Abstract
Background
Myopic corneal refractive surgery is one of the most prevalent ophthalmic procedures for correcting ametropia. This study aimed to perform a bibliometric analysis of research in the field of corneal refractive surgery over the past 40 years in order to describe the current international status and to identify most influential factors, while highlighting research hotspots.
Methods
A bibliometric analysis based on the Web of Science Core Collection (WoSCC) was used to analyze the publication trends in research related to myopic corneal refractive surgery. VOSviewer v.1.6.10 was used to construct the knowledge map in order to visualize the publications, distribution of countries, international collaborations, author productivity, source journals, cited references, keywords, and research hotspots in this field.
Results
A total of 4,680 publications on myopic corneal refractive surgery published between 1979 and 2022 were retrieved. The United States has published the most papers, with Emory University contributing to the most citations. The Journal of Cataract and Refractive Surgery published the greatest number of articles, and the top 10 cited references mainly focused on outcomes and wound healing in refractive surgery. Previous research emphasized “radial keratotomy (RK)” and excimer laser-associated operation methods. The keywords containing femtosecond (FS) laser associated with “small incision lenticule extraction (SMILE)” and its “safety” had higher burst strength, indicating a shift of operation methods and coinciding with the global trends in refractive surgery. The document citation network was clustered into five groups: (1) outcomes of refractive surgery: (2) preoperative examinations for refractive surgery were as follows: (3) complications of myopic corneal refractive surgery; (4) corneal wound healing and cytobiology research related to photorefractive laser keratotomy; and (5) biomechanics of myopic corneal refractive surgery.
Conclusion
The bibliometric analysis in this study may provide scholars with valuable to information and help them better understand the global trends in myopic corneal refractive surgery research frontiers. Two stages of rapid development occurred around 1991 and 2013, shortly after the innovation of PRK and SMILE surgical techniques. The most cited articles mainly focused on corneal wound healing, clinical outcomes, ocular aberration, corneal ectasia, and corneal topography, representing the safety of the new techniques.
Keywords: bibliometrics analysis, corneal refractive surgery, global trends, VOSviewer, CiteSpace
1. Introduction
Myopia is the leading cause of visual impairment worldwide (1). Thus, refractive surgery has become one of the most commonly performed ophthalmologic surgical procedures (2, 3). Over the last decades, the reliance on spectacles and contact lenses has decreased worldwide owing to the low risk and high success rates of refractive surgery (4). Since the cornea contributes to approximately two-thirds of the eye’s total optical power while being the most accessible part of the eye, myopic corneal refractive surgery is the mainstay of myopic correction. In 1896, Lendeer Jans Lans of Holland was the first to publish the idea of changing the cornea’s shape to correct refractive errors. In 1948, Jose Ignacio Barraquer Moner suggested that changing the corneal curvature could correct refractive error by sculpting corneal stromal tissue (5).
Radial keratotomy (RK) was first developed by the former Soviet scholar Svyatoslav N. Fyodrov in the 1970s (6) and was introduced in the United States in 1979. Since then, RK has been widely used as a treatment for myopia (7). Unfortunately, the initial surgical techniques were inherently imprecise, but the discovery of excimer laser photoablation in 1980 has brought precision to the process and has become the root of modern refractive surgery. The use of ocular excimer lasers was first reported in 1981 by Taboada, Mikesell, and Reed in United States (8). Stephen Trokel, a physical engineer and ophthalmologist, was the first to experiment with the use of an excimer laser on the cornea for refractive correction in 1983 (9). In 1985, Seiler performed the first procedure using an excimer laser on a sighted human eye (10). Subsequently, another technique known as in situ keratomileusis was developed in 1988 (11). In 1989, Ioannis Pallikaris from Greece was the first to perform laser in situ keratomileusis (LASIK) on a blind human eye at the University of Crete (12). Since then, there has been rapid progression in the use of excimer lasers for refractive and therapeutic purposes. Significant technological advances have led to refractive surgery becoming one of the fastest-evolving fields in healthcare. In recent years, clinical outcomes have improved remarkably due to advances in surgical methods and technology, such as laser epithelial keratomileusis (LASEK), femtosecond (FS) lasers, thin flap LASIK (sub-Bowman’s keratomileusis), enhanced laser delivery systems, improvement of eye trackers, and wavefront-guided and topography-guided lasers. Enhanced visual outcomes, refractive predictability, and low occurrence of complications, combined with these significant technical advances, have led to widespread acceptance of laser refractive surgery as a replacement for spectacles and contact lenses (10). A new technique called Small-incision lenticule extraction (SMILE) uses a FS laser to shape a refractive lenticule and then remove it through a minor wound. The potential advantages of SMILE over other techniques include lower laser energy requirements (13), less induction of higher-order aberrations (14), a significant decrease in corneal inflammation and keratocyte damage (15, 16), shorter duration and lower suction pressure during the procedure (17), greater tectonic strength, and a decreased occurrence of dry eye (18, 19).
Citation analysis is a widely used type of bibliometric method utilizing mathematical and statistical methods to explore and analyze the citation and reference patterns of both academic and scientific journals, papers, and authors. In this study, citation analysis was applied to study research impact, knowledge flows, and citation networks. Citation analysis has been used to visually highlight and provide a valuable overview of current academic literature, and to predict research trends (20). With the development of analysis software and the expansion of easily accessible online databases, citation analysis is gaining more and more attention worldwide. Although many studies on clinical outcome, efficacy, safety, stability, pathology, and biomechanical changes in surgery have been published, to our knowledge, the global research trend in myopic corneal refractive surgery has not yet been explored using bibliometric analysis. Therefore, this study aimed to investigate the prevailing status of myopic corneal refractive surgery research using Web of Science Core Collection (WoSCC) data. Bibliometric analysis was applied to analyze the existing international status of myopic corneal refractive surgery research, determine the highly influential factors in this field, and investigate hotspots in the research. Overall, this study provides not only a complete and extensive reference but also a promising reference for researchers.
2. Materials and methods
The publications were downloaded and extracted from the WoSCC provided by Thomson Reuters (Philadelphia, PA, United States). Data were acquired on August 21, 2022. The keywords used for the search were “myopic corneal refractive surgery” or some specific myopic corneal refractive surgery in the title or abstract because we were concerned with myopic corneal refractive surgery per se rather than related terminology. The search strategy was as follows: TI = “myopic corneal refractive surgery” or AB = “myopic corneal refractive surgery” or TI = “laser-assisted in situ keratomileusis” or AB = “laser-assisted in situ keratomileusis” or TI = “femtosecond laser-assisted LASIK” or AB = “femtosecond laser-assisted LASIK” or TI = “photorefractive keratectomy” or AB = “photorefractive keratectomy” or TI = “femtosecond laser-assisted LASIK” or AB = “femtosecond laser-assisted LASIK” or TI = “femotosecond lamellar extraction” or AB = “femotosecond lamellar extraction” OR TI = “epipolis laser in situ keratomileusis” OR AB = “epipolis laser in situ keratomileusis” or TI = “small incision lenticule extraction” or AB = “small incision lenticule extraction” or TI = “transepithelial photo-refractive keratectomy” or AB = “transepithelial photo-refractive keratectomy” or TI = “laser epithelial keratomileusis” or AB = “laser epithelial keratomileusis” or TI = “radial keratotomy” or AB = “radial keratotomy” or TI = “excimer laser refractive surgery” or AB = “excimer laser refractive surgery.” To increase accuracy, we restricted our search strategy to terms related to myopia in the title or abstract. The reason is that many of the reported publications were not associated with myopia when other search fields were used, such as keywords. As opposed to title, abstract, or keywords search queries, the title or abstract search is recommended in bibliometric studies because it significantly increases specificity with minimal loss of sensitivity.
Among the document types included were only articles and reviews (other types of documents, such as meeting abstracts, editorial materials, proceedings papers, and letters, were excluded). For the analysis, journal articles were used since they contain complete research ideas and results. Downloaded data from WoSCC are in “plain text” format, with “full records and cited references.”
The use of visualization software can create node-link maps that make it intuitive to observe publication outputs, hotspots, and other aspects of a research field. This study used VOSviewer v.1.6.101 to analyze the data systematically. VOSviewer generated knowledge maps that represented items as nodes and links. There were nodes and labels corresponding to countries, organizations, authors, co-citation literature, and keywords based on their weights. Nodes are linked together based on their relationships. We used CiteSpace IV (Drexel University, Philadelphia, PA, United States) to identify keywords with strong citation bursts, which may indicate research frontiers. To evaluate the development of scientific research within a particular field, CitNetExplorer Software2 was used to visualize the citation networks and the connections between them. This software can perform cluster analysis and show the main research content of the research field.
3. Results
3.1. Yearly quantitative distribution of publications
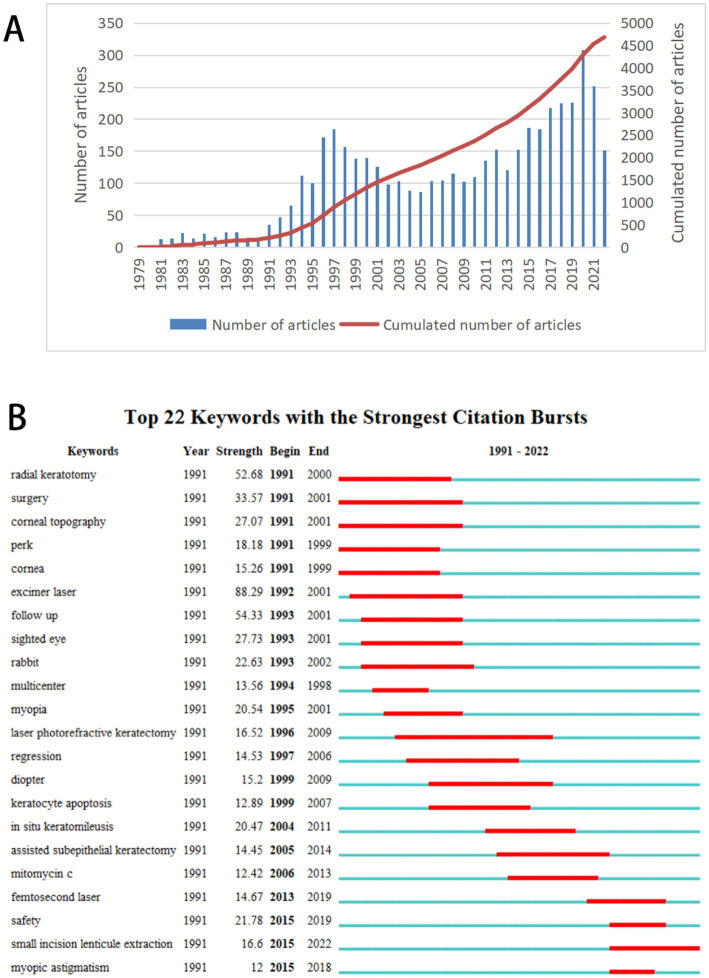
The WoSCC database search resulted in a total of 4,680 articles. The first article on myopic corneal refractive surgery was published in 1979 (Figure 1A). In 2020, 308 articles were published, which was the highest yearly number (Figure 1A). In 2022, 152 articles had been published by August (Figure 1A), and the number is expected to rise significantly by the end of the year. We identified 22 keywords representing citation bursts by keyword burst detection analysis (Figure 1B). The first keyword was “radial keratotomy” detected in 1991 and lasted for 9 years. “Excimer laser” had the highest burst intensity (88.29) among the 22 keywords during the rapid development stage. “small incision lenticule extraction,” “femtosecond laser,” “safety,” and “myopic astigmatism” were the latest keywords in the rapid development stage.
Figure 1.
Annual publications and citation bursts analysis. (A) Number and Cumulated number of publications on myopic corneal refractive surgery per year (1979–2022). (B) Top 22 keywords with the strongest citation bursts on myopic corneal refractive surgery. The blue lines represent the base timeline, while the red segments represent the burst duration of the keywords.
3.2. Distribution of productive countries
The search results showed that 4,680 articles were found from 83 countries. As shown in Table 1, the top 10 cited countries involved in myopic corneal refractive surgery research published a total of 3,699 articles, accounting for 79.04% of the total number of publications. The United States had the largest number of publications (1,490 articles, 31.84%), followed by China (537 articles, 11.47%) and Germany (318 articles, 6.79%). The citation analysis showed that the United States had 41,020 citations, followed by Germany (7536) and the United Kingdom (6747).
Table 1.
