Abstract
Background:
Whether brain-derived neurotrophic factor (BDNF) Met carriage impacts the risk or progression of Alzheimer’s disease (AD) is unknown.
Objective:
To evaluate the interaction of BDNF Met and APOE4 carriage on cerebral metabolic rate for glucose (CMRgl), amyloid burden, hippocampus volume, and cognitive decline among cognitively unimpaired (CU) adults enrolled in the Arizona APOE cohort study.
Methods:
114 CU adults (mean age 56.85 years, 38% male) with longitudinal FDG PET, magnetic resonance imaging and cognitive measures were BDNF and APOE genotyped. A subgroup of 58 individuals also had Pittsburgh B (PiB) PET imaging. We examined baseline CMRgl, PiB PET amyloid burden, CMRgl and hippocampus volume change over time, and rate of change in cognition over an average of 15 years.
Results:
Among APOE4 carriers, BDNF Met carriers had significantly increased amyloid deposition and accelerated CMRgl decline in regions typically affected by AD, but without accompanying acceleration of cognitive decline or hippocampal volume changes and with higher baseline frontal CMRgl and slower frontal decline relative to the Val/Val group. The BDNF effects were not found among APOE4 non-carriers.
Conclusion:
Our preliminary studies suggest that there is a weak interaction between BDNF Met and APOE4 on amyloid-β plaque burden and longitudinal PET measurements of AD-related CMRgl decline in cognitively unimpaired late-middle-aged and older adults, but with no apparent effect upon rate of cognitive decline. We suggest that cognitive effects of BDNF variants may be mitigated by compensatory increases in frontal brain activity—findings that would need to be confirmed in larger studies.
Keywords: BDNF, APOE4, Positron-Emission Tomography, Fluorodeoxyglucose F18, Amyloid, cognition
Introduction
Apolipoprotein (APOE) ɛ4 allele is a major genetic risk factor for AD. APOE4 carriage (APOE4c) is associated with increased Aβ accumulation [1, 2]. Cognitively unimpaired (CU) APOE4 individuals exhibit cerebral Aβ accumulation as early as in their third or fourth decade [1, 3]. Age-related cognitive decline in APOE4 carriers also begins earlier relative to non-carriers [4], although the increased rate of memory decline in CU APOE4c was found to be associated with high Aβ levels compared with APOE4 non-carriers (APOE4nc) with low Aβ [5]. In addition, CU APOE4 carriers show lower cerebral metabolic rate of glucose (CMRgl) as measured by FDG-PET in regions known to be affected in AD [6] such as the posterior cingulate, precuneus, parietotemporal, and prefrontal cortex [7–11] cross-sectionally, had greater rate of CMRgl decline longitudinally in these same regions than APOE4nc CU [12], and nonsignificant trends for smaller hippocampal volumes as measured by MRI [13].
It is unknown whether brain derived neurotrophic factor (BDNF), a neurotrophin involved in synaptic modulation, is a genetic risk factor for AD. A common single nucleotide polymorphism (SNP) in the human BDNF gene (Val66Met; rs6265) substitutes a valine to methionine in the 5’ pro-domain of the BDNF protein which attenuates the activity-dependent form of BDNF secretion without affecting its constitutive secretion [14, 15]. In vitro and in vivo model systems describing the molecular and cellular characteristics of the polymorphism have demonstrated that Val66Met differentially impacts BDNF protein availability, neuronal survival and morphology, and altered neuronal function [14, 16]. Literature regarding Val66Met describes diverse, conflicting patterns of effects. BDNF Met carriage has been linked with a positive effect in healthy adults for cognitive control function such as response inhibition [17], was associated with reduced cognitive decline in patients with multiple sclerosis and [18] systemic lupus erythematosus [19], and preservation of general intelligence following traumatic brain injury [20]. With regard to AD, the literature on whether BDNF genetic variants are an AD susceptibility factor or mitigates the effects of the APOE4 risk for developing AD is divided, ranging from: 1) no evidence of increased risk for AD [21]; 2) modulation effects of aging on working memory but no interaction with APOE4 on hippocampal volumes or memory performance[22] to 3) increased rates of cognitive decline among those BDNF Met Carriers with APOE4c and higher Aβ load [23–26] and 4) significantly higher amyloid load in BDNF Met carriers than Val/Val homozygotes only among APOE4 carriers[27].
The objective of our study was to better understand the role of BDNF and its interaction with APOE4c on the endophenotypes of AD in CU adults by examining the interaction between BDNF Met and APOE4 carriage in baseline and longitudinal glucose metabolism, baseline and longitudinal hippocampal volume, baseline amyloid burden, and longitudinal cognitive decline in CU adults enrolled in the Arizona APOE cohort study. We tested the hypothesis that within the APOE4 carrier group, individuals with BDNF Met carriage would have higher Aβ burden at baseline, differing glucose metabolism, differing hippocampal volumes, and increased cognitive decline over time compared to individuals with BDNF Val/Val. Our results reveal an intriguing interaction between BDNF Met and APOE4 in AD pathogenesis and disease progression.
Methods
Study Participants:
The included participants (N=114) were drawn from the longitudinal Arizona APOE cohort study [4, 28–30], specifically those who were both APOE and BDNF genotyped and had undergone longitudinal FDG PET and magnetic resonance (MR) imaging and cognitive testing. The Arizona APOE cohort study began in 1994 and is composed of CU individuals residing in Maricopa County, Arizona, mostly 47–68 years old, recruited through local media advertisements for inclusion in a study of cognitive aging. At entry, participants must score at least a 27/30 on the Mini-Mental State Examination (with at least 1of 3 on the recall subject) and exhibit no evidence of depression quantified by 10 points or less on the Hamilton Depression Rating Scale. The participants must also have perfect scores on the Functional Activities Questionnaire and Instrumental Activities of Daily Living Questionnaire, absence of a current psychiatric or vascular disease, normal neurological examination, and no clinically significant imaging abnormalities. All participants had a family history of dementia, were APOE genotyped, completed a full battery of neuropsychological testing (including the Auditory Verbal Learning Test, Controlled Oral Word Association Test, Mini-Mental State Exam), and had both FDG PET and T1 MRI measurements at every two-year visit as initially designed. In year 2007, we also added PiB PET for the amyloid measurements. Study participants were followed and seen in the clinic also every 2 years. All individuals gave written informed consent to participate in the study and the study protocol was approved by the institutional review boards of Banner Good Samaritan Medical Center (now Banner-University Medical Center, Phoenix, AZ, USA) and the Mayo Clinic.
APOE/BDNF genotyping:
Blood for plasma analysis was collected in tubes containing ethylenediaminetetraacetic acid (EDTA). Samples were centrifuged at 2000x g at 4C for 10 minutes. Centrifuged samples were aliquoted and immediately frozen at −80C in polypropylene vials pending biochemical analysis. Single nucleotide polymorphisms (SNPs) for APOE (rs429358, rs7412) were genotyped as described elsewhere [31]. BDNF genotypes (rs6265, Val66Met variant, located on chromosome 11:27,658,369 in human genome build GRCh38 38.1/142) were generated using KASP chemistry (LGC Biosearch Technologies, Teddington, Middlesex, United Kingdom). KASP reactions were comprised of sample DNA, KASP Master Mix, and KASP Assay Mix which contains competitive, allele-specific forward primers with differing FRET tags and one common reverse primer. KASP-based polymerase chain reaction amplification results in fluorescent signals that indicate genotypes for the Val66Met variant.
Brain Imaging:
PiB-PET:
58 participants who were both APOE and BDNF genotyped also had one-time PiB PET imaging. Amyloid PET imaging was performed using a HR+ scanner (Siemens, Knoxville, TN) in a three-dimensional mode after intravenous injection of approximately 15 mCi of 11C-PiB for a 90-minute dynamic sequence of emission scans. For quantification of amyloid burden, PiB PET images between 50 to 70 minutes post-injection were summed and normalized to the cerebellum to generate cerebral-to-cerebellar standard uptake value ratio (SUVR). All the quantification was performed using SPM8 in the MNI template space. For the cross-sectional PiB-PET voxel-wise analysis, we used general linear model (GLM) procedure with uncorrected p-value of 0.005 for brain regions known to be associated with beta amyloid. To address possible inflated type I error in the examination of group differences in the voxel wise analysis, we used the same post hoc Monte Carlo simulation procedure with 1000 iterations as in our previous study [32]. Using this procedure, we tested the hypothesis that the number of PiB SUVR differences observed in the postulated direction (i.e., SUVR higher in the APOE4c-BDNF Met carriers than in the APOE4nc-BDNF Val/Val group) was significantly greater than the number of voxels with elevations in the opposite direction (i.e., SUVR higher in the APOE4nc-BDNF Val/Val group than in the APOE4c-BDNF Met carriers group). We note this Monte-Carlo simulation based test is global and had no localization power for group difference for any brain region.
