Abstract
Study Design:
Retrospective cohort study.
Objectives:
The impact of modifiable risk factors (MRFs) on complications, costs, and readmission rates at 30, 90, and 180-days following lumbar spine fusion.
Methods:
Patients with lumbar spine fusions within the 2016-2017 Nationwide Readmissions Database (NRD). Patients were stratified by the following MRFs: Alcohol use, tobacco/nicotine use, nutritional malnourishment, dyslipidemia, and primary hypertension. Differences in complications, non-elective readmission rates, costs, and length of stay were compared between MRFs and the non-MRF group. Statistical analysis was conducted using Tukey multiple comparisons of means, 1-way ANOVA, Wald testing, unpaired Welch 2-sample t-tests, multivariate analysis, and predictive modeling.
Results:
The final analysis included 297,579 lumbar fusion patients. At 30 and 90 days, patients with nutritional malnutrition, dyslipidemia, and primary hypertension had significantly greater readmission rates than patients without MRFs (all P<0.01). At 180-days, all MRFs had significantly greater readmission rates than the non-MRF group (all P<0.001). Dyslipidemia demonstrated significantly greater rates of myocardial infarction at 90 days compared to all groups (all P<0.02). Nutritional malnutrition was associated with a significantly greater mortality rate than primary hypertension, non-MRF, and tobacco/nicotine use at 90 days (P<0.001) and only tobacco/nicotine use at 180 days (P=0.007). Predictive modeling showed increases of 0.77%, 1.70%, and 2.44% risk of readmission at 30, 90, and 180-days respectively per additional MRF (all P<0.001).
Conclusions:
These findings highlight the negative impact each MRF has on patients following lumbar spinal fusion. Further longitudinal research is necessary to comprehensively characterize the effects of various MRFs on spine surgery outcomes.
Keywords: modifiable risk factors, lumbar, predictive model, spinal fusion, postoperative, complications
Introduction
Complications and readmissions following spine surgery are chief concerns shared between hospitals, surgeons, and patients alike. In 2010, a systematic review of 105 spine surgery studies containing a total 79,471 patients found an overall complication rate of 16.4%. 1 These unexpected setbacks contribute heavily to cost and clinical burdens for all, especially patients.
From 2001 to 2010, the cost of spine fusion-related admissions increased from $13.3 billion to $49.9 billion with a significant portion of costs drawing from complications and readmissions. 2 Surgical site infections (SSI), which are considered the most common complication following lumbar spine fusion, average an overall direct cost of $15,817 per SSI. 3 Furthermore, Patil et al. found substantial increases in hospital charges, length of stay (LOS), and mortality for patients by number of inpatient complications during spinal fusion for adult spinal deformity. 4 Such complications often leave a lasting impact by conferring negative psychosocial outcomes in patients. 5
Readmissions exhibited a similar effect in producing exorbitant charges, with unplanned readmissions accounting for $17.4 billion of $102.6 billion Medicare payments in 2004. 6 Likewise, readmissions negatively affect patient outcomes and must be attenuated to optimize surgical efficacy. 7 Therefore, reducing complications and readmissions to curb costs and improve patient outcomes for all procedures remains a nation-wide priority.
Patient characteristics play a multifactorial role in influencing complication and readmission rates. Understanding the extent to which risk factors might negatively impact outcomes is essential to determining patients’ eligibility for surgery and guide medical optimization strategies to limit adverse outcomes. For example, the associations between increased age and diabetes with increased complication and readmission rates have been well-established in lumbar fusions.8,9
While understanding this relationship could help providers postpone surgeries that would otherwise lead to poor outcomes, the fixed and often progressive nature of many well-studied risk factors leave patients without a solution. Thus, further understanding of modifiable risk factors (MRFs) not only helps providers better weigh the risks and benefits associated with surgical treatment and quantify the relative impact of MRFs, but also offers patients a role in optimizing their outcomes. To date, no study has been performed with a specified focus on MRFs, comparing each as independent predictors of postoperative morbidity and mortality in lumbar spinal fusion. Therefore, the present study analyzes differences in several MRFs as independent predictors of complications, readmissions, and costs, following lumbar spine fusion.
Methods
Data Source
The Nationwide Readmissions Database (NRD) is an annually published database within the Healthcare Cost and Utilization Project (HCUP) which encompasses approximately 18 million inpatient discharges across the United States. The database provides nationally representative information on inpatient hospital stays, readmissions, and demographic features. All diagnoses and procedures per admission are documented using the International Classification of Diseases, Tenth Revision (ICD-10) codes. To preserve granularity in our analysis, NRD years prior to 2016 were not incorporated due to the use of nonspecific ICD-9 coding in 2015 and earlier. Institutional review board approval was not required for this study as patient data within the NRD is de-identified.
Patient Selection
Patients who had received an elective lumbar spine fusion (n=454,070) from the 2016-2017 NRD were identified using ICD-10 codes. Patients were then stratified into the following 5 MRF groups: Alcohol use, tobacco/nicotine use, nutritional malnutrition, dyslipidemia, and primary hypertension. ICD-10 codes for MRFs were confirmed through comparison with prior studies analyzing MRFs. 10 Nutritional malnutrition, which included nutritional, caloric, vitamin, or mineral deficiencies, was defined using the corresponding ICD-10 codes established by the Centers for Medicare and Medicaid Services.11,12 Exclusion criteria included surgical indications for relevant trauma and neoplasms. Additionally, patients with nutritional malnutrition and dyslipidemia secondary to common non-modifiable disorders (Crohn’s disease, celiac disease, familial hypercholesterolemia, etc.) were excluded. All ICD-10 codes used to define MRFs as well as exclusion codes are listed in Table 1 . Demographic characteristics and inpatient hospitalization information were recorded for all groups ( Table 2 ).
Table 1.
ICD-10 Inclusion and Exclusion Codes for MRF Groups.
