Abstract
This review aimed to identify and describe individual-level behavioral interventions for children 0–18 years of age with sickle cell disease (SCD). PRISMA guidelines were followed at each stage of this review. Twenty-seven studies were included, representing six intervention types: disease knowledge (n = 7), self-management (n = 7), pain management (n = 4), school functioning (n = 4), cognitive health (n = 4), and mental health (n = 2). Most interventions targeted older children (5+ years), while only two examined interventions for children 0–3 years. This review suggests that offering education about disease knowledge, self-management, and pain management interventions can be beneficial for this population. Future research is needed to understand interventions to support young children and the impact of SCD on development.
Keywords: child development, developmental delay, intervention, rehabilitation, sickle cell disease
1 |. INTRODUCTION
Sickle cell disease (SCD) is the most common monogenic condition in the world, affecting approximately 100,000 individuals in the United States (U.S.) and 300,000–400,000 worldwide.1–3 SCD is a hematologic disorder resulting in abnormally shaped red blood cells that disrupt blood flow and contribute to acute episodes of severe pain and an elevated risk of stroke across the lifespan, with SCD-associated complications increasing with age.4,5 Acute pain crises contribute to increased hospitalization rates and absences from work or school.6,7 In the U.S., many children with SCD live in low-income households with limited learning opportunities, which can exacerbate the harmful consequences of SCD on health-related quality of life, including physical, emotional, social, and school functioning.8,9 Cognitive impairments are common in children with SCD, even when adjusting for socioeconomic status and the presence of cerebral infarcts.10–12 Psychological disorders, including anxiety and depression, are also more common among children and adolescents with SCD when compared with peers.13 Children with SCD have an elevated risk for deficits in attention, executive function, cognition, and language.11
Medical advancements have decreased the morbidity of SCD, and there is a critical need to identify interventions that can ameliorate the impact of the disease and improve quality of life into adulthood.14–16 Children with SCD are limited in participating fully in everyday activities (e.g., school, sports, socializing) because of symptoms and complications associated with the disease. Currently, children with SCD are not regularly accessing therapeutic services, yet they would likely benefit from interventions to address the elevated risk for developmental delay, cognitive deficits, and behavioral and psychological concerns.17–20 The American Society of Hematology 2020 Clinical Guidelines recommends screening for developmental delay and cognitive impairment in children with SCD prior to entering school and providing rehabilitative therapies in early childhood to address modifiable risk factors.21 While some interventions have been explored for supporting adults with SCD, little has been done to determine how best to intervene early in life to reduce the impact of disability starting in childhood.
Individual-level behavioral interventions for SCD that address both biological and environmental factors have the potential to modify the developmental trajectory and improve long-term outcomes. While some research on interventions for children and adolescents with SCD exists, little to no research includes interventions specifically designed to address the elevated rate of cognitive deficits and developmental delay in this population. Prior reviews on interventions specific to this population have been limited to eHealth or have not used a systematic review approach.22–24 Interventions to support positive outcomes are not well understood. Thus, the purpose of this systematic review is to identify and describe evidence-based individual-level behavioral interventions to support the optimal development of children, ages 0–18 years, with SCD.
2 |. METHODS
2.1 |. Protocol and registration
The standards and guidelines for conducting and reporting systematic reviews set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses,25 the National Academies Standards for Systematic Reviews,26 the Cochrane Handbook of Systematic Reviews,27,28 and Peer Review of Systematic Search Strategies29 guided the creation of the search strategies and written methodology for the review. The study was registered with PROSPERO with number CRD42021249345, and the literature review was completed May 4, 2021, with assistance from a medical librarian (LHY) at Washington University School of Medicine. The search was duplicated on December 20, 2021, to identify any additional citations.
2.2 |. Eligibility criteria
Studies that included a behavioral intervention for children ages 0–18 years with SCD were included (Table 1). Behavioral interventions were defined as those that included outcomes related to motor, cognitive, language, medication management, and transition programming. Articles published after 1990 were included, aligning with advances in medical care and increased life expectancy for those with SCD.14–16 Studies that did not provide outcomes related to the child’s developmental progress or behavior were excluded (e.g., newborn screening programs). Studies were also excluded if no outcome data were reported (e.g., feasibility studies) or if they only reported outcomes related to medical interventions (e.g., blood pressure, neuroimaging). Abstracts, dissertations, systematic reviews, interviews, and study protocols were excluded.
TABLE 1.
