUKOFRANCE cohort included 83 (90.2%) patients among the 92 identified within the registry. After the 2-stepped linkage, 77 patients were successfully matched, leading to a linkage rate of 92.8%, with 6 patients unmatched. Finally, 52 patients with at least one CCALD-related event during study period and sufficient data depth were included into the LEUKOFRANCE-CCALD cohort, composing the initial study population (Fig. 1).
Fig. 1.
Patient Disposition (N = 52)
ICD-10 ALD codes (E71.3, G13, G31.8, G31.9, G37.8, G37.9, G53.8, G64, G94, G95, G99)
ALD events: hospitalization or Long Duration Disease record with ICD-10 ALD codes
Among these patients, mean (SD) follow-up duration after CCALD diagnosis was 89.4 (77.8) months, with 68.1 (42.7) months during study period.
Over study period, median annual incidence of CCALD was estimated at 4 patients, ranging between 2 and 6, leading to 35 incident cases during study period and 17 prevalent ones. Median age at CCALD diagnosis was 7.0 years, ranging from 0.5 (screened case) to 13 years of age, while more than half (51.9%) of patients were diagnosed between 5 and 8 years of age. A majority of patients did not have a family history for CCALD (Table 1).
Table 1.
Patients’ characteristics
| Characteristics | LEUKOFRANCE CCALD cohort (N = 52) | |
|---|---|---|
| Age (y) | median (Q1-Q3) | 7.0 (6.0–9.0) |
| min-max | 0.5–13.0 | |
| Age classes (y) | ||
| < 3 years old | N (%) | 2 (3.8) |
| [3–5[ years old | 2 (3.8) | |
| [5–8[ years old | 27 (51.9) | |
| [8–12[ years old | 17 (32.7) | |
| [12–15[ years old | 4 (7.7) | |
| [15–18[ years old | 0 (0.0) | |
| ALD family history | ||
| yes | N (%) | 15 (29.4) |
| no | 36 (70.6) | |
| Incident cases | ||
| 2009 | N | 2 |
| 2010 | 3 | |
| 2011 | 5 | |
| 2012 | 2 | |
| 2013 | 4 | |
| 2014 | 2 | |
| 2015 | 6 | |
| 2016 | 3 | |
| 2017 | 4 | |
| 2018 | 4 | |
| 10-year study period | N (%) | 35 (67.3) |
| Prevalent cases | N (%) | 17 (32.7) |
| Inclusion status | ||
| Familial screen cases | N (%) | 14 (26.9) |
| Index cases | N (%) | 38 (73.1) |
| Hematopoietic stem cell transplant (HSCT) status | ||
| Patients with allogenic HSCT | N (%) | 19 (36.5) |
| Patients without allogenic HSCT | N (%) | 29 (55.8) |
| Patients with gene and auto HSCT therapy | N (%) | 3 (5.8) |
| Missing value | N (%) | 1 (1.9) |
ALD: adrenoleukodystrophy; CCALD: childhood cerebral ALD
CCALD natural history (objective B)
Among the 52 patients included in this study, 47 (90.4%) had at least one year of clinical history before index date and sufficient follow-up (i.e. 6 months minimum) (Fig. 1). Twenty-nine (29, 61.7%) of them did not undergo HSCT, while 18 (38.3%) had HSCT.
Median ages at ALD diagnosis (VLCFA dosage, ABCD1 mutation detection – detection of every ALD subtypes) and CCALD diagnosis (clinical description) were both 7.0 years.
Major functional disability (MFD) gathers different CCALD-related complications including loss of communication, use of a wheelchair, incontinence, blindness, use of enteral feeding (nasogastric/gastrostomy) and loss of movement (Table 2).
Table 2.
CCALD natural history, symptomatology and survival
| Characteristics | No HSCT (N = 29) | HSCT (N = 18) | Total (N = 47) | |
|---|---|---|---|---|
| Diagnoses | ||||
| Age at ALD diagnosis (y) |
Median (Q1-Q3) |
7.0 (6.0–10.0) | 6.0 (5.0–8.0) | 7.0 (5.0–9.0) |
| Age at CCALD diagnosis (y) | 7.0 (6.0–10.0) | 7.0 (6.0–9.0) | 7.0 (6.0–10.0) | |
| Time between diagnoses (months) | 0.0 (0.0–0.0) | 1.3 (0.0–16.1) | 0.0 (0.0–0.0) | |
| Symptoms | ||||
| Patients with CCALD symptoms | N (%) | 27 (93.1) | 11 (61.1) | 38 (80.9) |