Top 10 cited countries in myopic corneal refractive surgery study.
| Rank | Country | Number of citations (%) | Number of publications (%) |
|---|---|---|---|
| 1 | United States | 41,020 (36.21%) | 1,490 (31.84%) |
| 2 | Germany | 7,536 (6.65%) | 318 (6.79%) |
| 3 | England | 6,747 (5.96%) | 236 (5.04%) |
| 4 | China | 5,506 (4.86%) | 537 (11.47%) |
| 5 | Italy | 5,455 (4.81%) | 283 (6.05%) |
| 6 | Spain | 4,769 (4.21%) | 237 (5.06%) |
| 7 | France | 3,989 (3.52%) | 157 (3.35%) |
| 8 | South Korea | 3,226 (2.85%) | 184 (3.93%) |
| 9 | Brazil | 2,986 (2.64%) | 135 (2.88%) |
| 10 | Japan | 2,969 (2.62%) | 122 (2.61%) |
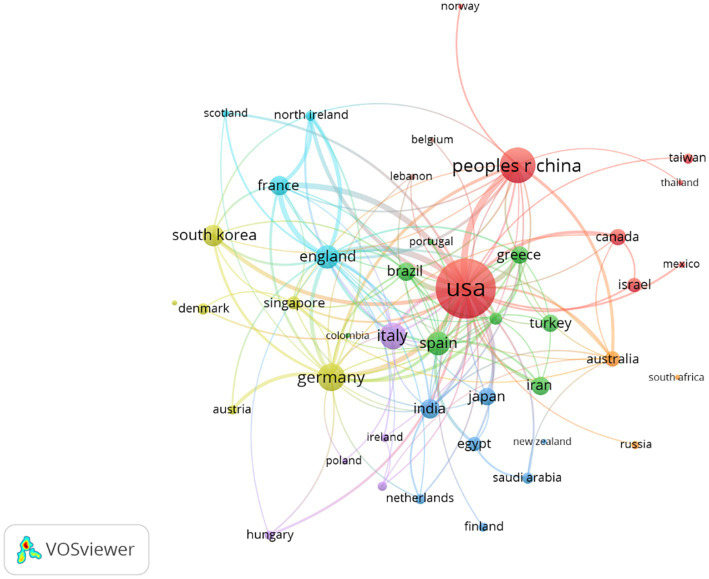
The degree of communication between countries and the most influential countries in the field is reflected by country co-authorship analysis. The larger the node, the greater the country’s influence; the thickness and distance of the links between nodes define the strength of the cooperative relationships between countries. Figure 2 shows that the United States cooperated closely with numerous countries in the field of myopic corneal refractive surgery, including United Kingdom, China, France, Germany, and South Korea.
Figure 2.
Network visualization map of countries’ collaboration in myopic corneal refractive surgery research. The size of the node represents the number of publications of the country and the thickness of the lines signifies the size of collaboration between the countries. The minimum number of documents of a country was set as 10. Of the 83 countries that were involved in myopic corneal refractive surgery research, 43 countries met the threshold.
3.3. Distribution of prominent research organizations
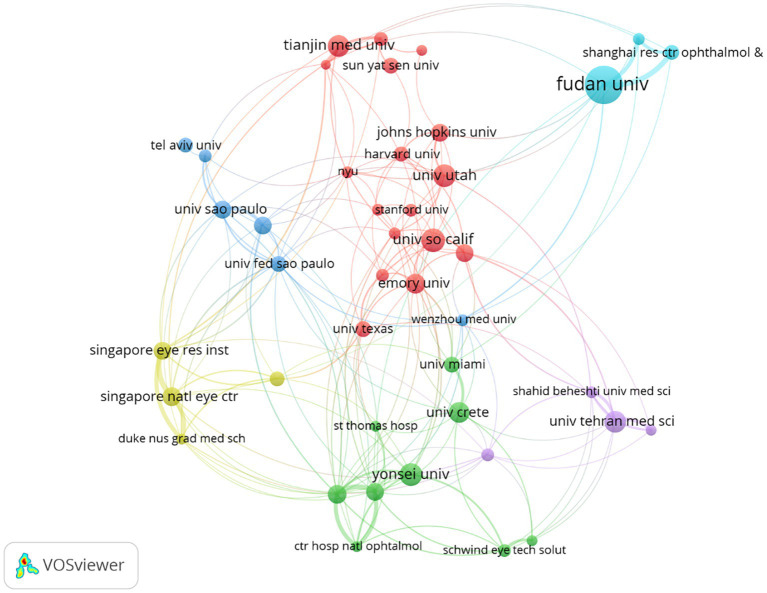
The search results showed that the top 10 cited institutions published 573 articles, accounting for 12.24% of the total number of publications (Table 2); seven of the 10 most cited institutions for myopic corneal refractive surgery were from the United States. Citation analysis showed that Emory University had 4,037 citations and was ranked first. The knowledge domain distribution map of myopic corneal refractive surgery research institutions is shown in Figure 3 and is based on co-authorship analysis. The size of each node corresponds to the number of published articles. The links between nodes represent collaborations. The stronger the node link, the closer the collaboration between the two organizations.
Table 2.
Top 10 cited organizations in myopic corneal refractive surgery study.
| Rank | Organizations | Country | Number of citations (%) | Number of publications (%) |
|---|---|---|---|---|
| 1 | Emory University | United States | 4,073 (8.21%) | 60 (2.93%) |
| 2 | University of Texas | United States | 3,466 (6.99%) | 46 (2.25%) |
| 3 | University of California | United States | 2,713 (5.47%) | 74 (3.62%) |
| 4 | Louisiana State University | United States | 2,420 (4.88%) | 38 (1.86%) |
| 5 | University of California Los Angeles | United States | 2,199 (4.43%) | 54 (2.64%) |
| 6 | Aarhus University | Denmark | 2082 (4.20%) | 43 (2.10%) |
| 7 | University of São Paulo | Brazil | 1789 (3.61%) | 54 (2.64%) |
| 8 | Harvard University | United States | 1,694 (3.42%) | 43 (2.10%) |
| 9 | Cleveland Clinic | United States | 1,679 (3.39%) | 37 (1.81%) |
| 10 | Fudan University | China | 1,613 (3.25%) | 124 (6.06%) |
Figure 3.
Network visualization map of main research organizations in myopic corneal refractive surgery study. The size of each node is determined by the number of publications from each institution. The width of each line represents the strength of links between institutions. The minimum number of documents of an organization was set as 30. Of the 3,084 organizations that were involved in myopic corneal refractive surgery research, 43 organizations met the threshold.
3.4. Distribution of authors and co-authorship of research groups
According to the search results, more than 20,728 authors have contributed to myopic corneal refractive surgery research. Table 3 lists the 10 most productive authors in myopic corneal refractive surgery. Among all authors, Zhou and Xingtao (104 publications) ranked first, followed by Wang and Yan (68 publications) and Reinstein and Dan Z (54 publications), reflecting their abundant contribution to the research of myopic corneal refractive surgery. The authors’ co-citation status was also analyzed. The results showed that Reinstein, DZ (1,430 co-citations), was the most commonly cited author, followed by Seiler, T (1,263 co-citations) and Wilson, SE (908 co-citations), indicating their relative influence on myopic corneal refractive surgery research.
Table 3.
Top 10 productive authors and co-cited authors in myopic corneal refractive surgery study.
| Rank | Author | Number of publications | Citation ratio | Co-cited author | Number of citations | Citation ratio |
|---|---|---|---|---|---|---|
| 1 | Zhou, Xingtao | 104 | 13.73 | Reinstein, DZ | 1,430 | 26.48 |
| 2 | Wang, Yan | 68 | 12.81 | Seiler, T | 1,263 | 37.14 |
| 3 | Reinstein, Dan Z | 54 | 26.48 | Wilson, SE | 908 | 22.70 |
| 4 | Moshirfar, Majid | 52 | 10.46 | Kanellopoulos, AJ | 875 | 28.22 |
| 5 | Archer, Timothy J | 48 | 25.04 | Alio, JL | 803 | 25.90 |
| 6 | Kymionis, George D | 43 | 23.67 | Sekundo, W | 767 | 28.40 |
| 7 | Jhanji, Vishal | 42 | 7.86 | Waring, GO | 763 | 29.35 |
| 8 | Mehta, Jodhbir S | 42 | 24.71 | Randleman, JB | 586 | 22.63 |
| 9 | Wilson, Steven E | 40 | 22.70 | Hersh, PS | 547 | 26.05 |
| 10 | Li, Meiyan | 39 | 20.67 | Gartry, DS | 544 | 23.65 |
Citation ration = Number of citations/number of publications.
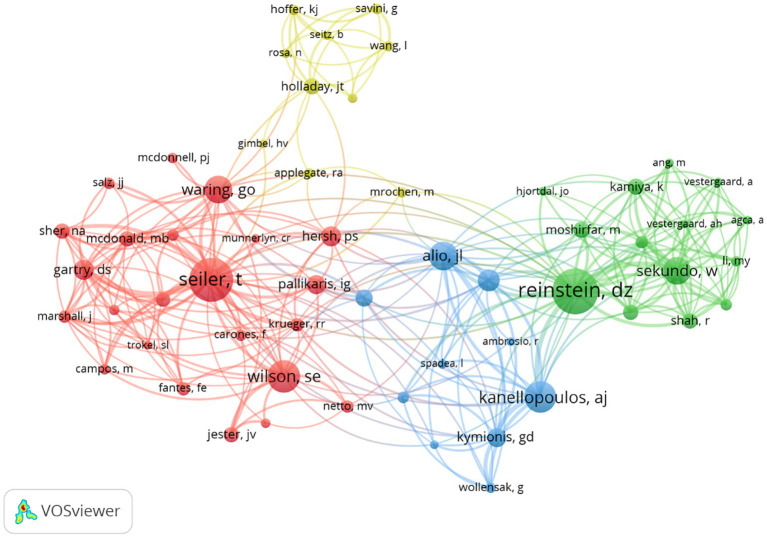
Based on the co-authorship analysis, the knowledge domain map of authors of myopic corneal refractive surgery research is shown in Figure 4. There was a close collaboration between high-yielding authors, except for the group marked in yellow. Different co-authorship groups have different cores, such as the red group with Seiler T and the green group with Reinstein DZ as the core.
Figure 4.
Co-cited authorship network in myopic corneal refractive surgery study. The size of the frame represents the number of publications of the author and the thickness of lines signifies the size of collaboration between the authors. The minimum number of documents of an author was set as 200. Of the 20,728 authors that were involved in myopic corneal refractive surgery research, 57 authors met the threshold.
3.5. Top prolific source journals
Based on the retrieved results, articles on myopic corneal refractive surgery were published in 96 journals. Table 4 lists the top 10 journals with publications related to myopic corneal refractive surgery. According to the citations results, the citations of the Journal of Cataract and Refractive Surgery ranked first and the Journal of Refractive Surgery ranked second. The Journal of Refractive Surgery published the most significant number of articles (793, 16.94%), followed by the Journal of Cataract and Refractive Surgery (710, 15.17%) and Ophthalmology (237, 5.06%). These three journals accounted for 39.80% of the total number of articles published in this field. Considering the countries of publication, seven of the top 10 journals were from the United States, two were from the United Kingdom, and one was from the Netherlands.
Table 4.
Top 10 cited source journals in myopic corneal refractive surgery study.
| Rank | Journal | Publishing country | Citations (%) | Count (%) |
|---|---|---|---|---|
| 1 | Journal of Cataract and Refractive Surgery | United States | 17,280 (18.87%) | 710 (15.17%) |
| 2 | Journal of Refractive Surgery | United States | 16,261 (17.76%) | 793 (16.94%) |
| 3 | Ophthalmology | Netherlands | 13,154 (14.37%) | 237 (5.06%) |
| 4 | Archives of Ophthalmology | United States | 6,144 (6.71%) | 116 (2.48%) |
| 5 | American Journal of Ophthalmology | United States | 5,718 (6.24%) | 162 (3.46%) |
| 6 | Investigative Ophthalmology & Visual Science | United States | 4,269 (4.66%) | 80 (1.71%) |
| 7 | Cornea | United States | 4,220 (4.61%) | 263 (5.62%) |
| 8 | British Journal of Ophthalmology | United Kingdom | 2,215 (2.42%) | 70 (1.50%) |
| 9 | Experimental Eye Research | United States | 1,632 (1.78%) | 30 (0.64%) |
| 10 | Clinical Ophthalmology | United Kingdom | 1,533 (1.67%) | 134 (2.86%) |
3.6. Top-cited publications
A myopic corneal refractive surgery research knowledge base can be efficiently constructed through the co-citation analysis of cited references. The minimum number of citations for a cited reference was set to 50 of the 34,267 cited references, and 136 met the threshold. Table 5 lists the top 10 co-cited references The top 10 papers were co-cited over 4,200 times, and the first was co-cited 570 times. The top 10 cited references primarily concentrated on outcomes and wound healing in refractive surgery.
Table 5.
