Abstract
Background
Acute pancreatitis, a sudden inflammation of the pancreas, can result in severe complications. The presence and volume of ascites, an abnormal accumulation of fluid in the abdomen, has been linked to disease severity. Our study investigates ascites volume, quantified via abdominal CT scans, as a potential predictive tool for disease severity.
Material/Methods
In this retrospective analysis, patients diagnosed with acute pancreatitis were evaluated. Patients were categorized into groups with and without ascites, with comparisons made regarding clinical characteristics. We further compared the mean ascitic volume against various outcome parameters in patients with ascites. Ascites volume and other predictive systems were assessed through receiver operating characteristic (ROC) curves, with the area under the ROC curve (AUC) for different predictive systems being analyzed.
Results
The ascites group had higher severity scores and related serological indexes (P<0.05 for all). Among patients with ascites, a significant correlation was observed between ascites volume and outcome parameters (P<0.05 for all). The area under the ROC curve for predicting severe acute pancreatitis was 0.896, with 93% sensitivity and 79% specificity. Ascites volume yielded the highest diagnostic odds ratio (53.1; 95% confidence interval: 13.2,199.6).
Conclusions
Early-stage acute pancreatitis patients with ascites are indicative of severe illness and poor prognosis. An increase in ascites volume correlates with adverse clinical outcomes, thus highlighting the significance of ascites volume as a prognostic marker. This underscores the importance of abdominal CT in measuring ascites volume to predict disease severity.
Keywords: Ascites; Coinfection; Multiple Organ Failure; Pancreatitis; Tomography, X-Ray Computed
Background
Acute pancreatitis (AP) is a common digestive system disease in clinical practice, with a high incidence rate and an increasing trend year by year [1–3]. In the past, AP was thought to be mild and self-limiting, but now research has found that AP is a disease that cannot be regarded as self-limiting, because it has serious early and long-term effects [4]. Approximately 10–20% of these patients develop severe acute pancreatitis (SAP). SAP has many complications, high mortality, and unpredictable prognosis [5]. During the course of acute pancreatitis, organ failure, infection, and persistent systemic inflammatory response syndrome (SIRS) are determinants of the severity of the disease [3,6]. It is of great importance to identify and predict the above decisive factors early, prevent further progression of the disease, carry out risk stratification management for AP patients, and improve the prognosis [7].
At present, the commonly used clinical assessment and prediction systems for the severity of AP include serological indicators, such as C-reactive protein (CRP) level and procalcitonin (PCT) level [8–11], and scoring systems, such as acute physiology and chronic health evaluation II (APACHE II) score and Bedside Index of Severity in Acute Pancreatitis (BISAP) score [12–14]. However, ascites in AP is not involved in these indicators.
It has been reported that the development of ascites, as a common complication in the natural history of AP, has an incidence of 30–40% [15], and the incidence of ascites in SAP patients is over 60% [16]. Nikhil Bush, Reza Mofidi, and other scholars have noted that patients with ascites in AP have higher mortality and complication rates [15]. Recent studies have also shown that ascites is a marker of poor prognosis in AP and that reducing the volume of ascites improves prognosis [17,18]. However, the impact of ascites produced during the course of AP on clinical outcomes such as organ failure, infection, and SIRS has not been reported in detail in the literature to date, and the predictive role of ascites volume for disease severity is uncertain.
The objectives of this study were to: (1) assess the incidence of ascites in patients with AP; (2) analyze the relationship between ascites volume and severity of AP; and (3) to evaluate the reliability of ascites volume in predicting severe clinical outcome of AP.
Material and Methods
Patient and Clinical Data
A retrospective study was conducted on AP patients who visited our Pancreatitis Department from January 2018 to December 2021. The diagnostic criteria of AP met 2 of the following 3 characteristics according to the Atlanta criteria [19]: (1) persistent pain in the upper abdomen; (2) serum amylase and/or lipase concentrations at least 3 times higher than the upper normal limit; and (3) abdominal imaging examination results consistent with the imaging changes of acute pancreatitis. Exclusion criteria were: (1) previous ascites, (2) previous history of chronic pancreatitis, (3) not having received an abdominal computed tomography (CT) scan early (2 to 6 days after the onset of symptoms), and (4) patients age <18 or >80 years. The imaging, laboratory, and demographic data of the included patients were collected. This study was approved by our Ethics Committee (no: QYFYWZLL26817) and was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments, and the included patients gave their consent.