FDG-PET:
Cerebral glucose metabolism was measured with 18F-fluorodeoxyglucose (FDG) PET imaging on the same HR+ scanner (Siemens, Knoxville, TN), with an intravenous injection of approximately 10 mCi of FDG and a 60-min dynamic sequence of emission scans as the subjects, who had fasted for at least 4 hours, lay quietly in a darkened room with their eyes closed and directed forward. For quantification of brain glucose metabolic rate (CMRgl) for this study, the last 30 minutes of the dynamic FDG PET images were summed and proportionally scaled (normalized) by whole brain average counts to allow assessment of relative CMRgl at regional and voxel level. For baseline FDG-PET voxel-wise analysis, we used general linear model (GLM) procedure with uncorrected p-value of 0.005 with the brain regions known to be affected by AD[8, 33]. For longitudinal FDG-PET voxel-wise analysis, assuming the CMRgl changes over time are linear, we estimated the voxel-wise rate of changes for each of those subjects who had multiple time points FDG PET scans using linear regression with subject’s visit ages as the independent variable. Thus, a slope image was created for each subject. Two sample independent t-test was then used to compare the difference of the longitudinal change rates between APOE4-carrier + BDNF-met and APOE4-Carrier + BDNF-val. For the FDG-PET analyses, the same Monte-Carlo simulation test described above was also performed.
T1-MRI:
T1-weighted MRI scans were acquired on a 3T GE Discovery MR750 system. They were preprocessed with FreeSurfer 5.3. Cortical and subcortical regions were labeled and the automated segmentations were manually inspected and corrected as needed (surfer.nmr.mgh.harvard.edu/fswiki/).[34, 35] The relative hippocampal volume was calculated by taking the sum of the left and right hippocampal volume and dividing by the intracranial volume (ICV) for each subject.
Statistical analysis:
Descriptive data results are shown as mean +/− standard deviation. For baseline comparisons under the ANOVA framework, we examined the differences between the following groups: BDNF Met carriers versus non-carriers, APOE4 carriers versus non-carriers, and especially APOE4c-BDNF Met carriers/APOE4nc-BDNF Val/Val. Equivalent to the interaction test under the general two-factor ANOVA framework, the interaction examined in this study is with the SPM contrast settings under the general linear model to examine the difference between BDNF Met versus Val/Val groups in APOE4c minus the difference between BDNF Met versus Val/Val groups in APOE4nc (the difference of difference). Likewise, the same interaction can be examined by the APOE4 difference in BDNF Met vs the APOE4 difference in BDNF Val/Val group (the difference of difference). To illustrate, using subscript 1 for APOE4 carriers and 2 for non-carriers, the difference between BDNF Met and Val/Val in APOE4 carriers is (BDNFm1 - BDNFv1). The same difference in APOE4 non-carriers is (BDNFm2 – BDNFv2). The difference of difference is, in this case, (BDNFm1 - BDNFv1) - (BDNFm2 – BDNFv2), which is examining the BDNF differential effects between APOE4 carriers vs non-carriers. The same expression can be re-arranged equivalently as (BDNFm1-BDNFm2)- (BDNFv1-BDNFv2), which is examining the APOE4 differential effects between BDNF Met vs BDNF Val/Val. For longitudinal cognitive measures, we used linear mixed effect modeling approach taking the APOE4 by BDNF interactions into consideration. Linear mixed effect models were adjusted for baseline age, sex, and education. Only findings with values of p≤0.05 (2-tailed) were considered significant. Analyses were conducted with R software (www.r-project.org/).
To correct for the multiple comparisons associated with the voxel-wise analyses, we adopted an omnibus Monte Carlo Simulation (MCS) strategy previously developed in our laboratory.[32] This Monte Carlo simulation procedure assumes that a) the null hypothesis is that there is no difference between the two groups everywhere in the brain (using the PiB-PET as an example, the mean SUVR at a given voxel in one group is the same as in another group); b) the noise is of Gaussian at each voxel for each subject; c) the measurements are inter-voxel correlated with the smoothness resulted from preprocessing steps. The simulation procedure then generated the group difference t-score map based on the same GLM model used in the analysis of the real data, number of subjects in each of the two groups and the assumptions above, repeated such t-score map generations N times (in our study, N=1,000). For each of such maps, we counted the number of voxels at one direction vs. the number of voxels in the opposite direction, all at uncorrected p=0.05 level (note this p-value is not for statistical inferences about regional changes). Over N iterations, we then counted the number of times, referred to n, the simulated t-score had the number of voxels in the hypothesized direction equal or exceed the observed in the real data. Finally, the ratio of n/N is the type-I error of interest. Our simulation procedure is conceptually very similar to the widely used Alpha-Sim approach https://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf), but we deliberately decided not to consider the individual cluster sizes, rather to compare over the whole spatial extent (number of voxels), noting the low resolution of the PET data.
Results
Study Participant Characteristics
Table 1 summarizes the demographics of the entire study cohort (N=114) with BDNF status (BDNF Met carrier, BDNF Val/Val) and APOE4 subgroups. Note that the overall BDNF Met carrier group included both Met/Met homozygotes (n=4) and Val/Met heterozygotes (n=36). The average age in the entire cohort was 56.85 years old and 38% of participants were men. No significant differences (p≤0.05) were identified in baseline characteristics of age, sex, education, and the cardiovascular risk factors of diabetes, hypertension, hyperlipidemia, and smoking for the BDNF and the APOE4 main effects. We however observed APOE4 effects on AVLT-LTM, AVLT-STM and AVLT-TL (p<0.05) and no significant BDNF/APOE4 interaction effects for baseline characteristics. Similarly, Table 2 summarizes the demographics and baseline characteristics of the PiB-PET cohort (N=58) subgrouped by BDNF and APOE4 status. However, we found no significant main or interactive effects in this cohort, most likely due to the much-reduced sample size.
Table 1.
Entire Cohort (n=114) Demographics
| Baseline Characteristics | APOE4c/BDNF Met (N=20) | APOE4c/BDNF Val/Val (N= 39) | APOE4nc/BDNF Met (N=20) | APOE4nc/BDNF Val/Val (N= 35) | APOE4 effect p-value | BDNF effect p-value | APOE4×BDNF p-value |
|---|---|---|---|---|---|---|---|
| Sex male/female, (%) | 6/14 (30%/70%) | 14/25 (35.9%/64.1%) | 11/9 (55%/45%) | 12/23 (34.3%/65.7%) | 0.11 | 0.65 | 0.17 |
| APOE4 (HM/HT/NC) | 11/9/0 | 12/27/0 | 0/0/20 | 0/0/35 | NA | NA | NA |
| Education, years mean (SD) | 16.1 (1.8) | 15.5 (2.1) | 58.3 (4.2) | 57.0 (4.5) | 0.25 | 0.31 | 0.58 |
| Age, years mean (SD) | 55.0 (4.0) | 56.8 (4.5) | 16.3 (2.1) | 16.1 (2.1) | 0.18 | 0.70 | 0.07 |
| MMSE mean (SD) | 29.6 (0.7) | 29.9 (0.4) | 29.7 (0.7) | 29.6 (0.7) | 0.16 | 0.21 | 0.18 |
| AVLT STM mean (SD) | 10.6 (2.8) | 10.1 (2.7) | 8.3 (1.9) | 9.5 (2.5) | 0.01* | 0.42 | 0.07 |
| AVLT LTM mean (SD) | 9.9 (3.4) | 9.8 (2.5) | 7.3 (2.5) | 8.9 (3.1) | 0.01* | 0.17 | 0.12 |
| AVLT TL mean (SD) | 51.3 (9.5) | 48.6 (7.7) | 43.9 (5.8) | 47.0 (8.8) | 0.02* | 0.86 | 0.07 |
| COWAT mean (SD) | 45.8 (10.3) | 44.9 (12.0) | 43.6 (9.3) | 43.4 (10.3) | 0.39 | 0.82 | 0.88 |
| Diabetes n (%) | 0 | 1 (2.6%) | 0 | 0 | 1.00 | 1.00 | 1.00 |
| Hypertension n (%) | 3 (15.0%) | 11 (28.2%) | 2 (10.0%) | 3 (8.6%) | 0.65 | 0.27 | 0.41 |
| Smoking n (%) | 3 (15.0%) | 7 (17.9%) | 6 (30.0%) | 10 (28.6%) | 0.26 | 0.78 | 0.77 |
| Hyperlipidemia n (%) | 3 (15.0%) | 11 (28.2%) | 2 (10.0%) | 3 (8.6%) | 0.38 | 0.24 | 0.05 |
| Any cardiovascular n (%) | 0 | 1 (2.6%) | 0 | 0 | 1.00 | 1.00 | 1.00 |
Abbreviations: APOE4 HM: apolipoprotein e4 homozygote, APOE4/4; APOE4 HT: apolipoprotein e4 heterozygote, APOE3/4; APOE4 NC: apolipoprotein e4 non-carrier, APOE 3/3, 2/3; MMSE: Mini-Mental State Examination; AVLT: Auditory-Verbal Learning Test; STM: short term memory; LTM: long term memory; TL: total learning; COWAT: Controlled Oral Word Association Test. Note: COWAT tests executive function and language skills, on a scale with a lower limit of 0 and no upper limit, with higher scores indicating better performance. Data are given as mean +/‒ standard deviation unless otherwise indicated, p-values for continuous variables are from two-way ANOVA and p-values for categorical data are from logistics regression test as developed and reported in Hosmer & Lemeshow, 1989.[44]
Table 2.