Top 10 co-cited references in myopic corneal refractive surgery research.
| Rank | Title | Author | Year | Citations |
|---|---|---|---|---|
| 1 | Wound-healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys | Fantes, FE | 1990 | 570 |
| 2 | Photorefractive keratectomy-a technique for laser refractive surgery | Munnerlyn, CR | 1988 | 529 |
| 3 | Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study | Sekundo, Walter | 2011 | 511 |
| 4 | Risk assessment for ectasia after corneal refractive surgery | Randleman, J | 2008 | 444 |
| 5 | Results of small incision lenticule extraction: All-in-one femtosecond laser refractive surgery | Shah, Rupa | 2011 | 397 |
| 6 | Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing | Moreno-Barriuso, E | 2001 | 365 |
| 7 | Corneal stromal wound healing in refractive surgery: the role of myofibroblast | Jester, JV | 1999 | 362 |
| 8 | Excimer-laser in-situ keratomileusis and photorefractive keratectomy for correction of high myopia | Pallikaris, IG | 1994 | 362 |
| 9 | Myopic photorefractive keratectomy with the excimer laser - one-year follow-up | Seiler, T | 1990 | 346 |
| 10 | Comparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusis | Oshika, T; Klyce, SD | 1999 | 315 |
3.7. Myopic corneal refractive surgery research themes, frequent topics, and trends
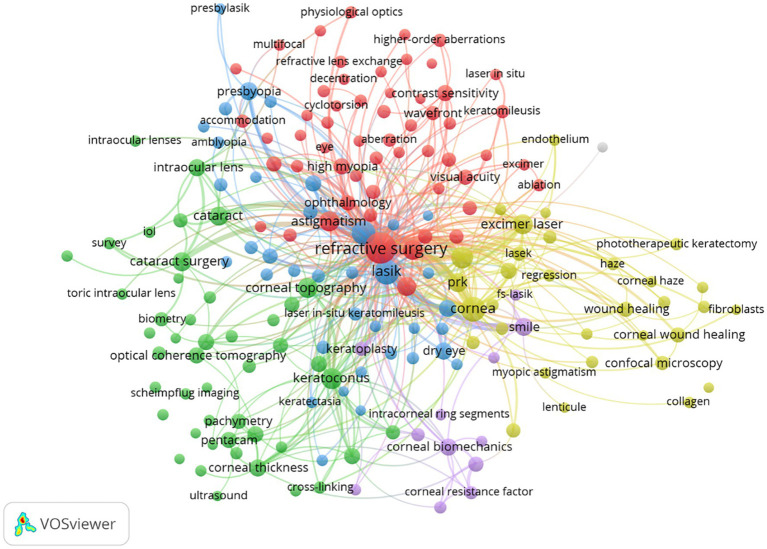
The research hotspots of myopic corneal refractive surgery have been identified by high-frequency keyword co-occurrence analysis the minimum number of occurrences of each keyword was set to eight. Of the 4,133 keywords related to myopic corneal refractive surgery research, 184 met the threshold. Keywords with similarity were clustered based on the network, and five major clusters were represented by red, green, yellow, purple, and blue (Figure 5), and Table 6 lists the top 10 keywords for each cluster.
Figure 5.
Co-occurrence network of keywords in myopic corneal refractive surgery study. The size of the points represents the frequency, and the keywords are grouped into five clusters: (Cluster 1 – Red) Outcome of refractive surgery; (Cluster 2 – Green) Preoperative examinations of refractive surgery; (Cluster 3 – Blue) Complications of myopic corneal refractive surgery; (Cluster 4 – Yellow) Corneal wound-healing and cytobiology research related to photorefractive laser keratotomy; and (Cluster 5 – Purple) biomechanics in myopic corneal refractive surgery. The minimum number of occurrences of a keyword was set as 8. Of the 4,133 keywords that were involved in myopic corneal refractive surgery research, 184 keywords met the threshold.
Table 6.
Co-occurence analysis of keywords.
| Clustrer 1 (Red) | OF | Clustrer 2 (Green) | OF | Clustrer 3 (Blue) | OF | Clustrer 4 (Yellow) | OF | Clustrer 5 (Violet) | OF |
|---|---|---|---|---|---|---|---|---|---|
| Refractive surgery | 731 | Keratoconus | 127 | Lasik | 278 | Cornea | 259 | SMILE | 65 |
| Astigmatism | 108 | Corneal topography | 94 | Myopia | 208 | Photorefractive keratectomy | 158 | Corneal biomechanics | 48 |
| Femtosecond laser | 103 | Corneal thickness | 88 | Presbyopia | 51 | Excimer laser | 95 | Corneal hysteresis | 30 |
| Laser in situ keratomileusis | 82 | Glaucoma | 78 | Dry eye | 41 | PRK | 89 | Keratoplasty | 26 |
| High myopia | 45 | Intraocular pressure | 55 | Small incision lenticule extraction | 39 | Radial keratotomy | 44 | Corneal resistance factor | 21 |
| Contrast sensitivity | 41 | Optical coherence tomography | 51 | Hyperopia | 39 | Corneal wound healing | 33 | Corneal ectasia | 19 |
| Visual acuity | 37 | Pentacam | 41 | Refractive error | 39 | Wound healing | 31 | Ocular response analyzer | 16 |
| Ophthalmology | 32 | Central corneal thickness | 39 | Contact lenses | 38 | Confocal microscopy | 27 | Astigmatic keratotomy | 10 |
| Phakic intraocular lens | 31 | Keratometry | 39 | Contact lens | 38 | LASEK | 25 | Corvis St. | 10 |
| Laser | 28 | Pachymetry | 39 | Laser in-situ keratomileusis | 32 | Biomechanics | 23 | Intracorneal ring segments | 10 |
Top 10 keywords in the 5 clusters. OF, occurrence frequency.
4. Discussion
The purpose of bibliometric analysis is to answer a specific research question based on a large number of publications (21) even without having access to special data collections, in contrast to systematic reviews, which attempt to address research questions based on a small number of publications (22, 23). Recently, bibliometric analysis has become widely recognized as an alternative tool for academically detailed information assessment in the information and library sciences. Although there have been many studies concerning myopic corneal refractive surgery, they were limited to a single aspect or had a short study period and did not include keyword bursts in their analyses (10, 18, 24, 25). In this study, we conducted a comprehensive bibliometric analysis of 4,680 available literatures concerning myopic corneal refractive surgery from 1979 to 2022; five groups were recognized in citation network, and keyword burst detection was carried out.
4.1. Global contribution to research on myopic corneal refractive surgery
A change in the number of academic papers is an important research index reflecting a growing trend in the relevant field. As shown in Figure 1A, 4,680 articles were retrieved for myopic corneal refractive surgery from 1979 to 2022, and the annual research output increased with time. The number of literatures on myopic corneal refractive surgery has been divided into four stages: the inception phase (from 1979 to 1991), the rapid development phase (from 1991 to 2001), the steady development phase (from 2001 to 2013), and another fast development phase (after 2013). At the start of the inception phase, RK was introduced in the United States in 1979 (7) and received widespread attention as a treatment option for myopia. The use of excimer lasers for refractive and therapeutic purposes has grown rapidly since the 1980s, with the advent of excimer lasers for photorefractive keratectomy (PRK) and LASIK. In 1990, Pallikaris and Buratto developed LASIK based on Barraquer’s lamellar incision practice 40 years earlier (26), which became a significant milestone and ushered in the first rapid development stage of myopic corneal refractive surgery. The first laser epithelial keratomileusis (LASEK) procedure was performed by Azar et al. in United States in 1996 (27). In 2000, the Food and Drug Administration (FDA) approved an FS laser for lamellar corneal surgery (28). Surgical techniques have evolved from FS lenticule extraction and pseudo SMILE to the most commonly used surgery, SMILE, which the FDA has approved for treating myopia and astigmatism (29). SMILE was first utilized by Sekundo and Blum in 2008 (30). The results of these inchoate studies were published in 2008 (30) and 2011 (31). Subsequently, the number of articles published annually increased. As of 2017, more than a million SMILE procedures had been performed worldwide (32).
International cooperation among countries in scientific research is becoming increasingly important. As shown in Table 1, the United States ranked first in the number of publications and citations, at 31.84 and 36.21%, respectively. Meanwhile, China contributed 537 articles, accounting for 11.47% of publications. At the same time, the United States and China teamed up intensely with many other countries in the myopic corneal refractive surgery field, as shown in Figure 2; the United States and China are international scientific centers of myopic corneal refractive surgery research and play an important role in academic exchanges and cooperation. Adequate funding, the sharing of advanced techniques, and equipment settings are also essential. The most productive organizations and collaboration within the groups can be recognized and are shown in Table 2; the most influential research institute was Fudan University (124 documents), followed by the University of California (74 documents), and Emory University (60 documents). According to the citation analysis, Emory University had 4,037 citations and was ranked first. These organizations are at the heart of the research network. Among all authors, Zhou Xingtao contributed 104 publications and was ranked first, followed by Wang Yan, the first surgeon to report the clinical results of SMILE surgery in China (33). Information on author co-citations was also analyzed. Regarding authors’ analysis, Reinstein DZ was the most cited author, and Seiler T had the highest citation ratio.
Valuable information for researchers seeking collaboration opportunities can be provided by establishing a co-authorship network knowledge map. Figure 4 shows the co-authorship groups. The red-colored team includes Professor Seiler who first applied excimer lasers to the human eye in 1985 (10). Seiler performed the first wavefront-guided myopic corneal refractive surgery putting forward the concept of personalized cutting as the focus. The green-colored team centers on Professor Reinstein, a pioneer in corneal measurement techniques and instruments, who invented laser-blended vision therapy to treat the reading vision of patients with presbyopia. The yellow-colored team centers on Professor Holladay, the inventor of Holladay’s formula. The blue-colored team has Professor Alio as the focus.
A distribution analysis of academic journals helps to identify the core journals in particular field of research. We found that the Journal of Refractive Surgery has published the maximum number of articles, whereas, the Journal of Cataract and Refractive Surgery has the highest number of citations and is most influential.
4.2. Intellectual base
We used citation parameters to describe relevant topics in the selected articles on the premise that high-quality research will be widely cited. Through co-citation analysis, a large number of cited references can effectively display the research background. As shown in Table 5, the top-cited article is “Wound-healing after excimer laser keratomileusis (PRK) in monkeys,” published in 1990 in the Archives of Ophthalmology with 544 citations. This study deduced that the monkey anterior cornea showed a mild, typical wound-healing response after an excimer laser keratomileusis procedure (34). The second top-cited article is “PRK-a technique for laser refractive surgery,” published in 1988 in the Journal of Cataract and Refractive Surgery with 512 citations. In this paper (35), the conditions for PRK, which utilizes tissue ablation with far-ultra-violet radiation to directly reshape the central optical zone of the cornea, were described, and the equations for the tissue ablation to accomplish the required refractive corrections were presented. The third top-cited article is “Small incision myopic corneal refractive surgery using the SMILE procedure for the correction of myopia and myopic astigmatism: results of a six-month prospective study,” published in 2011 in the British Journal of Ophthalmology with 456 citations. This study suggests that SMILE is a hopeful new flapless and minimally invasive technique for correcting myopia and myopic astigmatism (31).
4.3. Focus on myopic corneal refractive surgery
Keyword co-occurrence analysis can be used to represent the search topic and reveal the internal structure of the involved literatures and frontal subjects. As given in Table 6, the themes of corneal refractive surgery mainly formed five clusters. Combined with the characteristics and present status of corneal refractive surgery research, the following five groups are discussed:
4.3.1. Outcome of myopic corneal refractive surgery
In recent years, with the accumulation of experience and continuous improvements in technology, the safety and efficacy of refractive surgery have significantly improved. RK can be used to treat myopia, but it has been replaced by corneal laser surgery due to its lower safety and fecklessness (10). With the application of excimer lasers for myopic corneal refractive surgery, the effectiveness, accuracy, security, predictability, and stability of refractive surgery have been greatly improved. A review (36) of nearly 100 studies published since 2008 revealed that up to 99.5% of patients who underwent laser refractive surgery had uncorrected distance vision better than 20/40. 98.6% of these patients had a refractive target within ±1.0 diopter, and almost 98.8% of patients were satisfied with their results. Seiler and Wollensak (37) reported that PRK is an effective technique to rectify myopia by up to −7.0 D. Nevertheless, corneal haze and myopic regression may usually appear after PRK procedure (38). Although improvement in uncorrected visual acuity is faster with LASIK than with PRK, the long-term effectiveness outcomes are generally similar between the two procedures (39). LASIK is more efficient, safe, and stable than PRK in the correction of higher myopia and creates less postoperative corneal haze (40, 41). Furthermore, the results of LASIK surgery are three times more predictable than PRK (40). In a meta-analysis, LASEK-treated eyes showed no significant benefit over PRK-treated eyes in terms of clinical outcome (42). All-in-one FS refractive correction is safer, predictable, and is more efficient in treating myopia and myopic astigmatism due to its small incision technique (43). SMILE also achieved similar effectiveness, predictability, and safety in comparison to FS-LASIK (44). Corneal biomechanical strength and corneal nerve protection were significantly improved with SMILE compared to LASIK (45) or PRK (44). However, this technique lacks automated centration and cyclotorsion control, so several concerns have been raised regarding its capability to correct moderate or high levels of astigmatism (46).