Abdominal CT and Ascites Measurement
All CT examinations were performed by using a 16-detector row CT scanner (Activion 16; Toshiba Medical Systems, Japan or Sensation 16; Siemens, Erlangen, Germany). The imaging parameters were as follows: tube voltage 120 kV; tube current, 200 mAs per section; reconstruction thickness, 5 mm; and matrix, 512×512. Some patients underwent enhanced scanning at the same time: iopromide was injected intravenously with a flow rate of 3–5 ml/s. Scanning range was from diaphragm fornix to ischial level. Ascites definition was free-flowing fluid collection, primarily for the abdominal or pelvic cavity [20–22]. 3D-slicer (3D Slicer image computing platform | 3D Slicer) [23]. Open-source software of medical imaging was used for measurement: abnormal free-flowing fluid accumulated in the abdominal cavity was the area of interest, and the volume of all voxels in the region of interest, which was the volume of ascites, was measured. CT images of the included patients were reviewed and calculated by an observer who was unaware of the clinical outcomes and experimental data, and then reviewed and calculated again 2 months later by disrupting the original order of observation.
Result Parameters and Severity Evaluation System
The following result parameters were collected from the hospital system: the length of hospital stay (in days), the need for intervention (including surgery and interventional puncture), the need for continuous renal replacement therapy (CRRT), the occurrence of SIRS, and various clinical outcomes (eg, infection, organ failure, SAP, death) in the course of disease. The classification of AP was based on the revised Atlanta classification [19] and was divided into mild, moderately severe, and severe, as well as mild without organ failure and local or systemic complications, moderately severe with transient (≤48 h) organ failure and/or local complications, and severe with persistent (> 48 h) organ failure. Organ failure was defined using the modified Marshall scoring system: Marshall score of any organ of heart, lung and kidney ≤2 points [24]. The infection was judged to meet 1 of the following 3 conditions [25]: (1) Fever ≥37.8°C and white blood cell count ≥15 000/mm3; (2) Gram staining positive result; and (3) Gram culture positive results. Systemic inflammatory response syndrome (SIRS) is defined as having at least 2 of the following 4 points [26]: (1) temperature >38°C or <36°C; (2) heart rate >90 beats/minute; (3) breathing >20 breaths/minute or PCO2 <32.33 mmHg; (4) White blood cell count of >12×109/L or <4×109/L(>12000/μl or <4000/μl or immature granulocyte >10%).
Severity assessment and prediction system used the APACHE II score, usually divided into mild (0–7 points) and severe (≥8 points) [27]; BISAP scores are generally divided into mild (0–2 points) and severe (≥3 points) [7], as well as CRP and PCT levels.
Statistical Analysis
SPSS software (version 26.0, IBM, USA) and MedCalc 11.4.2.0 (MedCalc, Mariakerke, Belgium) were used to analyze the data.
Continuous variables are expressed as the mean±standard deviation, and classification variables and rank variables are expressed as percentages. The data were checked for normal distribution by the Kolmogorov-Smirnov test. For normally distributed data, a t test was used for continuous variables, and nonparametric tests were used for skewed data. The chi-square test was used for the second-category data or ascites volume, length of stay, and various outcome parameters that did not conform to a normal distribution, and the Pearson correlation coefficient was used to test the correlation between them.
A receiver operating characteristic (ROC) curve was constructed to determine the optimal threshold for ascites volume to predict SAP, organ failure, and infection. ROC curves of BISAP score, APACHE II score, CRP level, and PCT level were also constructed. The area under the ROC curve (AUC) was calculated, and the z test was used to compare them in pairs. The sensitivity, specificity, positive likelihood ratio, positive predictive value, and diagnostic odds ratio in the same population were calculated. P<0.05 was considered statistically significant.
Results
Population
A total of 258 AP patients were included, including 5 patients with tumors, 11 patients with a previous history of chronic pancreatitis, and 16 patients with missing data, and 226 patients were included in the study. Among them, 88 patients (38.9%) had ascites in the early course of disease, and 146 patients (61.1%) had no ascites. The demographic and clinical characteristics of the 2 groups are shown in Table 1.
Table 1.
Comparison of clinical characteristics and outcome parameters between patients with and non-ascites.
