Abstract
OBJECTIVE
To evaluate the efficacy of non-pharmacological interventions for antipsychotic-associated weight gain.
METHODS
Systematic literature search and meta-analysis of randomized controlled trials comparing behavioral interventions with control groups to ameliorate antipsychotic-associated weight gain.
RESULTS
Across 17 studies (n=810, mean age: 38.8 years, 52.7% male, 40.8% White, 85.6% with schizophrenia-spectrum disorders), non-pharmacological interventions led to a significant reduction in weight (−3.12kg; CI: −4.03, −2.21, p<0.0001) and body mass index (BMI) (−0.94kg/m2; CI: −1.45, −0.43, p=0.0003) compared with control groups. Intervention benefits extended to all secondary outcomes, except for high density-lipoprotein-cholesterol and systolic blood pressure. Compared to controls, intervention patients experienced significant decreases in waist circumference (WMD=−3.58cm, CI:−5.51,−1.66, p=0.03), percent body fat (WMD=−2.82%, CI:−5.35,−0.30, p=0.03), glucose (WMD=−5.79mg/dL, CI:−9.73,−1.86, p=0.004), insulin (WMD=−4.93uIU/ml, CI:−7.64,−2.23, p=0.0004), total cholesterol (WMD=−20.98mg/dL, CI:−33.78,−8.19; p=0.001), low density-lipoprotein-cholesterol (WMD=−22.06mg/dL, CI:−37.80,−6.32, p=0.006) and triglycerides (WMD=−61.68mg/dL, CI:−92.77,−30.59, p=0.0001), and less weight gain ≥7% (29.7% vs. 61.3%; RR=−0.52, CI:−0.35,−0.78, p=0.002; number-needed-to-treat=4). Up to 12 months after the intervention ended (mean=3.6 months), benefits endured regarding weight (WMD=−3.48kg, CI: −6.37, −0.58, p=0.02), but not BMI (p=0.40). Subgroup analyses showed superiority of non-pharmacological interventions irrespective of treatment duration, individual or group, cognitive behavioral or nutritional interventions, or prevention versus intervention trials. However, weight and BMI were significantly improved only in outpatient trials (p<0.0001), but not in inpatient or mixed samples (p=0.09–0.96).
CONCLUSION
Behavioral interventions effectively prevented and reduced antipsychotic-associated weight gain and cardiometabolic perturbations, at least in outpatients agreeing to participate in trials aimed at improving physical health. Effective treatments ranged from nutritional interventions to cognitive behavioral therapy.
Keywords: antipsychotics, adverse effects, weight gain, metabolic syndrome, behavioral, diet, exercise, healthy lifestyle, intervention, treatment, CBT, nutritional interventions
1. INTRODUCTION
Antipsychotic efficacy for psychotic disorders (Leucht et al., 2009), bipolar disorder (Correll et al., 2010), major depressive disorders (Nelson and Papakostas, 2009) and irritability/aggression associated with autism (Correll et al., 2011a), as well as off–label use in other psychiatric conditions (Maher et al., 2011) is counterbalanced by significant weight gain and cardiometabolic risk (Allison et al., 1999; American Diabetes Association, 2004; Lieberman et al., 2005; De Hert et al., 2011a; Kahn et al., 2008). This weight gain is problematic as it may adversely affect adherence, quality of life (Allison, 2003), and especially, cardiovascular morbidity and mortality (Newcomer, 2005; Correll et al., 2011b; De Hert et al., 2011b).
Pharmacologic interventions to ameliorate antipsychotic weight gain have had moderate success. Out of 15 agents examined in a recent meta-analysis, only five showed significant benefit versus placebo, and three were already taken off the market due to adverse effects (fenfluramine, sibutramine, reboxetine). Metformin and topiramate reduced antipsychotic-related weight gain compared to placebo by 2.5–3 kg after 8–12 weeks of treatment (Maayan et al., 2010). Pharmacologic interventions can cause additional side effects (Maayan and Correll, 2010). Metformin carries a risk of lactic acidosis, particularly in the elderly and in those with compromised renal function (Chang et al., 2002), and its use can be limited by nausea, vomiting and diarrhea. Topiramate has been associated with cognitive blunting (Narula et al., 2010).
Conversely, non-pharmacological interventions do not have such side effects and have shown promise. In a meta-analysis of 10 randomized controlled (RCTs) (n=482) Alvarez-Jimenez et al. (2008) reported that non-pharmacological interventions led to 2.56 kg less weight gain and 0.91kg/m2 less BMI increase than the control condition and that nutritional counseling was equivalent to cognitive behavioral therapy (CBT). While this meta-analysis provided support for non-pharmacological interventions, the small number of studies precluded secondary analyses regarding metabolic outcomes, and the mediating impact of treatment duration. A more recent, systematic review included the same10 RCTs plus 6 additional, but non-randomized studies (Gabriele et al., 2009), reporting similar results.
The current study expands upon the prior publications (Alvarez-Jimenez et al., 2008; Gabriele et al., 2009) by (1) including additional RCTs , and (2) analyzing effects on insulin, glucose, high density lipoprotein (HDL), low density lipoprotein (LDL), total cholesterol, triglycerides, and systolic blood pressure. addition we also aimed to assess maintenance effects after the behavioral interventions ended.
2. MATERIALS AND METHODS
2.1. Search
A literature search was conducted in PsycInfo, Medline, PubMed, CINAHL, and the Cochrane Library using the following search terms: ‘weight,’ ‘antipsychotic,’ and ‘intervention’ plus ‘behavioral,’ ‘psychoeducation,’ ‘exercise,’ or ‘cognitive’. Reference lists of relevant articles were searched for additional studies. When required data was missing, first/corresponding authors were contacted for additional information.
Included in this meta-analysis were RCTs of non-pharmacological interventions aimed at preventing or reducing antipsychotic associated weight gain (see Figure 1 for the search results and flow).
Figure 1:
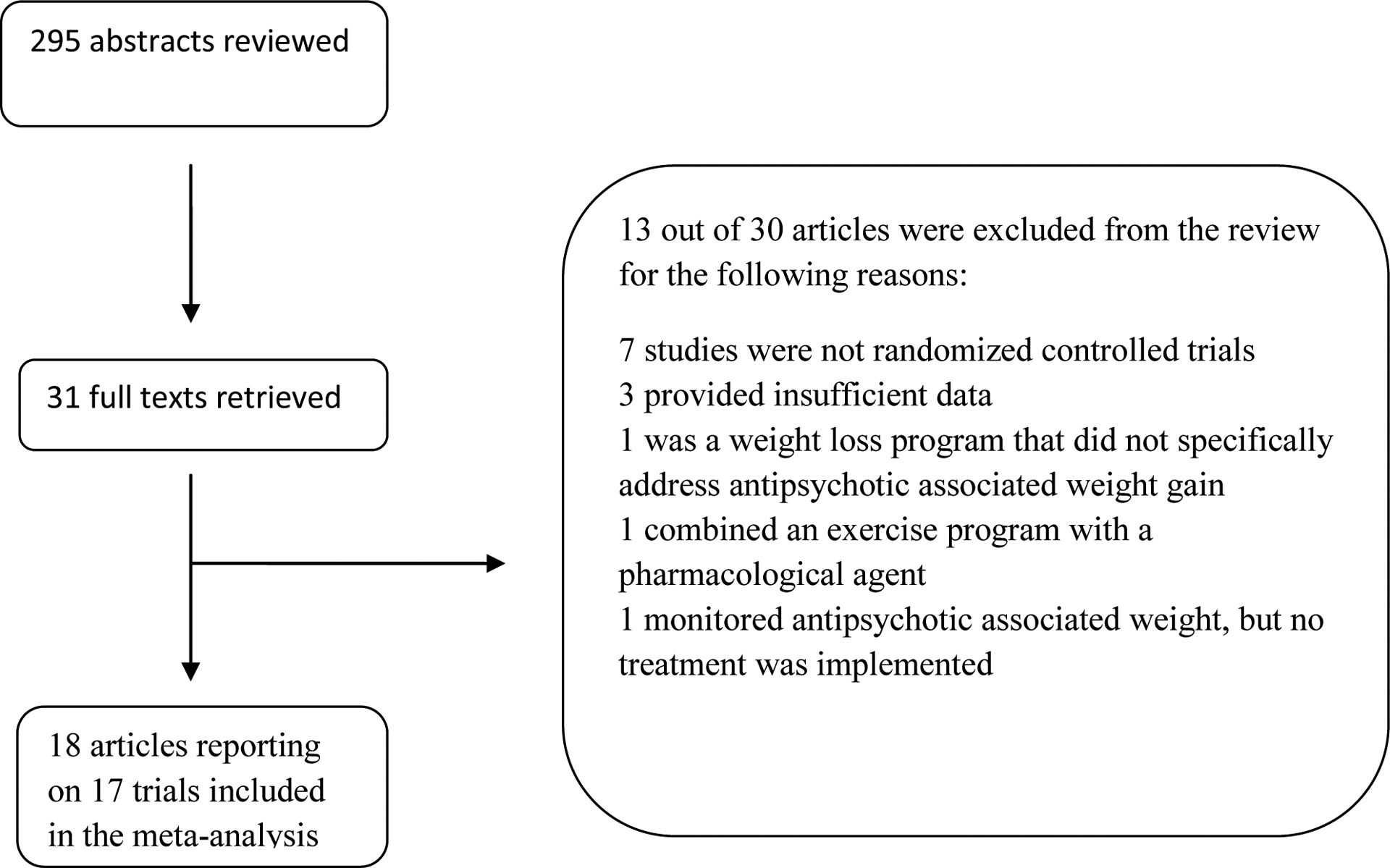
Flow chart of systematic review
2.2. Data Extraction and Outcomes
All data were extracted by one author and verified at a later point by a second author. Inconsistencies were reviewed and resolved.