Additionally, a short-tear breakup time-type dry eye may appear in patients treated with SMILE (47), while the postoperative dry eye syndrome incidence was lower in SMILE than in FS-LASIK (44). A network meta-analysis compared the postoperative efficiency, predictability, safety, and visual quality of all dominating modes of laser corneal refractive surgeries for correcting myopia in 2017. These procedures can be broadly divided into 3 categories: corneal surface ablation surgery, corneal stromal ablation surgery, and refractive corneal lenticule extraction. Surface ablation procedures include photorefractive keratectomy (PRK), transepithelial photorefractive keratectomy (T-PRK), laser epithelial keratomileusis (LASEK), and epipolis laser in situ keratomileusis (Epi-LASIK) (48). Corneal stromal ablation procedures include laser in situ keratomileusis (LASIK) with the flap created with either a mechanical microkeratome or femtosecond-based microkeratome (FS-LASIK). Refractive corneal lenticule extraction procedures include femtosecond lenticule extraction (FLEx) and small incision lenticule extraction (SMILE) (49). No statistically significant differences in either visual outcomes or visual quality between FS-LASIK, LASIK, Epi-LASIK, PRK, T-PRK, LASEK, FLEx and SMILE were found (50). However, in predictability of outcome, FS-LASIK performed better than other surgeries (50).
Patients who have undergone refractive surgery usually have some visual complaints even if their visual acuity was 20/20. The human eye normally has a substantial number of aberrations (51). It was shown that high-order aberrations (HOAs) increased after surgery, and a high correlation was revealed between HOAs, corneal spherical aberration, and visual performance (52, 53). The total wavefront error increased by a factor of 17.65 on average in any treated individual (54). Correspondingly, refractive surgery can induce a mass of third- and higher-order aberrations, with spherical aberrations increasing the most (52). Wavefront aberrations of the cornea were increased and the relative contribution of coma-and spherical-like aberrations was changed in PRK and LASIK procedures (55). In Wen’s study (50), LASIK appeared to induce a higher degree of HOAs than other surgical methods, regardless of pupil size, and the surface group (PRK, T-PRK and LASEK) was better than the stromal group (FS-LASIK, LASIK and Epi-LASIK), especially for 6-mm pupils. Previous research showed that optimal ablation algorithms and procedures are essential to avoiding new aberrations and eliminating existing high-order aberrations while obtaining the desired correction of refractive error.
4.3.2. Preoperative and postoperative examinations of myopic corneal refractive surgery
Refractive surgery aims to obtain good postoperative visual acuity and patient satisfaction. Therefore, effective patient selection is essential. A thorough preoperative examination and evaluation must be performed with full consideration of the motivation and expectations of the patient as well as the possibilities, complications, and contraindications of each operative technique. Routine procedures for myopic corneal refractive surgery include measurement of intraocular pressure (IOP), vision acuity, slit-lamp examination, fundus examination, dry eye screening, refractive status examination, corneal curvature and corneal topography, wavefront aberration, and ocular ultrasound. Randleman et al., using retrospective comparative and case–control studies, reported that the risk factors for corneal ectasia after myopic corneal refractive surgery included topographic abnormalities, low preoperative corneal thickness, inadequate residual stromal bed thickness, high myopia, and young in age (56). Other factors may also predict postoperative corneal ectasia, such as erratic refractions, eye rubbing history, a family history of ectatic corneal disease, and an underlying increase in corneal elasticity (56). Nevertheless, unacknowledged preoperative corneal ectasia is another major risk factor of postoperative corneal ectasia (57). Ectasia can occur after an otherwise uncomplicated laser in situ keratomileusis procedure, even in the absence of apparent preoperative risk factors (58).
Additionally, it is necessary to eliminate early keratoconus or forme fruste keratoconus before surgery, as rapidly progressive corneal ectasia can occur in such cases (59). Keratoconus is a non-inflammatory disease of corneal thinning characterized by anterior protrusion of the cornea and stromal attenuation (60). Since the first report of keratectasia in 1998 (61), many cases have been reported. In some situations, ignoring topographic abnormalities will result in serious consequences. For example, LASIK or PRK performed in the eye when true keratoconus or early keratoconus is present but not detected can lead to severe corneal damage and result in progressive corneal thinning and eventual need for corneal transplantation (62). Thus, detecting keratoconus is essential to avoid unpredictable results in refractive surgery.
The most challenging determinations clinicians must make involve distinguishing true early keratoconus from similar patterns, such as contact lens-induced warpage or normal corneas with asymmetric bowtie. Corneal topography is one of the most sensitive methods for detecting early keratoconus (63, 64). Topography can often provide characteristic clues to the presence of this disease before the cornea becomes significant thinner or other signs appear, such as abnormalities detectable by slit-lamp biometrics or light reflection under funduscopy. Unlike corneal topographers, tomographers generate a three-dimensional recreation of the anterior segment and provide information about the corneal thickness, which can evaluate the whole cornea by obtaining information from both anterior and posterior corneal surfaces (65). Progression is one of the most important markers of true keratoconus (62). In case of uncertainty (e.g., when considering whether to proceed with myopic corneal refractive surgery), the best course of action is to follow the topography and other indicators, such as central corneal thickness, over at least a year. Increased steepening over time is highly suggestive of true keratoconus (62). In recent years, machine learning has been applied to the detection of keratoconus (66) and in the analysis of corneal images (67, 68). Furthermore, corneal biomechanics have been employed for keratoconus detection. Wang et al. provided a new potential approach for diagnosing keratoconus solely from the perspective of corneal biomechanics (69). Without corneal topographic examination, machine learning makes rapid and accurate keratoconus diagnosis possible (69).
Determining the corneal thickness is a prerequisite for avoiding complications from refractive surgical procedures. Accurate measurement of corneal thickness helps to detect and manage corneal pathology associated with corneal thinning and to distinguish keratoconus from contact lens-induced corneal thinning (70); this allows the surgeon to calculate the depth of the residual corneal tissue and determine the safe limits for a given procedure (71). Preoperatively, the corneal thickness measurement was equivalent using Orbscan II, conventional ultrasound, or confocal techniques, but ultrasound biomicroscopy readings thicker (72). Pentacam was more accurate than Orbscan II, especially after refractive surgery (73).
In refractive surgery, pupil size is considered to be an essential factor of optical quality. Numerous of studies have analyzed the connection between the ablation zone and mesovisual pupil size in night vision problems after laser correction (74–77). Therefore, it is advisable to accurately measure preoperative pupil diameter under low-light illumination conditions. The most commonly used devices for scotopic and low-mesopic pupil size measurements are handheld infrared pupillometer (Colvard, Oasis, CA) and digital pupillometer(Procyon, United Kingdom), which allow coinstantaneous or close measurements (78).
It is common for patients to have lower IOP after corneal refractive surgery than before. The IOP measured after PRK and LASIK for myopia may be reduced because of reduced corneal thickness and curvature and, possibly, tissue softening after natural healing (79). The IOP in the central part of the cornea after PRK is lower than that in the corneal periphery (80). The reduction in measured IOP following refractive surgery, by about 0.5 mmHg/D of myopic correction, needs to be remembered when possible abnormality of IOP in such patients is being considered (79). Meanwhile, IOP is influenced by body position and different devices (81). It was shown that IOP increase in the supine and standing positions (81).
IOL power calculation after corneal refractive surgery is important and challenging due to inaccurate measurement of anterior keratometry, keratometric index variation, and wrong effective lens position (ELP) estimation (82, 83). An Advanced Lens Measurement Approach (ALMA) method (84) which was published by Rosa et al. can improve R Factor (85) and ALxK (86).
4.3.3. Complications of myopic corneal refractive surgery
Laser refractive corneal surgery is a common operation with a low complication rate (38). However, because this is an elective procedure that improves the quality of life by restoring uncorrected visual acuity, any adverse events could seriously affect patient satisfaction. Patients who experience halos, increased glare, irregular astigmatism, corneal scarring or residual ametropia, are often unsatisfied (42). Dry eye is another common side effect due to reduced tear secretion from nerve damage and inflammation of the cornea. Fortunately, dry eye is generally an interim problem and can be treated effectively with lubricating eye drops or other methods. However, without proper treatment, pre-existing dry eye can get worsen (87). Iatrogenic keratectasia is one of the most severe complications. Rarely, this procedure weakens the corneal biomechanical strength, and results in corneal ectasia (88). Since the first report in 1998 (89), the characteristics of patients who developed corneal ectasia after surgery in a small case series were reported (58, 90–106).
There are few postoperative complications with PRK except for corrections greater than six diopters (107). Typically, a corneal haze appears in the first month after surgery and is the most severe at three to 6 months, after which it gradually decreases (108). Corneal haze continus to improve during long-term follow-up (108). At 12 months postoperatively, 3% of patients had haze, and 3.6% reported glare or halos (109). Ptosis occurred in 0.4% of eyes, and intraocular pressure increased significantly in 3.5% of eyes due to corticosteroid treatment (109). During the 18-year follow-up period, there was no evidence of a progressive hyperopic metastasis, corneal ectasia, or late-onset corneal haze (108).
Complications of LASIK in the papers published in peer-reviewed journals included night vision problem (14.0%), haze (8.7%), reoperation (8.2%), interface debris (6.8%), wrinkle (5.9%), central island (5.3%), induced astigmatism (5.1%), free cap (4.9%), decentration (4.7%), epithelial ingrowth (4.3%), irregular flap (4.0%), short flap (3.0%), perforated lenticule (2.6%), incomplete cut (2.5%) and sliding flap (1.4%) (110). Flap displacement (111), diffuse lamellar keratitis (112), and epithelial ingrowth (113) are flap-related complications, all of which can be treated with topical eye drops or, in rare cases, by relifting the flap.
SMILE has some advantages, including the absence of a corneal flap and better corneal biomechanical stability (114). However, some intraoperative complications, such as incisional bleeding, suction loss, subconjunctival hemorrhage, opaque bubble layer, black areas, lenticule tearing, unintended posterior plane dissection and incision abrasion (115), are observed in some cases. Although intraoperative complications inevitably occur, patients may achieve satisfactory visual outcomes although intraoperative complications inevitably occur, with appropriate management techniques (115). Some of the postoperative complications, including diffuse lamellar keratitis (DLK), punctate epithelial erosions, corneal infiltrates, foreign interface body, interface debris/secretion, interface haze, corneal edema, corneal striae and epithelial ingrowth, may temporarily affect visual recovery; however, most are resolved with appropriate management (116). Irregular corneal topography occurred in 1.0% of eyes, leading to ghost images or reduced corrected distance visual acuity at 3 months or ghost images (117).
4.3.4. Corneal wound healing and cytobiology research related to myopic corneal refractive surgery
As shown in Table 5, the top-cited articles concerning wound healing after excimer laser keratomileusis. The predictability and stability of refractive surgery were undermined by wound-healing properties of the cornea, which lead to discrepancies between attempted and achieved visual outcomes after PRK, LASIK, and other keratorefractive procedures (118). With extensive research on wound healing after myopic corneal refractive surgery, achieved by the mechanical responses of the cornea to injury and dominated by the stroma, awareness of its role in refractive surgery has developed. Any mechanical or biological response to injury influences optical properties; in some cases, a tendency to mechanical instability or abnormal healing regulation can result in severe complications such as corneal ectasia or loss of corneal clarity. Variations in wound healing are the underlying cause of the varied refractive outcomes and progressive effect of incisional keratotomy (119–121), and these variations determine regression and complications following PRK (122, 123).
The wound healing process begins with epithelial injury, which may take the form of microknife, femtosecond laser disruption, mechanical scratching, or alcohol exposure. Subsequently, damaged epithelium cells and epithelial basement membrane release cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1 (124), bone morphogenic proteins two and four, platelet-derived growth factor (PDGF), and epidermal growth factor (EGF) (125). These factors, along with others derived from the tears, trigger various responses in underlying stromal keratocytes. The keratin Fas ligand binds to the Fas receptor on nearby keratocytes and leads to apoptosis (124). After this, additional cells experience the pro-inflammatory process of necrosis (126). The remaining keratocytes begin to proliferate and migrate within 12–24 h, producing activated keratocytes, fibroblasts, and possibly myofibroblasts responsible for repopulating the depleted stroma (127). In addition, pro-inflammatory chemokines of epithelial cells or keratocytes respond to IL-1 and TNF-α within 24 h of injury, triggering stromal infiltration of T cells, macrophages/monocytes, and polymorphonuclear cells. These cells arrive via the limbal blood supply and tear film, and then, play a part in the phagocytosis of apoptotic and necrotic debris and possibly serve other functions in the stroma (128). Myofibroblasts stained with antibodies against alpha-smooth muscle actin (α-SMA) can be observed in the anterior stroma directly below the damaged areas of epithelial basement membrane one to 2 weeks after injury, depending on the surface irregularity, correction level, and other factors (129). Owing to altered corneal crystalline production, these cells revealed reduced transparency and play a comprehensive role in collagen and extracellular matrix remodeling through the production of collagen, collagenases, matrix metalloproteinases, gelatinases and glycosaminoglycans (130). The appearance of myofibroblasts is associated with a significant increase in a stromal haze (122, 123). The anterior cornea of monkey eyes after excimer laser keratomileusis showed a mild, typical wound healing response (35). Marshall et al. discovered that all corneas were clear immediately after 3 mm diameter discs were excided from the optical zone of the monkey corneas using an excimer laser at 193 nm at various depths up to 130 μm, except for the deepest ablation (131). The haze gradually resolved over 6 months, but the deepest discs were still identifiable on slit-lamp examination (131). The morphology was nearly normal at 8 months, except for the absence of Bowman’s membrane and the immediate subepithelial stromal fibers that still had some degree of disorder (131).