| Modifiable risk factors | ICD-10 Codes |
|---|---|
| Alcohol Use | |
| F1010, F10120, F10121, F10129, F1014, F10150, F10151, F10159, F10180, F10181, F10182, F10188, F1019, F1020, F10220, F10221, F10229, F10230, F10231, F10232, F10239, F1024, F10250, F10251, F10259, F1026, F1027, F10280, F10281, F10282, F10288, F1029, F10920, F10921, F10929, F1094, F10950, F10951, F10959, F1096, F1097, F10980, F10981, F10982, F10988, F1099 | |
| Tobacco/Nicotine Use | |
| Z720, F17200, F17208, F17209, F17210, F17213, F17218, F17219, F17220, F17223, F17228, F17229, F17290, F17293, F17298, F17299 |
|
| Malnourishment | |
| E43, E440, E441, E46, E500, E501, E502, E503, E504, E505, E506, E507, E508, E509, E5111, E5112, E512, E518, E519, E52, E530, E531, E538, E539, E534, E550, E559, E560, E561, E568, E569, E58, E59, E60, E610, E611, E612, E613, E614, E615, E616, E617, E618, E619, E630, E631, E638, E639, E640, E641, E642, E643, E648, E649, D530, D531, D532, D538, D539 | |
| Hyperlipidemia | |
| E7800, E782, E783, E781, E7841, E7849, E785 | |
| Primary Hypertension | |
| I10 | |
| Lumbar Spine Fusion Cohort | |
| 0SG0070, 0SG0071, 0SG007 J, 0SG00A0, 0SG00AJ, 0SG00J0, 0SG00J1, 0SG00JJ, 0SG00K0, 0SG00K1, 0SG00KJ, 0SG0370, 0SG0371, 0SG037 J, 0SG03A0, 0SG03AJ, 0SG03J0, 0SG03J1, 0SG03JJ, 0SG03K0, 0SG03K1, 0SG03KJ, 0SG0470, 0SG0471, 0SG047 J, 0SG04A0, 0SG04AJ, 0SG04J0, 0SG04J1, 0SG04JJ, 0SG04K0, 0SG04K1, 0SG04KJ, 0SG1070, 0SG1071, 0SG107 J, 0SG10A0, 0SG10AJ, 0SG10J0, 0SG10J1, 0SG10JJ, 0SG10K0, 0SG10K1, 0SG10KJ, 0SG1370, 0SG1371, 0SG137 J, 0SG13A0, 0SG13AJ, 0SG13J0, 0SG13J1, 0SG13JJ, 0SG13K0, 0SG13K1, 0SG13KJ, 0SG1470, 0SG1471, 0SG147 J, 0SG14A0, 0SG14AJ, 0SG14J0, 0SG14J1, 0SG14JJ, 0SG14K0, 0SG14K1, 0SG14KJ, 0SG3070, 0SG3071, 0SG307 J, 0SG30A0, 0SG30AJ, 0SG30J0, 0SG30J1, 0SG30JJ, 0SG30K0, 0SG30K1, 0SG30KJ, 0SG3370, 0SG3371, 0SG337 J, 0SG33A0, 0SG33AJ, 0SG33J0, 0SG33J1, 0SG33JJ, 0SG33K0, 0SG33K1, 0SG33KJ, 0SG3470, 0SG3471, 0SG347 J, 0SG34A0, 0SG34AJ, 0SG34J0, 0SG34J1, 0SG34JJ, 0SG34K0, 0SG34K1, 0SG34KJ | |
| Exclusion Codes | ICD-10 Codes |
| Malabsorptive Disorders | |
| K900, K901, K902, K903, K9041, K9049, K9081, K9089, K909, K5000, K50011, K50012, K50013, K50014, K50018, K50019, K5080, K50811, K50812 K50813, K50814, K50818, K50819, K5090, K50911, K50912, K50913, K50914, K50918, K50919, D510, Z90410, Z90411, Z9049, Z903, K861, K8681 | |
| Lipid Metabolism Disorders | |
| E7870, E7801, E7871, E7872, E7879 | |
| Malignancy | |
| C412, C414, D166, D168 | |
| Trauma | |
| S32002A, S32002B, S32002D, S32002G, S32002 K, S32002 S, S32012A, S32012B, S32012D, S32012G, S32012 K, S32012 S, S32022, S32022B, S32022D, S32022G, S32022 K, S32022 S, S32032A, S32032B, S32032D, S32032G, S32032 K, S32032 S, S32042A, S32042B, S32042D, S32042G, S32042 K, S32042 S, S32052A, S32052B, S32052D, S32052G, S32052 K, S32052 S, S32009A, S32009B, S32009D, S32009G, S32009 K, S32009 S, S32019A, S32019B, S32019D, S32019G, S32019 K, S32019 S, S32029A, S32029B, S32029D, S32029G, S32029 K, S32029 S, S32039A, S32039B, S32039D, S32039G, S32039 K, S32039 S, S32049A, S32049B, S32049D, S32049G, S32049 K, S32049 S, S32059A, S32059B, S32059D, S32059G, S32059 K, S32059 S, S32008A, S32008B, S32008D, S32008G, S32008 K, S32008 S, S32018A, S32018B, S32018D, S32018G, S32018 K, S32018 S, S32028A, S32028B, S32028D, S32028G, S32028 K, S32028 S S32038A, S32038B, S32038D, S32038G, S32038 K, S32038 S, S32048A, S32048B, S32048D, S32048G, S32048 K, S32048 S, S32058A, S32058B, S32058D, S32058G, S32058 K, S32058 S, S32001A, S32001B, S32001D, S32001G, S32001 K, S32001 S, S32019A, S32011B, S32011D, S32011G, S32011 K, S32011 S, S32021A, S32021B, S32021D, S32021G, S32021 K, S32021 S, S32031A, S32031B, S32031D, S32031G, S32031 K, S32031 S, S32041A, S32041B, S32041D, S32041G, S32041 K, S32041 S, S32051A, S32051B, S32051D, S32051G, S32051 K, S32051S | |
Table 2.
Demographic and Hospitalization Information.
| Control (n=139,864) | Alcohol use (n=707) | Tobacco/ nicotine use (n=22,715) | Malnutrition (n=3,533) | Dyslipidemia (n=37,171) | Primary hypertension (n=93,589) | |
|---|---|---|---|---|---|---|
| Mean Age (years), ± SD | 51.4 + 18.5 | 54.2 + 14.2 | 49.3 + 12.2 | 52.7 + 21.2 | 65.7 + 10.8 | 63.0 + 11.5 |
| Sex | ||||||
| Female, n (%) | 81,700, 58.4% | 269, 38.1% | 11,527, 50.7%, | 2,267, 64.2% | 19,435, 52.3% | 52,810, 56.4% |
| Male, n (%) | 58,164, 41.6% | 438, 61.9% | 11,188, 49.3% | 1,266, 35.8% | 17,736, 47.7% | 40,779, 43.6%, |
| Charlson Comorbidity Index Score, mean ± SD | 2.1 + 1.6 | 2.3 + 1.7 | 1.7 + 1.4 | 2.6 + 1.9 | 3.6 + 1.5 | 3.2 + 1.3 |
| Hospital type | ||||||
| Metropolitan non-teaching, n (%) | 29,749, 21.3% | 202, 28.6% | 5,544, 24.4% | 508, 14.4% | 8,198, 22.1% | 23,230, 24.8% |
| Metropolitan teaching, n (%) | 106,286, 76% | 502, 71% | 16,286, 71.7% | 2,931, 83% | 27,840, 74.9% | 67,056, 71.6% |
| Non-metropolitan, n (%) | 3,829, 2.74% | 3, 0.42% | 886, 3.9% | 95, 2.69% | 1,133, 3.05% | 3,303, 3.53% |
| Discharge location | ||||||
| Routine/Home, n (%) | 101,430, 72.5% | 425, 60.1% | 17,149, 75.5% | 1,765, 50% | 21,017, 56.5% | 57,689, 61.6% |
| Short-term hospital, n (%) | 288, 0.21% | 2, 0.28% | 42, 0.18% | 38, 1.08% | 85, 0.23% | 213, 0.23% |
| Skilled nursing facility, n (%) | 13,354, 9.54% | 128, 18.1% | 1,537, 6.77% | 841, 23.8% | 7,347, 19.8% | 14,868, 15.9% |
| Home health care, n (%) | 24,497, 17.5% | 145, 20.5% | 3,912, 17.2% | 849, 24% | 8,610, 23.2% | 20,640, 22.1% |
| Levels of fusion | ||||||
| Single-level fusion, n (%) | 98,285, 70.3% | 462, 65.3% | 17,637, 77.6% | 2,021, 57.2% | 24,547, 66% | 62,951, 67.3% |
| Multi-level fusion, n (%) | 41,573, 29.7% | 243, 34.4% | 5,079, 22.4% | 1,509, 42.7% | 12,622, 34% | 30,633, 32.7% |
Statistical Analysis
Statistical analysis was conducted using Rstudio. Differences in complications, readmission rates, costs, and LOS were compared across all MRFs using Tukey multiple comparisons of means, 1-way ANOVA, Wald testing, and unpaired Welch 2-sample t-tests. Univariate analysis was used to determine significant correlations and variables for multivariate analysis, which was then used to confirm statistical significance after controlling for confounds. Using a binomial regression model, multivariate logistic analysis was performed using the ‘glm’ function to determine the relationship between each MRF and several different complication and readmission rates, all of which were established as dependent variables when comparing between MRFs. MRF group was set as the primary independent variable while established confounders such as age, sex, and diabetes mellitus status were set as covariates. Post-hoc odds ratios, 95% confidence intervals, and P-values were calculated to determine statistical significance.