2.3. Calculations and Analyses
Data were analyzed using randomized effects models in Review Manager 5.0 (RevMan 5.0.24 (PC version), Cochrane Collaboration, Oxford, UK). All tests were two-sided and α was set at 0.05. For continuous outcomes, the weighted mean difference (WMD) with 95% confidence intervals (CI) was calculated. For dichotomous outcomes, Risk Ratio (RR) +/− CI was calculated and number-needed-to-treat (NNT) was derived by dividing 1 by the risk difference. Study heterogeneity was measured using the I-squared statistic, with I-squared > 50% indicating significant heterogeneity.
The co-primary outcomes were (a) body weight and (b) body mass index (BMI). Secondary outcomes included change in waist circumference, body fat percentage, total, HDL-, and LDL-cholesterol, triglycerides, fasting glucose, insulin, and systolic sitting blood pressure and all-cause discontinuation.
To examine potential moderator variables, five a priori planned sensitivity analyses were conducted: (1) CBT (N=6) vs. nutritional and/or exercise interventions (N=11); (2) trial duration ≤ 3 months (N=9) vs. trial duration >3 months (N=8); (3) prevention trials (i.e., non-pharmacologic intervention initiated within 4 weeks of starting the antipsychotic: N=6) vs. intervention trials (i.e., non-pharmacologic intervention initiated after antipsychotic weight gain had occurred: N=11); (4) individual interventions (N=5) vs. group interventions (N=12); and (5) inpatient status (N=3) vs. mixed (N=2) vs. outpatient status (N=12).
3. RESULTS
We identified 17 RCTs, including 810 participants, that had a comparison group (Table 1). Data were extracted from an 18th publication (Alvarez-Jimenez et al. 2010) for follow-up information on an active treatment study (Alvarez-Jimenez et al. 2006). Treatment duration ranged from 8–72 weeks (8 studies (47%) with >12 week duration, mean: 19.6 weeks). Treatment involved CBT (N=7, 41%) and nutritional and/or exercise interventions (N=10, 59%). Participants’ mean age was 38.1 years in the intervention group and 37.2 years in the control group. Overall, 52.3% of the participants in the intervention group and 54.9% in the control group were men. In the intervention group, 47% of the participants were Caucasian, 36% were Asian, 10.7% were African American, 0.8% were Hispanic, and 5.5% were reported as “Other”. In the control group, 51.3% of the participants were Caucasian, 31.7% were Asian, 10.9% were African American, 3% were Hispanic, and 3.9% were reported as “Other” (8 studies with data). Trials were conducted in the USA (N=6), Europe (N=5), Asia (N=3), Australia (N=1), Canada (N=1), and Israel (N=1). The mean BMI was 29.6 kg/m2 for the intervention group and 28.5 kg/m2 for the control group. Out of the 810 participants, 423 (52.2%) were diagnosed with schizophrenia, schizoaffective disorder, or schizophreniform (85.6% of patients with diagnostic information), 46 (5.7%) were diagnosed with unipolar or bipolar disorder, and in 341 patients (42.1%) diagnostic information was missing.
Table 1.
Design and Patient Characteristics of Studies of Non-pharmacologic Interventions for Antipsychotic-related Weight Gain and Metabolic Abnormalities
| Study | Design | Duration (weeks) | Group | N2 | Age (yrs) | Male (%) | White (%) | Diagnosis [nt/np]3 | Anti-psychotic Dose (daily dose) | Weight Δ (kg) or BMI Δ (kg/m2) [n]4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive Behavioral Therapy | — | Psychotic disorder [28/33] | Olanzapine 13.1 ±3. 3 mg/day, Risperidone 4.2±0.9 mg/day, Haloperidol 4.9±1.4 mg/day | |||||||
| Álvarez-Jiménez et al., 2010 | Control | 33 | 27.5±8.5 | 78.8 | 6.98±4.50 [33] after 24 week follow up: 8.98±5.61 | |||||
| Bebee et al., 2005 | Exercise Program | 16 | Total: 80 | Total: 80 | Schizophrenia | Atypicals (90%), typicals (20%) | ||||
| Control | 6 | 63 | Baseline: 30.07±4.54 End point: 29.93±4.74 [6] | |||||||
| Brar et al., 2005 | Cognitive Behavioral Therapy | 14 | Schizophrenia and schizoaffective disorders [34/37] | Switched from olanzapine to risperidone | ||||||
| Control | 37 | 40.5±10.6 | 35.1 | 45.9 | −1. 1±3. 11 [37] | |||||
| Cordes et al., 2011 | Weight Management Program | 24 + 24 week follow up | Schizophrenia and schizoaffective disorders | Started new treatment with olanzapine | ||||||
| Control | 18 | 40.7±11.7 | 61.1 | - | Baseline: 79.0±15.0 Endpoint (week 48 after 24 week follow up): 89.0±12.1 Change during intervention (week 24): 4.5±6.1[18] |
|||||
| Evans et al., 2005 | Nutritional Counseling Program | 12 +12 week follow up | Schizophrenia [9/3], schizoaffective disorder [4/6], schizophrenoform psychosis [4/6], bipolar disorder [4/4], depression [2/3] | Olanzapine 13.9±3.3 mg/day 10.6±4.8 mg/day | ||||||
| Control | 22 | 33.6±11.6 | 50 | 6.0±2.6 [22] After Follow up: 9.9±7.4* [8] |
||||||
| Khazaal et al., 2007 | Cognitive Behavioral Therapy | 12 + 12 week follow-up | Schizophrenia and schizoaffective [25/20], bipolar [1/4], schizotypal [2/2], depression and personality disorders [3/4] | Olanzapine, risperidone, clozapine, quetiapine, amisulpride | ||||||
| Control | 30 | 38.3±10.4 | 50 | — | Baseline: 84.3±17.2 After 12 week intervention: 83.5±17.2 [30] Endpoint after 12 week follow up: 83.5±17.4 [30] | |||||
| Kwon et al., 2006 | Cognitive Behavioral Therapy | 12 | Schizophrenia and schizoaffective disorders [33/15] | Olanzapine 5–20 mg/day | ||||||
| Control | 15 | 29.8±6.1 | 33.3 | −1.48±1.88 [15] | ||||||
| Littrell et al., 2003 | Nutritional Counseling Program | 16 + 8 week follow up | Schizophrenia and schizoaffective disorders [35/35] | Olanzapine [35/35] 5–20 mg/day | ||||||
| Control | 35 | 34.5±10.0 | 60.0 | 74.3 | 9.57±12.98 [35] | |||||