PiB-PET Cohort (N=58) Demographics
| Baseline Characteristics | APOE4c/BDNF Met (N=14) | APOE4c/BDNF Val/Val (N= 20) | APOE4nc/BDNF Met (N=10) | APOE4nc/BDNF Val/Val (N= 14) | APOE4 effect p-value | BDNF Met effect p-value | APOE4×BDNF p-value |
|---|---|---|---|---|---|---|---|
| Sex male/female, (%) | 4/10 (28.6%/71.4%) | 8/12 (40%/60%) | 3/7 (30%/70%) | 5/9 (35.7%/64.3%) | 0.94 | 0.49 | 0.83 |
| APOE4 (HM/HT/NC) | 8/6/0 | 5/15/0 | 0/0/10 | 0/0/14 | NA | NA | NA |
| Education, years mean (SD) | 15.8 (1.6) | 15.7 (2.3) | 16 (2.3) | 15.7 (2.5) | 0.78 | 0.87 | 0.87 |
| Age, years mean (SD) | 55.3 (5.5) | 57.4 (4.4) | 57.1 (5.3) | 57.5 (4.1) | 0.28 | 0.56 | 0.43 |
| MMSE mean (SD) | 29.6 (0.8) | 29.8 (0.4) | 29.7 (0.7) | 29.6 (0.7) | 0.64 | 0.96 | 0.50 |
| AVLT STM mean (SD) | 10.3 (2.9) | 9.4 (3.1) | 8.8 (2) | 9.6 (2.4) | 0.79 | 0.55 | 0.24 |
| AVLT LTM mean (SD) | 9.6 (3.5) | 8.8 (3.3) | 9 (2.4) | 9.4 (2.9) | 0.69 | 0.89 | 0.49 |
| AVLT TL mean (SD) | 51.8 (9.3) | 46.5 (9.5) | 46.7 (7.8) | 48.3 (8.3) | 0.30 | 0.66 | 0.16 |
| COWAT mean (SD) | 43.9 (7.1) | 46.7 (11.4) | 40.6 (7.9) | 48.9 (13.1) | 0.07 | 0.98 | 0.34 |
| Diabetes (%) | 0 | 0 | 0 | 0 | 1.00 | 1.00 | 1.00 |
| Hypertension (%) | 4 (20.0%) | 6 (15.4%) | 0 | 1 (2.9%) | 0.99 | 0.93 | 0.99 |
| Smoking (%) | 4 (20.0%) | 4 (10.3%) | 3 (15.0%) | 1 (2.9%) | 0.94 | 0.56 | 0.40 |
| Hyperlipidemia (%) | 2 (10.0%) | 6 (15.4%) | 3 (15.0%) | 0 | 0.36 | 0.30 | 0.99 |
| Any cardiovascular (%) | 1 (5.0%) | 2 (5.1%) | 1 (5.0%) | 0 | 0.80 | 0.77 | 1.00 |
Abbreviations: APOE4 HM: apolipoprotein e4 homozygote, APOE4/4; APOE4 HT: apolipoprotein e4 heterozygote, APOE3/4; APOE4 NC: apolipoprotein e4 non-carrier, APOE 3/3, 2/3; MMSE: Mini-Mental State Examination; AVLT: Auditory-Verbal Learning Test; STM: short term memory; LTM: long term memory; TL: total learning; COWAT: Controlled Oral Word Association Test. Note: COWAT tests executive function and language skills, on a scale with a lower limit of 0 and no upper limit, with higher scores indicating better performance. Data are given as mean +/‒ standard deviation unless otherwise indicated, p-values for continuous variables are from two-way ANOVA and p-values for categorical data are from logistics regression test as developed and reported in Hosmer & Lemeshow, 1989.[44]
Cognitive Change over Time
Table 3 summarizes the rate of cognitive change over time from baseline for the entire cohort (N=114, subgrouped both by APOE4 and BDNF status) as determined by linear mixed effects model. The mean rate of change for a given group is the fixed slope together with its standard deviation for that group. The only statistically significant difference was identified between the APOE4c and APOE4nc groups, where the APOE4c group had a greater decline in the rate of cognitive change over time as compared to APOE4nc in the AVLT TL (−0.45 vs −0.22 (p=0.02)), AVLT STM (−0.14 vs −0.04 (p=0.01)), AVLT LTM (−0.17 vs −0.06 (p=0.01)) and number of subjects who progressed to MCI (p=0.0004). However, the APOE4 by BDNF interaction for the cognitive longitudinal change was not statistically significant.
Table 3.
Rate of Cognitive Change over Time from Baseline for Entire Cohort (N=114) and APOE4c (N=59) Subgrouped by BDNF Status
| Total N=114 | Total N=114 | Total N=59 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| APOE4c (N=59) | APOE4nc (N=55) | P-Value | BDNF Met Carrier (N=40) | BDNF Val/Val (N=74) | P-Value | APOE4c/BDNF Met Carrier (N=20) | APOE4c/BDNF Val/Val (N=39) | P-Value | APOE by BDNF P-Value | |
| Time from Baseline, years Mean (SD) | 15 (4.76) | 15.04 (5.69) | 0.97 | 13.93 (5.29) | 15.61 (5.10) | 0.10 | 13.4 (4.76) | 15.82 (4.60) | 0.06 | 0.62 |
| FDG follow up time in years Mean (SD) | 7.34 (3.60) N=50 | 8.56 (3.43) N=46 | 0.09 | 8.31 (3.52) N=32 | 7.73 (3.58) N=64 | 0.45 | 7.26 (3.99) N=17 | 7.38 (3.44) N=33 | 0.91 | 0.33 |
| MMSE (SD) | −0.03 (0.04) | −0.01 (0.04) | 0.06 | −0.03 (0.04) | −0.02 (0.04) | 0.21 | −0.04 (0.05) | −0.02 (0.04) | 0.10 | 0.28 |
| AVLT TL (SD) | −0.45 (0.55) | −0.22 (0.48) | 0.02* | −0.31 (0.58) | −0.36 (0.50) | 0.64 | −0.52 (0.75) | −0.43 (0.44) | 0.65 | 0.22 |
| AVLT STM (SD) | −0.14 (0.22) | −0.04 (0.13) | 0.01* | −0.10 (0.19) | −0.09 (0.18) | 0.72 | −0.17 (0.25) | −0.12 (0.20) | 0.43 | 0.13 |
| AVLT LTM (SD) | −0.17 (0.25) | −0.06 (0.18) | 0.01* | −0.09 (0.21) | −0.12 (0.23) | 0.56 | −0.15 (0.30) | −0.17 (0.23) | 0.86 | 0.08 |
| COWAT (SD) | 0.11 (0.46) | 0.19 (0.50) | 0.34 | 0.16 (0.47) | 0.15 (0.49) | 0.93 | 0.21 (0.61) | 0.08 (0.41) | 0.44 | 0.54 |
| # of subjects who developed incident MCI | 17 | 4 | 0.0004* | 7 | 14 | 0.85 | 6 | 11 | 0.89 | 0.89 |
Table 3: Rate of cognitive change over time from baseline for entire cohort (N=114) and APOE4c (N=59) subgrouped by BDNF status was determined in the framework of linear mixed effects model for each subject who had at least two visits. Abbreviations: APOE4c: apolipoprotein e4 carrier, APOE4/4, 3/4; APOE4nc: apolipoprotein e4 non-carrier, APOE 3/3, 2/3; MMSE: Mini-Mental State Examination; AVLT: Auditory-Verbal Learning Test; STM: short term memory; LTM: long term memory; TL: total learning; COWAT: Controlled Oral Word Association Test. Note: COWAT tests executive function and language skills, on a scale with a lower limit of 0 and no upper limit, with higher scores indicating better performance. Data are given as mean +/‒ standard deviation unless otherwise indicated, p-values were computed under the linear mixed effect modeling with APOE4 and BDNF interaction term (p≤0.05)
Hippocampal Volume (MRI)
We found no hippocampus volume differences at baseline for any of the two main effects and their interaction. We also did not see significant longitudinal changes.