The large sample size of lumbar spinal fusion patients included in our analysis normalizes discrepancies between cohorts and increases the power of our statistical analysis. Significance was defined as alpha < 0.05.
Predictive Modeling
Predictive models were constructed using generalized gaussian linear regression models and were visually represented as dose-response curves to demonstrate the effect of additional MRFs on readmission rates. Wald testing was performed to assess the effect of the weighted distance between the estimated value and the hypothesized true value under the null hypothesis on the statistical parameters within the models.
Results
Demographic and Hospitalization Characteristics
A total of 297,579 patients (56.5% female, 43.5% male) who underwent lumbar spinal fusion (69% single-level, 31% multi-level) from 2016-2017 were included in the final analysis. The mean age for the overall cohort was 56.72 + 16.53 years. Of these patients, 22.66% were admitted to a metropolitan non-teaching hospital, 74.23% to a metropolitan teaching hospital, and 3.11% to a non-metropolitan hospital. Additionally, a majority of patients received a routine/home discharge (67.03%) while the remaining were either discharged to a short-term hospital (0.22%), skilled nursing facility (12.79%), or with home health care (19.71%). The demographic and hospitalization information stratified by MRF groups is depicted in Table 2 .
Readmissions, Complications, LOS, and Cost,
The overall cumulative readmission rates at 30, 90, and 180-days were 5.46%, 9.23%, and 12.93% respectively. At the 30-day interval, significant differences in readmission rates were found when comparing nutritional malnutrition (OR: 1.35, 95%CI: 1.10 -1.64, P=0.003), dyslipidemia (OR: 1.11, 95%CI: 1.03 -1.19, P=0.005), and primary hypertension (OR: 1.07, 95%CI: 1.02 -1.14, P=0.010) against the non-MRF group. Similarly, at 90-days, readmission rates were significantly different between nutritional malnutrition (OR: 1.72, 95%CI: 1.46-2.02, P<0.001), dyslipidemia (OR: 1.14, 95%CI: 1.06 -1.21, P<0.001), primary hypertension (OR: 1.10, 95%CI: 1.05 -1.16, P<0.001) and the non-MRF group. Within MRF groups, nutritional malnutrition had significantly greater 90-day readmission rates compared to tobacco/nicotine use (OR: 1.47, 95%CI: 1.21 -1.75, P<0.001). With further stratification, nutritional malnutrition was found to have 90-day readmission rates significantly greater than primary hypertension (OR: 1.55, 95%CI: 1.31 -1.83, P<0.001) and dyslipidemia (OR: 1.56, 95%CI: 1.31 -1.86, P<0.001). Significant differences in 180-day readmission rates were found between all MRF groups and the non-MRF group (11.01%) as shown: alcohol use (OR: 1.86, 95%CI: 1.26-2.67, P=0.001), tobacco/nicotine use (OR:1.27, 95%CI: 1.17 -1.38, P<0.001), nutritional malnourishment (OR: 1.82, 95%CI: 1.52-2.17, P<0.001), dyslipidemia (OR: 1.15, 95%CL: 1.07 -1.23, P<0.001), and primary hypertension (OR: 1.13, 95%CI: 1.07 -1.18, P<0.001). Amongst MRF groups, 180-day readmission rates continued to be significantly greater in nutritional malnutrition (OR: 1.49, 95%CI: 1.22 -1.80, P<0.001) and primary hypertension (OR:1.13, 95%CI: 1.03 -1.24, P=0.007) compared to tobacco/nicotine use. Delving further, nutritional malnutrition was associated with significantly greater readmission rates at 180 days than primary hypertension (OR: 1.61, 95%CI: 1.34 -1.92, P<0.001) and dyslipidemia (OR: 1.59, 95%CI: 1.31 -1.91, P<0.001).
Complication rates were analyzed in correspondence to readmissions — at 30, 90, and 180-days. At 30-days, dyslipidemia was associated with significantly greater rates of readmission for myocardial infarction (MI) and acute kidney injury (AKI) compared to the non-MRF group (OR: 2.13, 95%CI: 2.00-2.28, P<0.001) (OR:2.34, 95%CI: 2.11-2.59, P<0.001), primary hypertension (OR: 1.59, 95%CI: 1.31 -1.91, P<0.001) (OR:2.66, 95%CI: 2.41-2.94, P<0.001), and tobacco/nicotine use (OR: 1.56, 95%CI: 1.38 -1.76, P<0.001) (OR: 2.79, 95%CI: 2.21-3.56, P<0.001) groups. Primary hypertension was associated with significantly greater rates of AKI relative to the tobacco/nicotine use (OR: 1.38, 95%CI: 1.10 -1.76, P=0.007). Malnourished patients also experienced greater rates of AKI at 30-day readmission compared to patients with tobacco/nicotine use (OR: 4.76, 95%CI: 3.45-6.57, P<0.001).
At 90-days, dyslipidemia was shown to have significantly greater rates of MI against all MRF groups: non-MRF (OR: 2.17, 95%CI: 2.01-2.34, P<0.001), primary hypertension (OR: 1.80, 95%CI: 1.67 -1.94, P<0.001), tobacco/nicotine use (OR: 1.59, 95%CI: 1.38 -1.83, P<0.001), alcohol use (OR: 5.02, 95%CI: 2.30-14.16, P<0.001), and nutritional malnutrition (OR: 1.35, 95%CI: 1.07 -1.74, P=0.016). Dyslipidemia continued to demonstrate significantly greater rates of AKI at 90 days in comparison to the non-MRF group (OR: 2.50, 95%CI: 2.23-2.80, P<0.001), primary hypertension (OR: 2.81, 95%CI: 2.51-3.13, P<0.001), and tobacco/nicotine use (OR: 2.88, 95%CI: 2.22-3.77, P<0.001). Similarly, primary hypertension was associated with significantly greater rates of AKI at 90 days compared to the non-MRF group (OR: 1.73, 95%CI: 1.58 -1.90, P<0.001) tobacco/nicotine use cohorts (OR: 1.40, 95%CI: 1.08 -1.82, P=0.010). Nutritional malnutrition demonstrated a significantly greater mortality rate at 90-day readmission relative to the non-MRF group (OR: 8.16, 95%CI: 4.45-14.05, P<0.001), tobacco/nicotine use (OR: 7.77, 95%CI: 2.89-22.45, P<0.001), and primary hypertension (OR: 12.33, 95%CI: 6.57-21.93, P<0.001).
