Abstract
Study Design:
Prospective observational study.
Objectives:
Studying the effect of degenerative cervical spondylosis(CS) on blood flow velocity of vertebral artery (VA) during cervical spine rotation in different head positions and its association with vertigo.
Introduction:
Vertigo is one of the most common complaints seen in an out-patient clinic. Its association with CS remains an enigma for a treating physician. This study planned to systematically analyze the association between vertigo and CS by evaluating VA blood flow dynamics in different head positions.
Methods:
100 patients with ages ranging from 20-80 years were recruited. First group of 50 patients with CS with vertigo were compared with second study group of 50 patients having CS without vertigo. Cervical radiographs were used to evaluate CS using cervical degenerative index (CDI). Color doppler was used to measure VA blood flow with head in neutral position and 60° lateral rotation with 30° extension. Same procedure was repeated on opposite side. Measurements performed included peak systolic blood flow velocity(PSV) and end diastolic blood flow velocity (EDV).
Results:
Among patients with CS, patients having vertigo showed significantly more evident degenerative changes (CDI ≥25) (P=<0.001). High grade CS patients (CDI ≥25) with vertigo had statistically significant lower blood flow parameters with head rotation in the left and right VAs as compared to CS patients without vertigo.
Conclusion:
This study highlights important pathophysiological mechanism of vertigo observed in patients of CS. The magnitude of reduction in VA blood flow was significantly higher in patients with advanced CS presenting as vertigo.
Keywords: vertigo, cervical spine, spondylosis, vertebral artery
Introduction
Vertigo is often a hidden complaint and causes serious handicap with considerable psychological morbidity. 1 Vertigo patients with all the described causes systematically investigated and excluded are always referred from ENT surgeons, ophthalmologists and neuro-physicians to orthopaedic surgeons with radiologically evident cervical spondylosis (CS). However, CS leading to vertigo is an inadequately understood phenomenon with not many studies available in literature. A previously published study conducted in Indian population showed prevalence rate of vertigo was around 0.71% in the general community of rural adult population in India with highest prevalence (3.52%) found in the sixth decade and lowest (0.15%) in the third decade of life. 2 Despite these numbers, the etiology, pathogenesis, symptomatology, prevention and management of vertigo in CS has remained inconclusive and there is limited literature reviewing their association. This study aimed at systematically analyzing the basic pathophysiological mechanism of vertigo in CS.
Methods
This prospective-observational study with a duration of 3 years was conducted at a tertiary care hospital. Approval from the Institutional Review Board (IRB) was taken prior to the commencement of this study (IRB approval number-4180/18). Written informed consent was obtained from all study participants. We investigated a total of 100 patients arranged in 2 groups with age range of 20- 80 years. First group (Group I) comprised of 50 patients (30 females and 20 males) having clinically & radiologically proven CS with complaints of vertigo for more than 6 weeks duration. Second group (Group II) consisted of 50 patients (27 females and 23 males) having clinically & radiologically proven CS but without complaints of vertigo. Control group was selected from patients in outpatient department from year 2017 to 2020 consecutively, who presented with CS without vertigo. Thus control group patients were clinically & radiologically proven CS patients who presented to our outpatient department without complaints of vertigo. In addition to this, other common established causes of vertigo were thoroughly studied and examined by corresponding pathology specialist. In this way, patients with otological and vestibular disorders, ocular disorders, neurological disorders (except for the radicular symptoms related to cervical segments), psychiatric disorders and cardiac arrhythmias were excluded from the study.
Inclusion Criteria
Patients aged >20 years and <80 year with clinically & radiologically proven CS with or without radiculopathy, with or without vertigo.
Exclusion Criteria
Patients, otherwise meeting the inclusion criteria, were ineligible in case of any of the following criteria.
Patient with otological and vestibular disorders.
Patient with ocular disorders resulting in vertigo.
Patients with neurological disorders (except for the radicular symptoms related to cervical segments).
Patient with history of stroke / demyelinating disorders / Parkinson’s disease / Psychiatric disorders.
Patient those on Anticholinergics / Anti-depressants / Sedatives / Hypnotics / Antipsychotics.
Patients with Cardiac Arrhythmias & Arterial atherosclerosis disorders.