| Parameter | Ascites (n=88) | Non-ascites (n=138) | P value |
|---|---|---|---|
| Patient characteristics | |||
| Age (y)* | 48.4±16.3 | 50.5±17.5 | 0.359 |
| Cause of acute pancreatitis | |||
| Gallstone | 29 (33.0) | 46 (33.3) | |
| Ampullary tumor | 26 (29.5) | 39 (28.3) | |
| Alcohol abuse | 15 (17.0) | 16 (11.6) | |
| Other | 18 (20.5) | 37 (26.8) | |
| Result argument | |||
| Duration of hospitalization (d)* | 29.5±27.0 | 11.5±7.4 | <0.05 |
| Organ failure | 65 (73.9) | 39 (28.3) | <0.05 |
| Infection | 66 (75.0) | 35 (25.4) | <0.05 |
| SIRS | 62 (70.5) | 62 (44.9) | <0.05 |
| Need for intervention | 59 (67.0) | 10 (7.2) | <0.05 |
| CRRT | 60 (68.2) | 25 (18.1) | <0.05 |
| Atlanta classification | |||
| MAP | 0 (0) | 93 (61.90) | <0.05 |
| MSAP | 28 (31.8) | 36 (29.25) | <0.05 |
| SAP | 60 (68.2) | 9 (8.84) | <0.05 |
| Severity assessment scoring system | |||
| BISAP* | 1.9±0.7 | 0.8±0.7 | <0.05 |
| APACHE II* | 8.1±4.8 | 6.9±3.4 | <0.05 |
| Serological indicator | |||
| CRP* | 135.2±117.2 | 54.7±79.6 | <0.05 |
| PCT* | 3.0±7.8 | 1.0±2.4 | <0.05 |
| CRE | 19.7±13.0 | 14.3±6.6 | <0.05 |
| HCT | 41.9±8.6 | 40.9±5.8 | <0.05 |
| WBC | 13.8±5.6 | 12.1±5.5 | <0.05 |
| BUN | 90.7±78.7 | 64.7±37.9 | <0.05 |
| Body temperature | 37.2±0.7 | 36.9±0.6 | <0.05 |
Unless otherwise specified, the data are the number of patients, and the percentage in brackets.
The data are the mean±standard deviation.
Comparing the 2 groups of patients (Table 1: ascites group and non-ascites group), ascites in the early course of the disease was not significantly associated with patient age or etiology (P>0.05). However, there was a significant relationship with length of hospital stay, organ failure, infection, Atlanta grading system, APACHE II scores, BISAP scores and other related scores, CRP level, PCT level, and other serological indicators (P<0.05 for all). The hospitalization time of the ascites group was longer (P<0.05), and the occurrence of organ failure (73.9% >28.3%, P<0.05), infection (75% >25.4%, P<0.05), SIRS (70.5% >44.9%, P <0.05), need for CRRT (68.2% >18.1%, P<0.05) and intervention (67.0% >7.2%, P<0.05) was higher. Various severity assessment and prediction parameters at admission, such as BISAP score, APACHE II score, and serological indicators such as PCT, CRP, CRE, and HCT levels, were significantly higher in the ascites group than in the non-ascites group (P<0.05 for all). The incidence of SAP in patients with ascites was also significantly higher than that in patients without ascites (P<0.05).
Ascites Volume
Ascites Volume and Result Parameters
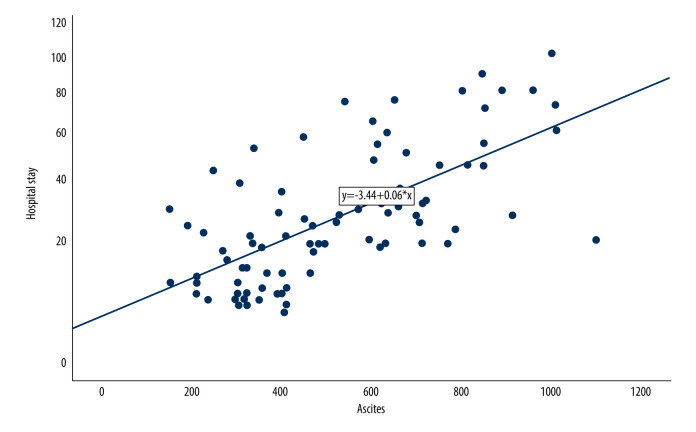
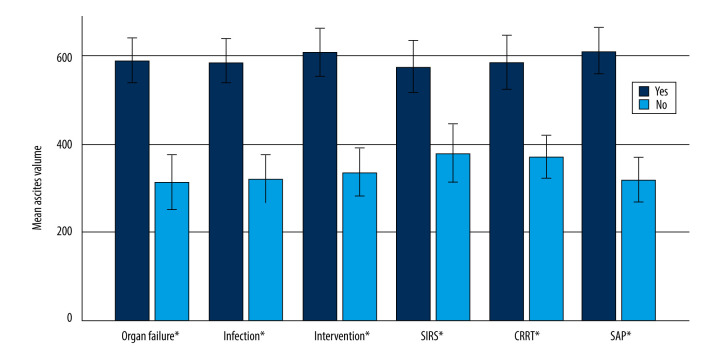
Eighty-eight patients with ascites were analyzed. The average volume of ascites in patients with ascites was 519.0 ml±231.5 ml (the range was 150–1100 ml). A positive correlation between ascites volume and length of stay was found in this study (Spearman correlation coefficient r=0.66, P<0.05) (Figure 1), as well as a positive correlation between ascites volume and the occurrence of SAP, organ failure, infection, SIRS, the need for intervention, and the need for CRRT (Table 2, Figure 2).