| Mauri et al., 2008 | Psychoeducational Program | 12 | Bipolar I [41], Bipolar II [2], Schizoaffective [5], Depression [1] | Olanzapine 5–20 mg/day | ||||||
| Control | 18 | 60 | 38.9 | 0.2±2.9 | ||||||
| McKibbin et al., 2006 | Cognitive Behavioral Therapy | 24 | Schizophrenia and schizoaffective disorder and type-2 diabetes mellitus [29/28] | Typical or atypical with low weight liability (aripiprazole, ziprasidone) [7/6]; Atypical with moderate weight liability (risperidone, quetiapine) [13/14]; Atypical with high weight gain liability (clozapine, olanzapine) [8/9] | ||||||
| Control | 29 | 54.8±8.2 | 62.1 | 72.4 | 3.10±4.60 [29] | |||||
| Khazaal et al., 2007 | Cognitive Behavioral Therapy | 12 + 12 week follow-up | Schizophrenia and schizoaffective [25/20], bipolar [1/4], schizotypal [2/2], depression and personality disorders [3/4] | Olanzapine, risperidone, clozapine, quetiapine, amisulpride | ||||||
| Control | 30 | 38.3±10.4 | 50 | — | Baseline: 84.3±17.2 After 12 week intervention: 83.5±17.2 [30] Endpoint after 12 week follow up: 83.5±17.4 [30] | |||||
| Kwon et al., 2006 | Cognitive Behavioral Therapy | 12 | Schizophrenia and schizoaffective disorders [33/15] | Olanzapine 5–20 mg/day | ||||||
| Control | 15 | 29.8±6.1 | 33.3 | −1.48±1.88 [15] | ||||||
| Littrell et al., 2003 | Nutritional Counseling Program | 16 + 8 week follow up | Schizophrenia and schizoaffective disorders [35/35] | Olanzapine [35/35] 5–20 mg/day | ||||||
| Control | 35 | 34.5±10.0 | 60.0 | 74.3 | 9.57±12.98 [35] | |||||
| Mauri et al., 2008 | Psychoeducational Program | 12 | Bipolar I [41], Bipolar II [2], Schizoaffective [5], Depression [1] | Olanzapine 5–20 mg/day | ||||||
| Control | 18 | 60 | 38.9 | 0.2±2.9 | ||||||
| McKibbin et al., 2006 | Cognitive Behavioral Therapy | 24 | Schizophrenia and schizoaffective disorder and type-2 diabetes mellitus [29/28] | Typical or atypical with low weight liability (aripiprazole, ziprasidone) [7/6]; Atypical with moderate weight liability (risperidone, quetiapine) [13/14]; Atypical with high weight gain liability (clozapine, olanzapine) [8/9] | ||||||
| Control | 29 | 54.8±8.2 | 62.1 | 72.4 | 3.10±4.60 [29] | |||||
| Melamed et al., 2008 | Nutrition & Exercise Program | 12 + 52 week follow-up | Total mean: 46.2±11.9 | Total: 72.9 | Schizophrenia and schizoaffective disorders [−/−] | First or second generation or both | ||||
| Control | 31 | Baseline: 30.6±3.2 After 12 week intervention: 31.4±4.4 Endpoint after 52 week follow-up: 30.4±3.4 [31] |
||||||||
| Poulin et al., 2007 | Nutritional & Exercise Program | 72 | Schizophrenia [19/15], Schizoaffective [19/17], Bipolar [21/19] | Clozapine, Olanzapine, Risperidone, Quetiapine | ||||||
| Control | 51 | 35.3±5.2 | 51 | 100 | 3.6 [51] | |||||
| Scocco et al., 2006 | Psychoeducational & Nutritional Counseling | 8 | Schizophrenia and schizoaffective disorders [9/8] | Olanzapine | ||||||
| Control | 8 | 39.2±9.9 | 87.5 | 2.96±3.08 [8] | ||||||
| Skrinar et al., 2005 | Psychoeducational & Exercise Program | Mood or psychotic disorder | Any antipsychotic | |||||||
| Control | 11 | 36.3±11.3 | Baseline: 31.8±3.9 Endpoint: 32.3±4.1 [11] | |||||||
| Weber & Wyne, 2006 | Cognitive Behavioral Therapy | 16 | Schizophrenia and schizoaffective disorders [8/9] | Olanzapine, Risperidone, Ziprasidone, Quetiapine | ||||||
| Control | 9 | - | 22.2 | 33.3 | −0.62±3.34 [9] | |||||
| Wu et al., 2007 | Nutritional & Exercise Program | 24 | Schizophrenia [28/25] | Clozapine [28/25] ≥ 300 mg/day | ||||||
| Control | 25 | 39.0±6.7 | 44 | 0 | 1.0±3.4 [25] | |||||
| Wu et al., 2008 | Nutritional & Exercise Lifestyle Program | 12 | Schizophrenia [32/32] | Clozapine [9/10], Olanzapine [10/7], Risperidone [6/8], Sulpiride [7/7] | ||||||
| Control | 32 | 25.8±1.7 | 50 | 0 | 3.1±0.7 [32] |
All values represent mean ± standard deviation, unless otherwise stated or it was not reported.
N= total number of subjects randomized in the study.
[nt/np] = number of subjects in the treatment group/number of subjects in the placebo group with the diagnosis.
[n] = number of subjects included in analysis.
“—” indicates data were not provided.
Summary data are presented as total number of participants in treatment and placebo groups for the above intervention.
The overall percentage in % Male and % White categories is calculated using studies with available data only. If the data was not reported for that trial, the trial was not utilized in the summary statistic and its population was not included in the overall calculation.
Bebee et al. (2005), Melamed et al. (2008), Skrinar (2005) reported only means for BMI endpoint data, and not weight mean change.
All but three studies included weight data (82%), with 57% of those studies (N=8) reporting weight change data and 43% (N=6) providing endpoint weight. Similarly, 94% of the studies (N=16) reported BMI data, with 44% of the studies (N=7) reporting BMI change data and 56% (N=9) reporting endpoint BMI. Only 6 studies (35%) reported change or endpoint for glucose and waist circumference, 5 studies (29%) provided total cholesterol endpoint data, 4 (24%) reported end point data in HDL-cholesterol and triglycerides, 3 (18%) reported change or end point in LDL-cholesterol, insulin, body fat percentage, and weight gain ≥7%, and 2 studies (12%) reported endpoint for systolic sitting blood pressure.
In six studies, changes in weight and/or BMI change were reported 2–12 months (mean: 3.6 months for weight, 5.8 months for BMI) after the intervention ended.
3.1. Co-primary Outcomes
Non-pharmacological interventions were associated with a pooled weight change of −3.12 kg (CI: −4.03, −2.21; p<0.0001; I2: 42%) (Figure 2) and a pooled BMI change of −0.94 kg/m2 (CI: −1.45, −0.43; p=0.0003; I2: 75%) compared to controls (see Figure 3).