Readmission at 180 days continued to demonstrate significantly higher rates of MI for dyslipidemia in relation to the following groups: non-MRF (OR: 2.18, 95%CI: 1.98-2.40, P<0.001), primary hypertension (OR: 1.73, 95%CI: 1.58 -1.90, P<0.001), tobacco/nicotine use (OR: 1.56, 95%CI: 1.32 -1.85, P<0.001), and alcohol use (OR: 2.50, 95%CI: 2.52-47.96, P<0.001). As with previous readmissions, dyslipidemia was associated with significantly higher rates of AKI in comparison to the non-MRF group (OR: 2.72, 95%CI: 2.37-3.13, P<0.001) and primary hypertension (OR: 2.96, 95%CI: 2.59-3.38, P<0.001). At 180-day readmission, only the malnourished group was shown to have significantly greater mortality compared to tobacco/nicotine use (OR: 5.99, 95%CI: 1.63-23.02, P=0.007). No statistically significant differences in thromboembolic events, hematoma formation, wound disruption, neurological injury, or hardware failure were found between any of the groups for any readmission.
The average LOS and cost of index admission was significantly different across all groups (all P<0.001) except when solely comparing cost between dyslipidemia and the non-MRF group. The malnourished group had by far the highest average LOS and cost of index admission (9.07 + 13.29 days, $59,936 + $57,341) with alcohol use following after (6.53 + 8.32 days, $45,279 + $39 726). Readmission, complication, and LOS and cost data are listed in Table 3 .
Table 3.
30-day, 90-Day, 180-Day Complication Rates, Length of Stay, and Readmission Rates.
| 30-days | Control (n=125,527) |
Alcohol use (n=657) |
Tobacco/nicotine use (n=20,531) |
Malnutrition (n=3,183) |
Dyslipidemia (n=33,121) |
Primary hypertension (n=85,103) |
|---|---|---|---|---|---|---|
| Complications, n (%) | ||||||
| Myocardial infarction | 475, 0.39% | 0, 0% | 111, 0.54% | 210, 0.79% | 337, 1.02% | 403, 0.47% |
| Acute renal failure | 564, 0.45% | 3, 0.46% | 49, 0.24% | 25, 0.85% | 381, 1.15% | 651, 0.76% |
| Thromboembolic events | 386, 0.31% | 3, 0.46% | 38, 0.19% | 4, 0.13% | 113, 0.34% | 278, 0.33% |
| Death | 78, 0.06% | 1, 0.15% | 14, 0.07% | 2, 0.06% | 34, 0.10% | 54, 0.06% |
| Infection | 1691, 1.35% | 11, 1.67% | 315, 1.53% | 71, 2.20% | 562, 1.70% | 1572, 1.85% |
| Wound disruption | 594, 0.47% | 5, 0.76% | 83, 0.40% | 32, 1.00% | 189, 0.57% | 508, 0.60% |
| Neurological injury | 199, 0.16% | 0, 0% | 42, 0.20% | 10, 0.31% | 50, 0.15% | 115, 0.14% |
| Hardware failure | 447, 0.36% | 1, 0.15% | 51, 0.25% | 7, 0.22% | 114, 0.34% | 307, 0.36% |
| Hematoma formation | 280, 0.22% | 5, 0.76% | 52, 0.25% | 6, 0.19% | 66, 0.20% | 253, 0.30% |
| 90-days | Control (n=100,650) |
Alcohol Use (n=496) |
Tobacco/Nicotine Use (n=16,580) |
Malnutrition (n=2,557) |
Dyslipidemia (n=25,674) |
Primary Hypertension (n=69,246) |
| Complications, n (%) | ||||||
| Myocardial infarction | 710, 0.71% | 0, 0% | 133, 0.8% | 33, 1.29% | 442, 1.72% | 624, 0.9% |
| Acute renal failure | 688, 0.68% | 12, 2.42% | 95, 0.57% | 36, 1.41% | 463, 1.8% | 827, 1.19% |
| Thromboembolic events | 391, 0.39% | 3, 0.6% | 65, 0.39% | 15, 0.59% | 113, 0.44% | 355, 0.51% |
| Death | 110, 0.11% | 8, 1.61% | 11, 0.066% | 11, 0.43% | 41, 0.16% | 85, 0.12% |
| Wound disruption | 718, 0.71% | 6, 1.21% | 112, 0.68% | 43, 1.68% | 237, 0.92% | 624, 0.9% |
| Neurological injury | 192, 0.19% | 0, 0% | 41, 0.25% | 6, 0.23% | 58, 0.23% | 125, 0.18% |
| Hardware failure | 668, 0.66% | 6, 1.21% | 125, 0.75% | 16, 0.63% | 201, 0.78% | 510, 0.74% |
| Hematoma formation | 324, 0.32% | 3, 0.6% | 64, 0.38% | 7, 0.27% | 76, 0.3% | 294, 0.43% |
| 180-days | Control (n=66,980) |
Alcohol Use (n=310) |
Tobacco/Nicotine Use (n=11,312) |
Malnutrition (n=1,689) |
Dyslipidemia (n=16,971) |
Primary Hypertension (n=46,425) |
| Complications, n (%) | ||||||
| Myocardial infarction | 618, 0.92% | 0, 0% | 129, 1.14% | 24, 1.41% | 436, 2.57% | 633, 1.36% |
| Acute renal failure | 641, 0.96% | 6, 1.84% | 99, 0.88% | 28, 1.68% | 402, 2.37% | 703, 1.51% |
| Thromboembolic events | 270, 0.4% | 3, 0.91% | 34, 0.3% | 3, 0.19% | 93, 0.55% | 276, 0.60% |
| Death | 105, 0.16% | 3, 0.92% | 11, 0.09% | 9, 0.54% | 42, 0.25% | 82, 0.18% |
| Wound disruption | 410, 0.61% | 3, 1.05% | 80, 0.71% | 31, 1.84% | 161, 0.95% | 389, 0.84% |
| Neurological injury | 124, 0.19% | 0, 0% | 38, 0.34% | 10, 0.57% | 42, 0.25% | 92, 0.20% |
| Hardware failure | 608, 0.91% | 4, 1.26% | 113, 1% | 11, 0.63% | 193, 1.14% | 471, 1.01% |
| Hematoma formation | 233, 0.35% | 3, 0.99% | 43, 0.38% | 6, 0.33% | 63, 0.37% | 217, 0.47% |
| Length of Stay, Cost, and Readmissions | Control | Alcohol Use | Tobacco/Nicotine Use | Malnutrition | Dyslipidemia | Primary Hypertension |
