Abstract
Background
We assessed whether hepatic steatosis with or without significant fibrosis (determined by validated non-invasive biomarkers) is associated with an increased 10-year estimated risk for cardiovascular disease (CVD) in people with type 1 diabetes mellitus (T1DM).
Methods
We conducted a retrospective, multicenter, cross-sectional study involving 1,254 adults with established T1DM without pre-existing CVD. We used the hepatic steatosis index (HSI) and fibrosis (FIB)-4 index for non-invasively detecting hepatic steatosis (defined as HSI > 36), with or without coexisting significant fibrosis (defined as FIB-4 index ≥ 1.3 or < 1.3). We calculated the Steno type 1 risk engine and the atherosclerotic CVD (ASCVD) risk score to estimate the 10-year risk of developing a first fatal or nonfatal CVD event.
Results
Using the Steno type 1 risk engine, a significantly greater proportion of patients with hepatic steatosis and significant fibrosis (n = 91) had a high 10-year estimated CVD risk compared to those with hepatic steatosis alone (n = 509) or without steatosis (n = 654) (75.8% vs. 23.2% vs. 24.9%, p < 0.001). After adjustment for sex, BMI, diabetes duration, hemoglobin A1c, chronic kidney disease, and lipid-lowering medication use, patients with hepatic steatosis and significant fibrosis had an increased 10-year estimated risk of developing a first fatal or nonfatal CVD event (adjusted-odds ratio 11.4, 95% confidence interval 3.54–36.9) than those without steatosis. We observed almost identical results using the ASCVD risk calculator.
Conclusions
The 10-year estimated CVD risk is remarkably greater in T1DM adults with hepatic steatosis and significant fibrosis than in their counterparts with hepatic steatosis alone or without steatosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-01945-x.
Keywords: NAFLD, Non-alcoholic fatty liver disease, T1DM, Type 1 diabetes, CVD, Cardiovascular disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become a widespread and fast-growing public health threat, causing substantial social and economic costs and reduced health-related quality of life [1, 2]. NAFLD affects almost a third of the world’s adult population [3], up to ~ 70% of individuals with type 2 diabetes mellitus (T2DM) [4], and up to ~ 40% of adults with type 1 diabetes mellitus (T1DM) [5]. NAFLD is a systemic disorder [6], which is associated not only with liver-related morbidity and mortality [7] but also with an increased risk of developing important extrahepatic complications [8], such as fatal and nonfatal cardiovascular disease (CVD) events and new-onset heart failure (HF) [9, 10]. Using the Korean National Health Insurance dataset, Park et al. recently reported that hepatic steatosis and/or advanced fibrosis as non-invasively assessed by fatty liver index and BARD score was significantly associated with an increased risk of HF and mortality [11] as well as with an increased risk of CVD events and mortality in new-onset T2DM [12]. Moreover, improvement in hepatic steatosis (assessed by changes in fatty liver index or BARD score) was significantly associated with decreased risk for HF and liver-related mortality as well as with decreased risk of CVD outcomes and mortality in new-onset T2DM [13, 14]. Strong evidence indicates that CVD is the leading cause of mortality in people with NAFLD, followed by extrahepatic malignancies and liver-related complications [15, 16].
CVD also represents the predominant cause of morbidity and mortality in adults with T1DM [17, 18], with an estimated loss of life expectancy at age 20 years of approximately 11 years for men and 13 years for women compared with the general population without T1DM [19]. Although NAFLD is associated with a substantial economic and health burden in individuals with T2DM, especially in terms of CVD mortality and morbidity [8], little information is currently available about the adverse effect of NAFLD on CVD risk in people with T1DM. Only a few small single-center studies examined the association of NAFLD with markers of subclinical atherosclerosis [20] or the risk of CVD outcomes in adults with T1DM [21–23].
Using quantitative risk assessment tools for estimating the risk of developing a first ‘hard’ CVD event in individuals with T1DM and NAFLD is an important starting point for clinicians to guide decision-making in the primary prevention of CVD. Many CVD risk scores have been proposed to estimate the 10-year risk for a first fatal and nonfatal CVD event, including the Steno type 1 risk engine [24], i.e., a CVD prediction calculator that is specific for adults with T1DM, and the atherosclerotic cardiovascular disease (ASCVD) risk calculator [25] that can be used for both adults with and without diabetes.
Thus, in this multicenter cross-sectional study, we aimed to explore whether hepatic steatosis (NAFLD) with and without significant fibrosis (as determined by validated non-invasive biomarkers) was associated with an increased 10-year estimated CVD risk in a large cohort of adults with T1DM without pre-existing CVD.
Methods
Participants
We performed a retrospective, multicenter, cross-sectional study on 11 Italian diabetes primary care outpatient clinics, all participating sites in the Study Group on Diabetes and Atherosclerosis of the Italian Society of Diabetes. More details about the recruitment methods of the study have been described elsewhere [26]. Briefly, all data were retrospectively retrieved from electronic medical records and patients’ medical charts in each participating center during 2018 and 2019 [26]. The inclusion criteria of the study were adult (age ≥ 18 years) individuals with established T1DM, according to the American Diabetes Association criteria [27]. Participants with T2DM or other specific types of diabetes, active cancer, and a history of chronic viral hepatitis or cirrhosis of any etiology were excluded. Participants with pre-existing ischemic heart disease, stroke, coronary or peripheral revascularization procedures, or missing data on platelet count, serum aminotransferase concentrations, and other variables that were used for calculating CVD risk scores and non-invasive biomarkers of hepatic steatosis and fibrosis (as described below), were also excluded. After excluding participants who did not meet inclusion criteria, the final sample for analysis consisted of 1,254 adult individuals (691 men and 563 women) with established T1DM (Supplementary Fig. 1). The study protocol was approved by the “Comitato etico per la Sperimentazione Clinica della Provincia di Padova” (code #63,553, October 2018) and by the ethics committee of each participating center. Written informed consent was collected accordingly to the requests of each local ethics committee [26].
Clinical and laboratory data
Extracted electronic data were sex, age, diabetes duration, body mass index (BMI), blood pressure, and biochemical parameters, such as complete blood count, plasma lipids, glucose, hemoglobin A1c (HbA1c), creatinine and liver enzymes (aspartate aminotransferase [AST], alanine aminotransferase [ALT] and gamma-glutamyl transferase [GGT]). The estimated glomerular filtration rate (e-GFR) was calculated using the CKD Epidemiology Collaboration (CKD-EPI) equation. [28]. Abnormal albuminuria was defined as urine albumin-to-creatinine ratio (ACR) ≥ 3.0 mg/mmol. Smoking history was dichotomized as current (yes) or no smoker (no or former > 1 year) and regular physical exercise using a cut-point of > 3.5 h/week [26]. Significant alcohol intake was defined as ≥ 2 alcohol units per day in men and ≥ 1 alcohol unit per day in women, respectively. In all participants, information was also recorded on chronic kidney disease (CKD) (defined as e-GFR < 60 mL/min/1.73 m2 or urine ACR ≥ 3.0 mg/mmol), diabetic retinopathy of any degree, as well as total daily insulin doses and use of other concomitant drug treatments (including antihypertensive, antiplatelet or lipid-lowering medications) [26].