| Patients with HSCT-preventable symptoms | 27 (93.1) | 3 (16.7) | 30 (63.8) | |
| Patients without symptoms | 2 (6.9) | 7 (38.9) | 9 (19.1) | |
| Age at 1st CCALD symptom (y) |
Median (Q1-Q3) |
7.0 (6.0–9.0) | 7.0 (6.0–9.0) | 7.0 (6.0–9.0) |
| CCALD main symptoms | ||||
| Cognitive symptoms | N (%) | 15 (51.7) | 1 (5.6) | 16 (34.0) |
| Motor symptoms | 8 (27.6) | 0 (0.0) | 8 (17.0) | |
| Adrenal insufficiency | 0 (0.0) | 9 (50.0) | 9 (19.1) | |
| Seizures | 1 (3.4) | 0 (0.0) | 1 (2.1) | |
| Other symptoms | 5 (17.2) | 2 (11.1) | 7 (14.9) | |
| Major Functional Disabilities (MFD) | ||||
| Patients with MFD | N (%) | 18 (62.1) | 3 (16.7) | 21 (44.7) |
| Patients without MFD | 11 (31.9) | 15 (83.3) | 26 (55.3) | |
| Age at 1st MFD |
Median (Q1-Q3) |
7.0 (6.0–12.0) | 10.0 (10.0–21.0) | 8.0 (6.0–12.0) |
| Main MFDs | ||||
| Communication loss | N (%) | 16 (80.0) | 0 (0.0) | 16 (42.1) |
| Use of wheelchair | 14 (48.3) | 0 (0.0) | 14 (29.8) | |
| Incontinence | 7 (24.1) | 0 (0.0) | 7 (14.9) | |
| Blindness | 10 (34.5) | 3 (16.7) | 13 (27.7) | |
| Enteral feeding | 11 (37.9) | 0 (0.0) | 11 (23.4) | |
| Movement loss | 9 (31.0) | 0 (0.0) | 9 (19.1) | |
| MFD-free survival* | ||||
| Median MFD-free survival (months) |
Median (Q1-Q3) |
20.0 (3.3–84.0) |
NE (NE – NE) |
41.9 (20.0 – NE) |
| % at 12 months after diagnosis |
% [CI95%] |
51.1 [31.8 ; 67.5] |
94.4 [66.6;99.2] |
67.9 [52.5;79.3] |
| % at 60 months after diagnosis |
31.9 [14.6;50.6] |
72.2 [45.6;87.4] |
48.4 [33.0;62.2] |
|
| Overall survival* | ||||
| Median age at death (y)** |
Median (Q1-Q3) |
10.0 (8.0–16.0) | 9.0 (9.0–9.0) | 10.0 (8.0–16.0) |
| 5-year overall survival |
% [CI95%] |
66.6 [41.0;83.2] |
94.4 [66.6;99.2] |
80.0 [63.8;89.5] |
| End of study overall survival |
30.5 [6.2;60.3] |
94.4 [66.6;99.2] |
65.5 [44.2;80.3] |
|
* Survivals were assessed based on Kaplan-Meier estimates
**Only one patient with HSCT died
ALD: adrenoleukodystrophy; CCALD: childhood cerebral ALD; HSCT: allogenic stem cell transplantation; MFD: major functional disabilities
CCALD symptoms gather: cognitive symptoms, adrenal insufficiency, motor symptoms, convulsions and other symptoms
MFD gathers: loss of communication, wheelchair, incontinence, blindness, enteral feeding (nasogastric/gastrostomy) and loss of movement
HSCT only refers to allogenic transplantations
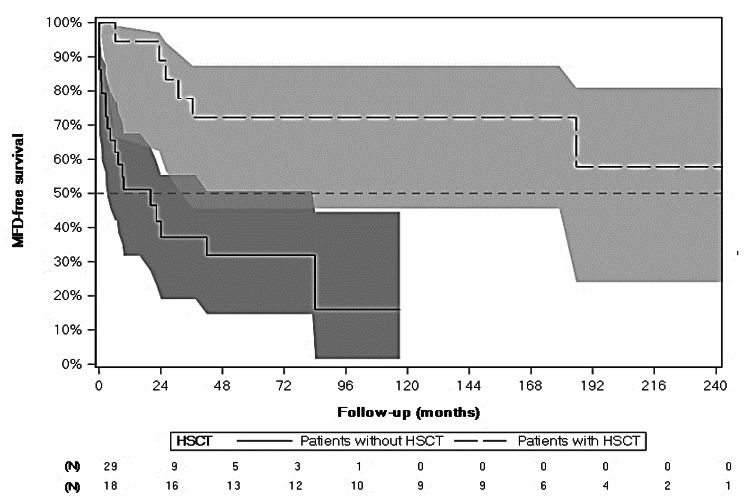
Almost half of patients (21, 44.7%) presented at least one MFD, with a MFD-free survival [CI95%] of 94.4% [66.6%; 99.2%] 12 months after CCALD diagnosis decreasing to 72.2% [45.6%; 87.4%] 60 months after CCALD diagnosis. Overall crude mortality rate was 23.4% (Table 2 – Fig. 2).
Fig. 2.
MFD-free Survival (months) from CCALD Diagnosis for Patients with and without HSCT - Kaplan-Meier (N = 47)
MFD gathers: loss of communication, use of wheelchair, incontinence, blindness, use of enteral feeding (nasogastric/gastrostomy) and loss of movement. HSCT only refers to allogenic transplantations
CCALD patients without HSCT
Among the 29 patients without HSCT, median (Q1-Q3) ages at ALD and CCALD diagnoses were both 7.0 (6.0–10.0) years of age, ranging from 5 to 13. These two diagnoses were concomitant for the entirety of the cohort without HSCT.
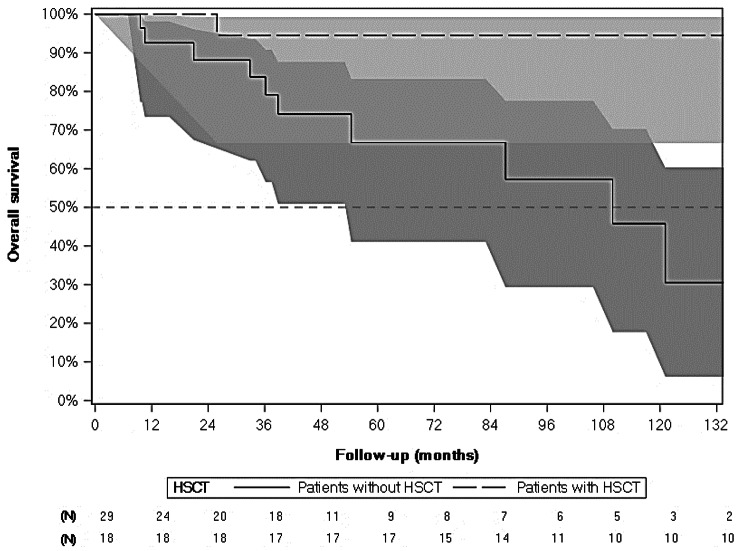
Crude mortality rate was 34.5%, with 10 patients dying during study period. Median age at death was 10 years, with two patients dying within the year after diagnosis. Five-year overall survival [CI95%] was 66.6% [41.0%; 83.2%], while overall survival at the end of follow-up was 30.5% [6.2%; 60.3%] based on Kaplan-Meier estimate (Table 2 – Fig. 3).