Amyloid Deposition (PiB-PET)
Within the PiB-PET imaging subgroup (N=58), an interaction between APOE4c and BDNF Met carriage was identified but only at uncorrected p=0.05 level. Figure 1 shows regions of higher amyloid deposition in APOE4c than in APOE4nc in BDNF Met group as compared to that same APOE4c/APOE4nc contrast in the BDNF Val/Val group. Equivalent to the interaction in the general two-factor ANOVA framework, the interaction examined in this study is the APOE4 difference in BDNF Met vs the APOE4 difference in BDNF Val/Val group (the difference of difference). Using our Monte-Carlo computer simulation to assess overall global significance, we found that there were 2827 voxels in the APOE4c>APOE4nc direction in the BDNF Met group (in contrast to that in the BDNF Val/Val group) and 377 voxels in the opposite direction. The overall global significance was estimated to be p<0.001 over 1000 simulations. The locations where we observed the significances are provided in the Table in Figure 1. Between the APOE4c and APOE4nc, only the APOE4c showed higher amyloid deposition in frontal regions (image not shown), consistent with our previous findings.[2]
Figure 1:
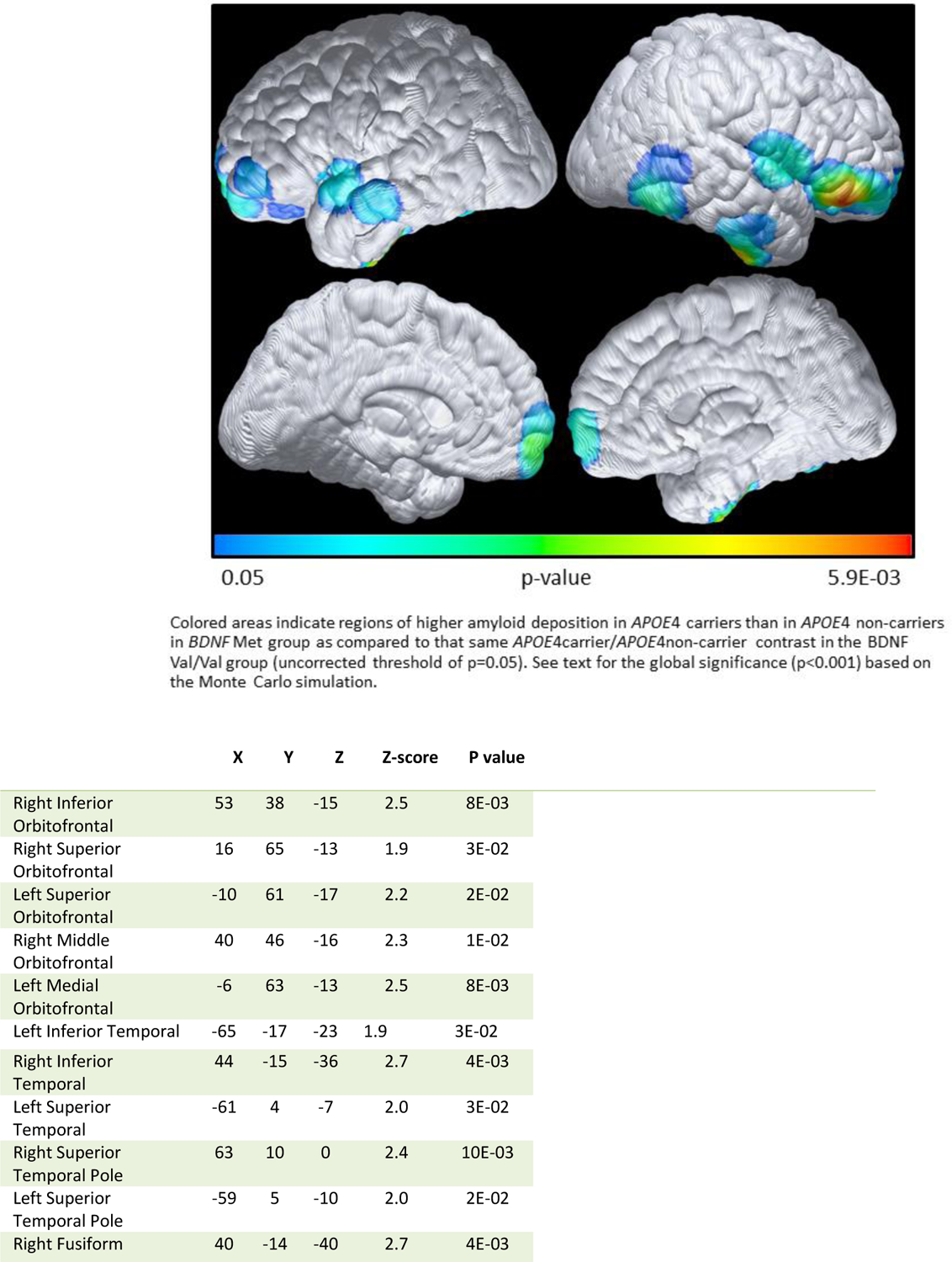
Higher amyloid deposition in APOE4c than in APOE4nc in BDNF Met group, as compared to the same directional difference in the BDANF Val/Val group
Note: The data were extracted from voxels associated with maximally significant in association with BDNF Met carriage and Met Val/Val ANOVA based analysis. At each location listed above, we observed higher amyloid deposition in APOE4c than in APOE4nc among BDNF Met individuals compared to the APOE4c/APOE4nc differences in the BDNF Val/Val individuals. Listed locations correspond to the brain maps shown in Figure 1, corresponding to p<=0.05, uncorrected. Coordinates were obtained from Talairach, X is the distance to the right or left of the midline, Y is the distance anterior or posterior to the anterior commissure, and Z is the distance superior or inferior to a horizontal plane through the anterior and posterior commissures.
Glucose Metabolism (FDG-PET)
Figure 2 shows the APOE4 and BDNF interactive effects (the difference of difference) at baseline. We found higher CMRgl in BDNF Met carriers than in BDNF Val/Val groups among APOE4 carriers, as compared to the same difference among APOE4 non-carriers, with the interaction appearing primarily in the frontal regions. For assessing the overall global significance for the APOE4 by BDNF interaction, we found that there were 7216 voxels in the direction of BDNF Met> BDNF Val/Val among APOE4 carriers and 267 voxels in the opposite direction. The global significance is also p<0.001 with 1000 simulations. Consistent with our prior studies, Figure 3 shows lower CMRgl uptake in APOE4 carriers than non-carriers in various brain regions known to be affected by AD. For post-hoc assessing the overall global significance using the Monte Carlo simulation, we found that there were 2097 voxels in this hypothesized direction and 223 voxels in opposite direction. The global significance is again p<0.001 with 1000 simulations.
