Abstract
Study Design:
Retrospective cohort study.
Objectives:
To investigate the usefulness of selective single-level lumbar endoscopic unilateral laminotomy for bilateral decompression (LE-ULBD) in patients with radiological multilevel lumbar spinal stenosis (LSS) and clarify the predictive factors of reoperation.
Methods:
A total of 128 patients who underwent LE-ULBD of radiological multilevel LSS were retrospectively examined. Single-level decompression was selected clinically and supplemented radiologically. Clinical outcomes were assessed with the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOABPEQ), numeric rating scale (NRS), and Macnab criteria (mean follow-up period, 28.6 months [range, 24-63 months]). Stenosis severity was classified as grades M (moderate) and S (severe) based magnetic resonance imaging findings. Multilevel LSS was classified as SS, SM, and MM according to the number of grade S levels.
Results:
The follow-up rate was 74.2%. All domains of the JOABPEQ and NRS significantly improved during follow-up. The Macnab outcome classification was “excellent” or “good” in 77.9% of the patients. The reoperation rate was 10.2%. None of the patients with unilateral symptoms required reoperation. The SS type was a significant risk factor of reoperation for multilevel LSS with bilateral symptoms. Additional LE-ULBD was performed for all the reoperation with the “excellent” or “good” results of the Macnab criteria in 69% of the patients.
Conclusions:
Selective single-level LE-ULBD provided favorable results for multilevel LSS. However, information about the risks of reoperation for multilevel severe stenosis with bilateral symptoms should be shared between surgeons and patients.
Keywords: full-endoscopic spine surgery, lumbar spinal stenosis, decompression, multilevel, reoperation
Introduction
Lumbar spinal stenosis (LSS) is diagnosed clinically, supplemented with radiological examination. Radiologic multilevel stenotic findings often show non-specific or atypical clinical features. 1 In the surgical treatment of radiologic single-level LSS, decompression with or without fusion of the stenotic level has been the gold standard procedure. In the absence of instability or deformity, fusion procedures are generally not recommended. 2 For multilevel lumbar spinal stenosis, especially multilevel lumbar spinal stenosis with degenerative spondylolisthesis, the choice of surgical procedure remains controversial.
Some surgeons choose extensive laminectomy, although it may cause segmental instability. Therefore, other surgeons choose multisegmental decompression and fusion to avoid iatrogenic spinal instability and ensure long-term efficacy. However, Takahashi et al suggested in their study that multilevel laminectomy delays the resolution of cauda equina adhesions, which may lead to the development of certain clinical symptoms. 3 Furthermore, radiological stenosis is not always the cause of the symptoms because it can be present to a certain degree in asymptomatic subjects.4,5 Therefore, decompression is not necessary for all stenotic spinal levels found radiologically, and surgery should be decided on the basis of the patient’s clinical findings. Precise decompression is advocated to maximize the clinical improvement and minimize the risk of iatrogenic instability with minimally invasive surgery.6-8
Some surgeons choose selective decompression and fusion. 6 However, adjacent segment disease (ASD) is common after posterior lumbar spinal fusion. Preservation of more spinal motion segments with shorter fusion may prevent ASD.9,10 Ulrich et al compared the results of decompression alone and those of decompression with fusion in patients with degenerative LSS and spondylolisthesis, and reported that both procedures provided comparable favorable outcomes. 11 Therefore, selective decompression with a minimally invasive procedure might be an ideal surgical option for multilevel LSS without segmental instability.
Lumbar endoscopic unilateral laminotomy for bilateral decompression (LE-ULBD) is a full-endoscopic spine surgery for posterior decompression of LSS that uses an 8-mm diameter cannula and full-endoscopic instruments. 12 In a clear and magnified surgical field under saline irrigation, central-to-bilateral lateral recess decompression via a unilateral approach can be performed. Owing to its less invasiveness to the spinal structures, including the facet joint and ligaments, LE-ULBD confers a low risk of iatrogenic postoperative instability. In our practice, we have adopted a selective one-level LE-ULBD for multilevel LSS. However, information on clinical efficacy, the risk of complication, and the incidence of recurrence of symptoms related to postoperative instability or ASD has been limited.
This study aimed to retrospectively examine the clinical results of LE-ULBD for patients with multilevel LSS by using patient-reported outcome measures (PROM), a numeric rating scale (NRS), and the Macnab criteria. We hypothesized that LE-ULBD provides favorable results with multilevel LSS. We also investigated the predictive factors of reoperation due to symptom relapse.