Fig. 3.
Overall Survival (months) from CCALD Diagnosis for Patients with and without HSCT - Kaplan-Meier (N = 47)
HSCT only refers to allogenic transplantations
Considering CCALD symptoms, the majority of them (27, 93.1%) presented at least one, with a median age at onset of 7.0 years, ranging from 3 to 12. Most of them (25, 92.6%) had symptoms before CCALD diagnosis, with a median period of 6.9 months between symptom occurrence and CCALD diagnosis. Cognitive and behavioral symptoms were the most frequent first CCALD symptoms to occur, encountered among half of patients (15, 51.7%). Motor symptoms were second, occurring in 27.6% (n = 8) of cases, while other symptoms were encountered in 17.2% (n = 5) of patients. Only two (6.9%) patients did not present any symptom (Table 2).
Eighteen (18, 62.1%) patients without HSCT presented at least one MFD, occurring at 7 years of age in median, either before or within the year after CCALD diagnosis. A median of 5 MFDs was encountered among them. Communication loss was the most frequent MFD, with 16 patients (34.0%). Need for a wheelchair and enteral feeding came second and third, with 14 (29.8%) and 11 (23.4%) patients, respectively. Communication loss was also the MFD with the earliest onset, with a median age at onset of 7.0 years, while movement loss and incontinence were of the latest onset with 13 years of age in median (Table 2). Median MFD-free survival was 20 (3.3–84.0) months. It was 51.1% [31.8%; 67.5%] 12 months after CCALD diagnosis and decreased to 31.9% [14.6%; 50.6%] 60 months after CCALD diagnosis (Table 2 – Fig. 2).
CCALD patients with HSCT
Among the 18 patients who underwent HSCT, median (Q1-Q3) age at ALD diagnosis was 6.0 (5.0–8.0) years and was 7.0 (6.0–9.0) years at CCALD diagnosis, all comprised between 0.5 and 13 years of age. Unlike patients without HSCT, ALD and CCALD diagnoses were not concomitant, with a median (Q1-Q3) time between them of 1.3 (0.0–16.1)months.
Crude mortality rate was 5.6%, as only one patient with HSCT died during study period at the age of 9, 26 months after CCALD diagnosis. Five-year overall survival [CI95%] was 94.4% [66.6%; 99.2%], which remained stable until the end of the study (Table 2 – Fig. 3).
Only 2 patients with HSCT (11.1%) presented HSCT-preventable CCALD symptoms (i.e. excluding adrenal insufficiency – encountered in 9 patients – which is not impacted by HSCT). Symptoms encountered were cognitive and behavioral symptoms. The remaining 16 (88.9%) patients did not present symptoms (Table 2).
Similarly to symptoms, only 3 (16.7%) patients with HSCT presented a MFD, all being blindness, occurring at 10 years of age in median, 3 years after CCALD diagnosis. Median MFD-free survival was not calculable as less than 50% of patients with HSCT either presented a MFD, needed a second HSCT or died during study period. MFD-free survival was 94.4% [66.6%;99.2%] 12 months after CCALD diagnosis and decreased to 72.2% [45.6%;87.4%] after 60 months after CCALD diagnosis (Table 2 – Fig. 2).
HSCT use according to diagnosis settings (objective C)
Among the 52 patients included in this study, 48 (92.3%) had a sufficient follow-up (i.e. 6 months minimum) after CCALD diagnosis (Fig. 1).
Of them, 19 (39.6%) patients had an allograft after CCALD diagnosis; 10 being screened cases after family analysis, and 9 being index cases. Proportions of HSCT among screened and index cases were 83.3% and 25.0%, respectively. Two (2) patients received at least one subsequent HSCT during study period.
Median (Q1 – Q3) age at HSCT was 7.0 (6.0–10.0) and 8.0 (7.0–10.0) among screened and index cases, respectively. Time between CCALD diagnosis and HSCT was 4.9 (1.4–9.2) months in median among index cases. It was doubled among screened cases with 10.3 (3.0–51.4) months (Table 3).
Table 3.
Allogenic Stem Cell Transplantation (HSCT) use
| Characteristics | Familial screen cases (N = 10) | Index cases (N = 9) | Total (N = 19) | |
|---|---|---|---|---|
| Allo-HSCT | ||||
| Age at 1st HSCT (y) |
median (Q1-Q3) |
7.0 (6.0–10.0) | 8.0 (7.0–10.0) | 7.0 (6.0–10.0) |
| Time between diagnosis and HSCT (months) | 10.3 (3.0–51.4) | 4.9 (1.4–9.2) | 4.9 (1.4–19.9) | |
Due to an important proportion of missing data concerning HSCT conditions among patients with HSCT (12, 63.2%), HSCT complications could not be presented in this study.
CCALD healthcare resource use (objective D)
Among the 52 patients included in this study, 48 (92.3%) had a sufficient follow-up period (i.e. 6 months minimum) after CCALD diagnosis and were followed-up until end of study or death, with a median (Q1-Q3) duration of 5.9 (2.3–9.9) years. Twenty-nine (29, 60.4%) of them were patients without HSCT, while the 19 (39.6%) remaining patients underwent HSCT (Fig. 1).
CCALD patients without HSCT
Considering inpatient healthcare resources, almost every patient without HSCT (93.1%) were hospitalized at least once during study period, with an annual median (Q1 – Q3) of 5.4 (1.1–8.0) hospitalizations, accounting for 23.8 (2.8–121.3) days, each year. Twenty-five (25, 86.2%) patients had at least one ER visit, with an annual median of 1.2 ER visits. Among patients without HSCT who had an ICU stay (< 11), annual length of stay in ICU was 4.5 (2.1–6.0) days in median.