Figure 2:
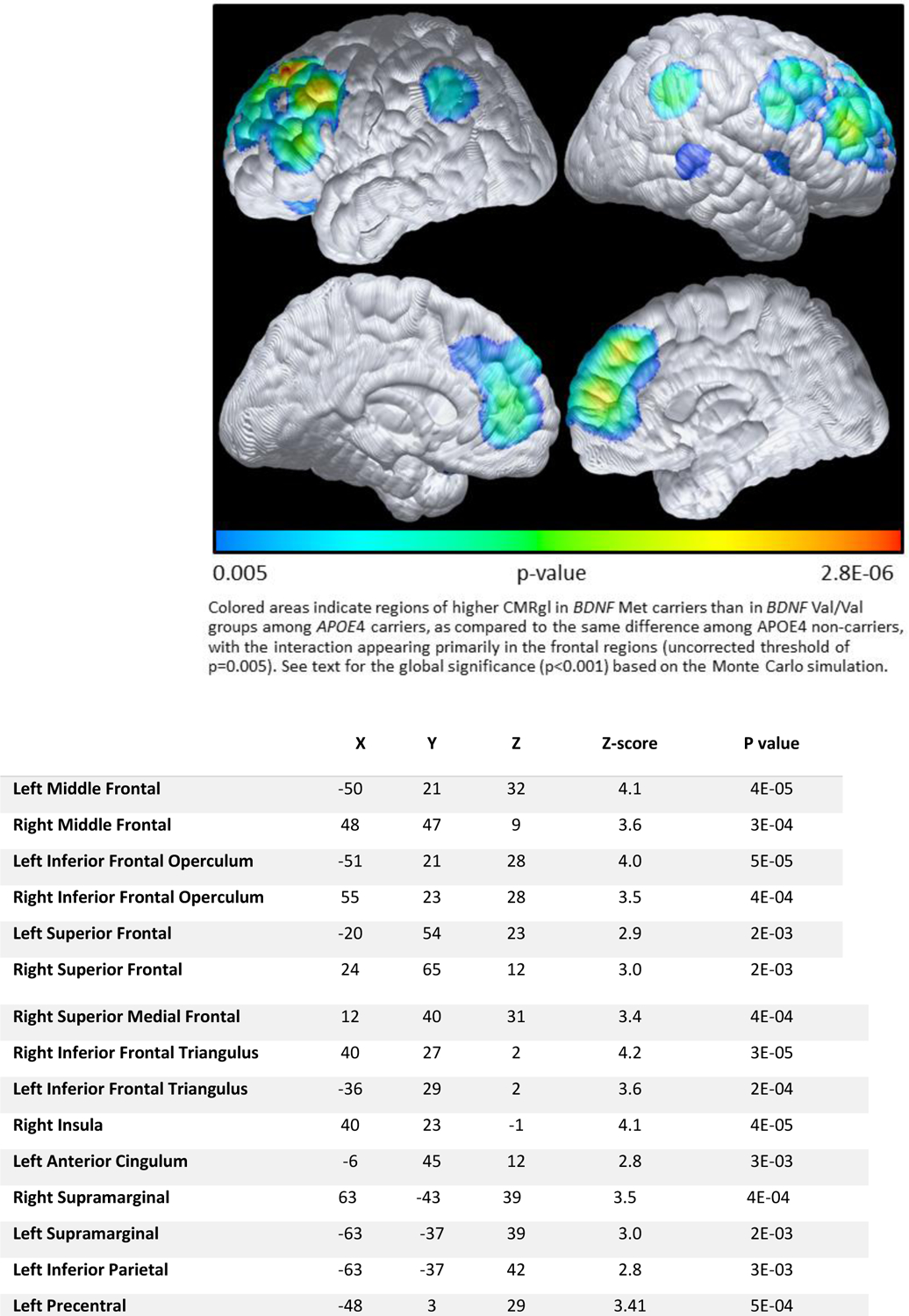
Higher CMRgl in BDNF Met carriers than in BDNF Val/Val group among АРОЕ4с, as compared to the same directional difference among АРОЕ4nс
Note: The data were extracted from voxels where maximally significantly higher CMRgl in BDNF Met than in BDNF Val/Val groups among APOE4 carriers, as compared to the same difference among APOE4 non-carriers were observed. Listed locations correspond to the brain maps shown in Figure 2, corresponding to p<=0.005, uncorrected. Coordinates were obtained from Talairach, X is the distance to the right or left of the midline, Y is the distance anterior or posterior to the anterior commissure, and Z is the distance superior or inferior to a horizontal plane through the anterior and posterior commissures.
Figure 3:
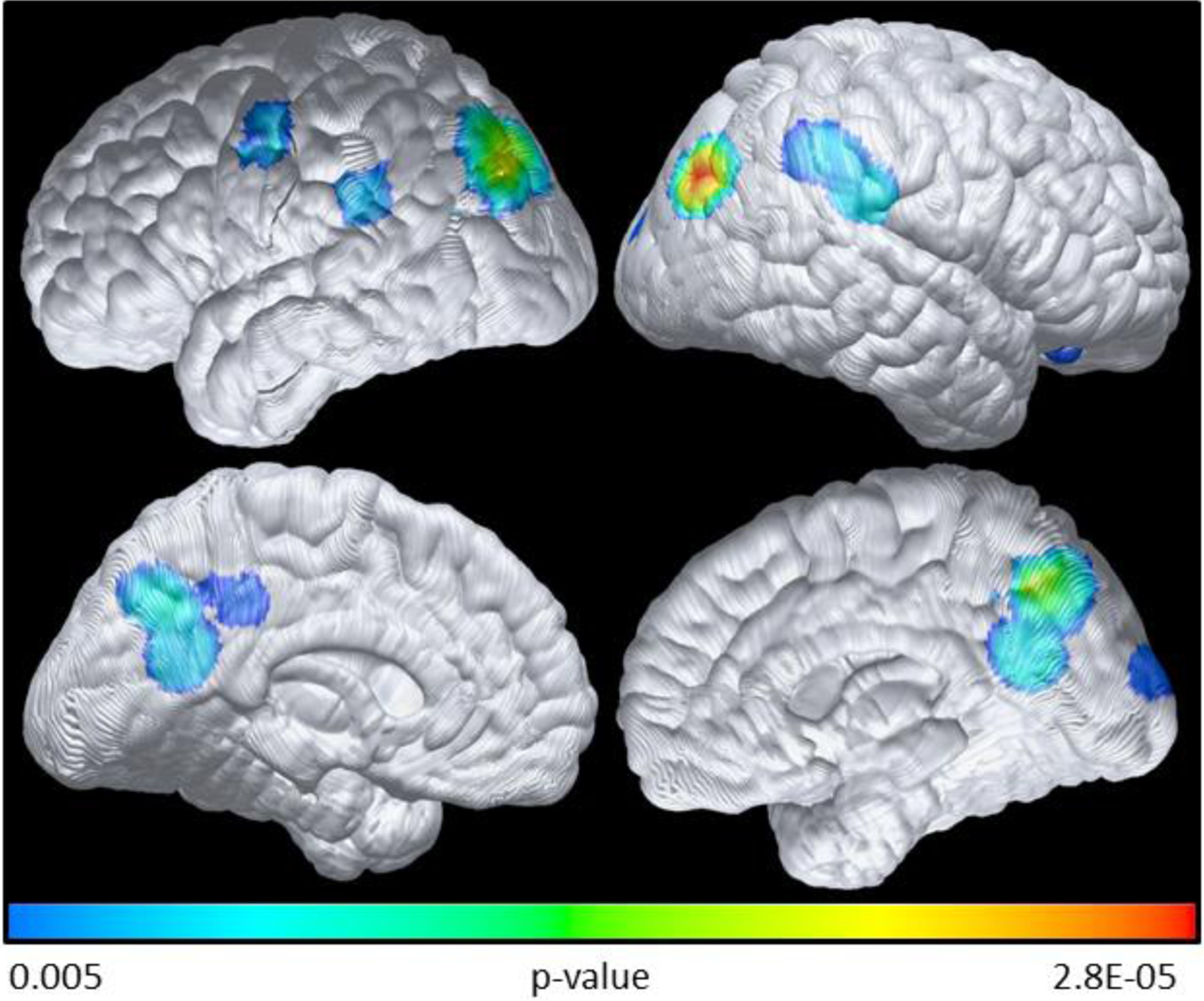
Lower baseline CMRgl in APOE4 carriers compared with APOE4 non-carriers
Statistical difference brain maps (uncorrected threshold of p=0.005) of the metabolic reduction between APOE4 carriers and non-carriers based on voxel-wise FDG analysis. As expected, compared with APOE4 non-carriers there is a reduction in CMRgl uptake in the APOE4 carriers. Colored areas indicate regions of lower CMRgl inAPOE4 carriers compared to non-carriers. See text for the global significance (p<0.001) based on the Monte Carlo simulation.