Inclusion and exclusion criteria for studies identified in search
| Include | Exclude |
|---|---|
| Children 0–18 years of age | Medical interventions (e.g., medication) |
| Confirmed diagnosis of sickle cell disease | Newborn screening programs |
| Intervention with outcome data associated with behavior/child development• Cognitive • Motor • Language • Medication management • Transition programming |
Publications without peer-reviewed outcome data • Abstracts • Dissertations • Book chapters • Systematic reviews • Commentary • Interviews • Study protocols |
2.3 |. Search
A medical librarian (LHY) searched the literature for records including the concepts of SCD, behavioral interventions, and children. The librarian created search strategies using a combination of keywords and controlled vocabulary in embase.com 1947-, Ovid Medline 1946-, Scopus 1823-, Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus 1937-, APA PsycINFO 1800s -, and Clinicaltrials.gov 1997-. All searches were completed May 4, 2021 and were limited to articles published between 1990 and 2021. A total of 2347 results were found. A de-duplication process was used to eliminate 978 duplicate records,30 resulting in 1404 unique citations included in this review. The search was duplicated in December 2021. No additional citations were identified. Fully reproducible search strategies for each database can be found in the supplement.
2.4 |. Study selection
Two authors (C. R. H. and S. H.) independently reviewed titles, abstracts, and full manuscripts based on eligibility criteria to determine studies to include in the final review (Table 1). Any discrepancies were discussed until a consensus was reached.
2.5 |. Data collection process and data items
The same two authors independently extracted data from included studies using Covidence systematic review software.31 The following information was extracted from each study: (1) study identification information including author, sponsorship source, country, setting, conflicts of interest, study purpose, and relevant results; (2) methods including study design and outcome measures; and (3) inclusion and exclusion criteria, participant age, SCD subtypes, the total sample size, caregiver involvement, group differences, intervention descriptions, and outcomes.
2.6 |. Risk of bias in individual and across studies
Authors evaluated the risk of bias for randomized studies and nonrandomized studies separately and recorded decisions using Covidence software.27,31 Randomized studies were evaluated using the Cochrane Risk of Bias Tool 2.0 (ROB2). The risk of bias for nonrandomized studies was assessed using the Risk of Bias in Non-Randomized Studies-of Interventions (ROBINS-I) tool.32 Risk of bias was assessed for each study independently by two authors (C. R. H. and S. H.), and discrepancies were discussed until consensus was reached. All studies were reviewed for publication bias within the study and across studies.
2.7 |. Summary measures
The primary outcome of this study was to summarize interventions that addressed behavior and development for children with SCD and describe the overall effectiveness of interventions.
2.8. | Synthesis of results
Due to the variability in outcomes and intervention types of the studies included, the authors synthesized outcome measures that were used across multiple studies.
The Oxford Centre for Evidence-Based Medicine Levels of Evidence33 was used to appraise the evidence of each article. Each article was classified into intervention categories based on its primary outcome, including disease knowledge, self-management, pain management, school functioning, mental health, and cognitive interventions. Articles with two primary outcomes were reflected in both intervention categories.
After classifying each article by intervention theme, the strength of evidence of each intervention theme was determined with the U.S. Preventive Services Task Force grade definitions34 criteria (Table S1).