Materials and Methods
From June 2014 to November 2018, 313 consecutive patients who underwent single-level LE-ULBD for LSS in the first author’s institute were retrospectively reviewed for this study. The surgical inclusion criteria for LE-ULBD of LSS were as follows: (1) neurogenic claudication or radiculopathy symptoms with or without lower back pain; (2) patients who failed to respond to conservative treatments for at least 3 months; (3) LSS including grade I spondylolisthesis without instability; and (4) LSS including intracanalicular disk herniation. The exclusion criteria were as follows: (1) patients with segmental instability defined as translation of >4 mm or 10° of angular motion between flexion and extension on upright lateral radiographs; (2) LSS with grade ≥II spondylolisthesis; (3) severe foraminal stenosis; (4) severe degenerative scoliosis with Cobb’s angle of >30°; (5) dominant back pain without lower limb symptoms; (6) patients with a history of previous spine surgery or referred leg pain due to other pathologies such as polyneuritis; and (7) patients with multilevel LSS with severe cauda equina syndrome such as numbness around the anus and loss of bowel or bladder control.
Among the 313 patients who underwent LE-ULBD on the basis of the above-mentioned criteria, 57 had concomitant LDH. In this study, we excluded patients with LDH to unify the pathology. Among the remaining 256 patients, 128 patients who showed radiological single-level LSS were also excluded from this study.Consequently, 128 patients who had radiological multilevel LSS were included in this study. The demographic data of the patients is presented in Table 1.
Table 1.
Demographic Data of Multilevel Lumbar Spinal Stenosis Patients.
| Total | Follow-up | Lost to follow-up | ||
|---|---|---|---|---|
| Number of patients | 128 | 95 | 33 | |
| Number of patients without DS | 82 | 65 | 17 | |
| Number of patients with DS | 46 | 30 | 16 | |
| Sex, male/female | 75/53 | 53/42 | 22/11 | |
| Age, years (range) | 72.1 (45-90) | 72.5 (45-90) | 70.8 (45-85) | |
| Duration of symptoms before surgery, mon. (S.D.) | 41.1 (49.5) | 35.6 (37.0) | 57.1 (73.2) | |
| Type of symptoms | Unilateral symptoms | 21 | 21 | 0 |
| Bilateral symptoms | 107 | 74 | 33 | |
| Number of levels of decompression / radiological spinal stenosis | L1-L2 | 0/5 | 0/3 | 0/2 |
| L2-L3 | 2/38 | 2/27 | 0/11 | |
| L3-L4 | 21/116 | 15/89 | 6/27 | |
| L4-L5 | 105/124 | 78/92 | 27/32 | |
| L5-S1 | 0/21 | 0/12 | 0/9 | |
| Operative time, min (S.D.) | 67.9 (15.1) | 67.6 (15.5) | 68.7 (14.2) | |
| Preoperative comorbidities | 98 | 75 | 23 | |
| Perioperative complications | 14 | 10 | 4 | |
| CSF leakage | 5 | 5 | 0 | |
| epidural hematoma | 1 | 0 | 1 | |
| drain tube trouble | 2 | 2 | 0 | |
| transient motor weakness | 6 | 3 | 3 | |
Abbreviations: LSS: lumber spinal stenosis, DS: degenerative spondylolisthesis, CSF: cerebrospinal fluid.
The patients were further classified into 2 groups as those with bilateral and unilateral symptoms, depending on whether neurogenic claudication due to pain, weakness, fatigue, tingling, weakness, heaviness, and/or paresthesia extended into the bilateral lower extremities or unilateral lower extremity.There were 21 patients (14.4%) with unilateral symptoms and 107 patients (83.6%) with bilateral symptoms.
Decompression was performed at a selected level on each patient. Radiological LSS was observed on magnetic resonance imaging (MRI) at 293 levels in total as follows: L1/L2, 5; L2/L3, 38; L3/L4, 116; L4/L5, 124; and L5/S1, 11. The mean number of LSS levels in each patient was 2.3. Decompression was performed at the following levels: L1/L2, 0; L2/L3, 2; L3/L4, 21; L4/L5, 105; and L5/S1, 0.