For longitudinal change, Figure 4 shows faster CMRgl decline in BDNF Met than BDNF Val/Val among APOE4c, as compared to BDNF Met versus BDNF Val/Val groups among APOE4nc (difference of difference) over an average of 8 years’ time. We observed significantly faster CMRgl decline in parahippocampus (p=0.002), precuneus (p=0.003), temporal (p=0.0001), and thalamus (p=0.0007) regions. In addition to areas of faster CMRgl decline, we looked for areas of slower CMRgl decline over time. We observed BDNF Met carriers had slower CMRgl decline in frontal regions than the BDNF Val/Val group among APOE4 carriers, as compared to BDNF Met/BDNF Val/Val groups among APOE4 non-carriers (difference of difference, image not shown).
Figure 4:
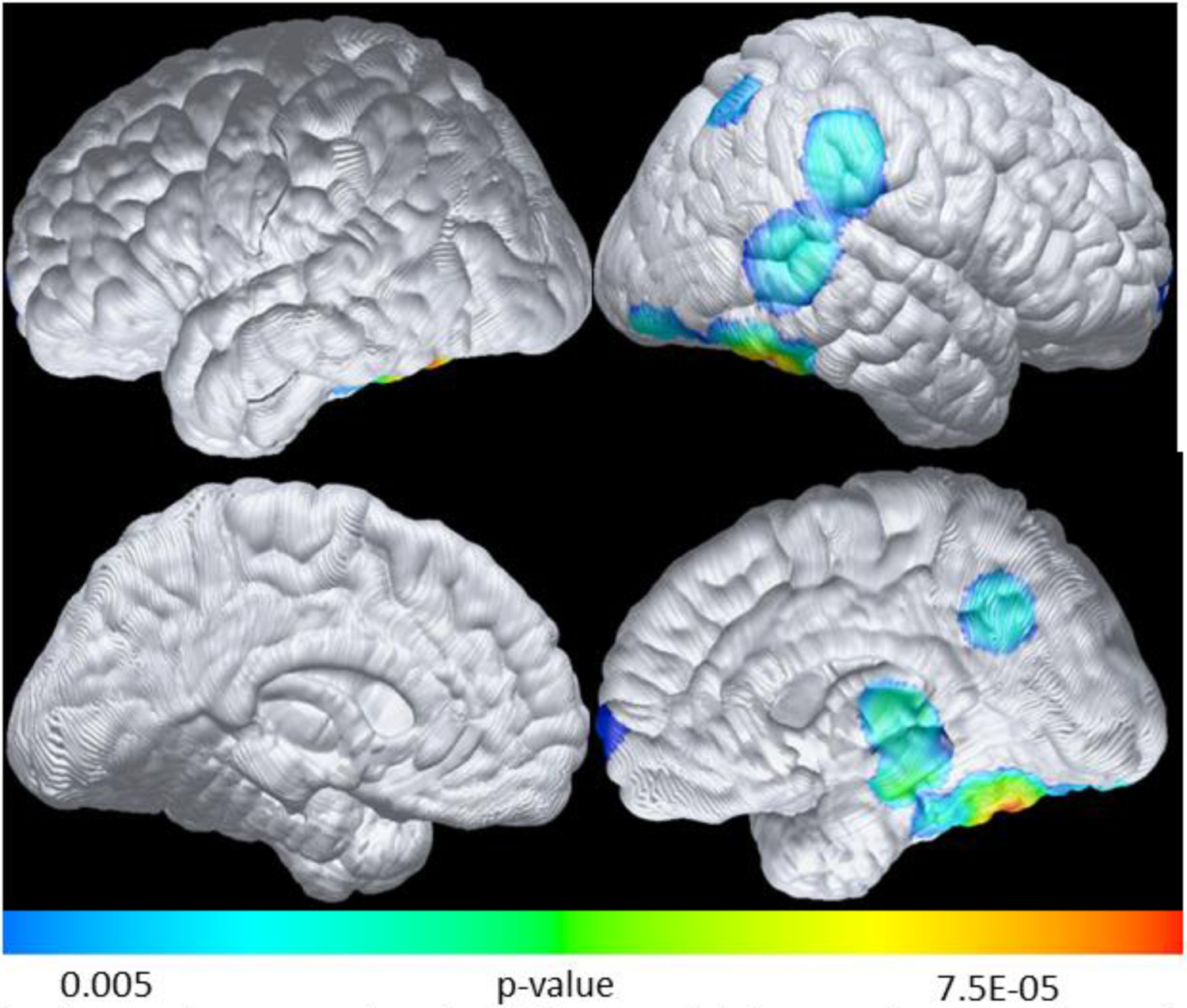
Faster CMRgl decline in BDNF Met than BDNF Val/Val among АРОЕ4с, as compared to same directional difference among АРОЕ4nс over an average of 8 years’ time
Colored areas indicate regions of significantly faster CMRgl decline in parahippocampus (p=0.002), precuneus (p=0.003), temporal (p=0.0001), and thalamus (p=0.0007) regions (uncorrected threshold of p=0.005) for BDNF Met than ßD/VF Val/Val among АРОЕ4 carriers, as compared to BDNF Met versus BDNF Val/Val groups among АРОЕ4 non-carriers, over an average of 8 years’ time.
Although not statistically significant, there were a higher proportion of APOE4 homozygotes in the BDNF Met carriers than in the BDNF Val/Val group. We therefore did a post-hoc analysis to examine the BDNF effects by covarying out APOE4 gene allele, and interactive effects remained even when covarying for APOE4 allele gene dose.
Discussion
Our initial hypothesis that in contrast to BDNF Val/Val and APOE4 non-carriers, BDNF Met and APOE4 carriage will be associated with higher Aβ burden, differing glucose metabolism, and greater cognitive decline was only partially supported. Among APOE4 carriers, BDNF met carriage was associated with increased amyloid deposition and accelerated CMRgl decline in regions typically affected by AD, but without accompanying acceleration of cognitive decline or hippocampal volume change over a nearly 15 year follow up period in our study. In contrast, these Met carriers had increased baseline frontal CMRgl and reduced frontal decline. Thus, while the BDNF Met and APOE4 carriage interaction of increased amyloid deposition and greater decline of glucose metabolism in regions typically affected by AD does suggest increased risk for AD, the preserved frontal metabolism may have been compensatory so that cognitive decline was not observed at this earlier, presymptomatic stage.
With regard to the PiB-PET results, when comparing APOE4 carriers and APOE4 non-carriers, the APOE4 carriers had significantly higher frontal amyloid deposition, a result that is consistent with previous literature [1, 2]. Consistent with our hypothesis and in line with a previous study by Adamczuk et al [27], we found an interaction between BDNF Met and APOE4 carriage associated with increased amyloid deposition and that the presence of BDNF Met in the context of APOE4 non-carriers does not result in increased amyloid deposition. With FDG-PET, we also found an interaction between BDNF Met and APOE4 carriage. Although, as expected [36, 37], the APOE4 carriers had significantly decreased CMRgl in a pattern similar to AD hypometabolism compared to APOE4nc, among APOE4 carriers, BDNF Met carriers had significantly higher frontal CMRgl and faster decline of parahippocampus, precuneus, temporal, and thalamus CMRgl over an 8-year period than the BDNF Val/Val group. We also showed, consistent with the amyloid imaging results, the baseline BDNF effects of higher CMRgl with slower longitudinal decline (average 8 years) in the frontal regions for BDNF Met carriage as compared to BDNF Val/Val individuals were not found among APOE4 non-carriers. This reflects a different, although not initially unfavorable, glucose metabolism mechanism that APOE4 BDNF Met carriers employ, as we did not observe statistically significant cognitive deficits related to APOE4c BDNF Met carriers relative to the APOE4c Val/Val group, nor did we observe hippocampal volume decline.