Demographic data and medical history including age, gender, history of trauma, medications, and presenting symptoms were collected and a case record form was prepared. The symptom of vertigo was evaluated in detail. Patients were asked whether they have experienced falls, hearing loss, or ringing in the ears, worsening of balance during neck movements and its direction. In our study, Cervical vertigo was a diagnosis of exclusion. By default patient with vertigo complaints had to be examined by different specialists of Otolaryngology/Ophthalmology/Neurology/Psychiatry/Cardiology, so that patients were screened for all established causes of vertigo and systematically ruled out. Only when all possible causes were ruled out, vertigo was considered to be cervical in origin and cases were considered for our study. Thus, patients were referred to our clinic with a prior diagnosis of cervical vertigo by exclusion. If any patient of vertigo presented to our clinic without exclusion then he/she was referred to the other specialists for ruling out other causes of vertigo. Total duration of symptoms also took into account the time spent while seeking exclusion of other possible causes as patient had active complaints during that period.
All patients underwent radiographs of cervical spine and degenerative changes were graded as per the Cervical Degenerative Index (CDI) 3 described in Table 1. The CDI assesses 4 factors: (1) narrowing of disc space, (2) endplate/facet sclerosis, (3) osteophyte formation, and (4) listhesis (either anterior or posterior). The assessment is derived from a standard antero-posterior(AP) and lateral cervical spine radiograph (Figure 1 & its assessment in Table 2). A quantitative score for each of the 4 factors for each level (C2-C3 to C6-C7) was added to achieve an overall cumulative score of CDI. For every factor, a normal appearance yields a score of “0” and worst spondylotic change yields a score of “3.” Thus the CDI score ranges from 0 (completely normal) to 60 (maximum degeneration at each level).
Table 1.
Cervical Degenerative Index (CDI).
| Factor | CDI score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Disc space narrowing (%) | None–25 | 25–50 | 50–75 | 75–100 |
| End plate sclerosis | None | Minimal | Moderate | Severe |
| Osteophytes | None | Small, <2 mm | Moderate, 2–4 mm | Large, >4 mm |
| Listhesis | None | <3 mm | 3–5 mm | >5 mm |
Figure 1.
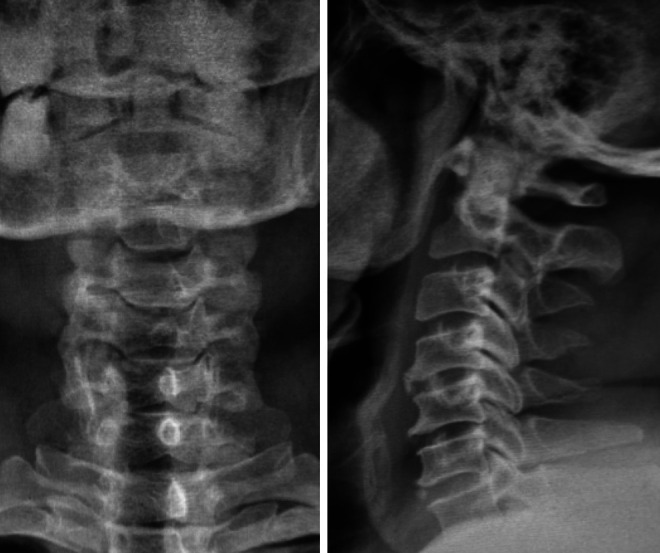
Cervical spine antero-posterior (AP) and lateral radiograph of a 50 years old female showing cervical spondylotic changes which can be graded by cervical degenerative index (CDI) as shown in Table 2.
Table 2.
Cervical Degenerative Index (CDI) Calculation of Cervical Radiograph in Figure 1.
| Factors | Cervical Vertebral level | Total factor score | ||||
|---|---|---|---|---|---|---|
| C2-C3 | C3-C4 | C4-C5 | C5-C6 | C6-C7 | ||
| Disc space narrowing | 1 | 1 | 3 | 2 | 2 | 9 |
| End plate sclerosis | 1 | 1 | 2 | 2 | 1 | 7 |
| Osteophytes | 1 | 2 | 3 | 3 | 2 | 11 |
| Listhesis | 1 | 0 | 0 | 0 | 0 | 1 |
| Total level score | 4 | 4 | 8 | 7 | 5 | |
| CDI Score | 28 | |||||
Arterial color doppler examination of vertebral artery (VA) on both sides was performed on all patients. The examination was done at multi-frequencies (7.5–10 MHz) using a linear array transducer (Figure 2). Evaluation was made initially with the subject’s head in neutral position (NP) in which the head was slightly in extension (Figure 3). Later the subject’s head was turned toward one side by 60° and extended at the angle of 30° for measurements of VA. 4 The same procedure was repeated on opposite side. Measurements were performed both on right & left VA. The Peak Systolic maximal blood flow Velocity (PSV) & End Diastolic blood flow Velocity (EDV) were calculated. All participants underwent arterial color doppler of carotid artery to rule out atherosclerosis. Carotid artery stenosis due to atherosclerosis is an important factor which can cause vertigo. Hence, all patients were screened for carotid artery stenosis before proceeding for VA doppler. Though carotid arterial stenosis of less than 50% do not cause any reduction in flow or pressure distal to the lesion, 5 we excluded all the patients with thickened carotid intima or carotid plaque from the study since it may act as a potential confounding factor. As all patients with doppler findings suggestive of atherosclerosis were excluded from the study, cut-off was not described for the same.