Non-invasive biomarkers of hepatic steatosis and fibrosis
The hepatic steatosis index (HSI) was used to identify individuals with a high probability of hepatic steatosis. HSI was calculated as follows: HSI = 8 × (ALT/AST ratio) + BMI (+ 2, if female; +2, if presence of diabetes) [29]. An HSI value > 36 was indicative of hepatic steatosis, according to the cohort study published by Lee et al., who first developed and validated HSI against liver ultrasonography in over 10,000 Korean individuals [29]. In this cohort study, HSI had an area under the receiver-operating curve of 0.81 (95% confidence interval [CI] 0.80–0.82). At HSI < 30 or > 36 values, HSI ruled out NAFLD with a sensitivity of 92.5% (95% CI 91.4–93.5%) or detected NAFLD with a specificity of 92.4% (95% CI 91.3–93.4%). Recently, HSI was also validated against liver ultrasonography in Italian patients with T2DM [30] and against magnetic resonance imaging in adults with T1DM, showing a sensitivity of 86%, specificity of 66%, positive predictive value of 0.50, and negative predictive value of 0.92 [31]. Notably, in a subset of our participants (n = 326, 26% of total), in whom we also had data on liver ultrasonography, we performed a receiver-operating characteristic curve analysis to identify hepatic steatosis according to the HSI. The area under the receiver-operating curve (AUROC) for HSI was 0.72 (95% CI 0.63–0.75).
We have also calculated the fibrosis (FIB)-4 index by using the following formula: FIB-4 index = age × AST (IU/L)/platelet count (×109/L) × √ALT (IU/L). The FIB-4 index is one of the most widely used non-invasive scores of advanced fibrosis [32]. A FIB-4 cut-off ≥ 1.3 was suggestive of significant liver fibrosis [33].
The 10-year risk of CVD risk estimates
We used the Steno type 1 risk engine [24] and the ASCVD risk calculator [25] to estimate the 10-year risk of developing a first fatal or nonfatal CVD event. It is known that the ASCVD risk calculator is not a specific tool for people with T1DM, while the Steno type 1 risk engine is a specific risk-estimation tool for people with T1DM as it has been implemented and validated in a cohort of 4,306 Danish adults with T1DM attending the Steno Diabetes center [24]. The Steno type 1 risk engine estimates the 10-year risk of developing a first fatal or nonfatal CVD event (ischemic heart disease, ischemic stroke, heart failure, and peripheral artery disease). It includes the following 10 variables: age, sex, diabetes duration, systolic blood pressure, LDL-cholesterol, HbA1c, albuminuria, e-GFR, smoking, and regular exercise. The Steno type 1 risk engine allows the 10-year estimated CVD risk to be categorized as follows: low risk (< 10%), moderate risk (10-19.9%), and high risk (≥ 20%) [24]. The ASCVD risk calculator was proposed in 2013 by the American College of Cardiology/American Heart Association Task Force on Practice and is based on data derived from representative community-based cohorts with White and Black individuals. In particular, this risk calculator estimates the 10-year risk of developing a first hard CVD event (coronary heart disease mortality, nonfatal myocardial infarction, and fatal or nonfatal stroke) and includes the following 9 variables: age, sex, race, total cholesterol, HDL-cholesterol, systolic blood pressure, hypertension treatment, smoking, and diabetes status [25]. The ASCVD risk calculator allows the 10-year estimated CVD risk to be categorized as follows: low risk (< 5%), borderline risk (5-7.4%), intermediate risk (7.5-19.9%), and high risk (≥ 20%) [25]. For this study, we merged the borderline and intermediate CVD risk groups into a single group called the intermediate ASCVD risk group.
Statistical analysis
Continuous variables were expressed as means ± SD or medians (interquartile ranges), and categorical variables as proportions. Differences in the main clinical and biochemical characteristics of participants stratified either by the presence of hepatic steatosis (with or without coexisting significant fibrosis) or by 10-year estimated CVD risk categories were tested by the one-way ANOVA for normally distributed continuous variables, the Kruskal-Wallis test for non-normally distributed variables, and the chi-squared test for categorical variables. Univariable and multivariable logistic regression analyses were performed to examine the associations between hepatic steatosis (with or without coexisting significant fibrosis) and the 10-year CVD risk estimates. In these logistic regression models, the dependent dichotomous variable was as follows: (a) the high or moderate Steno type 1 risk groups combined vs. the low Steno type 1 risk group; or (b) the high or intermediate ASCVD risk groups combined vs. the low ASCVD risk group. Specifically, we performed unadjusted logistic regression models and two forced-entry adjusted regression models. The first model was unadjusted; the second model was adjusted for sex, BMI, diabetes duration, HbA1c, presence of CKD (defined as e-GFR < 60 mL/min/1.73 m2 or abnormal albuminuria), and lipid-lowering medication use; and, finally, the third model included the same model 2’s covariates after excluding individuals with significant alcohol intake. Notably, as age was included both in the FIB-4 index and in the two CVD risk equations, and hypertension and smoking were also included in the two CVD risk equations, we decided not to include age, smoking, and hypertension status among the covariates of these multivariable regression models to reduce possible multicollinearity problems.
All statistical tests were two-sided and a P-value < 0.05 was considered statistically significant. Statistical analyses were performed using STATA software, version 17.0 (STATA, College Station, Texas, USA).
Results
Baseline characteristics
Among the 1,254 adult outpatients with established T1DM included in the study (55% men; mean [± SD] age 46 ± 14 years; diabetes duration 22 ± 12 years; BMI 25.1 ± 4.1 kg/m2; HbA1c 7.8 ± 1.1%), 654 (52.2%) patients had HSI ≤ 36 (i.e., indicative of absent hepatic steatosis), 509 (40.6%) had HSI > 36 and FIB-4 score < 1.3 (suggestive of steatosis without significant fibrosis) and 91 (7.3%) had HSI > 36 and FIB-4 score ≥ 1.3 (suggestive of steatosis with significant fibrosis). When we stratified participants according to low, intermediate, and high HSI values, 115 (9.2%) subjects had HSI < 30, 539 (43%) had intermediate HSI values between 30 and 36, and 600 (47.8%) had HSI > 36. Among the 91 subjects with FIB-4 index ≥ 1.3, about a third (n = 30) had FIB-4 index > 2.67 (indicative of advanced liver fibrosis). Using the Steno type 1 risk engine, 545 (43.5%) patients were classified as having a low 10-year estimated CVD risk, 359 (28.6%) had a moderate CVD risk, and 350 (27.9%) had a high CVD risk. Similarly, using the 10-year ASCVD risk calculator, 703 (56.0%) patients were classified as having a low risk, 348 (27.8%) had intermediate risk, and 203 (16.2%) had a high CVD risk.
Table 1 summarizes the main clinical and biochemical characteristics of participants stratified by the presence or absence of hepatic steatosis with or without coexisting significant fibrosis. Compared to patients with or without steatosis, those with steatosis and significant fibrosis were more likely to be older, overweight/obese, and less likely to be smokers. They also had a longer duration of diabetes, higher values of HbA1c, blood pressure, serum triglycerides, and liver enzymes, as well as lower platelet count, lower LDL-cholesterol, and lower e-GFRCKD−EPI. Patients with hepatic steatosis and significant fibrosis achieved more frequently a plasma LDL-cholesterol level < 1.8 mmol/L (< 70 mg/dL) (although the proportion, i.e., 15.4%, was extremely low, considering the high 10-year estimated CVD risk of this patient subgroup), and had a greater prevalence of hypertension, diabetic retinopathy of any degree, abnormal albuminuria, or CKD. The total daily insulin doses and the proportion of those treated with antihypertensive medications (i.e., diuretics, beta-blockers, calcium-channel blockers, or renin-angiotensin system inhibitors), antiplatelet or lipid-lowering agents were also higher in patients with hepatic steatosis and significant fibrosis. Sex distribution, regular exercise, daily alcohol intake, total cholesterol and glucose levels did not significantly differ among the patient groups. None of these patients with T1DM were treated with metformin or other glucose-lowering agents in addition to insulin treatment.