Figure 2:
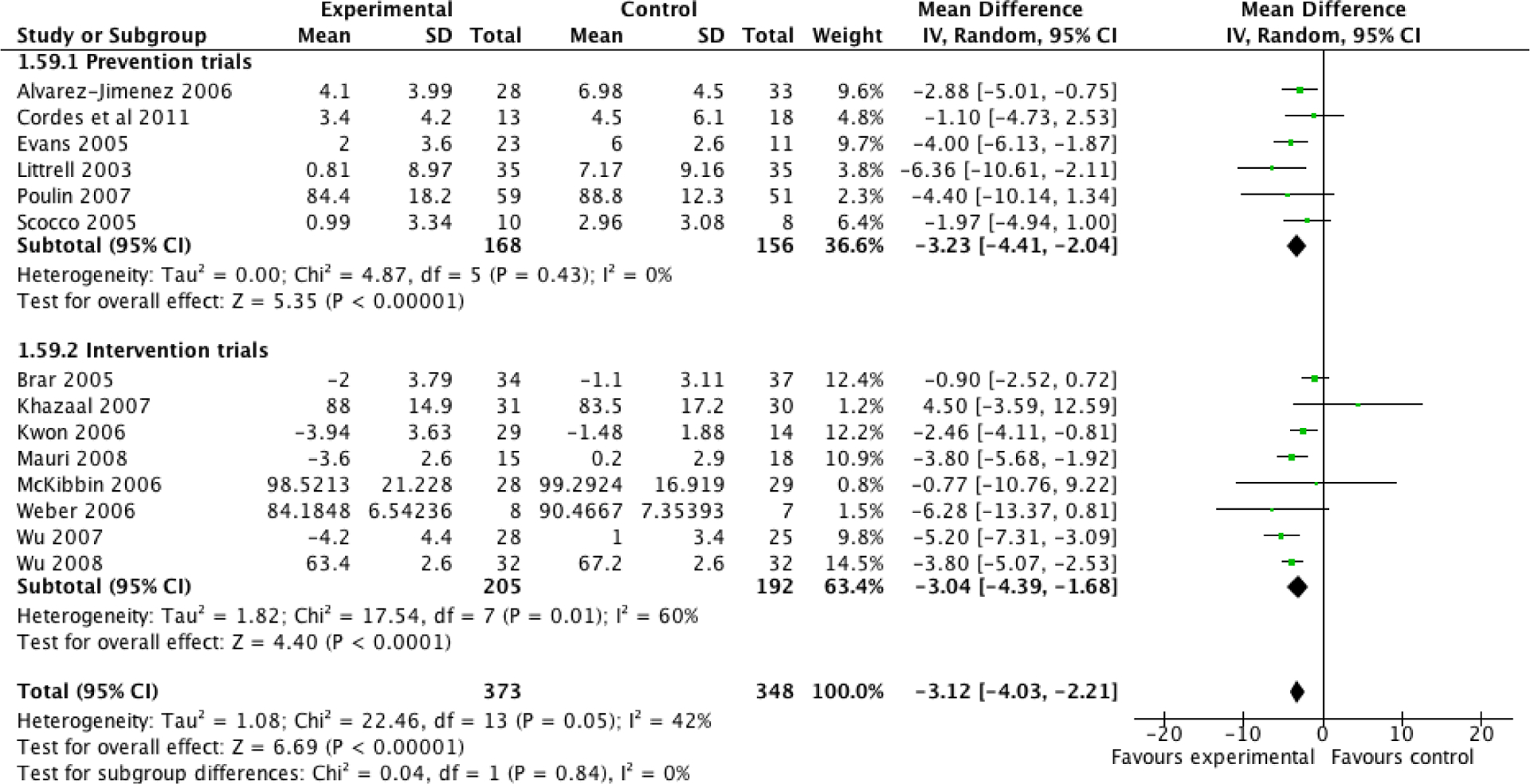
Differences in weight (kg) between behavioral interventions and control groups and separately analyzed by prevention and intervention trials.
Figure 3:
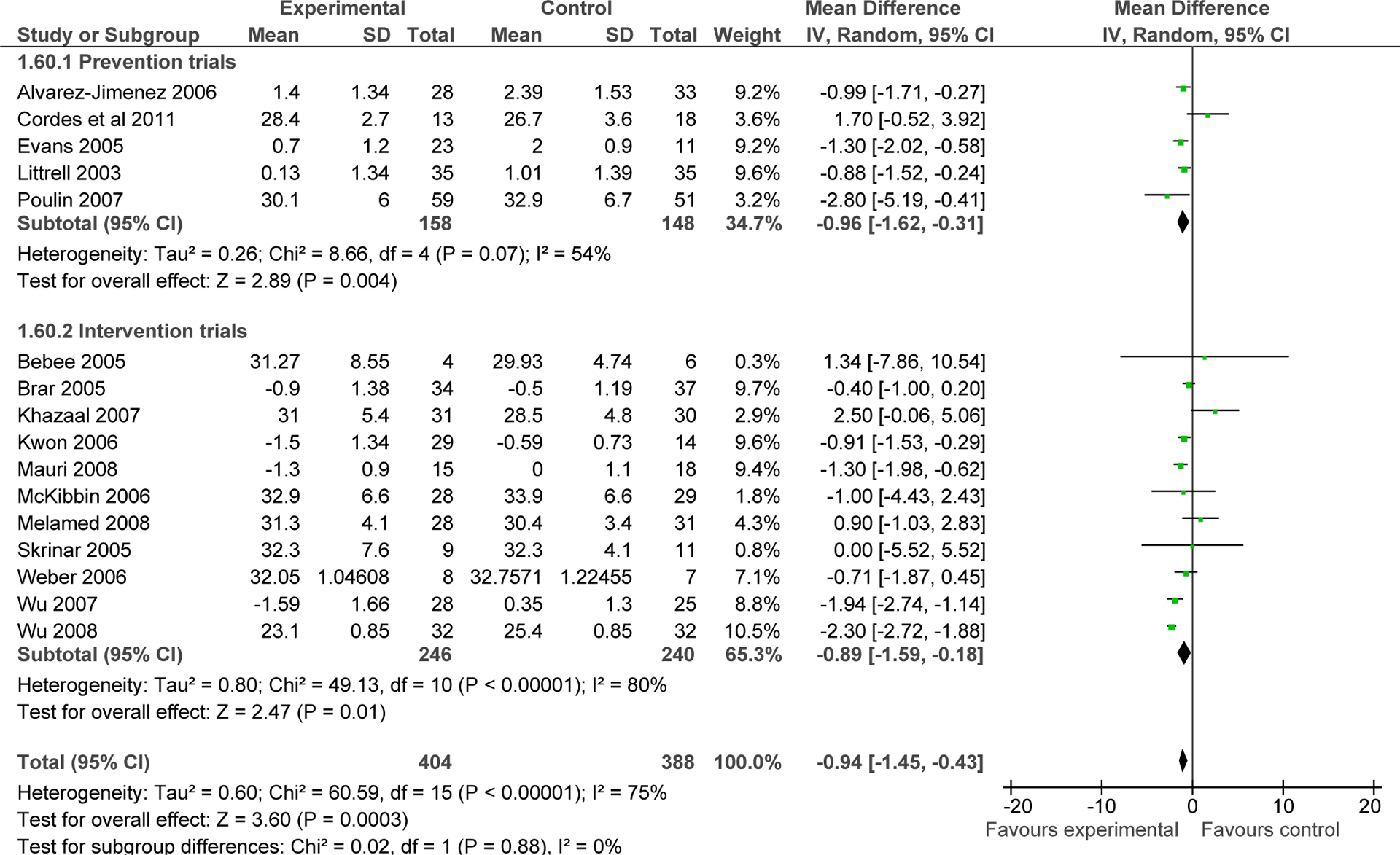
Differences in body mass index (kg/m2) between behavioral interventions and control groups and separately analyzed by prevention and intervention trials.
3.2. Secondary Outcomes
3.2.1. Waist circumference, percent body fat, and weight gain ≥7%,
The waist circumference in the intervention group decreased significantly compared with the control group (N=6, n=349, WMD= −3.58 cm, CI: −5.51, −1.66, p=0.03; I2=65%). Percentage of body fat decreased significantly in the intervention group compared with controls (N= 3, n=83, WMD= −2.82 %, CI: −5.35, −0.30, p=0.03; I2=0%). Lastly, in the intervention group significantly less patients gained ≥7% compared to the control group (N= 3, n=126, 29.7% vs. 61.3%; RR= −.52, CI: −0.35, −0.78, p=0.002; I2=67%, NNT=4).
3.2.2. Insulin and Glucose
Compared with the control group, insulin levels (N=3, n=150, WMD= −4.93 uIU/ml, CI: −7.64, −2.23, p=0.0004; I2=0%) and fasting glucose levels (N=6, n=348, WMD= −5.79 mg/dL, CI: −9.73, −1.86, p=0.004; I2=58%) were significantly lower in the intervention group.
3.2.3. Blood lipids
Total cholesterol decreased significantly in the intervention group compared to control group (N=5, n=273, WMD= −20.98 mg/dL, CI: −33.78, −8.19; p=0.001; I2=41%) (Figure 4). The same was true for LDL-cholesterol (N=3, n=200, WMD= −22.06 mg/dL, CI: −37.80, −6.32; p=0.006; I2=58%) (Figure 4) and triglycerides (N=4, n=253, WMD= −61.68 mg/dL, CI: −92.77, −30.59; p=0.0001; I2=0%) (Figure 4). Conversely, group differences were not significant regarding HDL-cholesterol (N=4, n=220, WMD=2.89 mg/dL, CI: −5.67, 11.45, p=0.51; I2=85%) (Figure 4).