Grading of Spinal Stenosis Severity Based on Dural Sac Morphology on MRI
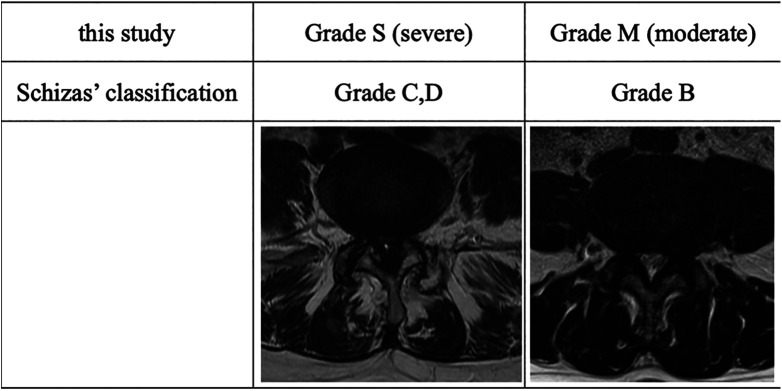
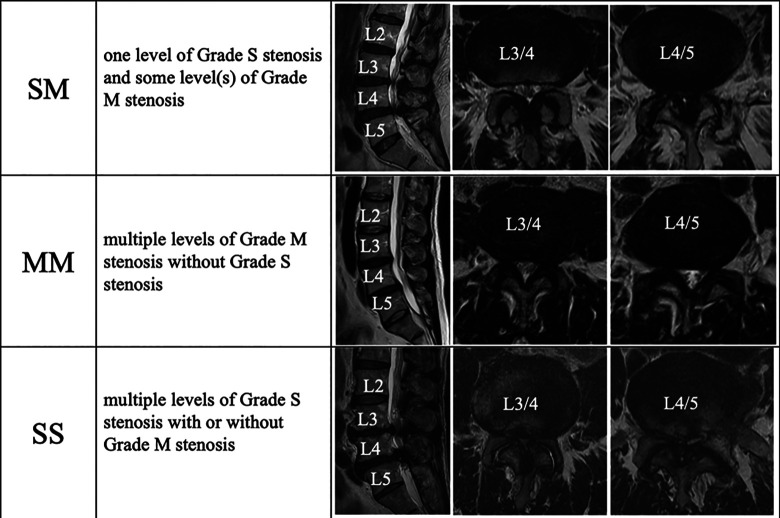
With reference to the qualitative grading system based on the morphological appearance of the dural sac on T2-weighted axial images of the lumbar spine, taking into account the cerebrospinal fluid (CSF)/rootlet content designed by Schizas et al, 13 we defined grade M (moderate) as stenosis with rootlets occupying the whole dural sac but that can still be individualized. Some CSF is still present, giving a grainy appearance to the sac. Grade S (severe) was defined as stenosis without recognizable rootlets and with a dural sac demonstrating a homogeneous gray signal but no visible CSF signal (Figure 1). Then, each patient was classified into 3 types as follows: those with the SM type with one level of grade S stenosis and some level(s) of grade M stenosis, those with the SS type with multiple levels of grade S stenosis, and those with MM type with multiple levels of grade M stenosis without grade S stenosis (Figure 2).
Figure 1.
Qualitative grading system based on the morphological appearance dictated by the cerebrospinal fluid-to-rootlet ratio as observed on axial T2 images at disc levels. The grading was based on the following observation of different patterns, according to which the rootlets were disposed within the dural sac while the patient rested supine during magnetic resonance imaging scan acquisition.
Figure 2.
Multilevel LSS was classified into 3 types as follows: SM, with one level of grade S stenosis and some level with grade M stenosis; SS, with multiple levels of grade S stenosis; and MM, with multiple grade M stenosis without grade S stenosis.
Selection of Decompression Level in the Patients With Multilevel LSS
Decompression was performed at one site corresponding to the selected stenotic level, taking into account the patient’s clinical picture, associated pain patterns on physical examination, and radiological diagnostic measures. From among multiple stenotic levels, the level that matched the most severe neurological deficit was selected. In the bilateral symptom type, when multiple levels of involvement were suspected, the level that was neurologically consistent with the most proximal disorder was selected.