Figure 2.
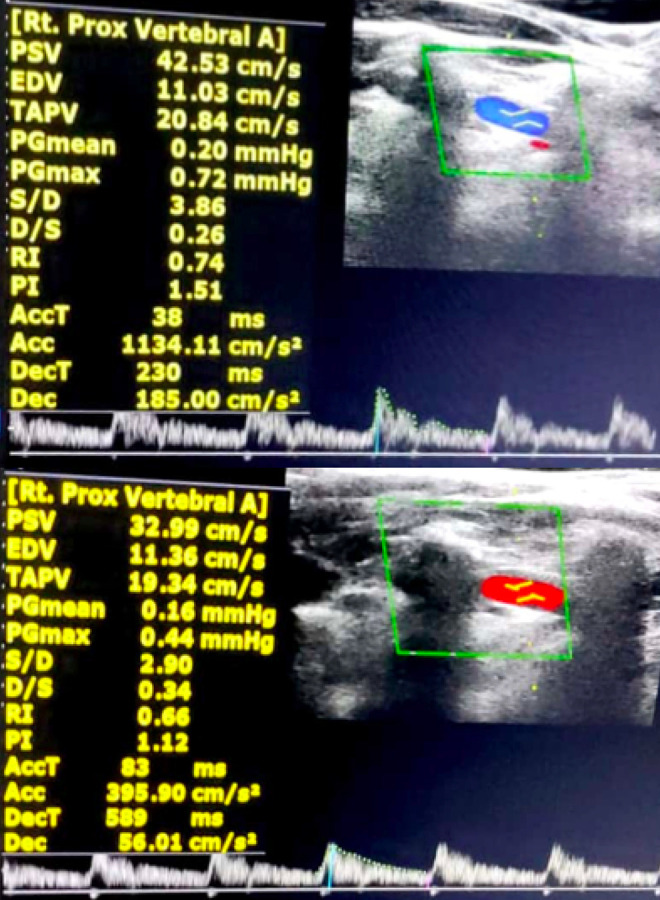
Evaluation of right vertebral artery of cervical spondylotic patient with vertigo in neutral head position (top image) and 60 ° head rotation to left & 30° extension position (bottom image); depicting decrease in PSV & EDV values on rotation.
Figure 3.
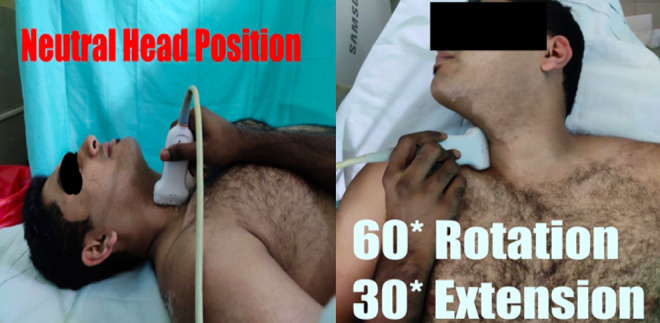
Positioning of patient for evaluation of right vertebral artery, initially with the subject’s head in neutral position with extension (left image). The subject’s head is turned toward right side by 60° and extended at an angle of 30° for measurements in the right vertebral artery (right image). Same procedure repeated for opposite side.
As per our Institutional Research Board standard protocol, all investigations which contribute to results need to be blinded. Hence to avoid interobserver error and bias in the study, grading of all radiographs to calculate cervical degenerative index was done by single expert radiologist who was blinded to all clinical variables, including the status of vertigo. Similarly, all the doppler studies were done by the single expert sonologist whose was blinded to vertigo status of all patients.