3 |. RESULTS
3.1 |. Study selection
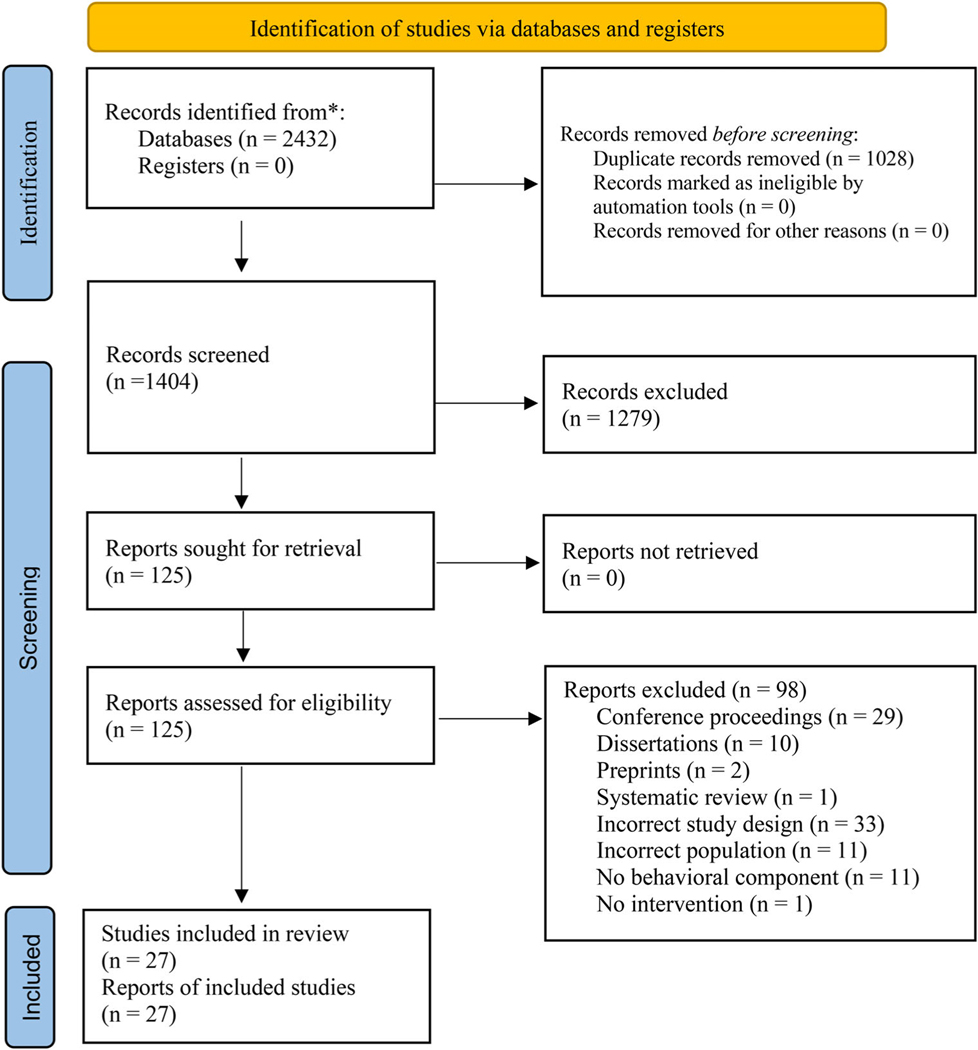
A total of 1404 records were identified and exported to Endnote; 1279 records were excluded. Full exclusion reasons are outlined in Figure 1. Of the 125 full texts that were reviewed, 98 were excluded, leaving 27 articles included in this review.35–61
FIGURE 1.
PRISMA flow diagram
3.2 |. Study characteristics
Twenty-seven studies are included in this review of behavioral interventions for children 0–18 years of age with SCD. The majority of studies (n = 24) were conducted in the U.S. One study was conducted in Jamaica,56 one in Iran,37 and one in Saudi Arabia.50 The majority of interventions occurred in an outpatient clinic setting (n = 17). 35,37–41,44,48,50–52,55–57,59–61 The remaining interventions were conducted in participants’ homes (n = 7),36,42–45,54,57 through the use of technology (e.g., computer games, mobile applications) (n = 4),35,45,47,49 in an inpatient hospital setting (n = 2),46,58 or in a school (n = 1).53 Eight studies involved caregivers in the intervention beyond solely completing outcome measures.37,39,41,43,45,51,54,55 Two studies involved children younger than school-age (<5 years),43,55 and the remaining 25 studies involved school-age children.
3.3 |. Risk of bias within and across studies
Risk of bias was assessed for each study using ROB2 and ROBINS-I (Tables S2 and S3).27,56,62 Overall, the majority of included studies had a low overall risk of bias. Nearly half (n = 12, 44%) had a high risk of bias. The most common sources of bias were in the selection and reporting of outcome measures. The majority of studies (n = 24) were conducted in the U.S., limiting the generalizability of findings for international populations. The limited retention rates in many included intervention studies may also limit the generalizability of presented findings.
3.4 |. Results of individual studies
This review included 27 studies describing behavioral interventions for children ages 0–18 years with SCD. Studies included children with all genotypes of SCD. Each study and its results are described in Tables S4 and S5.
3.4.1 |. Participant characteristics
Two studies included participants under the age of 5 years,43,55 and the remaining (n = 25) involved school-age children. Of the 27 included articles, 11 reported data on race. Of those 11, six had a participant population that was 100% African American35,40,44,47,48,52; the remaining five reported an African American population between 68 and 98%.36,42,46,55,57
3.4.2 |. Outcome measures
Assessments used for pretesting and posttesting of participants in included studies varied greatly. Assessments used in two or more studies included the Pediatric Quality of Life Inventory (PedsQL) (n = 5),35,40,41,45,49 Children’s Depression Inventory (n = 4), 48,52,53,58 Coping Strategies Questionnaire (n = 3),36,44,57 Wechsler Abbreviated Scale of Intelligence (n = 3),41,51,52 Children’s Memory Scale (n = 3),51,52,60 Sickle Cell Self-Efficacy Scale (n = 2),37,42 Revised Children’s Manifest Anxiety Scale (n = 2),39,44 Self-Perception Profile for Children (n = 2),39,53 Transition Readiness Assessment Questionnaire (n = 2),40,47 Woodcock-Johnson III (n = 2),41,45 and the Patient Activation Measure (n = 2).40,47 The PedsQL measures quality of life, the Children’s Depression Inventory and Revised Children’s Manifest Anxiety Scale assess depressive and anxiety symptoms, the Sickle Cell Self-Efficacy Scale and Self-Perception Profile for Children measure self-efficacy and self-esteem, the Weschler Abbreviated Scale of Intelligence and Woodcock-Johnson III are tests of intelligence and achievement, the Children’s Memory Scale assesses memory abilities, and the Transition Readiness Assessment Questionnaire and Patient Activation Measure assess health management in adolescents.