Surgical Procedures and Postoperative Treatment
LE-ULBD is bilateral decompression via a unilateral approach under general anesthesia with intraoperative monitoring. Through an approximately 8-mm skin incision slightly paramedian to the entry side, the cannula is placed on the interlaminar window. Entry side was chosen on the dominant side of the symptoms. All the surgical procedures are performed under full endoscopy with 25°-angled optics. Shaving the bony structures of the cranial lamina with a high-speed drill is started from the base of the spinous process to the bilateral sides until the attachments of the ligamentum flavum are exposed. The attachment to the caudal lamina is resected at the enthesis by using Kerrison punches or a high-speed drill. After bony decompression, the ligamentum flavum can be detached from the inner surface of the laminae on the cranial, ipsilateral, and contralateral sides and floated for careful removal. After confirming hemostasis in the epidural space, a drain tube is placed. Patients are allowed to walk again 3 h after operation, and the drain tube is removed 48 h after operation.
Evaluations
Medical records, surgical records, intraoperative videos, radiographs, computed tomography images, and MRI findings were retrospectively reviewed to determine the perioperative complications, prognosis, and reoperation rate.
The patients’ clinical outcomes were evaluated with the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOABPEQ) and a NRS before surgery and at final follow-up. A surgeon-performed retrospective assessment using the Macnab classification was performed for overall treatment outcome at the final follow-up. JOABPEQ is a PROM used to evaluate the health-related quality of life of patients with lumbar spine disorders. 14 It includes the following 5 domains, with each domain comprising of 25 questions: low back pain, lumbar function, walking ability, social life function, and mental health. Effectiveness was defined as an improvement in the score of each domain by >20 points. The NRS scores (rated from 0 to 10), with 0 indicating the absence of no pain/numbness and 10 indicating extremely intense pain/numbness imaginable, for low back pain, lower limb pain, and lower limb numbness were recorded by the patients.
Statistical Analyses
The power analysis revealed that when we assumed a power of 0.8, an alpha of 0.05, and a standard deviation (SD) of 20 points, the sample size allowed the detection of a difference of 20 points using the JOABPEQ, with 10 patients in a paired test and 34 in an unpaired test of. The Shapiro-Wilk test was used to determine the normality of continuous variables. The Wilcoxon signed-rank test was used for the preoperative and postoperative comparisons of variables with non-normal distribution. The Mann-Whitney U test was used to compare the means between the 2 groups. The chi-square or Fisher exact test was used to compare the categorical variables. A logistic regression analysis was performed to detect the factors related to the incidence of reoperation. The JMP ver. 15 software was used to conduct all the statistical analyses.
Ethical Consideration
Our institutional ethical review board approved the present study (approval No. 202 008 003). Informed consent was obtained using the opt-out form on the center’s website. All data was handled in accordance with the ethical standards of the Declaration of Helsinki.
Results
Clinical Evaluations
The mean operative time was 67.9 min. Intraoperative blood loss was too small to measure. Patients who 1) completed the preoperative and postoperative evaluations and 2) were followed up for ≥24 months were clinically evaluated. However, 33 patients could not complete the clinical evaluations, of whom 5 had incomplete preoperative clinical data; 12 were followed up for <24 months; and 3 underwent surgery on other spinal sites, which might have affected the clinical results. Reoperation was performed within 24 months after the initial surgery in 13 patients. As a result, 95 completed follow-up ≥24 months (follow-up rate: 74.2%, 53 men and 42 women, Table 1). The mean follow-up period was 28.6 months (range, 24-57 months). There were no significant differences in sex and age at time of surgery between the patients who did and did not complete the clinical follow-up. The mean follow-up period of the patients who did not complete the clinical follow-up was 21.8 months (range, 12-64 months).
Table 2 shows clinical results of the patients who completed the follow-up for ≥24 months. The scores in all the domains of the JOABPEQ and NRS were significantly improved at follow-up as compared with those at baseline (P < 0.0001).
Table 2.
Clinical Results of Patients Who Completed the Follow-Up for ≥24 Months (n = 95).a
| Preoperative, Mean (SD) | Follow-up, Mean (SD) | P-value | Effectiveness rate (%) of JOABPEQ | ||
|---|---|---|---|---|---|
| NRS | Low-back pain | 6.3 (2.9) | 3.0 (2.9) | <0.0001 | |
| Lower-limb pain | 7.5 (2.4) | 3.5 (3.2) | <0.0001 | ||
| Lower-limb numbness | 7.1 (2.8) | 3.6 (3.3) | <0.0001 | ||
| JOABPEQ | Low-back pain | 36.3 (30.7) | 70.4 (32.3) | <0.0001 | 71.1 |
| Lumbar function | 51.5 (29.6) | 67.0 (30.0) | <0.0001 | 50.0 | |
| Walking ability | 25.4 (25.0) | 57.6 (33.9) | <0.0001 | 58.9 | |
| Social life function | 32.6 (18.4) | 55.8 (25.5) | <0.0001 | 56.3 | |
| Mental health | 39.5 (16.2) | 54.6 (20.1) | <0.0001 | 35.9 |
a Wilcoxon signed-rank test.