| Average length of stay (days) ± SD | 3.84 + 4.20 | 6.53 + 8.32 | 3.54 + 4.41 | 9.07 + 13.29 | 4.32 + 4.53 | 4.04 + 4.15 |
| Average cost of index admission (USD) + SD | $36,649 + $26,351 | $45,279 + $39,727 | $32,488 + $21,071 | $59,936 + $57,341 | $35,556 + $24,047 | $34,353 + $23,587 |
| Mean 30-day readmission rate, n (%) | 6,709, 4.84% | 39, 5.94% | 1,057, 5.14% | 210, 6.57% | 2,076, 6.26% | 5,162, 6.07% |
| Mean 90-day readmission rate, n (%) | 8,067, 8.01% | 63, 12.70% | 1,532, 9.24% | 329, 12.87% | 2,768, 10.78% | 7,102, 10.26% |
| Mean 180-day readmission rate, n (%) | 7,375, 11.01% | 56, 18.06% | 1,495, 13.22% | 312, 18.47% | 2,609, 15.37% | 6,732, 14.50% |
Predictive Models
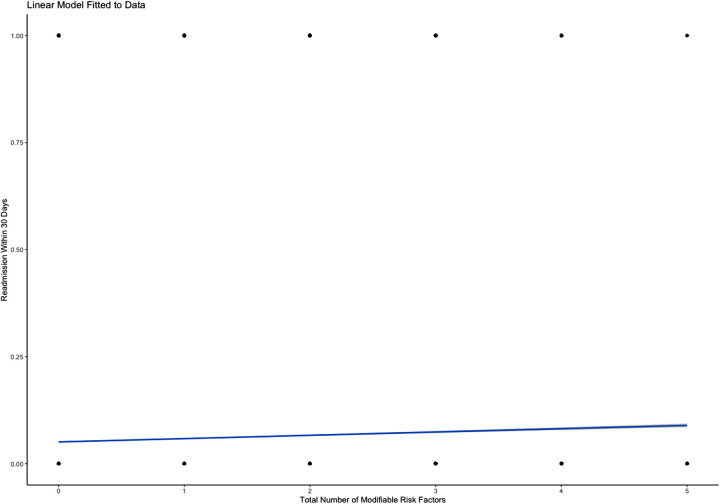
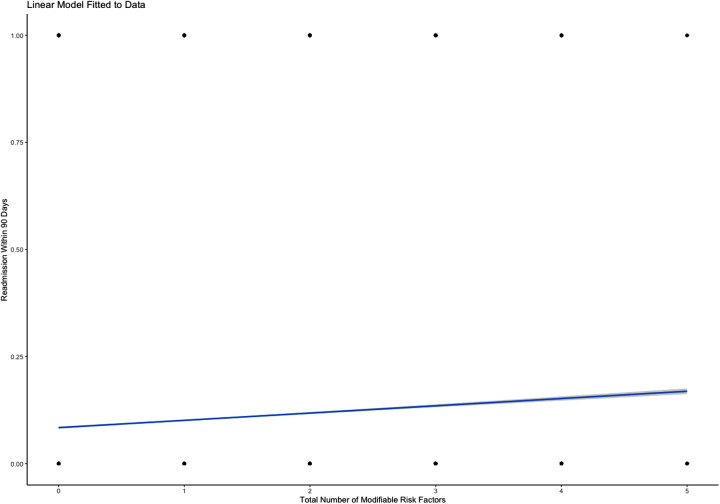
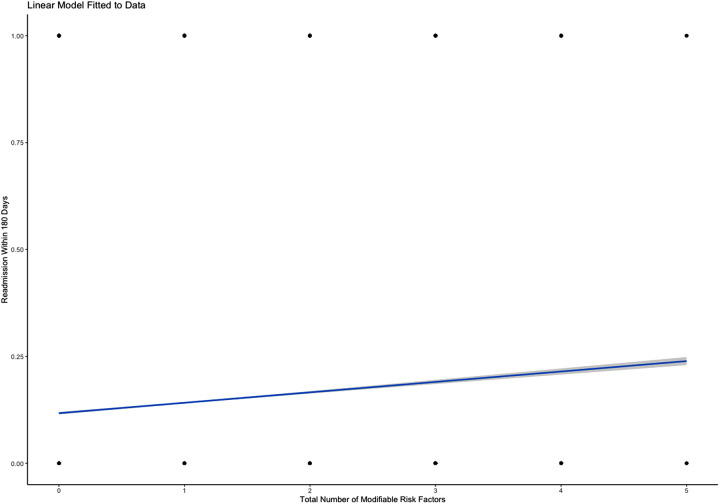
Predictive modeling revealed statistically significant differences between the number of MRFs present in any given individual and readmission rates at the 30-day, 90-day, and 180-day intervals. Predictive analysis demonstrated a 0.77% increase in risk of readmission within 30 days for every additional MRF (P<0.001; Figure 1 ). Similarly, predictive analytics showed that each additional MRF was associated with a 1.70% increased risk of readmission in 90-days (P<0.001; Figure 2 ). The same analysis outlined the largest effect with the longest time interval, describing an additional 2.44% increase in risk of readmission within 180 days with each subsequent MRF (P<0.001; Figure 3 ).
Figure 1.
Predictive linear regression model showing increased risk of 30-day readmission in the presence of each additional MRF.
Figure 2.
Predictive linear regression model showing increased risk of 90-day readmission in the presence of each additional MRF.
Figure 3.
Predictive linear regression model showing increased risk of 180-day readmission in the presence of each additional MRF.
Discussion
Our results revealed significantly greater readmission rates in malnourished, dyslipidemic, and primary hypertensive patients than those without any MRFs in question through the 30, 90, and 180-day readmission intervals. Malnourished patients consistently had the highest readmission rates (30-day: 6.57%, 90-day: 12.87%, and 180-day: 18.47%) as well as the highest associated LOS (9.07+13.29 days) and cost of index admission ($59 936 + $57 341). Our findings remain in accordance with the literature, which has firmly established poor nutritional status as a significant predictor of increased readmissions, mortality, LOS, and cost in all settings.13,14 In multivariate analysis of 29 covariates, Hydrick et al. found diabetes with chronic complications, which commonly includes nutritional malnourishment, dyslipidemia, and hypertension due to metabolic dysfunction and vascular inflammation, to be the only comorbidity associated with increased odds of readmission at 90 days. 15 Another retrospective study of 2,236 patients, reinforced the applicability of this notion within surgical treatment for ASD, reporting higher rates of mortality and any given complication in those with a nutritionally insufficient status. 16 Other studies have reported additional associations such as wound infection and dehiscence that were not found in the present study. Their findings, however, are likely from defining malnutrition entirely as hypoalbuminemia while neglecting to account for other comorbid etiologies that may cause both hypoalbuminemia and increased predisposition to infection such as liver disease and existing infectious processes. 17
Just as with nutritional malnutrition, primary hypertension and dyslipidemia are widely-accepted risk factors for poor outcomes following hospitalization. Nonetheless, the 2 variables are rarely assessed as independent risk factors in spine surgery but instead are merged together with related conditions and collectively analyzed as metabolic syndrome (MetS).18,19 In an analysis of 18,605 patients, Lovecchio et al. isolated obesity as a confound and was still able to find increased rates of readmission, wound complications, and extended LOS following elective lumbar fusion. 20 Because dyslipidemia and primary hypertension are the 2 most modifiable components of MetS outside of obesity, which has already been extensively reviewed in spine surgery, it is imperative to better define and quantify their associated risks as independent predictors. 21 Although our study found no significant differences between the 2 MRFs with regard to readmission rates, discrepancies in complication rates can be seen.