3.4.3 |. Interventions described in included studies
Of the 27 intervention studies included in this review, seven targeted SCD disease knowledge as the primary intervention outcome.35,40,46,48,49,56,59 Seven interventions aimed to improve self-management skills and self-efficacy.37,38,40,42,47,50,61 Four interventions targeted pain and pain management.36,44,57,58 Four studies examined school functioning,41,52,53,55 two interventions focused on mental health,39,54 and four targeted cognitive outcomes.43,45,51,60 Three studies examined two primary outcomes and are therefore reflected in two of the preceding categories (making a total of 30 outcomes evaluated).40,41,52 Each study and the associated interventions are described in Tables S4 and S5.
Disease knowledge interventions (n = 7)
Seven studies focused on disease knowledge.35,40,46,48,49,56,59 Five were conducted in an outpatient clinic setting with clinic personnel. The other two knowledge interventions took place in an inpatient hospital46 or at home.49 Three utilized technologies as the core of the intervention (e.g., mobile application, computer program).35,40,49 Four of the disease knowledge interventions included educational sessions.35,40,46,48 Five of the seven interventions using disease knowledge as a primary outcome reported a statistically significant increase in disease knowledge for participants who received the intervention.35,40,46,48,56
Self-management and self-efficacy interventions (n = 7)
Seven interventions included self-management or self-efficacy as an outcome.37,38,40,42,47,50,61 Five of these interventions occurred in an outpatient clinic setting,37,38,40,47,50,61 three used technology (e.g., mobile application, CD-ROM game),40,47,61 and one took place in the home.42 Four interventions involved group education sessions,37,40,47,50 one distributed educational handouts,38 one implemented the use of a CD-ROM game,61 and one focused on guided imagery training.42
Three of the studies using self-management and self-efficacy as primary outcomes reported a statistically significant improvement in self-efficacy following the intervention.37,40,42 Two studies showed improvements in self-management skills such as laundry, housekeeping, health care, and sexual development38; one found an increase in confidence50,61; and one found that the number of mobile application logins predicted self-management skills.47
Pain management interventions (n = 4)
Pain management was an outcome in four of the included studies measured by pain scales, pain diary entries, days of pain, and health service utilization rates.36,44,57,58 Three were primarily conducted in the home setting,36,44,57 and one was an intervention for children hospitalized with symptoms associated with SCD.58 One study found no significant change in outcomes following a pain management intervention teaching deep breathing, guided imagery, and coping statement techniques.36 Gil et al.44 taught deep breathing, imagery, and self-talk techniques and reported a reduction in health care contact rates, school absences, and interference with household activities on pain days when participants used learned strategies.44 Schatz57 used a home-based coping skills training intervention with cognitive-behavioral principles and found that participants receiving the intervention reported a decrease in pain intensity the day after using the cognitive-behavioral skills, as well as an increase in active psychological coping attempts.57 The inpatient hospital intervention reported suboptimal feasibility but high acceptance of a pain management intervention involving a workbook, videos, and relaxation exercises and found significant improvements in participant knowledge of pain management skills.58
School functioning interventions (n = 4)
Four interventions targeted school-related outcomes, including school functioning, educational attainment, absence rates, and caregiver knowledge regarding schooling for their children.41,52,53,55 Daniel41 provided a full-day workshop for participants at an outpatient clinic but found no significant effect on school functioning or health-related quality of life, yet the families involved found the intervention acceptable.41 King et al.52 implemented a cognitive training intervention to improve educational attainment among school-age children and found no significant decrease in grade retention and absenteeism rate.52 However, a school-related intervention that applied a teacher and peer education program resulted in significantly fewer absences in the intervention group, indicating the importance of social support and community-based interventions.53 A recent study by Miller et al.55 tested a school-based intervention to provide education on school selection, school quality, and individualized education plans/504 plans to caregivers of children under 5 years, which found an increase in caregiver empowerment and knowledge of choosing a school for their children.55
Cognitive interventions (n = 4)
Only four studies included cognitive domains as an outcome.43,45,51,60 Fields et al.43 tested a home-based education program for parents of children 3–36 months and observed a significant improvement in cognitive and language development.43 Another study was a home-based tablet training program for children to improve working memory and short-term memory, which resulted in an improvement in working memory among participants, although adherence to the program was low.45 King et al. 51 aimed to facilitate memory skills and academic achievement in children with SCD-related infarcts with a 2-year tutoring and memory rehabilitation training program. The intervention resulted in an improvement in scores on memory measures for the intervention group.51 A fourth cognitive intervention also involved academic tutoring and memory training; better memory and learning outcomes were reported for the intervention group.60
Mental health interventions (n = 2)