Figure 1.
Correlation between ascites volume and hospital stay (SPSS: Statistical Product and Service Solutions, SPSS 26.0, IBM; Photoshop CC, Adobe systems).
Table 2.
Correlation between ascites volume and various result parameters.
| variables | Ascites | |
|---|---|---|
| r | P value | |
| SAP | 0.639 | <0.05 |
| Organ failure | 0.583 | <0.05 |
| Infection | 0.522 | <0.05 |
| SIRS | 0.401 | <0.05 |
| CRRT | 0.446 | <0.05 |
| Intervention | 0.601 | <0.05 |
Figure 2.
Average ascites volume of different results under each result parameter (error bar: 95% confidence interval; * P<0.05) (SPSS: Statistical Product and Service Solutions, SPSS 26.0, IBM; Photoshop CC, Adobe systems).
Ascites Volume Prediction System
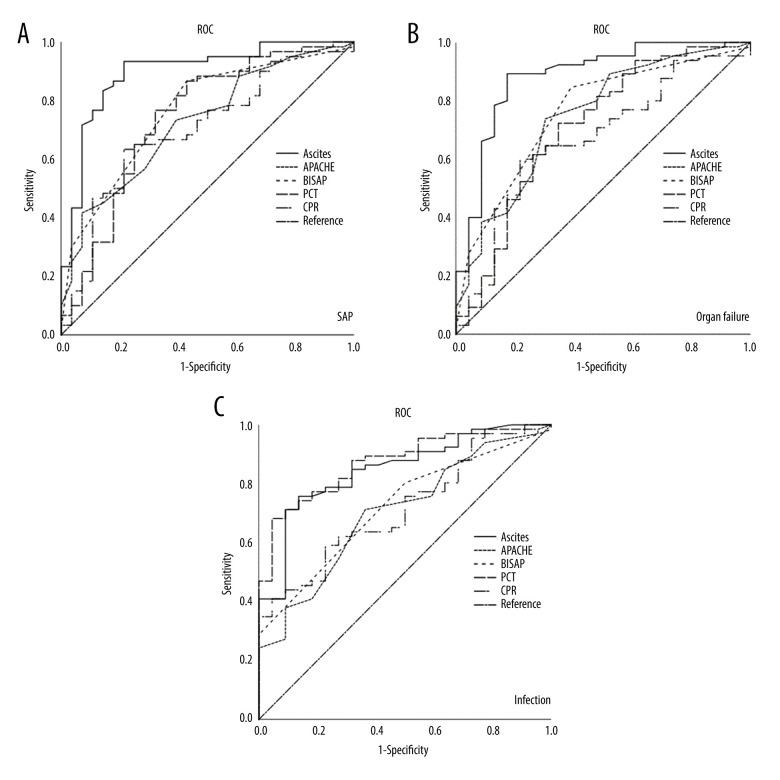
The occurrence of organ failure and infections was focused on to observe the severity of the disease and to predict the occurrence of SAP and death. ROC analysis of in-hospital mortality was not performed because the data collected included only 1 patient in the ascites group who died in the hospital. We performed ROC analysis of ascites volume to predict the predictive utility of organ failure, infection, and SAP. Because the collected data included 1 patient who died in the hospital, there was no ROC analysis of inpatient mortality. The best threshold for predicting SAP by ascites volume was 344 ml. Statistical analysis showed that there was a significant difference in each clinical result between the 2 groups with the ascites volume threshold as the dividing point (P<0.05) (Table 3). The AUC of ascites volume predicting organ failure was 0.883 (95% confidence interval: 0.795, 0.971), the AUC of predicting infection was 0.848 (95% confidence interval: 0.761, 0.935), and the AUC of predicting SAP was 0.896 (95% confidence interval: 0.821, 0.971) (Table 4).
Table 3.
Grading of ascites.
| Clinical outcome | Ascites | |
|---|---|---|
| ≥344, n=62 | <344, n=26 | |
| Hospital stay#,* | 35.4±23.6 | 16.5±12.1 |
| SAP# | 56 (90.3) | 4 (15.4) |
| Organ failure# | 58 (93.5) | 7 (26.9) |
| Infection# | 55 (88.7) | 11 (42.3) |
| Intervention# | 53 (85.5) | 6 (23.1) |
| CRRT# | 48 (77.4) | 12 (46.2) |
| SIRS# | 51 (82.3) | 11 (42.3) |
P is less than 0.05;
The data are the mean±standard deviation.