In line with other studies, our results showed a decline in the rate of cognitive change over 15 years’ time in the APOE4 carriers as compared with the APOE4 non-carriers. However, we found no differences in the rate of cognitive decline between the BDNF Met and Val/Val groups either as a whole or within the APOE4 group, which did not support our initial hypothesis that the combination of APOE4 and BDNF Met carriage would result in greater cognitive decline. The only statistically significant difference was found on the baseline MMSE mean score (p=0.04), where among BDNF Met carriers the mean (SD) was 29.6 (0.68) and BDNF Val/Val was 29.9 (0.38). While this achieved statistical significance, it is not clinically relevant as both groups scored well within the normal limits for normal cognition for the MMSE test (30 possible points). A recent study by Xia et al 2019 [38] investigated the influence of BDNF Val66Met on cognition, CSF and neuroimaging markers in the non-demented elderly using the ADNI cohort. While this study discusses non-demented elderly, it is important to note that this group included both CU and MCI (CDR 0.5, MMSE 23–30) together and did not analyze the groups separately. They found in this combined group of average age of 74 years that there was an interaction between Aβ load and BDNF Val66Met in cognition. The BDNF Val66Met polymorphism had significant association with atrophy of the entorhinal cortex and MMSE scores in the non-demented elderly and the A+ (abnormal Aβ) subgroup, while no association was found in the A-subgroup. Another recent study of very mild amnestic MCI patients (average age 72) found that the combination of BDNF Met and APOE4 carriage is associated with memory dysfunction but not with structural brain changes [39]. In contrast, Gomar and colleagues did see a trend for thinner posterior cingulate and precuneus cortices but, consistent with our findings, no significant differences in cognitive measures in CU APOE4c Met carriers compared to Val homozygotes (average age 76) [24]. Lim and colleagues [26] observed accelerated memory decline in CU amyloid positive/APOE4c/BDNF Met carriers (average age 72). They and Boots et al, who also observed accelerated cognitive decline in a similar but younger aged cohort [23], did not report on FDG PET data. Our study included CU participants who, with the exception of the study by Boots and colleagues, were younger than the participants in the aforementioned studies and had no evidence of MCI. These demographic differences may account for the lack of association with memory decline for the BDNF Met/APOE4c group in our study. Our baseline FDG PET findings further support the conclusion that there may be some initial compensatory brain activity in this younger, CU cohort to offset dysfunction and accelerated decline associated with Aβ deposition.
Our FDG PET findings compliment findings from Xu et al [40], where the BDNF Met allele affects glucose metabolism in some specific regions with both hyper- and hypometabolism in cognitively unimpaired adults. While Xu and colleagues did not combine BDNF Met and APOE4 carriage nor report on differences in cognitive measures over time as our study did, they did investigate BDNF Met carriage and used age, sex, and APOE4 status as covariates. Hypermetabolism in BDNF Met carriers compared to Val/Val group in CU was found in the superior and middle frontal gyrus cortex. Another study reported hyperactivity in frontal and posterior parietal cortexes with fMRI in healthy BDNF Met carriers in comparison to the Val/Val group during a spatial working memory task [41].
The strength of our study is the longitudinal nature and addition of FDG PET and MRI data to help explain varying results reported in the literature. One limitation of our study is that study participants all had a family history of dementia, so it is possible that the findings may not generalize to those without a family history. Additionally, our small sample size may have affected the variability of the results. Indeed, with association studies carried out on a relatively small sample size, Type 1 error may exist and thus, further replication of this study is required before we have a more defined answer regarding the interaction between BDNF Met carriage and APOE4 on amyloid burden, glucose metabolism, hippocampal volume, and change in cognitive scores over time. In addition to an overall small sample size, we did not separate Met carriers into heterozygotes and homozygotes. In a population of European ancestry, 64% of individuals are Val homozygotes (Val/Val), another 3% are Met homozygotes (Met/Met), and the 34% that remain are the heterozygotes (Val/Met) [42]. It is possible that previous studies may have suffered from small effect sizes with few Met homozygotes. Owing to the low frequency of the Met allele, studies, including ours, have combined Met/Met and Val/Met subjects, or only compared Val/Met and Val/Val subjects and excluded Met/Met due to the low sample size. Further studies are required to determine the consequences of BDNF Met homozygosity. Also, as with any study such as this, we cannot exclude the possibility of unexamined confounders such as medications for unrelated conditions. Our cohort was largely healthy and cognitively unimpaired and most likely confounders such as cardiovascular risk factors, which may differentially influence age-related memory decline in APOE4 homozygotes,[43] were evenly balanced among groups. We are unaware of cardiovascular risk factor influences with BDNF Val66Met. Although not statistically significant, there were more APOE4 homozygotes in the BDNF Met group than the BDNF Val/Val group, which could confound our conclusions, particularly with the small sample size. However the effects remained even when covarying for APOE4 allele gene dose, and, unlike the statistically greater proportion of APOE4 carriers than non-carriers who developed incident MCI, there were less in the BDNF Met group than the BDNF Val/Val who developed incident MCI (also not statistically significant). It is interesting to note the absence of significant hippocampal volume baseline and longitudinal differences in the presence of some significant PET and cognitive findings especially for APOE and for APOE4/BDNF interaction. We did not observe any significant differences in the hippocampal volume, even between APOE4c and APOE4nc groups, let alone the BDNF interactions. We previously reported the same insignificant findings for hippocampus volume.[13] The insignificant MRI based structural changes also contributed to the results of our one early report that CMRgl correlations with APOE4 gene dose remained the same with or without the correction for partial-volume averaging.[9]
Conclusion
We observed a weak interaction between BDNF Met and APOE4 carriage with the baseline PIB PET, baseline FDG PET, and longitudinal FDG PET findings. The increased baseline CMRgl in APOE4/BDNF Met carriers may reflect a compensatory response to offset the dysfunction resulting from higher frontal amyloid deposition. This reflects perhaps a different, although not initially unfavorable, glucose metabolism mechanism that APOE4c BDNF Met carriers employ, as we noticed preservation in cognition over time with no significant differences in decline of cognitive scores identified in either APOE4/BDNF Met carriers or the BDNF Met carriers alone. However, due to the small sample size, we cannot reach any definite conclusions regarding whether BDNF Met carriage is an AD genetic risk or protective factor. Further studies should examine BDNF Met carriage in both homozygotes and heterozygotes.
Acknowledgments
Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Numbers R01AG031581, P30AG019610, UF1AG046150 and RF1AG041705, Arizona DHS Grant No. CTR040636 (previously ADHS Grant No. ADHS14–052688), and the Arizona Alzheimer’s Consortium. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- BDNF
brain-derived neurotropic factor
- APOE
apolipoprotein E
- Val
valine
- Met
methionine
- MRI
magnetic resonance imaging
- PiB
Pittsburgh Compound B
- PET
positron emission tomography
- FDG
Fluorodeoxyglucose
- CMRgl
cerebral metabolic rate for glucose
- AD
Alzheimer’s disease
- CU
cognitively unimpaired
Footnotes
Conflict of Interest/Disclosure statement
The authors have no conflict of interest to report.