Two interventions targeted mental health outcomes, including adjustment, anxiety, depression, and overall mental health.39,54 One mental health intervention was a community-based support program that consisted of in-home and community family visits, phone calls, and group events and found a significant effect on adjustment.39 The second mental health intervention was a massage therapy intervention in which researchers taught caregivers how to give massages to their children. Children reported higher levels of functional status and lower levels of depression, anxiety, and pain following the intervention, but caregivers notably reported higher levels of anxiety and depression.54
3.5 |. Synthesis of results
This review identified 27 studies testing six types of behavioral intervention in children with SCD, using a total of 48 standardized behavioral outcome measures (Table S5). Based on the Oxford Centre for Evidence-Based Medicine Levels of Evidence,33 17 studies in the review were classified as Level 2B,35,36,38,39,41–43,46,49–52,55,56,58,59,61 indicating a low-level randomized controlled trial (RCT), nine were classified as Level 1B,37,40,44,45,47,48,53,54,57 indicating an RCT and high level of evidence, and one case series was classified as Level 4.60 Level 1B studies consisted of self-management, disease knowledge, pain management, cognitive, school functioning, and mental health interventions. Level 2B studies consisted of disease knowledge, self-management, pain management, school functioning, mental health, and cognitive interventions. The strength of evidence of each intervention theme is summarized in Table S6.
The strength of evidence indicates support for the reviewed interventions targeting disease knowledge, self-management and self-efficacy, and applying strategies to cope with pain (referred to as pain management). Further research is needed to determine efficacious interventions to improve school functioning, mental health, and cognitive outcomes.
4 |. DISCUSSION
This systematic review identified 27 studies that explored behavioral interventions for children, 0–18 years of age, with SCD. The majority of behavioral interventions targeted disease knowledge (n = 7) or self-management (n = 7) as a primary outcome. Other types of individual-level behavioral interventions reviewed included self-efficacy, pain management, school functioning, cognition, and mental health. Some interventions targeted disease-specific characteristics, such as sickle cell pain and disease knowledge. Other studies focused on applying interventions to complications not directly specific to but commonly associated with SCD like developmental delay and mental health concerns.
This review found strong evidence supporting interventions for disease knowledge, self-management/self-efficacy, and using guided approaches to cope with pain. The early childhood interventions examined in this review reflect recommendations to screen for developmental delay and provide early intervention in the first years of life offered by the American Society of Hematology 2020 Clinical Guidelines (Recommendations 8, 9).21 There was moderate evidence supporting school functioning interventions, indicating a need for more research in this area. Results from included studies were varied, and a number of the school functioning interventions found no significant intervention effects. There was a low strength of evidence supporting mental health and cognitive interventions. Further research is required to determine optimal intervention strategies and methods to target cognitive and mental health outcomes among children with SCD.
The included studies often lacked data related to feasibility and acceptability outcomes for study participants. Retention rates were less than 80% for many of the studies, indicating a need to receive input from the SCD community in the process of developing interventions to gain insight into strategies for participant buy-in, needs, wants, and motivating factors. Additionally, researchers must examine acceptability prior to examining feasibility. The lack of community-driven, versus science-driven, research on interventions for this population is a likely contributor to low retention rates in many included studies.
In addition to examining intervention acceptability and feasibility and receiving community input, behavioral interventions for children younger than 5 years were nearly nonexistent in this review, especially among intervention themes with high strength of evidence. Only two of the total 27 studies analyzed included an intervention for children with SCD under 5 years of age. Interventions for developmental delay, offered at the earliest possible opportunity, can influence the child’s developmental trajectory. Interventions specific to infants and toddlers are necessary and should be explored in future studies. Each child’s development and disease progression should be considered so that beneficial interventions can be appropriately provided.
For school-age children, caregiver involvement in interventions showed mixed results among studies. Children living with SCD in the U.S. more often come from families and communities of lower socioeconomic status. Interventions that include the child’s environment (family, living space, community) and that are offered at a young age will more likely have a lasting impact. Caregiver-focused and home-based interventions have demonstrated efficacy, which was corroborated by this review as a meaningful way to improve outcomes for children with SCD.43,63
4.1 |. Limitations
While the studies included in this review had limitations, we believe that the interventions were reviewed for their quality and were described thoroughly. To avoid the risk of bias in the initial search, we used an experienced research librarian with expertise in systematic reviews to ensure an effective and inclusive search and screening process. To minimize the impact of bias on the part of the review team, each team member thoroughly reviewed and discussed literature related to SCD and health equity, reflecting at each stage to ensure that researchers considered components related to historically excluded communities. Three papers were represented more than once in the results because they had more than one distinct outcome. While we recognize that this may overrepresent specific studies, we felt it was important to describe every intervention.