Table 4.
Ascites volume and AUC of SAP, organ failure, and infection predicted by different evaluation and prediction systems.
| Prediction system | SAP | Organ failure | Infection |
|---|---|---|---|
| Ascites volume | 0.896 (0.820, 0.972) | 0.883 (0.794, 0.972) | 0.848 (0.760, 0.935) |
| APACHE II | 0.725 (0.615, 0.835) | 0.747 (0.627, 0.867) | 0.703 (0.583, 0.822) |
| BISAP | 0.763 (0.668, 0.859) | 0.759 (0.654, 0.863) | 0.720 (0.621, 0.818) |
| CRP | 0.702 (0.584, 0.820) | 0.670 (0.540, 0.800) | 0.715 (0.601, 0.828) |
| PCT | 0.742 (0.622, 0.862) | 0.712 (0.578, 0.847) | 0.873 (0.798, 0.949) |
The confidence interval in brackets is 95%.
Ascites Volume Compared with Other Assessment Systems
Ascites volume showed the highest AUC in all scoring systems in predicting SAP and organ and tube failure (Figure 3, Table 4), with values of 0.896 and 0.883, respectively, but PCT level showed the highest AUC in predicting infection. When comparing the AUC of each evaluation system statistically, it was found that when predicting the occurrence of SAP, the ascites volume was significantly different from APACHE II, CRP level, and PCT level (P<0.05), but there was no significant difference between the ascites volume and BISAP score (P=0.051). There was no significant difference between BISAP, APACHE II, CRP level, and PCT level (P<0.05). When predicting organ failure, there was no significant difference between ascites volume and BISAP, APACHE II (P=0.106, P=0.074), but there was a significant difference between ascites volume and CRP level and PCT level (P<0.05), and there was no significant difference between BISAP, APACHE II, CRP level, and PCT level (P<0.05). There was a significant difference between PCT level and BISAP and APACHE II in predicting infection (P=0.012), but there was no significant difference between PCT level and ascites volume (P=0.660) (Table 5).
Figure 3.
(A–C) ROC curves of different evaluation and prediction systems (SPSS: Statistical Product and Service Solutions, SPSS 26.0, IBM; Photoshop CC, Adobe systems).
Table 5.
Paired comparison of AUCs.
| Prediction system | SAP | Organ failure | Infection | |||
|---|---|---|---|---|---|---|
| Z value | P value | Z value | P value | Z value | P value | |
| Ascites volume – APACHE II | 2.508 | 0.012 | 1.784 | 0.074 | 1.897 | 0.058 |
| Ascites volume – BISAP | 1.947 | 0.051 | 1.617 | 0.106 | 1.851 | 0.064 |
| Ascites volume – CRP | 2.983 | 0.003 | 2.881 | 0.004 | 1.971 | 0.049 |
| Ascites volume – PCT | 2.171 | 0.030 | 2.187 | 0.029 | 0.440 | 0.660 |
| APACHE II – BISAP | 0.630 | 0.529 | 0.175 | 0.861 | 0.282 | 0.778 |
| APACHE II – CRP | 0.255 | 0.799 | 0.782 | 0.434 | 0.126 | 0.900 |
| APACHE II – PCT | 0.235 | 0.814 | 0.445 | 0.657 | 2.516 | 0.012 |
| BISAP – CRP | 0.793 | 0.428 | 1.078 | 0.281 | 0.062 | 0.950 |
| BISAP – PCT | 0.289 | 0.773 | 0.601 | 0.548 | 2.505 | 0.012 |
| CRP – PCT | 0.486 | 0.627 | 0.461 | 0.645 | 2.594 | 0.009 |
Comparison of Prediction Effectiveness of Various Evaluation Systems for SAP
Thresholds for ROC curves and our usual thresholds were selected for predicting clinical outcomes, ascites volume ≥344 ml, BISAP score ≥2, APACHE II score ≥10 or ≥8, CRP level ≥115.015 or ≥150, and PCT level ≥0.428 (Table 6). The ascites volume showed the highest sensitivity of 93% (84%, 98%), APACHE II score ≥10 showed the highest specificity, positive predictive value, and positive likelihood ratio, and the specificity of CRP level ≥150mg/L was also higher than the ascites volume. However, when APACHE II score ≥10 and CRP level ≥150 mg/L were the threshold points, the sensitivity and specificity had the opposite trend, with APACHE II: 42% (29%, 55%) and 93% (77%, 99%), respectively; CRP ≥150 mg/L: 48% (35%, 62%) and 86% (67%, 96%) (Table 6). These thresholds will lead to too many false-negative cases. Compared with other evaluation systems, ascites volume has the best diagnostic advantage ratio of 51.3 (13.2, 199.6) for predicting SAP.