This data was compiled and evaluated using Epi-info Version 7.2. The qualitative variables were expressed in terms of proportions and to test the difference between 2 proportions chi square or fisher exact test was used. The quantitative variables were expressed in terms of mean and standard deviation. To test the difference between the 2 means, student t-test was used. All analysis was 2 tailed and the significance level was set at P < 0.05. Power analysis of the study was done prior to commencement of the study. Expecting a difference of 30% in proportions of CS in vertigo and non-vertigo group, with 95% confidence interval and 90% power, we found a minimum sample size to be 40 in each group. So considered 50 in each group for our convenience as a part of convenient sampling. The following formula 6 was used
where Zα/2 is the critical value of the Normal distribution at α/2 (e.g. for a confidence level of 95%, α is 0.05 and the critical value is 1.96), Zβ is the critical value of the Normal distribution at β (e.g. for a power of 80%, β is 0.2 and the critical value is 0.84) and p1 and p2 are the expected sample proportions of the 2 groups.
Results
In our study, a total of 100 patients with age ranging from 20-80 years were assessed. Mean age of the study subjects in non-vertigo group was 46.34 ± 10.79 years and in vertigo group it was 50.72 ± 10.86 years and this difference was statistically not significant. Among the non-vertigo group, 46% were females and 54% were males and among the vertigo group 60% were females and 40% were males. There was no statistically significant difference among the 2 groups in terms of gender. Among the non-vertigo group the proportion of the subjects having radiculopathy was 54% which was significantly lower than vertigo group (76%). Among the vertigo group 6% cases exhibited neurological deficit and none in non-vertigo group and this difference was not statistically significant.
Radiograph findings
Patients in group I showed significantly higher prevalence of CS as per CDI score when compared to group II (mean CDI score of 27.84 [± 4.7] and 17.20 [± 3.8] in group I and group II respectively; [P < 0.001]). Among the factors of the CDI score, the most remarkable difference was in osteophyte score (Table 3). The mean osteophyte score in group I was 12.1 (±3.2) vs. 6.1 (±1.7) in group II (P < 0.001). Another factor which showed statistical significance was disc space narrowing; with mean score of 7.5( ± 1.8) in group I and 5.7 (±1.6) in group II (P < 0.001). Remaining factors of CDI (sclerosis and listhesis) did not show any significant statistical association. Among the vertigo group, the subjects having CDI ≥25 were 80% of the cases and among the non-vertigo group, the patients having CDI ≥25 were only 20%. This difference was statistically significant.
Table 3.
Comparison of Cervical Degenerative Index (CDI) Score Between 2 Groups.
| CDI Factors | Group I (Vertigo) | Group II (Non Vertigo) | P value |
|---|---|---|---|
| Osteophytes score | 12.1 ± 3.2 | 6.1 ± 1.7 | <0.001 |
| Disc space narrowing score | 7.5 ± 1.8 | 5.7 ± 1.8 | <0.001 |
| Sclerosis score | 5.1 ± 1.5 | 3.8 ± 1.2 | 0.1227 |
| Listhesis score | 3.1 ± 0.6 | 1.6 ± 0.4 | 0.2255 |
| Total score | 27.84 + 4.7 | 17.2 ± 3.8 | <0.001 |
Arterial color doppler findings
To avoid inter-observer error, doppler ultrasound examination of all 100 patients was performed by the single expert sonologist who was blinded to vertigo status of all patients. No statistically significant difference was observed between group I and group II with respect to doppler ultrasound parameters in neutral head position. Although all doppler parameters were decreased on head rotation as compared to neutral head position in all patients, these parameters were significantly lower in group I than group II at 60° head rotation, specifically in subjects with high CDI score (Figure 4). Logistic regression analysis in a multivariate model showed that osteophyte score and total CDI score were associated with the decreased PSV & EDV in the right and left VAs at the 60° rotation of the cervical vertebrae in the group I. (Table 4 & 5).
Figure 4.