Nevertheless, there are important differences in the speed, intensity, and spatial distribution of the wound-healing activity among different surgical approaches of laser vision correction. Extensive injury and removal of the epithelial cells, epithelial basement membrane, Bowman’s layer, and part of the anterior stroma were involved in PRK; these structures were left relatively undisturbed in LASIK, except at the edge of the flap under the stromal-epithelial flap (118). The essential determinants of corneal wound healing may be the level and distribution of keratocyte apoptosis and subsequent activation of stromal keratocytes regeneration, which were associated with variability and regression after PRK and LASIK (128). This difference in the degree of central epithelial injury is a major factor in the clinical and histological differences observed after PRK and LASIK. The wound healing response in PRK was amplified and higher rates of regression and haze were generated owing to the destruction of the central corneal epithelial basement membrane. The haze development after PRK is directly related to increased cell reflectance of many wound-healing keratocytes (132). Apoptosis and proliferation of keratocyte and myofibroblast generation were observed significantly greater after PRK for high myopia (-9D) than after LASIK for equivalent myopia in rabbit corneas (133). Wound-healing responses may be more obvious after FS flap creation than after mechanical microknife surgery. Due to the fulminic cavitation procedure related with plasma formation, the interface irregularity may be higher after FS flap formation (134, 135), while, DLK and flap-edge DLK increased (136). Fewer cellular ultrastructural changes have been observed after SMILE (137). Except for the side-cut incision, the corneas did not display any opacity at any time (138). Unlike the PRK surgery, no epithelial and endothelial cell damage was observed after SMILE (137). Corneal stromal wound healing after SMILE and FS-LASIK was almost identical to keratocyte proliferation and apoptosis in a human donor eye model (139). Nonetheless, Liu et al. found that SMILE induced significantly less acute inflammation in the cornea and aqueous humor in rabbit models than FS-LASIK (140). However, reactive fibrosis near the laser application site was less pronounced after SMILE, and the surface texture of stromal bed was smoother after LASIK (139).
4.3.5. Biomechanics in myopic corneal refractive surgery
Biomechanics has also been a concern in recent years. Morphological changes and thinning of the cornea after refractive surgery may affect corneal biomechanics. Accordingly, linking the morphology of the cornea to its mechanical behavior is crucial to understanding its mechanical properties. The cornea is defined as a complex anisotropic composite with biomechanical properties characterized by highly nonlinear elasticity and viscoelasticity. In terms of corneal biomechanical behavior, several structural features of the cornea have been speculated or proven to play an essential role in corneal biomechanical behavior. The stroma accounts for more than 90% of corneal thickness and controls corneal biomechanics (141). In particular, the anterior stroma consists of dense, regularly packed interwoven collagen lamellae known to be inserted vertically into Bowman’s layer. The anterior stroma is the strongest part of the cornea due to the high tensile strength provided by this collagen network structure, making it 50% stiffer compared to the mid or posterior stroma (135). The anterior stroma is also resistant to swelling owing to its structure, enabling the preservation of corneal curvature (142). However, corneal refractive surgery not only gives a corneal flattening, thinning and biomechanical changes, but also induces anterior chamber depth (ACD) and axial length (AL) decrease (143, 144).
Worldwide, more than 4 million people undergo elective refractive surgery each year to correct their vision; Studies have shown that postoperative corneal ectasia occurs in 0.04–0.6% of patients undergoing refractive surgery, and that, LASIK accounts for 96% of all cases (130). However, with this low incidence rate, the safety of refractive surgeries is relatively high. Therefore, understanding and utilizing corneal biomechanical properties to evaluate the suitability of patients for myopic corneal refractive surgery can help to predict its outcome and avoid complications, as well as help to improve the efficacy and safety of the surgery. Keratoconus is associated with corneal biomechanical abnormalities. These abnormalities lead to progressive thinning of the cornea and focal curvature changes in the cornea, due to loss of the collagen matrix and surrounding components, resulting in reduced biomechanical integrity (145). In most cases, the disease must progress into advanced stages that affect vision before it can be diagnosed by relying on changes in thickness, topography, and other morphological features. Therefore, it is desirable to look for other methods to aid in the diagnosis before these changes occur, and one of the most effective methods is to identify biomechanical abnormalities.
Corneal biomechanics can be affected by external and environmental stimuli such as variety of injuries and surgeries, different hydration statuses, and hypoxia. Studies have shown that the clinical manifestations of these biomechanical changes are immediate corneal shape changes, shape instability over time and increased sensitivity to shape changes (118). Notably, the same study indicated that the discrepancy between the expected outcomes after corneal refractive surgeries and the actual outcomes achieved after these surgeries is due to the biomechanical properties and wound healing properties of the cornea, which clearly impairs the predictability and stability of surgery (118). These patients had corneal biomechanical abnormalities before surgery, which manifested as subclinical keratoconus (145). In any refractive surgical procedure involving corneal surface ablation such as LASIK and PRK, the corneal lamellae are immediately transected in a circumferential direction. It is important to determine that the lamellar tension in the remaining peripheral lamellar segments is reduced due to central ablation, thereby reducing the local resistance to swelling, and resulting in peripheral stromal thickening (146). At the margin of the ablation zone, centripetal stress may be generated in the underlying lamellae through the dense interlaminar connections caused by peripheral stroma expansion (146). Empirically, hyperopic responses predominate when ablation is limited to the anterior stroma, whereas gradually deepening circumferential damage leads to corneal steepening (147, 148).
Regarding changes in the biomechanical strength of the cornea, a number of studies have been conducted to compare these changes after flap lifting procedures and intrastromal flapless procedures (149, 150). It is noteworthy that different studies have found a wide difference in biomechanical effects between individuals (151). Compared to other procedures, the anterior stroma contains much more collagen fibers during LASIK. Models predict that LASIK results in a 55–65% reduction in corneal elasticity (152), whereas PRK results in about a 20% reduction (153). A study by Hassan et al. comparing some biomechanical parameters measured with corneal visualization Scheimpflug technology (CorVis ST) before and after LASIK and PRK procedure has reported significant changes in the early postoperative period. However, there were no significant differences between most of the biomechanical parameters preoperatively and 1 month after the surgeries (154). SMILE may lower the risk of complications compared to LASIK, and has been shown to have similar outcomes to LASIK while tending to provide advantages regarding epithelium maintenance. Furthermore, SMILE has biomechanical advantages over LASIK due to its smaller incision size and reduced number of collagen fibers involved in the cutting process (145). In addition, further benefits derive from the removal of tissue deep within the stroma, which helps to maintain a strong anterior corneal stroma and Bowman’s layer (145). A previous study reported a 49% mean reduction in the stiffness of stromal collagen fibers in the flap area after flap-based procedures. Loading increase effects were observed in flap-based cases and SMILE cases, and results showed lower stromal bed displacements and stresses in SMILE cases.
5. Strengths and limitations
The present study is the first bibliometric analysis of myopic corneal refractive surgery performed using the literature from the 1970s. To acquire deep insight into myopic corneal refractive surgery research, VOSviewer was used to identify the hotspots and major clusters in this field. But this study has some limitations. First, the publications were extracted from the WoSCC from 1979 to 2022, which may not sufficiently represent all myopic corneal refractive surgery research topics. Second, because most publications in WoSCC were in English, a linguistic bias may exist. Third, the collaboration network analysis successfully displayed the co-occurrence (distance between the two nodes/items) and the institutions’ co-authorship (the links’ strength). But bibliometrics software could not distinguish the real author contribution among complicated partnership, which requires researchers to read the original literature themselves. Finally, though analysis was done by software objectively, the way to interpret these results will have inherent subjective bias by individuals.
6. Conclusion
A scientific map of myopic corneal refractive surgery research was constructed, including annual publications, national distribution, international cooperation, author publications, source journals, cited articles, and keywords. The results of this study can provide a reference for ophthalmologists to choose appropriate journals for publication, institutions, or authors for collaboration. The extracted keywords allow researchers to identify new topics and help predict research directions.
Based on the bibliometric analysis, myopic corneal refractive surgery had two rapid development stages, from 1991 to 2001 and after 2013. United States and China are the international scientific centers of myopic corneal refractive surgery research. The most productive institution was Fudan University. The most cited institution was Emory University. Reinstein DZ, Seiler T, Zhou XT, and Wang Y are the key researchers in this field. Journal of Refractive Surgery is the most prolific journal on myopic corneal refractive surgery research and Journal of Cataract and Refractive Surgery is the most influential journal in this field. The priority themes involved wound healing, cytobiology, and biomechanics, which are central to the safety and efficacy of refractive surgery. Taken together, the results of these analyses will help researchers understand the current state of research and provide promising directions for future research. New studies should consider exploring specific aspects of myopic corneal refractive surgery research, such as cytobiology and corneal biomechanics. We aim to study other aspects of myopic corneal refractive surgery using different literature databases and bibliometric methods such as bibliographic coupling analysis. Altmetrics is a new comprehensive bibliometric method used to assess the academic and social impact of research results and can also be applied in conjunction with scientometric analysis to better understand trends and new field research areas.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FY and YD performed the bibliometrics analysis and drafted the manuscript. FY and MA organized the manuscript writing. CB oversaw the search strategy. YW reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China, grant no: 82271118, and the Young and Middle-Aged Talents Project of Hubei Provincial Department of Education of China, grant no: Q20222112.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing. The authors sincerely treasured the comments and suggestions from reviewers.