Table 6.
The main characteristics of different grading systems.
| Grading System | Sensitivity(%) | Specificity(%) | PPV | PLR | DOR |
|---|---|---|---|---|---|
| SAP | |||||
| Ascites volume ≥344 ml | 93 (84, 98) | 79 (59, 92) | 90 (80, 96) | 4.4 (2.1, 8.9) | 51.3 (13.2, 199.6) |
| BISAP ≥2 | 87 (75, 94) | 57 (37, 76) | 81 (70, 90) | 2.0 (1.3, 3.1) | 8.7 (3.0, 24.9) |
| APACHE II | |||||
| ≥8 | 57 (43, 69) | 71 (51, 87) | 81 (66, 91) | 2.0 (1.1, 3.7) | 3.3 (1.2, 8.6) |
| ≥10 | 42 (29, 55) | 93 (77, 99) | 93 (76, 99) | 5.8 (1.5, 22.9) | 9.3 (2.0, 42.8) |
| CRP level | |||||
| ≥150 mg/L | 48 (35, 62) | 86 (67, 96) | 88 (72, 97) | 3.4 (1.3, 8.7) | 5.6 (1.7, 18.1) |
| ≥115 mg/L | 63 (50, 75) | 79 (59, 92) | 86 (73, 95) | 3.0 () | 6.3 (2.2, 18.0) |
| PCT level | |||||
| ≥0.43ng/L | 77 (64, 87) | 68 (48, 84) | 84 (71, 92) | 2.4 (1.4, 4.2) | 6.9 (2.6, 18.7) |
The confidence interval in brackets is 95%. PPV – positive predictive value; PLR – positive likelihood ratio; DOR – diagnostic advantage ratio.
Discussion
To the best of our knowledge, this is the first study to examine the severity of acute pancreatitis using ascites volume. Our study strictly followed the criteria of a retrospective clinical study and showed realistic feasibility.
Factors contributing to poor AP outcomes include organ failure, infection, persistent SIRS [28], and intra-abdominal hypertension, and ascites is an important contributor to these factors [29]. AP ascites originates from many factors, including: (1) Inflammatory exudation, inflammation in the AP, especially peritoneal inflammation, leading to increased vascular permeability and the formation of a “capillary leak” phenomenon [30]; (2) Ductal disruption, where the ducts associated with pancreatic ascites are disrupted, causing pancreatic secretions to leak into the abdominal cavity and accumulate in the peritoneal cavity [31]; (3) Chylous ascites, such as high triglycerides can cause obstruction of lymphatic flow, leading to dilated and leaking subplasmic lymphatic vessels[32]; (4) Fluid leakage, such as acute portal or mesenteric vein thrombosis due to pancreatitis, resulting in portal hypertension and the formation of leaky ascites [33]; (5) Consumptive ascites [34].
AP ascites contains many toxic substances, such as cytokines, amylase, and lipid plum [35], which not only directly induce cellular tissue necrosis, but also interfere with anti-inflammatory pathways, promote the deterioration of macrophage activation in pancreatitis, aggravate the inflammatory response [17], and interfere with apoptosis-related proteins and signaling pathways, thus aggravating the condition of AP [36]. Many studies have shown that reducing the volume of AP ascites can reduce the inflammatory response, reduce the occurrence of further interventions or multi-organ dysfunction [37,38], reduce damage to the intestinal mucosal barrier, and also reduce costs and length of hospital stay [39], and even reduce all-cause mortality [40,41].
The assessment of the clinical status of AP patients currently relies more on symptoms and serological indicators. PCT levels and CRP levels are the most widely used single serological indicators to predict AP severity [8–11]. However, our study found that CRP levels had the smallest AUC in predicting SAP, organ failure, and infection, whereas PCT levels had a relative advantage in predicting infection only, and their AUC in predicting SAP and organ failure was also significantly smaller than that of ascites volume. For scoring systems such as BISAP and APACHE II, which are involved in current clinical work, the AUC area for predicting SAP and organ failure and infection in this study was also less than the ascites volume.
Although this study confirms the usefulness of ascites volume for grading the severity of early acute pancreatitis, there are still several limitations in the study. First, because some patients had only plain CT scans without enhanced CT sweeps, the comparison of CT scores for acute pancreatitis was not addressed in this study, but this did not affect the results and conclusions related to ascites volume and had no impact on the results of this study. Second, the characteristics of ascites routine and ascites biochemical indicators were not further explored in this study. In addition, issues related to the selection of the mode and timing of AP ascites intervention and the application of antibiotics during the intervention remain to be resolved.