In the assessment with the Macnab criteria, 14 patients who underwent reoperation were included, and they were classified as “poor” (Table 3). “Excellent” or “good” criteria were obtained in 84 patients (77.8%). As a worst-case scenario, all the patients who did not complete the clinical follow-up was classified as “poor,” the ratio of “excellent” and “good” was 65.6%.
Table 3.
Macnab Outcome Classification.a
| Followed-up + reoperation n = 108 | Worst-case scenario n = 128 | ||
|---|---|---|---|
| Macnab outcome classification | Excellent | 39 | 39 |
| Good | 45 | 45 | |
| Fair | 11 | 11 | |
| Poor | 13 | 33 |
a Patients who required reoperation were included in the “poor” classification. The worst-case scenario is defined as all patients who did not complete the follow-up were classified as “poor.”
Perioperative Complications
The patients reported preoperative comorbidities such as hypertension (43%), diabetes mellitus (17%), and cardiovascular disease (21%). In spite of the high prevalence of preoperative comorbidities, we found no major complications related to the comorbidities.
Operation-related complications were identified in 15 (11.7%) of the 128 patients. The incidence and features of the complications are shown in Table 1. Intraoperative leakage of cerebrospinal fluid was observed in 5 patients. The injury to the dural sac was the size of a pinhole, so a repair was not performed and a suction drain was not placed. Epidural breeding was controlled with a bi-polar coagulator. All complications were treated conservatively, and the symptoms were relieved. No other serious complications such as wound infection, dural nerve root injury, blood vessel injury, and deep vein thrombosis were observed.
Reoperation Cases
Reoperation was performed in 13 (10.2%) of the 128 patients at a mean (SD) of 19 (13) months after initial operation because of symptom recurrence during the follow-up period. In all the cases, single-level LE-ULBD was performed at the residual stenotic level, in addition to the initial decompression.
The clinical results of the revision surgery are shown in Table 4. A mean follow-up of 14.3 months (range, 4-24 months) after the second LE-ULBD, and a significant improvement was observed in NRS-low back pain, NRS-lower leg pain, NRS-lower leg numbness, and walking ability of JOABPEQ. According to the Macnab criteria, excellent and good results were obtained in 9 (69%) patients.
Table 4.
Clinical Results of Reoperation (n = 13).a
| Preoperative, Mean (SD) | Follow-up, Mean (SD) | P-value | Effectiveness rate (%) | ||
|---|---|---|---|---|---|
| NRS | Low back pain | 6.5 (3.4) | 3.8 (3.6) | 0.023 | |
| Lower-limb pain | 6.6 (3.3) | 2.9 (3.2) | 0.031 | ||
| Lower-limb numbness | 7.5 (2.2) | 4.0 (4.1) | 0.035 | ||
| JOABPEQ | Low back pain | 50.5 (40.2) | 67.0 (47.2) | 0.53 | 55.6 |
| Lumber function | 56.3 (33.2) | 69.8 (29.1) | 0.26 | 33.3 | |
| Walking ability | 29.2 (28.5) | 47.2 (28.5) | 0.011 | 53.8 | |
| Social life function | 38.3 (22.4) | 54.5 (28.0) | 0.083 | 33.3 | |
| Mental health | 43.2 (18.6) | 47.7 (22.7) | 0.63 | 15.4 |
a Wilcoxon signed-rank test.
To investigate the risk of reoperation, the 128 patients were neurologically classified into 2 groups, those with bilateral symptoms (107 patients) and those with unilateral symptoms (21 patients). Reoperation was performed in 13 (14.9%) of 107 patients with bilateral symptoms but was not required in any of the 21 patients with unilateral symptoms. Table 5 shows the classification of the morphological severity of spinal stenosis. The patients were classified into those with the SM (80 patients), MM (22 patients), and SS types (26 patients). The reoperation rate was significantly higher in the SS type (P = 0.0078). No significant differences in age, sex, follow-up period, symptom duration, and incidence of DS were found between the patients with or without reoperation. Multivariable logistic regression analysis to identify the factors related to the incidence of reoperation revealed that stenosis severity is a significant factor (P = 0.0281). The SS type was associated with increased risk of reoperation as compared with the SM type (odds ratio = 5.93, 95% confidence interval: 1.56-22.6, P = 0.0090).