Footnotes
References
- 1.Fricke TR, Jong M, Naidoo KS, Sankaridurg P, Naduvilath TJ, Ho SM, et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol. (2018) 102:855–62. doi: 10.1136/bjophthalmol-2017-311266, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naidoo KS, Leasher J, Bourne RR, Flaxman SR, Jonas JB, Keeffe J, et al. Global vision impairment and blindness due to uncorrected refractive error, 1990–2010. Optom Vis Sci. (2016) 93:227–34. doi: 10.1097/OPX.0000000000000796, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Lou L, Yao C, Jin Y, Perez V, Ye J. Global patterns in health burden of uncorrected refractive error. Invest Ophthalmol Vis Sci. (2016) 57:6271–7. doi: 10.1167/iovs.16-20242, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Fu L, Patel BC. Radial keratotomy correction. In: StatPearls. eds. Fu L, Patel BC. Treasure Island (FL): StatPearls Publishing; (2022) [PubMed] [Google Scholar]
- 5.Barraquer JI. Autokeratoplasty with optical carving for the correction of myopia (keratomileusis). An Med Espec. (1965) 51:66–82. PMID: [PubMed] [Google Scholar]
- 6.Fyodorov SN, Durnev VV. Operation of dosaged dissection of corneal circular ligament in cases of myopia of mild degree. Ann Ophthalmol. (1979) 11:1885. [PubMed] [Google Scholar]
- 7.Sawelson H, Marks RG. Ten-year refractive and visual results of radial keratotomy. Ophthalmology. (1995) 102:1892–901. [DOI] [PubMed] [Google Scholar]
- 8.Taboada J, Mikesell GJ, Reed RD. Response of the corneal epithelium to KrF excimer laser pulses. Health Phys. (1981) 40:677–83. doi: 10.1097/00004032-198105000-00006, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Trokel SL, Srinivasan R, Braren B. Excimer laser surgery of the cornea. Am J Ophthalmol. (1983) 96:710–5. doi: 10.1016/S0002-9394(14)71911-7 [DOI] [PubMed] [Google Scholar]
- 10.Mcalinden C. Corneal refractive surgery: past to present. Clin Exp Optom. (2012) 95:386–98. doi: 10.1111/j.1444-0938.2012.00761.x [DOI] [PubMed] [Google Scholar]
- 11.Reinstein DZ, Archer TJ, Gobbe M. The history of LASIK. J Refract Surg. (2012) 28:291–8. doi: 10.3928/1081597X-20120229-01 [DOI] [PubMed] [Google Scholar]
- 12.Pallikaris IG, Papatzanaki ME, Stathi EZ, Frenschock O, Georgiadis A. Laser in situ keratomileusis. Lasers Surg Med. (1990) 10:463–8. doi: 10.1002/lsm.1900100511 [DOI] [PubMed] [Google Scholar]
- 13.Riau AK, Angunawela RI, Chaurasia SS, Lee WS, Tan DT, Mehta JS. Early corneal wound healing and inflammatory responses after refractive lenticule extraction (ReLEx). Invest Ophthalmol Vis Sci. (2011) 52:6213–21. doi: 10.1167/iovs.11-7439, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Ganesh S, Gupta R. Comparison of visual and refractive outcomes following femtosecond laser-assisted LASIK with SMILE in patients with myopia or myopic astigmatism. J Refract Surg. (2014) 30:590–6. doi: 10.3928/1081597X-20140814-02 [DOI] [PubMed] [Google Scholar]
- 15.Dong Z, Zhou X, Wu J, Zhang Z, Li T, Zhou Z, et al. Small incision lenticule extraction (SMILE) and femtosecond laser LASIK: comparison of corneal wound healing and inflammation. Br J Ophthalmol. (2014) 98:263–9. doi: 10.1136/bjophthalmol-2013-303415, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Teo EPW, Lwin NC, Yam GHF, Mehta JS. Early corneal wound healing and inflammatory responses after SMILE: comparison of the effects of different refractive corrections and surgical experiences. J Refract Surg. (2016) 32:346–53. doi: 10.3928/1081597X-20160217-05 [DOI] [PubMed] [Google Scholar]
- 17.Ang M, Chaurasia SS, Angunawela RI, Poh R, Riau A, Tan D, et al. Femtosecond lenticule extraction (FLEx): clinical results, interface evaluation, and intraocular pressure variation. Invest Ophthalmol Vis Sci. (2012) 53:1414–21. doi: 10.1167/iovs.11-8808, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Kim T, Alió del Barrio JL, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. (2019) 393:2085–98. doi: 10.1016/S0140-6736(18)33209-4 [DOI] [PubMed] [Google Scholar]
- 19.Vestergaard AHM, Grauslund J, Ivarsen AR, Hjortdal J. Efficacy, safety, predictability, contrast sensitivity, and aberrations after femtosecond laser lenticule extraction. J Cataract Refract Surg. (2014) 40:403–11. doi: 10.1016/j.jcrs.2013.07.053 [DOI] [PubMed] [Google Scholar]
- 20.Agarwal A, Durairajanayagam D, Tatagari S, Esteves SC, Harlev A, Henkel R, et al. Bibliometrics: tracking research impact by selecting the appropriate metrics. Asian J Androl. (2016) 18:296–309. doi: 10.4103/1008-682X.171582, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallin JA. Bibliometric methods: pitfalls and possibilities. Basic Clin Pharmacol Toxicol. (2005) 97:261–75. doi: 10.1111/j.1742-7843.2005.pto_139.x [DOI] [PubMed] [Google Scholar]
- 22.Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. (2015) 13:141–6. doi: 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 23.O’Gorman CS, Macken AP, Cullen W, Saunders J, Dunne C, Higgins MF. What are the differences between a literature search, a literature review, a systematic review and a meta-analysis? And why is a systematic review considered to be so good? Ir Med J. (2013) 106:8. [PubMed] [Google Scholar]
- 24.Kohnen T, Remy M. Complications of corneal lamellar refractive surgery. Ophthalmologe. (2015) 112:982–9. doi: 10.1007/s00347-015-0172-x, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Moussa S, Dietrich M, Lenzhofer M, Ruckhofer J, Reitsamer HA. Femtosecond laser in refractive corneal surgery. Photochem Photobiol Sci. (2019) 18:1669–74. doi: 10.1039/c9pp00039a [DOI] [PubMed] [Google Scholar]
- 26.Pallikaris IG, Papatzanaki ME, Siganos DS, Tsilimbaris MK. A corneal flap technique for laser in situ keratomileusis: human studies. Arch Ophthalmol. (1991) 109:1699–702. doi: 10.1001/archopht.1991.01080120083031 [DOI] [PubMed] [Google Scholar]
- 27.Azar DT, Ang RT, Lee JB, Kato T, Chen CC, Jain S, et al. Laser subepithelial keratomileusis: electron microscopy and visual outcomes of flap photorefractive keratectomy. Curr Opin Ophthalmol. (2001) 12:323–8. doi: 10.1097/00055735-200108000-00014, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Gonzalez M, Bouza-Miguens C, Parafita-Fernandez A, Gros-Otero J, Cañones-Zafra R, Villa-Collar C, et al. Comparison of visual outcomes and flap morphology using 2 femtosecond-laser platforms. J Cataract Refract Surg. (2018) 44:78–84. doi: 10.1016/j.jcrs.2017.10.041, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Ang M, Mehta JS, Chan C, Htoon HM, Koh JCW, Tan DT. Refractive lenticule extraction: transition and comparison of 3 surgical techniques. J Cataract Refract Surg. (2014) 40:1415–24. doi: 10.1016/j.jcrs.2013.12.026 [DOI] [PubMed] [Google Scholar]
- 30.Sekundo W, Kunert K, Russmann C, Gille A, Bissmann W, Stobrawa G, et al. First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia: six-month results. J Cataract Refract Surg. (2008) 34:1513–20. doi: 10.1016/j.jcrs.2008.05.033, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Sekundo W, Kunert KS, Blum M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol. (2011) 95:335–9. doi: 10.1136/bjo.2009.174284 [DOI] [PubMed] [Google Scholar]
- 32.Shah R. History and results; indications and contraindications of SMILE compared with LASIK. Asia Pac J Ophthalmol (Phila). (2019) 8:371–6. doi: 10.1097/01.APO.0000580132.98159.fa, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Bao XL, Tang X, Zuo T, Geng WL, Jin Y. Clinical study of femtosecond laser corneal small incision lenticule extraction for correction of myopia and myopic astigmatism. Zhonghua Yan Ke Za Zhi. (2013) 49:292–8. doi: 10.3760/cma.j.issn.0412-4081.2013.04.002 PMID: [DOI] [PubMed] [Google Scholar]
- 34.Fantes FE, Hanna KD, Waring GO, 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. (1990) 108:665. doi: 10.1001/archopht.1990.01070070051034 [DOI] [PubMed] [Google Scholar]
- 35.Munnerlyn CR, Koons SJ, Marshall J. Photorefractive keratectomy: a technique for laser refractive surgery. J Cataract Refract Surg. (1988) 14:46–52. doi: 10.1016/S0886-3350(88)80063-4 [DOI] [PubMed] [Google Scholar]
- 36.Sandoval HPMM, Donnenfeld ED, Kohnen T, Lindstrom RL, Potvin R, Tremblay DM, et al. Modern laser in situ keratomileusis outcomes. J Cataract Refract Surg. (2016) 42:1224–34. doi: 10.1016/j.jcrs.2016.07.012, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Seiler T, Wollensak J. Myopic photorefractive keratectomy with the excimer laser. One-year follow-up. Ophthalmology. (1991) 98:1156–63. doi: 10.1016/S0161-6420(91)32157-2, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Na KS, Chung SH, Kim JK, Jang EJ, Lee NR, Joo CK. Comparison of LASIK and surface ablation by using propensity score analysis: a multicenter study in Korea. Invest Ophthalmol Vis Sci. (2012) 53:7116–21. doi: 10.1167/iovs.12-9826, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Hersh PS, Brint SF, Maloney RK, Durrie DS, Gordon M, Michelson MA, et al. Photorefractive keratectomy versus laser in situ keratomileusis for moderate to high myopia. Ophthalmology. (1998) 105:1512–23, discussion 1522-3. doi: 10.1016/S0161-6420(98)98038-1 [DOI] [PubMed] [Google Scholar]
- 40.Pallikaris IG, Siganos DS. Excimer laser in situ keratomileusis and photorefractive keratectomy for correction of high myopia. J Refract Corneal Surg. (1994) 10:498–510. doi: 10.3928/1081-597X-19940901-07 [DOI] [PubMed] [Google Scholar]
- 41.Shortt AJ, Allan BDS, Evans JR. Laser-assisted in-situ keratomileusis (LASIK) versus photorefractive keratectomy (PRK) for myopia. Cochrane Database Syst Rev. (2013) 1:CD005135. doi: 10.1002/14651858.CD005135.pub3 [DOI] [PubMed] [Google Scholar]
- 42.Zhao L, Wei RL, Cheng JW, Li Y, Cai JP, Ma XY. Meta-analysis: clinical outcomes of laser-assisted subepithelial keratectomy and photorefractive keratectomy in myopia. Ophthalmology. (2010) 117:1912–22. doi: 10.1016/j.ophtha.2010.02.004, PMID: [DOI] [PubMed] [Google Scholar]
- 43.Shah RM, Shah SMT, Sengupta SD. Results of small incision lenticule extraction: all-in-one femtosecond laser refractive surgery. J Cataract Refract Surg. (2011) 37:127–37. doi: 10.1016/j.jcrs.2010.07.033 [DOI] [PubMed] [Google Scholar]
- 44.Lee JK, Chuck RS, Park CY. Femtosecond laser refractive surgery. Curr Opin Ophthalmol. (2015) 26:260–4. doi: 10.1097/ICU.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 45.Wu D, Wang Y, Zhang L, Wei S, Tang X. Corneal biomechanical effects: small-incision lenticule extraction versus femtosecond laser–assisted laser in situ keratomileusis. J Cataract Refract Surg. (2014) 40:954–62. doi: 10.1016/j.jcrs.2013.07.056, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Alió del Barrio JL, Vargas V, al-Shymali O, Alió JL. Small incision lenticule extraction (SMILE) in the correction of myopic astigmatism: outcomes and limitations - an update. Eye Vis. (2017) 4:26. doi: 10.1186/s40662-017-0091-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H, Wang Y. Dry eye evaluation and correlation analysis between tear film stability and corneal surface regularity after small incision lenticule extraction. Int Ophthalmol. (2018) 38:2283–8. doi: 10.1007/s10792-017-0717-x, PMID: [DOI] [PubMed] [Google Scholar]
- 48.McAlinden C, Moore J. Laser-assisted subepithelial keratectomy retreatment surgery. J Cataract Refract Surg. (2011) 37:358–63. doi: 10.1016/j.jcrs.2010.11.009, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Chansue E, Tanehsakdi M, Swasdibutra S, McAlinden C. Efficacy, predictability and safety of small incision lenticule extraction (SMILE). Eye Vis. (2015) 2:14–4. doi: 10.1186/s40662-015-0024-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen D, McAlinden C, Flitcroft I, Tu R, Wang Q, Alió J, et al. Postoperative efficacy, predictability, safety, and visual quality of laser corneal refractive surgery: a network meta-analysis. Am J Ophthalmol. (2017) 178:65–78. doi: 10.1016/j.ajo.2017.03.013, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Liang J, Williams DR. Aberrations and retinal image quality of the normal human eye. J Opt Soc Am A Opt Image Sci Vis. (1997) 14:2873–83. doi: 10.1364/JOSAA.14.002873 [DOI] [PubMed] [Google Scholar]