Table 1.
Clinical and biochemical characteristics of adults with type 1 diabetes, stratified by the presence of hepatic steatosis with or without coexisting significant liver fibrosis (non-invasively assessed by HSI and FIB-4 scores)
| Patients with HSI ≤ 36 (n = 654) | Patients with HSI > 36 and FIB4 < 1.3 (n = 509) | Patients with HSI > 36 and FIB4 ≥ 1.3 (n = 91) | P-value | |
|---|---|---|---|---|
| Age (years) | 45 ± 16 | 44 ± 13 | 63 ± 12 | < 0.001 |
| Male sex (%) | 55.1 | 56.6 | 47.3 | 0.258 |
| BMI (kg/m2) | 22.7 ± 2.3 | 27.9 ± 4.0 | 29.3 ± 4.7 | < 0.001 |
| Current smokers (%) | 29.4 | 22.6 | 13.2 | < 0.001 |
| Regular physical exercise (≥ 3.5 h/week) (%) | 48.3 | 46.6 | 35.2 | 0.061 |
| Alcohol intake (%) | 18.2 | 14.7 | 12.1 | 0.251 |
| Diabetes duration (years) | 22 ± 12 | 21 ± 11 | 30 ± 14 | < 0.001 |
| Glucose (mg/dL) | 175 ± 72 | 185 ± 70 | 178 ± 68 | 0.242 |
| HbA1c (%) | 7.7 ± 1.2 | 7.9 ± 1.3 | 8.2 ± 1.0 | < 0.001 |
| Systolic blood pressure (mmHg) | 126 ± 18 | 129 ± 16 | 140 ± 20 | < 0.001 |
| Diastolic blood pressure (mmHg) | 75 ± 8 | 77 ± 9 | 78 ± 10 | < 0.001 |
| Total cholesterol (mg/dL) | 180 ± 34 | 184 ± 33 | 179 ± 43 | 0.167 |
| HDL cholesterol (mg/dL) | 61 ± 16 | 55 ± 14 | 50 ± 18 | < 0.001 |
| LDL cholesterol (mg/dL) | 103 ± 28 | 108 ± 29 | 99 ± 36 | 0.001 |
| LDL cholesterol < 70 mg/dL (%) | 10.1 | 6.7 | 15.4 | 0.012 |
| Triglycerides (mg/dL) | 70 (56–95) | 83 (62–120) | 89 (66–131) | < 0.001 |
| AST (IU/L) | 16 (12–22) | 17 (12–28) | 24 (15–30) | < 0.001 |
| ALT (IU/L) | 17 (13–22) | 21 (16–29) | 22 (17–31) | < 0.001 |
| GGT (IU/L) | 15 (11–21) | 18 (13–29) | 21 (14–40) | < 0.001 |
| Platelet count (x 100,000/mm3) | 241 ± 68 | 258 ± 63 | 191 ± 57 | < 0.001 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.9 ± 0.5 | 1.1 ± 0.9 | < 0.001 |
| eGFRCKD−EPI (mL/min/1.73 m2) | 98 ± 20 | 97 ± 19 | 79 ± 26 | < 0.001 |
| Abnormal albuminuria (%) | 11.3 | 13.9 | 30.8 | < 0.001 |
| CKD (%) | 13.5 | 14.9 | 37.4 | < 0.001 |
| Diabetic retinopathy (%) | 27.2 | 33.7 | 51.1 | < 0.001 |
| Hypertension (%) | 32.9 | 39.9 | 74.7 | < 0.001 |
| Total daily insulin dose (IU/day) | 38 ± 15 | 49 ± 20 | 45 ± 17 | < 0.001 |
| Antiplatelet drug users (%) | 11.5 | 10.8 | 39.6 | < 0.001 |
| Diuretic users (%) | 5.5 | 9.3 | 37.4 | < 0.001 |
| Beta-blocker users (%) | 5.5 | 6.1 | 26.4 | < 0.001 |
| Calcium-channel blocker users (%) | 4.4 | 7.5 | 24.2 | < 0.001 |
| ACE-i/ARB users (%) | 24.2 | 33.2 | 67.0 | < 0.001 |
| Statin users (%) | 27.4 | 33.9 | 62.6 | < 0.001 |
Cohort size: n = 1,254. Data are expressed as means ± SD, medians, and interquartile ranges (IQRs) or percentages. Differences among the three patient groups were tested by the Chi-squared test for categorical variables, the one-way ANOVA for normally distributed continuous variables, and the Kruskal-Wallis test for non-normally distributed variables (i.e., serum liver enzymes and triglycerides)
Note: Alcohol intake was defined as ≥ 2 alcohol units per day in men and ≥ 1 alcohol unit per day in women, respectively. Abnormal albuminuria was defined as urine albumin-to-creatinine ratio ≥ 3.0 mg/mmol. CKD was defined as eGFRCKD−EPI <60 mL/min/1.73 m2 or abnormal albuminuria.
Abbreviations: ACE, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; eGFRCKD−EPI, estimated glomerular filtration rate calculated by the CKD-Epidemiology Collaboration study equation; GGT, gamma-glutamyl-transferase; FIB-4, fibrosis 4; HSI, hepatic steatosis index
Table 2 shows the main clinical and biochemical characteristics of participants stratified by 10-year estimated CVD risk categories using the Steno type 1 risk engine. Patients with a high 10-year estimated CVD risk were more likely to be older, overweight/obese, and less likely to be engaged in regular physical activity than those with low or moderate CVD risk. The former also had longer diabetes duration, higher values of HbA1c, blood pressure, serum triglycerides, total cholesterol, liver enzymes, and lower platelet count and e-GFRCKD−EPI. Compared to the other two patient subgroups, patients at high 10-year estimated CVD risk also had a greater prevalence of hypertension, diabetic retinopathy of any degree, abnormal albuminuria, and CKD. Furthermore, the proportion of patients treated with antihypertensive, antiplatelet, or lipid-lowering agents was greater among those at high CVD risk. Sex distribution, smoking history, and daily alcohol intake, as well as total daily insulin doses and the proportion of those achieving a plasma LDL-cholesterol level < 1.8 mmol/L did not significantly differ among the patient groups.
Table 2.