Table 5.
Reoperation Rate Among the 3 Types of Stenosis Severity: SM, MM, and SS in Multilevel LSS.a
| Total | Unilateral symptom | Bilateral symptom | ||||
|---|---|---|---|---|---|---|
| Case | Reoperation | Case | Reoperation | Case | Reoperation | |
| Total | 128 | 13 (10.2%) | 21 | 0 | 107 | 13 (12.1%) |
| SM | 80 | 4 (5%) | 15 | 0 | 65 | 4 (6.2%) |
| MM | 22 | 2 (9.1%) | 5 | 0 | 17 | 2 (11.8%) |
| SS | 26 | 7 (26.9%) | 1 | 0 | 25 | 7 (28%) |
| P-value | 0.0078 | 0.0178 | ||||
Abbreviations: S, severe; M, mild.
a Fisher exact test.
Discussion
On the basis of our study, selective single-level LE-ULBD appears to be an effective surgical procedure for patients with multilevel LSS. The preoperative JOABPEQ and NRS scores significantly improved postoperatively. The “excellent” or “good” result of the assessment with the Macnab criteria was obtained in 77.8% of the patients who completed the follow-up. These results were comparable with those reported previously. 15 However, reoperation was required owing to symptom recurrence in 13 patients (10%). Factors related to the risk of reoperation for multilevel LSS were revealed to be dependent on whether the preoperative symptom occurred in the lower leg bilaterally or unilaterally, and stenosis severity was classified on the basis of MRI findings.
Lee et al reported that the reoperation rate after LE-ULBD was 1.9% in their meta-analysis in which selective single-level surgery was performed for single- and multilevel LSS in total of 156 patients from 5 studies. 16 However, the number of patients with multiple stenosis was only 26 in their study. In our study, reoperation was performed in 10.2% of the patients with multilevel LSS who underwent selective single-level decompression. All the reoperations were performed in the patients with bilateral symptoms. In the patients with unilateral symptoms, the responsible pathology was assumed to be a single nerve root rather than the entire dural tube at the stenotic level. Therefore, nerve root decompression was effective for improving the symptom even though the radiological findings of stenosis were found at another level that was not related to the patient’s symptom. However, in the patients with bilateral symptoms, the responsible pathology was assumed to be bilateral nerves compressed at the nerve root level or within the dural tube. Therefore, symptoms due to stenosis at other spinal levels might have been hidden by the most severe symptom at the decompressed level. In fact, the risk of reoperation was high in the patients with stenosis severity classified as SS, which means that severe stenosis occurred in at least 2 levels.
In minimally invasive surgery, the combination of neurological examination and radiological findings can guide in determining the surgical site for efficient outcomes. Amundsen et al found no relationship between the degree of stenosis (measured on myelography and CT) and clinical symptoms in 100 patients selected from a neurology department on the basis of clinical symptoms of LSS. 8 Sirvanci et al examined the correlation between imaging and Oswestry disability index (ODI) in 63 surgical candidates with LSS. They studied cross-sectional areas and subjective criteria of lateral recess and foraminal stenosis on axial MRI scans but found no significant correlation between those parameters and ODI. 17 On the other hand, Schizas et al designed a qualitative grading system based on the morphological appearance dictated by the CSF-to-rootlet ratio as observed on axial T2 images at disc levels. The grading was based on the different patterns observed, according to which the rootlets were disposed within the dural sac while the patient rested supine during the MRI acquisition. The grades were defined as follows: grade A, no or minor stenosis (CSF clearly visible inside the dural sac); grade B, moderate stenosis (rootlets occupy the whole of the dural sac with some residual CSF); grade C, severe stenosis (no CSF signal visible); and grade D, extreme stenosis (no CSF signal visible and no epidural fat found posteriorly; Figure 1). They reported that in patients with grades C and D stenosis, conservative treatment is more likely to fail. 13 Soman et al also reported that morphological grading is a useful tool in deciding whether to perform surgery for multilevel LSS. Grade C and D stenosis should be decompressed, whereas grades A and B should not be decompressed, unless clinically justified. 1 In this study we defined Schizas’ grade B as grade M (moderate) and grades C and D as grade S (severe).