- 52.McAlinden C, Moore JE. The change in internal aberrations following myopic corneal laser refractive surgery. Graefes Arch Clin Exp Ophthalmol. (2010) 249:775–81. doi: 10.1007/s00417-010-1459-x [DOI] [PubMed] [Google Scholar]
- 53.McAlinden C, Moore JE. Comparison of higher order aberrations after LASIK and LASEK for myopia. J Refract Surg. (2010) 26:45–51. doi: 10.3928/1081597X-20101215-07 [DOI] [PubMed] [Google Scholar]
- 54.Seiler T, Kaemmerer M, Mierdel P, Krinke HE. Ocular optical aberrations after photorefractive keratectomy for myopia and myopic astigmatism. Arch Ophthalmol. (2000) 118:17. doi: 10.1001/archopht.118.1.17 [DOI] [PubMed] [Google Scholar]
- 55.Oshika T, Klyce SD, Applegate RA, Howland HC, el Danasoury MA. Comparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. (1999) 127:1–7. doi: 10.1016/S0002-9394(98)00288-8, PMID: [DOI] [PubMed] [Google Scholar]
- 56.Randleman JBM, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. (2008) 115:37–50.e4. doi: 10.1016/j.ophtha.2007.03.073 [DOI] [PubMed] [Google Scholar]
- 57.Moshirfar M, Tukan AN, Bundogji N, Liu HY, McCabe SE, Ronquillo YC, et al. Ectasia after corneal refractive surgery: a systematic review. Ophthalmol Therapy. (2021) 10:753–76. doi: 10.1007/s40123-021-00383-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klein SR, Epstein RJ, Randleman JB, Stulting RD. Corneal ectasia after laser in situ keratomileusis in patients without apparent preoperative risk factors. Cornea. (2006) 25:388–403. doi: 10.1097/01.ico.0000222479.68242.77 [DOI] [PubMed] [Google Scholar]
- 59.Speicher L, Göttinger W. Progressive Keratektasie nach Laser-in-situ-Keratomileusis (LASIK). Klin Monatsbl Augenheilkd. (1998) 213:247–51. doi: 10.1055/s-2008-1034982 [DOI] [PubMed] [Google Scholar]
- 60.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. (1984) 28:293–322. doi: 10.1016/0039-6257(84)90094-8, PMID: [DOI] [PubMed] [Google Scholar]
- 61.Seiler T, Koufala K, Richter G. Iatrogenic keratectasia after laser in situ keratomileusis. J Refract Surg. (1998) 14:312–7. doi: 10.3928/1081-597X-19980501-15 [DOI] [PubMed] [Google Scholar]
- 62.Wilson SE, Ambrosio R. Computerized corneal topography and its importance to wavefront technology. Cornea. (2001) 20:441–54. doi: 10.1097/00003226-200107000-00001, PMID: [DOI] [PubMed] [Google Scholar]
- 63.Maguire LJ, Bourne WM. Corneal topography of early keratoconus. Am J Ophthalmol. (1989) 108:107–12. doi: 10.1016/0002-9394(89)90001-9 [DOI] [PubMed] [Google Scholar]
- 64.Wilson SE, Lin DT, Klyce SD. Corneal topography of keratoconus. Cornea. (1991) 10:2–8. doi: 10.1097/00003226-199101000-00002 [DOI] [PubMed] [Google Scholar]
- 65.Fan R, Chan TCY, Prakash G, Jhanji V. Applications of corneal topography and tomography: a review. Clin Exp Ophthalmol. (2018) 46:133–46. doi: 10.1111/ceo.13136, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Lin SR, Ladas JG, Bahadur GG, al-Hashimi S, Pineda R. A review of machine learning techniques for keratoconus detection and refractive surgery screening. Semin Ophthalmol. (2019) 34:317–26. doi: 10.1080/08820538.2019.1620812 [DOI] [PubMed] [Google Scholar]
- 67.Ruiz Hidalgo I, Rodriguez P, Rozema JJ, Ní Dhubhghaill S, Zakaria N, Tassignon MJ, et al. Evaluation of a machine-learning classifier for keratoconus detection based on Scheimpflug tomography. Cornea. (2016) 35:827–32. doi: 10.1097/ICO.0000000000000834, PMID: [DOI] [PubMed] [Google Scholar]
- 68.Arbelaez MC, Versaci F, Vestri G, Barboni P, Savini G. Use of a support vector machine for keratoconus and subclinical keratoconus detection by topographic and tomographic data. Ophthalmology. (2012) 119:2231–8. doi: 10.1016/j.ophtha.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 69.Tan Z, Chen X, Li K, Liu Y, Cao H, Li J, et al. Artificial intelligence-based diagnostic model for detecting keratoconus using videos of corneal force deformation. Transl Vis Sci Technol. (2022) 11:32. doi: 10.1167/tvst.11.9.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pflugfelder SC, Liu Z, Feuer W, Verm A. Corneal thickness indices discriminate between keratoconus and contact lens-induced corneal thinning. Ophthalmology. (2002) 109:2336–41. doi: 10.1016/S0161-6420(02)01276-9 [DOI] [PubMed] [Google Scholar]
- 71.Price FJ, Koller DL, Price MO. Central corneal pachymetry in patients undergoing laser in situ keratomileusis11The authors have no proprietary or financial interest in any product mentioned in this article. Ophthalmology. (1999) 106:2216–20. doi: 10.1016/S0161-6420(99)90508-0 [DOI] [PubMed] [Google Scholar]
- 72.Javaloy J, Vidal MT, Villada JR, Artola A, Alió JL. Comparison of four corneal pachymetry techniques in corneal refractive surgery. J Refract Surg. (2004) 20:29–34. doi: 10.3928/1081-597X-20040101-06, PMID: [DOI] [PubMed] [Google Scholar]
- 73.Hashemi H, Mehravaran S. Central corneal thickness measurement with Pentacam, Orbscan II, and ultrasound devices before and after laser refractive surgery for myopia. J Cataract Refract Surg. (2007) 33:1701–7. doi: 10.1016/j.jcrs.2007.05.040, PMID: [DOI] [PubMed] [Google Scholar]
- 74.Schallhorn SC, Kaupp SE, Tanzer DJ, Tidwell J, Laurent J, Bourque LB. Pupil size and quality of vision after LASIK. Ophthalmology. (2003) 110:1606–14. doi: 10.1016/S0161-6420(03)00494-9 [DOI] [PubMed] [Google Scholar]
- 75.Haw WW, Manche EE. Effect of preoperative pupil measurements on glare, halos, and visual function after photoastigmatic refractive keratectomy. J Cataract Refract Surg. (2001) 27:907–16. doi: 10.1016/S0886-3350(01)00871-9, PMID: [DOI] [PubMed] [Google Scholar]
- 76.Chan A, Manche EE. Effect of preoperative pupil size on quality of vision after wavefront-guided LASIK. Ophthalmology. (2011) 118:736–41. doi: 10.1016/j.ophtha.2010.07.030, PMID: [DOI] [PubMed] [Google Scholar]
- 77.Lee Y, Hu F, Wang IJ. Quality of vision after laser in situ keratomileusis: influence of dioptric correction and pupil size on visual function. J Cataract Refract Surg. (2003) 29:769–77. doi: 10.1016/S0886-3350(02)01844-8 [DOI] [PubMed] [Google Scholar]
- 78.Linke SJ, Baviera J, Munzer G, Fricke OH, Richard G, Katz T. Mesopic pupil size in a refractive surgery population (13,959 eyes). Optom Vis Sci. (2012) 89:1156–64. doi: 10.1097/OPX.0b013e318263c165, PMID: [DOI] [PubMed] [Google Scholar]
- 79.Montés-Micó R, Neil Charman W. Intraocular pressure after excimer laser myopic refractive surgery. Ophthalmic Physiol Opt. (2001) 21:228–35. doi: 10.1046/j.1475-1313.2001.00581.x [DOI] [PubMed] [Google Scholar]
- 80.Schipper I, Senn P, Thomann U, Suppiger M. Intraocular pressure after excimer laser photorefractive keratectomy for myopia. J Refract Surg. (1995) 11:366–406. doi: 10.3928/1081-597X-19950901-13 [DOI] [PubMed] [Google Scholar]
- 81.de Bernardo M, Abbinante G, Borrelli M, di Stasi M, Cione F, Rosa N. Intraocular pressure measurements in standing, sitting, and supine position: comparison between Tono-pen Avia and Icare pro Tonometers. J Clin Med. (2022) 11:6234. doi: 10.3390/jcm11216234, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Bernardo M, Salerno G, Cornetta P, Rosa N. Axial length shortening after cataract surgery: New approach to solve the question. Transl Vis Sci Technol. (2018) 7:34. doi: 10.1167/tvst.7.6.34, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrara G, Cennamo G, Marotta G, Loffredo E. New formula to calculate corneal power after refractive surgery. J Refract Surg. (2004) 20:465–71. doi: 10.3928/1081-597X-20040901-09 [DOI] [PubMed] [Google Scholar]
- 84.Rosa N, Cione F, Pepe A, Musto S, de Bernardo M. An advanced lens measurement approach (ALMA) in post refractive surgery IOL power calculation with unknown preoperative parameters. PLoS One. (2020) 15:e0237990. doi: 10.1371/journal.pone.0237990, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosa N, Capasso L, Romano A. A New method of calculating intraocular lens power after photorefractive keratectomy. J Refract Surg. (2002) 18:720–4. doi: 10.3928/1081-597X-20021101-09 [DOI] [PubMed] [Google Scholar]
- 86.Rosa N, de Bernardo M, Borrelli M, Lanza M. New factor to improve reliability of the clinical history method for intraocular lens power calculation after refractive surgery. J Cataract Refract Surg. (2010) 36:2123–8. doi: 10.1016/j.jcrs.2010.07.017, PMID: [DOI] [PubMed] [Google Scholar]
- 87.Wallerstein A, Jackson WB, Chambers J, Moezzi AM, Lin H, Simmons PA. Management of post-LASIK dry eye: a multicenter randomized comparison of a new multi-ingredient artificial tear to carboxymethylcellulose. Clin Ophthalmol. (2018) 12:839–48. doi: 10.2147/OPTH.S163744, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bohac M, Koncarevic M, Pasalic A, Biscevic A, Merlak M, Gabric N, et al. Incidence and clinical characteristics of post LASIK ectasia: a review of over 30,000 LASIK cases. Semin Ophthalmol. (2018) 33:869–77. doi: 10.1080/08820538.2018.1539183, PMID: [DOI] [PubMed] [Google Scholar]
- 89.Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg. (1998) 24:1007–9. doi: 10.1016/S0886-3350(98)80057-6 [DOI] [PubMed] [Google Scholar]
- 90.Geggel HS, Talley AR. Delayed onset keratectasia following laser in situ keratomileusis. J Cataract Refract Surg. (1999) 25:582–6. doi: 10.1016/S0886-3350(99)80060-1, PMID: [DOI] [PubMed] [Google Scholar]
- 91.Amoils SP, Deist MB, Gous P, Amoils PM. Iatrogenic keratectasia after laser in situ keratomileusis for less than −4.0 to −7.0 diopters of myopia. J Cataract Refract Surg. (2000) 26:967–77. doi: 10.1016/S0886-3350(00)00434-X, PMID: [DOI] [PubMed] [Google Scholar]
- 92.Joo CK, Kim TG. Corneal ectasia detected after laser in situ keratomileusis for correction of less than −12 diopters of myopia. J Cataract Refract Surg. (2000) 26:292–5. doi: 10.1016/S0886-3350(99)00340-5, PMID: [DOI] [PubMed] [Google Scholar]
- 93.McLeod SD, Kisla TA, Caro NC, McMahon TT. Iatrogenic keratoconus: corneal ectasia following laser in situ keratomileusis for myopia. Arch Ophthalmol. (2000) 118:282. [PubMed] [Google Scholar]
- 94.Schmitt-Bernard CF, Lesage C, Arnaud B. Keratectasia induced by laser in situ keratomileusis in keratoconus. J Refract Surg. (2000) 16:368–70. doi: 10.3928/1081-597X-20000501-12, PMID: [DOI] [PubMed] [Google Scholar]
- 95.Haw WW, Manche EE. Iatrogenic keratectasia after a deep primary keratotomy during laser in situ keratomileusis. Am J Ophthalmol. (2001) 132:920–1. doi: 10.1016/S0002-9394(01)01148-5, PMID: [DOI] [PubMed] [Google Scholar]
- 96.Lafond G, Bazin R, Lajoie C. Bilateral severe keratoconus after laser in situ keratomileusis in a patient with forme fruste keratoconus. J Cataract Refract Surg. (2001) 27:1115–8. doi: 10.1016/S0886-3350(00)00805-1, PMID: [DOI] [PubMed] [Google Scholar]
- 97.Pallikaris IG, Kymionis GD, Astyrakakis NI. Corneal ectasia induced by laser in situ keratomileusis. J Cataract Refract Surg. (2001) 27:1796–802. doi: 10.1016/S0886-3350(01)01090-2 [DOI] [PubMed] [Google Scholar]
- 98.Ou RJ, Shaw EL, Glasgow BJ. Keratectasia after laser in situ keratomileusis (LASIK): evaluation of the calculated residual stromal bed thickness. Am J Ophthalmol. (2002) 134:771–3. doi: 10.1016/S0002-9394(02)01656-2, PMID: [DOI] [PubMed] [Google Scholar]
- 99.Rao SN, Epstein RJ. Early onset ectasia following laser in situ keratomileusus: case report and literature review. J Refract Surg. (2002) 18:177–84. doi: 10.3928/1081-597X-20020301-13 [DOI] [PubMed] [Google Scholar]
- 100.Binder PS. Ectasia after laser in situ keratomileusis. J Cataract Refract Surg. (2003) 29:2419–29. doi: 10.1016/j.jcrs.2003.10.012 [DOI] [PubMed] [Google Scholar]
- 101.Chiang RK, Park AJ, Rapuano CJ, Cohen EJ. Bilateral keratoconus after LASIK in a keratoconus patient. Eye Contact Lens. (2003) 29:90–2. doi: 10.1097/01.ICL.0000060780.24132.51 [DOI] [PubMed] [Google Scholar]