Conclusions
Our findings suggest that patients with ascites are more severely ill and have a worse prognosis compared to those without ascites, and the volume of ascites was positively correlated with the severity of AP. Ascites volume predicts the onset of SAP, organ failure, and infection, and offers a higher diagnostic advantage ratio over current scoring systems.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: Natural Science Foundation of China (no. 81870440)
References
- 1.Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1(1):45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 2.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(8):479–96. doi: 10.1038/s41575-019-0158-2. [DOI] [PubMed] [Google Scholar]
- 3.Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis. Lancet. 2020;396(10252):726–34. doi: 10.1016/S0140-6736(20)31310-6. [DOI] [PubMed] [Google Scholar]
- 4.Szatmary P, Grammatikopoulos T, Cai W, et al. Acute pancreatitis: Diagnosis and treatment. Drugs. 2022;82(12):1251–76. doi: 10.1007/s40265-022-01766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trikudanathan G, Wolbrink DRJ, van Santvoort HC, et al. Current concepts in severe acute and necrotizing pancreatitis: An evidence-based approach. Gastroenterology. 2019;156(7):1994–2007. doi: 10.1053/j.gastro.2019.01.269. [DOI] [PubMed] [Google Scholar]
- 6.Gunjaca I, Zunic J, Gunjaca M, et al. Circulating cytokine levels in acute pancreatitis – model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation. 2012;35(2):758–63. doi: 10.1007/s10753-011-9371-z. [DOI] [PubMed] [Google Scholar]
- 7.Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: A large population-based study. Gut. 2008;57(12):1698. doi: 10.1136/gut.2008.152702. [DOI] [PubMed] [Google Scholar]
- 8.Meher S, Mishra TS, Sasmal PK, et al. Role of biomarkers in diagnosis and prognostic evaluation of acute pancreatitis. J Biomark. 2015;2015:519534. doi: 10.1155/2015/519534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leppäniemi A, Tolonen M, Tarasconi A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. doi: 10.1186/s13017-019-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Chen Z, Li L, et al. Interleukin-6 is better than C-reactive protein for the prediction of infected pancreatic necrosis and mortality in patients with acute pancreatitis. Front Cell Infect Microbiol. 2022;12:933221. doi: 10.3389/fcimb.2022.933221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staubli SM, Oertli D, Nebiker CA. Laboratory markers predicting severity of acute pancreatitis. Crit Rev Clin Lab Sci. 2015;52(6):273–83. doi: 10.3109/10408363.2015.1051659. [DOI] [PubMed] [Google Scholar]
- 12.Arif A, Jaleel F, Rashid K. Accuracy of BISAP score in prediction of severe acute pancreatitis. Pak J Med Sci. 2019;35(4):1008–12. doi: 10.12669/pjms.35.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagjer S, Kumar N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis – a prospective observational study. Int J Surg. 2018;54(Pt A):76–81. doi: 10.1016/j.ijsu.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Harshit Kumar A, Singh Griwan M. A comparison of APACHE II, BISAP, Ranson’s score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol Rep (Oxf) 2018;6(2):127–31. doi: 10.1093/gastro/gox029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samanta J, Rana A, Dhaka N, et al. Ascites in acute pancreatitis: Not a silent bystander. Pancreatology. 2019;19(5):646–52. doi: 10.1016/j.pan.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Bush N, Rana SS. Ascites in Acute Pancreatitis: Clinical Implications and Management. Dig Dis Sci. 2022;67(6):1987–93. doi: 10.1007/s10620-021-07063-6. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez PT, Folch-Puy E, Bulbena O, et al. Oxidised lipids present in ascitic fluid interfere with the regulation of the macrophages during acute pancreatitis, promoting an exacerbation of the inflammatory response. Gut. 2008;57(5):642–48. doi: 10.1136/gut.2007.127472. [DOI] [PubMed] [Google Scholar]
- 18.Singh AK, Samanta J, Dawra S, et al. Reduction of intra-abdominal pressure after percutaneous catheter drainage of pancreatic fluid collection predicts survival. Pancreatology. 2020;20(4):772–77. doi: 10.1016/j.pan.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis – 2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 20.Kerschbaum M, Schurr LA, Riedl M, et al. Clinical value of CT for differentiation between ascites and hemorrhage: An experimental in-vitro study. J Clin Med. 2020;10(1):76. doi: 10.3390/jcm10010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bydder GM, Kreel L. Attenuation values of fluid collections within the abdomen. J Comput Assist Tomogr. 