Figure 4:
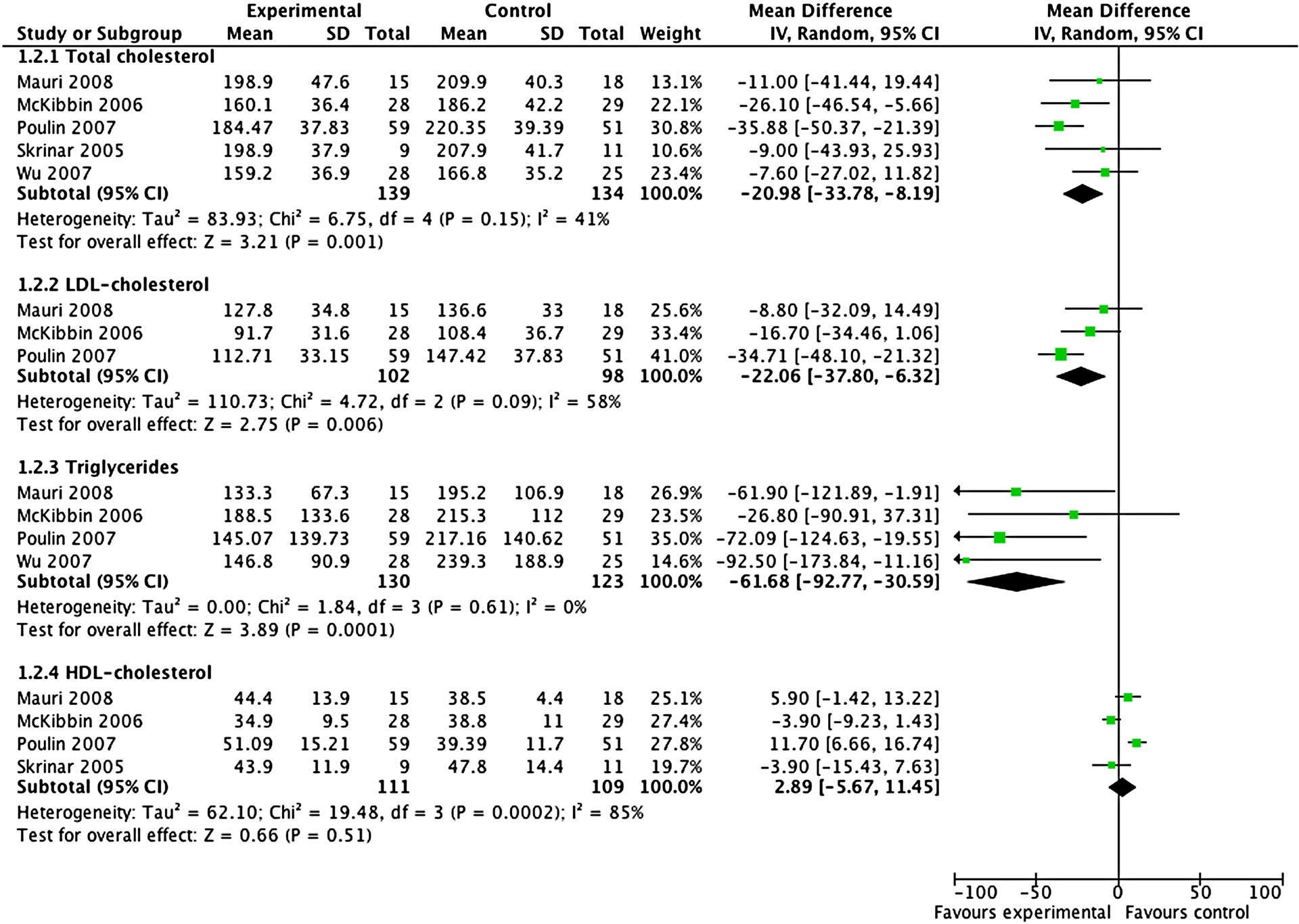
Differences in total, LDL- and HDL-cholesterol as well as triglycerides change (mg/dL) between behavioral interventions and control groups.
3.2.4. Systolic blood pressure
No significant group differences existed for systolic blood pressure (N=2, n=128, WMD= −3.88, CI: −8.79, 1.03, p=0.12; I2=0%).
3.2.5. All-cause discontinuation
All-cause discontinuation rates were similar between treatment (16.6%) and control groups (15.1%) (N=15, n=858; RR: 1.03, CI: 0.68–1.56, p=0.88; I=30%).
3.3. Sensitivity Analyses
Across five sensitivity analyses to determine the effects of potential moderators, no significant subgroup differences emerged, except that weight and BMI were only significantly improved in outpatient trials (p<0.0001), but not in inpatient or mixed samples (p=0.09–0.96) (Table 2).
Table 2:
Five Sensitivity Analyses of Potential Moderator Variables
| Sensitivity Analysis | Outcomes | Number of Studies | n | Mean Difference | 95% C.I. | P value |
|---|---|---|---|---|---|---|
| Cognitive-behavioral | Weight (kg) | 6 | 308 | −1.95 | −3.26, −0.64 | 0.003 |
| BMI (kg/m2) | 6 | 308 | −0.64 | −1.14, −0.14 | 0.01 | |
| Nutrition and/or exercise | Wt (kg) | 8 | 413 | −3.78 | −4.57, −2.98 | <0.00001 |
| BMI (kg/m2) | 10 | 484 | −1.22 | −1.87, −0.56 | 0.003 | |
| Trial duration < / = 3 months | Weight (kg) | 7 | 314 | −3.23 | −4.04, −2.42 | <0.00001 |
| BMI (kg/m2) | 8 | 375 | −0.96 | −1.67, −0.25 | 0.008 | |
| Trial duration > 3 months | Weight (kg) | 7 | 407 | −2.96 | −5.09, −0.82 | 0.007 |
| BMI (kg/m2) | 8 | 417 | −0.89 | −1.58, −0.19 | 0.01 | |
| Prevention | Weight (kg) | 6 | 324 | −2.98 | −4.20, −1.76 | <0.00001 |
| BMI (kg/m2) | 5 | 306 | −0.96 | −1.62, −0.31 | 0.004 | |
| Intervention | Weight (kg) | 8 | 397 | −3.04 | −4.39, −1.68 | <0.00001 |
| BMI (kg/m2) | 11 | 486 | −0.89 | −1.59, −0.18 | 0.01 | |
| Group treatment | Weight (kg) | 9 | 501 | −2.82 | −4.58, −1.05 | 0.002 |
| BMI (kg/m2) | 12 | 590 | −0.66 | −1.29, −0.04 | 0.04 | |
| Individual treatment | Weight (kg) | 5 | 220 | −3.24 | −4.05, −2.44 | <0.00001 |
| BMI (kg/m2) | 4 | 202 | −1.40 | −2.16, −0.65 | 0.0003 | |
| Inpatients | Weight (kg) | 2 | 84 | −3.43 | −7.41, 0.55 | 0.09 |
| BMI (kg/m2) | 3 | 143 | 0.06 | −2.44, 2.57 | 0.96 | |
| Mixed (Both inpatients and outpatients) | Weight (kg) | 1 | 71 | −0.90 | −2.52, 0.72 | 0.28 |
| BMI (kg/m2) | 2 | 91 | −0.40 | −0.99, 0.20 | 0.20 | |
| Outpatients | Weight (kg) | 11 | 566 | −3.30 | −4.03, −2.58 | <0.00001 |
| BMI (kg/m2) | 11 | 596 | −1.29 | −1.81, −0.77 | <0.00001 |
There were numerically larger reductions for nutritional and/or exercise interventions compared to CBT regarding weight (−3.76kg (CI: −4.78, −2.74) vs. −1.95kg (−3.26, −0.64)) and BMI (−1.04 kg/m2 (−1.66, −0.42) vs. −0.64 kg/m2 (−1.14, −0.14)).
3.4. Maintenance Effects after Discontinuation of the Intervention
Across 5 studies with follow-up data after the end of the weight loss intervention, significantly greater weight loss persisted in favor of the intervention group compared to control group after a mean of 3.6 months (range: 2–12 months) (N=5, n=220, WMD=−3.48kg, CI: −6.37, −0.58, p=0.02; I2=4%) (Figure 5). However, the five studies which included BMI follow-up data (two of which were different from those reporting weight outcomes), did not show continued benefit for treatment over the control group (N=5 n=211 WMD= −0.72 kg/m2, CI: −2.36, 0.93, p=0.40, I2=53%) (Figure 5).