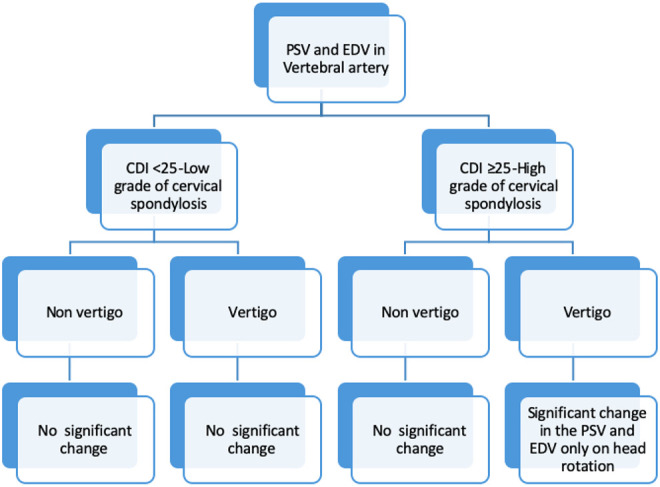
Brief summary of the changes in peak systolic velocity (PSV) and end diastolic velocity (EDV) in vertebral artery in both groups. CDI, cervical degenerative index.
Table 4.
Comparison of the Doppler Findings of Right Vertebral Artery Between 2 Groups.
| Right vertebral artery | P value | |||||
|---|---|---|---|---|---|---|
| Head Positions | Neutral | Right Lateral | Left Lateral | Neutral | Right Lateral | Left Lateral |
| CDI <25 | ||||||
| PSV | 0.9769 | 0.2255 | 0.1227 | |||
| Group I | 48.30 + 21.11 | 44.10 + 18.69 | 43.11 + 19.10 | |||
| Group II | 48.05 + 25.01 | 47.75 + 22.20 | 47.65 + 20.11 | |||
| EDV | 0.8766 | 0.2321 | 0.6543 | |||
| Group I | 19.80 + 7.65 | 17.20 + 8.59 | 18.22 + 5.57 | |||
| Group II | 18.98 + 7.87 | 18.53 + 6.98 | 17.83 + 11.76 | |||
| CDI >25 | ||||||
| PSV | 0.8812 | < 0.001 | < 0.001 | |||
| Group I | 52.43 + 27.10 | 41.12 + 22.10 | 40.89 + 23.10 | |||
| Group II | 51.60 + 26.32 | 49.34 + 27.32 | 48.66 + 28.18 | |||
| EDV | 0.2133 | < 0.001 | < 0.001 | |||
| Group I | 20.90 + 9.11 | 14.56 + 7.77 | 13.58 + 6.72 | |||
| Group II | 21.33 + 9.21 | 20.32 + 7.78 | 19.30 + 8.26 | |||
PSV, peak systolic blood flow velocity; EDV, end diastolic blood flow velocity; CDI, cervical degenerative index.
Table 5.
Comparison of the Doppler Findings of Left Vertebral Artery Between 2 Groups.
| Left vertebral artery | P value | |||||
|---|---|---|---|---|---|---|
| Head Positions | Neutral | Right Lateral | Left Lateral | Neutral | Right Lateral | Left Lateral |
| CDI <25 | ||||||
| PSV | 0.6783 | 0.4666 | 0.5322 | |||
| Group I | 43.46 + 12.75 | 41.30 + 15.94 | 40.60 + 9.48 | |||
| Group II | 44.41 + 13.73 | 43.43 + 15.84 | 42.85 + 19.74 | |||
| EDV | 0.2439 | 0.5818 | 0.3883 | |||
| Group I | 18.00 + 7.62 | 15.60 + 5.06 | 16.20 + 3.85 | |||
| Group II | 21.31 + 7.17 | 18.68 + 5.58 | 19.85 + 13.04 | |||
| CDI >25 | ||||||
| PSV | 0.5818 | < 0.001 | < 0.001 | |||
| Group I | 40.60 + 13.78 | 31.15 + 12.34 | 30.20 + 10.67 | |||
| Group II | 45.60 + 14.49 | 44.0 + 16.81 | 43.45 + 17.23 | |||
| EDV | 0.3851 | < 0.001 | < 0.001 | |||
| Group I | 16.55 + 4.38 | 10.23 + 4.15 | 11.24 + 3.93 | |||
| Group II | 20.34 + 7.05 | 18.80 + 6.03 | 19.58 + 7.81 | |||
PSV, peak systolic blood flow velocity; EDV, end diastolic blood flow velocity; CDI, cervical degenerative index.