LE-ULBD was selectively conducted at the level that matched the most severe neurological deficit from multiple stenotic levels. In the patients with bilateral symptoms, additional LE-ULBD was necessary at another level of the initial surgery in 12% of the patients. For the SS type, single-level LE-ULBD was selectively performed at one level among the multiple levels of grade S. The remaining stenotic level of grade S caused relapse of neurogenic claudication and reoperation in 28% of the patients. For the SM type, LE-ULBD was selectively performed at the grade S level. In 11.8% of the patients with the SM type and 6.2% of the patients with the MM type after initial LE-ULBD, the remaining grade M level caused relapse of neurogenic claudication. No statistically significant difference in mean duration from initial operation to reoperation was found among the 3 types. However, the reoperation rate for SS was significantly higher than those for the other types (Table 5). In the patients with the SS type, multiple stenotic levels are suspected to be involved in the neurogenic symptom simultaneously, and selecting one decompression level from multiple stenotic levels is difficult. Therefore, simultaneous multiple decompression at levels that neurologically match all the physical examination results can be a surgical option for patients with multilevel LSS who show bilateral symptoms. However, from the opposite point of view, selective single-level decompression did not require reoperation in 88% of the patients with bilateral symptoms. Successful results were obtained in 78% of the patients on the basis of the Macnab criteria. Therefore, decision making based on these findings should be shared between surgeons and patients, taking into account the balance of risk and benefit when considering surgery for multilevel LSS.
In fact, perioperative complications occurred in 15 patients (11.7%). All of the complications were treated conservatively with success. However, if the dura was severely damaged and the cauda equina erupted, the surgery was converted to an open operation for repair. Shibayama et al reported the successful repair of an incidental dural tear without suturing by applying a polyglactin sheet that was soaked in fibrinogen under microendoscopic surgery. 18 In case of a pinhole injury, it is left untreated without any postoperative complications.
This study had several limitations. First, it was retrospective in design and analyzed only short-term outcomes. Reoperations were performed at a mean of 20 months (range, 4-52 months) after initial operation. The mean follow-up period was 28.6 months (range, 24-63 months); however, in some patients, symptoms relapsed owing to another residual stenotic level later than 2 years after surgery. Guigui et al reported that regrowth of bone occurred in 7 to 11% of the patients in 8 years after decompressive spine surgery. 19 Moreover, our study did not directly compare different techniques (selective single-level LE-ULBD vs. multilevel LE-ULBD or conventional open decompression with or without fusion) to determine the superiority of a certain technique. Further long-term prospective randomized studies are needed to compare LE-ULBD with other techniques to determine its long-term durability and superiority as a treatment method for multilevel LSS.
Conclusions
Selective single-level LE-ULBD for patients with multilevel LSS had favorable clinical outcomes, with excellent and good outcomes according to Macnab’s criteria in 78% of the patients. Reoperation was performed in 12.1% of the patients with bilateral symptoms but in none of the patients with unilateral symptoms. Multilevel severe stenosis can be a risk factor of reoperation in patients with bilateral symptoms. Information about the risks of reoperation should be shared between surgeons and patients when considering surgery for multilevel LSS.