Figure 5:
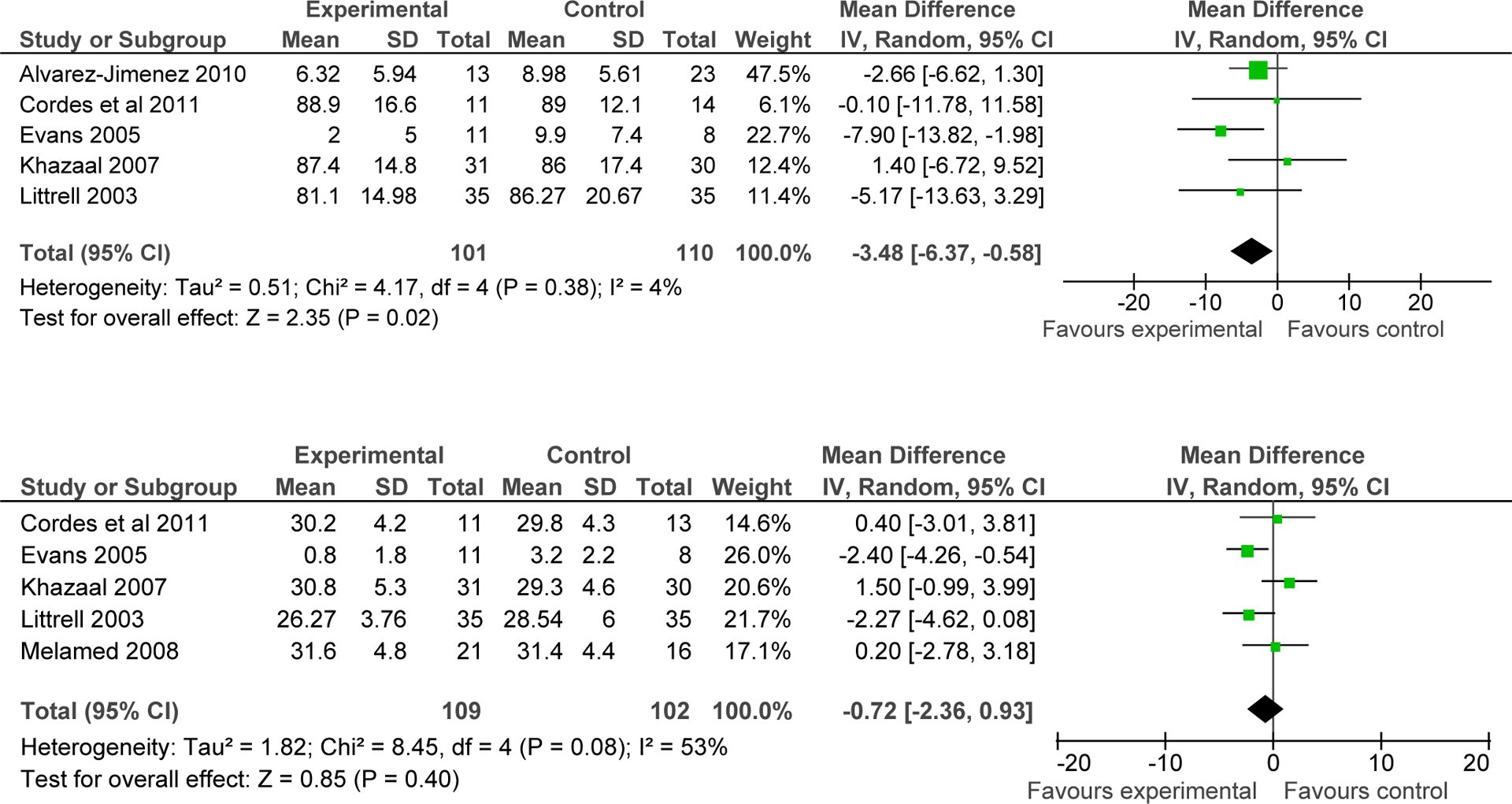
Differences in weight (kg) and body mass index (kg/m2) between behavioral interventions and control groups during follow up after the end of the intervention.
3.5. Publication Bias
Funnel plots for studies reporting on weight change (Supplemental Figure 1) or BMI change (Supplemental Figure 2) showed no evidence of publication bias.
4. DISCUSSION
In this to date largest meta-analysis of 17 studies, including 810 participants, non-pharmacologic interventions were significantly more effective than the respective control condition regarding the reduction in weight and all metabolic parameters, except for HDL-cholesterol and systolic blood pressure. The interventions resulted in 3.12 kg less weight gain and a 0.94 kg/m2 lower BMI unit increase compared to the control conditions. In addition, at least regarding body weight, beneficial effects endured for a mean of 3.6 months after the intervention ended. Although the same benefit was not shown for BMI, data on maintenance effects were relatively scarce, suggesting that additional studies are needed to further clarify the degree and duration of potentially enduring benefits of non-pharmacologic interventions beyond the active treatment phase.
Although study methodologies and samples differed, the magnitude of weight and BMI advantage for behavioral interventions was generally comparable to that achieved with the two available weight loss medications, metformin and topiramate, when added to antipsychotic medications. In a recent meta-analysis, metformin (N=7, n=334) was associated with 2.94 kg less weight gain and 1.36 kg/m2 less BMI increase than placebo (Maayan et al. 2010). For topiramate, the weight loss was 2.52 kg compared to placebo (Maayan et al., 2010). In addition, the NNT of 4 for less weight gain of at least 7% with behavioral interventions is also similar to the NNT of 3 for metformin (Maayan et al., 2010). However, the only direct head-to-head comparison of a non-pharmacologic intervention with metformin showed that metformin outperformed the behavioral intervention (−3.2 kg [95% CI: −3.9, −2.5] vs. −1.4 kg [95% CI: −2.0, −0.7]), while combined metformin and behavioral intervention was even more effective (−4.7 kg [95% CI: −5.7, −3.4]) (Wu et al. 2008). As this was an inpatient study and most non-pharmacologic intervention trials were conducted in outpatient settings, this research deserves replication.
In addition to the significant results regarding change in weight and BMI, our study demonstrated for the first time in a pooled meta-analysis that non-pharmacological interventions decrease waist circumference, total body fat percentage, glucose levels, insulin levels, total and LDL-cholesterol, and triglyceride levels compared to the control conditions. These differences are not only statistically, but also clinically relevant, especially for waist circumference and triglyceride changes, because of their role as cardiometabolic risk factors (Haffner, 2006). For example, insulin resistance and increased LDL-cholesterol are primary targets of coronary artery disease prevention (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, 2001). Unfortunately, only five studies measured HDL-cholesterol and two measured systolic blood pressure. This made it difficult to fully explore these factors and the results for these two metabolic outcomes were non-significant. However, it is also known that HDL-cholesterol is less readily affected by changes in weight, as exercise levels seem to affect HDL-cholesterol the most (Correll et al., 2011b). Nevertheless, it is crucial for future studies to examine the impact of interventions on all cardiometabolic factors. This should also include measures of insulin resistance, inflammatory markers and coagulation factors, which were generally not assessed.
Importantly, the superiority of behavioral interventions for antipsychotic-associated weight gain was independent of treatment modality, treatment duration, group versus individual treatment, and prevention versus intervention design. We cannot exclude that some of these factors may show differences, given larger groups, as a non-significant trend was observed in that nutritional interventions produced a slightly larger effect size than CBT. While this speaks favorably for simpler nutritional interventions, a closer analysis reveals overlapping aspects of each intervention. Specifically, components of CBT included psychoeducation, self-monitoring, teaching behavioral change strategies and/or cognitive restructuring. Nutritional and/or exercise interventions consisted of supervised exercise programs, psychoeducation regarding healthy lifestyles, and/or nutritionist and dietician consultations. However, many CBT programs also included dietary and even exercise management, while many of the nutritional and/or exercise programs utilized behavioral techniques, such as reinforcers to encourage compliance and self-monitoring. Therefore, the distinction between the two treatment modalities is not clearly demarcated, making comparisons difficult.