The purpose of this review was to evaluate the published literature related to individual-level behavioral interventions for children with SCD to understand the breadth and effectiveness of interventions. However, we did not directly examine the implementation context or implementation strategies used. It is likely that factors related to how an intervention was implemented and evaluated (e.g., outcome measures) influenced intervention efficacy. Additionally, the authors recognize that robust interventions targeting issues not specific to SCD but commonly associated with the disease, like developmental delay and mental health, have been published previously with youth in general. These interventions were not included in this review if the studies did not include children with SCD. Researchers interested in providing interventions for children with SCD should consider what has worked in the general population and pilot with individuals with SCD to determine population-specific feasibility, acceptability, and efficacy.
5 |. CONCLUSIONS
We identified 27 published behavioral interventions for children with SCD. The majority of interventions addressed increasing disease knowledge and self-management skills among school-age children and adolescents. Interventions targeting cognitive and mental health outcomes demonstrated low strength of evidence. Only two studies included children less than 5 years of age. Results from this review suggest that more work is needed to study and implement interventions that can improve the lives of individuals with SCD. Specifically, interventions targeting cognition, mental health, and early childhood development among individuals with SCD should be explored.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all members of our research team for their support, Megen Devine for her editorial support, and Becker Medical Library for their support in conducting this search.
Abbreviations:
- PedsQL
Pediatric Quality of Life Inventory
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
randomized controlled trial
- ROB2
Cochrane Risk of Bias Tool 2.0
- ROBINS-I
Risk of Bias in Non-Randomized Studies of Interventions
- SCD
sickle cell disease
- U.S.
United States
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet North Am Ed. 2010;376(9757):2018–2031. [DOI] [PubMed] [Google Scholar]
- 2.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4):S512–S521. [DOI] [PubMed] [Google Scholar]
- 3.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4(1):18010. [DOI] [PubMed] [Google Scholar]
- 4.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet North Am Ed. 2017;390(10091):311–323. [DOI] [PubMed] [Google Scholar]
- 5.Feliu MH, Crawford RD, Edwards L, et al. Neurocognitive testing and functioning in adults sickle cell disease. Hemoglobin. 2011;35(5–6):476–484. [DOI] [PubMed] [Google Scholar]
- 6.Eaton ML, Have JS, Armstrong FD, Pegelow CH, Thomas M. Hospitalizations for painful episodes: association with school absenteeism and academic performance in children and adolescents with sickle cell anemia. Issues Compr Pediatr Nurs. 1995;18(1):1–9. [DOI] [PubMed] [Google Scholar]
- 7.Gil KM, Porter L, Ready J, Workman E, Sedway J, Anthony KK. Pain in children and adolescents with sickle cell disease: an analysis of daily pain diaries. Children’s Health Care. 2000;29(4):225–241. [Google Scholar]
- 8.Panepinto JA, Pajewski NM, Foerster LM, Sabnis S, Hoffmann RG. Impact of family income and sickle cell disease on the health-related quality of life of children. Qual Life Res. 2008;18(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panepinto JA, Foerster LM, Sabnis S, Pajewski N, Hoffmann R. Impact of poverty and sickle cell disease on the health-related quality of life of children. Blood. 2007;110(11):83–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro IPS, Viana MB. Cognitive profile of children with sickle cell anemia compared to healthy controls. J Pediatr (Rio J). 2019;95(4):451–457. [DOI] [PubMed] [Google Scholar]
- 11.Prussien KV, Jordan LC, DeBaun MR, Compas BE. Cognitive function in sickle cell disease across domains, cerebral infarct status, and the lifespan: a meta-analysis. J Pediatr Psychol. 2019;44(8):948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schatz J, Finke RL, Kellett JM, Kramer JH. Cognitive functioning in children with sickle cell disease: a meta-analysis. J Pediatr Psychol. 2002;27(8):739–748. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran G, Krishnakumar P, Feroze M, Gireeshan VK. Cognitive functions and psychological problems in children with Sickle cell anemia. Indian Pediatr. 2016;53(6):485–488. [DOI] [PubMed] [Google Scholar]
- 14.Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Network Open. 2019;2(11):e1915374-e1915374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease – life expectancy and risk factors for early death. N EnglJ Med. 1994;330(23):1639–1644. [DOI] [PubMed] [Google Scholar]