Clinical and biochemical characteristics of patients with type 1 diabetes, stratified by categories of the Steno type 1 risk engine
| Patients with low CVD risk (n = 545) | Patients with intermediate CVD risk (n = 359) | Patients with high CVD risk (n = 350) | P-value | |
|---|---|---|---|---|
| Age (years) | 33 ± 8 | 49 ± 7 | 64 ± 10 | < 0.001 |
| Male sex (%) | 55.2 | 57.7 | 52.3 | 0.355 |
| BMI (kg/m2) | 24.6 ± 3.9 | 25.5 ± 4.1 | 26.2 ± 5.0 | < 0.001 |
| Current smokers (%) | 27.9 | 25.6 | 21.4 | 0.095 |
| Regular physical exercise (≥ 3.5 h/week) (%) | 56.9 | 46.2 | 31.1 | < 0.001 |
| Alcohol intake (%) | 15.7 | 17.5 | 15.7 | 0.138 |
| Diabetes duration (years) | 16 ± 8 | 22 ± 11 | 31 ± 13 | < 0.001 |
| Glucose (mg/dL) | 175 ± 71 | 175 ± 69 | 187 ± 72 | 0.149 |
| HbA1c (%) | 7.7 ± 1.1 | 7.9 ± 1.2 | 8.2 ± 1.4 | < 0.001 |
| Systolic blood pressure (mmHg) | 120 ± 14 | 127 ± 14 | 141 ± 19 | < 0.001 |
| Diastolic blood pressure (mmHg) | 75 ± 9 | 77 ± 9 | 77 ± 10 | < 0.001 |
| Total cholesterol (mg/dL) | 177 ± 33 | 186 ± 33 | 185 ± 36 | < 0.001 |
| HDL cholesterol (mg/dL) | 56 ± 14 | 60 ± 15 | 61 ± 18 | < 0.001 |
| LDL cholesterol (mg/dL) | 104 ± 27 | 108 ± 29 | 103 ± 32 | 0.064 |
| LDL cholesterol < 70 mg/dL (%) | 9.0 | 7.8 | 10.6 | 0.436 |
| Triglycerides (mg/dL) | 71 (55–103) | 74 (59–102) | 85 (65–119) | < 0.001 |
| AST (IU/L) | 20 ± 9 | 22 ± 9 | 25 ± 15 | < 0.001 |
| ALT (IU/L) | 17 (12–23) | 19 (12–26) | 23 (12–28) | 0.001 |
| GGT (IU/L) | 15 (11–20) | 17 (12–26) | 20 (14–36) | < 0.001 |
| Platelet count (x 100,000/mm3) | 244 ± 63 | 248 ± 66 | 241 ± 76 | 0.337 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 0.8 | < 0.001 |
| eGFRCKD−EPI (mL/min/1.73 m2) | 108 ± 14 | 96 ± 13 | 79 ± 22 | < 0.001 |
| Abnormal albuminuria (%) | 4.2 | 9.8 | 32.9 | < 0.001 |
| CKD (%) | 4.2 | 10.0 | 39.7 | < 0.001 |
| Diabetic retinopathy (%) | 17.7 | 34.9 | 49.6 | < 0.001 |
| Hypertension (%) | 14.9 | 39.6 | 75.1 | < 0.001 |
| Total daily insulin dose (IU/day) | 43 ± 18 | 43 ± 17 | 42 ± 20 | 0.629 |
| Antiplatelet drug users (%) | 2.0 | 8.7 | 35.5 | < 0.001 |
| Diuretic users (%) | 1.7 | 6.7 | 24.0 | < 0.001 |
| Beta-blocker users (%) | 1.7 | 6.2 | 17.1 | < 0.001 |
| Calcium-channel blocker users (%) | 0.9 | 5.0 | 18.9 | < 0.001 |
| ACE-i/ARB users (%) | 7.2 | 32.3 | 66.6 | < 0.001 |
| Statin users (%) | 8.3 | 37.3 | 65.7 | < 0.001 |
Cohort size: n = 1,254. Data are expressed as means ± SD, medians, and interquartile ranges (IQRs) or percentages. Differences among the three patient groups were tested by the Chi-squared test for categorical variables, the one-way ANOVA for normally distributed continuous variables, and the Kruskal-Wallis test for non-normally distributed variables (i.e., serum liver enzymes and triglycerides)
Note: Alcohol intake was defined as ≥ 2 alcohol units per day in men and ≥ 1 alcohol unit per day in women, respectively. Abnormal albuminuria was defined as urine albumin-to-creatinine ratio ≥ 3.0 mg/mmol. CKD was defined as eGFRCKD−EPI <60 mL/min/1.73 m2 or abnormal albuminuria.
Abbreviations: ACE, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; eGFRCKD−EPI, estimated glomerular filtration rate calculated by the CKD-Epidemiology Collaboration study equation; GGT, gamma-glutamyl-transferase; FIB-4, fibrosis 4; HSI, hepatic steatosis index.
Table 3 shows the main clinical and biochemical characteristics of participants stratified by 10-year estimated CVD risk categories using the ASCVD risk calculator. Compared to those at low or intermediate risk, patients at high 10-year estimated CVD risk were more likely to be older, men, overweight/obese, and less likely to be engaged in regular physical activity. In addition, patients at high CVD risk also had longer diabetes duration, higher blood pressure and serum liver enzymes, lower platelet count, and lower e-GFRCKD−EPI. These patients also had a higher prevalence of hypertension, retinopathy of any degree, abnormal albuminuria, and CKD. The proportion of those treated with antihypertensive, antiplatelet drugs, or lipid-lowering agents was also higher in patients at high 10-year estimated CVD risk. Alcohol intake, HbA1c, glucose, HDL-cholesterol, and total daily insulin doses did not significantly differ among the patient groups.
Table 3.
Clinical and biochemical characteristics of patients with type 1 diabetes, stratified by 10-year ASCVD risk calculator categories
| Patients with low CVD risk (n = 703) | Patients with intermediate CVD risk (n = 348) | Patients with high CVD risk (n = 203) | P-value | |
|---|---|---|---|---|
| Age (years) | 37 ± 10 | 52 ± 10 | 66 ± 13 | < 0.001 |
| Male sex (%) | 50.0 | 61.9 | 57.7 | < 0.001 |
| BMI (kg/m2) | 24.7 ± 3.9 | 25.9 ± 4.8 | 25.8 ± 4.4 | < 0.001 |
| Current smokers (%) | 20.3 | 38.1 | 25.0 | < 0.001 |
| Regular physical exercise (≥ 3.5 h/week) (%) | 50.4 | 46.5 | 32.3 | < 0.001 |
| Alcohol intake (%) | 13.9 | 18.4 | 15.5 | 0.318 |
| Diabetes duration (years) | 19 ± 10 | 23 ± 13 | 29 ± 15 | < 0.001 |
| Glucose (mg/dL) | 175 ± 73 | 181 ± 70 | 183 ± 67 | 0.499 |