Treatment setting however, did play a role in outcome, as only outpatients, but not inpatients and mixed in- and outpatient samples, showed significant benefits from the behavioral interventions compared to the control conditions in the pooled analyses. This discrepancy could be due to the lower number of inpatient trials and participants compared to outpatient trials, reducing the power to find significant differences. Conversely, it is also possible that a more controlled food delivery in inpatient settings may confer advantages for the control condition. However, it is also possible that more acutely ill patients are less likely to comply with the behavioral intervention or that there are less opportunities for exercise during an inpatient stay.
Although our results suggest that behavioral treatments are potentially comparable to metformin and topiramate in reducing antipsychotic related weight gain, it needs to be borne in mind that this is an indirect comparison only and that patients agreeing to each of these interventions may differ. However, our results also show that individuals with schizophrenia and other psychiatric illnesses can respond well to behavioral weight loss programs and that interventions may not need to be much more complicated than nutritional counseling. This suggests further, that at least in individuals motivated to initiate a non-pharmacologic intervention program, non-pharmacologic interventions should be tried first, before attempting pharmacologic augmentation strategies. In fact, a recent 1-year study suggested that compared to the obese in the general population, psychiatric outpatients with psychotropic medication related obesity referred to a cognitive behavioral weight management program were less likely to drop out of that program, leading to greater weight loss, at least in last-observation-carried-forward analyses (Zhang et al., in press). In our analyses, the all-cause discontinuation rates were low in both the behavioral intervention and control groups (17% and 15%), almost 50% lower than the rates found for pharmacologic weight loss interventions in antipsychotic treated patients (23% and 22%, respectively) (Maayan et al., 2010). This finding underscores that there are at least subgroups of psychiatric patients in whom behavioral weight management approaches are acceptable and effective. For some patients, especially those in whom a switch to a lower risk antipsychotic (Mukundan et al., 2010; Stroup et al., 2011) may not be an option and who do not normalize cardiovascular risk markers sufficiently with non-pharmacologic interventions alone, combined treatment with a pharmacologic agent might also be an option (Wu et al., 2008).
The results of this meta-analysis have to be interpreted in light of its limitations. Although we were able to add another 7 RCTs (70.0%) and 328 patients (68.0%) to the previous meta-analysis, the number of studies and participants is still relatively small. In addition, studies only lasted between 8 and 24 weeks and while there were data to assess enduring maintenance effects for weight and BMI 2–12 months following the cessation of treatment, these came from only five studies for each of these outcomes. Moreover, while we were able to report on metabolic outcomes, our conclusions are clearly limited in that area because only 6/17 studies reported these and other secondary outcomes. Furthermore, there are no behavioral studies in youth, a group particularly vulnerable to antipsychotic weight gain (Correll et al., 2009; De Hert et al., 2011; Maayan and Correll, 2011). We were also not able to investigate the effect of diagnosis on outcome, as in 42.1% of patients diagnostic information was not available. In addition, the sensitivity analyses found that only outpatients benefitted from the intervention. This may be partially explained by the lack of insight and possible lower likelihood of agreement of some acutely ill patients, who are more likely to hospitalized, to participate in behavioral interventions. Therefore, motivational factors predicting agreement and success with behavioral weight loss interventions should be investigated in future studies. Further, treatment adherence was not formally assessed. Finally, relevant outcomes, including insulin resistance, inflammatory and coagulation factors, were not reported and only one study compared directly non-pharmacologic vs. pharmacologic weight loss interventions in patients treated with antipsychotics (Wu et al., 2008). Nevertheless, this is the largest meta-analysis of non-pharmacologic interventions for antipsychotic-induced weight gain and metabolic complications, an important area in the clinical care of patients requiring antipsychotics.
Future studies of behavioral interventions should include larger samples, last longer and assess participants’ maintenance of weight loss after cessation of the intervention, possibly compared against infrequent booster sessions. Maintenance studies also need to focus on metabolic outcomes. Moreover, a broader range of cardiometabolic risk markers, as well as mechanisms and predictors of weight loss should also be investigated. In addition, it would be valuable to assess if an improvement in aspects of the physical health of those affected by antipsychotic-associated weight gain has a positive effect on psychiatric outcomes. As mentioned above, more studies are needed in high-risk samples, such as first episode and pediatric patients, and motivational factors predicting or preventing successful participation in behavioral weight management programs for antipsychotic related weight gain require investigation. Fianlly, studies are needed that directly compare the effectiveness of pharmacological, non-pharmacological, and combined treatments. At a minimum, more placebo-controlled, pharmacologic intervention trials should have a behavioral intervention arm for comparison in order to further inform clinical practice.
Supplementary Material
Acknowledgements:
The authors wish to thank Allison Larr of the Nathan Kline Institute and Constance Moussouris of the Columbia University Postbaccalaureate Premedical Program for their assistance with the preparation of this manuscript.
Conflict of Interest:
Mrs. Caemmerer has nothing to disclose.
Dr. Maayan has received research support from Eli Lilly and Pfizer.
Dr. Correll has been a consultant and/or advisor to or has received honoraria from: Actelion, Alexza; AstraZeneca, Biotis, Bristol-Myers Squibb, Cephalon, Desitin, Eli Lilly, GSK, IntraCellular Therapies, Lundbeck, Medavante, Medscape, Merck, Novartis, Ortho-McNeill/Janssen/J&J, Otsuka, Pfizer, ProPhase, and Sunovion. He has received grant support from BMS, Feinstein Institute for Medical Research, Janssen/J&J, National Institute of Mental Health (NIMH), National Alliance for Research in Schizophrenia and Depression (NARSAD), and Otsuka.
ROLE OF FUNDING SOURCE:
Supported in parts by The Zucker Hillside Hospital National Institute of Mental Health (NIMH) Advanced Center for Intervention and Services Research for the Study of Schizophrenia (MH MH090590) and by the Stanley Medical Research Institute Award 07TGF-1112. The NIMH and Stanley Medical Research Institute had no further role in study design, collection, analysis, interpretation of data, writing of the report, and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allison DB, Mackell JA, McDonnell DD 2003. The impact of weight gain on quality of life among persons with schizophrenia. Psychiatr. Serv 54 (4) 565–567. [DOI] [PubMed] [Google Scholar]
- Allison DB, Mentore JL, Heo M et al. 1999. Antipsychotic-induced weight gain: A comprehensive research synthesis. Am J Psychiatry. 156 (11) 1686–1696. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jimenez M, Martinez-Garcia O, Perez-Iglesias R, Ramirez ML, Vazquez-Barquero JL, Crespo-Facorro B, 2010. Prevention of Antipsychotic-Induced Weight Gain with Early Behavioral Intervention in First Episode Psychosis: 2-Year Results if a Randomized Controlled Trial. Schizophrenia Research 116 16–19. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jimenez M, Hetrick SE, Gonzalez-Blanch C, Gleeson JF, McGorry PD, 2008. Non-pharmacological management of antipsychotic-induced weight gain: Systematic review and meta-analysis of randomised controlled trials. Br. J. Psychiatry 193 101–07. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jimenez M, Gonzalez-Blanch C, Vazquez-Barquero JL, Perez-Iglesias R, Martinez-Garcia O, Perez-Pardal T, Ramirez-Bonilla ML, Crespo-Facorro B, 2006. Attenuation of antipsychotic-inducted weight gain with early behavioral intervention in drug-naïve first-episode psychosis patients: A randomized controlled trial, J. Clin. Psychiatry 67 1253–1260. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity, 2004. Consensus development conference on antipsychotic drugs and obesity and diabetes. J. Clin. Psychiatry 65(2) 267–272. [DOI] [PubMed] [Google Scholar]