- 17.Hogan AM, Kirkham FJ, Prengler M, et al. An exploratory study of physiological correlates of neurodevelopmental delay in infants with sickle cell anaemia. Br J Haematol. 2006;132(1):99–107. [DOI] [PubMed] [Google Scholar]
- 18.Brown RT, Armstrong FD, Eckman JR. Neurocognitive Aspects of Pediatric Sickle Cell Disease. J Learn Disabil. 1993;26(1):33–45. [DOI] [PubMed] [Google Scholar]
- 19.Schatz J Brief report: academic attainment in children with sickle cell disease. J Pediatr Psychol. 2004;29(8):627–633. [DOI] [PubMed] [Google Scholar]
- 20.Brown RT, Kaslow NJ, Doepke K, et al. Psychosocial and family functioning in children with sickle cell syndrome and their mothers. Journal of the American Academy of Child & Adolescent Psychiatry. 1993;32(3):545–553. [DOI] [PubMed] [Google Scholar]
- 21.DeBaun MR, Jordan LC, King AA, et al. American Society of Hematology 2020 guidelines for sickle cell disease: prevention, diagnosis, and treatment of cerebrovascular disease in children and adults. Blood Advances. 2020;4(8):1554–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badawy SM, Cronin RM, Hankins J, et al. Patient-centered eHealth interventions for children, adolescents, and adults with sickle cell disease: systematic review. Journal of medical Internet research. 2018;20(7):e10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins M, Kaslow N, Doepke K, Eckman J, Johnson M. Psychosocial interventions for children and adolescents with sickle cell disease (SCD). Journal of Black Psychology. 1998;24(4):432–454. [Google Scholar]
- 24.Karkoska KA, Haber K, Elam M, Strong S, McGann PT. Academic challenges and school service utilization in children with sickle cell disease. J Pediatr. 2021;230:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, Shamseer L, Fau-Clarke M, Clarke M Fau - Ghersi D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. (2046–4053 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton S, Berg A, Levit L, Eden J, editors. Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC): National Academies Press (US);2011. [PubMed] [Google Scholar]
- 27.Higgins J, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane; 2021. [Google Scholar]
- 28.Cumpston M, Li T, Fau-Page MJ, Page Mj Fau - Chandler J, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. (1469–493X (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. (1878–5921 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 30.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. Deduplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Covidence systematic review software. Veritas Health; Accessed. http://www.Innovation.covidence.org [Google Scholar]
- 32.Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Oxford Levels of Evidence 2. In: Oxford Centre for Evidence-Based Medicine; 2011. [Google Scholar]
- 34.Definitions Grade. U.S. Preventive Services Task Force. Published 2018. Accessed. [Google Scholar]
- 35.Anderson LM, Leonard S, Jonassaint J, Lunyera J, Bonner M, Shah N. Mobile health intervention for youth with sickle cell disease: Impact on adherence, disease knowledge, and quality of life. Pediatr Blood Cancer. 2018;65(8). [DOI] [PubMed] [Google Scholar]
- 36.Barakat LP, Schwartz LA, Salamon KS, Radcliffe J. A family-based randomized controlled trial of pain intervention for adolescents with sickle cell disease. J Pediatr Hematol Oncol. 2010;32(7):540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borimnejad L, Parvizy S, Haghaani H, Sheibani B. The effect of family centered empowerment program on self-efficacy of adolescents with thalassemia major: a randomized controlled clinical trial. International Journal of Community Based Nursing & Midwifery. 2018;6(1):29–38. [PMC free article] [PubMed] [Google Scholar]
- 38.Calhoun CL, Abel RA, Pham HA, Thompson S, King AA. Implementation of an educational intervention to optimize self-management and transition readiness in young adults with sickle cell disease. Pediatr Blood Cancer. 2019;66(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chernoff RG, Ireys HT, DeVet KA, Kim YJ. A randomized, controlled trial of a community-based support program for families of children with chronic illness: Pediatric outcomes. Arch Pediatr Adolesc Med. 2002;156(6):533–539. [DOI] [PubMed] [Google Scholar]
- 40.Crosby LE, Hood A, Kidwell K, et al. Improving self-management in adolescents with sickle cell disease. Pediatr Blood Cancer. 2020;67(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniel LC, Li Y, Smith K, et al. Lessons learned from a randomized controlled trial of a family-based intervention to promote school functioning for school-age children with sickle cell disease. J Pediatr Psychol. 2015;40(10):1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobson C Outcome results of self-efficacy in children with sickle disease pain who were trained to use guided imagery. Appl Nurs Res. 2015;28(4):384–390. [DOI] [PubMed] [Google Scholar]