Footnotes
Authors’ Note: Ethical Approval code: 202 008 003. The institutional ethical board of Kitakyushu Municipal Medical Center. Informed consent was obtained using the opt-out form on the center’s website. All data was handled in accordance with the ethical standards of the Declaration of Helsinki.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ken Okazaki, MD, PhD  https://orcid.org/0000-0003-1274-8406
https://orcid.org/0000-0003-1274-8406
References
- 1.Soman SM, Chokshi J, Chhatrala N, Tharadara GH, Prabhakar M. Qualitative grading as a tool in the management of multilevel lumbar spine stenosis. Asian Spine J. 2017;11(2):278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Försth P, Ólafsson G, Carlsson T, et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374(15):1413–1423. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi N, Konno S, Kikuchi S. A histologic and functional study on cauda equina adhesion induced by multiple level laminectomy. Spine (Phila Pa 1976). 2003;28(1):4–8. [DOI] [PubMed] [Google Scholar]
- 4.Amundsen T, Weber H, Lilleås F, Nordal HJ, Abdelnoor M, Magnaes B.Lumbar spinal stenosis. Clinical and radiologic features. Spine (Phila Pa 1976). 1995;20(10):1178–1186. [DOI] [PubMed] [Google Scholar]
- 5.Park DK, An HS, Lurie JD, et al. Does multilevel lumbar stenosis lead to poorer outcomes? A subanalysis of the spine patient outcomes research trial (SPORT) lumbar stenosis study. Spine (Phila Pa 1976). 2010;35(4):439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun W, Xue C, Tang XY, et al. Selective versus multi-segmental decompression and fusion for multi-segment lumbar spinal stenosis with single-segment degenerative spondylolisthesis. J Orthop Surg Res. 2019;14(1):46. doi:10.1186/s13018-019-1092-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smorgick Y, Park DK, Baker KC, et al. Single-versus multilevel fusion for single-level degenerative spondylolisthesis and multilevel lumbar stenosis four-year results of the spine patient outcomes research trial. Spine (Phila Pa 1976). 2013;38(10):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleâs F. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine (Phila Pa 1976). 2000;25(11):1424–1435; discussion 1435. [DOI] [PubMed] [Google Scholar]
- 9.Lee CS, Chung SS, Shin SK, Park SJ, Lee HI, Kang KC. Differences in postoperative functional disability and patient satisfaction between patients with long (three levels or more) and short (less than three) lumbar fusions. J Bone Joint Surg Br. 2011;93(10):1400–1404. [DOI] [PubMed] [Google Scholar]
- 10.Hamawandi SA, Sulaiman II, Al-Humairi AK. Microdecompression versus open laminectomy and posterior stabilization for multilevel lumbar spine stenosis: a randomized controlled trial. Pain Res Manag. 2019;2019:7214129. doi:10.1155/2019/7214129. eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulrich NH, Burgstaller JM, Pichierri G, et al. ; LSOS Study Group. Decompression surgery alone versus decompression plus fusion in symptomatic lumbar spinal stenosis: a Swiss prospective multicenter cohort study with 3 years of follow-up. Spine (Phila Pa 1976). 2017;42(18):E1077–E1086. [DOI] [PubMed] [Google Scholar]
- 12.Hofstetter CP, Ahn Y, Choi G, et al. AO spine consensus paper on nomenclature for working-channel endoscopic spinal procedures. Global Spine J. 2020;10(2 suppl):111S–121S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of dural sac on magnetic resonance images. Spine (Phila Pa 1976). 2010;35(21):1919–1924. [DOI] [PubMed] [Google Scholar]
- 14.Fukui M, Chiba K, Kawakami M, et al. ; Subcommittee of the Clinical Outcome Committee of the Japanese Orthopaedic Association on Low Back Pain and Cervical Myelopathy Evaluation. JOA back pain evaluation questionnaire (JOABPQ)/JOA cervical myelopathy evaluation questionnaire (JOACMEQ) the report on the development of revised versions April 16, 2007. J Orthop Sci. 2009;14(3):348–365. [DOI] [PubMed] [Google Scholar]
- 15.Yoshikane K, Kikuchi K, Okazaki K. Lumbar endoscopic unilateral laminotomy for bilateral decompression for lumbar spinal stenosis provides comparable clinical outcomes in patients with and without degenerative spondylolisthesis. World Neurosurg. 2021;150:e361–e371. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Choi M, Ryu DS, et al. Efficacy and safety of full-endoscopic decompression via interlaminar approach for central or lateral recess stenosis of the lumbar spine. A meta-analysis. Spine (Phila Pa 1976). 2018;43(24):1756–1764. [DOI] [PubMed] [Google Scholar]
- 17.Sirvanci M, Bhatia M, Ganiyusufoglu KA, et al. Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J. 2008;17(5):679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibayama M, Mizutani J, Takahashi I, Nagao S, Ohta H, Otsuka T. Patch technique for repair of a dural tear in microendoscopic spinal surgery. J Bone Joint Surg Br. 2008:90(8):1066–1067. [DOI] [PubMed] [Google Scholar]
- 19.Guigui P, Barre E, Benoist M, Deburge A. Radiologic and computed tomography image evaluation of bone regrowth after wide surgical decompression for lumbar stenosis. Spine (Phila Pa 1976). 1999;24(3):281–288. [DOI] [PubMed] [Google Scholar]