References
- [1].Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA (2010) APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ (2009) Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 106, 6820–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ (2009) Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol 65, 650–657. [DOI] [PubMed] [Google Scholar]
- [4].Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DE, Snyder CH, Alexander GE, Rademakers R, Reiman EM (2009) Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med 361, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, Rentz DM, Johnson KA, Sperling RA, Alzheimer’s Disease Neuroimaging I, Australian Imaging B , Lifestyle Flagship Study of A, Harvard Aging Brain S (2014) Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology 82, 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nielsen HM, Chen K, Lee W, Chen Y, Bauer RJ 3rd, Reiman E, Caselli R, Bu G (2017) Peripheral apoE isoform levels in cognitively normal APOE epsilon3/epsilon4 individuals are associated with regional gray matter volume and cerebral glucose metabolism. Alzheimers Res Ther 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J (2001) Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci U S A 98, 3334–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D (1996) Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 334, 752–758. [DOI] [PubMed] [Google Scholar]
- [9].Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J (2005) Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A 102, 8299–8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, Mazziotta JC, Saxena S, Wu HM, Mega MS, Cummings JL, Saunders AM, Pericak-Vance MA, Roses AD, Barrio JR, Phelps ME (2000) Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 97, 6037–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA, Kaplan A, La Rue A, Adamson CF, Chang L, et al. (1995) Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 273, 942–947. [PubMed] [Google Scholar]
- [12].Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM (2010) APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 67, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, De Santi S, Convit A, Osborne D, Weaver A, Thibodeau SN (1998) Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s disease. Ann Neurol 44, 288–291. [DOI] [PubMed] [Google Scholar]
- [14].Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS (2004) Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci 24, 4401–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269. [DOI] [PubMed] [Google Scholar]
- [16].Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ, Lee FS (2010) Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann N Y Acad Sci 1208, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Beste C, Baune BT, Domschke K, Falkenstein M, Konrad C (2010) Paradoxical association of the brain-derived-neurotrophic-factor val66met genotype with response inhibition. Neuroscience 166, 178–184. [DOI] [PubMed] [Google Scholar]
- [18].Zivadinov R, Weinstock-Guttman B, Benedict R, Tamano-Blanco M, Hussein S, Abdelrahman N, Durfee J, Ramanathan M (2007) Preservation of gray matter volume in multiple sclerosis patients with the Met allele of the rs6265 (Val66Met) SNP of brain-derived neurotrophic factor. Hum Mol Genet 16, 2659–2668. [DOI] [PubMed] [Google Scholar]
- [19].Oroszi G, Lapteva L, Davis E, Yarboro CH, Weickert T, Roebuck-Spencer T, Bleiberg J, Rosenstein D, Pao M, Lipsky PE, Goldman D, Lipsky RH, Illei GG (2006) The Met66 allele of the functional Val66Met polymorphism in the brain-derived neurotrophic factor gene confers protection against neurocognitive dysfunction in systemic lupus erythematosus. Ann Rheum Dis 65, 1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barbey AK, Colom R, Paul E, Forbes C, Krueger F, Goldman D, Grafman J (2014) Preservation of general intelligence following traumatic brain injury: contributions of the Met66 brain-derived neurotrophic factor. PLoS One 9, e88733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nacmias B, Piccini C, Bagnoli S, Tedde A, Cellini E, Bracco L, Sorbi S (2004) Brain-derived neurotrophic factor, apolipoprotein E genetic variants and cognitive performance in Alzheimer’s disease. Neurosci Lett 367, 379–383. [DOI] [PubMed] [Google Scholar]
- [22].Richter-Schmidinger T, Alexopoulos P, Horn M, Maus S, Reichel M, Rhein C, Lewczuk P, Sidiropoulos C, Kneib T, Perneczky R, Doerfler A, Kornhuber J (2011) Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm (Vienna) 118, 249–257. [DOI] [PubMed] [Google Scholar]
- [23].Boots EA, Schultz SA, Clark LR, Racine AM, Darst BF, Koscik RL, Carlsson CM, Gallagher CL, Hogan KJ, Bendlin BB, Asthana S, Sager MA, Hermann BP, Christian BT, Dubal DB, Engelman CD, Johnson SC, Okonkwo OC (2017) BDNF Val66Met predicts cognitive decline in the Wisconsin Registry for Alzheimer’s Prevention. Neurology 88, 2098–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gomar JJ, Conejero-Goldberg C, Huey ED, Davies P, Goldberg TE, Alzheimer’s Disease Neuroimaging I (2016) Lack of neural compensatory mechanisms of BDNF val66met met carriers and APOE E4 carriers in healthy aging, mild cognitive impairment, and Alzheimer’s disease. Neurobiol Aging 39, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, Harrington KD, Bourgeat P, Salvado O, Darby D, Snyder PJ, Bush AI, Martins RN, Masters CL, Rowe CC, Nathan PJ, Maruff P, Australian Imaging B, Lifestyle Research G (2013) BDNF Val66Met, Abeta amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol Aging 34, 2457–2464. [DOI] [PubMed] [Google Scholar]
- [26].Lim YY, Villemagne VL, Laws SM, Pietrzak RH, Snyder PJ, Ames D, Ellis KA, Harrington K, Rembach A, Martins RN, Rowe CC, Masters CL, Maruff P (2015) APOE and BDNF polymorphisms moderate amyloid beta-related cognitive decline in preclinical Alzheimer’s disease. Mol Psychiatry 20, 1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Adamczuk K, De Weer AS, Nelissen N, Chen K, Sleegers K, Bettens K, Van Broeckhoven C, Vandenbulcke M, Thiyyagura P, Dupont P, Van Laere K, Reiman EM, Vandenberghe R (2013) Polymorphism of brain derived neurotrophic factor influences beta amyloid load in cognitively intact apolipoprotein E epsilon4 carriers. Neuroimage Clin 2, 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Caselli RJ, Dueck AC, Locke DE, Hoffman-Snyder CR, Woodruff BK, Rapcsak SZ, Reiman EM (2011) Longitudinal modeling of frontal cognition in APOE epsilon4 homozygotes, heterozygotes, and noncarriers. Neurology 76, 1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Caselli RJ, Reiman EM, Locke DE, Hutton ML, Hentz JG, Hoffman-Snyder C, Woodruff BK, Alexander GE, Osborne D (2007) Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol 64, 1306–1311. [DOI] [PubMed] [Google Scholar]
- [30].Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG (2004) Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology 62, 1990–1995. [DOI] [PubMed] [Google Scholar]
- [31].Liang WS, Chen K, Lee W, Sidhar K, Corneveaux JJ, Allen AN, Myers A, Villa S, Meechoovet B, Pruzin J, Bandy D, Fleisher AS, Langbaum JB, Huentelman MJ, Jensen K, Dunckley T, Caselli RJ, Kaib S, Reiman EM (2011) Association between GAB2 haplotype and higher glucose metabolism in Alzheimer’s disease-affected brain regions in cognitively normal APOEepsilon4 carriers. Neuroimage 54, 1896–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stern RA, Adler CH, Chen K, Navitsky M, Luo J, Dodick DW, Alosco ML, Tripodis Y, Goradia DD, Martin B, Mastroeni D, Fritts NG, Jarnagin J, Devous MD Sr., Mintun MA, Pontecorvo MJ, Shenton ME, Reiman EM (2019) Tau Positron-Emission Tomography in Former National Football League Players. N Engl J Med 380, 1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Langbaum JB, Chen K, Lee W, Reschke C, Bandy D, Fleisher AS, Alexander GE, Foster NL, Weiner MW, Koeppe RA, Jagust WJ, Reiman EM, Alzheimer’s Disease Neuroimaging I (2009) Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Neuroimage 45, 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- [35].Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM (2004) Sequence-independent segmentation of magnetic resonance images. Neuroimage 23 Suppl 1, S69–84. [DOI] [PubMed] [Google Scholar]
- [36].Lowe VJ, Weigand SD, Senjem ML, Vemuri P, Jordan L, Kantarci K, Boeve B, Jack CR Jr., Knopman D, Petersen RC (2014) Association of hypometabolism and amyloid levels in aging, normal subjects. Neurology 82, 1959–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Knopman DS, Jack CR Jr., Lundt ES, Weigand SD, Vemuri P, Lowe VJ, Kantarci K, Gunter JL, Senjem ML, Mielke MM, Machulda MM, Roberts RO, Boeve BF, Jones DT, Petersen RC (2016) Evolution of neurodegeneration-imaging biomarkers from clinically normal to dementia in the Alzheimer disease spectrum. Neurobiol Aging 46, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xia H, Wang M, Li JQ, Tan CC, Cao XP, Tan L, Yu JT, Alzheimer’s Disease Neuroimaging I (2019) The Influence of BDNF Val66Met Polymorphism on Cognition, Cerebrospinal Fluid, and Neuroimaging Markers in Non-Demented Elderly. J Alzheimers Dis 68, 405–414. [DOI] [PubMed] [Google Scholar]
- [39].Cechova K, Andel R, Angelucci F, Chmatalova Z, Markova H, Laczo J, Vyhnalek M, Matoska V, Kaplan V, Nedelska Z, Ward D, Hort J (2019) Impact of APOE and BDNF Val66Met Gene Polymorphisms on Cognitive Functions in Patients with Amnestic Mild Cognitive Impairment. J Alzheimers Dis. [DOI] [PubMed] [Google Scholar]
- [40].Xu C, Wang Z, Fan M, Liu B, Song M, Zhen X, Jiang T, Alzheimer’s Disease Neuroimaging I (2010) Effects of BDNF Val66Met polymorphism on brain metabolism in Alzheimer’s disease. Neuroreport 21, 802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cerasa A, Tongiorgi E, Fera F, Gioia MC, Valentino P, Liguori M, Manna I, Zito G, Passamonti L, Nistico R, Quattrone A (2010) The effects of BDNF Val66Met polymorphism on brain function in controls and patients with multiple sclerosis: an imaging genetic study. Behav Brain Res 207, 377–386. [DOI] [PubMed] [Google Scholar]
- [42].Lamb YN, Thompson CS, McKay NS, Waldie KE, Kirk IJ (2015) The brain-derived neurotrophic factor (BDNF) val66met polymorphism differentially affects performance on subscales of the Wechsler Memory Scale - Third Edition (WMS-III). Front Psychol 6, 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Caselli RJ, Dueck AC, Locke DE, Sabbagh MN, Ahern GL, Rapcsak SZ, Baxter LC, Yaari R, Woodruff BK, Hoffman-Snyder C, Rademakers R, Findley S, Reiman EM (2011) Cerebrovascular risk factors and preclinical memory decline in healthy APOE epsilon4 homozygotes. Neurology 76, 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hosmer DW Jr., Lemeshow S (1989) Applied logistic regression., Wiley, New York. [Google Scholar]