Regarding outpatient healthcare resources, patients without HSCT had a median of 2.9 (0.9–6.3) medical visits per year, mostly represented by hospital specialist visits. Twenty-five patients (86.2%) had at least one paramedical care during study period (including nursing care, physiotherapy, podology, speech therapy, orthoptist), almost exclusively represented by nursing care and physiotherapy. Median annualized number of days with any paramedical care, and for nursing care and physiotherapy were 12.7 (1.7–107.9), 1.5 (0.0–10.3) and 1.5 (0.0–97.9), respectively (Table 4).
Table 4.
Healthcare Resource Use
| Characteristics | No HSCT (N = 29) | HSCT (N = 19) | Total (N = 48) | |
|---|---|---|---|---|
| Hospitalizations | ||||
| At least one hospitalization | N (%) | 27 (93.1) | 18 (94.7) | 45 (93.8) |
| Annualized number of hospitalizations |
median (Q1-Q3) |
5.4 (1.1–8.0) | 1.6 (0.5–3.7) | 2.9 (0.6–6.2) |
| Annualized cumulative length of stay (d) |
median (Q1-Q3) |
21.1 (2.8–89.6) | 6.2 (1.0–13.6) | 10.9 (1.4 − 24.1) |
| Emergency room (ER) | ||||
| At least one ER visit | N (%) | 25 (86.2) | 14 (73.7) | 39 (81.3) |
| Annualized number of ER visits |
median (Q1-Q3) |
1.2 (0.3–1.9) | 0.4 (0.0–1.0) | 0.9 (0.2–1.6) |
| Intensive care unit (ICU) | ||||
| At least one ICU stay | N (%) | < 11 | < 11 | 18 (40.0) |
| Annualized cumulative length of stay (d) |
median (Q1-Q3) |
4.5 (2.1–6.0) | 7.4 (5.0–9.6) | 5.9 (2.1–9.6) |
| Magnetic Resonance Imaging | ||||
| At least one brain MRI | N (%) | 21 (72.4) | 14 (73.7) | 35 (72.9) |
| Overall Number of MRI |
median (Q1-Q3) |
2.0 (0.0–6.0) | 3.0 (0.0–11.0) | 2.0 (0.0–6.0) |
| Medical visits* | ||||
| At least one medical visit | N (%) | 27 (93.1) | 17 (89.5) | 44 (91.7) |
| Annualized number of medical visits |
median (Q1-Q3) |
2.9 (0.9–6.3) | 3.9 (1.2–6.5) | 3.2 (1.0–6.4) |
| Paramedical care** | ||||
| At least one paramedical care | N (%) | 25 (86.2) | 17 (89.5) | 42 (87.5) |
| Annualized number of paramedical care |
median (Q1-Q3) |
12.7 (1.7–107.9) | 5.9 (0.7–11.1) | 6.1 (1.3–83.4) |
| Medical devices | ||||
| Medical bed | N (%) | 14 (48.3) | < 11 | NE |
| Wheelchair | N (%) | 12 (41.4) | < 11 | NE |
| Orthesis | N (%) | < 11 | < 11 | NE |
| Medical transportations | ||||
| At least one medical transport | N (%) | 19 (65.5) | < 11 | NE |
| Annualized number of medical transport |
median (Q1-Q3) |
1.1 (0.0–11.2) | 0.0 (0.0–0.5) | 0.3 (0.0–5.1) |
*Medical visits gather: rehabilitation specialist, neurologist, urologist, pediatrician, general practitioner, hospital specialist
**Paramedical care gathers: nursing care, physiotherapist, podologist, speech therapist, orthoptist
HSCT only refers to allogenic transplantations
CCALD patients with HSCT
Considering inpatient healthcare resources, almost every patient with HSCT (94.7%) were hospitalized at least once during study period, with an annual median (Q1 – Q3) of 1.6 (0.5–3.7) hospitalizations, accounting for 6.2 (1.0–13.6) days, each year. Fourteen (14, 73.7%) patients had at least one ER visit, with an annual median of 0.4 ER visits. Among patients with HSCT who had an ICU stay (< 11), annual length of stay in ICU was 7.4 (5.0–9.6) days in median.
Regarding outpatient healthcare resources, patients with HSCT had a median of 3.9 (1.2–6.5) medical visits per year, mostly represented by hospital specialist visits. Seventeen patients (89.5%) had at least one paramedical care during study period, almost exclusively represented by nursing care and physiotherapy. Median annualized number of days with paramedical care was 5.9 (0.7–11.1), 0.9 (0.2–2.0) and 1.1 (0.0–5.8) overall, and regarding nursing care and physiotherapy, respectively (Table 4).
Costs associated with CCALD management (objective D)
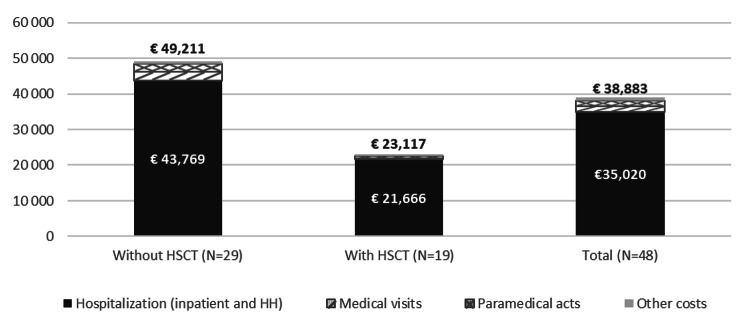
Annualized costs related to healthcare resources used during study period were 38,883€ (45,458€), with more than 90% (35,020€) for hospitalizations (including ER visits). Overall, paramedical care, transports and medications came in second, third and fourth, with mean annualized costs of 1,672€, 1,493€ and 267€, respectively. Medical procedures and laboratory tests were last with less than 100€ per year (Fig. 4).
Fig. 4.