In our study, alcohol users were shown to have higher rates of readmission only at the 180-day readmission interval. Consistent with our findings, Elsamadicy et al. observed no significant differences in 30-day readmission or postoperative complication rates in patients with a history of preoperative alcohol use versus those without. 22 Instead, studies have demonstrated impaired bone healing in association with alcohol consumption.23,24 In the context of spinal fusions, Passias et al. found alcohol use to be an independent predictor of pseudarthrosis, which typically becomes symptomatic, after 6 months. 25 The association of alcohol use and defective bone fusion, a process considered to be long-term, may explain why our study found increased rates of readmission only after 180 days and not at earlier time points.
Similar to alcohol users, our study observed significantly increased rates of readmission amongst tobacco/nicotine users only after 180 days. Glassman et al. showed that postoperative smoking cessation, rather than preoperative, was associated with increased fusion rates, satisfaction scores, and return to work rates in lumbar fusion patients. The same study found that 63.8% of smokers quit for at least 1 to 6 months and 40.4% quit for at least 6 months following their spinal fusion. 26 The progressive increase in amount of relapsed patients with time may explain why readmission rates for the tobacco/nicotine use group in our study was significant at 180 days but not 30 or 90 days. The relapsed patients not only stopped receiving the benefits of postoperative smoking cessation, but were also now incurring the negative effects of tobacco/nicotine use. Additionally, several studies found the principal complication of smoking to be pseudarthrosis, which has an insidious onset that often delays detection until beyond 6 months.25,27,28 Given that our analysis was limited to 180 days, this may explain why our study was unable to observe any significant increases in complications within the tobacco/nicotine use cohort. The cumulation of these findings should highlight the importance of constructing successful alcohol and smoking cessation strategies aimed at the postoperative period to improve patient outcomes.
With regard to perioperative complications, dyslipidemia was consistently shown to be associated with the greatest rates of MI and AKI at all readmission points. One national study showed that of prominent cardiovascular risk factors in patients undergoing noncardiac surgery, dyslipidemia was the second most prevalent next only to hypertension. 29 Although studies concerning cardiovascular complications in spine surgeries are scant, a recent study in 2019 by Harwin et al. found high cholesterol to be an significant preoperative risk factor for MI following surgery for the lumbar spine. 30 These findings in conjunction with our own are most likely explained by the high correlation between dyslipidemia and coronary artery disease (CAD) and silent myocardial ischemia.31,32 CAD in the setting of intraoperative myocardial ischemia, which may be exacerbated by significant blood loss volumes characteristic of lumbar spine fusion procedures, can damage the myocardium and increase predisposition to future MIs.23-35
The mechanism by which dyslipidemia can cause postoperative AKI is similar. In parallel to our findings, both dyslipidemia and hypertension have been thoroughly reported as major contributors to declining renal function.36,37 In a retrospective review of 726 patients who had undergone surgical treatment involving the thoracic and/or lumbar spine, Naik et al. found that the only risk factor (the study did not analyze dyslipidemia as a risk factor) associated with developing AKI was preoperative hypertension. 38 Furthermore, the risk of recurrent AKI is based heavily on the severity of the AKI episode at index admission and the presence of known premorbid risk factors such as dyslipidemia and hypertension. 39 The cumulation of these findings explains why patients with dyslipidemia and primary hypertension in our study continue to demonstrate significantly greater rates of AKI throughout all readmission intervals.
The development of AKI and MI following lumbar spinal fusion is associated with considerably increased short-term and long-term mortality and morbidity.38,39 Our results, along the aforementioned findings, suggest that dyslipidemia and primary hypertension play an integral role in the progression to these devastating medical conditions.
Of note, mortality rate surfaced as a differentiating complication only at the 90-day readmission mark and thereafter. Alcohol use showed a substantially increased mortality rate compared to all other groups except nutritional malnutrition on univariate analysis, but solely at 90-day readmission. Upon multivariate regression, there was no statistically significant difference. Thus, older patients with uncontrolled alcohol use should be approached with additional caution, as these findings underline the potentiating effect of increased age on alcohol use in mortality rates following lumbar spine fusions.
Overall, current discussions regarding alcohol use on outcomes in spine surgery are, however, contentious and range from alcohol having no clinical impact, deleterious effects, or even favorable outcomes.22,40,41 Our study found markedly increased LOS, cost of index admission, and 180-day readmission rates in alcohol users. These findings may be the result of preoperative and postoperative chronic opioid abuse, both of which are associated with increased morbidity and mortality. Over 70% of patients undergoing major spine surgeries report preoperative opioid use, and over 50% of these patients along with 18.3% of previously opioid-naive patients report continued use 12 months postoperatively. 42 Cauley et al. found that of the operation types, patients undergoing spine fusions were the second most likely to experience postoperative overdose. Patients presenting with postoperative overdose also had a higher frequency of nearly every comorbid condition with the exception of cerebrovascular diseases, most likely contributing to increased mortality rates. The same study also found substance abuse to be the strongest predictor of postoperative overdose. 43 Give that our MRF groups were formed by diagnosis at the index admission, it is likely that the patients in the alcohol use group were much more susceptible to postoperative opioid overdose than the other MRFs. Because prolonged opioid use, due to increased pharmacological tolerance, often prompts gradual escalation of opioid dosages to hazardous amounts, patients at risk may present with complications much later in their postoperative course. 44 This may explain why the alcohol use cohort in our study had significantly increased readmission rates only at 180 days.
Nutritional malnutrition demonstrated a lasting increase in mortality rate at both 90 and 180-day readmission. In a nationwide study from 2005-2012 using the PearlDiver database, Puvanesarajah et al. found nutritional malnutrition to be associated with increased odds of major complications at 90-days and a 6 fold increase in mortality at 1 year. 45 A previously mentioned study supports the present study’s findings, reporting higher rates of mortality, LOS, reoperation, and any complication. 15 As an increasing number of elderly patients undergo lumbar spinal fusion, it becomes important to consider the increased prevalence of nutritional malnourishment within this patient population. 46
Predictive modeling was used in this study to better quantify and visually depict the cumulative risk of readmission at 30, 90, and 180-days with each additional MRF. Other studies have shown the utility and validity of these models in predicting readmissions in the setting of elective spinal surgeries.47,48
Limitations
The use of a national de-identified database inherently presents a number of limitations, most notably the lack of patient-reported outcomes such as oswestry disability index scores. ICD-10 coding is also unable to incorporate quantitative descriptions or characterize the severity for a given diagnosis. For instance, a patient who has 14 drinks a week and a patient who has 28 drinks a week could both be categorized under “Alcohol abuse” per ICD-10 coding. The use of ICD-10 coding also limits any qualitative analysis, such as determining whether readmissions diagnoses were directly related to the presence of risk factors after a recent lumbar spinal fusion. Our study is thus limited in that it cannot outline causative relationships, but instead characterizes the degree of associations between modifiable risk factors and various primary outcome measures.