1980;4(2):145–50. doi: 10.1097/00004728-198004000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Rong Y, Smilowitz J, Tewatia D, et al. Dose calculation on kV cone beam CT images: An investigation of the Hu-density conversion stability and dose accuracy using the site-specific calibration. Med Dosim. Autumn. 2010;35(3):195–207. doi: 10.1016/j.meddos.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Parmar C, Rios Velazquez E, Leijenaar R, et al. Robust Radiomics feature quantification using semiautomatic volumetric segmentation. PLoS One. 2014;9(7):e102107. doi: 10.1371/journal.pone.0102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–52. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Meyrignac O, Lagarde S, Bournet B, et al. Acute pancreatitis: Extrapancreatic necrosis volume as early predictor of severity. Radiology. 2015;276(1):119–28. doi: 10.1148/radiol.15141494. [DOI] [PubMed] [Google Scholar]
- 26.Mofidi R, Duff MD, Wigmore SJ, et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93(6):738–44. doi: 10.1002/bjs.5290. [DOI] [PubMed] [Google Scholar]
- 27.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 28.Gardner TB. Acute pancreatitis. Ann Intern Med. 2021;174(2):ITC17–ITC32. doi: 10.7326/AITC202102160. [DOI] [PubMed] [Google Scholar]
- 29.Wen Y, Zhuo WQ, Liang HY, et al. Abdominal paracentesis drainage improves outcome of acute pancreatitis complicated with intra-abdominal hypertension in early phase. Am J Med Sci. 2023;365(1):48–55. doi: 10.1016/j.amjms.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Gukovskaya AS, Gukovsky I, Algül H, et al. Autophagy, inflammation, and immune dysfunction in the pathogenesis of pancreatitis. Gastroenterology. 2017;153(5):1212–26. doi: 10.1053/j.gastro.2017.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jagielski M, Jackowski M. The role of endoscopic transpapillary stenting of the main pancreatic duct during the endoscopic treatment of pancreatic fluid collections. J Clin Med. 2021;10(4):761. doi: 10.3390/jcm10040761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gómez-Martín JM, Martínez-Molina E, Sanjuanbenito A, et al. Chylous ascytes secondary to acute pancreatitis: A case report and review of literature. Nutr Hosp. 2012;27(1):314–18. doi: 10.1590/S0212-16112012000100044. [DOI] [PubMed] [Google Scholar]
- 33.Anis FS, Adiamah A, Lobo DN, et al. Incidence and treatment of splanchnic vein thrombosis in patients with acute pancreatitis: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37(3):446–54. doi: 10.1111/jgh.15711. [DOI] [PubMed] [Google Scholar]
- 34.Ocskay K, Vinkó Z, Németh D, et al. Hypoalbuminemia affects one third of acute pancreatitis patients and is independently associated with severity and mortality. Sci Rep. 2021;11(1):24158. doi: 10.1038/s41598-021-03449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramudo L, Manso MA, De Dios I. Biliary pancreatitis-associated ascitic fluid activates the production of tumor necrosis factor-alpha in acinar cells. Crit Care Med. 2005;33(1):143–48. doi: 10.1097/01.ccm.0000150654.13653.5b. discussion 248. [DOI] [PubMed] [Google Scholar]
- 36.Luo C, Huang Q, Yuan X, et al. Abdominal paracentesis drainage attenuates severe acute pancreatitis by enhancing cell apoptosis via PI3K/AKT signaling pathway. Apoptosis. 2020;25(3–4):290–303. doi: 10.1007/s10495-020-01597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu WH, Ren LN, Chen T, et al. Abdominal paracentesis drainage ahead of percutaneous catheter drainage benefits patients attacked by acute pancreatitis with fluid collections: A retrospective clinical cohort study. Crit Care Med. Jan. 2015;43(1):109–19. doi: 10.1097/CCM.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 38.Fujita M, Masamune A, Satoh A, et al. Ascites of rat experimental model of severe acute pancreatitis induces lung injury. Pancreas. May. 2001;22(4):409–18. doi: 10.1097/00006676-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Yuan X, Luo C, Wu J, et al. Abdominal paracentesis drainage attenuates intestinal mucosal barrier damage through macrophage polarization in severe acute pancreatitis. Exp Biol Med (Maywood) 2021;246(18):2029–38. doi: 10.1177/15353702211015144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Liu LY, Luo H, et al. Intra-abdominal pressure reduction after percutaneous catheter drainage is a protective factor for severe pancreatitis patients with sterile fluid collections. Pancreas. 2016;45(1):127–33. doi: 10.1097/MPA.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 41.Lu Z, Zhu X, Hua T, et al. Efficacy and safety of abdominal paracentesis drainage on patients with acute pancreatitis: A systematic review and meta-analysis. BMJ Open. 2021;11(8):e045031. doi: 10.1136/bmjopen-2020-045031. [DOI] [PMC free article] [PubMed] [Google Scholar]