| HbA1c (%) | 7.8 ± 1.2 | 8.0 ± 1.5 | 7.9 ± 1.0 | 0.052 |
| Systolic blood pressure (mmHg) | 121 ± 14 | 132 ± 17 | 143 ± 19 | < 0.001 |
| Diastolic blood pressure (mmHg) | 75 ± 9 | 78 ± 9 | 77 ± 10 | < 0.001 |
| Total cholesterol (mg/dL) | 178 ± 31 | 190 ± 36 | 182 ± 37 | < 0.001 |
| HDL cholesterol (mg/dL) | 59 ± 15 | 57 ± 17 | 59 ± 18 | 0.206 |
| LDL cholesterol (mg/dL) | 103 ± 25 | 109 ± 31 | 104 ± 36 | 0.001 |
| LDL cholesterol < 70 mg/dL (%) | 9.4 | 7.2 | 11.3 | 0.242 |
| Triglycerides (mg/dL) | 68 (55–94) | 87 (65–124) | 84 (64–112) | < 0.001 |
| AST (IU/L) | 20 ± 10 | 23 ± 10 | 25 ± 16 | < 0.001 |
| ALT (IU/L) | 21 ± 15 | 24 ± 14 | 23 ± 16 | 0.004 |
| GGT (IU/L) | 15 (11–20) | 20 (14–32) | 21 (14–38) | < 0.001 |
| Platelet count (x 100,000/mm3) | 248 ± 68 | 246 ± 71 | 233 ± 71 | 0.019 |
| Creatinine (mg/dL) | 0.80 (0.70–0.90) | 0.84 (0.72–0.95) | 0.81 (0.70-1.00) | 0.001 |
| eGFRCKD−EPI (mL/min/1.73 m2) | 104 ± 17 | 91 ± 20 | 81 ± 22 | < 0.001 |
| Abnormal albuminuria (%) | 8.0 | 17.9 | 28.5 | < 0.001 |
| CKD (%) | 7.9 | 20.0 | 34.1 | < 0.001 |
| Diabetic retinopathy (%) | 24.6 | 38.1 | 43.9 | < 0.001 |
| Hypertension (%) | 20.6 | 51.5 | 78.2 | < 0.001 |
| Total daily insulin dose (IU/day) | 42 ± 17 | 44 ± 19 | 41 ± 18 | 0.117 |
| Antiplatelet drug users (%) | 2.7 | 17.1 | 39.3 | < 0.001 |
| Diuretic users (%) | 2.6 | 12.3 | 26.8 | < 0.001 |
| Beta-blocker users (%) | 3.2 | 8.8 | 17.7 | < 0.001 |
| Calcium-channel blocker users (%) | 2.0 | 8.0 | 25.0 | < 0.001 |
| ACE-i/ARB users (%) | 13.5 | 44.0 | 64.1 | < 0.001 |
| Statin users (%) | 14.7 | 49.1 | 66.5 | < 0.001 |
Cohort size: n = 1,254. Data are expressed as means ± SD, medians and interquartile ranges (IQRs) or percentages. Differences among the three patient groups were tested by the Chi-squared test for categorical variables, the one-way ANOVA for normally distributed continuous variables, and the Kruskal-Wallis test for non-normally distributed variables (i.e., serum liver enzymes, creatinine, and triglycerides)
Note: Alcohol intake was defined as ≥ 2 alcohol units per day in men and ≥ 1 alcohol unit per day in women, respectively. Abnormal albuminuria was defined as urine albumin-to-creatinine ratio ≥ 3.0 mg/mmol. CKD was defined as eGFRCKD−EPI <60 mL/min/1.73 m2 or abnormal albuminuria.
Abbreviations: ACE, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; eGFRCKD−EPI, estimated glomerular filtration rate calculated by the CKD-Epidemiology Collaboration study equation; GGT, gamma-glutamyl-transferase; FIB-4, fibrosis 4; HSI, hepatic steatosis index.
The 10-year CVD risk estimates in NAFLD with or without significant fibrosis
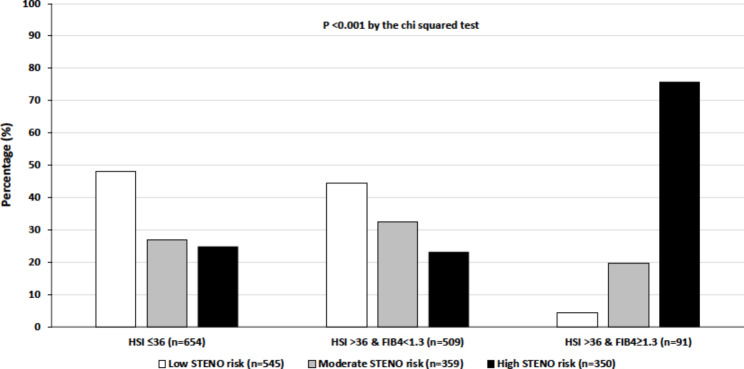
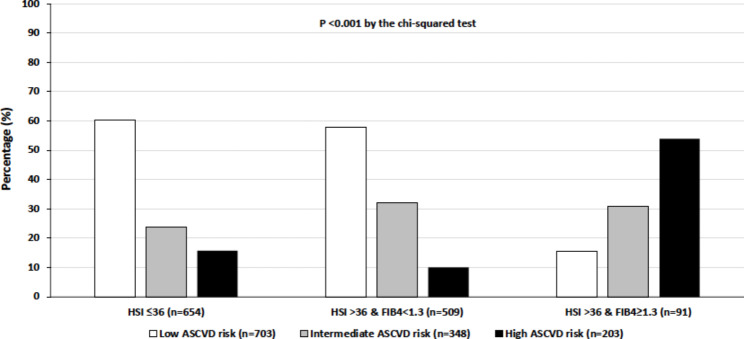
We calculated the prevalence rates of the 10-year estimated CVD risk categories, using the Steno type 1 risk engine (Fig. 1) or the ASCVD risk calculator (Fig. 2), in participants stratified by the presence or absence of hepatic steatosis with or without coexisting significant fibrosis. Using the Steno type 1 risk engine, we found that a remarkably higher proportion of patients with steatosis and significant fibrosis had a high 10-year estimated risk of developing a first fatal or nonfatal CVD event compared to their counterparts with steatosis alone or without steatosis (75.8% vs. 23.2% vs. 24.9%, p < 0.001 by the chi-squared test). Similarly, using the ASCVD risk calculator, we found that a significantly greater proportion of patients with steatosis and significant fibrosis had a high 10-year estimated CVD risk compared to those with steatosis alone or without steatosis (53.9% vs. 10.0% vs. 15.8%, p < 0.001).
Fig. 1.
Prevalence rates of the 10-year risk of developing a first fatal or nonfatal CVD (as estimated by the Steno type 1 risk engine) in adults with T1DM stratified by the presence or absence of hepatic steatosis with or without coexisting significant fibrosis (as determined by HSI and FIB-4 scores)
Fig. 2.
Prevalence rates of the 10-year risk of developing a first fatal or nonfatal CVD (as estimated by the ASCVD risk calculator) in adults with T1DM stratified by the presence or absence of hepatic steatosis with or without coexisting significant fibrosis (as determined by HSI and FIB-4 scores)
We performed subgroup analyses by median BMI values (< 24.8 vs. ≥24.8 kg/m2) (Supplementary Fig. 2), by median diabetes duration (< 20 vs. ≥20 years) (Supplementary Fig. 3), by median age (< 45 vs. ≥45 years) (Supplementary Fig. 4), by sex (Supplementary Fig. 5), or by hypertension status (Supplementary Fig. 6). These subgroup analyses confirmed that the 10-year estimated CVD risk (as calculated by the Steno type 1 risk engine or the ASCVD risk calculator) was remarkably greater in patients with hepatic steatosis and significant fibrosis than in those with steatosis alone or without steatosis, in both sexes and all other patient subgroups considered. Similar results were also found when we stratified our participants by median HbA1c (< 7.7% vs. ≥7.7%) or smoking status (data not shown).