Additionally, the lack of standardized criteria and in coding between hospitals may lead to inconsistencies in diagnoses or classifications of different conditions. For example, with regard to body mass index (BMI) scores, patients are more likely to be diagnosed with the nonspecific “obesity” ICD-10 code, which covers a broad range from 30-39.9, when they present on the higher end of the spectrum. 49 Given that this creates selection bias that would consequently lead to overstated effects of obesity on lumbar spine fusion outcomes, our study chose to not analyze it as a modifiable risk factor. As ICD-10 offers an alternative and more granular coding schema for BMI, coding in intervals of single BMI points (i.e 30-30.9, 31-31.9, etc.), we believe that a separate study dedicated to a more thorough analysis of BMI in the context of lumbar spine fusion outcomes is warranted.
The study is also limited by its analysis of a narrow time range (from 2016-2017). Our retrospective analysis was bounded by these timepoints due to the recent development and implementation of ICD-10 coding at the end of 2015. The granularity of ICD-10 codes was required to appropriately define the MRF groups used in this study. For example, in formulating the tobacco/nicotine use group, the ICD-10 code for “nicotine use, in remission” was explicitly avoided in order to capture patients with active preoperative use. Conversion to the corresponding ICD-9 code, however, inadequately classified it as “tobacco use disorder”. In spite of the decreased population size and shortened study period, the methodology was ultimately better refined to provide a more accurate analysis tailored to the study’s objectives.
Conclusions
Our results highlight the negative impact that each of the MRFs have on postoperative outcomes following lumbar spine fusion. Although all MRFs eventually led to increased 180-day readmissions, nutritional malnutrition, dyslipidemia, and primary hypertension was associated with increased readmissions much earlier. Dyslipidemia was notably associated with MI and AKI, complications predictive of significantly increased mortality. Interestingly, alcohol use revealed an increased mortality, but only at the 90-day readmission date. Thus, our study highlights the complex and distinctive nature of each MRF and their respective detrimental effects on patient outcomes. Further longitudinal research using prospective multicenter data is necessary to better characterize the effects of different MRFs on lumbar spine fusion outcomes. Doing so may help providers capitalize on the modifiable nature of these premorbid risk factors through the development of refined preoperative guidelines and interventions aimed at increasing patient autonomy while decreasing patient burden, morbidity, and mortality.
Footnotes
Authors’ Note: Disclosures outside of submitted work: JCW – Royalties – Biomet, Seaspine, Amedica, Synthes; Investments/Options – Bone Biologics, Pearldiver, Electrocore, Surgitech; Board of Directors – AO Foundation, Society for Brain Mapping and Therapeutics, Fellowship Funding (paid to institution): AO Foundation.
ZB – consultancy: Cerapedics (past), The Scripps Research Institute (past), Xenco Medical (past), AO Spine (past); Research Support: SeaSpine (past, paid to the institution), Next Science (paid directly to institution), Motion Metrics (paid directly to institution); North American Spine Society: committee member; Lumbar Spine Society: Co-chair Educational Committee, AOSpine Knowledge Forum Degenerative: Associate member; AOSNA Research committee-committee member.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zorica Buser, PhD  https://orcid.org/0000-0002-5680-0643
https://orcid.org/0000-0002-5680-0643
References
- 1.Nasser R, Yadla S, Maltenfort MG, et al. Complications in spine surgery: a review. J Neurosurg Spine. 2010;13(2):144–157. [DOI] [PubMed] [Google Scholar]
- 2.Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970–1976. [DOI] [PubMed] [Google Scholar]
- 3.McGirt MJ, Parker SL, Lerner J, Engelhart L, Knight T, Wang MY. Comparative analysis of perioperative surgical site infection after minimally invasive versus open posterior/transforaminal lumbar interbody fusion: analysis of hospital billing and discharge data from 5170 patients. J Neurosurg Spine. 2011;14(6):771–778. [DOI] [PubMed] [Google Scholar]
- 4.Patil CG, Santarelli J, Lad SP, Ho C, Tian W, Boakye M. Inpatient complications, mortality, and discharge disposition after surgical correction of idiopathic scoliosis: a national perspective. Spine J. 2008;8(6):904–910. [DOI] [PubMed] [Google Scholar]
- 5.Pinto A, Faiz O, Davis R, Almoudaris A, Vincent C. Surgical complications and their impact on patients’ psychosocial well-being: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e007224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Medicare & Medicaid Services. Medicare & Medicaid statistical supplement. Baltimore: Centers for Medicare & Medicaid Services; 2007. [Google Scholar]
- 7.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303(21):2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleh A, Thirukumaran C, Mesfin A, Molinari RW. Complications and readmission after lumbar spine surgery in elderly patients: an analysis of 2,320 patients. Spine J. 2017;17(8):1106–1112. doi:10.1016/j.spinee.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 9.Epstein NE. Predominantly negative impact of diabetes on spinal surgery: a review and recommendation for better preoperative screening. Surg Neurol Int. 2017;8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahrestani S, Ballatori AM, Chen XT, et al. Analysis of modifiable and nonmodifiable risk factors in patients undergoing pituitary surgery. J Neurosurg. 2020:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Phillips W. Coding for malnutrition in the adult patient: what the physician needs to know. Pract Gastroenterol. 2014;133:56–64. [Google Scholar]
- 12.International classification of diseases, tenth revision, clinical modification/procedural classification system Medicare severity-diagnostic related groups. Centers for Medicare and Medicaid Services Website. Accessed April 6, 2021. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0242.html [Google Scholar]
- 13.Lim SL, Ong KCB, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr. 2012;31(3):345–350. [DOI] [PubMed] [Google Scholar]
- 14.Hydrick TC, Rubel N, Renfree S, et al. Ninety-day readmission in elective revision lumbar fusion surgery in the inpatient setting. Global Spine J. 2010;10(8):1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phan K, Ranson W, White SJW, et al. Thirty-day perioperative complications, prolonged length of stay, and readmission following elective posterior lumbar fusion associated with poor nutritional status. Global Spine J. 2019;9(4):417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phan K, Kim JS, Xu J, et al. Nutritional insufficiency as a predictor for adverse outcomes in adult spinal deformity surgery. Global Spine J. 2018;8(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohl DD, Shen MR, Mayo BC, et al. Malnutrition predicts infectious and wound complications following posterior lumbar spinal fusion. Spine. 2016;41(21):1693–1699. doi:10.1097/brs.0000000000001591 [DOI] [PubMed] [Google Scholar]