Mean annualized Costs (€) for patients with and without HSCT during Study Period (N = 48)
HH: home hospitalization
Other costs gather medical devices, laboratory tests, procedures, medications and transportations
HSCT only refers to allogenic transplantations
CCALD patients without HSCT
Among the 29 patients without HSCT, mean (SD) annualized related costs were 49,211€ (52,623€), with 88% (43,769€) for hospitalizations. Medical transports were the second most important item of expenditure with 2,341€ (7,637€) (Fig. 4).
CCALD patients with HSCT
Among the 19 patients with allograft, mean (SD) annualized costs were 23,117€ (25,573€), with more than 93% (21,666€) for hospitalizations. These costs included the allograft stay fee, being more than 300,000€ by itself. Paramedical care was the second most important item of expenditure with 686€ (1,288€) (Fig. 4).
CCALD patients with HSCT – HSCT cost evolution
Among the patients who underwent HSCT between 2009 and 2018 (i.e. period with SNDS data available), seven presented sufficient historical period and data granularity to assess cost evolution around HSCT and up to end of study period. During the 6 months prior to HSCT, mean overall costs were 15,096€. During the first year following HSCT (HSCT included), costs peaked at 309,101€, with hospitalization accounting for 306,241€. Then a decrease was observed during the second and subsequent years post-HSCT, with mean costs of 9,289€ during the second year, and an annualized mean cost of 3,020€ after (Fig. 4).
Discussion
Study population
To the best of our knowledge, this study is the first one including this type of linkage in CCALD literature, allowing availability of both national database exhaustiveness and specific registry granularity. Based on literature, X-linked ALD incidence in France is estimated around 1/17,000 births, of whom 35–40% might develop CCALD, leading to an expected annual incidence between 5 and 6 young boys in France [1–4]. In current study, median annual incidence was 4, and reached expected incidence only twice. Apart from a limited underestimation due to partial linkage of patients (93%), the main cause for underestimation was expected to be a potential recruitment bias, highlighted by the rarity of the disease and its difficult diagnosis. As only patients referred to reference centers are included in LEUKOFRANCE registry, patients with very late diagnosis who already present severe CCALD symptoms or MFDs could not be referred to the registry, hence not captured in this study.
Patients’ characteristics and symptomatology
More than half of patients were diagnosed between 5 and 8 years of age, with a median age of 7. Mallack & al meta-analysis including 107 studies from 1970 to 2019, showed similar results, in line with CCALD clinical manifestations appearing between 3 and 12 years of age, usually [5, 29–31]. However, the absence of specific symptoms, notably for patients without family history, makes diagnosis more difficult and requiring for trained professionals. As HSCT is effective among youngest patients, neonatal diagnosis test would be a good option, even it is not implemented in France. In 2018, Bessey & al estimated the potential economic impact of implementing X-linked ALD within routine newborn screening program in United Kingdom. CCALD new-born screening has been shown as dominant in almost every tested scenario, when compared to no screening [32]. In current cohort, almost 25% of patients were screened, and more than 80% of them were transplanted and did not present CCALD symptoms during study period. In addition to newborn screening, a familial screening could allow an early detection of mutation carriers, targeted genetic counselling, and precocious therapies among patient’s family when needed. Several studies and case-reports showed the importance of familial screening. Hetman & al reported a CCALD diagnosis in the oldest boy of a brotherhood of 3 at an advanced stage. It led to a screening on his first younger brother still asymptomatic at age 7, who could undergo HSCT, and on the youngest one, at birth [33]. When analyzing time between ALD and CCALD diagnoses, it appeared that diagnoses were almost always concomitant among index cases, which is explained by the fact that index cases are mostly diagnosed after CCALD symptoms occurrence, then labelled as ALD. As HSCT is almost ineffective on adrenal insufficiency, more than 80% of patients with HSCT did not present any HSCT-preventable CCALD symptoms (i.e., excluding adrenal insufficiency). This result highlights the importance of HSCT in CCALD management, since almost no treatment can be proposed to patients with neurological defects, while oral therapies are available to compensate adrenal glands [34–36]. Only 3 patients with HSCT presented a MFD (cortical blindness). This finding shows the importance of early HSCT, as loss of eyesight in CCALD is progressive but irreversible, hence HSCT cannot repair present damages and is known to be fully effective after 6 to 9 months [37, 38]. In patients with HSCT, MFD-free survival was more than 70% 5 years after CCALD diagnosis. Half of patients without HSCT presented either MFD or death 20 months after CCALD diagnosis. In Kühl & al single center case study MFD-free survival was similar, with 64% after 10 years [31]. Five (5)-year overall survival was estimated at 66.6% for patients without HSCT, slightly higher than in the literature, which is around 55% [8, 39]. CCALD known high variability of both clinical presentation and management, can partly explain this discrepancy. Another hypothesis can be the presence of a recruitment bias, as described above, since the totality of patients included in our study come from LEUKOFRANCE registry. Among patients with HSCT, 5-year overall survival was estimated at 93%, in line with data from Raymond & al multicenter study [8].
CCALD management
One identified study limitation was the lack of information regarding HSCT donor. In Europe, EBMT report showed that less than 20% of CCALD patients who underwent allogenic HSCT did not have matched sibling donor with complete histocompatibility (HLA 10/10), leaving 80% with Matched Unrelated Donors or MisMatched Unrelated Donor [40]. In France, in order to maximize satisfying engraftment, HSCT are almost exclusively reserved to patients with complete histocompatibility. This could notably explain the longer period between diagnosis and HSCT among screened cases, as the early diagnosis decreases the urgency of the transplantation and allows a longer time for graft research. It is of note that GVHD and other complications such as infections, have been shown to have non-negligible impact on allografted patient evolution in literature. Raymond estimated that 45% and 21% of patients presented acute or chronic GVHD, respectively, with infections GVHD (either acute or chronic) recorded as cause of death among several patients [8]. Also, SNDS database does not provide information on the reasons of healthcare resources used, notably HSCT. Hence it is possible that some patients could have received HSCT for another indication, notably malignancies or myelodysplasia. However, in addition to accounting for a small proportion of the transplanted patients, the transplant would also have had a beneficial impact on CCALD, limiting the impact of this bias.