- 102.Fogla R, Rao SK, Padmanabhan P. Keratectasia in 2 cases with pellucid marginal corneal degeneration after laser in situ keratomileusis. J Cataract Refract Surg. (2003) 29:788–91. doi: 10.1016/S0886-3350(03)00047-6, PMID: [DOI] [PubMed] [Google Scholar]
- 103.Parmar D, Claoue C. Keratectasia following excimer laser photorefractive keratectomy. Acta Ophthalmol Scand. (2004) 82:102–5. doi: 10.1111/j.1395-3907.2003.0189b.x, PMID: [DOI] [PubMed] [Google Scholar]
- 104.Rad AS, Jabbarvand M, Saifi N. Progressive keratectasia after laser in situ keratomileusis. J Refract Surg. (2004) 20:S718–22. doi: 10.3928/1081-597X-20040903-18 [DOI] [PubMed] [Google Scholar]
- 105.Rao SK, Srinivasan B, Sitalakshmi G, Padmanabhan P. Photorefractive keratectomy versus laser in situ keratomileusis to prevent keratectasia after corneal ablation. J Cataract Refract Surg. (2004) 30:2623–8. doi: 10.1016/j.jcrs.2004.09.037, PMID: [DOI] [PubMed] [Google Scholar]
- 106.Leccisotti A. Corneal ectasia after photorefractive keratectomy. Graefes Arch Clin Exp Ophthalmol. (2007) 245:869–75. doi: 10.1007/s00417-006-0507-z [DOI] [PubMed] [Google Scholar]
- 107.Seiler T, Holschbach A, Derse M, Jean B, Genth U. Complications of myopic photorefractive keratectomy with the excimer laser. Ophthalmology. (1994) 101:153. [DOI] [PubMed] [Google Scholar]
- 108.Shalchi ZMBB, O’Brart DPS, McDonald RJ, Patel P, Archer TJ, Marshall J. Eighteen-year follow-up of excimer laser photorefractive keratectomy. J Cataract Refract Surg. (2015) 41:23–32. doi: 10.1016/j.jcrs.2014.05.034, PMID: [DOI] [PubMed] [Google Scholar]
- 109.Loewenstein A, Lipshitz I, Varssano D, Lazar M. Complications of excimer laser photorefractive keratectomy for myopia. J Cataract Refract Surg. (1997) 23:1174–6. doi: 10.1016/S0886-3350(97)80311-2 [DOI] [PubMed] [Google Scholar]
- 110.Farah SG, Azar DT, Gurdal C, Wong J. Laser in situ keratomileusis: literature review of a developing technique. J Cataract Refract Surg. (1998) 24:989–1006. doi: 10.1016/S0886-3350(98)80056-4 [DOI] [PubMed] [Google Scholar]
- 111.Clare G, Moore TCB, Grills C, Leccisotti A, Moore JE, Schallhorn S. Early flap displacement after LASIK. Ophthalmology. (2011) 118:1760–5. doi: 10.1016/j.ophtha.2011.01.053, PMID: [DOI] [PubMed] [Google Scholar]
- 112.Segev F, Mimouni M, Sela T, Munzer G, Kaiserman I. Risk factors for sporadic diffuse lamellar keratitis after microkeratome laser-assisted in situ keratomileusis: a retrospective large database analysis. Cornea. (2018) 37:1124–9. doi: 10.1097/ICO.0000000000001674, PMID: [DOI] [PubMed] [Google Scholar]
- 113.Yesilirmak N, Chhadva P, Cabot F, Galor A, Yoo SH. Post-laser in situ keratomileusis epithelial ingrowth: treatment, recurrence, and long-term results. Cornea. (2018) 37:1517–21. doi: 10.1097/ICO.0000000000001760, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Krueger RR, Meister CS. A review of small incision lenticule extraction complications. Curr Opin Ophthalmol. (2018) 29:292–8. doi: 10.1097/ICU.0000000000000494 [DOI] [PubMed] [Google Scholar]
- 115.Wang Y, Ma J, Zhang J, Dou R, Zhang H, Li L, et al. Incidence and management of intraoperative complications during small-incision lenticule extraction in 3004 cases. J Cataract Refract Surg. (2017) 43:796–802. doi: 10.1016/j.jcrs.2017.03.039, PMID: [DOI] [PubMed] [Google Scholar]
- 116.Wang Y, Ma J, Zhang L, Zou H, Li J, Zhang Y, et al. Postoperative corneal complications in small incision lenticule extraction: long-term study. J Refract Surg. (2019) 35:146–52. doi: 10.3928/1081597X-20190118-02, PMID: [DOI] [PubMed] [Google Scholar]
- 117.Ivarsen AMP, Asp SMD, Hjortdal JMD. Safety and complications of more than 1500 small-incision lenticule extraction procedures. Ophthalmol Retina. (2014) 121:822–8. doi: 10.1016/j.ophtha.2013.11.006, PMID: [DOI] [PubMed] [Google Scholar]
- 118.Dupps WJ, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. (2006) 83:709–20. doi: 10.1016/j.exer.2006.03.015, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garana RM, Petroll WM, Chen WT, Herman IM, Barry P, Andrews P, et al. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci. (1992) 33:3271–82. PMID: [PubMed] [Google Scholar]
- 120.Jester JV, Petroll WM, Feng W, Essepian J, Cavanagh HD. Radial keratotomy. 1. The wound healing process and measurement of incisional gape in two animal models using in vivo confocal microscopy. Invest Ophthalmol Vis Sci. (1992) 33:3255–70. PMID: [PubMed] [Google Scholar]
- 121.Petroll WM, New K, Sachdev M, Cavanagh HD, Jester JV. Radial keratotomy. III. Relationship between wound gape and corneal curvature in primate eyes. Invest Ophthalmol Vis Sci. (1992) 33:3283–91. PMID: [PubMed] [Google Scholar]
- 122.Møller-Pedersen T, Li HF, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. (1998) 39:487–501. PMID: [PubMed] [Google Scholar]
- 123.Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Neutralizing antibody to TGFβ modulates stromal fibrosis but not regression of photoablative effect following PRK. Curr Eye Res. (1998) 17:736–47. doi: 10.1076/ceyr.17.7.736.5163 [DOI] [PubMed] [Google Scholar]
- 124.Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. (1999) 18:293–309. doi: 10.1016/S1350-9462(98)00017-2, PMID: [DOI] [PubMed] [Google Scholar]
- 125.Tuominen IS, Tervo TMT, Teppo AM, Valle TU, Grönhagen-Riska C, Vesaluoma MH. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp Eye Res. (2001) 72:631–41. doi: 10.1006/exer.2001.0999, PMID: [DOI] [PubMed] [Google Scholar]
- 126.Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Jr, Hong JW, Lee JS. The corneal wound healing response:: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. (2001) 20:625–37. doi: 10.1016/S1350-9462(01)00008-8 [DOI] [PubMed] [Google Scholar]
- 127.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. (1999) 18:529–51. doi: 10.1016/S1350-9462(98)00033-0 [DOI] [PubMed] [Google Scholar]
- 128.Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. (1998) 39:276–83. PMID: [PubMed] [Google Scholar]
- 129.Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. (2006) 82:788–97. doi: 10.1016/j.exer.2005.09.021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, et al. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J Cell Sci. (1999) 112:613–22. doi: 10.1242/jcs.112.5.613, PMID: [DOI] [PubMed] [Google Scholar]
- 131.Marshall J, Trokel SL, Rothery S, Krueger RR. Long-term healing of the central cornea after photorefractive keratectomy using an excimer laser. Ophthalmology retina. (1988) 95:1411–21. doi: 10.1016/S0161-6420(88)32997-0, PMID: [DOI] [PubMed] [Google Scholar]
- 132.Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology retina. (2000) 107:1235–45. doi: 10.1016/S0161-6420(00)00142-1, PMID: [DOI] [PubMed] [Google Scholar]
- 133.Mohan RR, Hutcheon AEK, Choi R, Hong JW, Lee JS, Mohan RR, et al. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. (2003) 76:71–87. doi: 10.1016/S0014-4835(02)00251-8, PMID: [DOI] [PubMed] [Google Scholar]
- 134.Netto MV, Ambrósio R Jr, Chalita MR, Krueger RR, Wilson SE. Corneal wound healing response following different modalities of refractive surgical procedures. Arq Bras Oftalmol. (2005) 68:140–9. doi: 10.1590/s0004-27492005000100027, PMID: [DOI] [PubMed] [Google Scholar]
- 135.Winkler M, Chai D, Kriling S, Nien CJ, Brown DJ, Jester B, et al. Nonlinear optical macroscopic assessment of 3-D corneal collagen organization and axial biomechanics. Invest Ophthalmol Vis Sci. (2011) 52:8818–27. doi: 10.1167/iovs.11-8070, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg. (2004) 30:26–32. doi: 10.1016/S0886-3350(03)00578-9, PMID: [DOI] [PubMed] [Google Scholar]
- 137.Wei S, Wang Y, Wu D, Zu PP, Zhang H, Su X. Ultrastructural changes and corneal wound healing after SMILE and PRK procedures. Curr Eye Res. (2016) 41:1316–25. doi: 10.3109/02713683.2015.1114653, PMID: [DOI] [PubMed] [Google Scholar]
- 138.Sun Y, Zhang T, Liu M, Zhou Y, Weng S, Yang X, et al. Early corneal wound healing response after small incision lenticule extraction. Cornea. (2019) 38:1582–8. doi: 10.1097/ICO.0000000000002105, PMID: [DOI] [PubMed] [Google Scholar]
- 139.Luft N, Schumann RG, Dirisamer M, Kook D, Siedlecki J, Wertheimer C, et al. Wound healing, inflammation, and corneal ultrastructure after SMILE and femtosecond laser-assisted LASIK: a human ex vivo study. J Refract Surg. (2018) 34:393–9. doi: 10.3928/1081597X-20180425-02 [DOI] [PubMed] [Google Scholar]
- 140.Liu L, Cheng W, Wu D, Chen L, Yu S, Zuo T, et al. The differential expression of cytokines and growth factors after SMILE compared with FS-LASIK in rabbits. Invest Ophthalmol Vis Sci. (2020) 61:55. doi: 10.1167/iovs.61.5.55, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. (1991) 32:2244–58. PMID: [PubMed] [Google Scholar]
- 142.Müller LJ, Pels E, Vrensen GFJM. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. (2001) 85:437–43. doi: 10.1136/bjo.85.4.437, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Bernardo M, Pagliarulo S, Rosa N. Unexpected ocular morphological changes after corneal refractive surgery: a review. Front Med. (2022) 9:1014277–7. doi: 10.3389/fmed.2022.1014277, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Bernardo M, Borrelli M, Imparato R, Cione F, Rosa N. Anterior chamber depth measurement before and after photorefractive keratectomy. Comparison between IOLMaster and Pentacam. Photodiagn Photodyn Ther. (2020) 32:101976. doi: 10.1016/j.pdpdt.2020.101976, PMID: [DOI] [PubMed] [Google Scholar]
- 145.Wilson A, Marshall J. A review of corneal biomechanics: mechanisms for measurement and the implications for refractive surgery. Indian J Ophthalmol. (2020) 68:2679–90. doi: 10.4103/ijo.IJO_2146_20, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dupps WJ, Roberts C. Effect of acute biomechanical changes on corneal curvature after photokeratectomy. J Refract Surg. (2001) 17:658–69. doi: 10.3928/1081-597X-20011101-05 [DOI] [PubMed] [Google Scholar]
- 147.Gilbert ML, Roth AS, Friedlander MH. Corneal flattening by shallow circular trephination in human eye bank eyes. Refract Corneal Surg. (1990) 6:113–6. doi: 10.3928/1081-597X-19900301-08, PMID: [DOI] [PubMed] [Google Scholar]
- 148.Litwin KL, Moreira H, Ohadi C, McDonnell PJ. Changes in corneal curvature at different excimer laser ablative depths. Am J Ophthalmol. (1991) 111:382–4. doi: 10.1016/S0002-9394(14)72335-9, PMID: [DOI] [PubMed] [Google Scholar]
- 149.Kanellopoulos AJ. Comparison of corneal biomechanics after myopic small-incision lenticule extraction compared to LASIK: An ex vivo study. Clin Ophthalmol. (2018) 12:237–45. doi: 10.2147/OPTH.S153509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Francis M, Khamar P, Shetty R, Sainani K, Nuijts RMMA, Haex B, et al. In vivo prediction of air-puff induced corneal deformation using LASIK, SMILE, and PRK finite element simulations. Invest Ophthalmol Vis Sci. (2018) 59:5320–8. doi: 10.1167/iovs.18-2470, PMID: [DOI] [PubMed] [Google Scholar]
- 151.Seven I, Vahdati A, Pedersen IB, Vestergaard A, Hjortdal J, Roberts CJ, et al. Contralateral eye comparison of SMILE and flap-based corneal refractive surgery: computational analysis of biomechanical impact. J Refract Surg. (2017) 33:444–53. doi: 10.3928/1081597X-20170504-01, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sinha Roy A, Dupps JWJ. Patient-specific modeling of corneal refractive surgery outcomes and inverse estimation of elastic property changes. J Biomech Eng. (2011) 133:011002. doi: 10.1115/1.4002934, PMID: [DOI] [PubMed] [Google Scholar]
- 153.Pandolfi A, Fotia G, Manganiello F. Finite element simulations of laser refractive corneal surgery. Eng Comput. (2009) 25:15–24. doi: 10.1007/s00366-008-0102-5 [DOI] [Google Scholar]
- 154.Hassan Z, Modis L, Jr, Szalai E, Berta A, Nemeth G. Examination of ocular biomechanics with a new Scheimpflug technology after corneal refractive surgery. Cont Lens Anterior Eye. (2014) 37:337–41. doi: 10.1016/j.clae.2014.05.001, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.