Discussion
CS leading to vertigo is not a widely studied phenomenon. Claude Bernard in 1858 first described it, followed by Barré in 1926. Ryan & Cope in 1995 introduced the term “cervical vertigo”. 7 They found that in degenerative cervical myelopathy (DCM), vertigo is usually provoked by head movements. It presents as a to-and-fro vertigo and an unsteadiness of gait that can be induced by lesion in cervical spine on head movements. 7 The vestibular labyrinth, vestibulocochlear nerve, brain stem, cerebellum and occipital lobes are supplied by the vertebrobasilar circulation. Cervical osteophytes can occlude VA during turning of head to the same or opposite side8-11 leading to vertebrobasilar insufficiency. Commonest complaint in a patient with vertebrobasilar insufficiency is vertigo.9,10 The vascular supply to vestibulo-cochlear organ is by an end artery. Hence, it depends on vertebrobasilar circulation leading to increased susceptibility to vertebrobasilar insufficiency. 10 Neurons, axons, and hair cells in the vestibulocochlear system are known to respond to ischemia by depolarizing. This leads to transient hyper-excitability with ectopic discharges. Clinically it manifests as tinnitus, vertigo, and dizziness.
To the best of our knowledge, there is only one study done by Colledge et al 12 which assessed incidence of CS in vertigo patients. CS was the second cause of dizziness (65%) among their patients, based on clinical examination. Though this was one of the pilot studies looking at this symptom complex in CS, it had a few limitations. This study lacked a control group and no imaging analysis was done.
Petersen 10 in 1996 studied reduction in blood flow through basilar artery with head-turning in patients of vertebrobasilar ischemia. The authors studied mechanical compression of VA at the atlanto-axial joint during head rotation. Mitchell 13 in 2003 studied intracranial VA blood flow in normal male & female subjects aged 20-30-yrs, in neutral and maximally rotated cervical spinal positions. These studies introduced the concept of dynamic imaging of the VA. However, it is the sub-axial spine and ages beyond 30 years that are the most affected in CS. Thus, the second part of VA is the most susceptible on dynamic examination and we found significant statistical association between these 2 parameters.
Olszewski et al 14 in 2004 studied 80 CS patients with 40 patients having vertigo. All patients had transcranial doppler ultrasound with head rotations and it confirmed significant association between flow velocity in basilar artery after neck rotation and age, prevalence of vertigo and grade of radiological changes. The authors also concluded that, VA flow velocity in neutral position was not affected by degenerative changes in cervical spine. Strek et al 15 reported that during Doppler ultrasound examination, blood flow velocity in one or both of the VA showed a 50–70% reduction when cervical rotation was applied in patients with CS. VA changes in sub-axial spine on head rotation and using a scoring system (CDI) for grading severity CS, were some limitations of these study designs that we tried to address. Interestingly, we found that osteophyte score was the leading contributor to changes in the VA flow.
Bayrak et al 16 in 2007 evaluated the effect of CS on VA flow in 91 patients with different grades of degeneration on disks and apophysis. They concluded that CS did not correlate with the complaint of vertigo. Among all Doppler measurements, no significant difference was noted between male and female participants which is a consistent finding with our present study. As per total degeneration score, no difference was noted between patients with and without vertigo. But the major limitation of this study was evaluation of VAs with the head in the neutral position only & no dynamic measurements. We studied hemodynamic flow changes of VA in neutral, right lateral and left lateral head position and did dynamic doppler examinations which was found to show significant association.
In our study, we used Doppler sonography for measuring blood flow of VAs. Several techniques, such as MRI, digital subtraction angiography, invasive electromagnetic flow meter, and Doppler Ultra-sonography (US), have been used in the diagnosis of hemodynamic impairment that occurs in the vertebrobasilar system. 17 Because it is non-invasive, inexpensive, readily applicable, reliable, and reproducible, Doppler US has become investigation of choice for in assessing extra-cranial part of VA. The degree of the stenoses can be identified with high accuracy, especially when evaluated by an experienced sonologist.10,11
Doppler examination is routinely done with the head in neutral position. Limited studies14,15 in the literature have focused on the dynamic evaluation of vertebral arteries during head rotation. In our study, no significant decrease in blood flow in neutral head position is found among both groups. Decrease in peak systolic velocity (PSV) in VAs following 60° head rotation was detected in both groups but a more significant reduction was found in spondylotic patients with vertigo group who had higher total CDI score.