- 43.Fields ME, Hoyt-Drazen C, Abel R, et al. A pilot study of parent education intervention improves early childhood development among toddlers with sickle cell disease. Pediatr Blood Cancer. 2016;63(12):2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gil KM, Anthony KK, Carson JW,et al. Daily coping practice predicts treatment effects in children with sickle cell disease. J Pediatr Psychol. 2001;26(3):163–173. [DOI] [PubMed] [Google Scholar]
- 45.Hardy SJ, Bills SE, Meier ER, et al. A randomized controlled trial of working memory training in pediatric sickle cell disease. J Pediatr Psychol. 2021. [DOI] [PubMed] [Google Scholar]
- 46.Hazzard A, Celano M, Collins M, Markov Y. Effects of STARBRIGHT World on knowledge, social support, and coping in hospitalized children with sickle cell disease and asthma. Children’s Health Care. 2002;31(1):69–86. [Google Scholar]
- 47.Hood AM, Nwankwo C, McTate E, et al. Mobile health use predicts self-efficacy and self-management in adolescents with sickle cell disease. Blood. 2020;136(SUPPL 1):57–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaslow NJ, Collins MH, Rashid FL, et al. The efficacy of a pilot family psychoeducational intervention for pediatric Sickle Cell Disease (SCD). Families, Systems and Health. 2000;18(4):381–404. [Google Scholar]
- 49.Ketchen B, Hazzard A, Lassiter S, et al. STARBRIGHT World: A pilot study of a home-based sickle cell psychoeducational intervention. Children’s Health Care. 2006;35(4):321–338. [Google Scholar]
- 50.KhadigaAbdElgiedGomea H, AhmedQalawa SA. Impact of educational program for adolescents and young adults with sickle cell diseases on their knowledge, perception, self-care. Indian Journal of Public Health Research & Development. 2021;12(1):310–318. [Google Scholar]
- 51.King AA, White DA, McKinstry RC, Noetzel M, DeBaun MR. A pilot randomized education rehabilitation trial is feasible in sickle cell and strokes. Neurology. 2007;68(23):2008. [DOI] [PubMed] [Google Scholar]
- 52.King A, Herron S, McKinstry R, et al. A multidisciplinary health care team’s efforts to improve educational attainment in children with sickle-cell anemia and cerebral infarcts. J Sch Health. 2006;76(1):33–37. [DOI] [PubMed] [Google Scholar]
- 53.Koontz K, Short AD, Kalinyak K, Noll RB. A randomized, controlled pilot trial of a school intervention for children with sickle cell anemia. J Pediatr Psychol. 2004;29(1):7–17. [DOI] [PubMed] [Google Scholar]
- 54.Lemanek KL, Ranalli M, Lukens C. A randomized controlled trial of massage therapy in children with sickle cell disease. J Pediatr Psychol. 2009;34(10):1091–1096. [DOI] [PubMed] [Google Scholar]
- 55.Miller M, Landsman R, Scott JP, Heffelfinger AK. Fostering equity in education and academic outcomes in children with sickle cell disease. Clin Neuropsychol. 2021:1–19. [DOI] [PubMed] [Google Scholar]
- 56.Morrison-Levy N, Knight-Madden J, Royal-Thomas T, King L, Asnani M. Improving disease knowledge in 6- to 10-year-olds with sickle cell disease: A quasi-experimental study. Child Care Health Dev. 2018;44(3):501–506. [DOI] [PubMed] [Google Scholar]
- 57.Schatz J, Schlenz AM, McClellan CB, et al. Changes in coping, pain, and activity after cognitive-behavioral training: a randomized clinical trial for pediatric sickle cell disease using smartphones. Clin J Pain. 2015;31(6):536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sil S, Lee JL, Klosky J, et al. The comfort ability program for adolescents with sickle cell pain: Evaluating feasibility and acceptability of an inpatient group-based clinical implementation. Pediatr Blood Cancer. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith GM, Lewis VR, Whitworth E, Gold DT, Thornburg CD. Growing up with sickle cell disease: a pilot study of a transition program for adolescents with sickle cell disease. J Pediatr Hematol Oncol. 2011;33(5):379–382. [DOI] [PubMed] [Google Scholar]
- 60.Yerys BE, White DA, Salorio CF, McKinstry R, Moinuddin A, DeBaun M. Memory strategy training in children with cerebral infarcts related to sickle cell disease. J Pediatr Hematol Oncol. 2003;25(6). [DOI] [PubMed] [Google Scholar]
- 61.Yoon SL, Godwin A. Enhancing self-management in children with sickle cell disease through playing a CD-ROM educational game: a pilot study. Pediatr Nurs. 2007;33(1):60–72. [PubMed] [Google Scholar]
- 62.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019:l4898. [DOI] [PubMed] [Google Scholar]
- 63.Bann CM, Wallander JL, Do B, et al. Home-based early intervention and the influence of family resources on cognitive development. LID - e20153766 [pii] LID doi: 10.1542/peds.2015-3766 [doi] (1098–4275 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.