Table 4 shows the association between hepatic steatosis (with or without coexisting significant fibrosis) and the 10-year estimated CVD risk. In unadjusted regression models, patients with hepatic steatosis and significant fibrosis had a ~ 8-fold (for the ASCVD risk calculator) to ~ 20-fold (for the Steno type 1 risk engine) increased risk of having a high/moderate 10-year estimated CVD risk when compared to their counterparts without steatosis. Notably, this risk remained significant even after adjustment for sex, BMI, diabetes duration, HbA1c, presence of CKD, and lipid-lowering medication use (adjusted model 1). Conversely, the 10-year estimated CVD risk was comparable between patients with steatosis alone and those without steatosis. Excluding participants (n = 211) with estimated alcohol intake ≥ 2 alcohol units per day for men and ≥ 1 alcohol unit per day for women did not affect the results (adjusted model 2). In these multivariable logistic regression models, other variables that were independently associated with an increased 10-year estimated CVD risk were longer diabetes duration, CKD, and non-use of statins (for both CVD prediction models), as well as male sex (for the ASCVD risk calculator) and higher HbA1c (for the Steno type 1 risk engine) (p < 0.001 for all).
Table 4.
Association between hepatic steatosis with or without coexisting significant fibrosis and the 10-year estimated CVD risk (using the Steno type 1 risk engine or the ASCVD risk calculator)
| Logistic Regression Analyses | Odds Ratios (95% confidence intervals) | P-value |
|---|---|---|
| Y = High or moderate risk vs. low Steno type 1 risk score | ||
| Unadjusted model | ||
| Patients with HSI ≤ 36 (n = 654) | Ref. | - |
| Patients with HSI > 36 and FIB4 < 1.3 (n = 509) | 1.16 (0.92–1.47) | 0.202 |
| Patients with HSI > 36 and FIB4 ≥ 1.3 (n = 91) | 20.2 (7.33–55.7) | < 0.001 |
| Adjusted model 1 | ||
| Patients with HSI ≤ 36 (n = 654) | Ref. | - |
| Patients with HSI > 36 and FIB4 < 1.3 (n = 509) | 0.94 (0.65–1.36) | 0.753 |
| Patients with HSI > 36 and FIB4 ≥ 1.3 (n = 91) | 11.4 (3.54–36.9) | < 0.001 |
| Adjusted model 2 (n = 1,043) | ||
| Patients with HSI ≤ 36 (n = 532) | Ref. | - |
| Patients with HSI > 36 and FIB4 < 1.3 (n = 432) | 0.79 (0.52–1.18) | 0.244 |
| Patients with HSI > 36 and FIB4 ≥ 1.3 (n = 79) | 10.9 (2.99–39.9) | < 0.001 |
| Y = High or intermediate riskvs.low ASCVD risk score | ||
| Unadjusted model | ||
| Patients with HSI ≤ 36 (n = 654) | Ref. | - |
| Patients with HSI > 36 and FIB4 < 1.3 (n = 509) | 1.12 (0.88–1.41) | 0.364 |
| Patients with HSI > 36 and FIB4 ≥ 1.3 (n = 91) | 8.38 (4.64–15.1) | < 0.001 |
| Adjusted model 1 | ||
| Patients with HSI ≤ 36 (n = 654) | Ref. | - |
| Patients with HSI > 36 and FIB4 < 1.3 (n = 509) | 0.99 (0.71–1.39) | 0.982 |
| Patients with HSI > 36 and FIB4 ≥ 1.3 (n = 91) | 4.83 (2.39–9.78) | < 0.001 |
| Adjusted model 2 (n = 1,043) | ||
| Patients with HSI ≤ 36 (n = 532) | Ref. | - |
| Patients with HSI > 36 and FIB4 < 1.3 (n = 432) | 0.93 (0.64–1.36) | 0.697 |
| Patients with HSI > 36 and FIB4 ≥ 1.3 (n = 79) | 6.93 (3.00-15.9) | < 0.001 |
Cohort size, n = 1,254, except where indicated. Data are expressed as odds ratio (OR) and 95% confidence interval, assessed by univariable and multivariable logistic regression analyses. The dependent variable of logistic regression models was: (a) the high or moderate Steno type 1 risk groups combined vs. the low Steno type 1 risk group, or (b) the high or Intermediate ASCVD risk groups combined vs. the low ASCVD risk group. Regression model 1 was adjusted for sex, BMI, diabetes duration, HbA1c, presence of CKD (defined as e-GFR < 60 mL/min/1.73 m2 or abnormal albuminuria), and lipid-lowering medication use. Regression model 2 was adjusted for the same model’s 1 covariates after excluding those with significant alcohol intake (n = 211)
As sensitivity analyses, we also repeated the above-mentioned logistic regression models after excluding participants with intermediate HSI values (i.e., those with HSI ranging from 30 to 36) from the analysis (Supplementary Table 1) or after including these participants among those with hepatic steatosis (in such case, the diagnosis of NAFLD was defined as HSI > 30, instead of HSI > 36) (Supplementary Table 2). Notably, the results of the study remained unchanged.
Discussion
The main and novel findings of our large multicenter cross-sectional study that included 1,254 Italian adult outpatients with T1DM without pre-existing CVD (i.e., subjects in primary prevention of CVD) are as follows: (a) the 10-year estimated risk of developing a first fatal or nonfatal CVD event was markedly greater in patients with hepatic steatosis and significant fibrosis compared to those with steatosis alone or without steatosis; (b) this CVD risk remained statistically significant even after adjusting for sex, BMI, diabetes duration, HbA1c, CKD, and lipid-lowering medication use; and (c) subgroups analyses confirmed that the 10-year estimated CVD risk was greater in those with hepatic steatosis and liver fibrosis compared to the other two patient subgroups, regardless of age, sex, BMI, diabetes duration, HbA1c, smoking history, and hypertension status.
This is the most updated and largest cross-sectional study aimed at examining the association between NAFLD (with and without coexisting significant fibrosis) and the 10-year CVD risk estimates in adults with T1DM.
It is well known that NAFLD is a growing public health problem in people with T2DM, causing considerable healthcare costs, economic losses, and reduced health-related quality of life [1]. An updated meta-analysis reported a strong association between NAFLD and an increased risk of developing CVD events, i.e., the predominant cause of death in people with NAFLD [9]. To date, the clinical burden of NAFLD in people with T1DM has little been studied. There are few data (mostly derived from small single-center studies) regarding the magnitude of the clinical burden of NAFLD in adults with T1DM [20–23], especially regarding its possible adverse effects on CVD risk. For instance, in 2010, in a small cross-sectional study of 250 Italian outpatients with T1DM, Targher et al. [21]. reported for the first time that ultrasound-detected NAFLD (present in ~ 45% of patients) was associated, independently of common CVD risk factors, with the presence of asymptomatic/symptomatic CVD (assessed by patient history, chart review, electrocardiogram, and echo-Doppler scanning of carotid and lower limb arteries). In 2012, in a subsequent cross-sectional study of 343 Italian adult outpatients with T1DM (~ 52% of whom had NAFLD on ultrasonography), Targher et al. [22] reported that NAFLD was associated with an increased prevalence of asymptomatic/symptomatic coronary, cerebrovascular and peripheral vascular disease (adjusted-odds ratio 7.6, 95% CI 3.6–24.0), independent of multiple CVD risk factors. In 2016, in a single-center cross-sectional study of 722 Chinese adults with T1DM, Zhang et al. [20] reported that NAFLD on ultrasonography was associated with increased carotid-artery intimal medial thickness and an increased prevalence of carotid atherosclerotic plaques, independent of traditional CVD factors. Finally, and most interestingly, in a small retrospective longitudinal study involving 286 adult patients with T1DM followed for a mean period of 5.3 years, Mantovani et al. [23] reported that NAFLD on ultrasonography (present in ~ 52% of patients) was associated with an increased incidence of CVD events, independently of traditional CVD risk factors and diabetes-related variables.