VAs are vulnerable to compression by uncovertebral osteophytes, apophyseal osteophytes, or other degenerative changes of cervical spondylosis. The most susceptible is the second part of VA, that traverses through the transverse foramina of C2 to C6. 18 Compression by osteophytes with accompanying surrounding edema contiguous to the VA, in addition to its tortuosity, can diminish the VA flow. The movements of rotation and hyperextension of cervical spine diminish blood flow through the VA; however, this diminution in blood flow generally is compensated by increased blood flow through the opposite VA. But if one of the VAs shows compression by the extensive osteophyte formation in patients with high-grade degenerative changes, turning the head to the opposite side may lead to obstruction of flow in the only effective VA thereby eliciting the features of posterior circulation insufficiency including vertigo consequent to inadequate collateral flow. Our study excluded the patients with atherosclerosis since it may act as a confounding factor. Reduction in VAs blood flow may be attributed to atherosclerosis which prevent collaterals from compensating decreased blood flow in the VA after head rotation. Moreover, the relationship shown in the analysis between the grade of degenerative changes (indicated by the CDI score) and decreased blood flow velocity in the VAs during head rotation confirms the significant (P-value <0.001) effect of extensive spondylosis on VAs blood flow. Interestingly, among the individual components of the total CDI score, osteophyte score was associated with decreased PSV in right & left VAs at 60° rotation of the cervical vertebrae in CS patients with vertigo. Cervical vertigo is the manifestation of chronic vertebrobasilar insufficiency. Prolonged insufficiency leads to cervical vertigo. Patients complain of vertigo on keeping head in specific positions for variable time period which correlate with severity of degenerative changes. 21 patients of vertigo group had exacerbation of symptoms during neck turning, though doppler study showed reduction of VA blood flow in all patients of the group. This indicates that its complex interplay of multiple factors like spatial orientation of head in space, duration of head tilt, CDI apart from only reduction in VA blood flow which finally determines clinical manifestation of vertigo.
Our study has the limitation of a small sample size. Inclusion of non-spondylotic control group could have revealed status of PSV and EDV flow in normal patients which might have helped us to better understand the hemodynamics in normal population. The importance of analyzing the VA in various pathologies of the cervical spine is being increasingly recognized in current literature. 19 A multi centric study with a larger sample size and inclusion of non-spondylotic control group will further validate our findings of the pathophysiological association between CS and vertigo.
Conclusion
In patients with high-grade CS (CDI ≥ 25) and extensive osteophyte formation, VA compression becomes prominent. Head rotation aggravates the compression leading to vertigo due to vertebro-basilar Insufficiency. This highlights the possible pathophysiological association between vertigo and CS.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Chetan Shende, MS (Orthopaedics) https://orcid.org/0000-0003-3073-6329
https://orcid.org/0000-0003-3073-6329
Nandan Marathe, MS (Orthopaedics) https://orcid.org/0000-0002-8939-2690
https://orcid.org/0000-0002-8939-2690
Abhinandan Reddy Mallepally, MS (Orthopaedics) https://orcid.org/0000-0001-8153-7499
https://orcid.org/0000-0001-8153-7499
Ashwin Sathe, MS (Orthopaedics) https://orcid.org/0000-0002-1196-0694
https://orcid.org/0000-0002-1196-0694
References
- 1.Yardley L, Owen N, Nazareth I, Luxon L. Prevalence and presentation of dizziness in a general practice community sample of working age people. Br J Gen Pract. 1998;48(429):1131–1135. [PMC free article] [PubMed] [Google Scholar]