- Beebe LH, Tian L, Morris N, Goodwin A, Allen SS, Kuldau J, 2005. Effects of exercise on mental and physical health parameters of persons with schizophrenia. Issues in Ment. Health Nurs 26 661–676. [DOI] [PubMed] [Google Scholar]
- Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R, 2005. Effects of behavioral therapy on weight loss in overweight and obese patients with schizophreniaor schizoaffective disorder. J. Clin. Psychiatry 66, 205–212. [DOI] [PubMed] [Google Scholar]
- Colton CW, Manderscheid RW, 2006. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev. Chronic Dis 3 (2) A42. [PMC free article] [PubMed] [Google Scholar]
- Cordes J, Thunker J, Regenbrecht JZ, Correll CU, Schmidt-Kraepelin C, Lange-Asschenfeldt C, Agelink MW, Kahl KG, Gaebel W, Klimke A, Hauner H, In Press. Can an early weight management program (WMP) prevent olanzapine-induced disturbances in body weight, blood glucose and lipid metabolism? Twenty-four and 48-week results from a 6-month randomized trial. World J. of Biol. Psychia [DOI] [PubMed] [Google Scholar]
- Correll CU, Sheridan EM, DelBello MP 2010. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 12 (2): 116–141. [DOI] [PubMed] [Google Scholar]
- Correll CU, Kratochvil CJ, March J, 2011a. Developments in Pediatric Psychopharmacology: Focus on Stimulants, Antidepressants and Antipsychotics. J Clin Psychiatry. 72 (5):655–670. [DOI] [PubMed] [Google Scholar]
- Correll CU, Lencz T, Malhotra AK, 2011b. Antipsychotic drugs and obesity. Trends Mol Med. 17 (2) 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, Detraux J, Gautam S, Möller HJ, Ndetei DM, Newcomer JM, Uwakwe R, Leucht S, 2011a. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 10 (1) 52–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Detraux J, van Winkel R, Yu W, Correll CU, 2011b. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 8( 2) 114–26. [DOI] [PubMed] [Google Scholar]
- Evans S, Newton R, Higgins S, 2005. Nutritional intervention to prevent weight gain in patients commenced on olanzapine: A randomized controlled trial. Aust. N Z J. Psychiatry 39 479–486. [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. 2001. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol In Adults (adult treatment panel III). JAMA 285 (19) 2486–97. [DOI] [PubMed] [Google Scholar]
- Gabriele JM, Dubbert PM, Reeves RR, 2009. Efficacy of behavioural interventions in managing atypical antipsychotic weight gain. Obes. Rev 10 442–455. [DOI] [PubMed] [Google Scholar]
- Haffner SM, 2006. Relationship of metabolic risk factors and development of cardiovascular disease and diabetes. Obesity. 14 121–127. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Fleischhacker WW, Boter H et al. 2008. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: An open randomised clinical trial. Lancet. 371 (9618) 1085–1097. [DOI] [PubMed] [Google Scholar]
- Khazaal Y, Fresard E, Rabia S, Chatton A, Rothen S, Pomini V, Grasset F, Borgeat F, Zullino D, 2007. Cognitive behavioural therapy for weight gain associated with antipsychotic drugs. Schizophr. Res 169–177. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Choi JS, Bahk WM, Kim CY, Kim CH, Shin YC, Park BJ, Oh CG, 2006. Weight management program from treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: A 12-week randomized controlled clinical trial. J. Clin. Psychiatry 67 547–553. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP et al. , 2005. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med 353 (12) 1209–1223. [DOI] [PubMed] [Google Scholar]
- Leucht S, Arbter D, Engel RR, Kissling W, Davis JM, 2009. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 14 (4) 429–47. [DOI] [PubMed] [Google Scholar]
- Littrell KH, Hilligoss NM, Kirshner CD, Petty RG, Johnson CG, 2003. The effects of an educational intervention on antipsychotic-induced weight gain. J. of Nurs. Scholarship 3 (23) 7–241. [DOI] [PubMed] [Google Scholar]
- Maayan L, Correll CU, Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. 2011, J. Child and Adol. Psychopharmacology;21(6):517–35. [DOI] [PubMed] [Google Scholar]
- Maayan L, Correll CU, 2010. Management of antipsychotic-related weight gain. Expert Rev. of Neurother 10 175–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan L, Vakhrusheva J, Correll CU, 2010. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: A systematic review and meta-analysis. Neuropsychopharmacology. 35 1520–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher AR, Maglione M, Bagley S, Suttorp M, Hu JH, Ewing B, Wang Z, Timmer M, Sultzer D, Shekelle PG, 2011. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA 306 (12) 1359–69. [DOI] [PubMed] [Google Scholar]
- Mauri M, Simnocini M, Castrogiovianni S, Iovieno N, Dell’Agnello G, Quadrigli M, Rossi A, Donda P, Fagiolini A, Cassano GB, 2008. A psychoeducational program for weight loss in patients who have experienced weight gain during antipsychotic treatment with olanzapine. Pharmacopsychiatry. 41 17–23. [DOI] [PubMed] [Google Scholar]
- McKibbin CL, Patterson TL, Norman G, Patrick K, Jin H, Roesch S, Mudaliar S, Barrio C, O’Hanlon K, Griver K, Sirkin A, Jeste DV, 2006. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: A randomized controlled trial. Schizophr. Res 86 36–44. [DOI] [PubMed] [Google Scholar]
- Melamed Y, Stein-Reisner O, Gelkopf M, Levi G, Sivan T, Ilievici G, Rosenberg R, Weizman A, Bleich A, 2008. Multi-modal weight control intervention for people with persistent mental disorders. Psychiatr. Rehabil. J 31 194–200. [DOI] [PubMed] [Google Scholar]
- Mukundan A, Faulkner G, Cohn T, Remington G, 2010. Antipsychotic switching for people with schizophrenia who have neuroleptic-induced weight or metabolic problems. Cochrane Database Syst Rev. (12): CD006629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narula PK, Rehan HS, Unni KE, Gupta N 2010. Topiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: A double-blind, placebo-controlled trial. Schizophr. Res 118 (1–3) 218–223. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI., 2009. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 166 (9):980–91. [DOI] [PubMed] [Google Scholar]
- Newcomer JW 2005. Second-generation (atypical) antipsychotics and metabolic effects: A comprehensive literature review. CNS Drugs. 19 (Suppl. 1) 1–93. [DOI] [PubMed] [Google Scholar]
- Parks J, Svendsen D, Singer P, Forti M, 2006. Morbidity and Mortality in People with Serious Mental Illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council. [Google Scholar]
- Poulin MJ, Chaput JP, Simard V, Vicent P, Bernier J, Gauthier Y, Lanctot G, Saindon J, Vincent A, Gagnon S, Tremblay A, 2007. Management of antipsychotic-induced weight gain: Prospective naturalistic study of the effectiveness of a supervised exercise programme. Aust. N Z J. Psychiatry 41, 980–989. [DOI] [PubMed] [Google Scholar]
- Scocco P, Longo R, Caon F, 2006. Weight change in treatment with olanzapine and a psychoeducational approach. Eat. Behav 7, 115–124. [DOI] [PubMed] [Google Scholar]
- Skrinar GS, Huxley NA, Hutchinson DS, Menninger E, Glew P, 2005. The role of a fitness intervention on people with serious psychiatric disabilities. Psychiatr. Rehabil. J 29, 122–127. [DOI] [PubMed] [Google Scholar]
- Stroup TS, McEvoy JP, Ring KD, Hamer RH, LaVange LM, Swartz MS, Rosenheck RA, Perkins DO, Nussbaum AM, Lieberman JA; Schizophrenia Trials Network. 2011. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 168 (9) 947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Wyne K, 2006. A cognitive/behavioral group intervention for weight loss in patients treated with atypical antipsychotics. Schizophr. Res 83 95–101. [DOI] [PubMed] [Google Scholar]
- Wu RR, Zhao JP, Jin H, Fang MS, Guo XF, et al. , 2008. Lifestyle intervention and metformin for treatment of anti-psychotic induced weight gain: A randomized controlled trial. JAMA. 185–193. [DOI] [PubMed] [Google Scholar]
- Wu MK, Wang CK, Bai YM, Huang CY, Lee SD, 2007. Outcomes of obese, clozapine-treated inpatients with schizophrenia placed on a six-month diet and physical activity program. Psychiatr. Serv 58 544–550. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Weiss J, McCardle M, Kofman M, Rosendahl E, Maayan L, Convit A, Manu P, Kane JM, Correll CU, In Press. Effectiveness of a cognitive behavioral weight management intervention in obese patients with psychotic disorders compared to patients with non-psychotic disorders or no psychiatric disorders: Results from a 12-month, real-world study. J Clin Psychopharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