CCALD economic burden
This study is the first to directly assess HCRU and related costs through real-life data in France.
Patients who had HSCT spent less days per year hospitalized. This can be partly explained by the fact that most patients who did undergo HSCT might only encounter complications related to HSCT, and not to CCALD evolution. Despite the relative importance of HSCT-related complications, incidence of these events remains highly lower than those linked to CCALD which are part of natural disease expansion. Regardless of time period and HSCT status, a vast majority (> 90%) of CCALD economic burden seemed attributable to hospital care. As expected, peak cost of care was reached with HSCT. Despite the costs of stem cells themselves and infusion act, a non-negligible proportion of HSCT-related cost comes from a potential prolonged stay in hematological ICU, due to patient’s aplasia. Also, HSCT remains highly effective for long-term HCRU, and related cost decrease once discharged from HSCT stay, with mean annualized cost among study period almost divided by 2 when comparing patients with and without HSCT. Annualized costs remain a robust approach to estimate CCALD burden. For instance, when mean annual costs are multiplied by follow-up period, total CCALD related costs was estimated around 138,000€ and 273,000€ respectively for patients with and without HSCT, respectively, highlighting a consequent burden though life. When analyzing cost evolution with time and disease progression, two completely different patterns are expected between patients with HSCT and those without HSCT. As HSCT is usually performed within months after CCALD diagnosis, a peak could be reached with HSCT, followed by a drastic decrease and a low plateau. In the other hand, as disease progresses among patients without HSCT, costs might be quite low for the first months unlike with HSCT but will progressively and inexorably increase with symptoms and MFD onset. This study only estimated direct medical costs (e.g., inpatient related costs, transportations, laboratory test etc…). However, CCALD also has an important impact on non-medical, indirect, and intangible costs. Bessey et al. study included part of these indirect costs as social care costs, estimating a burden of 6.44 million £ for 18.3 X-ALD cases (9.7 CCALD cases), potentially leading to more than 360,000€ per child [32], overall. Caregivers are expected to take sick child leaves, or even fulltime care periods, strongly impacting their own productivity and quality of life. Also, among direct medical cost which were not available in SNDS data, some might be of significant importance for caregivers. Out of pocket expenses for partially reimbursed medications or medical devices, specialist fee overruns, use of alternative medicines, house adaptation for bedridden patients are several examples of other cost inputs not included in SNDS database, and which are at caregiver’s charge or covered by associations.
Finally, SNDS database does not include information on quality of life. However, Bessey et al. transposed ALD clinical score (ALD-DRS) into quality-of-life score (EQ-5D). For the lowest levels (ALD-DRS I / ALD-DRS II), corresponding to mild neuro-sensorial and behavioral impairments, the related EQ-5D scores were around 0.6, with 1.0 being the best health state possible. Non-transplanted CCALD patients are expected to present the highest scores, being ALD-DRS III and IV, the related EQ-5D were 0.11 and 0.031 respectively. It seems acceptable to assess that transplanted patients, if treated early enough, would present scores equal or higher to ALD-DRS I, highlighting the importance of HSCT on every outcomes of interest, either clinical, economic, or of quality of life [32].
Conclusion
X-linked ALD is a rare metabolic and neurodegenerative genetic disease, causing irreversible damages on peripheral nervous system and adrenal glands. Birth incidence of X-linked ALD is estimated around 1/17,000, of whom 40% will develop early symptoms, called Childhood Cerebral ALD (CCALD), the most devastating ALD form. This study estimated the number of CCALD patients in France, their clinical characteristics, therapeutic management and medium- and long-term evolution, through a follow-up analysis of 6 years in median. With 4 incident CCALD patients per year in France, and despite a potential underestimation due to patients’ non-exhaustive referral to LEUKOFRANCE registry, CCALD rarity has been highlighted. Until recently, HSCT was the only treatment able to stop CCALD progression but had to be performed as early as possible to prevent irreversible damages. Only a small amount of CCALD patients, less than 40% in our study, is eligible to HSCT. However, when performed, HSCT brings drastic improvement in terms of overall clinical presentation and overall lifespan. In addition, CCALD economic burden seemed highly linked to HSCT status, with a three-time increase of annual hospital length of stay (6.2 days vs. 21.1 days) and a two time increase of overall annual costs (23,117€ vs. 49,211€).
HSCT in CCALD disease management has been shown as primordial when performed early enough to prevent symptoms and MFDs. The necessity of a precocious management highlights the potential benefits of including an expanded screening program among newborns, coupled with family screenings when a mutation is detected.
To the best of our knowledge, this is the most up to date study analyzing CCALD epidemiology, clinical and economic burden in France. It is also the first one to link complete reimbursement database (SNIIRAM) to a clinical ALD specific registry (LEUKOFRANCE) in France. It allowed both precise clinical burden and disease evolution assessment through clinical data, as well as good estimates for CCALD economic burden among patients who did and did not undergo HSCT.
Acknowledgements
The LEUKOFRANCE study group for authorizing the use of patient-related data. Yann Dantal, responsible of the LEUKOFRANCE IT system, who supervised the transfer of data to SNDS secured platform.