Collectively, therefore, the findings of our large multicenter cross-sectional study corroborate and expand the results of the previously published studies, showing that T1DM patients with NAFLD and significant fibrosis had a remarkably higher 10-year estimated risk of developing a first fatal or nonfatal CVD event compared to those with hepatic steatosis alone or without steatosis. Notably, the sample size of our study was at least ~ 3–4 times greater than that of the previously published studies. Furthermore, this is the first large study to examine the association between the FIB-4 index (i.e., a reliable non-invasive biomarker of advanced liver fibrosis) and the CVD risk in people with T1DM. In the previously published studies, there was no information about the severity of liver fibrosis, which is one of the strongest predictors of all-cause mortality and adverse clinical outcomes in NAFLD [6, 8, 34].
Potential clinical implications
The findings of our study may have important clinical implications, as they support the assertion that the non-invasive identification of hepatic steatosis with coexisting significant fibrosis in individuals with T1DM can help to identify a subset of subjects at higher 10-year risk of developing a first fatal or nonfatal CVD event. Therefore, in the era of precision medicine, if further confirmed in large prospective studies on “hard” CVD endpoints, our findings point to the presence of hepatic steatosis with significant fibrosis as a possible CVD risk enhancer allowing to identify individuals with T1DM who might benefit the most from a more intensive control of the main modifiable CVD risk factors. In this regard, a complementary and interesting finding of our study was that the proportion of patients with T1DM who achieved a plasma LDL-cholesterol level < 1.8 mmol/L (i.e., a cut-off value strongly recommended by professional organizations in people with high-risk CVD without pre-existing CVD) [35, 36] was low (achieved in only 15.4%) among patients with hepatic steatosis and significant fibrosis, who were at increased 10-year estimated CVD risk. This finding further suggests that the true CVD risk of patients with T1DM is largely underappreciated in clinical practice, and statin therapy is often under-prescribed in this patient population.
Putative mechanisms underpinning the association between NAFLD and CVD risk
Growing clinical evidence supports that NAFLD is not an “innocent” bystander of CVD but may actively contribute to its pathogenesis [9, 37–40]. The magnitude of the CVD risk parallels the underlying severity of NAFLD (especially the level of liver fibrosis), thereby resulting in the worsening of systemic/hepatic insulin resistance, increased production of atherogenic lipids, and systemic release of multiple proinflammatory, prothrombogenic, and vasoactive mediators [9, 37–40]. All these NAFLD-related mediators may adversely influence the risk of CVD, thereby contributing to the development and progression of CVD complications in people with NAFLD [9, 37–40].
Study limitations and strengths
The current study has some important limitations. First, the cross-sectional design of the study does not allow us to establish any cause-and-effect relationships between NAFLD with significant fibrosis and the high 10-year estimated CVD risk. Second, we diagnosed hepatic steatosis using the HSI index (i.e., HSI > 36 vs. HSI ≤ 36) and not liver ultrasonography. Similarly, we used the FIB-4 index for non-invasively detecting significant fibrosis (FIB-4 index ≥ 1.3) and not vibration-controlled transient elastography (FibroScan®). In clinical practice, liver ultrasonography and FibroScan® are the two first-line imaging methods to detect hepatic steatosis and fibrosis non-invasively [41]. However, these two imaging methodologies are expensive and not easily applied in large epidemiological studies like this. That said, HSI showed a good performance in identifying hepatic steatosis when compared with liver ultrasonography or magnetic resonance imaging in adults of the general population [29], individuals with T2DM [29], and those with T1DM [31]. Furthermore, as reported in the Methods section above, we also found a satisfactory diagnostic performance of HSI (AUROC of 0.72, 95% CI 0.63–0.75) in identifying hepatic steatosis on ultrasonography in a subset of our participants. Although simple, inexpensive, and widely available serum biomarkers, such as HSI and FIB-4 scores, can be used as first-line tools, further studies using liver imaging methods for assessing hepatic steatosis and fibrosis are certainly needed to validate our findings in large cohorts of adults with T1DM. Future prospective cohort studies are also needed to confirm if NAFLD (with varying levels of fibrosis) increases the long-term risk of CVD outcomes in people with T1DM. Finally, we cannot exclude that other unmeasured factors might partly explain the observed associations.
Despite these limitations, our study has important strengths, such as the large sample size, the multicenter study design, the completeness of the database, and the exclusion of patients with important comorbidities (such as, for example, active cancer, cirrhosis and prior history of ischemic heart disease or stroke), as we believe that the inclusion of patients with such comorbidities might have confounded the interpretation of data.
Conclusions
The results of this large multicenter cross-sectional study involving individuals with T1DM without pre-existing CVD showed that NAFLD with liver fibrosis (assessed by validated non-invasive biomarkers) was significantly associated with an increased 10-year estimated risk of developing a first fatal or nonfatal CVD event. This association remained significant even after adjusting for common CVD risk factors, diabetes-related variables, and other potential confounders. Further research is required to corroborate these findings in other cohorts of individuals with T1DM from different countries and to better elucidate whether the non-invasive detection of NAFLD with varying levels of liver fibrosis could improve CVD risk prediction in people with T1DM. In the meantime, we believe that using CVD risk prediction models is an important step to support the clinical decision on the primary prevention of CVD in people with T1DM. In addition, a multidisciplinary, team-based approach to treating individuals with T1DM and advanced NAFLD, based on a careful evaluation of related risk factors and monitoring for liver and CVD complications, is warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
S.V.d.K, A.D.C., and G.T. were involved in the conception, design, and conduct of the study. S.V.d.K., A.D.C., and R.A. coordinated the data. All authors contributed to data collection. A.M. and G.T. wrote the first draft of the manuscript, and all authors edited, reviewed, and approved the final version of the manuscript. A.M. and G.T. conducted the statistical analysis. M.L.M. and S.V.d.K. contributed to the interpretation of the results. G.T. is the guarantor of this work and, as such, has full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
GT is partly supported by grants from the University School of Medicine of Verona, Verona, Italy.
Data Availability
The dataset supporting the conclusions of this article is included within the article (and its additional files).
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the “Comitato etico per la Sperimentazione Clinica della Provincia di Padova” (code #63553, October 18th, 2018) and by the ethics committee of each participating center. Written informed consent was collected accordingly to the requests of each local ethics committee.