- 2.Abrol R, Nehru VI, Venkatramana Y. Prevalence and etiology of vertigo in adult rural population. Indian J Otolaryngol Head Neck Surg. 2001;53(1):32–36. doi:10.1007/BF02910976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ofiram E, Garvey TA, Schwender JD, et al. Cervical degenerative index: a new quantitative radiographic scoring system for cervical spondylosis with interobserver and intraobserver reliability testing. J Orthop Traumatol. 2009;10(1):21–26. doi:10.1007/s10195-008-0041-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machaly S, Senna M, Sadek A. Vertigo is associated with advanced degenerative changes in patients with cervical spondylosis. Clin Rheumatol. 2011;30(12):1527–1534. doi:10.1007/s10067-011-1770-x [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Warlow CP. Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke. 2000;31(3):622–630. doi:10.1161/01.str.31.3.622 [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Chow S. Sample size calculation for comparing proportions. Encyclopedia of Statistical Sciences. 2005:1–14. doi:10.1002/0471667196.ess7211 [Google Scholar]
- 7.Ryan GM, Cope S. Cervical vertigo. Lancet. 1955;269(6905):1355–1358. doi:10.1016/s0140-6736(55)93159-7 [DOI] [PubMed] [Google Scholar]
- 8.Wrisley DM, Sparto PJ, Whitney SL, Furman JM. Cervicogenic dizziness: a review of diagnosis and treatment. J Orthop Sports Phys Ther. 2000;30(12): 755–766. doi:10.2519/jospt.2000.30.12.755) [DOI] [PubMed] [Google Scholar]
- 9.Hedera P, Bujdáková J, Traubner P. Blood flow velocities in basilar artery during rotation of the head. Acta Neurol Scand. 1993;88(3):229–233. doi:10.1111/j.1600-0404.1993.tb04224.x [DOI] [PubMed] [Google Scholar]
- 10.Petersen B, von Maravic M, Zeller JA, Walker ML, Kömpf D, Kessler C. Basilar artery blood flow during head rotation in vertebrobasilar ischemia. Acta Neurol Scand. 1996;94(4):294–301. doi:10.1111/j.1600-0404.1996.tb07068.x [DOI] [PubMed] [Google Scholar]
- 11.Olszewski J, Zalewski P, Machała W, Gaszyński W. Zastosowanie testu skretu szyi przy badaniu predkości przepływu krwi metoda doplerowska w układzie tetnic kregowych i podstawnej u osób ze zmianami zwyrodnieniowymi odcinka szyjnego kregosłupa [Administration of the cervical torsion test by the examination of Doppler’s blood flows in vertebral arteries and basilar artery in patients with degenerative cervical spine changes]. Otolaryngol Pol. 1994;48(6):549–555. [PubMed] [Google Scholar]
- 12.Colledge NR, Barr-Hamilton RM, Lewis SJ, Sellar RJ, Wilson JA. Evaluation of investigations to diagnose the cause of dizziness in elderly people: a community based controlled study. BMJ. 1996;313(7060):788–792. doi:10.1136/bmj.313.7060.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell JA. Changes in vertebral artery blood flow following normal rotation of the cervical spine. J Manipulative Physiol Ther. 2003;26(6):347–351. doi:10.1016/S0161-4754(03)00074-5 [DOI] [PubMed] [Google Scholar]
- 14.Olszewski J, Majak J, Pietkiewicz P, Luszcz C, Repetowski M. The association between positional vertebral and basilar artery flow lesion and prevalence of vertigo in patients with cervical spondylosis. Otolaryngol Head Neck Surg. 2006;134(4):680–684. doi:10.1016/j.otohns.2005.11.023 [DOI] [PubMed] [Google Scholar]
- 15.Strek P, Reroń E, Maga P, Modrzejewski M, Szybist N. A possible correlation between vertebral artery insufficiency and degenerative changes in the cervical spine. Eur Arch Otorhinolaryngol. 1998;255(9):437–440. doi:10.1007/s004050050094 [DOI] [PubMed] [Google Scholar]
- 16.Bayrak IK, Durmus D, Bayrak AO, Diren B, Canturk F. Effect of cervical spondylosis on vertebral arterial flow and its association with vertigo [published correction appears in Clin Rheumatol. 2009 Jan;28(1):107. Canturk, Feryal [corrected to Canturk, Ferhan]]. Clin Rheumatol. 2009;28(1):59–64. doi:10.1007/s10067-008-0983-0 [DOI] [PubMed] [Google Scholar]
- 17.Ozdemir H, Berilgen MS, Serhatlioglu S, et al. Examination of the effects of degeneration on vertebral artery by using neural network in cases with cervical spondylosis. J Med Syst. 2005;29(2):91–101. doi:10.1007/s10916-005-2998-2 [DOI] [PubMed] [Google Scholar]
- 18.Shinohara N, Kohno K, Takeda S, Ohta S, Sakaki S. [A case of bow hunter’s stroke caused by bilateral vertebral artery occlusive change on head rotation to the right]. No Shinkei Geka. 1998;26(5):417–422. [PubMed] [Google Scholar]
- 19.Rathod T, Garje V, Marathe N, et al. Incidence and outcome analysis of vertebral artery injury in posttraumatic cervical spine. Asian J Neurosurg. 2020;15(3):644–647. doi:10.4103/ajns.AJNS_45_20 [DOI] [PMC free article] [PubMed] [Google Scholar]