List of Abbreviations
- ALD
Adrenoleukodystrophy
- ALDP
Adrenoleukodystrophy Protein
- (Allo)-HSCT (allogeneic)
Hematopoietic Stem Cell Transplantation
- AMN
Adrenomyeloneuropathy
- ATC
Anatomical, Therapeutic and Chemical classification
- CCALD
Childhood Cerebral ALD
- CCAM
Medical procedures classification (Classification Commune des Actes Médicaux)
- CIP
French drug package code (Code Identifiant de Présentation)
- ER
Emergency Room
- GVHD
Graft Versus Host Disease
- ICD-10
International Classification of Diseases – 10th revision
- ICU
Intensive Care Unit
- INSEE
National institute for statistics (Institut National de la Statistique et des Etudes Economiques)
- LPP
Medical devices list (Liste des Produits et Prestations)
- MFD
Major Functional Disabilities
- MRI
Magnetic Resonance Imaging
- NABM
Laboratory test classification (Nomenclature des Actes de Biologie Médicale)
- NFS
Neurological Functional Score
- Q1-Q3
Quartiles
- SNDS
French National Health Data System (Système National des données de Santé)
- SD
Standard Deviation
Authors’ Contributions
CS and LC participated in the study design, interpretation of the data, manuscript drafting and revision and overall supervision. SH participated in the interpretation of the data, manuscript drafting and revision. AC, FBi, FBu and SB participated in the study design, interpretation of the data, revisions, and overall supervision. OBT participated in the study design, interpretation of the data, revisions, and led the study supervision. All authors read and approved the final Manuscript, and took responsibility for the accuracy, completeness, and protocol adherence of data and analyses.
Funding
This study was funded by Bluebird bio., Inc. Somerville, MA.
Data Availability
No additional data available.
Declarations
Ethics approval and consent to participate
Data are issued from the French National Health Insurance information system with agreement from the French National Data Protection Agency. The study protocol was approved on June 13, 2019, by the Expert Committee for Healthcare Data Research. During their inclusion in LEUKOFRANCE registry, patients or legal guardian signed a consent form for their data to be used.
Consent for publication
Not concerned.
Competing interests
CS has no conflict of interest to be disclosed. SH has no conflict of interest to be disclosed. AC has been employed by bluebird bio during this study and until October 2021. FBi has been employed by bluebird bio during this study and until January 2022. LC and FBu are employees of stève consultants, which has a research consultancy contract with Bluebird Bio. SB is the executive director of stève consultants, which has a research consultancy contract with Bluebird Bio. OBT has no conflict of interest to be disclosed.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kirk EP, Fletcher JM, Sharp P, Carey B, Poulos A. X-linked adrenoleukodystrophy: the Australasian experience. Am J Med Genet. 1998;76:420–3. doi: 10.1002/(SICI)1096-8628(19980413)76:5<420::AID-AJMG10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 2.Bezman L, Moser AB, Raymond GV, Rinaldo P, Watkins PA, Smith KD, et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol. 2001;49:512–7. doi: 10.1002/ana.101. [DOI] [PubMed] [Google Scholar]
- 3.Jardim LB, da Silva ACF, Blank D, Villanueva MM, Renck L, Costa MLB, et al. X-linked adrenoleukodystrophy: clinical course and minimal incidence in South Brazil. Brain Dev. 2010;32:180–90. doi: 10.1016/j.braindev.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Regelmann MO, Kamboj MK, Miller BS, Nakamoto JM, Sarafoglou K, Shah S, et al. Adrenoleukodystrophy: Guidance for adrenal surveillance in males identified by newborn screen. J Clin Endocrinol Metab. 2018;103:4324–31. doi: 10.1210/jc.2018-00920. [DOI] [PubMed] [Google Scholar]
- 5.Engelen M, Kemp S, de Visser M, van Geel BM, Wanders RJ, Aubourg P, et al. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis. 2012;7:51. doi: 10.1186/1750-1172-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aubourg P. Adrénoleucodystrophie liée à l’X. Ann Endocrinol. 2007;68:403–11. doi: 10.1016/j.ando.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Moser HW, Mahmood A, Raymond GV. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. 2007;3:140–51. doi: 10.1038/ncpneuro0421. [DOI] [PubMed] [Google Scholar]
- 8.Raymond GV, Aubourg P, Paker A, Escolar M, Fischer A, Blanche S, et al. Survival and functional outcomes in boys with cerebral adrenoleukodystrophy with and without hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:538–48. doi: 10.1016/j.bbmt.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 9.Moser HW, Loes DJ, Melhem ER, Raymond GV, Bezman L, Cox CS, et al. X-Linked adrenoleukodystrophy: overview and prognosis as a function of age and brain magnetic resonance imaging abnormality. A study involving 372 patients. Neuropediatrics. 2000;31:227–39. doi: 10.1055/s-2000-9236. [DOI] [PubMed] [Google Scholar]
- 10.Loes DJ, Hite S, Moser H, Stillman AE, Shapiro E, Lockman L, et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am J Neuroradiol. 1994;15:1761–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Engelen M, van Ballegoij WJC, Mallack EJ, Van Haren KP, Köhler W, Salsano E, et al. International Recommendations for the diagnosis and management of patients with adrenoleukodystrophy: a Consensus-Based Approach. Neurology. 2022;99:940–51. doi: 10.1212/WNL.0000000000201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh SH, Satwani P, Ahn KW, Sahr NA, Fretham C, Abraham AA, et al. Survival Trends in Infants undergoing allogeneic hematopoietic cell transplant. JAMA Pediatr. 2019;173:e190081. doi: 10.1001/jamapediatrics.2019.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichler F, Duncan C, Musolino PL, Orchard PJ, De Oliveira S, Thrasher AJ, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med Massachusetts Medical Society. 2017;377:1630–8. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.République française. Décret n° 2016 – 1871 du 26 décembre 2016 relatif au traitement de données à caractère personnel dénommé « système national des données de santé ». 2016 – 1871 Dec 26, 2016.
- 15.Système National des Données de Santé. Qu’est-ce que le SNDS ? | SNDS [Internet]. [cited 2021 Oct 20]. Available from: https://www.snds.gouv.fr/SNDS/Qu-est-ce-que-le-SNDS.
- 16.Direction de la Sécurité Sociale. CHIFFRES CLES 2020 ED2019.pdf [Internet]. [cited 2021 Oct 20]. Available from: https://www.securite-sociale.fr/files/live/sites/SSFR/files/medias/DSS/2020/CHIFFRES%20CLES%202020%20ED2019.pdf.