Consent for publication
We agree to the publication of the data; we also declare that the data are original and are not under review elsewhere.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Saula Vigili de Kreutzenberg and Giovanni Targher co-senior Investigators
References
- 1.Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022;19(1):60–78. doi: 10.1038/s41575-021-00523-4. [DOI] [PubMed] [Google Scholar]
- 2.Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399(10319):61–116. doi: 10.1016/S0140-6736(21)01701-3. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–47. doi: 10.1097/HEP.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. 2018;14(2):99–114. doi: 10.1038/nrendo.2017.173. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 2021;6(7):578–88. doi: 10.1016/S2468-1253(21)00020-0. [DOI] [PubMed] [Google Scholar]
- 7.Ajmera V, Kim BK, Yang K, Majzoub AM, Nayfeh T, Tamaki N, et al. Liver stiffness on magnetic resonance elastography and the MEFIB Index and Liver-Related outcomes in nonalcoholic fatty liver disease: a systematic review and Meta-analysis of individual participants. Gastroenterology. 2022;163(4):1079–89. doi: 10.1053/j.gastro.2022.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. 2020. S:154170. Epub 2020/02/02. [DOI] [PubMed]
- 9.Mantovani A, Csermely A, Petracca G, Beatrice G, Corey KE, Simon TG, et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(11):903–13. doi: 10.1016/S2468-1253(21)00308-3. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Petracca G, Csermely A, Beatrice G, Bonapace S, Rossi A, et al. Non-alcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut. 2022 doi: 10.1136/gutjnl-2022-327672. [DOI] [PubMed] [Google Scholar]
- 11.Park J, Kim G, Kim H, Lee J, Lee YB, Jin SM, et al. The association of hepatic steatosis and fibrosis with heart failure and mortality. Cardiovasc Diabetol. 2021;20(1):197. doi: 10.1186/s12933-021-01374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J, Kim G, Kim BS, Han KD, Kwon SY, Park SH, et al. The associations of hepatic steatosis and fibrosis using fatty liver index and BARD score with cardiovascular outcomes and mortality in patients with new-onset type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. 2022;21(1):53. doi: 10.1186/s12933-022-01483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Kim G, Kim BS, Han KD, Yoon Kwon S, Hee Park S, et al. The association between changes in hepatic steatosis and hepatic fibrosis with cardiovascular outcomes and mortality in patients with New-Onset type 2 diabetes: a nationwide cohort study. Diabetes Res Clin Pract. 2022;194:110191. doi: 10.1016/j.diabres.2022.110191. [DOI] [PubMed] [Google Scholar]
- 14.Park J, Kim G, Kim H, Lee J, Jin SM, Kim JH. The associations between changes in hepatic steatosis and heart failure and mortality: a nationwide cohort study. Cardiovasc Diabetol. 2022;21(1):287. doi: 10.1186/s12933-022-01725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–53. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 16.Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun. 2019;3(11):1459–71. doi: 10.1002/hep4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livingstone SJ, Looker HC, Hothersall EJ, Wild SH, Lindsay RS, Chalmers J, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: scottish registry linkage study. PLoS Med. 2012;9(10):e1001321. doi: 10.1371/journal.pmed.1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawshani A, Sattar N, Franzen S, Rawshani A, Hattersley AT, Svensson AM, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392(10146):477–86. doi: 10.1016/S0140-6736(18)31506-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, et al. Estimated life expectancy in a scottish cohort with type 1 diabetes, 2008–2010. JAMA. 2015;313(1):37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Guo K, Lu J, Zhao F, Yu H, Han J, et al. Nonalcoholic fatty liver disease is Associated with increased carotid intima-media thickness in type 1 Diabetic patients. Sci Rep. 2016;6:26805. doi: 10.1038/srep26805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Pichiri I, et al. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53(4):713–8. doi: 10.1016/j.jhep.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Targher G, Pichiri I, Zoppini G, Trombetta M, Bonora E. Increased prevalence of cardiovascular disease in type 1 diabetic patients with non-alcoholic fatty liver disease. J Endocrinol Invest. 2012;35(5):535–40. doi: 10.3275/7875. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A, Mingolla L, Rigolon R, Pichiri I, Cavalieri V, Zoppini G, 2016. :387 – 91. Epub 2016/10/22. [DOI] [PubMed]
- 24.Vistisen D, Andersen GS, Hansen CS, Hulman A, Henriksen JE, Bech-Nielsen H, et al. Prediction of First Cardiovascular Disease Event in Type 1 diabetes Mellitus: the Steno type 1 risk engine. Circulation. 2016;133(11):1058–66. doi: 10.1161/CIRCULATIONAHA.115.018844. [DOI] [PubMed] [Google Scholar]
- 25.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 26.Dei Cas A, Aldigeri R, Mantovani A, Masulli M, Palmisano L, Cavalot F et al. Sex differences in cardiovascular disease and cardiovascular risk estimation in patients with type 1 diabetes. J Clin Endocrinol Metab. 2023. Epub 2023/03/08. doi: 10.1210/clinem/dgad127. PubMed PMID: 36881927. [DOI] [PubMed]
- 27.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):19–S40. doi: 10.2337/dc23-S002. [DOI] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006. Epub 2009/05/06. [DOI] [PMC free article] [PubMed]
- 29.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–8. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Morieri ML, Vitturi N, Avogaro A, Targher G, Fadini GP, Society D-TDNotID. Prevalence of hepatic steatosis in patients with type 2 diabetes and response to glucose-lowering treatments. A multicenter retrospective study in Italian specialist care. J Endocrinol Invest. 2021;44(9):1879-89. Epub 2021/01/13. doi: 10.1007/s40618-021-01501-y. PubMed PMID: 33432553. [DOI] [PubMed]
- 31.Sviklane L, Olmane E, Dzerve Z, Kupcs K, Pirags V, Sokolovska J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J Gastroenterol Hepatol. 2018;33(1):270–6. doi: 10.1111/jgh.13814. [DOI] [PubMed] [Google Scholar]
- 32.Castera L, Friedrich-Rust M, Loomba R. 2019. 81 e4. Epub 2019/01/21. [DOI] [PMC free article] [PubMed]
- 33.Associazione Italiana per lo Studio del Fegato SIdD A, Italiana S, dO, Members of the guidelines p, Coordinator, Members A, Members SID Non-alcoholic fatty liver disease in adults 2021: a clinical practice guideline of the Italian Association for the study of the liver (AISF), the italian society of Diabetology (SID) and the italian society of obesity (SIO) Nutr Metab Cardiovasc Dis. 2021;32(1):1–16. doi: 10.1016/j.numecd.2021.04.028. [DOI] [PubMed] [Google Scholar]
- 34.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797–835. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 36.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, NLA/PCNA Guideline on the Management of Blood Cholesterol. 2019. 2018 AHA/ACC/AACVPR/AAPA. PubMed PMID: 30423393. [DOI]
- 37.Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–705. doi: 10.1136/gutjnl-2020-320622. [DOI] [PubMed] [Google Scholar]
- 38.Targher G, Corey KE, Byrne CD. NAFLD, and cardiovascular and cardiac diseases: factors influencing risk, prediction and treatment. Diabetes Metab. 2021;47(2):101215. doi: 10.1016/j.diabet.2020.101215. [DOI] [PubMed] [Google Scholar]
- 39.Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15(7):425–39. doi: 10.1038/s41575-018-0010-0. [DOI] [PubMed] [Google Scholar]
- 40.Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, et al. Nonalcoholic fatty liver Disease and Cardiovascular Risk: A Scientific Statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42(6):e168–e85. doi: 10.1161/ATV.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 41.Byrne CD, Patel J, Scorletti E, Targher G. 2018. :k2734. Epub 2018/07/14. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. 2020. S:154170. Epub 2020/02/02. [DOI] [PubMed]
- Mantovani A, Mingolla L, Rigolon R, Pichiri I, Cavalieri V, Zoppini G, 2016. :387 – 91. Epub 2016/10/22. [DOI] [PubMed]
- Castera L, Friedrich-Rust M, Loomba R. 2019. 81 e4. Epub 2019/01/21. [DOI] [PMC free article] [PubMed]
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, NLA/PCNA Guideline on the Management of Blood Cholesterol. 2019. 2018 AHA/ACC/AACVPR/AAPA. PubMed PMID: 30423393. [DOI]
- Byrne CD, Patel J, Scorletti E, Targher G. 2018. :k2734. Epub 2018/07/14. [DOI] [PubMed]
Supplementary Materials
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article (and its additional files).