- 18.Chung AS, Campbell D, Waldrop R, Crandall D. Metabolic syndrome and 30-day outcomes in elective lumbar spinal fusion. Spine. 2018;43(9):661–666. [DOI] [PubMed] [Google Scholar]
- 19.Memtsoudis SG, Kirksey M, Ma Y, et al. Metabolic syndrome and lumbar spine fusion surgery: epidemiology and perioperative outcomes. Spine. 2012;37(11):989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovecchio F, Fu MC, Iyer S, Steinhaus M, Albert T. Does obesity explain the effect of the metabolic syndrome on complications following elective lumbar fusion? a propensity score matched analysis. Global Spine J. 2018;8(7):683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grundy SM. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 22.Elsamadicy AA, Adogwa O, Vuong VD, et al. Impact of alcohol use on 30-day complication and readmission rates after elective spinal fusion (≥2 levels) for adult spine deformity: a single institutional study of 1,010 patients. J Spine Surg. 2017;3(3):403–410. doi:10.21037/jss.2017.08.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adogwa O, Parker SL, Shau D, et al. Long-term outcomes of revision fusion for lumbar pseudarthrosis. J Neurosurg Spine. 2011;15(4):393–398. [DOI] [PubMed] [Google Scholar]
- 24.Lauing KL, Roper PM, Nauer RK, Callaci JJ. Acute alcohol exposure impairs fracture healing and deregulates β-catenin signaling in the fracture callus. Alcohol Clin Exp Res. 2012;36(12):2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyquist F, Berglund M, Nilsson BE, Obrant KJ. Nature and healing of tibial shaft fractures in alcohol abusers. Alcohol Alcohol. 1997;32(1):91–95. [DOI] [PubMed] [Google Scholar]
- 26.Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 2000;25(20):2608–2615. [DOI] [PubMed] [Google Scholar]
- 27.Berman D, Oren JH, Bendo J, Spivak J. The Effect of smoking on spinal fusion. Int J Spine Surg. 2017;11(4):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YJ, Bridwell KH, Lenke LG, Cho KJ, Edwards CC, 2nd, Rinella AS. Pseudarthrosis in adult spinal deformity following multisegmental instrumentation and arthrodesis. J Bone Joint Surg Am. 2006;88(4):721–728. [DOI] [PubMed] [Google Scholar]
- 29.Smilowitz NR, Gupta N, Guo Y, Beckman JA, Bangalore S, Berger JS. Trends in cardiovascular risk factor and disease prevalence in patients undergoing non-cardiac surgery. Heart. 2018;104(14):1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harwin B, Formanek B, Spoonamore M, Robertson D, Buser Z, Wang JC. The incidence of myocardial infarction after lumbar spine surgery. Eur Spine J. 2019;28(9):2070–2076. [DOI] [PubMed] [Google Scholar]
- 31.Abd Alamir M, Goyfman M, Chaus A, et al. The Correlation of dyslipidemia with the extent of coronary artery disease in the multiethnic study of atherosclerosis. J Lipids. 2018;2018:5607349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valensi P, Avignon A, Sultan A, Chanu B, Nguyen MT, Cosson E. Atherogenic dyslipidemia and risk of silent coronary artery disease in asymptomatic patients with type 2 diabetes: a cross-sectional study. Cardiovasc Diabetol. 2016;15(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YH, Ou CY. Significant Blood Loss in Lumbar Fusion Surgery for Degenerative Spine. World Neurosurg. 2015;84(3):780–785. doi:10.1016/j.wneu.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 34.Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation. 2009;119(22):2936–2944. [DOI] [PubMed] [Google Scholar]
- 35.Priebe HJ. Perioperative myocardial infarction—aetiology and prevention. Br J Anaesth. 2005;95(1):3–19. doi:10.1093/bja/aei063 [DOI] [PubMed] [Google Scholar]
- 36.Mänttäri M, Tiula E, Alikoski T, Manninen V. Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension. 1995;26(4):670–675. doi:10.1161/01.hyp.26.4.670 [DOI] [PubMed] [Google Scholar]
- 37.Thallapureddy A, Migdal S, Crook ED, et al. Lipid abnormalities and renal disease: is dyslipidemia a predictor of progression of renal disease? Am J Med Sci. 2003;325(6):340–348. doi:10.1097/00000441-200306000-00005 [DOI] [PubMed] [Google Scholar]
- 38.Naik BI, Colquhoun DA, McKinney WE, et al. Incidence and risk factors for acute kidney injury after spine surgery using the RIFLE classification. J Neurosurg Spine. 2014;20(5):505–511. doi:10.3171/2014.2.spine13596 [DOI] [PubMed] [Google Scholar]
- 39.Fineberg SJ, Ahmadinia K, Patel AA, Oglesby M, Singh K. Incidence and mortality of cardiac events in lumbar spine surgery. Spine. 2013;38(16):1422–1429. [DOI] [PubMed] [Google Scholar]
- 40.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine. 2005;30(12):1460–1465. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen C. Lumbar disc herniation: favourable outcome associated with intake of wine. Eur Spine J. 1998;7(1):24–28. doi:10.1007/s005860050022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn LK, Yerra S, Fang S, et al. Incidence and risk factors for chronic postoperative opioid use after major spine surgery: a cross-sectional study with longitudinal outcome. Anesth Analg. 2018;127(1):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cauley CE, Anderson G, Haynes AB, Menendez M, Bateman BT, Ladha K. Predictors of in-hospital postoperative opioid overdose after major elective operations: a nationally representative cohort study. Ann Surg. 2017;265(4):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hser YI, Saxon AJ, Mooney LJ, et al. Escalating opioid dose is associated with mortality: a comparison of patients with and without opioid use disorder. J Addict Med. 2019;13(1):41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puvanesarajah V, Jain A, Kebaish K, et al. Poor nutrition status and lumbar spine fusion surgery in the elderly: readmissions, complications, and mortality. Spine. 2017;42(13):979–983. [DOI] [PubMed] [Google Scholar]
- 46.Sahyoun NR, Serdula MK, Galuska DA, Zhang XL, Pamuk ER. The epidemiology of recent involuntary weight loss in the United States population. J Nutr Health Aging. 2004;8(6):510–517. [PubMed] [Google Scholar]
- 47.Parker SL, Sivaganesan A, Chotai S, McGirt MJ, Asher AL, Devin CJ. Development and validation of a predictive model for 90-day readmission following elective spine surgery. J Neurosurg Spine. 2018;29(3):327–331. [DOI] [PubMed] [Google Scholar]
- 48.Sivaganesan A, Zuckerman S, Khan I, et al. Predictive model for medical and surgical readmissions following elective lumbar spine surgery. Spine. 2019;44(8):588–600. doi:10.1097/brs.0000000000002883 [DOI] [PubMed] [Google Scholar]
- 49.Ammann EM, Kalsekar I, Yoo A, et al. Assessment of obesity prevalence and validity of obesity diagnoses coded in claims data for selected surgical populations: a retrospective, observational study. Medicine (Baltimore). 2019;98(29):e16438. [DOI] [PMC free article] [PubMed] [Google Scholar]