- 17.Moulis G, Lapeyre-Mestre M, Palmaro A, Pugnet G, Montastruc J-L, Sailler L. French health insurance databases: what interest for medical research? Rev Médecine Interne. 2015;36:411–7. doi: 10.1016/j.revmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merlière Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286–90. doi: 10.1016/j.respe.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. ICD-10 Version:2019 [Internet]. [cited 2021 Oct 20]. Available from: https://icd.who.int/browse10/2019/en.
- 20.Raffray M, Bayat S, Lassalle M, Couchoud C. Linking disease registries and nationwide healthcare administrative databases: the french renal epidemiology and information network (REIN) insight. BMC Nephrol. 2020;21:25. doi: 10.1186/s12882-020-1692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perlbarg J, Allonier C, Boisnault P, Daniel F, Fur P, Szidon P, et al. Faisabilité et intérêt de l’appariement de données individuelles en médecine générale et de données de remboursement appliqué au diabète et à l’hypertension artérielle. Santé Publique. 2014;26:355. doi: 10.3917/spub.139.0355. [DOI] [PubMed] [Google Scholar]
- 22.Didier R, Gouysse M, Eltchaninoff H, Le Breton H, Commeau P, Cayla G, et al. Successful linkage of french large-scale national registry populations to national reimbursement data: improved data completeness and minimized loss to follow-up. Arch Cardiovasc Dis. 2020;113:534–41. doi: 10.1016/j.acvd.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Caisse Nationale d’Assurance Maladie. CCAM en ligne - CCAM [Internet]. [cited 2021 Jun 2]. Available from: https://www.ameli.fr/accueil-de-la-ccam/index.php.
- 24.Caisse Nationale d’Assurance Maladie. Présentation TNB [Internet]. [cited 2021 Oct 20]. Available from: http://www.codage.ext.cnamts.fr/codif/nabm/index_presentation.php?p_site=AMELI.
- 25.Caisse Nationale d’Assurance Maladie, Nomenclatures. NGAP et LPP [Internet]. [cited 2021 Sep 22]. Available from: https://www.ameli.fr/infirmier/exercice-liberal/facturation-remuneration/nomenclatures-ngap-lpp/nomenclatures-ngap-lpp.
- 26.World Health Organization. WHOCC – ATC/DDD Index [Internet]. [cited 2021 Jun 2]. Available from: https://www.whocc.no/atc_ddd_index/.
- 27.Ministère des Solidarités et de la Santé. Qu’est-ce que le code CIP 13 et le Datamatrix ? [Internet]. [cited 2021 Oct 20]. Available from: https://solidarites-sante.gouv.fr/soins-et-maladies/medicaments/professionnels-de-sante/suppression-de-la-vignette-pharmaceutique-questions-reponses-a-l-attention-des/article/qu-est-ce-que-le-code-cip-13-et-le-datamatrix.
- 28.Insee. Institut national de la statistique et des études économiques [Internet]. [cited 2021 Oct 20]. Available from: https://www.insee.fr/fr/accueil.
- 29.Turk BR, Theda C, Fatemi A, Moser AB. X-linked adrenoleukodystrophy: Pathology, pathophysiology, diagnostic testing, newborn screening and therapies. Int J Dev Neurosci Off J Int Soc Dev Neurosci. 2020;80:52–72. doi: 10.1002/jdn.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallack EJ, Turk BR, Yan H, Price C, Demetres M, Moser AB, et al. MRI surveillance of boys with X-linked adrenoleukodystrophy identified by newborn screening: Meta-analysis and consensus guidelines. J Inherit Metab Dis. 2021;44:728–39. doi: 10.1002/jimd.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kühl J-S, Kupper J, Baqué H, Ebell W, Gärtner J, Korenke C, et al. Potential risks to stable long-term outcome of allogeneic hematopoietic stem cell transplantation for children with cerebral X-linked adrenoleukodystrophy. JAMA Netw Open. 2018;1:e180769. doi: 10.1001/jamanetworkopen.2018.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bessey A, Chilcott JB, Leaviss J, Sutton A. Economic impact of screening for X-linked adrenoleukodystrophy within a newborn blood spot screening programme. Orphanet J Rare Dis. 2018;13:179. doi: 10.1186/s13023-018-0921-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman M, Jura M, Krakowska K, Barg E. X-linked adrenoleukodystrophy diagnosed in three brothers. Pediatr Endocrinol Diabetes Metab. 2019;25:95–8. doi: 10.5114/pedm.2019.85821. [DOI] [PubMed] [Google Scholar]
- 34.Cartier N, Aubourg P. Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-Linked adrenoleukodystrophy. Brain Pathol. 2010;20:857–62. doi: 10.1111/j.1750-3639.2010.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:364–89. doi: 10.1210/jc.2015-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanakis G, Kaltsas G. Adrenal Insufficiency Due to X-Linked Adrenoleukodystrophy. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000 [cited 2021 Sep 23]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK278944/.
- 37.Miller WP, Rothman SM, Nascene D, Kivisto T, DeFor TE, Ziegler RS, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. 2011;118:1971–8. doi: 10.1182/blood-2011-01-329235. [DOI] [PubMed] [Google Scholar]
- 38.Peters C, Charnas LR, Tan Y, Ziegler RS, Shapiro EG, DeFor T, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104:881–8. doi: 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- 39.Mahmood et al. Survival analysis of haematopoietic cell transplantation for childhood cerebral X-linked adrenoleukodystrophy: a comparison study. Available from: https://pubmed.ncbi.nlm.nih.gov/17618834/. [DOI] [PubMed]
- 40.Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies [Internet]. Cham: Springer International Publishing; 2019 [cited 2021 Jul 22]. Available from: http://link.springer.com/10.1007/978-3-030-02278-5. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data available.